

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Casein kinase I isoform delta (Homo sapiens (Human)) | BDBM50537592 (CHEMBL4632881) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 Y181C reverse transcriptase. | J Med Chem 62: 5298-5311 (2019) Article DOI: 10.1021/acs.jmedchem.9b00058 BindingDB Entry DOI: 10.7270/Q24170ZD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM521018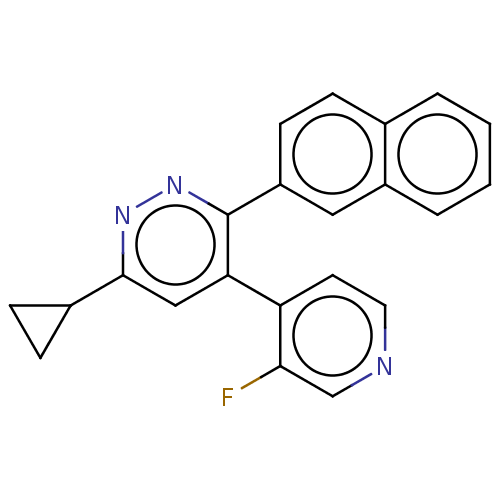 (US11149020, Compound 10 (MW-167)) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 86 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The concentration dependent ability of compounds to inhibit human p38α MAPK, p38β MAPK and CK-1δ were done essentially as described in... | Citation and Details BindingDB Entry DOI: 10.7270/Q2251NBP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM521021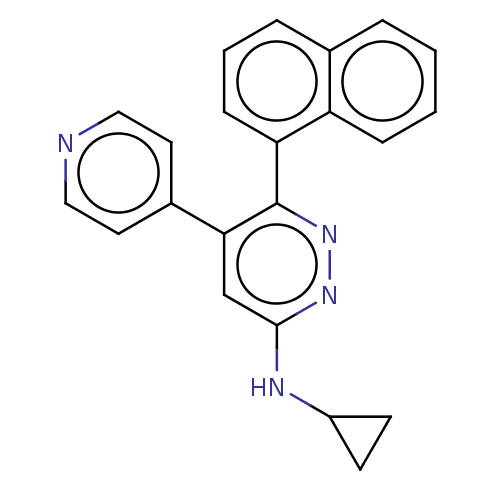 (US11149020, Compound 13 (MW-107)) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 91 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The concentration dependent ability of compounds to inhibit human p38α MAPK, p38β MAPK and CK-1δ were done essentially as described in... | Citation and Details BindingDB Entry DOI: 10.7270/Q2251NBP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM521019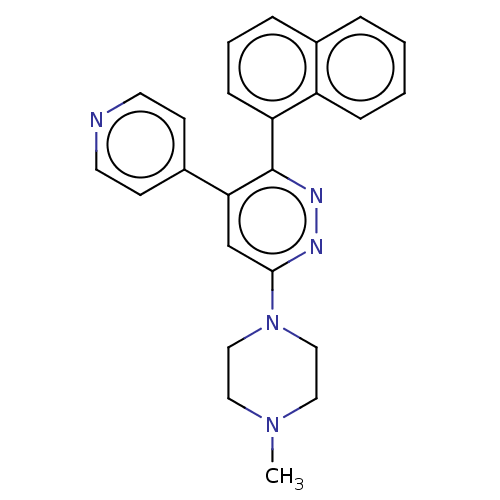 (US11149020, Compound 11 (MW-122)) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 98 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The concentration dependent ability of compounds to inhibit human p38α MAPK, p38β MAPK and CK-1δ were done essentially as described in... | Citation and Details BindingDB Entry DOI: 10.7270/Q2251NBP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM521025 (US11149020, Compound 16 (MW-200)) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The concentration dependent ability of compounds to inhibit human p38α MAPK, p38β MAPK and CK-1δ were done essentially as described in... | Citation and Details BindingDB Entry DOI: 10.7270/Q2251NBP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM50537600 (CHEMBL4129018 | US11149020, Compound 27 (MW-150)) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB Article PubMed | 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 Y188L reverse transcriptase. | J Med Chem 62: 5298-5311 (2019) Article DOI: 10.1021/acs.jmedchem.9b00058 BindingDB Entry DOI: 10.7270/Q24170ZD | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM50537600 (CHEMBL4129018 | US11149020, Compound 27 (MW-150)) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB US Patent | 101 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The concentration dependent ability of compounds to inhibit human p38α MAPK, p38β MAPK and CK-1δ were done essentially as described in... | Citation and Details BindingDB Entry DOI: 10.7270/Q2251NBP | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM521045 (US11149020, Compound 36 (MW-164)) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 101 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The concentration dependent ability of compounds to inhibit human p38α MAPK, p38β MAPK and CK-1δ were done essentially as described in... | Citation and Details BindingDB Entry DOI: 10.7270/Q2251NBP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM50537599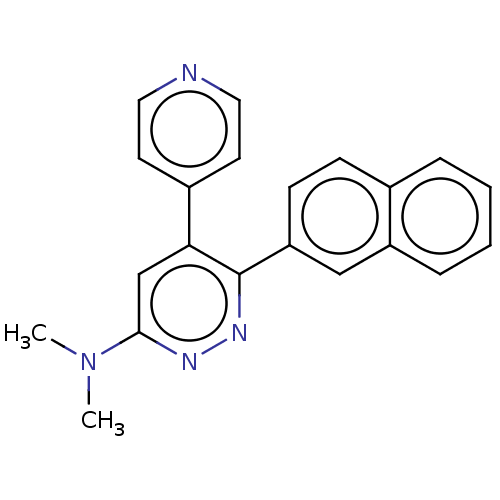 (CHEMBL4648060 | US11149020, Compound 2 (MW-108)) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | 110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 Mutant Reverse transcriptase G190A | J Med Chem 62: 5298-5311 (2019) Article DOI: 10.1021/acs.jmedchem.9b00058 BindingDB Entry DOI: 10.7270/Q24170ZD | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM50537599 (CHEMBL4648060 | US11149020, Compound 2 (MW-108)) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB US Patent | 114 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The concentration dependent ability of compounds to inhibit human p38α MAPK, p38β MAPK and CK-1δ were done essentially as described in... | Citation and Details BindingDB Entry DOI: 10.7270/Q2251NBP | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM521017 (US11149020, Compound 9 (MW-125)) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 127 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The concentration dependent ability of compounds to inhibit human p38α MAPK, p38β MAPK and CK-1δ were done essentially as described in... | Citation and Details BindingDB Entry DOI: 10.7270/Q2251NBP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM50537598 (CHEMBL4646628 | US11149020, Compound 1 (MW-181)) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | 180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 Mutant Reverse transcriptase P236L | J Med Chem 62: 5298-5311 (2019) Article DOI: 10.1021/acs.jmedchem.9b00058 BindingDB Entry DOI: 10.7270/Q24170ZD | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM50537598 (CHEMBL4646628 | US11149020, Compound 1 (MW-181)) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB US Patent | 184 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The concentration dependent ability of compounds to inhibit human p38α MAPK, p38β MAPK and CK-1δ were done essentially as described in... | Citation and Details BindingDB Entry DOI: 10.7270/Q2251NBP | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM521016 (US11149020, Compound 7 (MW-077)) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 186 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The concentration dependent ability of compounds to inhibit human p38α MAPK, p38β MAPK and CK-1δ were done essentially as described in... | Citation and Details BindingDB Entry DOI: 10.7270/Q2251NBP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM521024 (US11149020, Compound 15 (MW-156)) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 276 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The concentration dependent ability of compounds to inhibit human p38α MAPK, p38β MAPK and CK-1δ were done essentially as described in... | Citation and Details BindingDB Entry DOI: 10.7270/Q2251NBP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 11 (Homo sapiens (Human)) | BDBM50537598 (CHEMBL4646628 | US11149020, Compound 1 (MW-181)) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | 320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 Y188L reverse transcriptase. | J Med Chem 62: 5298-5311 (2019) Article DOI: 10.1021/acs.jmedchem.9b00058 BindingDB Entry DOI: 10.7270/Q24170ZD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM521047 (N,N-dimethyl-5-(naphthalen-1-yl)-6-(pyridin-4-yl)p...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 343 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The concentration dependent ability of compounds to inhibit human p38α MAPK, p38β MAPK and CK-1δ were done essentially as described in... | Citation and Details BindingDB Entry DOI: 10.7270/Q2251NBP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM50537592 (CHEMBL4632881) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | 620 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 Mutant Reverse transcriptase K103N | J Med Chem 62: 5298-5311 (2019) Article DOI: 10.1021/acs.jmedchem.9b00058 BindingDB Entry DOI: 10.7270/Q24170ZD | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM50537597 (CHEMBL4645737 | US11149020, Compound 6 (MW-105)) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 657 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The concentration dependent ability of compounds to inhibit human p38α MAPK, p38β MAPK and CK-1δ were done essentially as described in... | Citation and Details BindingDB Entry DOI: 10.7270/Q2251NBP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Papain (Carica papaya) | BDBM50437951 (CHEMBL2408922 | US9403843, 24a) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 1.62E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois College of Pharmacy Curated by ChEMBL | Assay Description Inhibition of papain after using SucLLVYAMC as substrate by FRET assay | J Med Chem 56: 6054-68 (2013) Article DOI: 10.1021/jm4006719 BindingDB Entry DOI: 10.7270/Q2VT1TG7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Calpain-1 catalytic subunit (Sus scrofa (pig)) | BDBM50437950 (CHEMBL2408917) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 2.59E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois College of Pharmacy Curated by ChEMBL | Assay Description Inhibition of pig full length Cal1 after using SucLLVYAMC as substrate by FRET assay | J Med Chem 56: 6054-68 (2013) Article DOI: 10.1021/jm4006719 BindingDB Entry DOI: 10.7270/Q2VT1TG7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Calpain-1 catalytic subunit (Sus scrofa (pig)) | BDBM240262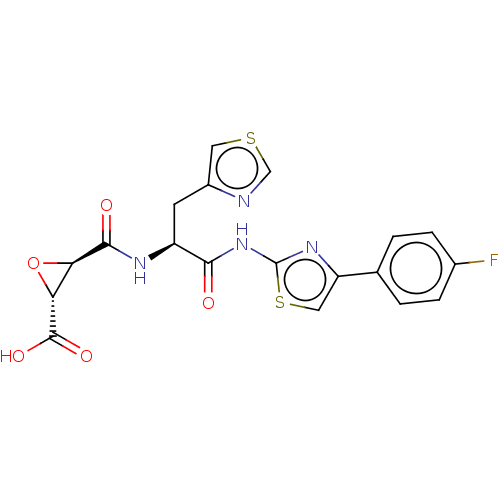 (US9403843, 33) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | US Patent | 2.60E+3 | -32.4 | n/a | n/a | n/a | n/a | n/a | 7.6 | 30 |
THE TRUSTEES OF COLUMBIA UNIVERSITY IN THE CITY OF NEW YORK; THE BOARD OF TRUSTEES OF THE UNIVERSITY OF ILLINOIS US Patent | Assay Description Full length porcine calpain (156 nM), or papain (236 pM) was added to a solution of 100 mM NaCl, 50 mM HEPES, pH 7.6, 1 mM TCEP, 30 μM Suc-LLVY-AM... | US Patent US9403843 (2016) BindingDB Entry DOI: 10.7270/Q2KP8124 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Calpain-1 catalytic subunit (Sus scrofa (pig)) | BDBM50437948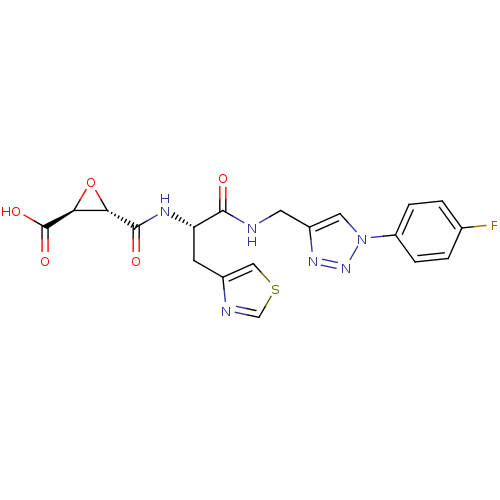 (CHEMBL2408899 | US9403843, 50) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem | Article PubMed | 2.89E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois College of Pharmacy Curated by ChEMBL | Assay Description Inhibition of pig full length Cal1 after using SucLLVYAMC as substrate by FRET assay | J Med Chem 56: 6054-68 (2013) Article DOI: 10.1021/jm4006719 BindingDB Entry DOI: 10.7270/Q2VT1TG7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Calpain-1 catalytic subunit (Sus scrofa (pig)) | BDBM50437948 (CHEMBL2408899 | US9403843, 50) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem | US Patent | 2.90E+3 | -32.1 | n/a | n/a | n/a | n/a | n/a | 7.6 | 30 |
THE TRUSTEES OF COLUMBIA UNIVERSITY IN THE CITY OF NEW YORK; THE BOARD OF TRUSTEES OF THE UNIVERSITY OF ILLINOIS US Patent | Assay Description Full length porcine calpain (156 nM), or papain (236 pM) was added to a solution of 100 mM NaCl, 50 mM HEPES, pH 7.6, 1 mM TCEP, 30 μM Suc-LLVY-AM... | US Patent US9403843 (2016) BindingDB Entry DOI: 10.7270/Q2KP8124 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Calpain-1 catalytic subunit (Sus scrofa (pig)) | BDBM50437949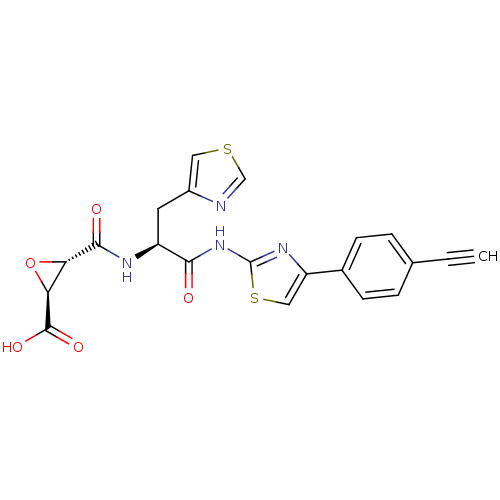 (CHEMBL2408918 | US9403843, 34) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | US Patent | 3.50E+3 | -31.7 | n/a | n/a | n/a | n/a | n/a | 7.6 | 30 |
THE TRUSTEES OF COLUMBIA UNIVERSITY IN THE CITY OF NEW YORK; THE BOARD OF TRUSTEES OF THE UNIVERSITY OF ILLINOIS US Patent | Assay Description Full length porcine calpain (156 nM), or papain (236 pM) was added to a solution of 100 mM NaCl, 50 mM HEPES, pH 7.6, 1 mM TCEP, 30 μM Suc-LLVY-AM... | US Patent US9403843 (2016) BindingDB Entry DOI: 10.7270/Q2KP8124 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Calpain-1 catalytic subunit (Sus scrofa (pig)) | BDBM50437949 (CHEMBL2408918 | US9403843, 34) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 3.53E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois College of Pharmacy Curated by ChEMBL | Assay Description Inhibition of pig full length Cal1 after using SucLLVYAMC as substrate by FRET assay | J Med Chem 56: 6054-68 (2013) Article DOI: 10.1021/jm4006719 BindingDB Entry DOI: 10.7270/Q2VT1TG7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Calpain-1 catalytic subunit (Sus scrofa (pig)) | BDBM50157741 (CHEMBL374508 | E-64 | E64) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 3.96E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois College of Pharmacy Curated by ChEMBL | Assay Description Inhibition of pig full length Cal1 after using SucLLVYAMC as substrate by FRET assay | J Med Chem 56: 6054-68 (2013) Article DOI: 10.1021/jm4006719 BindingDB Entry DOI: 10.7270/Q2VT1TG7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Calpain-1 catalytic subunit (Sus scrofa (pig)) | BDBM240232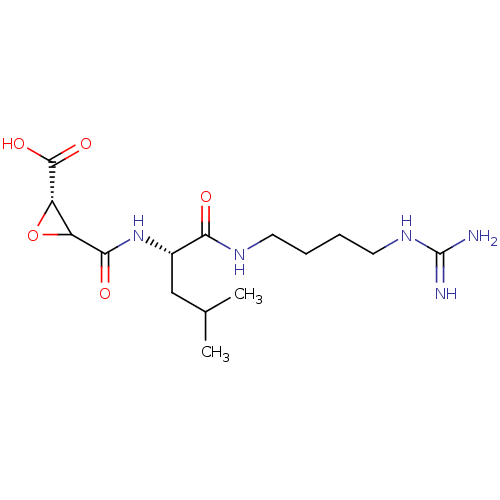 (US9403843, E64) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | US Patent | 4.00E+3 | -31.3 | n/a | n/a | n/a | n/a | n/a | 7.6 | 30 |
THE TRUSTEES OF COLUMBIA UNIVERSITY IN THE CITY OF NEW YORK; THE BOARD OF TRUSTEES OF THE UNIVERSITY OF ILLINOIS US Patent | Assay Description Full length porcine calpain (156 nM), or papain (236 pM) was added to a solution of 100 mM NaCl, 50 mM HEPES, pH 7.6, 1 mM TCEP, 30 μM Suc-LLVY-AM... | US Patent US9403843 (2016) BindingDB Entry DOI: 10.7270/Q2KP8124 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Papain (Carica papaya) | BDBM50157741 (CHEMBL374508 | E-64 | E64) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 5.08E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois College of Pharmacy Curated by ChEMBL | Assay Description Inhibition of papain after using SucLLVYAMC as substrate by FRET assay | J Med Chem 56: 6054-68 (2013) Article DOI: 10.1021/jm4006719 BindingDB Entry DOI: 10.7270/Q2VT1TG7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Papain (Carica papaya) | BDBM50437950 (CHEMBL2408917) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 5.31E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois College of Pharmacy Curated by ChEMBL | Assay Description Inhibition of papain after using SucLLVYAMC as substrate by FRET assay | J Med Chem 56: 6054-68 (2013) Article DOI: 10.1021/jm4006719 BindingDB Entry DOI: 10.7270/Q2VT1TG7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Calpain-1 catalytic subunit (Sus scrofa (pig)) | BDBM50437951 (CHEMBL2408922 | US9403843, 24a) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | US Patent | 6.00E+3 | -30.3 | n/a | n/a | n/a | n/a | n/a | 7.6 | 30 |
THE TRUSTEES OF COLUMBIA UNIVERSITY IN THE CITY OF NEW YORK; THE BOARD OF TRUSTEES OF THE UNIVERSITY OF ILLINOIS US Patent | Assay Description Full length porcine calpain (156 nM), or papain (236 pM) was added to a solution of 100 mM NaCl, 50 mM HEPES, pH 7.6, 1 mM TCEP, 30 μM Suc-LLVY-AM... | US Patent US9403843 (2016) BindingDB Entry DOI: 10.7270/Q2KP8124 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Calpain-1 catalytic subunit (Sus scrofa (pig)) | BDBM50437951 (CHEMBL2408922 | US9403843, 24a) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 6.02E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois College of Pharmacy Curated by ChEMBL | Assay Description Inhibition of pig full length Cal1 after using SucLLVYAMC as substrate by FRET assay | J Med Chem 56: 6054-68 (2013) Article DOI: 10.1021/jm4006719 BindingDB Entry DOI: 10.7270/Q2VT1TG7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Papain (Carica papaya) | BDBM50437949 (CHEMBL2408918 | US9403843, 34) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 9.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois College of Pharmacy Curated by ChEMBL | Assay Description Inhibition of papain after using SucLLVYAMC as substrate by FRET assay | J Med Chem 56: 6054-68 (2013) Article DOI: 10.1021/jm4006719 BindingDB Entry DOI: 10.7270/Q2VT1TG7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Papain (Carica papaya) | BDBM50437948 (CHEMBL2408899 | US9403843, 50) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem | Article PubMed | 1.92E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois College of Pharmacy Curated by ChEMBL | Assay Description Inhibition of papain after using SucLLVYAMC as substrate by FRET assay | J Med Chem 56: 6054-68 (2013) Article DOI: 10.1021/jm4006719 BindingDB Entry DOI: 10.7270/Q2VT1TG7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50241831 (CHEMBL4062273 | US10626113, Compound M | US1089975...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | n/a | n/a | 0.0440 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q2KH0SFD | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50241831 (CHEMBL4062273 | US10626113, Compound M | US1089975...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.0440 | n/a | n/a | n/a | n/a | n/a | n/a |
The Trustees of Columbia University in the City of New York US Patent | Assay Description A series of dilutions of the test compounds were prepared with 10% DMSO in assay buffer and 5 μl of the dilution was added to a 50 μl react... | US Patent US10899756 (2021) BindingDB Entry DOI: 10.7270/Q2RN3C0P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50241831 (CHEMBL4062273 | US10626113, Compound M | US1089975...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | n/a | n/a | 0.0560 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q2KH0SFD | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50241831 (CHEMBL4062273 | US10626113, Compound M | US1089975...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0560 | n/a | n/a | n/a | n/a | n/a | n/a |
Columbia University Curated by ChEMBL | Assay Description Inhibition of human recombinant PDE5A1 using FAM-labelled cGMP as substrate after 60 mins by fluorescence polarization assay | J Med Chem 60: 8858-8875 (2017) Article DOI: 10.1021/acs.jmedchem.7b00979 BindingDB Entry DOI: 10.7270/Q2J67K3P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50241831 (CHEMBL4062273 | US10626113, Compound M | US1089975...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.0560 | n/a | n/a | n/a | n/a | n/a | n/a |
The Trustees of Columbia University in the City of New York US Patent | Assay Description A series of dilutions of the test compounds were prepared with 10% DMSO in assay buffer and 5 μl of the dilution was added to a 50 μl react... | US Patent US10899756 (2021) BindingDB Entry DOI: 10.7270/Q2RN3C0P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50241840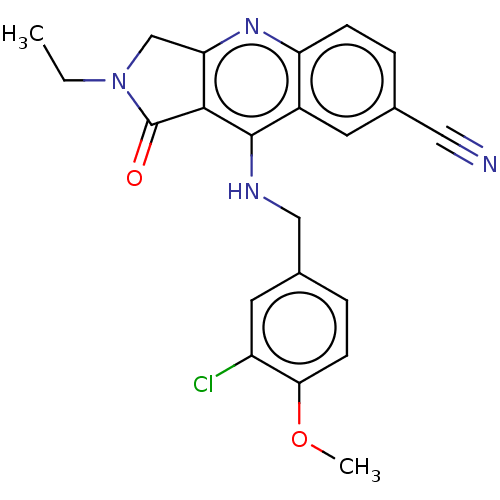 (CHEMBL4072903 | US10899756, Compound K) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.0590 | n/a | n/a | n/a | n/a | n/a | n/a |
The Trustees of Columbia University in the City of New York US Patent | Assay Description A series of dilutions of the test compounds were prepared with 10% DMSO in assay buffer and 5 μl of the dilution was added to a 50 μl react... | US Patent US10899756 (2021) BindingDB Entry DOI: 10.7270/Q2RN3C0P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50241840 (CHEMBL4072903 | US10899756, Compound K) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0590 | n/a | n/a | n/a | n/a | n/a | n/a |
Columbia University Curated by ChEMBL | Assay Description Inhibition of human recombinant PDE5A1 using FAM-labelled cGMP as substrate after 60 mins by fluorescence polarization assay | J Med Chem 60: 8858-8875 (2017) Article DOI: 10.1021/acs.jmedchem.7b00979 BindingDB Entry DOI: 10.7270/Q2J67K3P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM480487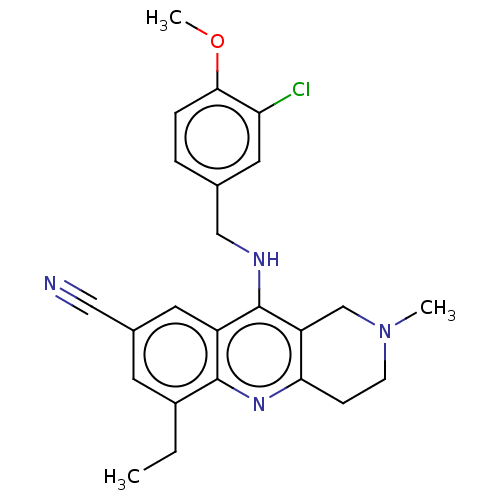 (US10626113, Compound D | US10899756, Compound D) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a |
The Trustees of Columbia University in the City of New York US Patent | Assay Description A series of dilutions of the test compounds were prepared with 10% DMSO in assay buffer and 5 μl of the dilution was added to a 50 μl react... | US Patent US10899756 (2021) BindingDB Entry DOI: 10.7270/Q2RN3C0P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM480487 (US10626113, Compound D | US10899756, Compound D) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q2KH0SFD | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50241832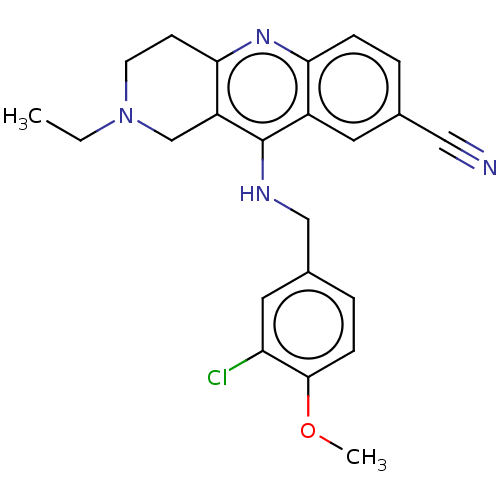 (CHEMBL4083986 | US10626113, Compound C | US1089975...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a |
Columbia University Curated by ChEMBL | Assay Description Inhibition of human recombinant PDE5A1 using FAM-labelled cGMP as substrate after 60 mins by fluorescence polarization assay | J Med Chem 60: 8858-8875 (2017) Article DOI: 10.1021/acs.jmedchem.7b00979 BindingDB Entry DOI: 10.7270/Q2J67K3P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50241832 (CHEMBL4083986 | US10626113, Compound C | US1089975...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q2KH0SFD | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50241832 (CHEMBL4083986 | US10626113, Compound C | US1089975...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | US Patent | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
The Trustees of Columbia University in the City of New York US Patent | Assay Description A series of dilutions of the test compounds were prepared with 10% DMSO in assay buffer and 5 μl of the dilution was added to a 50 μl react... | US Patent US10899756 (2021) BindingDB Entry DOI: 10.7270/Q2RN3C0P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50428976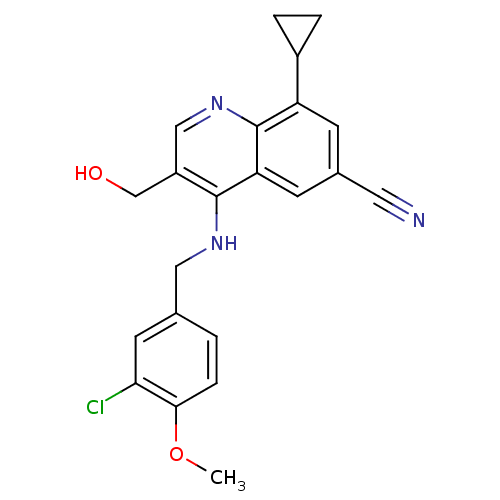 (CHEMBL2333219) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.277 | n/a | n/a | n/a | n/a | n/a | n/a |
Columbia University Curated by ChEMBL | Assay Description Inhibition of PDE5 (unknown origin) using FAM-cGMP as substrate after 60 mins by fluorescence assay | Eur J Med Chem 60: 285-94 (2013) Article DOI: 10.1016/j.ejmech.2012.12.009 BindingDB Entry DOI: 10.7270/Q22F7PSZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50241842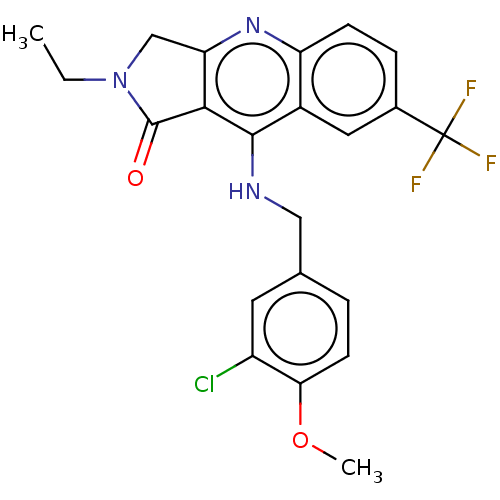 (CHEMBL4064315 | US10899756, Compound M) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.290 | n/a | n/a | n/a | n/a | n/a | n/a |
Columbia University Curated by ChEMBL | Assay Description Inhibition of adenylate cyclase via Adenosine A1 receptor in rat fat cell membranes | J Med Chem 60: 8858-8875 (2017) Article DOI: 10.1021/acs.jmedchem.7b00979 BindingDB Entry DOI: 10.7270/Q2J67K3P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50241842 (CHEMBL4064315 | US10899756, Compound M) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.290 | n/a | n/a | n/a | n/a | n/a | n/a |
The Trustees of Columbia University in the City of New York US Patent | Assay Description A series of dilutions of the test compounds were prepared with 10% DMSO in assay buffer and 5 μl of the dilution was added to a 50 μl react... | US Patent US10899756 (2021) BindingDB Entry DOI: 10.7270/Q2RN3C0P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50241835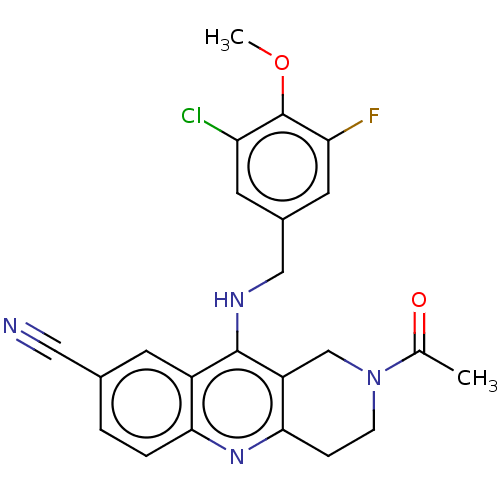 (CHEMBL4092717 | US10899756, Compound AC) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | US Patent | n/a | n/a | 0.320 | n/a | n/a | n/a | n/a | n/a | n/a |
The Trustees of Columbia University in the City of New York US Patent | Assay Description A series of dilutions of the test compounds were prepared with 10% DMSO in assay buffer and 5 μl of the dilution was added to a 50 μl react... | US Patent US10899756 (2021) BindingDB Entry DOI: 10.7270/Q2RN3C0P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 376 total ) | Next | Last >> |