

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Receptor tyrosine-protein kinase erbB-2 (Rattus norvegicus) | BDBM50099960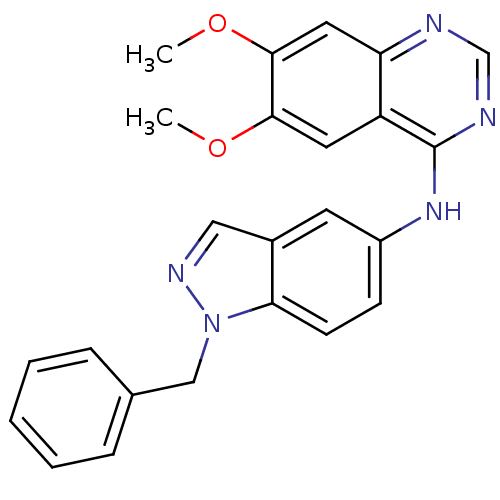 ((1-Benzyl-1H-indazol-5-yl)-(6,7-dimethoxy-quinazol...) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Research Biomet. Glaxo Wellcome Medicines Research Centre Curated by ChEMBL | Assay Description Inhibition of rat Receptor protein-tyrosine kinase erbB2 | Bioorg Med Chem Lett 11: 1401-5 (2001) BindingDB Entry DOI: 10.7270/Q2W37VKB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50099963 (CHEMBL30432 | N*4*-(1-Benzyl-1H-indazol-5-yl)-N*6*...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Research Biomet. Glaxo Wellcome Medicines Research Centre Curated by ChEMBL | Assay Description Inhibition of EGF receptor | Bioorg Med Chem Lett 11: 1401-5 (2001) BindingDB Entry DOI: 10.7270/Q2W37VKB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hematopoietic prostaglandin D synthase (Homo sapiens (Human)) | BDBM50526494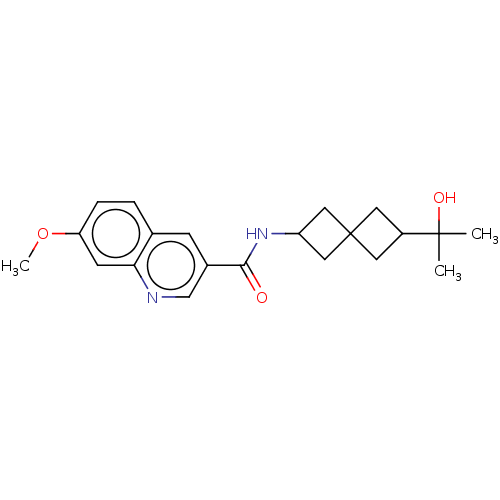 (CHEMBL4439454) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8.20 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of full-length human His6-tagged HPGDS expressed in Escherichia coli BL21 (DE3) using PGH2 as substrate measured after 90 to 120 secs by R... | Bioorg Med Chem 27: 1456-1478 (2019) Article DOI: 10.1016/j.bmc.2019.02.017 BindingDB Entry DOI: 10.7270/Q2QV3R00 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hematopoietic prostaglandin D synthase (Homo sapiens (Human)) | BDBM50526495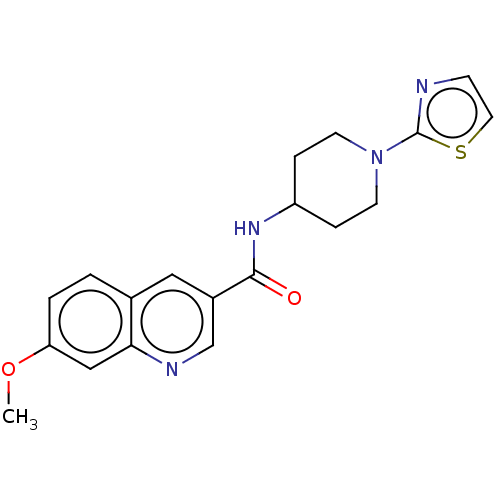 (CHEMBL4452633) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9.70 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of full-length human His6-tagged HPGDS expressed in Escherichia coli BL21 (DE3) using PGH2 as substrate measured after 90 to 120 secs by R... | Bioorg Med Chem 27: 1456-1478 (2019) Article DOI: 10.1016/j.bmc.2019.02.017 BindingDB Entry DOI: 10.7270/Q2QV3R00 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hematopoietic prostaglandin D synthase (Homo sapiens (Human)) | BDBM50526485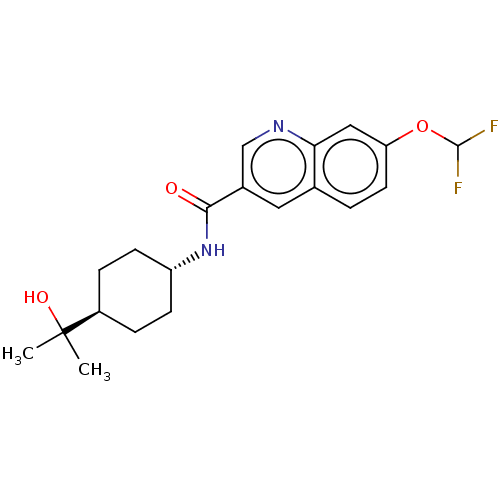 (CHEMBL4473072) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 9.90 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of full-length human His6-tagged HPGDS expressed in Escherichia coli BL21 (DE3) using PGH2 as substrate measured after 90 to 120 secs by R... | Bioorg Med Chem 27: 1456-1478 (2019) Article DOI: 10.1016/j.bmc.2019.02.017 BindingDB Entry DOI: 10.7270/Q2QV3R00 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Receptor tyrosine-protein kinase erbB-2 (Rattus norvegicus) | BDBM50099969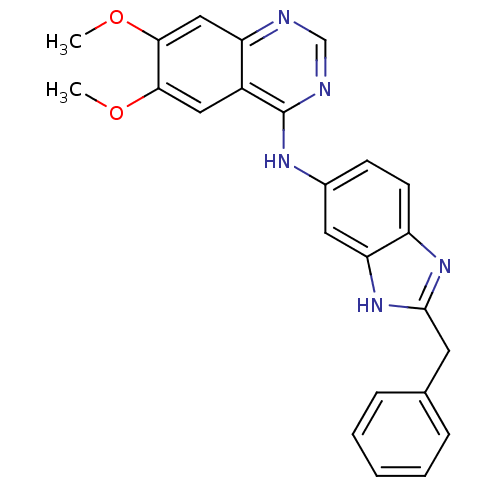 ((2-Benzyl-1H-benzoimidazol-5-yl)-(6,7-dimethoxy-qu...) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Research Biomet. Glaxo Wellcome Medicines Research Centre Curated by ChEMBL | Assay Description Inhibition of rat Receptor protein-tyrosine kinase erbB2 | Bioorg Med Chem Lett 11: 1401-5 (2001) BindingDB Entry DOI: 10.7270/Q2W37VKB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor tyrosine-protein kinase erbB-2 (Homo sapiens (Human)) | BDBM5459 (6-alkoxy-4-anilinoquinazoline 8e | N-{3-chloro-4-[...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | 7.5 | 23 |
GlaxoSmithKline | Assay Description Enzymatic reactions were initiated by adding kinase to the reaction mixture containing ATP, [gamma-33P] ATP, peptide substrate and test inhibitor com... | Bioorg Med Chem Lett 13: 637-40 (2003) Article DOI: 10.1016/s0960-894x(02)01047-8 BindingDB Entry DOI: 10.7270/Q2D21VS2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor tyrosine-protein kinase erbB-2 (Rattus norvegicus) | BDBM50099965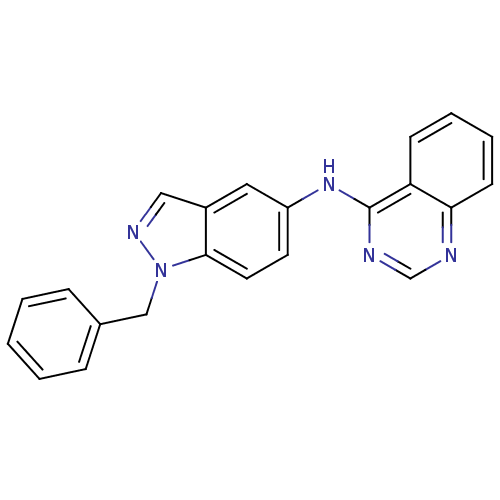 ((1-Benzyl-1H-indazol-5-yl)-quinazolin-4-yl-amine |...) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Research Biomet. Glaxo Wellcome Medicines Research Centre Curated by ChEMBL | Assay Description Inhibition of rat Receptor protein-tyrosine kinase erbB2 | Bioorg Med Chem Lett 11: 1401-5 (2001) BindingDB Entry DOI: 10.7270/Q2W37VKB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM5456 (6-alkoxy-4-anilinoquinazoline 8b | N-(3-ethynylphe...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | 7.5 | 23 |
GlaxoSmithKline | Assay Description Enzymatic reactions were initiated by adding kinase to the reaction mixture containing ATP, [gamma-33P] ATP, peptide substrate and test inhibitor com... | Bioorg Med Chem Lett 13: 637-40 (2003) Article DOI: 10.1016/s0960-894x(02)01047-8 BindingDB Entry DOI: 10.7270/Q2D21VS2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hematopoietic prostaglandin D synthase (Homo sapiens (Human)) | BDBM50526492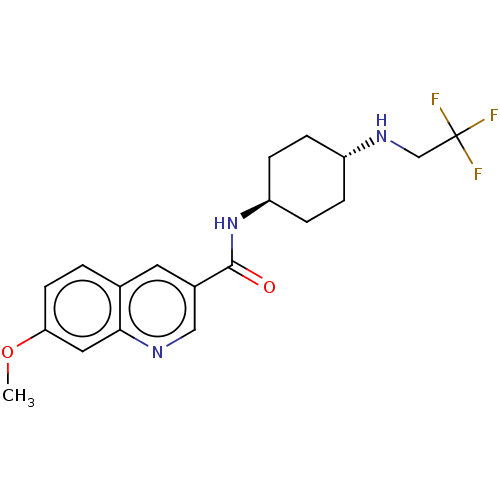 (CHEMBL4454901) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of full-length human His6-tagged HPGDS expressed in Escherichia coli BL21 (DE3) using PGH2 as substrate measured after 90 to 120 secs by R... | Bioorg Med Chem 27: 1456-1478 (2019) Article DOI: 10.1016/j.bmc.2019.02.017 BindingDB Entry DOI: 10.7270/Q2QV3R00 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hematopoietic prostaglandin D synthase (Homo sapiens (Human)) | BDBM50526486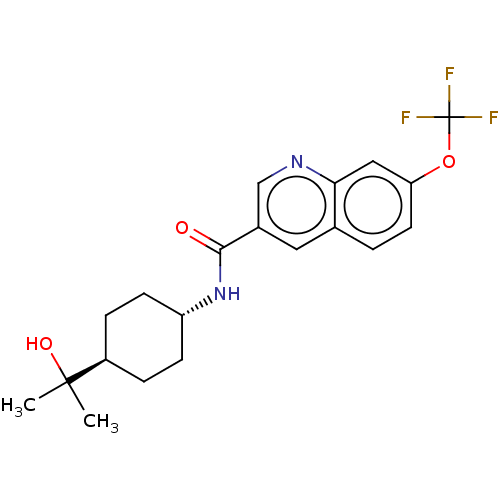 (CHEMBL4526974) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of full-length human His6-tagged HPGDS expressed in Escherichia coli BL21 (DE3) using PGH2 as substrate measured after 90 to 120 secs by R... | Bioorg Med Chem 27: 1456-1478 (2019) Article DOI: 10.1016/j.bmc.2019.02.017 BindingDB Entry DOI: 10.7270/Q2QV3R00 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hematopoietic prostaglandin D synthase (Homo sapiens (Human)) | BDBM50526498 (CHEMBL4530713) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of full-length human His6-tagged HPGDS expressed in Escherichia coli BL21 (DE3) using PGH2 as substrate measured after 90 to 120 secs by R... | Bioorg Med Chem 27: 1456-1478 (2019) Article DOI: 10.1016/j.bmc.2019.02.017 BindingDB Entry DOI: 10.7270/Q2QV3R00 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hematopoietic prostaglandin D synthase (Homo sapiens (Human)) | BDBM50526514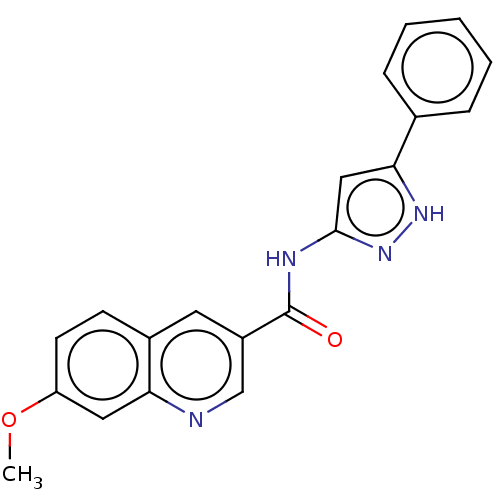 (CHEMBL4443093) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of full-length human His6-tagged HPGDS expressed in Escherichia coli BL21 (DE3) using PGH2 as substrate measured after 90 to 120 secs by R... | Bioorg Med Chem 27: 1456-1478 (2019) Article DOI: 10.1016/j.bmc.2019.02.017 BindingDB Entry DOI: 10.7270/Q2QV3R00 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor tyrosine-protein kinase erbB-2 (Homo sapiens (Human)) | BDBM5460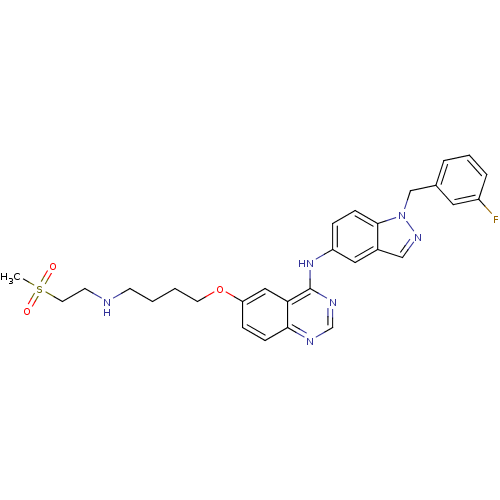 (6-alkoxy-4-anilinoquinazoline 8f | N-{1-[(3-fluoro...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | 7.5 | 23 |
GlaxoSmithKline | Assay Description Enzymatic reactions were initiated by adding kinase to the reaction mixture containing ATP, [gamma-33P] ATP, peptide substrate and test inhibitor com... | Bioorg Med Chem Lett 13: 637-40 (2003) Article DOI: 10.1016/s0960-894x(02)01047-8 BindingDB Entry DOI: 10.7270/Q2D21VS2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM5457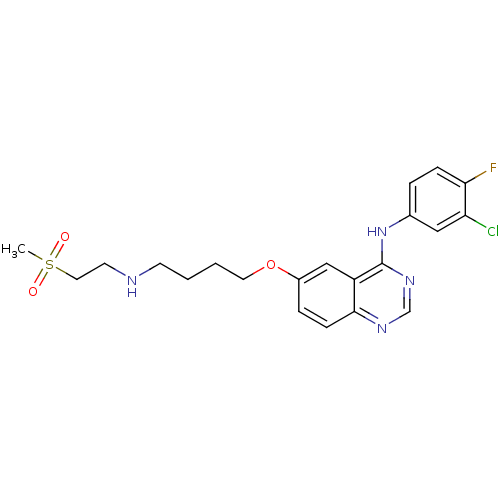 (6-alkoxy-4-anilinoquinazoline 8c | N-(3-chloro-4-f...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | 7.5 | 23 |
GlaxoSmithKline | Assay Description Enzymatic reactions were initiated by adding kinase to the reaction mixture containing ATP, [gamma-33P] ATP, peptide substrate and test inhibitor com... | Bioorg Med Chem Lett 13: 637-40 (2003) Article DOI: 10.1016/s0960-894x(02)01047-8 BindingDB Entry DOI: 10.7270/Q2D21VS2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hematopoietic prostaglandin D synthase (Homo sapiens (Human)) | BDBM50526487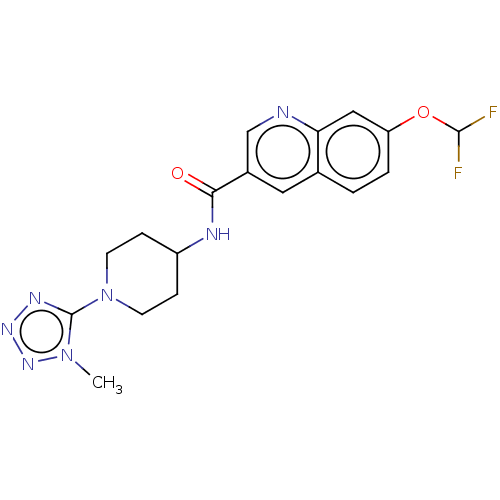 (CHEMBL4458862) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of full-length human His6-tagged HPGDS expressed in Escherichia coli BL21 (DE3) using PGH2 as substrate measured after 90 to 120 secs by R... | Bioorg Med Chem 27: 1456-1478 (2019) Article DOI: 10.1016/j.bmc.2019.02.017 BindingDB Entry DOI: 10.7270/Q2QV3R00 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor tyrosine-protein kinase erbB-4 (Homo sapiens (Human)) | BDBM50099963 (CHEMBL30432 | N*4*-(1-Benzyl-1H-indazol-5-yl)-N*6*...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Research Biomet. Glaxo Wellcome Medicines Research Centre Curated by ChEMBL | Assay Description Inhibition of erbB4 receptor | Bioorg Med Chem Lett 11: 1401-5 (2001) BindingDB Entry DOI: 10.7270/Q2W37VKB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hematopoietic prostaglandin D synthase (Homo sapiens (Human)) | BDBM50526528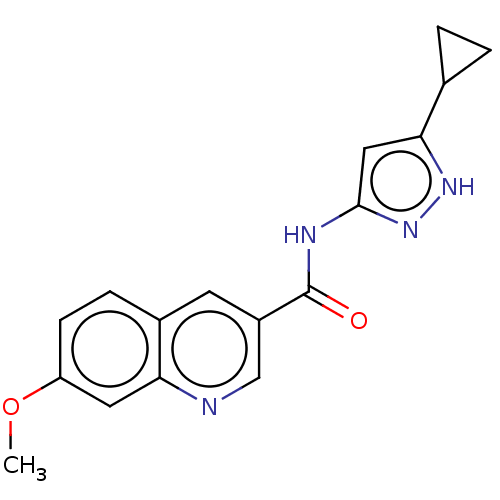 (CHEMBL4467696) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of full-length human His6-tagged HPGDS expressed in Escherichia coli BL21 (DE3) using PGH2 as substrate measured after 90 to 120 secs by R... | Bioorg Med Chem 27: 1456-1478 (2019) Article DOI: 10.1016/j.bmc.2019.02.017 BindingDB Entry DOI: 10.7270/Q2QV3R00 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM5460 (6-alkoxy-4-anilinoquinazoline 8f | N-{1-[(3-fluoro...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 24 | n/a | n/a | n/a | n/a | 7.5 | 23 |
GlaxoSmithKline | Assay Description Enzymatic reactions were initiated by adding kinase to the reaction mixture containing ATP, [gamma-33P] ATP, peptide substrate and test inhibitor com... | Bioorg Med Chem Lett 13: 637-40 (2003) Article DOI: 10.1016/s0960-894x(02)01047-8 BindingDB Entry DOI: 10.7270/Q2D21VS2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hematopoietic prostaglandin D synthase (Homo sapiens (Human)) | BDBM50526529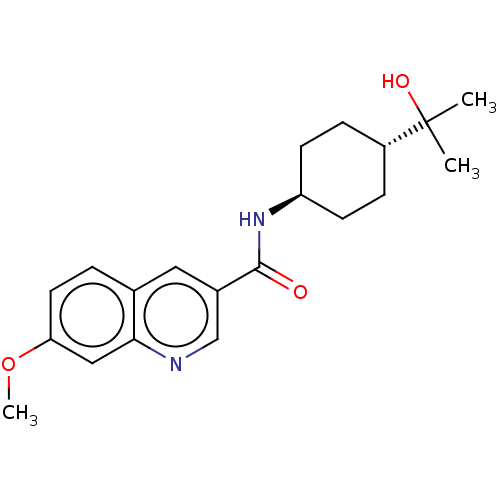 (CHEMBL4483631) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of full-length human His6-tagged HPGDS expressed in Escherichia coli BL21 (DE3) using PGH2 as substrate measured after 90 to 120 secs by R... | Bioorg Med Chem 27: 1456-1478 (2019) Article DOI: 10.1016/j.bmc.2019.02.017 BindingDB Entry DOI: 10.7270/Q2QV3R00 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor tyrosine-protein kinase erbB-2 (Rattus norvegicus) | BDBM50099971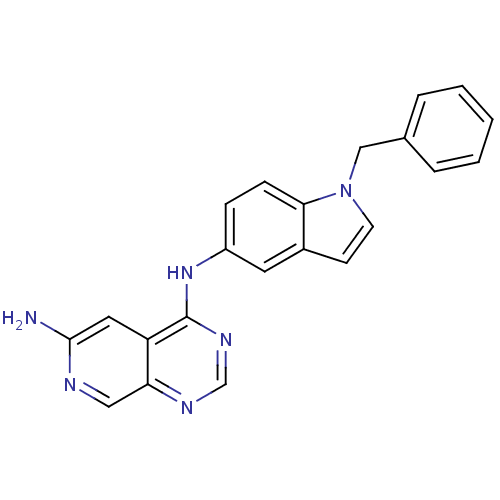 (CHEMBL288557 | N*4*-(1-Benzyl-1H-indol-5-yl)-pyrid...) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Research Biomet. Glaxo Wellcome Medicines Research Centre Curated by ChEMBL | Assay Description Inhibition of rat Receptor protein-tyrosine kinase erbB2 | Bioorg Med Chem Lett 11: 1401-5 (2001) BindingDB Entry DOI: 10.7270/Q2W37VKB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hematopoietic prostaglandin D synthase (Homo sapiens (Human)) | BDBM50526493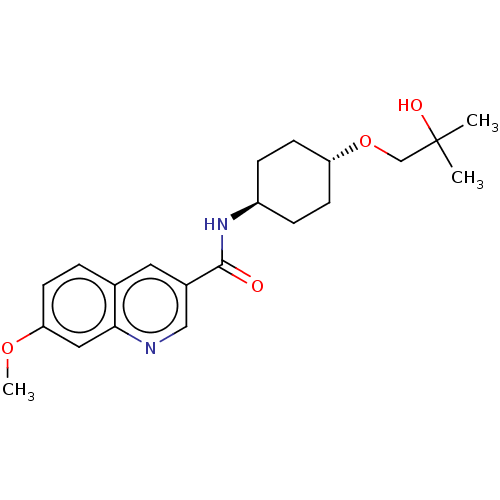 (CHEMBL4463046) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of full-length human His6-tagged HPGDS expressed in Escherichia coli BL21 (DE3) using PGH2 as substrate measured after 90 to 120 secs by R... | Bioorg Med Chem 27: 1456-1478 (2019) Article DOI: 10.1016/j.bmc.2019.02.017 BindingDB Entry DOI: 10.7270/Q2QV3R00 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM5459 (6-alkoxy-4-anilinoquinazoline 8e | N-{3-chloro-4-[...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 27 | n/a | n/a | n/a | n/a | 7.5 | 23 |
GlaxoSmithKline | Assay Description Enzymatic reactions were initiated by adding kinase to the reaction mixture containing ATP, [gamma-33P] ATP, peptide substrate and test inhibitor com... | Bioorg Med Chem Lett 13: 637-40 (2003) Article DOI: 10.1016/s0960-894x(02)01047-8 BindingDB Entry DOI: 10.7270/Q2D21VS2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor tyrosine-protein kinase erbB-2 (Rattus norvegicus) | BDBM50099966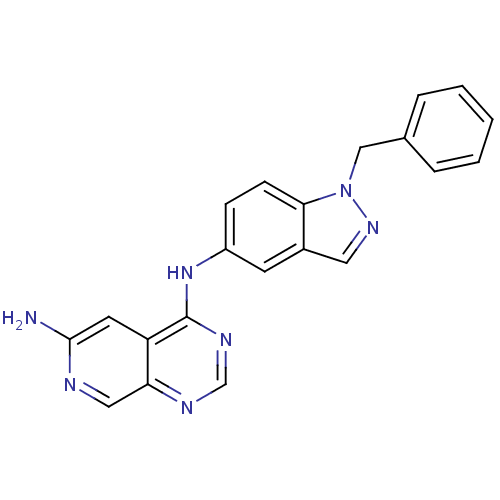 (CHEMBL281006 | N*4*-(1-Benzyl-1H-indazol-5-yl)-pyr...) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Research Biomet. Glaxo Wellcome Medicines Research Centre Curated by ChEMBL | Assay Description Inhibition of rat Receptor protein-tyrosine kinase erbB2 | Bioorg Med Chem Lett 11: 1401-5 (2001) BindingDB Entry DOI: 10.7270/Q2W37VKB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor tyrosine-protein kinase erbB-2 (Rattus norvegicus) | BDBM50099970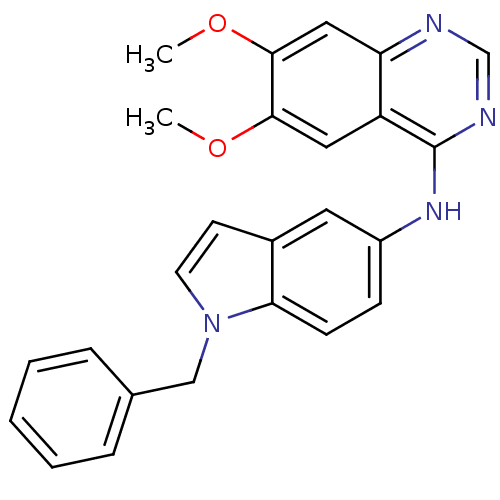 ((1-Benzyl-1H-indol-5-yl)-(6,7-dimethoxy-quinazolin...) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | n/a | n/a | 49 | n/a | n/a | n/a | n/a | n/a | n/a |
Research Biomet. Glaxo Wellcome Medicines Research Centre Curated by ChEMBL | Assay Description Inhibition of rat Receptor protein-tyrosine kinase erbB2 | Bioorg Med Chem Lett 11: 1401-5 (2001) BindingDB Entry DOI: 10.7270/Q2W37VKB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor tyrosine-protein kinase erbB-2 (Rattus norvegicus) | BDBM50099968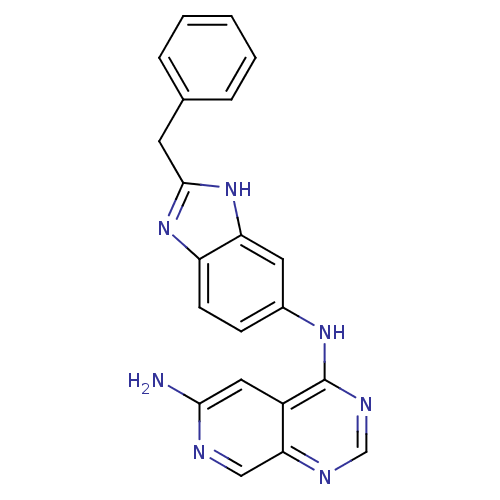 (CHEMBL31653 | N*4*-(2-Benzyl-1H-benzoimidazol-5-yl...) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 49 | n/a | n/a | n/a | n/a | n/a | n/a |
Research Biomet. Glaxo Wellcome Medicines Research Centre Curated by ChEMBL | Assay Description Inhibition of rat Receptor protein-tyrosine kinase erbB2 | Bioorg Med Chem Lett 11: 1401-5 (2001) BindingDB Entry DOI: 10.7270/Q2W37VKB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hematopoietic prostaglandin D synthase (Homo sapiens (Human)) | BDBM50526480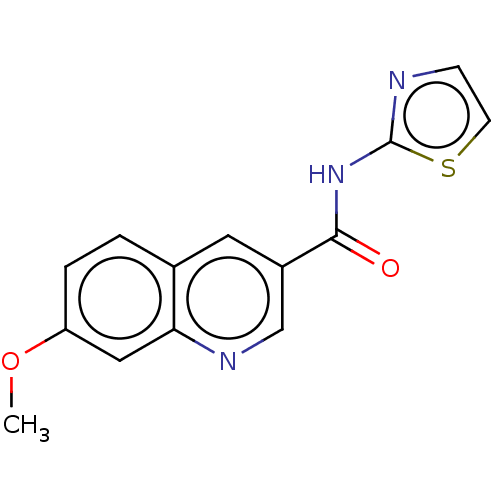 (CHEMBL4454083) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 49 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of full-length human His6-tagged HPGDS expressed in Escherichia coli BL21 (DE3) using PGH2 as substrate measured after 90 to 120 secs by R... | Bioorg Med Chem 27: 1456-1478 (2019) Article DOI: 10.1016/j.bmc.2019.02.017 BindingDB Entry DOI: 10.7270/Q2QV3R00 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hematopoietic prostaglandin D synthase (Homo sapiens (Human)) | BDBM50526488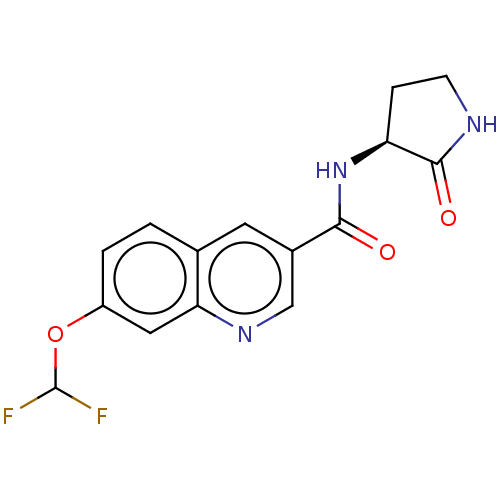 (CHEMBL4441669) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 52 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of full-length human His6-tagged HPGDS expressed in Escherichia coli BL21 (DE3) using PGH2 as substrate measured after 90 to 120 secs by R... | Bioorg Med Chem 27: 1456-1478 (2019) Article DOI: 10.1016/j.bmc.2019.02.017 BindingDB Entry DOI: 10.7270/Q2QV3R00 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hematopoietic prostaglandin D synthase (Homo sapiens (Human)) | BDBM50526527 (CHEMBL4483661) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 56 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of full-length human His6-tagged HPGDS expressed in Escherichia coli BL21 (DE3) using PGH2 as substrate measured after 90 to 120 secs by R... | Bioorg Med Chem 27: 1456-1478 (2019) Article DOI: 10.1016/j.bmc.2019.02.017 BindingDB Entry DOI: 10.7270/Q2QV3R00 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hematopoietic prostaglandin D synthase (Homo sapiens (Human)) | BDBM50526483 (CHEMBL4435792) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 57 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of full-length human His6-tagged HPGDS expressed in Escherichia coli BL21 (DE3) using PGH2 as substrate measured after 90 to 120 secs by R... | Bioorg Med Chem 27: 1456-1478 (2019) Article DOI: 10.1016/j.bmc.2019.02.017 BindingDB Entry DOI: 10.7270/Q2QV3R00 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hematopoietic prostaglandin D synthase (Homo sapiens (Human)) | BDBM50526517 (CHEMBL4564218) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 57 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of full-length human His6-tagged HPGDS expressed in Escherichia coli BL21 (DE3) using PGH2 as substrate measured after 90 to 120 secs by R... | Bioorg Med Chem 27: 1456-1478 (2019) Article DOI: 10.1016/j.bmc.2019.02.017 BindingDB Entry DOI: 10.7270/Q2QV3R00 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM5451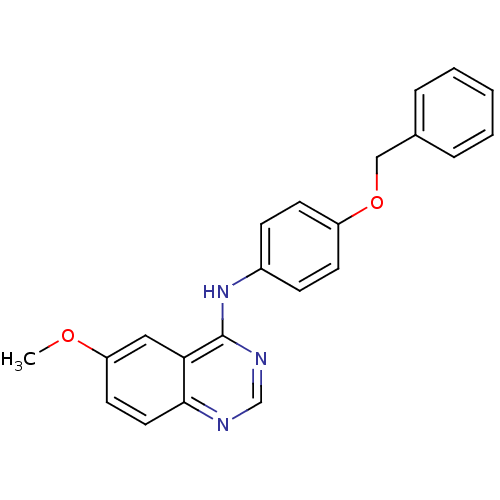 (6-alkoxy-4-anilinoquinazoline 9a | N-[4-(benzyloxy...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 68 | n/a | n/a | n/a | n/a | 7.5 | 23 |
GlaxoSmithKline | Assay Description Enzymatic reactions were initiated by adding kinase to the reaction mixture containing ATP, [gamma-33P] ATP, peptide substrate and test inhibitor com... | Bioorg Med Chem Lett 13: 637-40 (2003) Article DOI: 10.1016/s0960-894x(02)01047-8 BindingDB Entry DOI: 10.7270/Q2D21VS2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor tyrosine-protein kinase erbB-2 (Homo sapiens (Human)) | BDBM50099963 (CHEMBL30432 | N*4*-(1-Benzyl-1H-indazol-5-yl)-N*6*...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
Research Biomet. Glaxo Wellcome Medicines Research Centre Curated by ChEMBL | Assay Description Inhibition of erbB2 overexpressing BT 474 cell proliferation | Bioorg Med Chem Lett 11: 1401-5 (2001) BindingDB Entry DOI: 10.7270/Q2W37VKB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hematopoietic prostaglandin D synthase (Homo sapiens (Human)) | BDBM50526534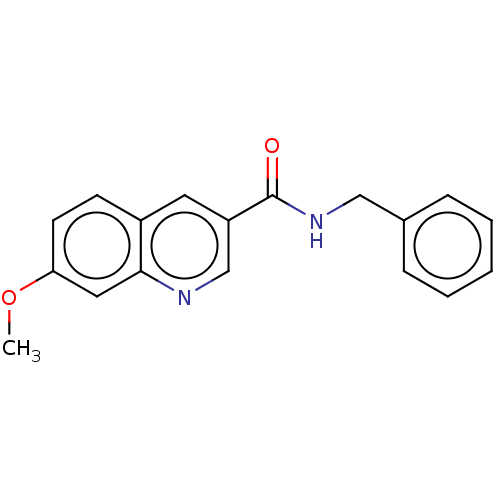 (CHEMBL4544169) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 71 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of full-length human His6-tagged HPGDS expressed in Escherichia coli BL21 (DE3) using PGH2 as substrate measured after 90 to 120 secs by R... | Bioorg Med Chem 27: 1456-1478 (2019) Article DOI: 10.1016/j.bmc.2019.02.017 BindingDB Entry DOI: 10.7270/Q2QV3R00 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hematopoietic prostaglandin D synthase (Homo sapiens (Human)) | BDBM50526515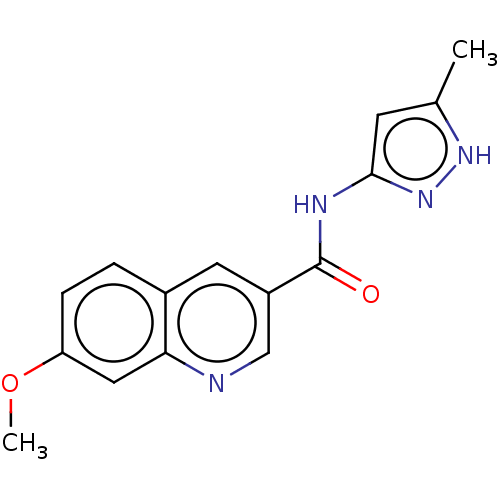 (CHEMBL4476572) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 73 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of full-length human His6-tagged HPGDS expressed in Escherichia coli BL21 (DE3) using PGH2 as substrate measured after 90 to 120 secs by R... | Bioorg Med Chem 27: 1456-1478 (2019) Article DOI: 10.1016/j.bmc.2019.02.017 BindingDB Entry DOI: 10.7270/Q2QV3R00 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM5455 (6-alkoxy-4-anilinoquinazoline 14a | N-{4-[(4-{[4-(...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 74 | n/a | n/a | n/a | n/a | 7.5 | 23 |
GlaxoSmithKline | Assay Description Enzymatic reactions were initiated by adding kinase to the reaction mixture containing ATP, [gamma-33P] ATP, peptide substrate and test inhibitor com... | Bioorg Med Chem Lett 13: 637-40 (2003) Article DOI: 10.1016/s0960-894x(02)01047-8 BindingDB Entry DOI: 10.7270/Q2D21VS2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM5450 (6-alkoxy-4-anilinoquinazoline 8a | N-[4-(benzyloxy...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 74 | n/a | n/a | n/a | n/a | 7.5 | 23 |
GlaxoSmithKline | Assay Description Enzymatic reactions were initiated by adding kinase to the reaction mixture containing ATP, [gamma-33P] ATP, peptide substrate and test inhibitor com... | Bioorg Med Chem Lett 13: 637-40 (2003) Article DOI: 10.1016/s0960-894x(02)01047-8 BindingDB Entry DOI: 10.7270/Q2D21VS2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hematopoietic prostaglandin D synthase (Homo sapiens (Human)) | BDBM50526548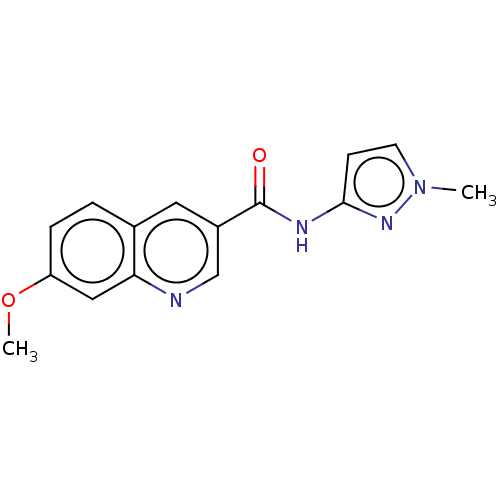 (CHEMBL4573375) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 78 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of full-length human His6-tagged HPGDS expressed in Escherichia coli BL21 (DE3) using PGH2 as substrate measured after 90 to 120 secs by R... | Bioorg Med Chem 27: 1456-1478 (2019) Article DOI: 10.1016/j.bmc.2019.02.017 BindingDB Entry DOI: 10.7270/Q2QV3R00 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM5452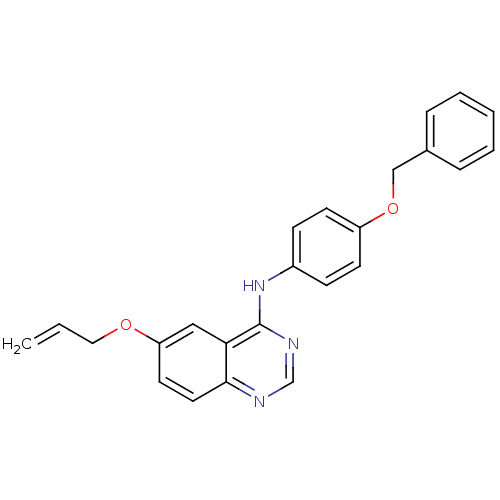 (6-alkoxy-4-anilinoquinazoline 10a | N-[4-(benzylox...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 79 | n/a | n/a | n/a | n/a | 7.5 | 23 |
GlaxoSmithKline | Assay Description Enzymatic reactions were initiated by adding kinase to the reaction mixture containing ATP, [gamma-33P] ATP, peptide substrate and test inhibitor com... | Bioorg Med Chem Lett 13: 637-40 (2003) Article DOI: 10.1016/s0960-894x(02)01047-8 BindingDB Entry DOI: 10.7270/Q2D21VS2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor tyrosine-protein kinase erbB-2 (Rattus norvegicus) | BDBM50099967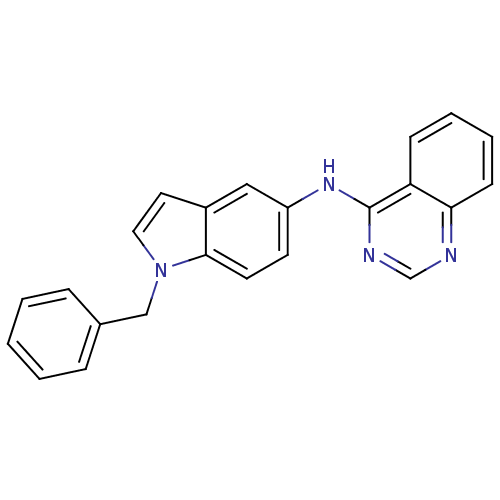 ((1-Benzyl-1H-indol-5-yl)-quinazolin-4-yl-amine | C...) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
Research Biomet. Glaxo Wellcome Medicines Research Centre Curated by ChEMBL | Assay Description Inhibition of rat Receptor protein-tyrosine kinase erbB2 | Bioorg Med Chem Lett 11: 1401-5 (2001) BindingDB Entry DOI: 10.7270/Q2W37VKB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM5458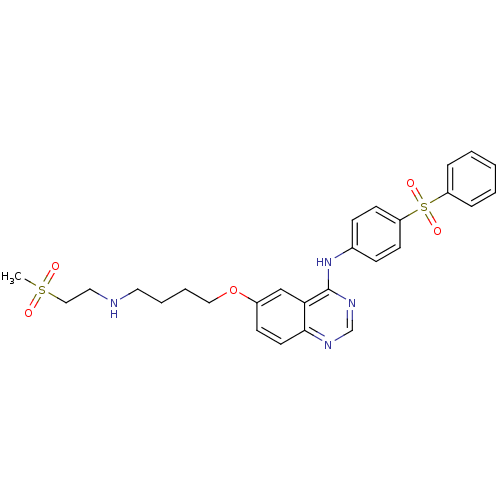 (6-alkoxy-4-anilinoquinazoline 8d | N-[4-(benzenesu...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 93 | n/a | n/a | n/a | n/a | 7.5 | 23 |
GlaxoSmithKline | Assay Description Enzymatic reactions were initiated by adding kinase to the reaction mixture containing ATP, [gamma-33P] ATP, peptide substrate and test inhibitor com... | Bioorg Med Chem Lett 13: 637-40 (2003) Article DOI: 10.1016/s0960-894x(02)01047-8 BindingDB Entry DOI: 10.7270/Q2D21VS2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor tyrosine-protein kinase erbB-2 (Homo sapiens (Human)) | BDBM5450 (6-alkoxy-4-anilinoquinazoline 8a | N-[4-(benzyloxy...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 95 | n/a | n/a | n/a | n/a | 7.5 | 23 |
GlaxoSmithKline | Assay Description Enzymatic reactions were initiated by adding kinase to the reaction mixture containing ATP, [gamma-33P] ATP, peptide substrate and test inhibitor com... | Bioorg Med Chem Lett 13: 637-40 (2003) Article DOI: 10.1016/s0960-894x(02)01047-8 BindingDB Entry DOI: 10.7270/Q2D21VS2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor tyrosine-protein kinase erbB-2 (Homo sapiens (Human)) | BDBM50099963 (CHEMBL30432 | N*4*-(1-Benzyl-1H-indazol-5-yl)-N*6*...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 97 | n/a | n/a | n/a | n/a | n/a | n/a |
Research Biomet. Glaxo Wellcome Medicines Research Centre Curated by ChEMBL | Assay Description Inhibition of erbB2 overexpressing HB4a.e5.2 cell proliferation | Bioorg Med Chem Lett 11: 1401-5 (2001) BindingDB Entry DOI: 10.7270/Q2W37VKB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor tyrosine-protein kinase erbB-2 (Homo sapiens (Human)) | BDBM50099966 (CHEMBL281006 | N*4*-(1-Benzyl-1H-indazol-5-yl)-pyr...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 97 | n/a | n/a | n/a | n/a | n/a | n/a |
Research Biomet. Glaxo Wellcome Medicines Research Centre Curated by ChEMBL | Assay Description Inhibition of c-erbB-2 overexpressing HB4a cell proliferation | Bioorg Med Chem Lett 11: 1401-5 (2001) BindingDB Entry DOI: 10.7270/Q2W37VKB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM5448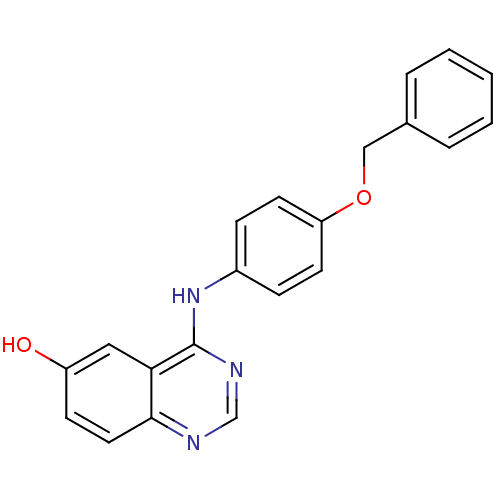 (4-anilinoquinazoline 4a | 4-{[4-(benzyloxy)phenyl]...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 98 | n/a | n/a | n/a | n/a | 7.5 | 23 |
GlaxoSmithKline | Assay Description Enzymatic reactions were initiated by adding kinase to the reaction mixture containing ATP, [gamma-33P] ATP, peptide substrate and test inhibitor com... | Bioorg Med Chem Lett 13: 637-40 (2003) Article DOI: 10.1016/s0960-894x(02)01047-8 BindingDB Entry DOI: 10.7270/Q2D21VS2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hematopoietic prostaglandin D synthase (Homo sapiens (Human)) | BDBM50526501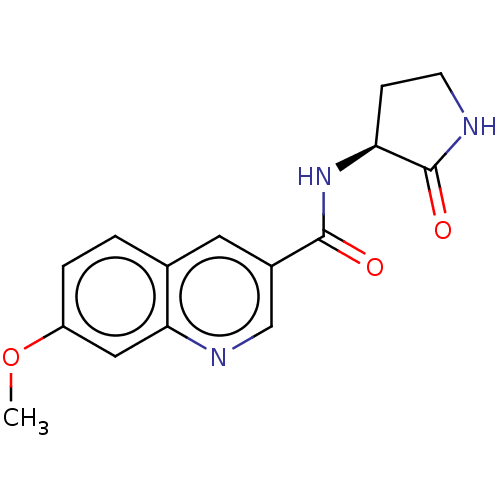 (CHEMBL4461589) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 98 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of full-length human His6-tagged HPGDS expressed in Escherichia coli BL21 (DE3) using PGH2 as substrate measured after 90 to 120 secs by R... | Bioorg Med Chem 27: 1456-1478 (2019) Article DOI: 10.1016/j.bmc.2019.02.017 BindingDB Entry DOI: 10.7270/Q2QV3R00 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hematopoietic prostaglandin D synthase (Rattus norvegicus) | BDBM50526486 (CHEMBL4526974) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of HPGDS in RBL cells assessed as reduction in A23187-induced PGD2 production preincubated for 30 mins followed by A23187 addition and mea... | Bioorg Med Chem 27: 1456-1478 (2019) Article DOI: 10.1016/j.bmc.2019.02.017 BindingDB Entry DOI: 10.7270/Q2QV3R00 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hematopoietic prostaglandin D synthase (Rattus norvegicus) | BDBM50526485 (CHEMBL4473072) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of HPGDS in RBL cells assessed as reduction in A23187-induced PGD2 production preincubated for 30 mins followed by A23187 addition and mea... | Bioorg Med Chem 27: 1456-1478 (2019) Article DOI: 10.1016/j.bmc.2019.02.017 BindingDB Entry DOI: 10.7270/Q2QV3R00 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hematopoietic prostaglandin D synthase (Homo sapiens (Human)) | BDBM50526507 (CHEMBL4438813) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of full-length human His6-tagged HPGDS expressed in Escherichia coli BL21 (DE3) using PGH2 as substrate measured after 90 to 120 secs by R... | Bioorg Med Chem 27: 1456-1478 (2019) Article DOI: 10.1016/j.bmc.2019.02.017 BindingDB Entry DOI: 10.7270/Q2QV3R00 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM5453 (6-alkoxy-4-anilinoquinazoline 12a | N-[4-(benzylox...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 104 | n/a | n/a | n/a | n/a | 7.5 | 23 |
GlaxoSmithKline | Assay Description Enzymatic reactions were initiated by adding kinase to the reaction mixture containing ATP, [gamma-33P] ATP, peptide substrate and test inhibitor com... | Bioorg Med Chem Lett 13: 637-40 (2003) Article DOI: 10.1016/s0960-894x(02)01047-8 BindingDB Entry DOI: 10.7270/Q2D21VS2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 140 total ) | Next | Last >> |