 Found 6638 hits with Last Name = 'an' and Initial = 'sj'
Found 6638 hits with Last Name = 'an' and Initial = 'sj' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Platelet-activating factor acetylhydrolase
(Homo sapiens (Human)) | BDBM50117772
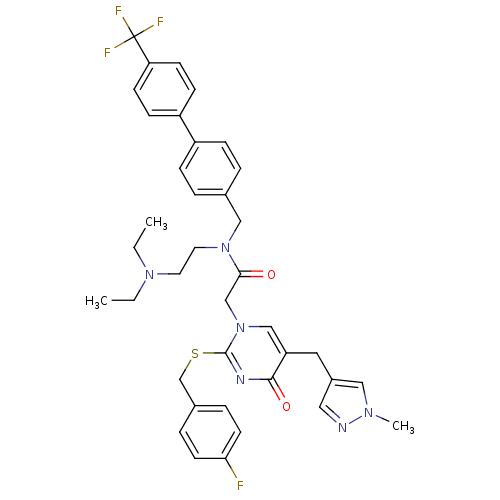
(CHEMBL10921 | N-(2-Diethylamino-ethyl)-2-[2-(4-flu...)Show SMILES CCN(CC)CCN(Cc1ccc(cc1)-c1ccc(cc1)C(F)(F)F)C(=O)Cn1cc(Cc2cnn(C)c2)c(=O)nc1SCc1ccc(F)cc1 Show InChI InChI=1S/C38H40F4N6O2S/c1-4-46(5-2)18-19-47(23-27-6-10-30(11-7-27)31-12-14-33(15-13-31)38(40,41)42)35(49)25-48-24-32(20-29-21-43-45(3)22-29)36(50)44-37(48)51-26-28-8-16-34(39)17-9-28/h6-17,21-22,24H,4-5,18-20,23,25-26H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Steady state and transient kinetics to a freely reversible, non-covalently bound, human recombinant Phospholipase A2 (rhLp-PLA2) was determined |
Bioorg Med Chem Lett 12: 2603-6 (2002)
BindingDB Entry DOI: 10.7270/Q2G44PNN |
More data for this
Ligand-Target Pair | |
Platelet-activating factor acetylhydrolase
(Homo sapiens (Human)) | BDBM50125265
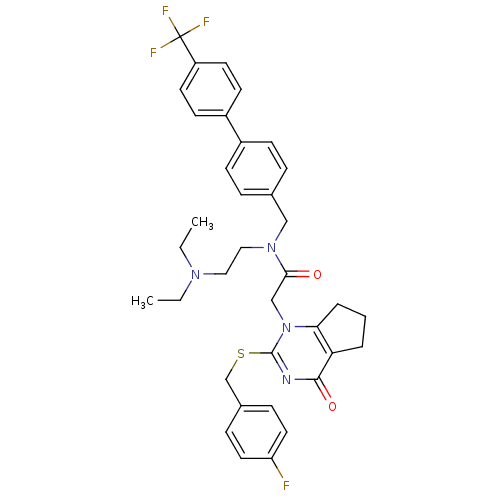
(CHEMBL204021 | N-(2-Diethylamino-ethyl)-2-[2-(4-fl...)Show SMILES CCN(CC)CCN(Cc1ccc(cc1)-c1ccc(cc1)C(F)(F)F)C(=O)Cn1c2CCCc2c(=O)nc1SCc1ccc(F)cc1 Show InChI InChI=1S/C36H38F4N4O2S/c1-3-42(4-2)20-21-43(22-25-8-12-27(13-9-25)28-14-16-29(17-15-28)36(38,39)40)33(45)23-44-32-7-5-6-31(32)34(46)41-35(44)47-24-26-10-18-30(37)19-11-26/h8-19H,3-7,20-24H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
PubMed
| 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibitory activity against recombinant human Lp-PLA2 |
Bioorg Med Chem Lett 13: 1067-70 (2003)
BindingDB Entry DOI: 10.7270/Q2S75FPD |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Serine/threonine-protein kinase ATR
(Homo sapiens (Human)) | BDBM350085
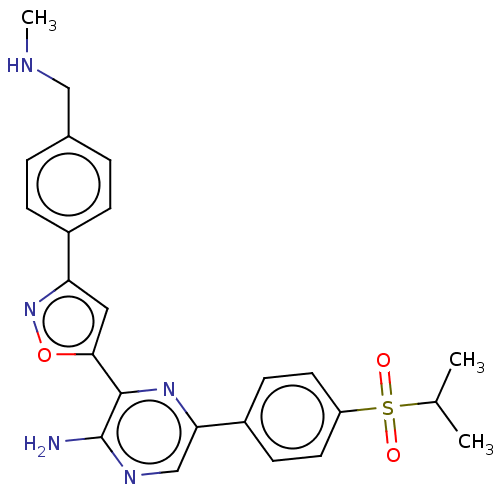
(3-[3-[4-[dideuterio(methylamino)methyl]phenyl]isox...)Show SMILES CNCc1ccc(cc1)-c1cc(on1)-c1nc(cnc1N)-c1ccc(cc1)S(=O)(=O)C(C)C Show InChI InChI=1S/C24H25N5O3S/c1-15(2)33(30,31)19-10-8-18(9-11-19)21-14-27-24(25)23(28-21)22-12-20(29-32-22)17-6-4-16(5-7-17)13-26-3/h4-12,14-15,26H,13H2,1-3H3,(H2,25,27) | PDB
Reactome pathway
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| US Patent
| 0.148 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
VERTEX PHARMACEUTICALS INCORPORATED
US Patent
| Assay Description
Compounds can be screened for their ability to inhibit ATR kinase using a radioactive-phosphate incorporation assay. Assays are carried out in a mixt... |
US Patent US10822331 (2020)
BindingDB Entry DOI: 10.7270/Q2V98C59 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase ATR
(Homo sapiens (Human)) | BDBM350085

(3-[3-[4-[dideuterio(methylamino)methyl]phenyl]isox...)Show SMILES CNCc1ccc(cc1)-c1cc(on1)-c1nc(cnc1N)-c1ccc(cc1)S(=O)(=O)C(C)C Show InChI InChI=1S/C24H25N5O3S/c1-15(2)33(30,31)19-10-8-18(9-11-19)21-14-27-24(25)23(28-21)22-12-20(29-32-22)17-6-4-16(5-7-17)13-26-3/h4-12,14-15,26H,13H2,1-3H3,(H2,25,27) | PDB
Reactome pathway
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| US Patent
| 0.148 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Incorporated
US Patent
| Assay Description
Compounds can be screened for their ability to inhibit ATR kinase using a radioactive-phosphate incorporation assay. Assays are carried out in a mixt... |
US Patent US10208027 (2019)
BindingDB Entry DOI: 10.7270/Q27S7QX5 |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM86492

(CAS_170713-75-4 | NSC_6324645 | Nociceptin)Show SMILES [#6]-[#6](-[#8])-[#6](-[#7]-[#6](=O)-[#6](-[#6]-c1ccccc1)-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6](-[#7])-[#6]-c1ccccc1)-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6](-[#6])-[#6](=O)-[#7]-[#6](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-[#6](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](=O)-[#7]-[#6](-[#6]-[#8])-[#6](=O)-[#7]-[#6](-[#6])-[#6](=O)-[#7]-[#6](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-[#6](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](-[#7])=O Show InChI InChI=1S/C61H100N22O15/c1-34(75-47(87)32-74-59(98)49(36(3)85)83-57(96)44(29-38-18-8-5-9-19-38)77-48(88)31-72-46(86)30-73-53(92)39(64)28-37-16-6-4-7-17-37)51(90)79-43(23-15-27-71-61(68)69)55(94)81-41(21-11-13-25-63)56(95)82-45(33-84)58(97)76-35(2)52(91)80-42(22-14-26-70-60(66)67)54(93)78-40(50(65)89)20-10-12-24-62/h4-9,16-19,34-36,39-45,49,84-85H,10-15,20-33,62-64H2,1-3H3,(H2,65,89)(H,72,86)(H,73,92)(H,74,98)(H,75,87)(H,76,97)(H,77,88)(H,78,93)(H,79,90)(H,80,91)(H,81,94)(H,82,95)(H,83,96)(H4,66,67,70)(H4,68,69,71) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue Pharma Discovery Research
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 310: 783-92 (2004)
Article DOI: 10.1124/jpet.103.063313
BindingDB Entry DOI: 10.7270/Q2J67FHD |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase ATR
(Homo sapiens (Human)) | BDBM350085

(3-[3-[4-[dideuterio(methylamino)methyl]phenyl]isox...)Show SMILES CNCc1ccc(cc1)-c1cc(on1)-c1nc(cnc1N)-c1ccc(cc1)S(=O)(=O)C(C)C Show InChI InChI=1S/C24H25N5O3S/c1-15(2)33(30,31)19-10-8-18(9-11-19)21-14-27-24(25)23(28-21)22-12-20(29-32-22)17-6-4-16(5-7-17)13-26-3/h4-12,14-15,26H,13H2,1-3H3,(H2,25,27) | PDB
Reactome pathway
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| US Patent
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
VERTEX PHARMACEUTICALS INCORPORATED
US Patent
| Assay Description
Compounds can be screened for their ability to inhibit ATR kinase using a radioactive-phosphate incorporation assay. Assays are carried out in a mixt... |
US Patent US10822331 (2020)
BindingDB Entry DOI: 10.7270/Q2V98C59 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase ATR
(Homo sapiens (Human)) | BDBM350085

(3-[3-[4-[dideuterio(methylamino)methyl]phenyl]isox...)Show SMILES CNCc1ccc(cc1)-c1cc(on1)-c1nc(cnc1N)-c1ccc(cc1)S(=O)(=O)C(C)C Show InChI InChI=1S/C24H25N5O3S/c1-15(2)33(30,31)19-10-8-18(9-11-19)21-14-27-24(25)23(28-21)22-12-20(29-32-22)17-6-4-16(5-7-17)13-26-3/h4-12,14-15,26H,13H2,1-3H3,(H2,25,27) | PDB
Reactome pathway
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| US Patent
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Incorporated
US Patent
| Assay Description
Compounds can be screened for their ability to inhibit ATR kinase using a radioactive-phosphate incorporation assay. Assays are carried out in a mixt... |
US Patent US10208027 (2019)
BindingDB Entry DOI: 10.7270/Q27S7QX5 |
More data for this
Ligand-Target Pair | |
Polycomb protein EED
(Homo sapiens (Human)) | BDBM50594944
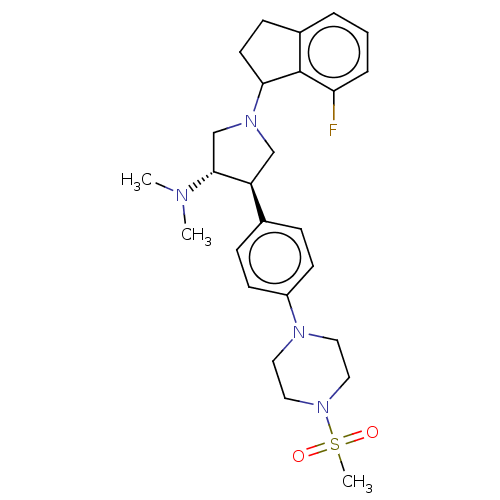
(CHEMBL5181703)Show SMILES CN(C)[C@@H]1CN(C[C@H]1c1ccc(cc1)N1CCN(CC1)S(C)(=O)=O)C1CCc2cccc(F)c12 |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114144
BindingDB Entry DOI: 10.7270/Q2DN4921 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(RAT) | BDBM50241132

(3-Hydroxy-2-phenyl-propionic acid 9-methyl-3-oxa-9...)Show SMILES C[N+]1(C)[C@H]2C[C@@H](C[C@@H]1[C@H]1O[C@@H]21)OC(=O)[C@H](CO)c1ccccc1 |r,TLB:9:8:4.5.6:1,9:10:4.5.6:1,THB:11:5:1:8.10| Show InChI InChI=1S/C18H24NO4/c1-19(2)14-8-12(9-15(19)17-16(14)23-17)22-18(21)13(10-20)11-6-4-3-5-7-11/h3-7,12-17,20H,8-10H2,1-2H3/q+1/t12-,13-,14-,15+,16-,17+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 256: 1173-81 (1991)
BindingDB Entry DOI: 10.7270/Q2BC3X18 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(RAT) | BDBM50241132

(3-Hydroxy-2-phenyl-propionic acid 9-methyl-3-oxa-9...)Show SMILES C[N+]1(C)[C@H]2C[C@@H](C[C@@H]1[C@H]1O[C@@H]21)OC(=O)[C@H](CO)c1ccccc1 |r,TLB:9:8:4.5.6:1,9:10:4.5.6:1,THB:11:5:1:8.10| Show InChI InChI=1S/C18H24NO4/c1-19(2)14-8-12(9-15(19)17-16(14)23-17)22-18(21)13(10-20)11-6-4-3-5-7-11/h3-7,12-17,20H,8-10H2,1-2H3/q+1/t12-,13-,14-,15+,16-,17+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 0.310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 256: 1173-81 (1991)
BindingDB Entry DOI: 10.7270/Q2BC3X18 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50113787

(CHEMBL81056 | S-(2-{2-[2-(4-Carbamimidoyl-phenoxy)...)Show SMILES NC(=N)c1ccc(OCC[C@@H]2CCCCN2C(=O)[C@@H](CC2CCCCC2)NCC(O)=O)cc1 Show InChI InChI=1S/C25H38N4O4/c26-24(27)19-9-11-21(12-10-19)33-15-13-20-8-4-5-14-29(20)25(32)22(28-17-23(30)31)16-18-6-2-1-3-7-18/h9-12,18,20,22,28H,1-8,13-17H2,(H3,26,27)(H,30,31)/t20-,22+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
In vitro inhibition of human thrombin. |
J Med Chem 45: 2432-53 (2002)
BindingDB Entry DOI: 10.7270/Q2S181VR |
More data for this
Ligand-Target Pair | |
Carboxypeptidase B
(Sus scrofa) | BDBM50201438
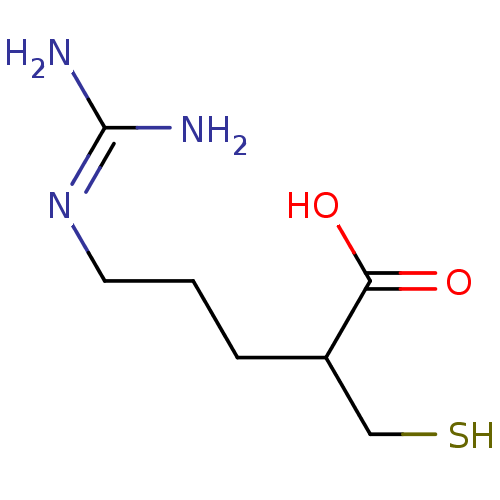
((+/-)-5-guanidino-2-(mercaptomethyl)pentanoic acid...)Show SMILES [#7]\[#6](-[#7])=[#7]\[#6]-[#6]-[#6]-[#6](-[#6]-[#16])-[#6](-[#8])=O Show InChI InChI=1S/C7H15N3O2S/c8-7(9)10-3-1-2-5(4-13)6(11)12/h5,13H,1-4H2,(H,11,12)(H4,8,9,10) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of porcine pancreatic carboxypeptidase B |
J Med Chem 50: 6095-103 (2007)
Article DOI: 10.1021/jm0702433
BindingDB Entry DOI: 10.7270/Q2T153CG |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(RAT) | BDBM50241132

(3-Hydroxy-2-phenyl-propionic acid 9-methyl-3-oxa-9...)Show SMILES C[N+]1(C)[C@H]2C[C@@H](C[C@@H]1[C@H]1O[C@@H]21)OC(=O)[C@H](CO)c1ccccc1 |r,TLB:9:8:4.5.6:1,9:10:4.5.6:1,THB:11:5:1:8.10| Show InChI InChI=1S/C18H24NO4/c1-19(2)14-8-12(9-15(19)17-16(14)23-17)22-18(21)13(10-20)11-6-4-3-5-7-11/h3-7,12-17,20H,8-10H2,1-2H3/q+1/t12-,13-,14-,15+,16-,17+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 0.460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 256: 1173-81 (1991)
BindingDB Entry DOI: 10.7270/Q2BC3X18 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(RAT) | BDBM50241132

(3-Hydroxy-2-phenyl-propionic acid 9-methyl-3-oxa-9...)Show SMILES C[N+]1(C)[C@H]2C[C@@H](C[C@@H]1[C@H]1O[C@@H]21)OC(=O)[C@H](CO)c1ccccc1 |r,TLB:9:8:4.5.6:1,9:10:4.5.6:1,THB:11:5:1:8.10| Show InChI InChI=1S/C18H24NO4/c1-19(2)14-8-12(9-15(19)17-16(14)23-17)22-18(21)13(10-20)11-6-4-3-5-7-11/h3-7,12-17,20H,8-10H2,1-2H3/q+1/t12-,13-,14-,15+,16-,17+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 0.460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 256: 1173-81 (1991)
BindingDB Entry DOI: 10.7270/Q2BC3X18 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50113790
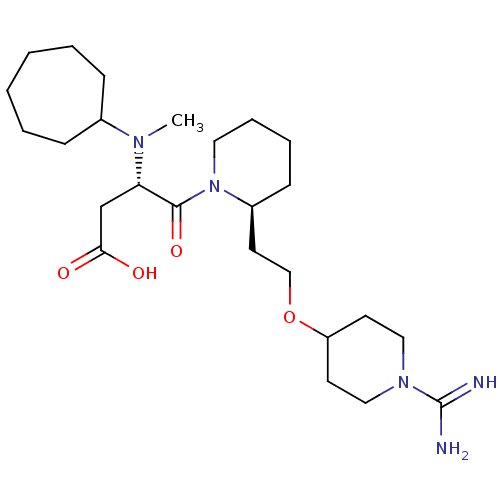
(CHEMBL84389 | S-4-{2-[2-(1-Carbamimidoyl-piperidin...)Show SMILES CN([C@@H](CC(O)=O)C(=O)N1CCCC[C@H]1CCOC1CCN(CC1)C(N)=N)C1CCCCCC1 Show InChI InChI=1S/C25H45N5O4/c1-28(19-8-4-2-3-5-9-19)22(18-23(31)32)24(33)30-14-7-6-10-20(30)13-17-34-21-11-15-29(16-12-21)25(26)27/h19-22H,2-18H2,1H3,(H3,26,27)(H,31,32)/t20-,22-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.530 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
In vitro inhibition of human thrombin. |
J Med Chem 45: 2432-53 (2002)
BindingDB Entry DOI: 10.7270/Q2S181VR |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM86491

(DiPOA | [8-(3,3-Diphenyl-propyl)-4-oxo-1-phenyl-1,...)Show SMILES OC(=O)CN1CN(c2ccccc2)C2(CCN(CCC(c3ccccc3)c3ccccc3)CC2)C1=O Show InChI InChI=1S/C30H33N3O3/c34-28(35)22-32-23-33(26-14-8-3-9-15-26)30(29(32)36)17-20-31(21-18-30)19-16-27(24-10-4-1-5-11-24)25-12-6-2-7-13-25/h1-15,27H,16-23H2,(H,34,35) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 0.760 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue Pharma Discovery Research
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 310: 783-92 (2004)
Article DOI: 10.1124/jpet.103.063313
BindingDB Entry DOI: 10.7270/Q2J67FHD |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50113791

(CHEMBL84229 | S-4-{2-[2-(1-Carbamimidoyl-piperidin...)Show SMILES CN([C@@H](CC(O)=O)C(=O)N1CCCC[C@H]1CCOC1CCN(CC1)C(N)=N)C1CCC=CCC1 |c:32| Show InChI InChI=1S/C25H43N5O4/c1-28(19-8-4-2-3-5-9-19)22(18-23(31)32)24(33)30-14-7-6-10-20(30)13-17-34-21-11-15-29(16-12-21)25(26)27/h2-3,19-22H,4-18H2,1H3,(H3,26,27)(H,31,32)/t20-,22-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
In vitro inhibition of human thrombin. |
J Med Chem 45: 2432-53 (2002)
BindingDB Entry DOI: 10.7270/Q2S181VR |
More data for this
Ligand-Target Pair | |
Receptor-interacting serine/threonine-protein kinase 1
(Homo sapiens (Human)) | BDBM50159507
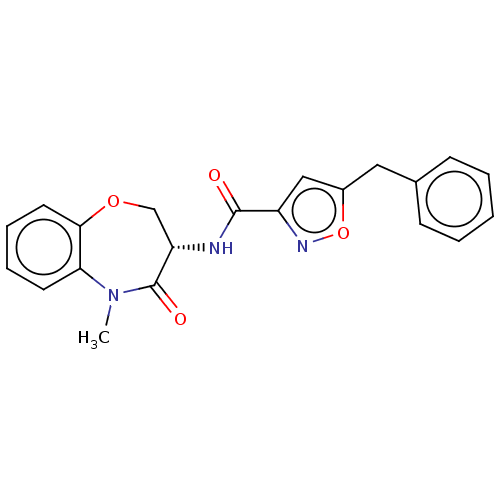
(CHEMBL3785703)Show SMILES CN1c2ccccc2OC[C@H](NC(=O)c2cc(Cc3ccccc3)on2)C1=O |r| Show InChI InChI=1S/C21H19N3O4/c1-24-18-9-5-6-10-19(18)27-13-17(21(24)26)22-20(25)16-12-15(28-23-16)11-14-7-3-2-4-8-14/h2-10,12,17H,11,13H2,1H3,(H,22,25)/t17-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Competitive inhibition of human RIP1 (1 to 375 residues) in presence of increasing ATP by ADP-Glo reagent based assay |
J Med Chem 59: 2163-78 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01898
BindingDB Entry DOI: 10.7270/Q26H4K97 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Muscarinic acetylcholine receptor M2
(RAT) | BDBM50176065
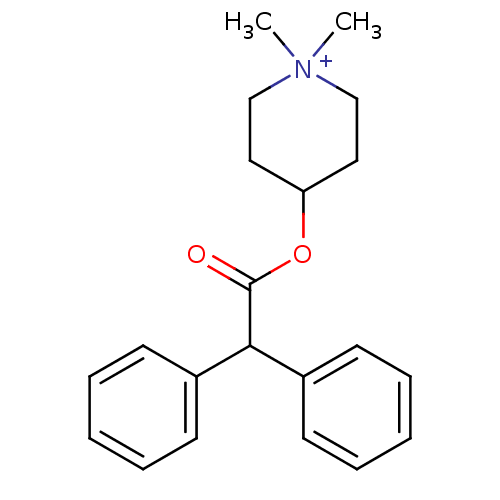
(4-DAMP | 4-Diphenylacetoxy-1,1-dimethyl-piperidini...)Show InChI InChI=1S/C21H26NO2/c1-22(2)15-13-19(14-16-22)24-21(23)20(17-9-5-3-6-10-17)18-11-7-4-8-12-18/h3-12,19-20H,13-16H2,1-2H3/q+1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.850 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 256: 1173-81 (1991)
BindingDB Entry DOI: 10.7270/Q2BC3X18 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50113785
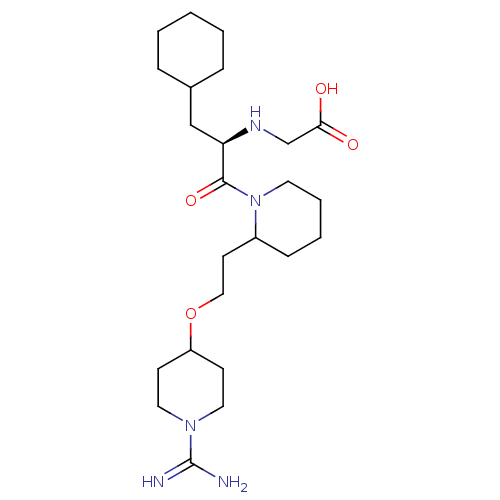
(CHEMBL309670 | RS-(2-{2-[2-(1-Carbamimidoyl-piperi...)Show SMILES NC(=N)N1CCC(CC1)OCCC1CCCCN1C(=O)[C@@H](CC1CCCCC1)NCC(O)=O Show InChI InChI=1S/C24H43N5O4/c25-24(26)28-13-9-20(10-14-28)33-15-11-19-8-4-5-12-29(19)23(32)21(27-17-22(30)31)16-18-6-2-1-3-7-18/h18-21,27H,1-17H2,(H3,25,26)(H,30,31)/t19?,21-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 0.960 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
In vitro inhibition of human thrombin. |
J Med Chem 45: 2432-53 (2002)
BindingDB Entry DOI: 10.7270/Q2S181VR |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase ATR
(Homo sapiens (Human)) | BDBM350085

(3-[3-[4-[dideuterio(methylamino)methyl]phenyl]isox...)Show SMILES CNCc1ccc(cc1)-c1cc(on1)-c1nc(cnc1N)-c1ccc(cc1)S(=O)(=O)C(C)C Show InChI InChI=1S/C24H25N5O3S/c1-15(2)33(30,31)19-10-8-18(9-11-19)21-14-27-24(25)23(28-21)22-12-20(29-32-22)17-6-4-16(5-7-17)13-26-3/h4-12,14-15,26H,13H2,1-3H3,(H2,25,27) | PDB
Reactome pathway
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| US Patent
| <1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Incorporated
US Patent
| Assay Description
Compounds can be screened for their ability to inhibit ATR kinase using a radioactive-phosphate incorporation assay. Assays are carried out in a mixt... |
US Patent US10208027 (2019)
BindingDB Entry DOI: 10.7270/Q27S7QX5 |
More data for this
Ligand-Target Pair | |
B1 bradykinin receptor
(Homo sapiens (Human)) | BDBM50151885

(1-[4-(2,2-Diphenyl-ethylamino)-3-(morpholine-4-car...)Show SMILES CN(C)CCCN(CCCN(C)C)C(=O)C1CCN(CC1)S(=O)(=O)c1ccc(NCC(c2ccccc2)c2ccccc2)c(c1)C(=O)N1CCOCC1 Show InChI InChI=1S/C41H58N6O5S/c1-43(2)21-11-23-45(24-12-22-44(3)4)40(48)35-19-25-47(26-20-35)53(50,51)36-17-18-39(37(31-36)41(49)46-27-29-52-30-28-46)42-32-38(33-13-7-5-8-14-33)34-15-9-6-10-16-34/h5-10,13-18,31,35,38,42H,11-12,19-30,32H2,1-4H3 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity for Bradykinin receptor B1 expressed in HEK293 cells |
J Med Chem 47: 4642-4 (2004)
Article DOI: 10.1021/jm049747g
BindingDB Entry DOI: 10.7270/Q2J965V7 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase ATR
(Homo sapiens (Human)) | BDBM350085

(3-[3-[4-[dideuterio(methylamino)methyl]phenyl]isox...)Show SMILES CNCc1ccc(cc1)-c1cc(on1)-c1nc(cnc1N)-c1ccc(cc1)S(=O)(=O)C(C)C Show InChI InChI=1S/C24H25N5O3S/c1-15(2)33(30,31)19-10-8-18(9-11-19)21-14-27-24(25)23(28-21)22-12-20(29-32-22)17-6-4-16(5-7-17)13-26-3/h4-12,14-15,26H,13H2,1-3H3,(H2,25,27) | PDB
Reactome pathway
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| US Patent
| <1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Incorporated
US Patent
| Assay Description
Compounds can be screened for their ability to inhibit ATR kinase using a radioactive-phosphate incorporation assay. Assays are carried out in a mixt... |
US Patent US10208027 (2019)
BindingDB Entry DOI: 10.7270/Q27S7QX5 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase ATR
(Homo sapiens (Human)) | BDBM350085

(3-[3-[4-[dideuterio(methylamino)methyl]phenyl]isox...)Show SMILES CNCc1ccc(cc1)-c1cc(on1)-c1nc(cnc1N)-c1ccc(cc1)S(=O)(=O)C(C)C Show InChI InChI=1S/C24H25N5O3S/c1-15(2)33(30,31)19-10-8-18(9-11-19)21-14-27-24(25)23(28-21)22-12-20(29-32-22)17-6-4-16(5-7-17)13-26-3/h4-12,14-15,26H,13H2,1-3H3,(H2,25,27) | PDB
Reactome pathway
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| US Patent
| <1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
VERTEX PHARMACEUTICALS INCORPORATED
US Patent
| Assay Description
Compounds can be screened for their ability to inhibit ATR kinase using a radioactive-phosphate incorporation assay. Assays are carried out in a mixt... |
US Patent US10822331 (2020)
BindingDB Entry DOI: 10.7270/Q2V98C59 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase ATR
(Homo sapiens (Human)) | BDBM350085

(3-[3-[4-[dideuterio(methylamino)methyl]phenyl]isox...)Show SMILES CNCc1ccc(cc1)-c1cc(on1)-c1nc(cnc1N)-c1ccc(cc1)S(=O)(=O)C(C)C Show InChI InChI=1S/C24H25N5O3S/c1-15(2)33(30,31)19-10-8-18(9-11-19)21-14-27-24(25)23(28-21)22-12-20(29-32-22)17-6-4-16(5-7-17)13-26-3/h4-12,14-15,26H,13H2,1-3H3,(H2,25,27) | PDB
Reactome pathway
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| US Patent
| <1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
VERTEX PHARMACEUTICALS INCORPORATED
US Patent
| Assay Description
Compounds can be screened for their ability to inhibit ATR kinase using a radioactive-phosphate incorporation assay. Assays are carried out in a mixt... |
US Patent US10822331 (2020)
BindingDB Entry DOI: 10.7270/Q2V98C59 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50113799
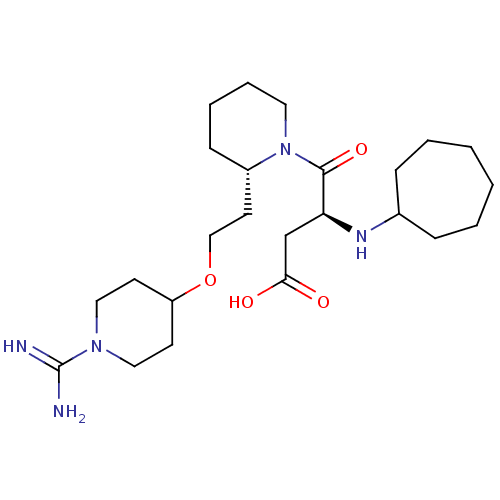
(CHEMBL309403 | S-4-{2-[2-(1-Carbamimidoyl-piperidi...)Show SMILES NC(=N)N1CCC(CC1)OCC[C@@H]1CCCCN1C(=O)[C@H](CC(O)=O)NC1CCCCCC1 Show InChI InChI=1S/C24H43N5O4/c25-24(26)28-14-10-20(11-15-28)33-16-12-19-9-5-6-13-29(19)23(32)21(17-22(30)31)27-18-7-3-1-2-4-8-18/h18-21,27H,1-17H2,(H3,25,26)(H,30,31)/t19-,21-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
In vitro inhibition of human thrombin. |
J Med Chem 45: 2432-53 (2002)
BindingDB Entry DOI: 10.7270/Q2S181VR |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(RAT) | BDBM50176065

(4-DAMP | 4-Diphenylacetoxy-1,1-dimethyl-piperidini...)Show InChI InChI=1S/C21H26NO2/c1-22(2)15-13-19(14-16-22)24-21(23)20(17-9-5-3-6-10-17)18-11-7-4-8-12-18/h3-12,19-20H,13-16H2,1-2H3/q+1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 256: 1173-81 (1991)
BindingDB Entry DOI: 10.7270/Q2BC3X18 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50113794
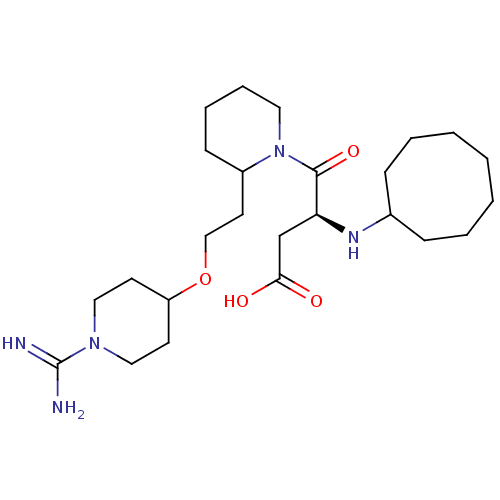
(CHEMBL82658 | RS-4-{2-[2-(1-Carbamimidoyl-piperidi...)Show SMILES NC(=N)N1CCC(CC1)OCCC1CCCCN1C(=O)[C@H](CC(O)=O)NC1CCCCCCC1 Show InChI InChI=1S/C25H45N5O4/c26-25(27)29-15-11-21(12-16-29)34-17-13-20-10-6-7-14-30(20)24(33)22(18-23(31)32)28-19-8-4-2-1-3-5-9-19/h19-22,28H,1-18H2,(H3,26,27)(H,31,32)/t20?,22-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
In vitro inhibition of human thrombin. |
J Med Chem 45: 2432-53 (2002)
BindingDB Entry DOI: 10.7270/Q2S181VR |
More data for this
Ligand-Target Pair | |
Integrin alpha-V/beta-5
(Homo sapiens (Human)) | BDBM50078714
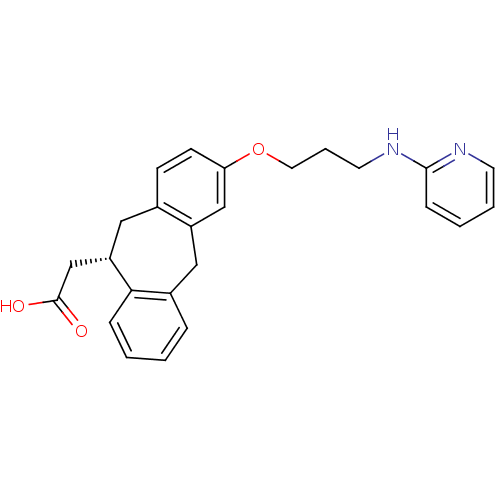
(CHEMBL288493 | SB-265123 | {(S)-3-[3-(Pyridin-2-yl...)Show SMILES OC(=O)C[C@@H]1Cc2ccc(OCCCNc3ccccn3)cc2Cc2ccccc12 Show InChI InChI=1S/C25H26N2O3/c28-25(29)17-21-14-18-9-10-22(16-20(18)15-19-6-1-2-7-23(19)21)30-13-5-12-27-24-8-3-4-11-26-24/h1-4,6-11,16,21H,5,12-15,17H2,(H,26,27)(H,28,29)/t21-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity for non-peptide Vitronectin receptor (alpha V beta 3) |
Bioorg Med Chem Lett 9: 1807-12 (1999)
BindingDB Entry DOI: 10.7270/Q2Q23ZFC |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50088301

((E)-N,N-dimethyl-N-(4-(2-p-tolyl-6,7-dihydro-5H-be...)Show SMILES Cc1ccc(cc1)-c1ccc2CCCC(=Cc2c1)C(=O)Nc1ccc(C[N+](C)(C)C2CCOCC2)cc1 |c:15| Show InChI InChI=1S/C33H38N2O2/c1-24-7-11-27(12-8-24)28-14-13-26-5-4-6-29(22-30(26)21-28)33(36)34-31-15-9-25(10-16-31)23-35(2,3)32-17-19-37-20-18-32/h7-16,21-22,32H,4-6,17-20,23H2,1-3H3/p+1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound against specific binding of [125I]-MIP-1 alpha to human CCR5 receptor |
Bioorg Med Chem Lett 11: 265-70 (2001)
BindingDB Entry DOI: 10.7270/Q2668CFZ |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 12
(Homo sapiens (Human)) | BDBM50226748
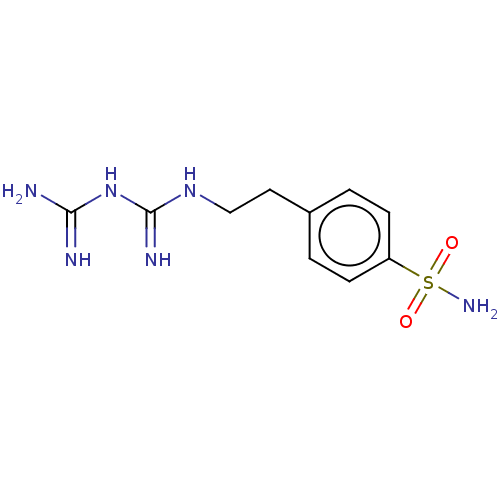
(CHEMBL4080055)Show InChI InChI=1S/C10H16N6O2S/c11-9(12)16-10(13)15-6-5-7-1-3-8(4-2-7)19(14,17)18/h1-4H,5-6H2,(H2,14,17,18)(H6,11,12,13,15,16) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Maastricht University Medical Centre
Curated by ChEMBL
| Assay Description
Binding affinity to recombinant human carbonic anhydrase 12 after 15 mins by stopped-flow CO2 hydration assay |
Eur J Med Chem 127: 691-702 (2017)
Article DOI: 10.1016/j.ejmech.2016.10.037
BindingDB Entry DOI: 10.7270/Q2MK6G3G |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50113802
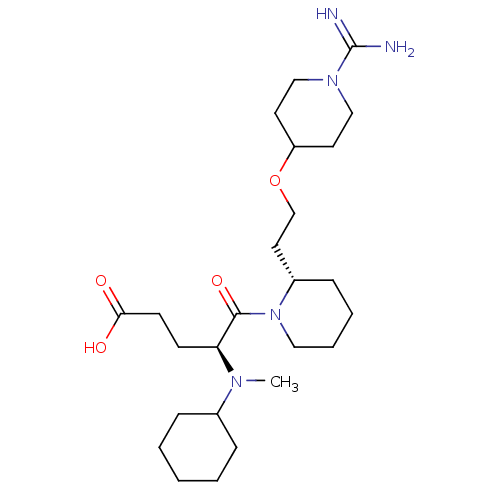
(5-{2-[2-(1-Carbamimidoyl-piperidin-4-yloxy)-ethyl]...)Show SMILES CN([C@@H](CCC(O)=O)C(=O)N1CCCC[C@H]1CCOC1CCN(CC1)C(N)=N)C1CCCCC1 Show InChI InChI=1S/C25H45N5O4/c1-28(19-7-3-2-4-8-19)22(10-11-23(31)32)24(33)30-15-6-5-9-20(30)14-18-34-21-12-16-29(17-13-21)25(26)27/h19-22H,2-18H2,1H3,(H3,26,27)(H,31,32)/t20-,22-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
In vitro inhibition of human thrombin. |
J Med Chem 45: 2432-53 (2002)
BindingDB Entry DOI: 10.7270/Q2S181VR |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 1
(RAT) | BDBM50197248
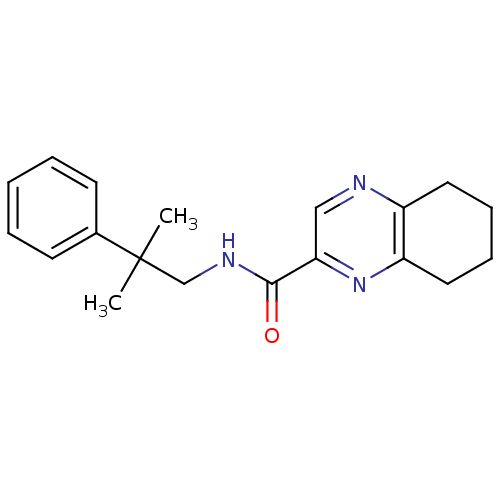
(CHEMBL246244 | N-(2-methyl-2-phenylpropyl)-5,6,7,8...)Show InChI InChI=1S/C19H23N3O/c1-19(2,14-8-4-3-5-9-14)13-21-18(23)17-12-20-15-10-6-7-11-16(15)22-17/h3-5,8-9,12H,6-7,10-11,13H2,1-2H3,(H,21,23) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Antagonist activity at rat mGluR1a expressed in CHO cells assessed as increase in calcium internalisation by FLIPR assay |
Bioorg Med Chem Lett 17: 486-90 (2007)
Article DOI: 10.1016/j.bmcl.2006.10.015
BindingDB Entry DOI: 10.7270/Q2VX0G5Q |
More data for this
Ligand-Target Pair | |
Carboxypeptidase N catalytic chain
(Homo sapiens (Human)) | BDBM50201438

((+/-)-5-guanidino-2-(mercaptomethyl)pentanoic acid...)Show SMILES [#7]\[#6](-[#7])=[#7]\[#6]-[#6]-[#6]-[#6](-[#6]-[#16])-[#6](-[#8])=O Show InChI InChI=1S/C7H15N3O2S/c8-7(9)10-3-1-2-5(4-13)6(11)12/h5,13H,1-4H2,(H,11,12)(H4,8,9,10) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human plasma carboxypeptidase N |
J Med Chem 50: 6095-103 (2007)
Article DOI: 10.1021/jm0702433
BindingDB Entry DOI: 10.7270/Q2T153CG |
More data for this
Ligand-Target Pair | |
Sigma non-opioid intracellular receptor 1
(Homo sapiens (Human)) | BDBM50048866

(1-Cyclohexyl-4-[3-(5-methoxy-1,2,3,4-tetrahydro-na...)Show InChI InChI=1S/C24H38N2O/c1-27-24-14-6-12-22-20(8-5-13-23(22)24)9-7-15-25-16-18-26(19-17-25)21-10-3-2-4-11-21/h6,12,14,20-21H,2-5,7-11,13,15-19H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
QBI COVID-19 Research Group (QCRG)
Curated by ChEMBL
| Assay Description
Displacement of [3H]-pentazocin from the Sigma1 receptor |
Nature 583: 459-468 (2020)
Article DOI: 10.1038/s41586-020-2286-9
BindingDB Entry DOI: 10.7270/Q29Z984K |
More data for this
Ligand-Target Pair | |
Sigma non-opioid intracellular receptor 1
(Homo sapiens (Human)) | BDBM50251208
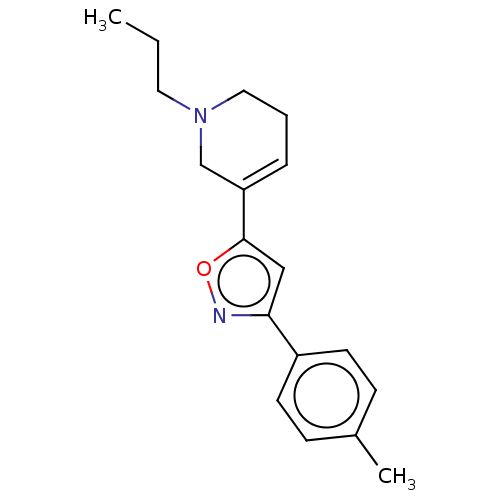
(CHEMBL4088272)Show InChI InChI=1S/C18H22N2O/c1-3-10-20-11-4-5-16(13-20)18-12-17(19-21-18)15-8-6-14(2)7-9-15/h5-9,12H,3-4,10-11,13H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
QBI COVID-19 Research Group (QCRG)
Curated by ChEMBL
| Assay Description
Displacement of [3H]-pentazocin from the Sigma1 receptor |
Nature 583: 459-468 (2020)
Article DOI: 10.1038/s41586-020-2286-9
BindingDB Entry DOI: 10.7270/Q29Z984K |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Metabotropic glutamate receptor 1
(RAT) | BDBM50197275

(CHEMBL246023 | N-(2-(4-fluorophenyl)-2-methylpropy...)Show InChI InChI=1S/C19H18FN3O/c1-19(2,13-7-9-14(20)10-8-13)12-22-18(24)17-11-21-15-5-3-4-6-16(15)23-17/h3-11H,12H2,1-2H3,(H,22,24) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Antagonist activity at rat mGluR1a expressed in CHO cells assessed as increase in calcium internalisation by FLIPR assay |
Bioorg Med Chem Lett 17: 486-90 (2007)
Article DOI: 10.1016/j.bmcl.2006.10.015
BindingDB Entry DOI: 10.7270/Q2VX0G5Q |
More data for this
Ligand-Target Pair | |
Integrin alpha-V/beta-3
(Homo sapiens (Human)) | BDBM50059133
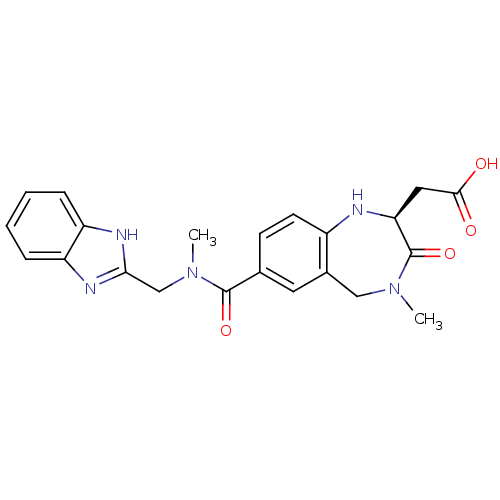
(CHEMBL50106 | SB-223245 | {(S)-7-[(1H-Benzoimidazo...)Show SMILES CN(Cc1nc2ccccc2[nH]1)C(=O)c1ccc2N[C@@H](CC(O)=O)C(=O)N(C)Cc2c1 Show InChI InChI=1S/C22H23N5O4/c1-26-11-14-9-13(7-8-15(14)23-18(22(26)31)10-20(28)29)21(30)27(2)12-19-24-16-5-3-4-6-17(16)25-19/h3-9,18,23H,10-12H2,1-2H3,(H,24,25)(H,28,29)/t18-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of HEK 293 cell adhesion to vitronectin by alpha V beta 3 |
Bioorg Med Chem Lett 9: 1807-12 (1999)
BindingDB Entry DOI: 10.7270/Q2Q23ZFC |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM86258
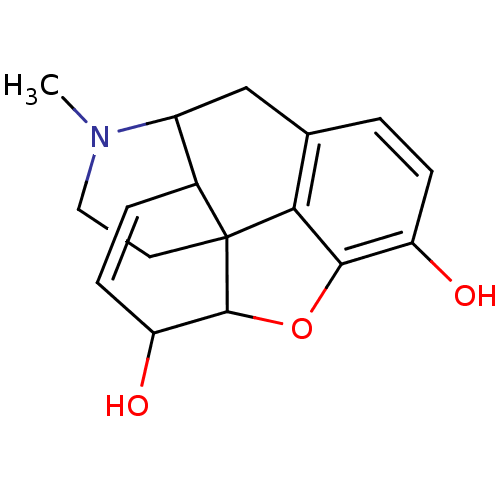
(CAS_23552-18-3 | Morphine | NSC_5980)Show SMILES CN1CCC23C4Oc5c2c(CC1C3C=CC4O)ccc5O |c:16,TLB:13:12:1.2.3:10.9.8| Show InChI InChI=1S/C17H19NO3/c1-18-7-6-17-10-3-5-13(20)16(17)21-15-12(19)4-2-9(14(15)17)8-11(10)18/h2-5,10-11,13,16,19-20H,6-8H2,1H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 2.06 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue Pharma Discovery Research
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 310: 783-92 (2004)
Article DOI: 10.1124/jpet.103.063313
BindingDB Entry DOI: 10.7270/Q2J67FHD |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prothrombin
(Homo sapiens (Human)) | BDBM50113792
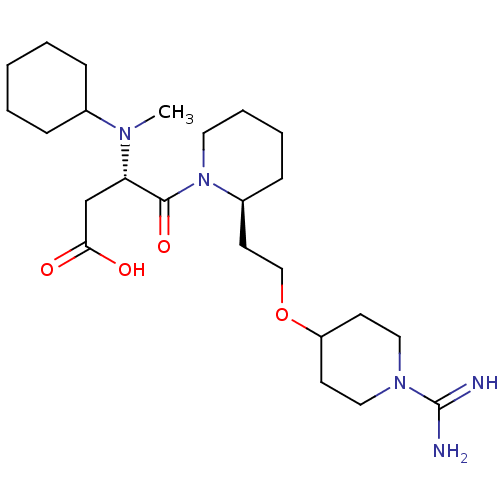
(CHEMBL81521 | S-4-{2-[2-(1-Carbamimidoyl-piperidin...)Show SMILES CN([C@@H](CC(O)=O)C(=O)N1CCCC[C@H]1CCOC1CCN(CC1)C(N)=N)C1CCCCC1 Show InChI InChI=1S/C24H43N5O4/c1-27(18-7-3-2-4-8-18)21(17-22(30)31)23(32)29-13-6-5-9-19(29)12-16-33-20-10-14-28(15-11-20)24(25)26/h18-21H,2-17H2,1H3,(H3,25,26)(H,30,31)/t19-,21-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 2.17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
In vitro inhibition of human thrombin. |
J Med Chem 45: 2432-53 (2002)
BindingDB Entry DOI: 10.7270/Q2S181VR |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM86493
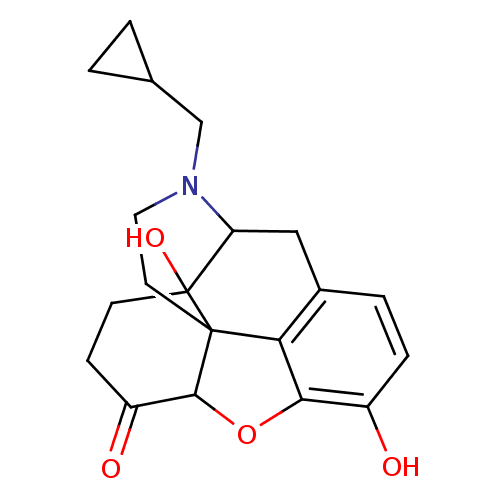
(CAS_27943 | NALTREXONE-HCl | NSC_27943 | Naltrexon...)Show SMILES Oc1ccc2CC3N(CC4CC4)CCC45C(Oc1c24)C(=O)CCC35O |TLB:22:23:7.12.13:5.4.18,THB:24:23:7.12.13:5.4.18| Show InChI InChI=1S/C20H23NO4/c22-13-4-3-12-9-15-20(24)6-5-14(23)18-19(20,16(12)17(13)25-18)7-8-21(15)10-11-1-2-11/h3-4,11,15,18,22,24H,1-2,5-10H2 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.39 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue Pharma Discovery Research
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 310: 783-92 (2004)
Article DOI: 10.1124/jpet.103.063313
BindingDB Entry DOI: 10.7270/Q2J67FHD |
More data for this
Ligand-Target Pair | |
Sigma intracellular receptor 2
(Homo sapiens (Human)) | BDBM50048866

(1-Cyclohexyl-4-[3-(5-methoxy-1,2,3,4-tetrahydro-na...)Show InChI InChI=1S/C24H38N2O/c1-27-24-14-6-12-22-20(8-5-13-23(22)24)9-7-15-25-16-18-26(19-17-25)21-10-3-2-4-11-21/h6,12,14,20-21H,2-5,7-11,13,15-19H2,1H3 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
QBI COVID-19 Research Group (QCRG)
Curated by ChEMBL
| Assay Description
Displacement of [3H]-DTG from the Sigma2 receptor |
Nature 583: 459-468 (2020)
Article DOI: 10.1038/s41586-020-2286-9
BindingDB Entry DOI: 10.7270/Q29Z984K |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM150251
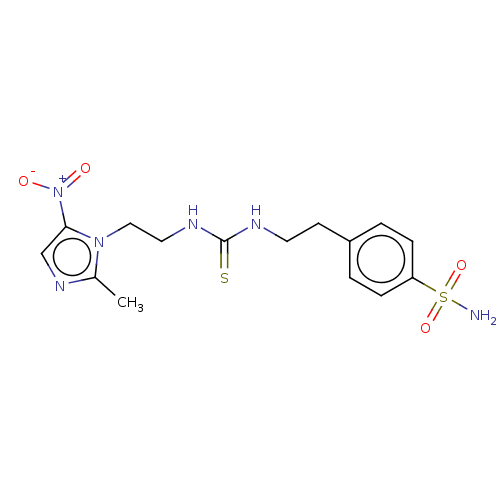
(US8980932, 6c (DH310))Show SMILES Cc1ncc(n1CCNC(=S)NCCc1ccc(cc1)S(N)(=O)=O)[N+]([O-])=O Show InChI InChI=1S/C15H20N6O4S2/c1-11-19-10-14(21(22)23)20(11)9-8-18-15(26)17-7-6-12-2-4-13(5-3-12)27(16,24)25/h2-5,10H,6-9H2,1H3,(H2,16,24,25)(H2,17,18,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ecole Nationale Sup£rieure de Chimie de Montpellier
Curated by ChEMBL
| Assay Description
Inhibition of full length human cytosolic carbonic anhydrase-2 preincubated for 15 mins by stopped-flow CO2 hydration assay |
J Med Chem 56: 8512-20 (2013)
Article DOI: 10.1021/jm4009532
BindingDB Entry DOI: 10.7270/Q2GQ71QP |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 1
(RAT) | BDBM50197249
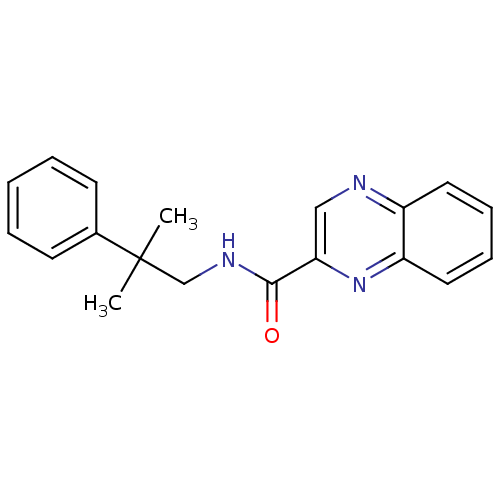
(CHEMBL245819 | N-(2-methyl-2-phenylpropyl)quinoxal...)Show InChI InChI=1S/C19H19N3O/c1-19(2,14-8-4-3-5-9-14)13-21-18(23)17-12-20-15-10-6-7-11-16(15)22-17/h3-12H,13H2,1-2H3,(H,21,23) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Antagonist activity at rat mGluR1a expressed in CHO cells assessed as increase in calcium internalisation by FLIPR assay |
Bioorg Med Chem Lett 17: 486-90 (2007)
Article DOI: 10.1016/j.bmcl.2006.10.015
BindingDB Entry DOI: 10.7270/Q2VX0G5Q |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 1
(RAT) | BDBM50197254
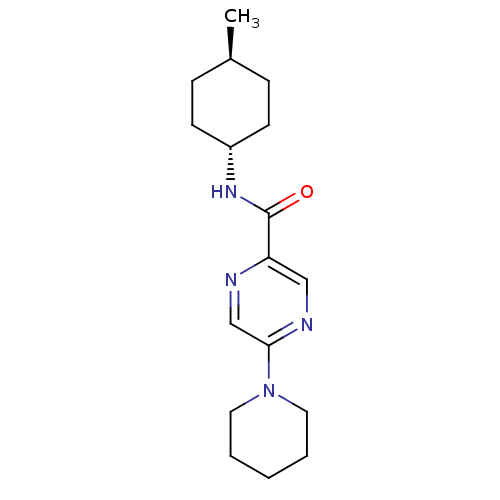
(CHEMBL246250 | N-((1r,4r)-4-methylcyclohexyl)-5-(p...)Show SMILES C[C@H]1CC[C@@H](CC1)NC(=O)c1cnc(cn1)N1CCCCC1 |wU:4.7,wD:1.0,(7.01,-30.31,;5.68,-31.07,;4.34,-30.31,;3.01,-31.08,;3.02,-32.61,;4.35,-33.38,;5.68,-32.61,;1.69,-33.39,;.35,-32.62,;.35,-31.08,;-.98,-33.4,;-.97,-34.95,;-2.31,-35.72,;-3.64,-34.95,;-3.64,-33.4,;-2.31,-32.63,;-4.97,-35.72,;-6.31,-34.95,;-7.64,-35.71,;-7.65,-37.25,;-6.31,-38.02,;-4.97,-37.26,)| Show InChI InChI=1S/C17H26N4O/c1-13-5-7-14(8-6-13)20-17(22)15-11-19-16(12-18-15)21-9-3-2-4-10-21/h11-14H,2-10H2,1H3,(H,20,22)/t13-,14- | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Antagonist activity at rat mGluR1a expressed in CHO cells assessed as increase in calcium internalisation by FLIPR assay |
Bioorg Med Chem Lett 17: 486-90 (2007)
Article DOI: 10.1016/j.bmcl.2006.10.015
BindingDB Entry DOI: 10.7270/Q2VX0G5Q |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 1
(RAT) | BDBM50278288
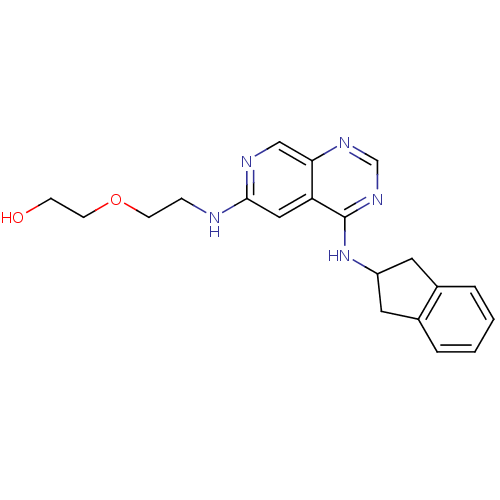
(2-(2-(4-(2,3-dihydro-1H-inden-2-ylamino)pyrido[3,4...)Show InChI InChI=1S/C20H23N5O2/c26-6-8-27-7-5-21-19-11-17-18(12-22-19)23-13-24-20(17)25-16-9-14-3-1-2-4-15(14)10-16/h1-4,11-13,16,26H,5-10H2,(H,21,22)(H,23,24,25) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical Research Council Technology
Curated by ChEMBL
| Assay Description
Antagonist activity at rat mGluR1a expressed in CHO cells assessed as inhibition of glutamate-evoked increase in calcium internalization preincubated... |
Bioorg Med Chem Lett 19: 2190-4 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.106
BindingDB Entry DOI: 10.7270/Q2057FSP |
More data for this
Ligand-Target Pair | |
Plasminogen
(Bos taurus) | BDBM50113792

(CHEMBL81521 | S-4-{2-[2-(1-Carbamimidoyl-piperidin...)Show SMILES CN([C@@H](CC(O)=O)C(=O)N1CCCC[C@H]1CCOC1CCN(CC1)C(N)=N)C1CCCCC1 Show InChI InChI=1S/C24H43N5O4/c1-27(18-7-3-2-4-8-18)21(17-22(30)31)23(32)29-13-6-5-9-19(29)12-16-33-20-10-14-28(15-11-20)24(25)26/h18-21H,2-17H2,1H3,(H3,25,26)(H,30,31)/t19-,21-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 3.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory effect on plasmin in bovine plasma |
J Med Chem 45: 2432-53 (2002)
BindingDB Entry DOI: 10.7270/Q2S181VR |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM21130
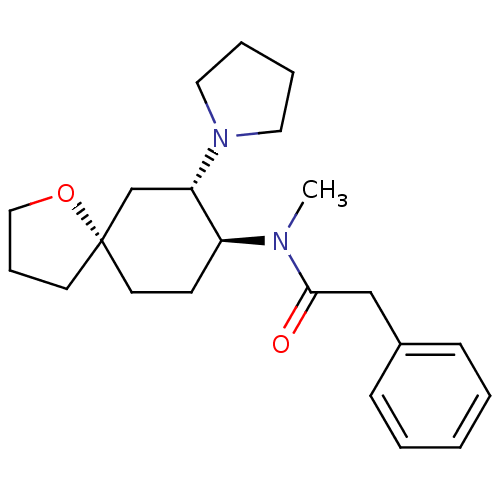
(N-methyl-2-phenyl-N-[(5R,7S,8S)-7-(pyrrolidin-1-yl...)Show SMILES CN([C@H]1CC[C@@]2(CCCO2)C[C@@H]1N1CCCC1)C(=O)Cc1ccccc1 Show InChI InChI=1S/C22H32N2O2/c1-23(21(25)16-18-8-3-2-4-9-18)19-10-12-22(11-7-15-26-22)17-20(19)24-13-5-6-14-24/h2-4,8-9,19-20H,5-7,10-17H2,1H3/t19-,20-,22-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3.69 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue Pharma Discovery Research
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 310: 783-92 (2004)
Article DOI: 10.1124/jpet.103.063313
BindingDB Entry DOI: 10.7270/Q2J67FHD |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM150254
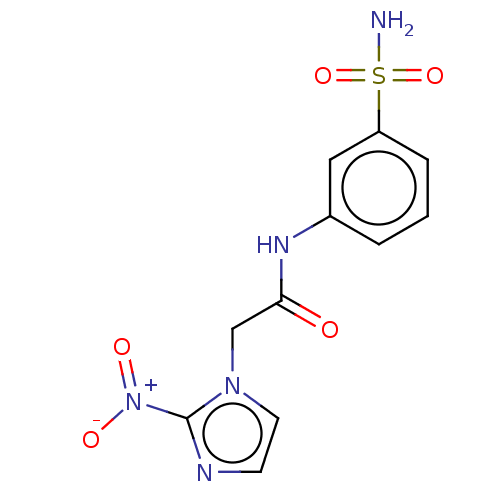
(US8980932, 4d (DH302))Show SMILES NS(=O)(=O)c1cccc(NC(=O)Cn2ccnc2[N+]([O-])=O)c1 Show InChI InChI=1S/C11H11N5O5S/c12-22(20,21)9-3-1-2-8(6-9)14-10(17)7-15-5-4-13-11(15)16(18)19/h1-6H,7H2,(H,14,17)(H2,12,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ecole Nationale Sup£rieure de Chimie de Montpellier
Curated by ChEMBL
| Assay Description
Inhibition of full length human cytosolic carbonic anhydrase-2 preincubated for 15 mins by stopped-flow CO2 hydration assay |
J Med Chem 56: 8512-20 (2013)
Article DOI: 10.1021/jm4009532
BindingDB Entry DOI: 10.7270/Q2GQ71QP |
More data for this
Ligand-Target Pair | |
Carboxypeptidase B2
(Homo sapiens (Human)) | BDBM50201438

((+/-)-5-guanidino-2-(mercaptomethyl)pentanoic acid...)Show SMILES [#7]\[#6](-[#7])=[#7]\[#6]-[#6]-[#6]-[#6](-[#6]-[#16])-[#6](-[#8])=O Show InChI InChI=1S/C7H15N3O2S/c8-7(9)10-3-1-2-5(4-13)6(11)12/h5,13H,1-4H2,(H,11,12)(H4,8,9,10) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human TAFIa |
J Med Chem 50: 6095-103 (2007)
Article DOI: 10.1021/jm0702433
BindingDB Entry DOI: 10.7270/Q2T153CG |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data