

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Monocarboxylate transporter 1 (Homo sapiens (Human)) | BDBM22000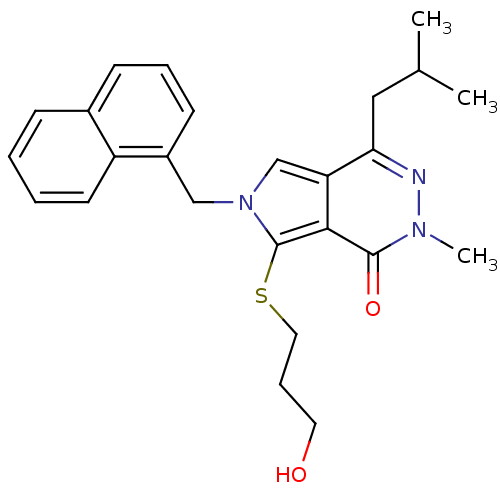 (7-[(3-hydroxypropyl)sulfanyl]-2-methyl-4-(2-methyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | PubMed | 0.0955 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Charnwood Curated by ChEMBL | Assay Description Displacement of [3H]5-[(3-hydroxypropyl)thio]-3-methyl-1-[2-(methyl-t)propyl-2,3-t2]-6-(1-naphthalenylmethyl)-1H-pyrrolo[3,4-d]pyrimidine-2,4(3H,6H)-... | Nat Chem Biol 1: 371-6 (2005) BindingDB Entry DOI: 10.7270/Q2MC9063 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monocarboxylate transporter 1 (Homo sapiens (Human)) | BDBM22001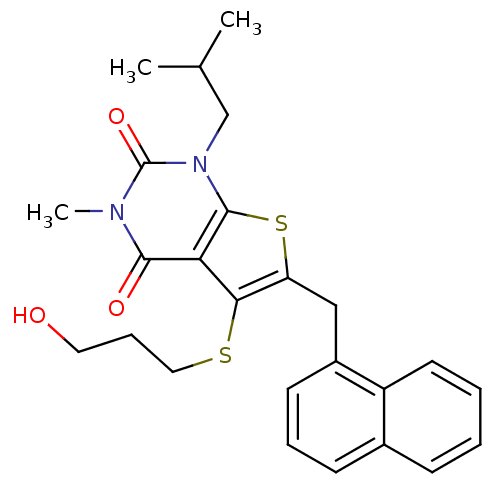 (5-[(3-hydroxypropyl)sulfanyl]-3-methyl-1-(2-methyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | PubMed | 0.275 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Charnwood Curated by ChEMBL | Assay Description Displacement of [3H]5-[(3-hydroxypropyl)thio]-3-methyl-1-[2-(methyl-t)propyl-2,3-t2]-6-(1-naphthalenylmethyl)-1H-pyrrolo[3,4-d]pyrimidine-2,4(3H,6H)-... | Nat Chem Biol 1: 371-6 (2005) BindingDB Entry DOI: 10.7270/Q2MC9063 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monocarboxylate transporter 1 (Homo sapiens (Human)) | BDBM22001 (5-[(3-hydroxypropyl)sulfanyl]-3-methyl-1-(2-methyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | PubMed | 0.302 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Charnwood Curated by ChEMBL | Assay Description Displacement of [3H]5-[(3-hydroxypropyl)thio]-3-methyl-1-[2-(methyl-t)propyl-2,3-t2]-6-(1-naphthalenylmethyl)-1H-pyrrolo[3,4-d]pyrimidine-2,4(3H,6H)-... | Nat Chem Biol 1: 371-6 (2005) BindingDB Entry DOI: 10.7270/Q2MC9063 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monocarboxylate transporter 1 (Homo sapiens (Human)) | BDBM21986 (5-[(3-hydroxypropyl)sulfanyl]-3-methyl-1-(2-methyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Charnwood Curated by ChEMBL | Assay Description Displacement of [3H]5-[(3-hydroxypropyl)thio]-3-methyl-1-[2-(methyl-t)propyl-2,3-t2]-6-(1-naphthalenylmethyl)-1H-pyrrolo[3,4-d]pyrimidine-2,4(3H,6H)-... | Nat Chem Biol 1: 371-6 (2005) BindingDB Entry DOI: 10.7270/Q2MC9063 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monocarboxylate transporter 1 (Homo sapiens (Human)) | BDBM21986 (5-[(3-hydroxypropyl)sulfanyl]-3-methyl-1-(2-methyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.331 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Charnwood Curated by ChEMBL | Assay Description Displacement of [3H]5-[(3-hydroxypropyl)thio]-3-methyl-1-[2-(methyl-t)propyl-2,3-t2]-6-(1-naphthalenylmethyl)-1H-pyrrolo[3,4-d]pyrimidine-2,4(3H,6H)-... | Nat Chem Biol 1: 371-6 (2005) BindingDB Entry DOI: 10.7270/Q2MC9063 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monocarboxylate transporter 1 (Homo sapiens (Human)) | BDBM22002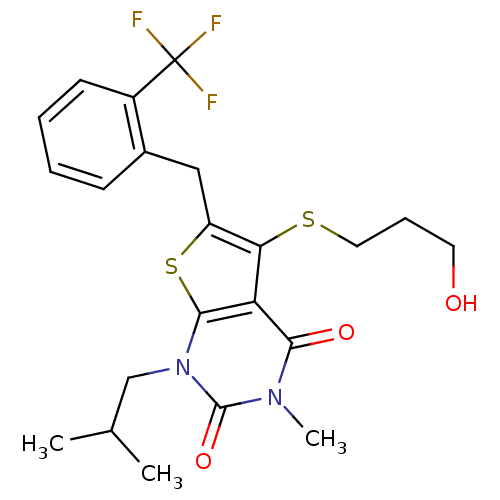 (5-[(3-hydroxypropyl)sulfanyl]-3-methyl-1-(2-methyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 0.355 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Charnwood Curated by ChEMBL | Assay Description Displacement of [3H]5-[(3-hydroxypropyl)thio]-3-methyl-1-[2-(methyl-t)propyl-2,3-t2]-6-(1-naphthalenylmethyl)-1H-pyrrolo[3,4-d]pyrimidine-2,4(3H,6H)-... | Nat Chem Biol 1: 371-6 (2005) BindingDB Entry DOI: 10.7270/Q2MC9063 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monocarboxylate transporter 1 (Homo sapiens (Human)) | BDBM22002 (5-[(3-hydroxypropyl)sulfanyl]-3-methyl-1-(2-methyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 0.355 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Charnwood Curated by ChEMBL | Assay Description Displacement of [3H]5-[(3-hydroxypropyl)thio]-3-methyl-1-[2-(methyl-t)propyl-2,3-t2]-6-(1-naphthalenylmethyl)-1H-pyrrolo[3,4-d]pyrimidine-2,4(3H,6H)-... | Nat Chem Biol 1: 371-6 (2005) BindingDB Entry DOI: 10.7270/Q2MC9063 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monocarboxylate transporter 1 (Homo sapiens (Human)) | BDBM22000 (7-[(3-hydroxypropyl)sulfanyl]-2-methyl-4-(2-methyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | PubMed | 0.708 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Charnwood Curated by ChEMBL | Assay Description Displacement of [3H]5-[(3-hydroxypropyl)thio]-3-methyl-1-[2-(methyl-t)propyl-2,3-t2]-6-(1-naphthalenylmethyl)-1H-pyrrolo[3,4-d]pyrimidine-2,4(3H,6H)-... | Nat Chem Biol 1: 371-6 (2005) BindingDB Entry DOI: 10.7270/Q2MC9063 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monocarboxylate transporter 1 (Homo sapiens (Human)) | BDBM21986 (5-[(3-hydroxypropyl)sulfanyl]-3-methyl-1-(2-methyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1.17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Charnwood Curated by ChEMBL | Assay Description Displacement of [3H]5-[(3-hydroxypropyl)thio]-3-methyl-1-[2-(methyl-t)propyl-2,3-t2]-6-(1-naphthalenylmethyl)-1H-pyrrolo[3,4-d]pyrimidine-2,4(3H,6H)-... | Nat Chem Biol 1: 371-6 (2005) BindingDB Entry DOI: 10.7270/Q2MC9063 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monocarboxylate transporter 1 (Homo sapiens (Human)) | BDBM22025 (5-{[(3R)-3-hydroxypyrrolidin-1-yl]carbonyl}-6-(1H-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Charnwood Curated by ChEMBL | Assay Description Displacement of [3H]5-[(3-hydroxypropyl)thio]-3-methyl-1-[2-(methyl-t)propyl-2,3-t2]-6-(1-naphthalenylmethyl)-1H-pyrrolo[3,4-d]pyrimidine-2,4(3H,6H)-... | Nat Chem Biol 1: 371-6 (2005) BindingDB Entry DOI: 10.7270/Q2MC9063 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monocarboxylate transporter 1 (Homo sapiens (Human)) | BDBM50416571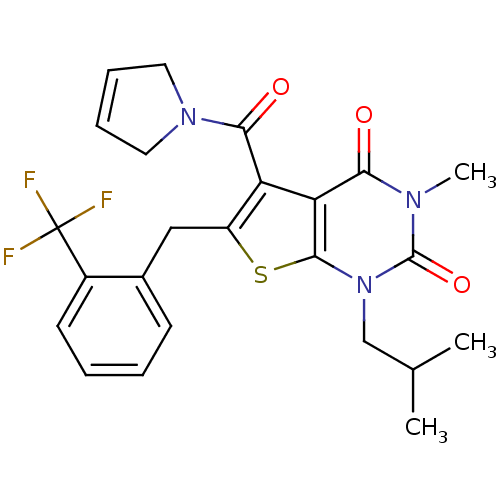 (CHEMBL1221551) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.39 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Charnwood Curated by ChEMBL | Assay Description Displacement of [3H]5-[(3-hydroxypropyl)thio]-3-methyl-1-[2-(methyl-t)propyl-2,3-t2]-6-(1-naphthalenylmethyl)-1H-pyrrolo[3,4-d]pyrimidine-2,4(3H,6H)-... | Nat Chem Biol 1: 371-6 (2005) BindingDB Entry DOI: 10.7270/Q2MC9063 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monocarboxylate transporter 1 (Homo sapiens (Human)) | BDBM50416571 (CHEMBL1221551) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Charnwood Curated by ChEMBL | Assay Description Displacement of [3H]5-[(3-hydroxypropyl)thio]-3-methyl-1-[2-(methyl-t)propyl-2,3-t2]-6-(1-naphthalenylmethyl)-1H-pyrrolo[3,4-d]pyrimidine-2,4(3H,6H)-... | Nat Chem Biol 1: 371-6 (2005) BindingDB Entry DOI: 10.7270/Q2MC9063 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monocarboxylate transporter 1 (Homo sapiens (Human)) | BDBM22017 (3-methyl-1-(2-methylpropyl)-5-(pyrrolidin-1-ylcarb...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Charnwood Curated by ChEMBL | Assay Description Displacement of [3H]5-[(3-hydroxypropyl)thio]-3-methyl-1-[2-(methyl-t)propyl-2,3-t2]-6-(1-naphthalenylmethyl)-1H-pyrrolo[3,4-d]pyrimidine-2,4(3H,6H)-... | Nat Chem Biol 1: 371-6 (2005) BindingDB Entry DOI: 10.7270/Q2MC9063 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monocarboxylate transporter 1 (Homo sapiens (Human)) | BDBM22017 (3-methyl-1-(2-methylpropyl)-5-(pyrrolidin-1-ylcarb...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 5.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Charnwood Curated by ChEMBL | Assay Description Displacement of [3H]5-[(3-hydroxypropyl)thio]-3-methyl-1-[2-(methyl-t)propyl-2,3-t2]-6-(1-naphthalenylmethyl)-1H-pyrrolo[3,4-d]pyrimidine-2,4(3H,6H)-... | Nat Chem Biol 1: 371-6 (2005) BindingDB Entry DOI: 10.7270/Q2MC9063 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monocarboxylate transporter 1 (Homo sapiens (Human)) | BDBM22025 (5-{[(3R)-3-hydroxypyrrolidin-1-yl]carbonyl}-6-(1H-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Charnwood Curated by ChEMBL | Assay Description Displacement of [3H]5-[(3-hydroxypropyl)thio]-3-methyl-1-[2-(methyl-t)propyl-2,3-t2]-6-(1-naphthalenylmethyl)-1H-pyrrolo[3,4-d]pyrimidine-2,4(3H,6H)-... | Nat Chem Biol 1: 371-6 (2005) BindingDB Entry DOI: 10.7270/Q2MC9063 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM8610 (1-[4-(4-{[(2R,4S)-2-(2,4-dichlorophenyl)-2-(1H-imi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of CYP3A4 activity in human liver microsomes using midazolam as a substrate by LC/MS analysis | Drug Metab Dispos 41: 814-26 (2013) Article DOI: 10.1124/dmd.112.048355 BindingDB Entry DOI: 10.7270/Q2NC62XH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM8610 (1-[4-(4-{[(2R,4S)-2-(2,4-dichlorophenyl)-2-(1H-imi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of CYP3A4 activity in human liver microsomes using nifedipine as a substrate by LC/MS analysis | Drug Metab Dispos 41: 814-26 (2013) Article DOI: 10.1124/dmd.112.048355 BindingDB Entry DOI: 10.7270/Q2NC62XH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monocarboxylate transporter 1 (Homo sapiens (Human)) | BDBM50031739 (1-Isobutyl-3-methyl-6-naphthalen-1-ylmethyl-1,6-di...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Charnwood Curated by ChEMBL | Assay Description Displacement of [3H]5-[(3-hydroxypropyl)thio]-3-methyl-1-[2-(methyl-t)propyl-2,3-t2]-6-(1-naphthalenylmethyl)-1H-pyrrolo[3,4-d]pyrimidine-2,4(3H,6H)-... | Nat Chem Biol 1: 371-6 (2005) BindingDB Entry DOI: 10.7270/Q2MC9063 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monocarboxylate transporter 1 (Homo sapiens (Human)) | BDBM50324668 (6-[[4-azido-3-(iodo-125I)phenyl]methyl]-2,6-dihydr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Charnwood Curated by ChEMBL | Assay Description Displacement of [3H]5-[(3-hydroxypropyl)thio]-3-methyl-1-[2-(methyl-t)propyl-2,3-t2]-6-(1-naphthalenylmethyl)-1H-pyrrolo[3,4-d]pyrimidine-2,4(3H,6H)-... | Nat Chem Biol 1: 371-6 (2005) BindingDB Entry DOI: 10.7270/Q2MC9063 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monocarboxylate transporter 1 (Homo sapiens (Human)) | BDBM21985 (5-{[(3R)-3-hydroxypyrrolidin-1-yl]carbonyl}-3-meth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | PubMed | 30.9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Charnwood Curated by ChEMBL | Assay Description Displacement of [3H]5-[(3-hydroxypropyl)thio]-3-methyl-1-[2-(methyl-t)propyl-2,3-t2]-6-(1-naphthalenylmethyl)-1H-pyrrolo[3,4-d]pyrimidine-2,4(3H,6H)-... | Nat Chem Biol 1: 371-6 (2005) BindingDB Entry DOI: 10.7270/Q2MC9063 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monocarboxylate transporter 1 (Homo sapiens (Human)) | BDBM21985 (5-{[(3R)-3-hydroxypyrrolidin-1-yl]carbonyl}-3-meth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | PubMed | 31 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Charnwood Curated by ChEMBL | Assay Description Displacement of [3H]5-[(3-hydroxypropyl)thio]-3-methyl-1-[2-(methyl-t)propyl-2,3-t2]-6-(1-naphthalenylmethyl)-1H-pyrrolo[3,4-d]pyrimidine-2,4(3H,6H)-... | Nat Chem Biol 1: 371-6 (2005) BindingDB Entry DOI: 10.7270/Q2MC9063 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monocarboxylate transporter 1 (Homo sapiens (Human)) | BDBM50031739 (1-Isobutyl-3-methyl-6-naphthalen-1-ylmethyl-1,6-di...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 47 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Charnwood Curated by ChEMBL | Assay Description Displacement of [3H]5-[(3-hydroxypropyl)thio]-3-methyl-1-[2-(methyl-t)propyl-2,3-t2]-6-(1-naphthalenylmethyl)-1H-pyrrolo[3,4-d]pyrimidine-2,4(3H,6H)-... | Nat Chem Biol 1: 371-6 (2005) BindingDB Entry DOI: 10.7270/Q2MC9063 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM8610 (1-[4-(4-{[(2R,4S)-2-(2,4-dichlorophenyl)-2-(1H-imi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 49 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of CYP3A4 activity in human liver microsomes using testosterone as a substrate by LC/MS analysis | Drug Metab Dispos 41: 814-26 (2013) Article DOI: 10.1124/dmd.112.048355 BindingDB Entry DOI: 10.7270/Q2NC62XH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monocarboxylate transporter 1 (Homo sapiens (Human)) | BDBM50416572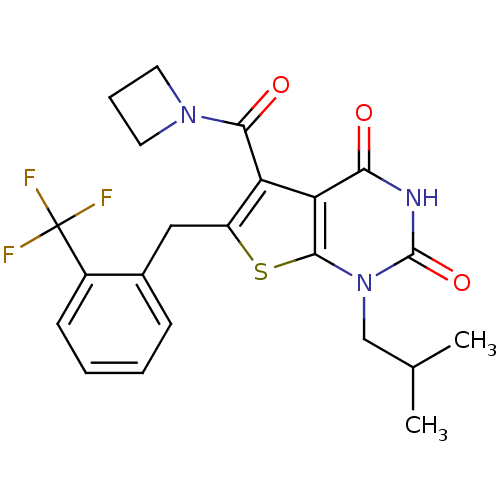 (CHEMBL1221510) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | <200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Charnwood Curated by ChEMBL | Assay Description Displacement of [3H]5-[(3-hydroxypropyl)thio]-3-methyl-1-[2-(methyl-t)propyl-2,3-t2]-6-(1-naphthalenylmethyl)-1H-pyrrolo[3,4-d]pyrimidine-2,4(3H,6H)-... | Nat Chem Biol 1: 371-6 (2005) BindingDB Entry DOI: 10.7270/Q2MC9063 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50088527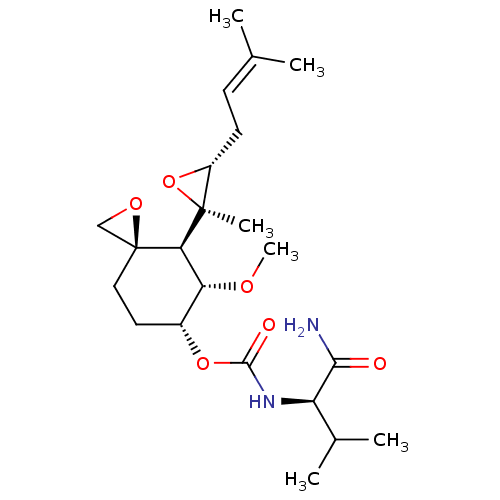 (CHEMBL3527358) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid UniChem Similars | Article PubMed | 5.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of CYP3A4 activity in human liver microsomes using midazolam as a substrate by LC/MS analysis | Drug Metab Dispos 41: 814-26 (2013) Article DOI: 10.1124/dmd.112.048355 BindingDB Entry DOI: 10.7270/Q2NC62XH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50088527 (CHEMBL3527358) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid UniChem Similars | Article PubMed | 2.87E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of CYP3A4 activity in human liver microsomes using testosterone as a substrate by LC/MS analysis | Drug Metab Dispos 41: 814-26 (2013) Article DOI: 10.1124/dmd.112.048355 BindingDB Entry DOI: 10.7270/Q2NC62XH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50088527 (CHEMBL3527358) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid UniChem Similars | Article PubMed | 4.03E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of CYP3A4 activity in human liver microsomes using nifedipine as a substrate by LC/MS analysis | Drug Metab Dispos 41: 814-26 (2013) Article DOI: 10.1124/dmd.112.048355 BindingDB Entry DOI: 10.7270/Q2NC62XH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM36462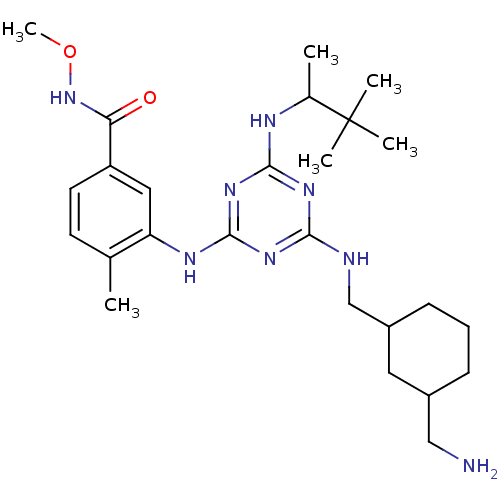 (3-(4-((3-(Aminomethyl)cyclohexyl)methylamino)-6-(3...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | <2 | n/a | n/a | n/a | n/a | 9.5 | 16 |
Praecis Pharmaceuticals | Assay Description Selection of DNA-encoded libraries (DELs), which are covalent attachment of encoding double stranded DNA to small-molecule created using a combinatio... | Nat Chem Biol 5: 647-54 (2009) Article DOI: 10.1038/nchembio.211 BindingDB Entry DOI: 10.7270/Q2MP51NX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM36463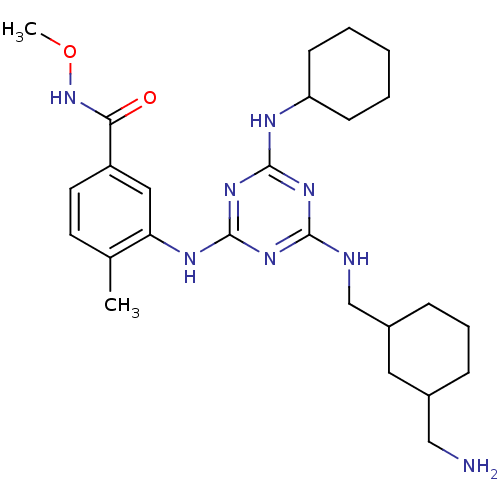 (3-(4-((3-(Aminomethyl)cyclohexyl)methylamino)-6-(3...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | <2 | n/a | n/a | n/a | n/a | 9.5 | 16 |
Praecis Pharmaceuticals | Assay Description Selection of DNA-encoded libraries (DELs), which are covalent attachment of encoding double stranded DNA to small-molecule created using a combinatio... | Nat Chem Biol 5: 647-54 (2009) Article DOI: 10.1038/nchembio.211 BindingDB Entry DOI: 10.7270/Q2MP51NX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-derived growth factor receptor alpha/beta (Homo sapiens (Human)) | BDBM50132388 ((3-Chloro-phenyl)-(6,7-dimethoxy-quinoxalin-2-yl)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of Platelet-derived growth factor receptor | Bioorg Med Chem Lett 13: 3091-5 (2003) BindingDB Entry DOI: 10.7270/Q2N8796B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-derived growth factor receptor alpha/beta (Homo sapiens (Human)) | BDBM50132386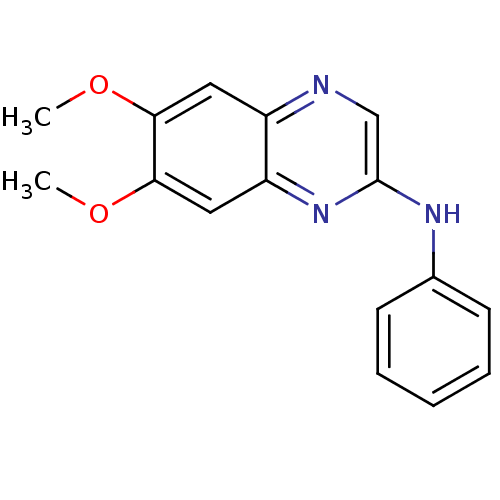 ((6,7-Dimethoxy-quinoxalin-2-yl)-phenyl-amine | CHE...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of Platelet-derived growth factor receptor | Bioorg Med Chem Lett 13: 3097-100 (2003) BindingDB Entry DOI: 10.7270/Q2HH6JGM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transcription factor p65 (Homo sapiens (Human)) | BDBM50543887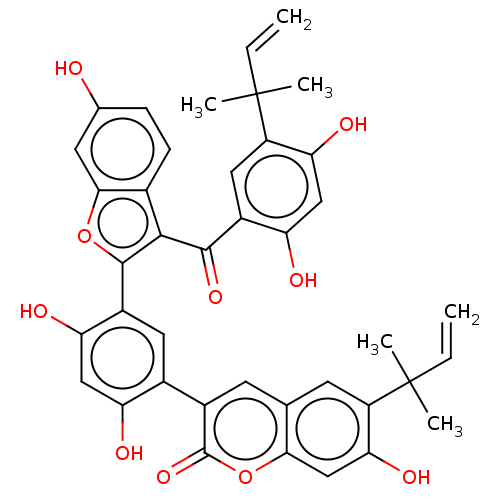 (CHEMBL4649115) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade de S£o Paulo Curated by ChEMBL | Assay Description Inhibition of NF-kappaB p65 in human HeLa cells nuclear extract by chemiluminescent assay | J Nat Prod 83: 1784-1793 (2020) Article DOI: 10.1021/acs.jnatprod.9b01136 BindingDB Entry DOI: 10.7270/Q2SX6HSV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-derived growth factor receptor alpha/beta (Homo sapiens (Human)) | BDBM50132386 ((6,7-Dimethoxy-quinoxalin-2-yl)-phenyl-amine | CHE...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of Platelet-derived growth factor receptor | Bioorg Med Chem Lett 13: 3091-5 (2003) BindingDB Entry DOI: 10.7270/Q2N8796B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM8610 (1-[4-(4-{[(2R,4S)-2-(2,4-dichlorophenyl)-2-(1H-imi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 9.80 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of CYP3A4 activity in human liver microsomes assessed as dibenzo fluuorescene oxidation up to 40 uM | Drug Metab Dispos 41: 814-26 (2013) Article DOI: 10.1124/dmd.112.048355 BindingDB Entry DOI: 10.7270/Q2NC62XH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM36464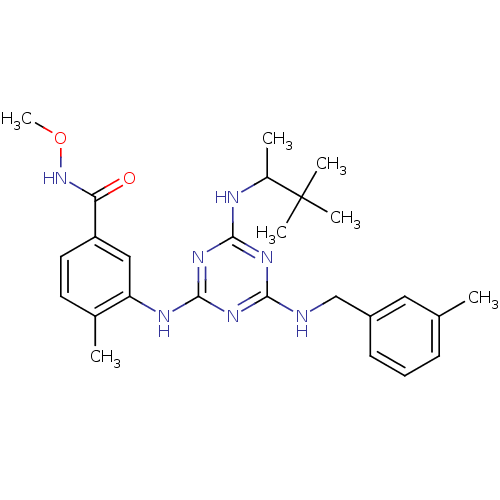 (3-(4-(3,3-Dimethylbutan-2-ylamino)-6-(3-methylbenz...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | 9.5 | 16 |
Praecis Pharmaceuticals | Assay Description Selection of DNA-encoded libraries (DELs), which are covalent attachment of encoding double stranded DNA to small-molecule created using a combinatio... | Nat Chem Biol 5: 647-54 (2009) Article DOI: 10.1038/nchembio.211 BindingDB Entry DOI: 10.7270/Q2MP51NX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM50156750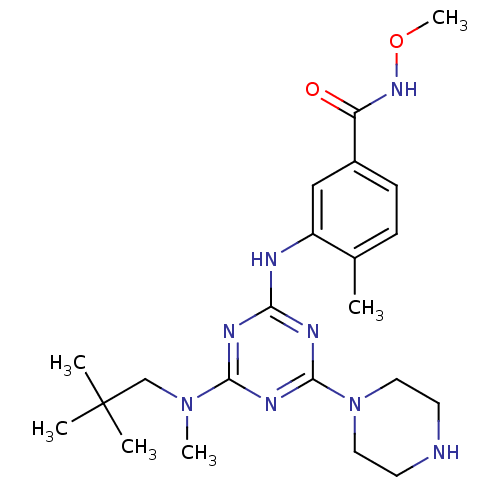 (CHEMBL376506 | DEL-A, 5 | N-methoxy-4-methyl-3-(4-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | 9.5 | 16 |
Praecis Pharmaceuticals | Assay Description Selection of DNA-encoded libraries (DELs), which are covalent attachment of encoding double stranded DNA to small-molecule created using a combinatio... | Nat Chem Biol 5: 647-54 (2009) Article DOI: 10.1038/nchembio.211 BindingDB Entry DOI: 10.7270/Q2MP51NX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-derived growth factor receptor alpha/beta (Homo sapiens (Human)) | BDBM50132406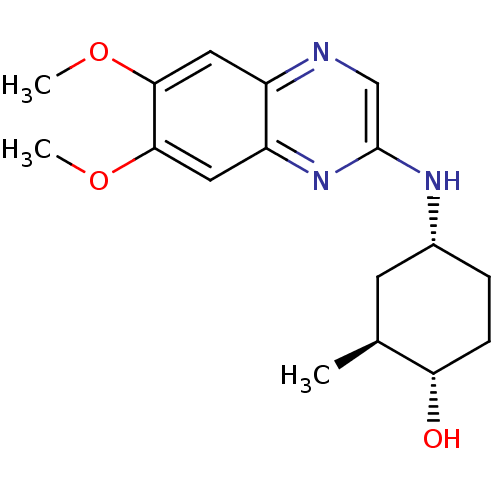 (4-(6,7-Dimethoxy-quinoxalin-2-ylamino)-2-methyl-cy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of Platelet-derived growth factor receptor | Bioorg Med Chem Lett 13: 3097-100 (2003) BindingDB Entry DOI: 10.7270/Q2HH6JGM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase PLK1 (Homo sapiens (Human)) | BDBM50615459 (CHEMBL5281562) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | UniChem | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-derived growth factor receptor alpha/beta (Homo sapiens (Human)) | BDBM50132392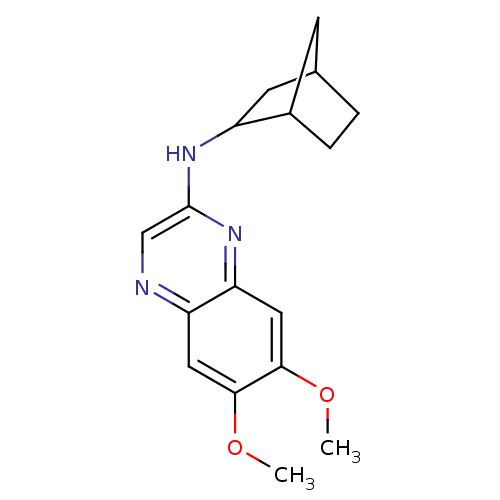 ((+/-)Bicyclo[2.2.1]hept-2-yl-(6,7-dimethoxy-quinox...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of Platelet-derived growth factor receptor | Bioorg Med Chem Lett 13: 3091-5 (2003) BindingDB Entry DOI: 10.7270/Q2N8796B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-derived growth factor receptor alpha/beta (Homo sapiens (Human)) | BDBM50132405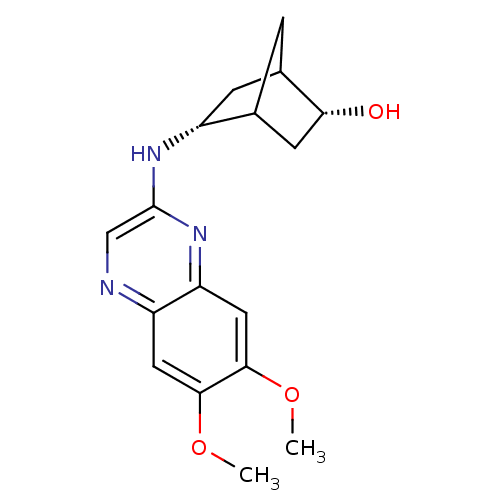 (5-(6,7-Dimethoxy-quinoxalin-2-ylamino)-bicyclo[2.2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of Platelet-derived growth factor receptor | Bioorg Med Chem Lett 13: 3097-100 (2003) BindingDB Entry DOI: 10.7270/Q2HH6JGM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-derived growth factor receptor alpha/beta (Homo sapiens (Human)) | BDBM50404045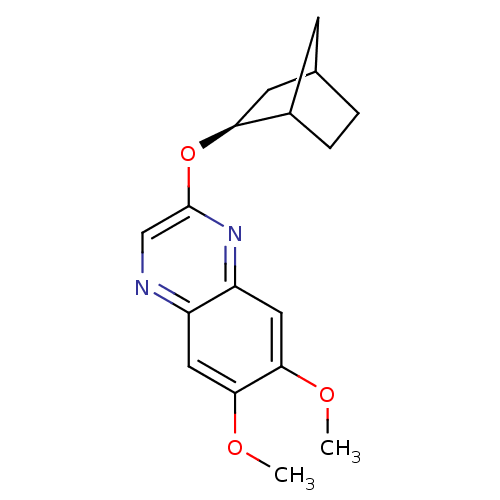 (CHEMBL2112463) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of Platelet-derived growth factor receptor | Bioorg Med Chem Lett 13: 3091-5 (2003) BindingDB Entry DOI: 10.7270/Q2N8796B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-derived growth factor receptor alpha/beta (Homo sapiens (Human)) | BDBM50132409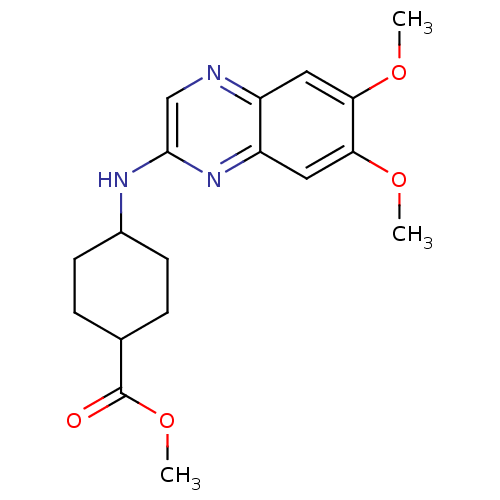 (4-(6,7-Dimethoxy-quinoxalin-2-ylamino)-cyclohexane...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of Platelet-derived growth factor receptor | Bioorg Med Chem Lett 13: 3097-100 (2003) BindingDB Entry DOI: 10.7270/Q2HH6JGM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity tyrosine-phosphorylation-regulated kinase 1A (Homo sapiens (Human)) | BDBM50615460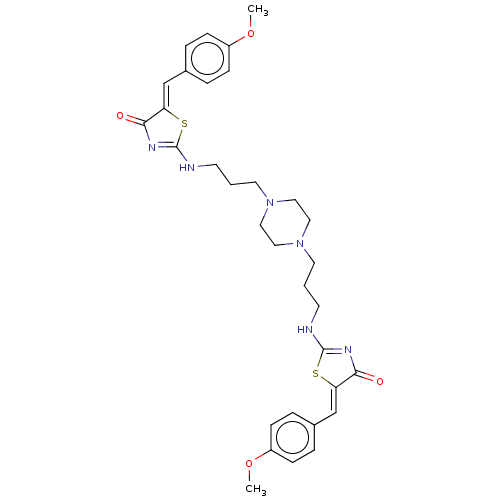 (CHEMBL5271428) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | UniChem | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-derived growth factor receptor alpha/beta (Homo sapiens (Human)) | BDBM50132395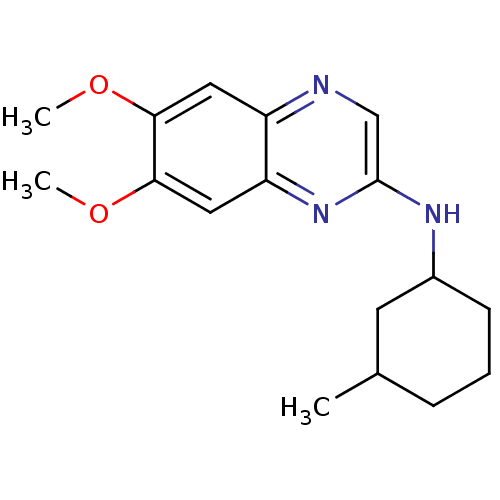 ((6,7-Dimethoxy-quinoxalin-2-yl)-(3-methyl-cyclohex...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of Platelet-derived growth factor receptor | Bioorg Med Chem Lett 13: 3097-100 (2003) BindingDB Entry DOI: 10.7270/Q2HH6JGM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-derived growth factor receptor alpha/beta (Homo sapiens (Human)) | BDBM50132395 ((6,7-Dimethoxy-quinoxalin-2-yl)-(3-methyl-cyclohex...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of Platelet-derived growth factor receptor | Bioorg Med Chem Lett 13: 3091-5 (2003) BindingDB Entry DOI: 10.7270/Q2N8796B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-derived growth factor receptor alpha/beta (Homo sapiens (Human)) | BDBM50132387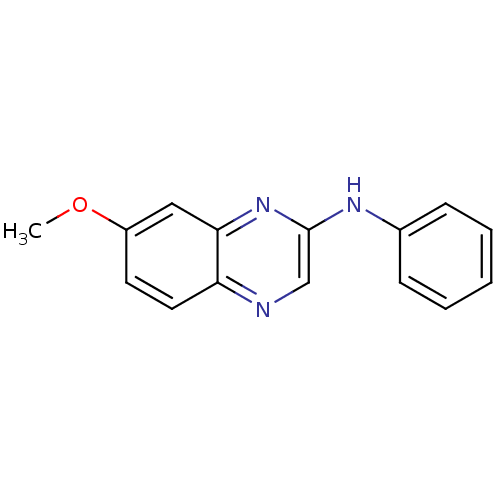 ((7-Methoxy-quinoxalin-2-yl)-phenyl-amine | CHEMBL1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 42 | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of Platelet-derived growth factor receptor | Bioorg Med Chem Lett 13: 3091-5 (2003) BindingDB Entry DOI: 10.7270/Q2N8796B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-derived growth factor receptor alpha/beta (Homo sapiens (Human)) | BDBM50132409 (4-(6,7-Dimethoxy-quinoxalin-2-ylamino)-cyclohexane...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 49 | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of Platelet-derived growth factor receptor | Bioorg Med Chem Lett 13: 3097-100 (2003) BindingDB Entry DOI: 10.7270/Q2HH6JGM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-derived growth factor receptor alpha/beta (Homo sapiens (Human)) | BDBM50132384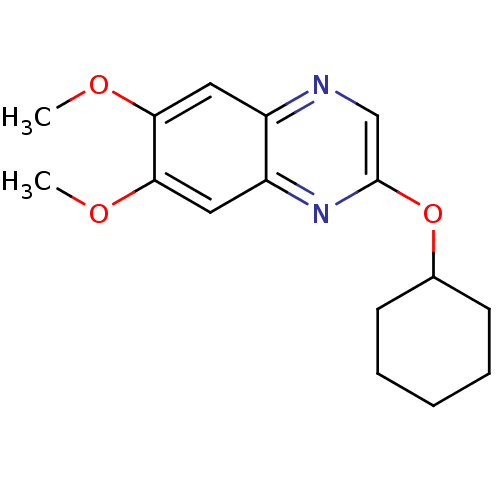 (2-Cyclohexyloxy-6,7-dimethoxy-quinoxaline | CHEMBL...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 65 | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of Platelet-derived growth factor receptor | Bioorg Med Chem Lett 13: 3091-5 (2003) BindingDB Entry DOI: 10.7270/Q2N8796B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-derived growth factor receptor alpha/beta (Homo sapiens (Human)) | BDBM50132407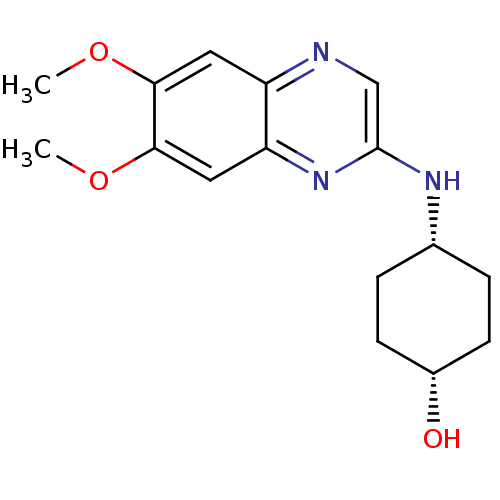 (4-(6,7-Dimethoxy-quinoxalin-2-ylamino)-cyclohexano...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 68 | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of Platelet-derived growth factor receptor | Bioorg Med Chem Lett 13: 3097-100 (2003) BindingDB Entry DOI: 10.7270/Q2HH6JGM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-derived growth factor receptor alpha/beta (Homo sapiens (Human)) | BDBM50132391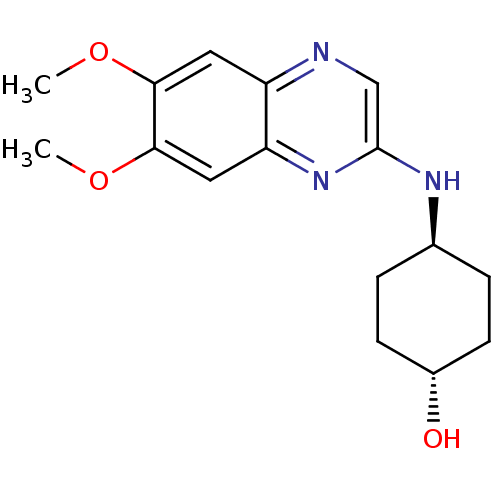 (4-((R)-6,7-Dimethoxy-quinoxalin-2-ylamino)-cyclohe...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 76 | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of Platelet-derived growth factor receptor | Bioorg Med Chem Lett 13: 3097-100 (2003) BindingDB Entry DOI: 10.7270/Q2HH6JGM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 113 total ) | Next | Last >> |