 Found 686 hits with Last Name = 'aubert' and Initial = 'j'
Found 686 hits with Last Name = 'aubert' and Initial = 'j' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Endothelin-1 receptor
(Homo sapiens (Human)) | BDBM50051007
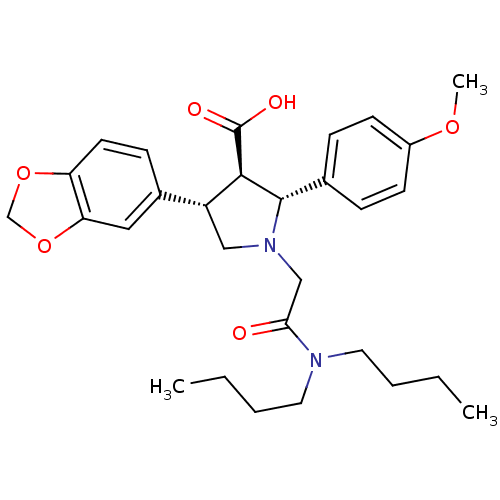
((2R,3R,4S)-4-Benzo[1,3]dioxol-5-yl-1-dibutylcarbam...)Show SMILES CCCCN(CCCC)C(=O)CN1C[C@@H]([C@H]([C@@H]1c1ccc(OC)cc1)C(O)=O)c1ccc2OCOc2c1 Show InChI InChI=1S/C29H38N2O6/c1-4-6-14-30(15-7-5-2)26(32)18-31-17-23(21-10-13-24-25(16-21)37-19-36-24)27(29(33)34)28(31)20-8-11-22(35-3)12-9-20/h8-13,16,23,27-28H,4-7,14-15,17-19H2,1-3H3,(H,33,34)/t23-,27-,28+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centre Hospitalier Universitaire Vaudois (CHUV)
Curated by ChEMBL
| Assay Description
Displacement of [125I]ET-1 from human ET-A receptor expressed in CHO cell membrane |
J Med Chem 59: 8168-88 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01781
BindingDB Entry DOI: 10.7270/Q22N55RM |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50007344
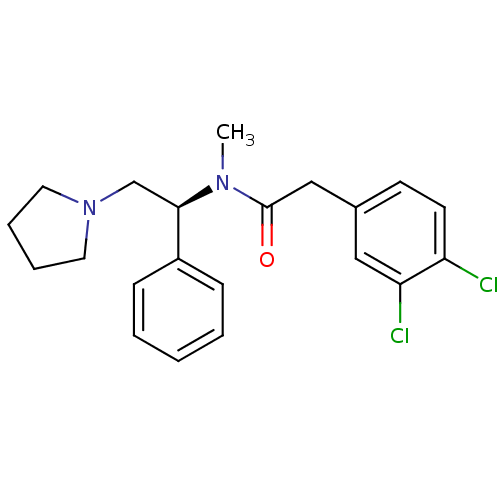
((S)-2-(3,4-Dichloro-phenyl)-N-methyl-N-(1-phenyl-2...)Show SMILES CN([C@H](CN1CCCC1)c1ccccc1)C(=O)Cc1ccc(Cl)c(Cl)c1 |r| Show InChI InChI=1S/C21H24Cl2N2O/c1-24(21(26)14-16-9-10-18(22)19(23)13-16)20(15-25-11-5-6-12-25)17-7-3-2-4-8-17/h2-4,7-10,13,20H,5-6,11-12,14-15H2,1H3/t20-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
In vitro binding affinity for human kappa opioid receptor was determined by using [3H]-diprenorphine as radioligand |
Bioorg Med Chem Lett 15: 1279-82 (2005)
Article DOI: 10.1016/j.bmcl.2005.01.038
BindingDB Entry DOI: 10.7270/Q2QF8SDJ |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50007344

((S)-2-(3,4-Dichloro-phenyl)-N-methyl-N-(1-phenyl-2...)Show SMILES CN([C@H](CN1CCCC1)c1ccccc1)C(=O)Cc1ccc(Cl)c(Cl)c1 |r| Show InChI InChI=1S/C21H24Cl2N2O/c1-24(21(26)14-16-9-10-18(22)19(23)13-16)20(15-25-11-5-6-12-25)17-7-3-2-4-8-17/h2-4,7-10,13,20H,5-6,11-12,14-15H2,1H3/t20-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0440 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Displacement of [3H]diprenorphine from human kappa opioid receptor expressed in CHO-K1 cells |
Bioorg Med Chem Lett 18: 3667-71 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.116
BindingDB Entry DOI: 10.7270/Q2TX3G7F |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50007344

((S)-2-(3,4-Dichloro-phenyl)-N-methyl-N-(1-phenyl-2...)Show SMILES CN([C@H](CN1CCCC1)c1ccccc1)C(=O)Cc1ccc(Cl)c(Cl)c1 |r| Show InChI InChI=1S/C21H24Cl2N2O/c1-24(21(26)14-16-9-10-18(22)19(23)13-16)20(15-25-11-5-6-12-25)17-7-3-2-4-8-17/h2-4,7-10,13,20H,5-6,11-12,14-15H2,1H3/t20-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.0540 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
In vitro binding affinity towards Opioid receptor kappa 1 by displacement of bound [3H]U69,593 |
Bioorg Med Chem Lett 10: 2567-70 (2001)
BindingDB Entry DOI: 10.7270/Q26H4GNG |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50463758
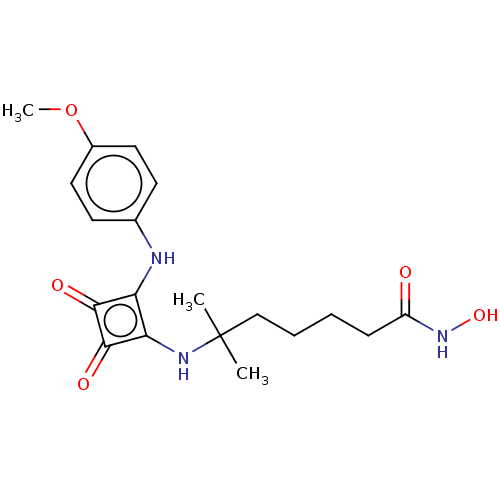
(CHEMBL4250302)Show SMILES COc1ccc(Nc2c(NC(C)(C)CCCCC(=O)NO)c(=O)c2=O)cc1 Show InChI InChI=1S/C19H25N3O5/c1-19(2,11-5-4-6-14(23)22-26)21-16-15(17(24)18(16)25)20-12-7-9-13(27-3)10-8-12/h7-10,20-21,26H,4-6,11H2,1-3H3,(H,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0560 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nestle Skin Health R&D
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC1 using Fluor de Lys as substrate after 2 hrs by fluorescence method |
Bioorg Med Chem Lett 28: 2985-2992 (2018)
Article DOI: 10.1016/j.bmcl.2018.06.029
BindingDB Entry DOI: 10.7270/Q2M0484T |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50463741
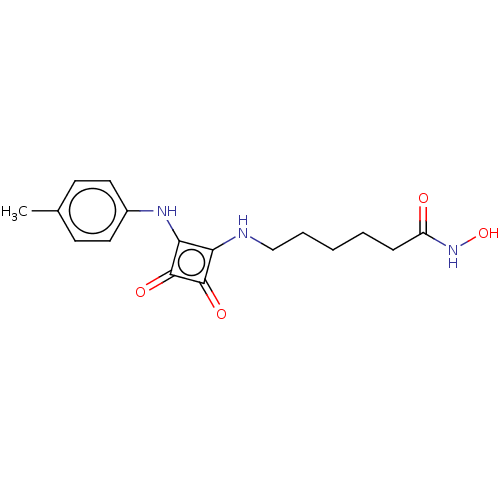
(CHEMBL4239232)Show InChI InChI=1S/C17H21N3O4/c1-11-6-8-12(9-7-11)19-15-14(16(22)17(15)23)18-10-4-2-3-5-13(21)20-24/h6-9,18-19,24H,2-5,10H2,1H3,(H,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0570 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nestle Skin Health R&D
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC1 using Fluor de Lys as substrate after 2 hrs by fluorescence method |
Bioorg Med Chem Lett 28: 2985-2992 (2018)
Article DOI: 10.1016/j.bmcl.2018.06.029
BindingDB Entry DOI: 10.7270/Q2M0484T |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50093965
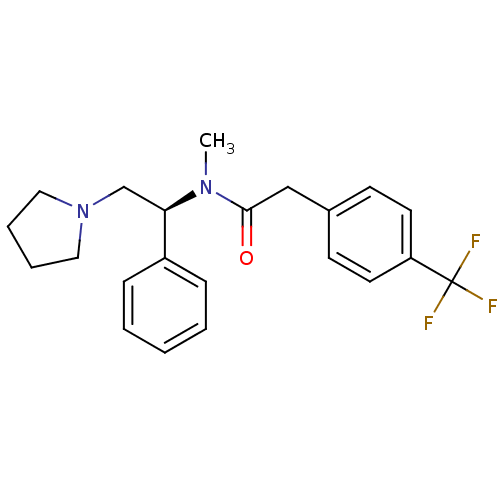
(CHEMBL86324 | N-Methyl-N-((S)-1-phenyl-2-pyrrolidi...)Show SMILES CN([C@H](CN1CCCC1)c1ccccc1)C(=O)Cc1ccc(cc1)C(F)(F)F Show InChI InChI=1S/C22H25F3N2O/c1-26(21(28)15-17-9-11-19(12-10-17)22(23,24)25)20(16-27-13-5-6-14-27)18-7-3-2-4-8-18/h2-4,7-12,20H,5-6,13-16H2,1H3/t20-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0580 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Displacement of bound [3H]-diprenorphine from membranes expressing cloned human kappa opioid receptor |
Bioorg Med Chem Lett 15: 1091-5 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.018
BindingDB Entry DOI: 10.7270/Q2JW8DCG |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50093965

(CHEMBL86324 | N-Methyl-N-((S)-1-phenyl-2-pyrrolidi...)Show SMILES CN([C@H](CN1CCCC1)c1ccccc1)C(=O)Cc1ccc(cc1)C(F)(F)F Show InChI InChI=1S/C22H25F3N2O/c1-26(21(28)15-17-9-11-19(12-10-17)22(23,24)25)20(16-27-13-5-6-14-27)18-7-3-2-4-8-18/h2-4,7-12,20H,5-6,13-16H2,1H3/t20-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.0580 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
In vitro binding affinity towards Opioid receptor kappa 1 by displacement of bound [3H]U69,593 |
Bioorg Med Chem Lett 10: 2567-70 (2001)
BindingDB Entry DOI: 10.7270/Q26H4GNG |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50090002
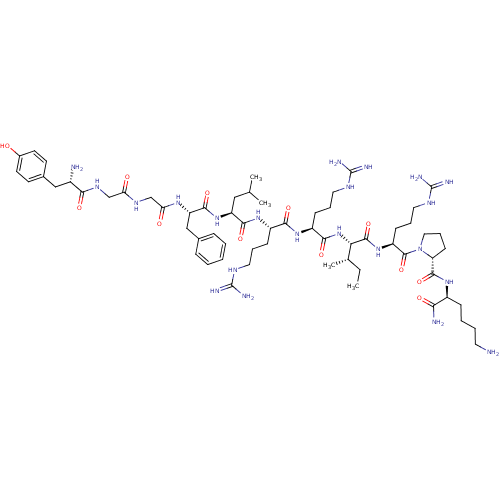
(CHEMBL411003 | Dynorphin A (1-11)-NH2H-Tyr-Gly-Gly...)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](Cc1ccccc1)NC(=O)CNC(=O)CNC(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N1CCC[C@@H]1C(=O)N[C@@H](CCCCN)C(N)=O Show InChI InChI=1S/C63H104N22O12/c1-5-37(4)51(59(96)82-45(20-13-29-75-63(71)72)60(97)85-30-14-21-48(85)58(95)79-42(52(66)89)17-9-10-26-64)84-55(92)44(19-12-28-74-62(69)70)80-54(91)43(18-11-27-73-61(67)68)81-56(93)46(31-36(2)3)83-57(94)47(33-38-15-7-6-8-16-38)78-50(88)35-76-49(87)34-77-53(90)41(65)32-39-22-24-40(86)25-23-39/h6-8,15-16,22-25,36-37,41-48,51,86H,5,9-14,17-21,26-35,64-65H2,1-4H3,(H2,66,89)(H,76,87)(H,77,90)(H,78,88)(H,79,95)(H,80,91)(H,81,93)(H,82,96)(H,83,94)(H,84,92)(H4,67,68,73)(H4,69,70,74)(H4,71,72,75)/t37-,41-,42-,43-,44-,45-,46-,47-,48+,51-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0590 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California
Curated by ChEMBL
| Assay Description
In vitro binding affinity towards human Opioid receptor kappa 1 on CHO cell membranes using [3H]diprenorphine displacement. |
J Med Chem 46: 2104-9 (2003)
Article DOI: 10.1021/jm020125+
BindingDB Entry DOI: 10.7270/Q2NZ88CF |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50463759
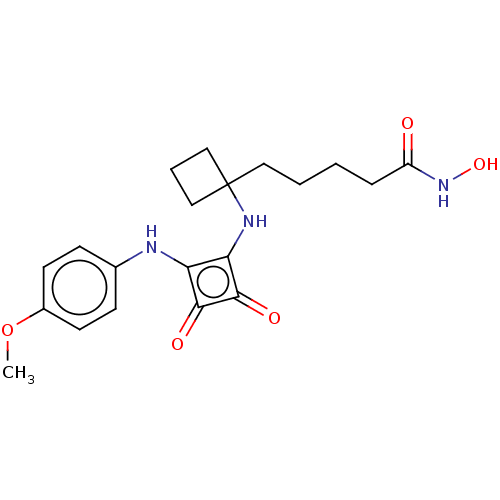
(CHEMBL4237636)Show SMILES COc1ccc(Nc2c(NC3(CCCCC(=O)NO)CCC3)c(=O)c2=O)cc1 Show InChI InChI=1S/C20H25N3O5/c1-28-14-8-6-13(7-9-14)21-16-17(19(26)18(16)25)22-20(11-4-12-20)10-3-2-5-15(24)23-27/h6-9,21-22,27H,2-5,10-12H2,1H3,(H,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0630 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nestle Skin Health R&D
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC1 using Fluor de Lys as substrate after 2 hrs by fluorescence method |
Bioorg Med Chem Lett 28: 2985-2992 (2018)
Article DOI: 10.1016/j.bmcl.2018.06.029
BindingDB Entry DOI: 10.7270/Q2M0484T |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50093964
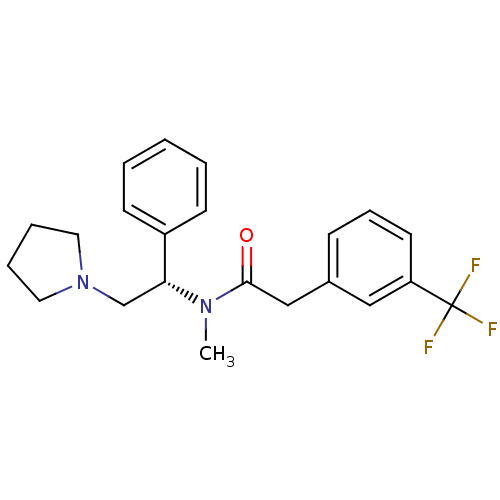
(CHEMBL313484 | N-Methyl-N-((S)-1-phenyl-2-pyrrolid...)Show SMILES CN([C@H](CN1CCCC1)c1ccccc1)C(=O)Cc1cccc(c1)C(F)(F)F Show InChI InChI=1S/C22H25F3N2O/c1-26(21(28)15-17-8-7-11-19(14-17)22(23,24)25)20(16-27-12-5-6-13-27)18-9-3-2-4-10-18/h2-4,7-11,14,20H,5-6,12-13,15-16H2,1H3/t20-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.0640 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
In vitro binding affinity towards Opioid receptor kappa 1 by displacement of bound [3H]U69,593 |
Bioorg Med Chem Lett 10: 2567-70 (2001)
BindingDB Entry DOI: 10.7270/Q26H4GNG |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50093969
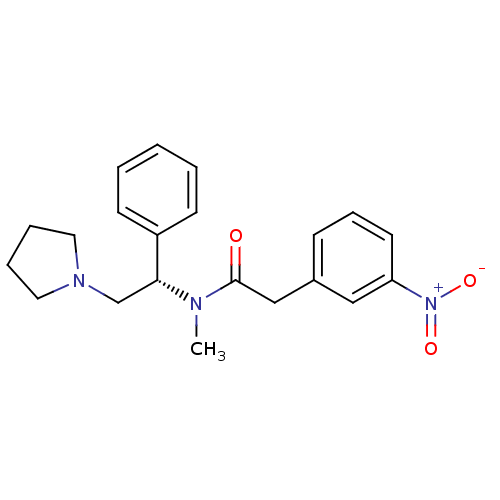
(CHEMBL82919 | N-Methyl-2-(3-nitro-phenyl)-N-((S)-1...)Show SMILES CN([C@H](CN1CCCC1)c1ccccc1)C(=O)Cc1cccc(c1)[N+]([O-])=O Show InChI InChI=1S/C21H25N3O3/c1-22(21(25)15-17-8-7-11-19(14-17)24(26)27)20(16-23-12-5-6-13-23)18-9-3-2-4-10-18/h2-4,7-11,14,20H,5-6,12-13,15-16H2,1H3/t20-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0650 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
In vitro binding affinity towards Opioid receptor kappa 1 by displacement of bound [3H]U69,593 |
Bioorg Med Chem Lett 10: 2567-70 (2001)
BindingDB Entry DOI: 10.7270/Q26H4GNG |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50090002

(CHEMBL411003 | Dynorphin A (1-11)-NH2H-Tyr-Gly-Gly...)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](Cc1ccccc1)NC(=O)CNC(=O)CNC(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N1CCC[C@@H]1C(=O)N[C@@H](CCCCN)C(N)=O Show InChI InChI=1S/C63H104N22O12/c1-5-37(4)51(59(96)82-45(20-13-29-75-63(71)72)60(97)85-30-14-21-48(85)58(95)79-42(52(66)89)17-9-10-26-64)84-55(92)44(19-12-28-74-62(69)70)80-54(91)43(18-11-27-73-61(67)68)81-56(93)46(31-36(2)3)83-57(94)47(33-38-15-7-6-8-16-38)78-50(88)35-76-49(87)34-77-53(90)41(65)32-39-22-24-40(86)25-23-39/h6-8,15-16,22-25,36-37,41-48,51,86H,5,9-14,17-21,26-35,64-65H2,1-4H3,(H2,66,89)(H,76,87)(H,77,90)(H,78,88)(H,79,95)(H,80,91)(H,81,93)(H,82,96)(H,83,94)(H,84,92)(H4,67,68,73)(H4,69,70,74)(H4,71,72,75)/t37-,41-,42-,43-,44-,45-,46-,47-,48+,51-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0740 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California
Curated by ChEMBL
| Assay Description
Binding affinity towards cloned human Opioid receptor kappa 1 in CHO cell membranes. |
J Med Chem 43: 2698-702 (2000)
BindingDB Entry DOI: 10.7270/Q25D8SJ1 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50463739
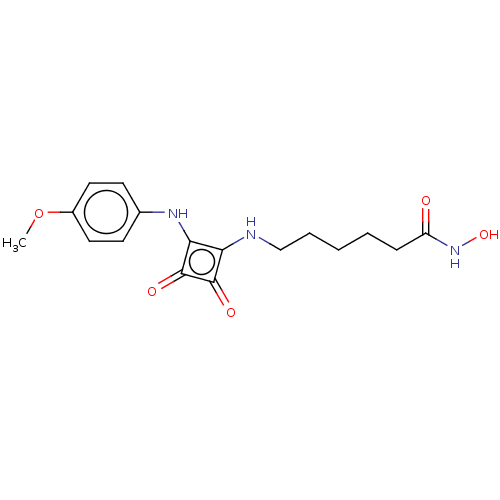
(CHEMBL4237803)Show InChI InChI=1S/C17H21N3O5/c1-25-12-8-6-11(7-9-12)19-15-14(16(22)17(15)23)18-10-4-2-3-5-13(21)20-24/h6-9,18-19,24H,2-5,10H2,1H3,(H,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0750 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nestle Skin Health R&D
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC1 using Fluor de Lys as substrate after 2 hrs by fluorescence method |
Bioorg Med Chem Lett 28: 2985-2992 (2018)
Article DOI: 10.1016/j.bmcl.2018.06.029
BindingDB Entry DOI: 10.7270/Q2M0484T |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50093958
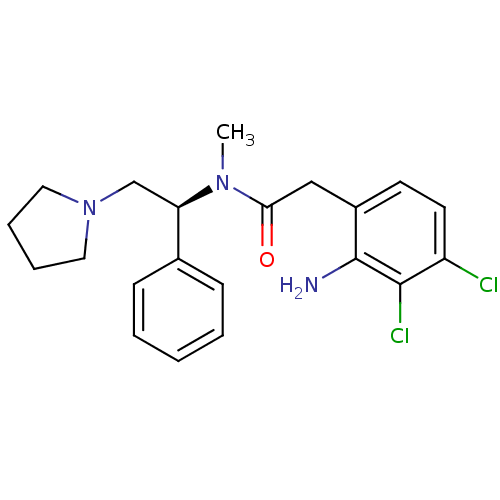
(2-(2-Amino-3,4-dichloro-phenyl)-N-methyl-N-((S)-1-...)Show SMILES CN([C@H](CN1CCCC1)c1ccccc1)C(=O)Cc1ccc(Cl)c(Cl)c1N Show InChI InChI=1S/C21H25Cl2N3O/c1-25(19(27)13-16-9-10-17(22)20(23)21(16)24)18(14-26-11-5-6-12-26)15-7-3-2-4-8-15/h2-4,7-10,18H,5-6,11-14,24H2,1H3/t18-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0860 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
In vitro binding affinity towards Opioid receptor kappa 1 by displacement of bound [3H]U69,593 |
Bioorg Med Chem Lett 10: 2567-70 (2001)
BindingDB Entry DOI: 10.7270/Q26H4GNG |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50093966
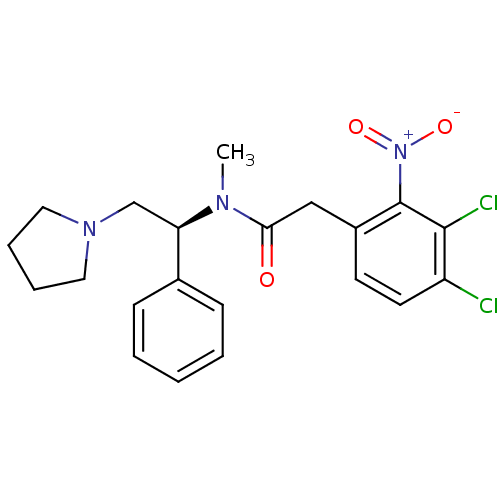
(2-(3,4-Dichloro-2-nitro-phenyl)-N-methyl-N-((S)-1-...)Show SMILES CN([C@H](CN1CCCC1)c1ccccc1)C(=O)Cc1ccc(Cl)c(Cl)c1[N+]([O-])=O Show InChI InChI=1S/C21H23Cl2N3O3/c1-24(18(14-25-11-5-6-12-25)15-7-3-2-4-8-15)19(27)13-16-9-10-17(22)20(23)21(16)26(28)29/h2-4,7-10,18H,5-6,11-14H2,1H3/t18-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 0.0910 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
In vitro binding affinity towards Opioid receptor kappa 1 by displacement of bound [3H]U69,593 |
Bioorg Med Chem Lett 10: 2567-70 (2001)
BindingDB Entry DOI: 10.7270/Q26H4GNG |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50093970
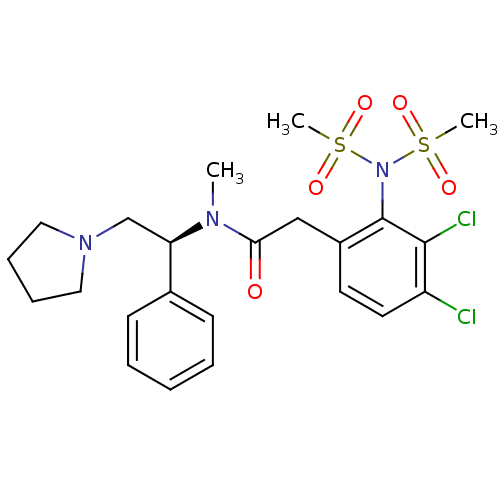
(2-(3,4-Dichloro-2-dimethanesulfonylamino-phenyl)-N...)Show SMILES CN([C@H](CN1CCCC1)c1ccccc1)C(=O)Cc1ccc(Cl)c(Cl)c1N(S(C)(=O)=O)S(C)(=O)=O Show InChI InChI=1S/C23H29Cl2N3O5S2/c1-26(20(16-27-13-7-8-14-27)17-9-5-4-6-10-17)21(29)15-18-11-12-19(24)22(25)23(18)28(34(2,30)31)35(3,32)33/h4-6,9-12,20H,7-8,13-16H2,1-3H3/t20-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0960 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
In vitro binding affinity towards Opioid receptor kappa 1 by displacement of bound [3H]U69,593 |
Bioorg Med Chem Lett 10: 2567-70 (2001)
BindingDB Entry DOI: 10.7270/Q26H4GNG |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50463736
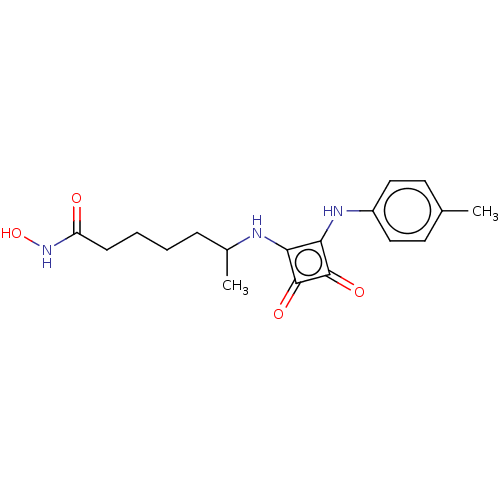
(CHEMBL4251203)Show InChI InChI=1S/C18H23N3O4/c1-11-7-9-13(10-8-11)20-16-15(17(23)18(16)24)19-12(2)5-3-4-6-14(22)21-25/h7-10,12,19-20,25H,3-6H2,1-2H3,(H,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0970 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nestle Skin Health R&D
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC1 using Fluor de Lys as substrate after 2 hrs by fluorescence method |
Bioorg Med Chem Lett 28: 2985-2992 (2018)
Article DOI: 10.1016/j.bmcl.2018.06.029
BindingDB Entry DOI: 10.7270/Q2M0484T |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50093962
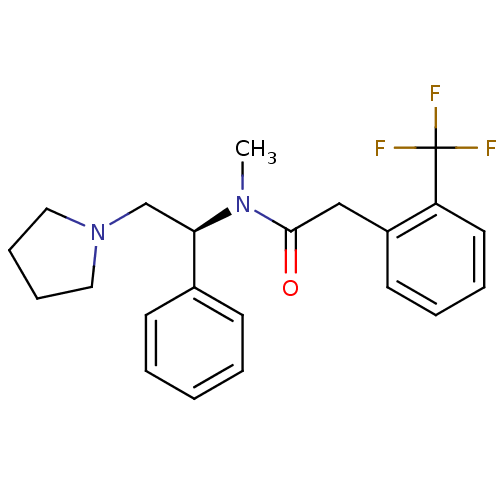
(CHEMBL87306 | N-Methyl-N-((S)-1-phenyl-2-pyrrolidi...)Show SMILES CN([C@H](CN1CCCC1)c1ccccc1)C(=O)Cc1ccccc1C(F)(F)F Show InChI InChI=1S/C22H25F3N2O/c1-26(21(28)15-18-11-5-6-12-19(18)22(23,24)25)20(16-27-13-7-8-14-27)17-9-3-2-4-10-17/h2-6,9-12,20H,7-8,13-16H2,1H3/t20-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
In vitro binding affinity towards Opioid receptor kappa 1 by displacement of bound [3H]U69,593 |
Bioorg Med Chem Lett 10: 2567-70 (2001)
BindingDB Entry DOI: 10.7270/Q26H4GNG |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50463756
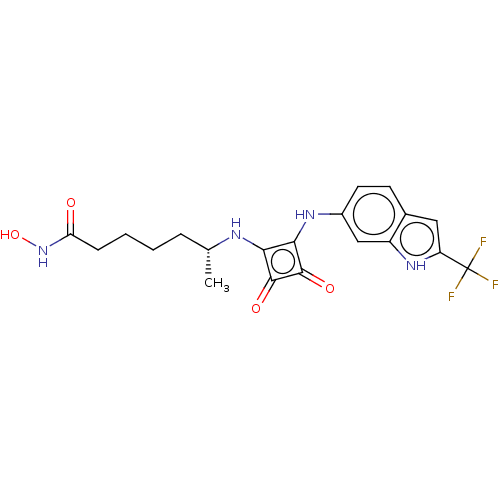
(CHEMBL4246561)Show SMILES C[C@H](CCCCC(=O)NO)Nc1c(Nc2ccc3cc([nH]c3c2)C(F)(F)F)c(=O)c1=O |r| Show InChI InChI=1S/C20H21F3N4O4/c1-10(4-2-3-5-15(28)27-31)24-16-17(19(30)18(16)29)25-12-7-6-11-8-14(20(21,22)23)26-13(11)9-12/h6-10,24-26,31H,2-5H2,1H3,(H,27,28)/t10-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nestle Skin Health R&D
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC1 using Fluor de Lys as substrate after 2 hrs by fluorescence method |
Bioorg Med Chem Lett 28: 2985-2992 (2018)
Article DOI: 10.1016/j.bmcl.2018.06.029
BindingDB Entry DOI: 10.7270/Q2M0484T |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50090003
(CHEMBL405190 | Tyr-Gly-[D-Ala]-Phe-Leu-Arg-Arg-Ile...)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@@H](C)NC(=O)CNC(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N1CCC[C@@H]1C(=O)N[C@@H](CCCCN)C(N)=O Show InChI InChI=1S/C64H106N22O12/c1-6-37(4)51(60(97)82-46(21-14-30-76-64(72)73)61(98)86-31-15-22-49(86)59(96)79-43(52(67)89)18-10-11-27-65)85-56(93)45(20-13-29-75-63(70)71)80-55(92)44(19-12-28-74-62(68)69)81-57(94)47(32-36(2)3)84-58(95)48(34-39-16-8-7-9-17-39)83-53(90)38(5)78-50(88)35-77-54(91)42(66)33-40-23-25-41(87)26-24-40/h7-9,16-17,23-26,36-38,42-49,51,87H,6,10-15,18-22,27-35,65-66H2,1-5H3,(H2,67,89)(H,77,91)(H,78,88)(H,79,96)(H,80,92)(H,81,94)(H,82,97)(H,83,90)(H,84,95)(H,85,93)(H4,68,69,74)(H4,70,71,75)(H4,72,73,76)/t37-,38+,42-,43-,44-,45-,46-,47-,48-,49+,51-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California
Curated by ChEMBL
| Assay Description
Human Opioid receptor kappa 1 mediated stimulation of [35S]- GTPgammaS binding in CHO cells (Agonist potency). |
J Med Chem 43: 2698-702 (2000)
BindingDB Entry DOI: 10.7270/Q25D8SJ1 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50377009
(CHEMBL257140)Show SMILES CN(Cc1ccccc1)S(=O)(=O)c1cc2OCOc2cc1CC(=O)N(C)[C@H](CN1CCCC1)c1ccccc1 Show InChI InChI=1S/C30H35N3O5S/c1-31(20-23-11-5-3-6-12-23)39(35,36)29-19-28-27(37-22-38-28)17-25(29)18-30(34)32(2)26(21-33-15-9-10-16-33)24-13-7-4-8-14-24/h3-8,11-14,17,19,26H,9-10,15-16,18,20-22H2,1-2H3/t26-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Displacement of [3H]diprenorphine from human kappa opioid receptor expressed in CHO-K1 cells |
Bioorg Med Chem Lett 18: 3667-71 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.116
BindingDB Entry DOI: 10.7270/Q2TX3G7F |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50010702
(CHEMBL411557 | DYNORPHIN(1-17)-OH | Dynorphin A (1...)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](Cc1ccccc1)NC(=O)CNC(=O)CNC(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CCC(N)=O)C(O)=O |r,wU:38.46,19.26,4.4,2.2,88.90,128.132,144.148,70.71,105.107,wD:57.58,30.34,8.15,84.87,97.99,114.116,136.140,(27.24,-21.93,;25.83,-21.28,;25.7,-19.76,;26.97,-18.87,;24.28,-19.11,;24.14,-17.56,;25.39,-16.68,;26.8,-17.34,;25.25,-15.13,;23.87,-14.49,;23.73,-12.97,;22.32,-12.32,;22.18,-10.79,;20.78,-10.14,;19.51,-11.03,;20.63,-8.6,;26.5,-14.25,;26.41,-12.7,;25.05,-12.02,;27.68,-11.87,;29.07,-12.56,;30.37,-11.71,;31.74,-12.4,;33.01,-11.53,;34.37,-12.24,;34.46,-13.77,;35.67,-11.39,;27.59,-10.31,;28.87,-9.48,;30.26,-10.18,;28.78,-7.92,;27.42,-7.24,;26.12,-8.09,;24.76,-7.41,;26.21,-9.64,;30.05,-7.09,;29.97,-5.53,;28.6,-4.85,;31.24,-4.7,;32.63,-5.4,;32.72,-6.92,;31.45,-7.77,;31.55,-9.31,;32.93,-9.99,;34.21,-9.14,;34.11,-7.6,;31.15,-3.14,;32.43,-2.31,;33.81,-3.01,;32.34,-.76,;33.61,.08,;33.52,1.63,;32.16,2.32,;34.8,2.47,;34.71,4.01,;36,4.86,;37.37,4.17,;35.91,6.4,;37.19,7.24,;34.53,7.1,;33.23,6.24,;33.34,4.69,;32.06,3.84,;30.68,4.52,;29.39,3.71,;30.58,6.06,;31.86,6.91,;23.04,-19.99,;21.65,-19.34,;23.18,-21.52,;21.92,-22.42,;22.06,-23.97,;20.82,-24.84,;19.43,-24.2,;18.16,-25.1,;16.78,-24.46,;16.64,-22.94,;15.51,-25.35,;20.12,-21.58,;18.85,-22.48,;19.94,-19.61,;21.09,-18.4,;20.31,-16.91,;18.66,-17.19,;18.69,-18.75,;17.33,-19.47,;17.28,-21.01,;16.02,-18.66,;14.66,-19.38,;14.61,-20.92,;13.25,-21.64,;13.2,-23.18,;11.84,-23.91,;11.79,-25.45,;13.36,-18.57,;13.41,-17.03,;12,-19.29,;10.69,-18.48,;10.74,-16.94,;9.44,-16.12,;9.49,-14.58,;8.08,-16.85,;9.33,-19.2,;9.28,-20.74,;8.03,-18.39,;6.67,-19.11,;6.61,-20.65,;5.26,-21.37,;5.2,-22.91,;3.85,-23.64,;3.79,-25.18,;5.36,-18.3,;5.41,-16.76,;4,-19.02,;2.69,-18.21,;2.75,-16.67,;1.44,-15.85,;.01,-16.43,;-.98,-15.25,;-.16,-13.95,;-.59,-12.47,;.48,-11.36,;1.97,-11.73,;2.4,-13.21,;1.33,-14.32,;1.34,-18.93,;1.28,-20.47,;.03,-18.12,;-1.33,-18.84,;-1.38,-20.38,;-2.74,-21.1,;-4.05,-20.29,;-2.79,-22.64,;-2.64,-18.03,;-2.59,-16.49,;-4,-18.75,;-5.3,-17.94,;-5.25,-16.4,;-6.56,-15.58,;-7.92,-16.31,;-6.51,-14.04,;-6.66,-18.66,;-6.71,-20.2,;-7.97,-17.85,;-9.33,-18.57,;-9.38,-20.11,;-10.74,-20.83,;-10.79,-22.37,;-9.48,-23.19,;-12.15,-23.1,;-10.63,-17.76,;-11.99,-18.48,;-10.58,-16.22,)| Show InChI InChI=1S/C99H155N31O23/c1-7-55(6)81(129-86(142)66(28-18-40-112-98(107)108)118-83(139)65(27-17-39-111-97(105)106)120-88(144)70(44-54(4)5)125-89(145)71(46-56-21-9-8-10-22-56)117-79(135)52-115-78(134)51-116-82(138)61(102)45-57-31-33-59(131)34-32-57)94(150)122-67(29-19-41-113-99(109)110)95(151)130-42-20-30-75(130)93(149)121-64(26-14-16-38-101)85(141)124-69(43-53(2)3)87(143)119-63(25-13-15-37-100)84(140)126-72(47-58-50-114-62-24-12-11-23-60(58)62)90(146)128-74(49-80(136)137)92(148)127-73(48-77(104)133)91(147)123-68(96(152)153)35-36-76(103)132/h8-12,21-24,31-34,50,53-55,61,63-75,81,114,131H,7,13-20,25-30,35-49,51-52,100-102H2,1-6H3,(H2,103,132)(H2,104,133)(H,115,134)(H,116,138)(H,117,135)(H,118,139)(H,119,143)(H,120,144)(H,121,149)(H,122,150)(H,123,147)(H,124,141)(H,125,145)(H,126,140)(H,127,148)(H,128,146)(H,129,142)(H,136,137)(H,152,153)(H4,105,106,111)(H4,107,108,112)(H4,109,110,113)/t55-,61-,63-,64-,65-,66-,67-,68-,69-,70-,71-,72-,73-,74-,75-,81-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Displacement of [3H]diprenorphine from human kappa opioid receptor expressed in CHO-K1 cells |
Bioorg Med Chem Lett 18: 3667-71 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.116
BindingDB Entry DOI: 10.7270/Q2TX3G7F |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50377005
(CHEMBL402135)Show SMILES CN(C)S(=O)(=O)c1cc2OCOc2cc1CC(=O)N(C)[C@H](CN1CCCC1)c1ccccc1 Show InChI InChI=1S/C24H31N3O5S/c1-25(2)33(29,30)23-15-22-21(31-17-32-22)13-19(23)14-24(28)26(3)20(16-27-11-7-8-12-27)18-9-5-4-6-10-18/h4-6,9-10,13,15,20H,7-8,11-12,14,16-17H2,1-3H3/t20-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Displacement of [3H]diprenorphine from human kappa opioid receptor expressed in CHO-K1 cells |
Bioorg Med Chem Lett 18: 3667-71 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.116
BindingDB Entry DOI: 10.7270/Q2TX3G7F |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50128091
(CHEMBL265813 | Tyr-Gly-Gly-Phe-Leu-Arg-Arg-Ile-Arg...)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](Cc1ccccc1)NC(=O)CNC(=O)CNC(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N1CCC[C@@H]1C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCCCN)C(O)=O Show InChI InChI=1S/C75H126N24O15/c1-7-45(6)61(70(111)94-53(25-17-35-87-75(83)84)71(112)99-36-18-26-58(99)69(110)93-50(21-11-13-31-76)64(105)96-56(38-44(4)5)67(108)95-54(72(113)114)22-12-14-32-77)98-65(106)52(24-16-34-86-74(81)82)91-63(104)51(23-15-33-85-73(79)80)92-66(107)55(37-43(2)3)97-68(109)57(40-46-19-9-8-10-20-46)90-60(102)42-88-59(101)41-89-62(103)49(78)39-47-27-29-48(100)30-28-47/h8-10,19-20,27-30,43-45,49-58,61,100H,7,11-18,21-26,31-42,76-78H2,1-6H3,(H,88,101)(H,89,103)(H,90,102)(H,91,104)(H,92,107)(H,93,110)(H,94,111)(H,95,108)(H,96,105)(H,97,109)(H,98,106)(H,113,114)(H4,79,80,85)(H4,81,82,86)(H4,83,84,87)/t45-,49-,50-,51-,52-,53-,54-,55-,56-,57-,58+,61-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California
Curated by ChEMBL
| Assay Description
In vitro binding affinity towards human Opioid receptor kappa 1 on CHO cell membranes using [3H]diprenorphine displacement. |
J Med Chem 46: 2104-9 (2003)
Article DOI: 10.1021/jm020125+
BindingDB Entry DOI: 10.7270/Q2NZ88CF |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50463750
(CHEMBL4241807)Show SMILES ONC(=O)CCCCCNc1c(Nc2ccc(cc2)C(F)(F)F)c(=O)c1=O Show InChI InChI=1S/C17H18F3N3O4/c18-17(19,20)10-5-7-11(8-6-10)22-14-13(15(25)16(14)26)21-9-3-1-2-4-12(24)23-27/h5-8,21-22,27H,1-4,9H2,(H,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nestle Skin Health R&D
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC1 using Fluor de Lys as substrate after 2 hrs by fluorescence method |
Bioorg Med Chem Lett 28: 2985-2992 (2018)
Article DOI: 10.1016/j.bmcl.2018.06.029
BindingDB Entry DOI: 10.7270/Q2M0484T |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50463743
(CHEMBL4241370)Show SMILES CC(C)(CCCCC(=O)NO)Nc1c(Nc2ccc(cc2)C(F)(F)F)c(=O)c1=O Show InChI InChI=1S/C19H22F3N3O4/c1-18(2,10-4-3-5-13(26)25-29)24-15-14(16(27)17(15)28)23-12-8-6-11(7-9-12)19(20,21)22/h6-9,23-24,29H,3-5,10H2,1-2H3,(H,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| 0.270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nestle Skin Health R&D
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC1 using Fluor de Lys as substrate after 2 hrs by fluorescence method |
Bioorg Med Chem Lett 28: 2985-2992 (2018)
Article DOI: 10.1016/j.bmcl.2018.06.029
BindingDB Entry DOI: 10.7270/Q2M0484T |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50463753
(CHEMBL4250739)Show SMILES COc1ccc(Nc2c(NC(C)CCCCC(=O)NO)c(=O)c2=O)cc1 Show InChI InChI=1S/C18H23N3O5/c1-11(5-3-4-6-14(22)21-25)19-15-16(18(24)17(15)23)20-12-7-9-13(26-2)10-8-12/h7-11,19-20,25H,3-6H2,1-2H3,(H,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nestle Skin Health R&D
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC1 using Fluor de Lys as substrate after 2 hrs by fluorescence method |
Bioorg Med Chem Lett 28: 2985-2992 (2018)
Article DOI: 10.1016/j.bmcl.2018.06.029
BindingDB Entry DOI: 10.7270/Q2M0484T |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50463752
(CHEMBL4247370)Show SMILES Cc1cc2ccc(Nc3c(NCCCCCC(=O)NO)c(=O)c3=O)cc2[nH]1 Show InChI InChI=1S/C19H22N4O4/c1-11-9-12-6-7-13(10-14(12)21-11)22-17-16(18(25)19(17)26)20-8-4-2-3-5-15(24)23-27/h6-7,9-10,20-22,27H,2-5,8H2,1H3,(H,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nestle Skin Health R&D
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC1 using Fluor de Lys as substrate after 2 hrs by fluorescence method |
Bioorg Med Chem Lett 28: 2985-2992 (2018)
Article DOI: 10.1016/j.bmcl.2018.06.029
BindingDB Entry DOI: 10.7270/Q2M0484T |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50463754
(CHEMBL4240635)Show SMILES CC(CCCCC(=O)NO)Nc1c(Nc2ccc(cc2)C(F)(F)F)c(=O)c1=O Show InChI InChI=1S/C18H20F3N3O4/c1-10(4-2-3-5-13(25)24-28)22-14-15(17(27)16(14)26)23-12-8-6-11(7-9-12)18(19,20)21/h6-10,22-23,28H,2-5H2,1H3,(H,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nestle Skin Health R&D
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC1 using Fluor de Lys as substrate after 2 hrs by fluorescence method |
Bioorg Med Chem Lett 28: 2985-2992 (2018)
Article DOI: 10.1016/j.bmcl.2018.06.029
BindingDB Entry DOI: 10.7270/Q2M0484T |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50463740
(CHEMBL4251365)Show InChI InChI=1S/C16H19N3O4/c20-12(19-23)9-5-2-6-10-17-13-14(16(22)15(13)21)18-11-7-3-1-4-8-11/h1,3-4,7-8,17-18,23H,2,5-6,9-10H2,(H,19,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nestle Skin Health R&D
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC1 using Fluor de Lys as substrate after 2 hrs by fluorescence method |
Bioorg Med Chem Lett 28: 2985-2992 (2018)
Article DOI: 10.1016/j.bmcl.2018.06.029
BindingDB Entry DOI: 10.7270/Q2M0484T |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50377016
(CHEMBL402820)Show SMILES COc1cc(CC(=O)N(C)[C@H](CN2CCCC2)c2ccccc2)c(cc1OC)S(=O)(=O)N1CCCC1 Show InChI InChI=1S/C27H37N3O5S/c1-28(23(20-29-13-7-8-14-29)21-11-5-4-6-12-21)27(31)18-22-17-24(34-2)25(35-3)19-26(22)36(32,33)30-15-9-10-16-30/h4-6,11-12,17,19,23H,7-10,13-16,18,20H2,1-3H3/t23-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Displacement of [3H]diprenorphine from human kappa opioid receptor expressed in CHO-K1 cells |
Bioorg Med Chem Lett 18: 3667-71 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.116
BindingDB Entry DOI: 10.7270/Q2TX3G7F |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50160834
(CHEMBL183248 | N-[(S)-1-(3-Amino-phenyl)-2-pyrroli...)Show SMILES CN([C@H](CN1CCCC1)c1cccc(N)c1)C(=O)Cc1ccc(cc1)C(F)(F)F Show InChI InChI=1S/C22H26F3N3O/c1-27(21(29)13-16-7-9-18(10-8-16)22(23,24)25)20(15-28-11-2-3-12-28)17-5-4-6-19(26)14-17/h4-10,14,20H,2-3,11-13,15,26H2,1H3/t20-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Displacement of bound [3H]-diprenorphine from membranes expressing cloned human kappa opioid receptor |
Bioorg Med Chem Lett 15: 1091-5 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.018
BindingDB Entry DOI: 10.7270/Q2JW8DCG |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50463739

(CHEMBL4237803)Show InChI InChI=1S/C17H21N3O5/c1-25-12-8-6-11(7-9-12)19-15-14(16(22)17(15)23)18-10-4-2-3-5-13(21)20-24/h6-9,18-19,24H,2-5,10H2,1H3,(H,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nestle Skin Health R&D
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC1 using fluorogenic HDAC substrate after 15 mins by fluorimetrc method |
Bioorg Med Chem Lett 28: 2985-2992 (2018)
Article DOI: 10.1016/j.bmcl.2018.06.029
BindingDB Entry DOI: 10.7270/Q2M0484T |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50128074
(CHEMBL269483 | Tyr-Gly-Gly-Phe-Leu-Arg-Arg-Ile-Arg...)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](Cc1ccccc1)NC(=O)CNC(=O)CNC(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N1CCC[C@@H]1C(O)=O Show InChI InChI=1S/C57H91N19O12/c1-5-33(4)46(52(85)73-40(17-11-25-67-57(63)64)53(86)76-26-12-18-43(76)54(87)88)75-49(82)39(16-10-24-66-56(61)62)71-48(81)38(15-9-23-65-55(59)60)72-50(83)41(27-32(2)3)74-51(84)42(29-34-13-7-6-8-14-34)70-45(79)31-68-44(78)30-69-47(80)37(58)28-35-19-21-36(77)22-20-35/h6-8,13-14,19-22,32-33,37-43,46,77H,5,9-12,15-18,23-31,58H2,1-4H3,(H,68,78)(H,69,80)(H,70,79)(H,71,81)(H,72,83)(H,73,85)(H,74,84)(H,75,82)(H,87,88)(H4,59,60,65)(H4,61,62,66)(H4,63,64,67)/t33-,37-,38-,39-,40-,41-,42-,43+,46-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California
Curated by ChEMBL
| Assay Description
In vitro binding affinity towards human Opioid receptor kappa 1 on CHO cell membranes using [3H]diprenorphine displacement. |
J Med Chem 46: 2104-9 (2003)
Article DOI: 10.1021/jm020125+
BindingDB Entry DOI: 10.7270/Q2NZ88CF |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM82551

(C18130 | CAS_105618-26-6 | NOR-BNI (HCI)2 | NORBNI)Show SMILES Oc1ccc2C[C@H]3N(CC4CC4)CC[C@@]45[C@@H](Oc1c24)c1[nH]c2[C@@H]4Oc6c7c(C[C@H]8N(CC9CC9)CC[C@@]47[C@@]8(O)Cc2c1C[C@@]35O)ccc6O |r| Show InChI InChI=1S/C40H43N3O6/c44-25-7-5-21-13-27-39(46)15-23-24-16-40(47)28-14-22-6-8-26(45)34-30(22)38(40,10-12-43(28)18-20-3-4-20)36(49-34)32(24)41-31(23)35-37(39,29(21)33(25)48-35)9-11-42(27)17-19-1-2-19/h5-8,19-20,27-28,35-36,41,44-47H,1-4,9-18H2/t27-,28-,35+,36+,37+,38+,39-,40-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California
Curated by ChEMBL
| Assay Description
Human Opioid receptor kappa 1 mediated stimulation of [35S]- GTPgammaS binding in CHO cells (Agonist potency). |
J Med Chem 43: 2698-702 (2000)
BindingDB Entry DOI: 10.7270/Q25D8SJ1 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50160840
(CHEMBL365486 | N-[(S)-1-(3-Methanesulfonylamino-ph...)Show SMILES CN([C@H](CN1CCCC1)c1cccc(NS(C)(=O)=O)c1)C(=O)Cc1ccc(cc1)C(F)(F)F Show InChI InChI=1S/C23H28F3N3O3S/c1-28(22(30)14-17-8-10-19(11-9-17)23(24,25)26)21(16-29-12-3-4-13-29)18-6-5-7-20(15-18)27-33(2,31)32/h5-11,15,21,27H,3-4,12-14,16H2,1-2H3/t21-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Displacement of bound [3H]-diprenorphine from membranes expressing cloned human kappa opioid receptor |
Bioorg Med Chem Lett 15: 1091-5 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.018
BindingDB Entry DOI: 10.7270/Q2JW8DCG |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM82551

(C18130 | CAS_105618-26-6 | NOR-BNI (HCI)2 | NORBNI)Show SMILES Oc1ccc2C[C@H]3N(CC4CC4)CC[C@@]45[C@@H](Oc1c24)c1[nH]c2[C@@H]4Oc6c7c(C[C@H]8N(CC9CC9)CC[C@@]47[C@@]8(O)Cc2c1C[C@@]35O)ccc6O |r| Show InChI InChI=1S/C40H43N3O6/c44-25-7-5-21-13-27-39(46)15-23-24-16-40(47)28-14-22-6-8-26(45)34-30(22)38(40,10-12-43(28)18-20-3-4-20)36(49-34)32(24)41-31(23)35-37(39,29(21)33(25)48-35)9-11-42(27)17-19-1-2-19/h5-8,19-20,27-28,35-36,41,44-47H,1-4,9-18H2/t27-,28-,35+,36+,37+,38+,39-,40-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California
Curated by ChEMBL
| Assay Description
In vitro binding affinity towards human Opioid receptor kappa 1 on CHO cell membranes using [3H]diprenorphine displacement. |
J Med Chem 46: 2104-9 (2003)
Article DOI: 10.1021/jm020125+
BindingDB Entry DOI: 10.7270/Q2NZ88CF |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50463763
(CHEMBL4248374)Show InChI InChI=1S/C17H21N3O4/c1-11-6-5-7-12(10-11)19-15-14(16(22)17(15)23)18-9-4-2-3-8-13(21)20-24/h5-7,10,18-19,24H,2-4,8-9H2,1H3,(H,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nestle Skin Health R&D
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC1 using Fluor de Lys as substrate after 2 hrs by fluorescence method |
Bioorg Med Chem Lett 28: 2985-2992 (2018)
Article DOI: 10.1016/j.bmcl.2018.06.029
BindingDB Entry DOI: 10.7270/Q2M0484T |
More data for this
Ligand-Target Pair | |
Type-2 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50156173
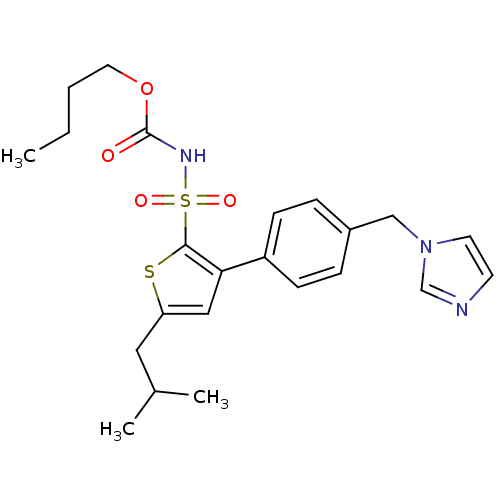
((1-((2'-(1H-tetrazol-5-yl)biphenyl-4-yl)methyl)-2-...)Show SMILES CCCCOC(=O)NS(=O)(=O)c1sc(CC(C)C)cc1-c1ccc(Cn2ccnc2)cc1 Show InChI InChI=1S/C23H29N3O4S2/c1-4-5-12-30-23(27)25-32(28,29)22-21(14-20(31-22)13-17(2)3)19-8-6-18(7-9-19)15-26-11-10-24-16-26/h6-11,14,16-17H,4-5,12-13,15H2,1-3H3,(H,25,27) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centre Hospitalier Universitaire Vaudois (CHUV) and University of Lausanne (UNIL)
Curated by ChEMBL
| Assay Description
Binding affinity to AT2 receptor (unknown origin) |
J Med Chem 61: 9811-9840 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00294
BindingDB Entry DOI: 10.7270/Q2XK8J7N |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50093968
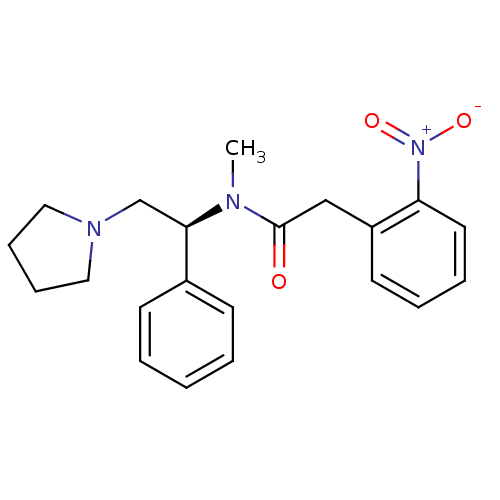
(CHEMBL87207 | N-Methyl-2-(2-nitro-phenyl)-N-((S)-1...)Show SMILES CN([C@H](CN1CCCC1)c1ccccc1)C(=O)Cc1ccccc1[N+]([O-])=O Show InChI InChI=1S/C21H25N3O3/c1-22(21(25)15-18-11-5-6-12-19(18)24(26)27)20(16-23-13-7-8-14-23)17-9-3-2-4-10-17/h2-6,9-12,20H,7-8,13-16H2,1H3/t20-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.410 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
In vitro binding affinity towards Opioid receptor kappa 1 by displacement of bound [3H]U69,593 |
Bioorg Med Chem Lett 10: 2567-70 (2001)
BindingDB Entry DOI: 10.7270/Q26H4GNG |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50160832
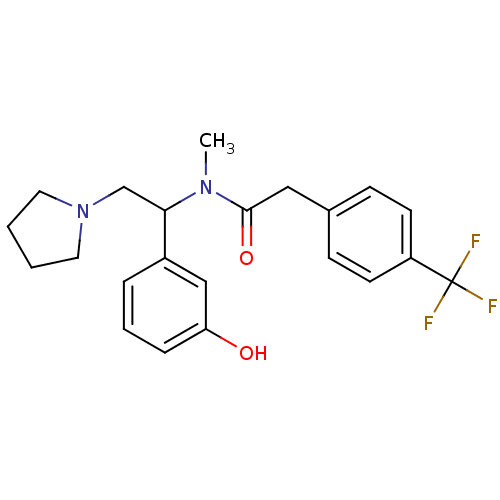
(CHEMBL181279 | N-[1-(3-Hydroxy-phenyl)-2-pyrrolidi...)Show SMILES CN(C(CN1CCCC1)c1cccc(O)c1)C(=O)Cc1ccc(cc1)C(F)(F)F Show InChI InChI=1S/C22H25F3N2O2/c1-26(21(29)13-16-7-9-18(10-8-16)22(23,24)25)20(15-27-11-2-3-12-27)17-5-4-6-19(28)14-17/h4-10,14,20,28H,2-3,11-13,15H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.440 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Displacement of bound [3H]-diprenorphine from membranes expressing cloned human kappa opioid receptor |
Bioorg Med Chem Lett 15: 1091-5 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.018
BindingDB Entry DOI: 10.7270/Q2JW8DCG |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50128078
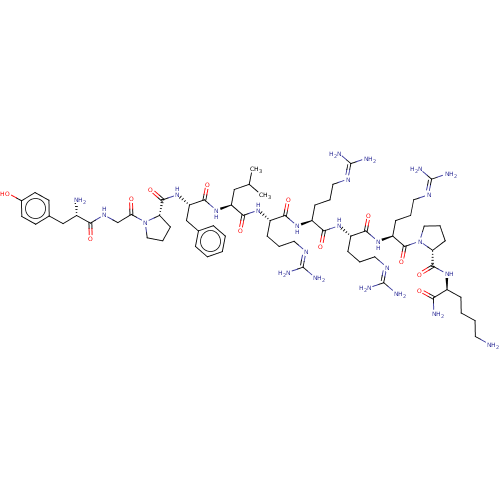
(CHEMBL2369408 | Tyr-Gly-Pro-Phe-Leu-Arg-Arg-Arg-Ar...)Show SMILES [#6]-[#6](-[#6])-[#6]-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccccc1)-[#7]-[#6](=O)-[#6@@H]-1-[#6]-[#6]-[#6]-[#7]-1-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6@@H](-[#7])-[#6]-c1ccc(-[#8])cc1)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@@H]-1-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](-[#7])=O Show InChI InChI=1S/C66H109N25O12/c1-38(2)34-48(88-59(100)49(36-39-14-4-3-5-15-39)89-60(101)50-21-12-32-90(50)52(93)37-82-54(95)42(68)35-40-23-25-41(92)26-24-40)58(99)86-45(18-9-29-79-64(72)73)56(97)84-44(17-8-28-78-63(70)71)55(96)85-46(19-10-30-80-65(74)75)57(98)87-47(20-11-31-81-66(76)77)62(103)91-33-13-22-51(91)61(102)83-43(53(69)94)16-6-7-27-67/h3-5,14-15,23-26,38,42-51,92H,6-13,16-22,27-37,67-68H2,1-2H3,(H2,69,94)(H,82,95)(H,83,102)(H,84,97)(H,85,96)(H,86,99)(H,87,98)(H,88,100)(H,89,101)(H4,70,71,78)(H4,72,73,79)(H4,74,75,80)(H4,76,77,81)/t42-,43-,44-,45-,46-,47-,48-,49-,50?,51+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.440 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California
Curated by ChEMBL
| Assay Description
In vitro binding affinity towards human Opioid receptor kappa 1 on CHO cell membranes using [3H]diprenorphine displacement. |
J Med Chem 46: 2104-9 (2003)
Article DOI: 10.1021/jm020125+
BindingDB Entry DOI: 10.7270/Q2NZ88CF |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50105327

(JNJ-26481585 | Quisinostat)Show SMILES Cn1cc(CNCC2CCN(CC2)c2ncc(cn2)C(=O)NO)c2ccccc12 Show InChI InChI=1S/C21H26N6O2/c1-26-14-17(18-4-2-3-5-19(18)26)11-22-10-15-6-8-27(9-7-15)21-23-12-16(13-24-21)20(28)25-29/h2-5,12-15,22,29H,6-11H2,1H3,(H,25,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 0.480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nestle Skin Health R&D
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC1 using Fluor de Lys as substrate after 2 hrs by fluorescence method |
Bioorg Med Chem Lett 28: 2985-2992 (2018)
Article DOI: 10.1016/j.bmcl.2018.06.029
BindingDB Entry DOI: 10.7270/Q2M0484T |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50160842
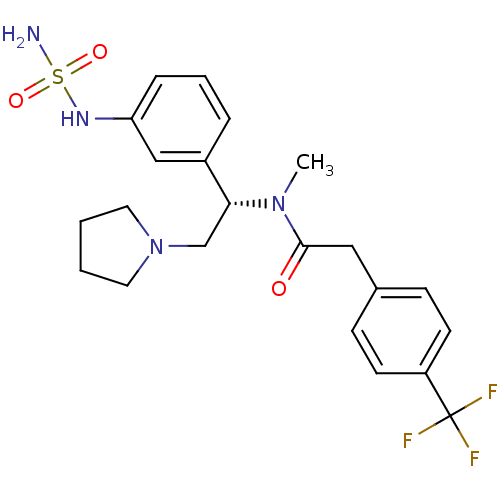
(CHEMBL362248 | N-[(S)-1-(3-aminosulfonylamino-phen...)Show SMILES CN([C@H](CN1CCCC1)c1cccc(NS(N)(=O)=O)c1)C(=O)Cc1ccc(cc1)C(F)(F)F Show InChI InChI=1S/C22H27F3N4O3S/c1-28(21(30)13-16-7-9-18(10-8-16)22(23,24)25)20(15-29-11-2-3-12-29)17-5-4-6-19(14-17)27-33(26,31)32/h4-10,14,20,27H,2-3,11-13,15H2,1H3,(H2,26,31,32)/t20-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Displacement of bound [3H]-diprenorphine from membranes expressing cloned human kappa opioid receptor |
Bioorg Med Chem Lett 15: 1091-5 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.018
BindingDB Entry DOI: 10.7270/Q2JW8DCG |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50377008
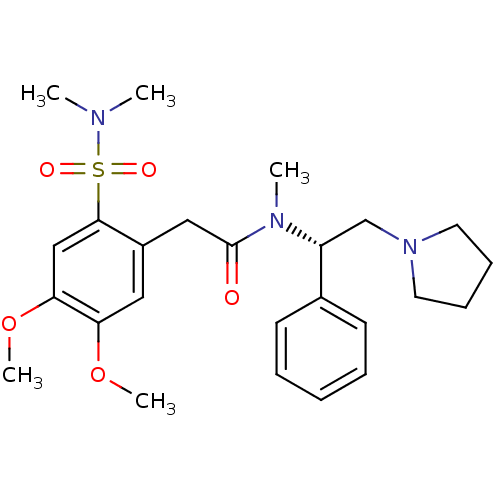
(CHEMBL254959)Show SMILES COc1cc(CC(=O)N(C)[C@H](CN2CCCC2)c2ccccc2)c(cc1OC)S(=O)(=O)N(C)C Show InChI InChI=1S/C25H35N3O5S/c1-26(2)34(30,31)24-17-23(33-5)22(32-4)15-20(24)16-25(29)27(3)21(18-28-13-9-10-14-28)19-11-7-6-8-12-19/h6-8,11-12,15,17,21H,9-10,13-14,16,18H2,1-5H3/t21-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Displacement of [3H]diprenorphine from human kappa opioid receptor expressed in CHO-K1 cells |
Bioorg Med Chem Lett 18: 3667-71 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.116
BindingDB Entry DOI: 10.7270/Q2TX3G7F |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50463751
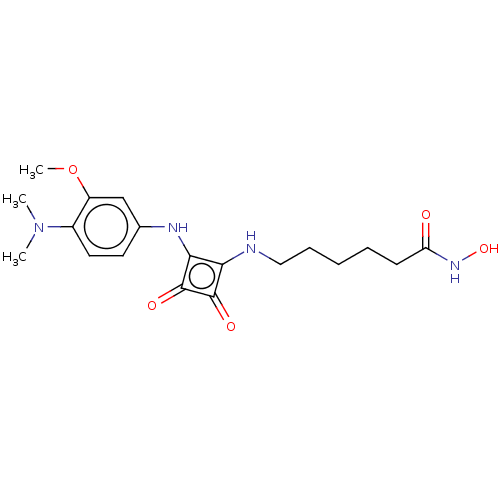
(CHEMBL4244350)Show SMILES COc1cc(Nc2c(NCCCCCC(=O)NO)c(=O)c2=O)ccc1N(C)C Show InChI InChI=1S/C19H26N4O5/c1-23(2)13-9-8-12(11-14(13)28-3)21-17-16(18(25)19(17)26)20-10-6-4-5-7-15(24)22-27/h8-9,11,20-21,27H,4-7,10H2,1-3H3,(H,22,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nestle Skin Health R&D
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC1 using Fluor de Lys as substrate after 2 hrs by fluorescence method |
Bioorg Med Chem Lett 28: 2985-2992 (2018)
Article DOI: 10.1016/j.bmcl.2018.06.029
BindingDB Entry DOI: 10.7270/Q2M0484T |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50463757
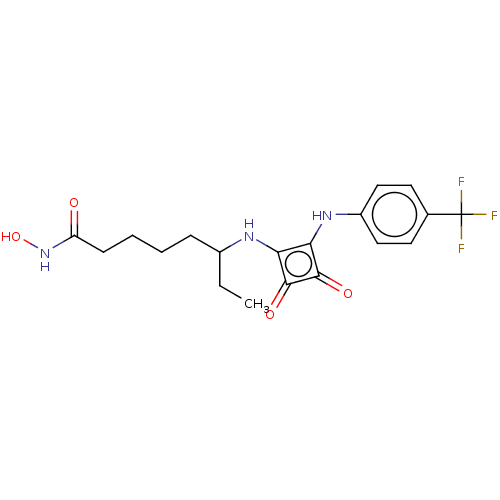
(CHEMBL4249385)Show SMILES CCC(CCCCC(=O)NO)Nc1c(Nc2ccc(cc2)C(F)(F)F)c(=O)c1=O Show InChI InChI=1S/C19H22F3N3O4/c1-2-12(5-3-4-6-14(26)25-29)23-15-16(18(28)17(15)27)24-13-9-7-11(8-10-13)19(20,21)22/h7-10,12,23-24,29H,2-6H2,1H3,(H,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.510 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nestle Skin Health R&D
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC1 using Fluor de Lys as substrate after 2 hrs by fluorescence method |
Bioorg Med Chem Lett 28: 2985-2992 (2018)
Article DOI: 10.1016/j.bmcl.2018.06.029
BindingDB Entry DOI: 10.7270/Q2M0484T |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50160836
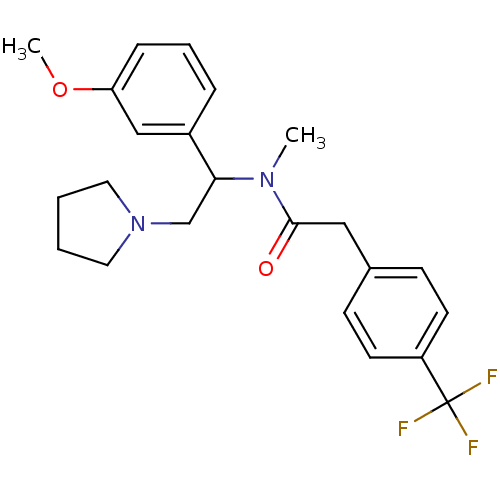
(CHEMBL180803 | N-[1-(3-Methoxy-phenyl)-2-pyrrolidi...)Show SMILES COc1cccc(c1)C(CN1CCCC1)N(C)C(=O)Cc1ccc(cc1)C(F)(F)F Show InChI InChI=1S/C23H27F3N2O2/c1-27(22(29)14-17-8-10-19(11-9-17)23(24,25)26)21(16-28-12-3-4-13-28)18-6-5-7-20(15-18)30-2/h5-11,15,21H,3-4,12-14,16H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.530 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Displacement of bound [3H]-diprenorphine from membranes expressing cloned human kappa opioid receptor |
Bioorg Med Chem Lett 15: 1091-5 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.018
BindingDB Entry DOI: 10.7270/Q2JW8DCG |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM29589

(Faridak | LBH-589 | LBH-589B | Panobinostat | US10...)Show SMILES Cc1[nH]c2ccccc2c1CCNCc1ccc(\C=C\C(=O)NO)cc1 Show InChI InChI=1S/C21H23N3O2/c1-15-18(19-4-2-3-5-20(19)23-15)12-13-22-14-17-8-6-16(7-9-17)10-11-21(25)24-26/h2-11,22-23,26H,12-14H2,1H3,(H,24,25)/b11-10+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.540 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nestle Skin Health R&D
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC1 using Fluor de Lys as substrate after 2 hrs by fluorescence method |
Bioorg Med Chem Lett 28: 2985-2992 (2018)
Article DOI: 10.1016/j.bmcl.2018.06.029
BindingDB Entry DOI: 10.7270/Q2M0484T |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data