 Found 244 hits with Last Name = 'avery' and Initial = 'ca'
Found 244 hits with Last Name = 'avery' and Initial = 'ca' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Coagulation factor XIII A chain
(Homo sapiens (Human)) | BDBM50083722
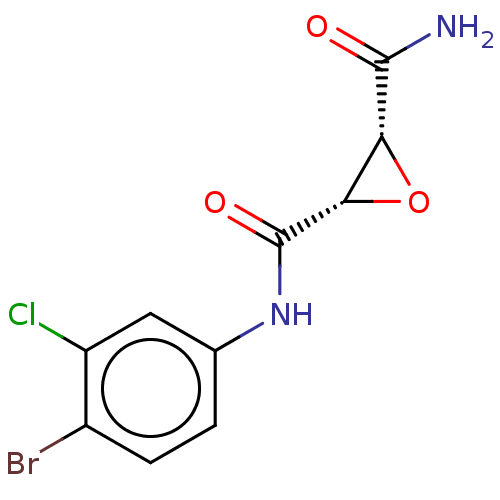
(CHEMBL3423198)Show SMILES NC(=O)[C@@H]1O[C@@H]1C(=O)Nc1ccc(Br)c(Cl)c1 |r| Show InChI InChI=1S/C10H8BrClN2O3/c11-5-2-1-4(3-6(5)12)14-10(16)8-7(17-8)9(13)15/h1-3,7-8H,(H2,13,15)(H,14,16)/t7-,8+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Leeds
Curated by ChEMBL
| Assay Description
Inhibition of thrombin-activated F13-A (unknown origin) in plasma assessed as inhibition of fibrin clot formation after 7 mins by biotin incorporatio... |
Eur J Med Chem 98: 49-53 (2015)
Article DOI: 10.1016/j.ejmech.2015.05.019
BindingDB Entry DOI: 10.7270/Q261121G |
More data for this
Ligand-Target Pair | |
Coagulation factor XIII A chain
(Homo sapiens (Human)) | BDBM50083725
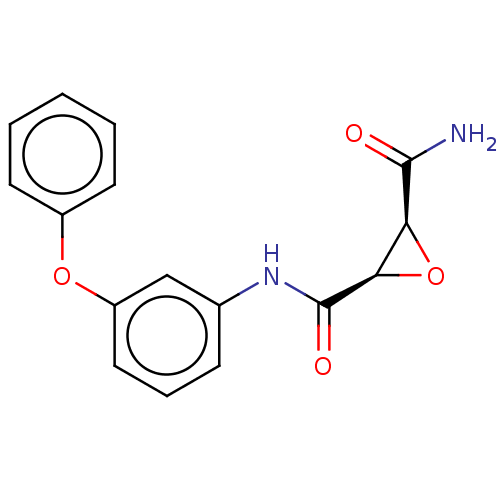
(CHEMBL3423195)Show SMILES NC(=O)[C@H]1O[C@H]1C(=O)Nc1cccc(Oc2ccccc2)c1 |r| Show InChI InChI=1S/C16H14N2O4/c17-15(19)13-14(22-13)16(20)18-10-5-4-8-12(9-10)21-11-6-2-1-3-7-11/h1-9,13-14H,(H2,17,19)(H,18,20)/t13-,14+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Leeds
Curated by ChEMBL
| Assay Description
Inhibition of thrombin-activated F13-A (unknown origin) in plasma assessed as inhibition of fibrin clot formation after 7 mins by biotin incorporatio... |
Eur J Med Chem 98: 49-53 (2015)
Article DOI: 10.1016/j.ejmech.2015.05.019
BindingDB Entry DOI: 10.7270/Q261121G |
More data for this
Ligand-Target Pair | |
Protein-glutamine gamma-glutamyltransferase 2
(Homo sapiens (Human)) | BDBM50083722

(CHEMBL3423198)Show SMILES NC(=O)[C@@H]1O[C@@H]1C(=O)Nc1ccc(Br)c(Cl)c1 |r| Show InChI InChI=1S/C10H8BrClN2O3/c11-5-2-1-4(3-6(5)12)14-10(16)8-7(17-8)9(13)15/h1-3,7-8H,(H2,13,15)(H,14,16)/t7-,8+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9.60 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Leeds
Curated by ChEMBL
| Assay Description
Inhibition of transglutaminase 2 (unknown origin) |
Eur J Med Chem 98: 49-53 (2015)
Article DOI: 10.1016/j.ejmech.2015.05.019
BindingDB Entry DOI: 10.7270/Q261121G |
More data for this
Ligand-Target Pair | |
Protein-glutamine gamma-glutamyltransferase 2
(Homo sapiens (Human)) | BDBM50083728

(CHEMBL3423193)Show SMILES NC(=O)[C@H]1O[C@H]1C(=O)Nc1ccc(Oc2ccccc2)cc1 |r| Show InChI InChI=1S/C16H14N2O4/c17-15(19)13-14(22-13)16(20)18-10-6-8-12(9-7-10)21-11-4-2-1-3-5-11/h1-9,13-14H,(H2,17,19)(H,18,20)/t13-,14+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 46 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Leeds
Curated by ChEMBL
| Assay Description
Inhibition of transglutaminase 2 (unknown origin) |
Eur J Med Chem 98: 49-53 (2015)
Article DOI: 10.1016/j.ejmech.2015.05.019
BindingDB Entry DOI: 10.7270/Q261121G |
More data for this
Ligand-Target Pair | |
Protein-glutamine gamma-glutamyltransferase 2
(Homo sapiens (Human)) | BDBM50083718
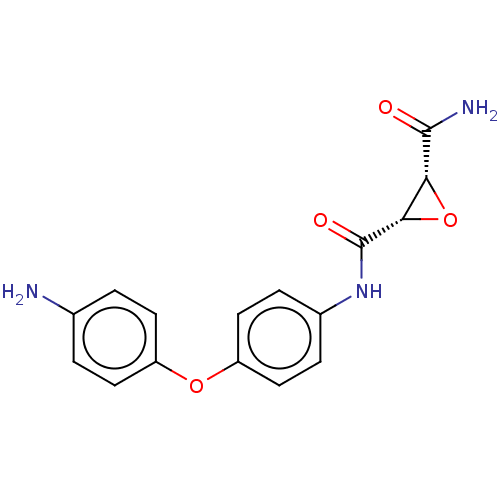
(CHEMBL3423202)Show SMILES NC(=O)[C@@H]1O[C@@H]1C(=O)Nc1ccc(Oc2ccc(N)cc2)cc1 |r| Show InChI InChI=1S/C16H15N3O4/c17-9-1-5-11(6-2-9)22-12-7-3-10(4-8-12)19-16(21)14-13(23-14)15(18)20/h1-8,13-14H,17H2,(H2,18,20)(H,19,21)/t13-,14+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 48 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Leeds
Curated by ChEMBL
| Assay Description
Inhibition of transglutaminase 2 (unknown origin) |
Eur J Med Chem 98: 49-53 (2015)
Article DOI: 10.1016/j.ejmech.2015.05.019
BindingDB Entry DOI: 10.7270/Q261121G |
More data for this
Ligand-Target Pair | |
Protein-glutamine gamma-glutamyltransferase 2
(Homo sapiens (Human)) | BDBM50083726
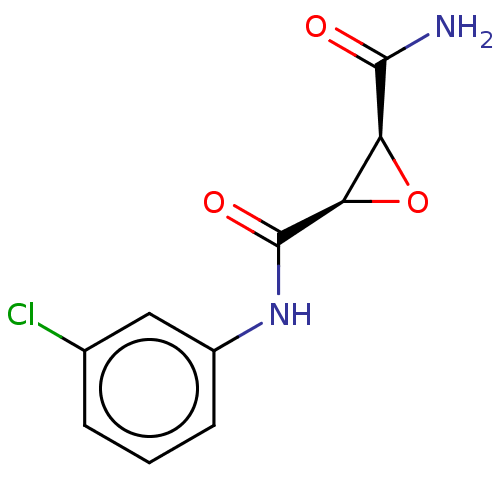
(CHEMBL3423194)Show InChI InChI=1S/C10H9ClN2O3/c11-5-2-1-3-6(4-5)13-10(15)8-7(16-8)9(12)14/h1-4,7-8H,(H2,12,14)(H,13,15)/t7-,8+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 48 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Leeds
Curated by ChEMBL
| Assay Description
Inhibition of transglutaminase 2 (unknown origin) |
Eur J Med Chem 98: 49-53 (2015)
Article DOI: 10.1016/j.ejmech.2015.05.019
BindingDB Entry DOI: 10.7270/Q261121G |
More data for this
Ligand-Target Pair | |
Coagulation factor XIII A chain
(Homo sapiens (Human)) | BDBM50083726

(CHEMBL3423194)Show InChI InChI=1S/C10H9ClN2O3/c11-5-2-1-3-6(4-5)13-10(15)8-7(16-8)9(12)14/h1-4,7-8H,(H2,12,14)(H,13,15)/t7-,8+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 48 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Leeds
Curated by ChEMBL
| Assay Description
Inhibition of thrombin-activated F13-A (unknown origin) in plasma assessed as inhibition of fibrin clot formation after 7 mins by biotin incorporatio... |
Eur J Med Chem 98: 49-53 (2015)
Article DOI: 10.1016/j.ejmech.2015.05.019
BindingDB Entry DOI: 10.7270/Q261121G |
More data for this
Ligand-Target Pair | |
Protein-glutamine gamma-glutamyltransferase 2
(Homo sapiens (Human)) | BDBM50083725

(CHEMBL3423195)Show SMILES NC(=O)[C@H]1O[C@H]1C(=O)Nc1cccc(Oc2ccccc2)c1 |r| Show InChI InChI=1S/C16H14N2O4/c17-15(19)13-14(22-13)16(20)18-10-5-4-8-12(9-10)21-11-6-2-1-3-7-11/h1-9,13-14H,(H2,17,19)(H,18,20)/t13-,14+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Leeds
Curated by ChEMBL
| Assay Description
Inhibition of transglutaminase 2 (unknown origin) |
Eur J Med Chem 98: 49-53 (2015)
Article DOI: 10.1016/j.ejmech.2015.05.019
BindingDB Entry DOI: 10.7270/Q261121G |
More data for this
Ligand-Target Pair | |
Coagulation factor XIII A chain
(Homo sapiens (Human)) | BDBM50083728

(CHEMBL3423193)Show SMILES NC(=O)[C@H]1O[C@H]1C(=O)Nc1ccc(Oc2ccccc2)cc1 |r| Show InChI InChI=1S/C16H14N2O4/c17-15(19)13-14(22-13)16(20)18-10-6-8-12(9-7-10)21-11-4-2-1-3-5-11/h1-9,13-14H,(H2,17,19)(H,18,20)/t13-,14+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 55 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Leeds
Curated by ChEMBL
| Assay Description
Inhibition of thrombin-activated F13-A (unknown origin) in plasma assessed as inhibition of fibrin clot formation after 7 mins by biotin incorporatio... |
Eur J Med Chem 98: 49-53 (2015)
Article DOI: 10.1016/j.ejmech.2015.05.019
BindingDB Entry DOI: 10.7270/Q261121G |
More data for this
Ligand-Target Pair | |
Protein-glutamine gamma-glutamyltransferase 2
(Homo sapiens (Human)) | BDBM50083723

(CHEMBL3423197)Show SMILES NC(=O)[C@@H]1O[C@@H]1C(=O)Nc1ccc(Br)cc1 |r| Show InChI InChI=1S/C10H9BrN2O3/c11-5-1-3-6(4-2-5)13-10(15)8-7(16-8)9(12)14/h1-4,7-8H,(H2,12,14)(H,13,15)/t7-,8+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 57 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Leeds
Curated by ChEMBL
| Assay Description
Inhibition of transglutaminase 2 (unknown origin) |
Eur J Med Chem 98: 49-53 (2015)
Article DOI: 10.1016/j.ejmech.2015.05.019
BindingDB Entry DOI: 10.7270/Q261121G |
More data for this
Ligand-Target Pair | |
Coagulation factor XIII A chain
(Homo sapiens (Human)) | BDBM50083718

(CHEMBL3423202)Show SMILES NC(=O)[C@@H]1O[C@@H]1C(=O)Nc1ccc(Oc2ccc(N)cc2)cc1 |r| Show InChI InChI=1S/C16H15N3O4/c17-9-1-5-11(6-2-9)22-12-7-3-10(4-8-12)19-16(21)14-13(23-14)15(18)20/h1-8,13-14H,17H2,(H2,18,20)(H,19,21)/t13-,14+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 86 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Leeds
Curated by ChEMBL
| Assay Description
Inhibition of thrombin-activated F13-A (unknown origin) in plasma assessed as inhibition of fibrin clot formation after 7 mins by biotin incorporatio... |
Eur J Med Chem 98: 49-53 (2015)
Article DOI: 10.1016/j.ejmech.2015.05.019
BindingDB Entry DOI: 10.7270/Q261121G |
More data for this
Ligand-Target Pair | |
Protein-glutamine gamma-glutamyltransferase 2
(Homo sapiens (Human)) | BDBM50083720
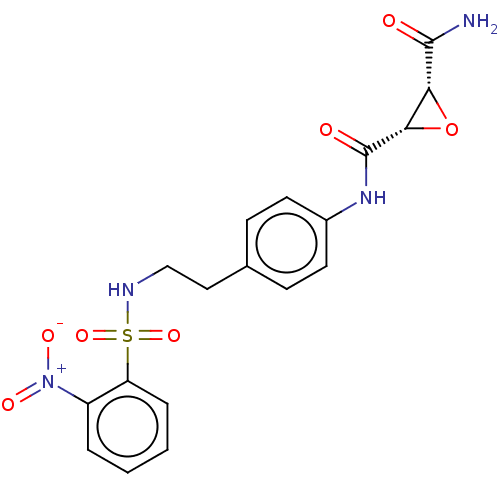
(CHEMBL3423200)Show SMILES NC(=O)[C@@H]1O[C@@H]1C(=O)Nc1ccc(CCNS(=O)(=O)c2ccccc2[N+]([O-])=O)cc1 |r| Show InChI InChI=1S/C18H18N4O7S/c19-17(23)15-16(29-15)18(24)21-12-7-5-11(6-8-12)9-10-20-30(27,28)14-4-2-1-3-13(14)22(25)26/h1-8,15-16,20H,9-10H2,(H2,19,23)(H,21,24)/t15-,16+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 88 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Leeds
Curated by ChEMBL
| Assay Description
Inhibition of transglutaminase 2 (unknown origin) |
Eur J Med Chem 98: 49-53 (2015)
Article DOI: 10.1016/j.ejmech.2015.05.019
BindingDB Entry DOI: 10.7270/Q261121G |
More data for this
Ligand-Target Pair | |
Coagulation factor XIII A chain
(Homo sapiens (Human)) | BDBM50083720

(CHEMBL3423200)Show SMILES NC(=O)[C@@H]1O[C@@H]1C(=O)Nc1ccc(CCNS(=O)(=O)c2ccccc2[N+]([O-])=O)cc1 |r| Show InChI InChI=1S/C18H18N4O7S/c19-17(23)15-16(29-15)18(24)21-12-7-5-11(6-8-12)9-10-20-30(27,28)14-4-2-1-3-13(14)22(25)26/h1-8,15-16,20H,9-10H2,(H2,19,23)(H,21,24)/t15-,16+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Leeds
Curated by ChEMBL
| Assay Description
Inhibition of thrombin-activated F13-A (unknown origin) in plasma assessed as inhibition of fibrin clot formation after 7 mins by biotin incorporatio... |
Eur J Med Chem 98: 49-53 (2015)
Article DOI: 10.1016/j.ejmech.2015.05.019
BindingDB Entry DOI: 10.7270/Q261121G |
More data for this
Ligand-Target Pair | |
Coagulation factor XIII A chain
(Homo sapiens (Human)) | BDBM50083717
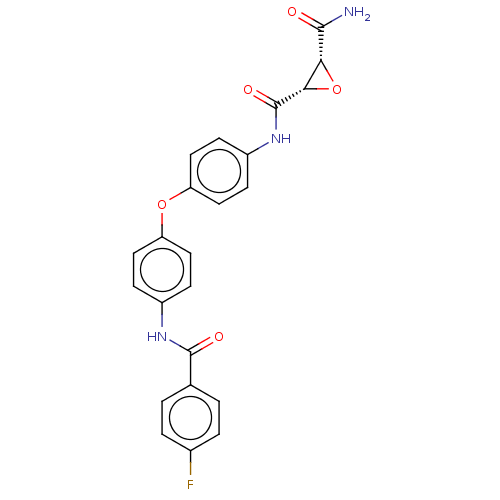
(CHEMBL3423203)Show SMILES NC(=O)[C@@H]1O[C@@H]1C(=O)Nc1ccc(Oc2ccc(NC(=O)c3ccc(F)cc3)cc2)cc1 |r| Show InChI InChI=1S/C23H18FN3O5/c24-14-3-1-13(2-4-14)22(29)26-15-5-9-17(10-6-15)31-18-11-7-16(8-12-18)27-23(30)20-19(32-20)21(25)28/h1-12,19-20H,(H2,25,28)(H,26,29)(H,27,30)/t19-,20+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Leeds
Curated by ChEMBL
| Assay Description
Inhibition of thrombin-activated F13-A (unknown origin) in plasma assessed as inhibition of fibrin clot formation after 7 mins by biotin incorporatio... |
Eur J Med Chem 98: 49-53 (2015)
Article DOI: 10.1016/j.ejmech.2015.05.019
BindingDB Entry DOI: 10.7270/Q261121G |
More data for this
Ligand-Target Pair | |
Coagulation factor XIII A chain
(Homo sapiens (Human)) | BDBM50083721
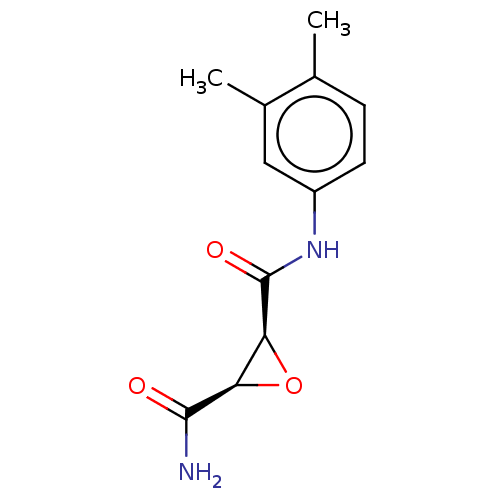
(CHEMBL3423199)Show InChI InChI=1S/C12H14N2O3/c1-6-3-4-8(5-7(6)2)14-12(16)10-9(17-10)11(13)15/h3-5,9-10H,1-2H3,(H2,13,15)(H,14,16)/t9-,10+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Leeds
Curated by ChEMBL
| Assay Description
Inhibition of thrombin-activated F13-A (unknown origin) in plasma assessed as inhibition of fibrin clot formation after 7 mins by biotin incorporatio... |
Eur J Med Chem 98: 49-53 (2015)
Article DOI: 10.1016/j.ejmech.2015.05.019
BindingDB Entry DOI: 10.7270/Q261121G |
More data for this
Ligand-Target Pair | |
Coagulation factor XIII A chain
(Homo sapiens (Human)) | BDBM50083723

(CHEMBL3423197)Show SMILES NC(=O)[C@@H]1O[C@@H]1C(=O)Nc1ccc(Br)cc1 |r| Show InChI InChI=1S/C10H9BrN2O3/c11-5-1-3-6(4-2-5)13-10(15)8-7(16-8)9(12)14/h1-4,7-8H,(H2,12,14)(H,13,15)/t7-,8+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Leeds
Curated by ChEMBL
| Assay Description
Inhibition of thrombin-activated F13-A (unknown origin) in plasma assessed as inhibition of fibrin clot formation after 7 mins by biotin incorporatio... |
Eur J Med Chem 98: 49-53 (2015)
Article DOI: 10.1016/j.ejmech.2015.05.019
BindingDB Entry DOI: 10.7270/Q261121G |
More data for this
Ligand-Target Pair | |
Protein-glutamine gamma-glutamyltransferase 2
(Homo sapiens (Human)) | BDBM50083721

(CHEMBL3423199)Show InChI InChI=1S/C12H14N2O3/c1-6-3-4-8(5-7(6)2)14-12(16)10-9(17-10)11(13)15/h3-5,9-10H,1-2H3,(H2,13,15)(H,14,16)/t9-,10+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Leeds
Curated by ChEMBL
| Assay Description
Inhibition of transglutaminase 2 (unknown origin) |
Eur J Med Chem 98: 49-53 (2015)
Article DOI: 10.1016/j.ejmech.2015.05.019
BindingDB Entry DOI: 10.7270/Q261121G |
More data for this
Ligand-Target Pair | |
Protein-glutamine gamma-glutamyltransferase 2
(Homo sapiens (Human)) | BDBM50083759

(CHEMBL3423192)Show SMILES CCCCc1ccc(NC(=O)[C@@H]2O[C@@H]2C(N)=O)cc1 |r| Show InChI InChI=1S/C14H18N2O3/c1-2-3-4-9-5-7-10(8-6-9)16-14(18)12-11(19-12)13(15)17/h5-8,11-12H,2-4H2,1H3,(H2,15,17)(H,16,18)/t11-,12+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Leeds
Curated by ChEMBL
| Assay Description
Inhibition of transglutaminase 2 (unknown origin) |
Eur J Med Chem 98: 49-53 (2015)
Article DOI: 10.1016/j.ejmech.2015.05.019
BindingDB Entry DOI: 10.7270/Q261121G |
More data for this
Ligand-Target Pair | |
Protein-glutamine gamma-glutamyltransferase 2
(Homo sapiens (Human)) | BDBM50083717

(CHEMBL3423203)Show SMILES NC(=O)[C@@H]1O[C@@H]1C(=O)Nc1ccc(Oc2ccc(NC(=O)c3ccc(F)cc3)cc2)cc1 |r| Show InChI InChI=1S/C23H18FN3O5/c24-14-3-1-13(2-4-14)22(29)26-15-5-9-17(10-6-15)31-18-11-7-16(8-12-18)27-23(30)20-19(32-20)21(25)28/h1-12,19-20H,(H2,25,28)(H,26,29)(H,27,30)/t19-,20+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Leeds
Curated by ChEMBL
| Assay Description
Inhibition of transglutaminase 2 (unknown origin) |
Eur J Med Chem 98: 49-53 (2015)
Article DOI: 10.1016/j.ejmech.2015.05.019
BindingDB Entry DOI: 10.7270/Q261121G |
More data for this
Ligand-Target Pair | |
Coagulation factor XIII A chain
(Homo sapiens (Human)) | BDBM50083759

(CHEMBL3423192)Show SMILES CCCCc1ccc(NC(=O)[C@@H]2O[C@@H]2C(N)=O)cc1 |r| Show InChI InChI=1S/C14H18N2O3/c1-2-3-4-9-5-7-10(8-6-9)16-14(18)12-11(19-12)13(15)17/h5-8,11-12H,2-4H2,1H3,(H2,15,17)(H,16,18)/t11-,12+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 220 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Leeds
Curated by ChEMBL
| Assay Description
Inhibition of thrombin-activated F13-A (unknown origin) in plasma assessed as inhibition of fibrin clot formation after 7 mins by biotin incorporatio... |
Eur J Med Chem 98: 49-53 (2015)
Article DOI: 10.1016/j.ejmech.2015.05.019
BindingDB Entry DOI: 10.7270/Q261121G |
More data for this
Ligand-Target Pair | |
Coagulation factor XIII A chain
(Homo sapiens (Human)) | BDBM50083719

(CHEMBL3423201)Show SMILES NC(=O)[C@@H]1O[C@@H]1C(=O)Nc1ccc(Oc2ccc(cc2)[N+]([O-])=O)cc1 |r| Show InChI InChI=1S/C16H13N3O6/c17-15(20)13-14(25-13)16(21)18-9-1-5-11(6-2-9)24-12-7-3-10(4-8-12)19(22)23/h1-8,13-14H,(H2,17,20)(H,18,21)/t13-,14+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 430 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Leeds
Curated by ChEMBL
| Assay Description
Inhibition of thrombin-activated F13-A (unknown origin) in plasma assessed as inhibition of fibrin clot formation after 7 mins by biotin incorporatio... |
Eur J Med Chem 98: 49-53 (2015)
Article DOI: 10.1016/j.ejmech.2015.05.019
BindingDB Entry DOI: 10.7270/Q261121G |
More data for this
Ligand-Target Pair | |
Coagulation factor XIII A chain
(Homo sapiens (Human)) | BDBM50083724

(CHEMBL3423196)Show InChI InChI=1S/C10H10N2O3/c11-9(13)7-8(15-7)10(14)12-6-4-2-1-3-5-6/h1-5,7-8H,(H2,11,13)(H,12,14)/t7-,8+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 720 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Leeds
Curated by ChEMBL
| Assay Description
Inhibition of thrombin-activated F13-A (unknown origin) in plasma assessed as inhibition of fibrin clot formation after 7 mins by biotin incorporatio... |
Eur J Med Chem 98: 49-53 (2015)
Article DOI: 10.1016/j.ejmech.2015.05.019
BindingDB Entry DOI: 10.7270/Q261121G |
More data for this
Ligand-Target Pair | |
Protein-glutamine gamma-glutamyltransferase 2
(Homo sapiens (Human)) | BDBM50083724

(CHEMBL3423196)Show InChI InChI=1S/C10H10N2O3/c11-9(13)7-8(15-7)10(14)12-6-4-2-1-3-5-6/h1-5,7-8H,(H2,11,13)(H,12,14)/t7-,8+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Leeds
Curated by ChEMBL
| Assay Description
Inhibition of transglutaminase 2 (unknown origin) |
Eur J Med Chem 98: 49-53 (2015)
Article DOI: 10.1016/j.ejmech.2015.05.019
BindingDB Entry DOI: 10.7270/Q261121G |
More data for this
Ligand-Target Pair | |
Coagulation factor XIII A chain
(Homo sapiens (Human)) | BDBM50009248
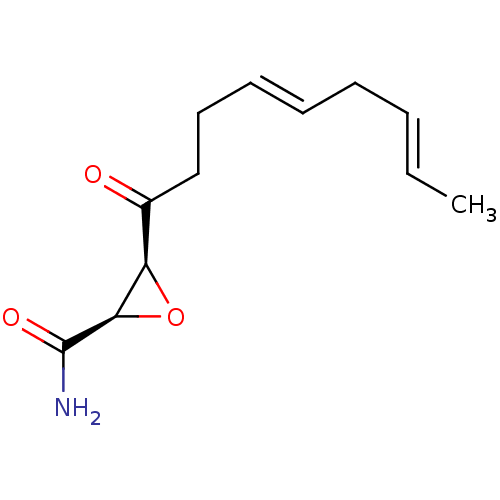
((2R,3S)-3-((4E,7E)-Nona-4,7-dienoyl)-oxirane-2-car...)Show InChI InChI=1S/C12H17NO3/c1-2-3-4-5-6-7-8-9(14)10-11(16-10)12(13)15/h2-3,5-6,10-11H,4,7-8H2,1H3,(H2,13,15)/b3-2+,6-5+/t10-,11-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
| Article
PubMed
| n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Leeds
Curated by ChEMBL
| Assay Description
Inhibition of thrombin-activated F13-A (unknown origin) in plasma assessed as inhibition of fibrin clot formation after 7 mins by biotin incorporatio... |
Eur J Med Chem 98: 49-53 (2015)
Article DOI: 10.1016/j.ejmech.2015.05.019
BindingDB Entry DOI: 10.7270/Q261121G |
More data for this
Ligand-Target Pair | |
Coagulation factor XIII A chain
(Homo sapiens (Human)) | BDBM50009248

((2R,3S)-3-((4E,7E)-Nona-4,7-dienoyl)-oxirane-2-car...)Show InChI InChI=1S/C12H17NO3/c1-2-3-4-5-6-7-8-9(14)10-11(16-10)12(13)15/h2-3,5-6,10-11H,4,7-8H2,1H3,(H2,13,15)/b3-2+,6-5+/t10-,11-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
| Article
PubMed
| n/a | n/a | 1.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Leeds
Curated by ChEMBL
| Assay Description
Inhibition of F13-A in human plasma assessed as inhibition of fibrin clot formation by biotin incorporation assay in presence of 1 mM reduced GSH |
Eur J Med Chem 98: 49-53 (2015)
Article DOI: 10.1016/j.ejmech.2015.05.019
BindingDB Entry DOI: 10.7270/Q261121G |
More data for this
Ligand-Target Pair | |
Nucleoprotein
(Human respiratory syncytial virus A (strain A2)) | BDBM614422
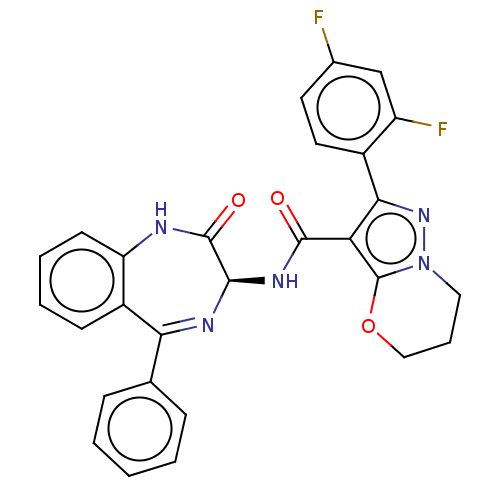
(2-(2,4-Difluorophenyl)-N-[(3S)-2-oxo-5-phenyl-1,3-...)Show SMILES Fc1ccc(-c2nn3CCCOc3c2C(=O)N[C@H]2N=C(c3ccccc3)c3ccccc3NC2=O)c(F)c1 |t:20| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | n/a | n/a | 33 | n/a | n/a | n/a | n/a |
Celgene Quanticel Research, Inc.
US Patent
| |
US Patent US9604961 (2017)
BindingDB Entry DOI: 10.7270/Q23B648H |
More data for this
Ligand-Target Pair | |
Nucleoprotein
(Human respiratory syncytial virus A (strain A2)) | BDBM614423
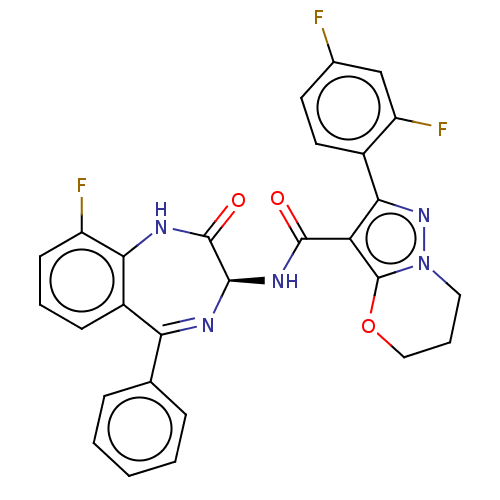
(2-(2,4-Difluorophenyl)- N-[(3S)-9-fluoro-2-oxo- 5-...)Show SMILES Fc1ccc(-c2nn3CCCOc3c2C(=O)N[C@H]2N=C(c3ccccc3)c3cccc(F)c3NC2=O)c(F)c1 |t:20| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | n/a | n/a | 22 | n/a | n/a | n/a | n/a |
Celgene Quanticel Research, Inc.
US Patent
| |
US Patent US9604961 (2017)
BindingDB Entry DOI: 10.7270/Q23B648H |
More data for this
Ligand-Target Pair | |
Nucleoprotein
(Human respiratory syncytial virus A (strain A2)) | BDBM614426
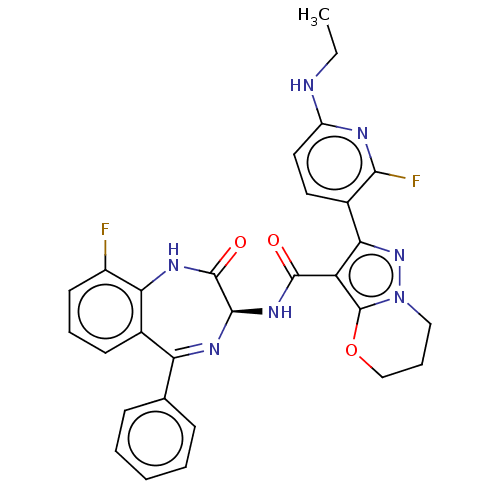
(2-[6-(Ethylamino)-2- fluoropyridin-3-yl]-N- [(3S)-...)Show SMILES CCNc1ccc(-c2nn3CCCOc3c2C(=O)N[C@H]2N=C(c3ccccc3)c3cccc(F)c3NC2=O)c(F)n1 |t:22| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | n/a | n/a | 20 | n/a | n/a | n/a | n/a |
Celgene Quanticel Research, Inc.
US Patent
| |
US Patent US9604961 (2017)
BindingDB Entry DOI: 10.7270/Q23B648H |
More data for this
Ligand-Target Pair | |
Nucleoprotein
(Human respiratory syncytial virus A (strain A2)) | BDBM614427
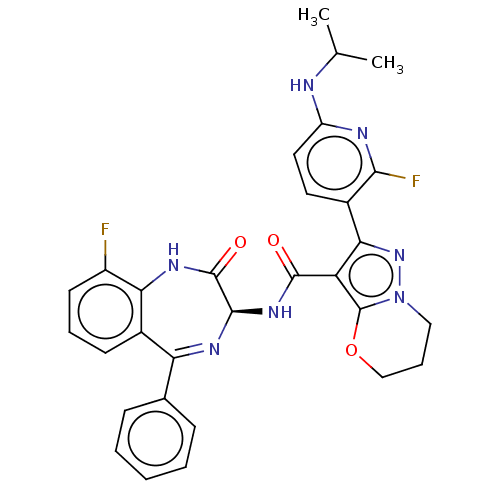
(N-[(3S)-9-Fluoro-2-oxo- 5-phenyl-1,3-dihydro-1,4- ...)Show SMILES CC(C)Nc1ccc(-c2nn3CCCOc3c2C(=O)N[C@H]2N=C(c3ccccc3)c3cccc(F)c3NC2=O)c(F)n1 |t:23| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | n/a | n/a | 29 | n/a | n/a | n/a | n/a |
Celgene Quanticel Research, Inc.
US Patent
| |
US Patent US9604961 (2017)
BindingDB Entry DOI: 10.7270/Q23B648H |
More data for this
Ligand-Target Pair | |
Nucleoprotein
(Human respiratory syncytial virus A (strain A2)) | BDBM614428
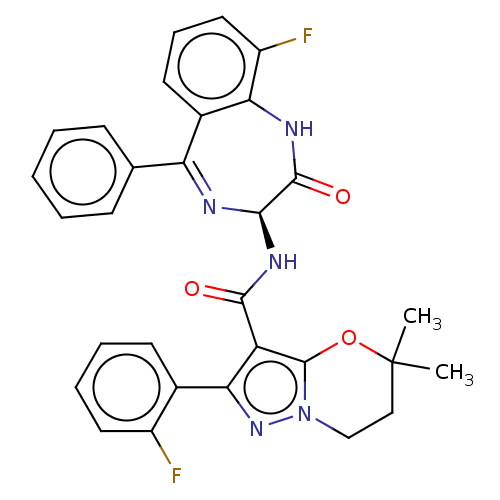
(N-[(3S)-9-fluoro-2-oxo-5-phenyl-2,3-dihydro-1H-1,4...)Show SMILES CC1(C)CCn2nc(c(C(=O)N[C@H]3N=C(c4ccccc4)c4cccc(F)c4NC3=O)c2O1)-c1ccccc1F |t:13| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | n/a | n/a | 54 | n/a | n/a | n/a | n/a |
Celgene Quanticel Research, Inc.
US Patent
| |
US Patent US9604961 (2017)
BindingDB Entry DOI: 10.7270/Q23B648H |
More data for this
Ligand-Target Pair | |
Nucleoprotein
(Human respiratory syncytial virus A (strain A2)) | BDBM614429
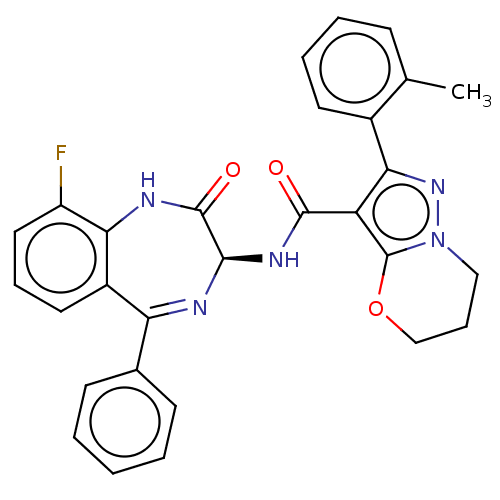
(N-[(3S)-9-Fluoro- 2-oxo-5-phenyl- 1,3-dihydro-1,4-...)Show SMILES Cc1ccccc1-c1nn2CCCOc2c1C(=O)N[C@H]1N=C(c2ccccc2)c2cccc(F)c2NC1=O |t:23| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | n/a | n/a | 165 | n/a | n/a | n/a | n/a |
Celgene Quanticel Research, Inc.
US Patent
| |
US Patent US9604961 (2017)
BindingDB Entry DOI: 10.7270/Q23B648H |
More data for this
Ligand-Target Pair | |
Nucleoprotein
(Human respiratory syncytial virus A (strain A2)) | BDBM614430
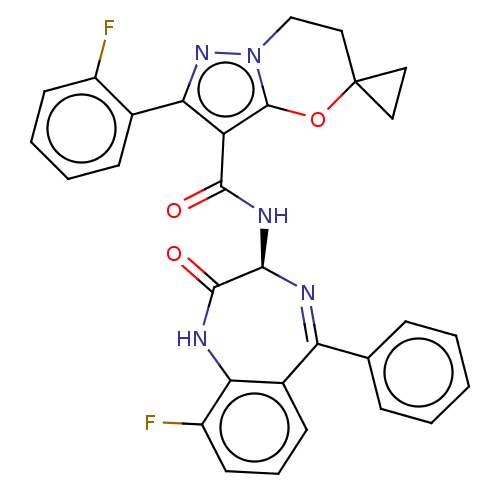
(N-[(3S)-9-fluoro-2- oxo-5-phenyl-1,3- dihydro-1,4-...)Show SMILES Fc1cccc2c1NC(=O)[C@@H](NC(=O)c1c(nn3CCC4(CC4)Oc13)-c1ccccc1F)N=C2c1ccccc1 |c:37| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | n/a | n/a | 32 | n/a | n/a | n/a | n/a |
Celgene Quanticel Research, Inc.
US Patent
| |
US Patent US9604961 (2017)
BindingDB Entry DOI: 10.7270/Q23B648H |
More data for this
Ligand-Target Pair | |
Nucleoprotein
(Human respiratory syncytial virus A (strain A2)) | BDBM614431
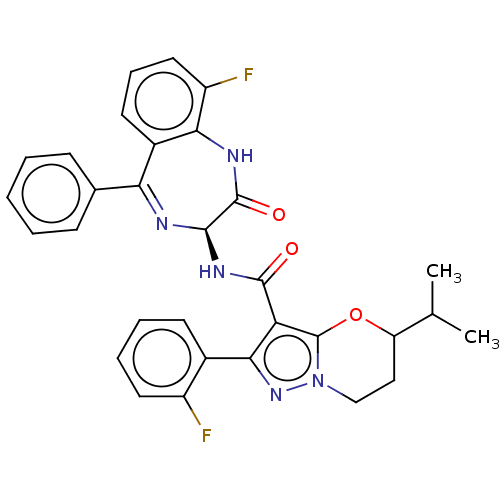
(N-[(3S)-9-fluoro-2- oxo-5-phenyl-2,3- dihydro-1H-1...)Show SMILES CC(C)C1CCn2nc(c(C(=O)N[C@H]3N=C(c4ccccc4)c4cccc(F)c4NC3=O)c2O1)-c1ccccc1F |t:14| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | n/a | n/a | 37 | n/a | n/a | n/a | n/a |
Celgene Quanticel Research, Inc.
US Patent
| |
US Patent US9604961 (2017)
BindingDB Entry DOI: 10.7270/Q23B648H |
More data for this
Ligand-Target Pair | |
Nucleoprotein
(Human respiratory syncytial virus A (strain A2)) | BDBM614432
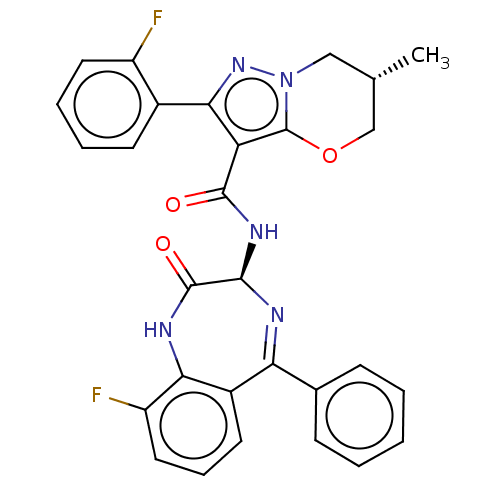
((6S)-N-[(3S)-9- fluoro-2-oxo-5- phenyl-1,3- dihydr...)Show SMILES C[C@@H]1COc2c(C(=O)N[C@H]3N=C(c4ccccc4)c4cccc(F)c4NC3=O)c(nn2C1)-c1ccccc1F |t:10| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | n/a | n/a | 30 | n/a | n/a | n/a | n/a |
Celgene Quanticel Research, Inc.
US Patent
| |
US Patent US9604961 (2017)
BindingDB Entry DOI: 10.7270/Q23B648H |
More data for this
Ligand-Target Pair | |
Nucleoprotein
(Human respiratory syncytial virus A (strain A2)) | BDBM614433
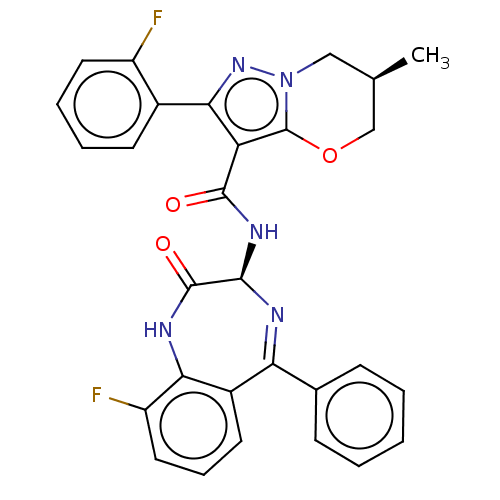
((6R)-N-[(3S)-9- fluoro-2-oxo-5- phenyl-1,3- dihydr...)Show SMILES C[C@H]1COc2c(C(=O)N[C@H]3N=C(c4ccccc4)c4cccc(F)c4NC3=O)c(nn2C1)-c1ccccc1F |t:10| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | n/a | n/a | 23 | n/a | n/a | n/a | n/a |
Celgene Quanticel Research, Inc.
US Patent
| |
US Patent US9604961 (2017)
BindingDB Entry DOI: 10.7270/Q23B648H |
More data for this
Ligand-Target Pair | |
Nucleoprotein
(Human respiratory syncytial virus A (strain A2)) | BDBM614434
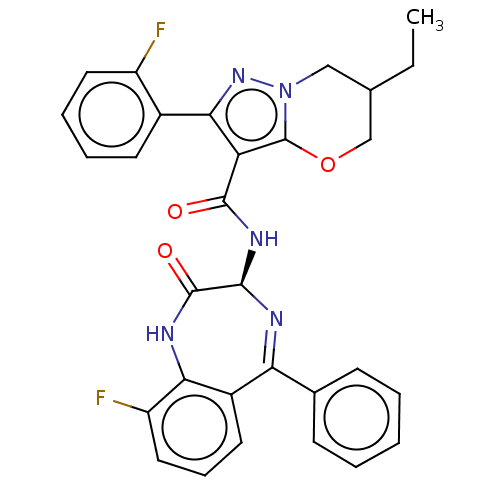
(6-Ethyl-N-[(3S)-9- fluoro-2-oxo-5- phenyl-2,3- dih...)Show SMILES CCC1COc2c(C(=O)N[C@H]3N=C(c4ccccc4)c4cccc(F)c4NC3=O)c(nn2C1)-c1ccccc1F |t:11| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | n/a | n/a | 33 | n/a | n/a | n/a | n/a |
Celgene Quanticel Research, Inc.
US Patent
| |
US Patent US9604961 (2017)
BindingDB Entry DOI: 10.7270/Q23B648H |
More data for this
Ligand-Target Pair | |
Nucleoprotein
(Human respiratory syncytial virus A (strain A2)) | BDBM614435
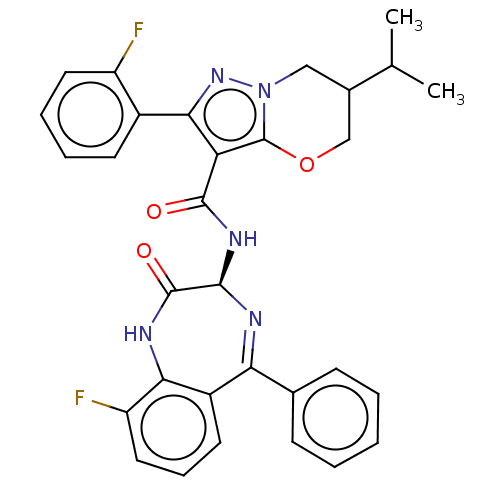
(N-[(3S)-9-Fluoro- 2-oxo-5-phenyl- 2,3-dihydro-1H- ...)Show SMILES CC(C)C1COc2c(C(=O)N[C@H]3N=C(c4ccccc4)c4cccc(F)c4NC3=O)c(nn2C1)-c1ccccc1F |t:12| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | n/a | n/a | 39 | n/a | n/a | n/a | n/a |
Celgene Quanticel Research, Inc.
US Patent
| |
US Patent US9604961 (2017)
BindingDB Entry DOI: 10.7270/Q23B648H |
More data for this
Ligand-Target Pair | |
Nucleoprotein
(Human respiratory syncytial virus A (strain A2)) | BDBM614436
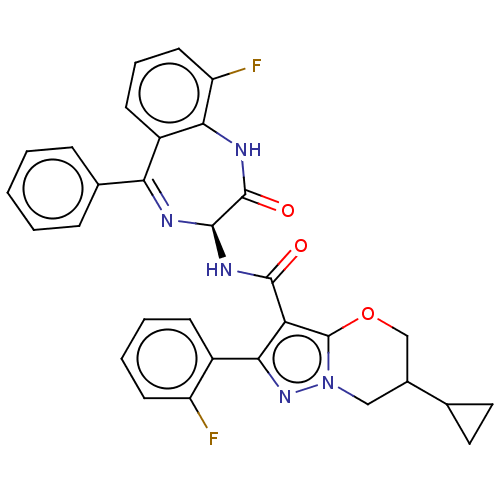
(6-Cyclopropyl-N- [(3S)-9-fluoro-2- oxo-5-phenyl-2,...)Show SMILES Fc1cccc2c1NC(=O)[C@@H](NC(=O)c1c3OCC(Cn3nc1-c1ccccc1F)C1CC1)N=C2c1ccccc1 |c:38| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | n/a | n/a | 28 | n/a | n/a | n/a | n/a |
Celgene Quanticel Research, Inc.
US Patent
| |
US Patent US9604961 (2017)
BindingDB Entry DOI: 10.7270/Q23B648H |
More data for this
Ligand-Target Pair | |
Nucleoprotein
(Human respiratory syncytial virus A (strain A2)) | BDBM614437
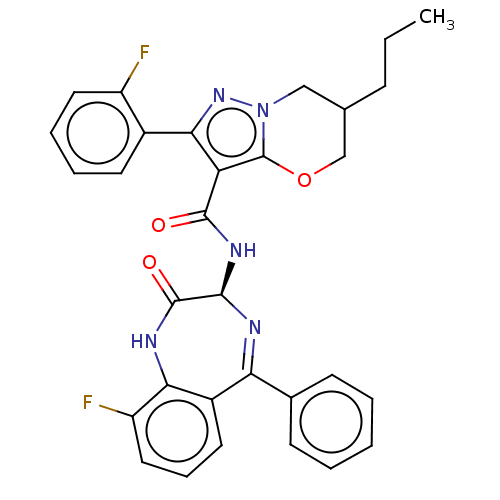
(N-[(3S)-9-fluoro-2- oxo-5-phenyl-2,3- dihydro-1H-1...)Show SMILES CCCC1COc2c(C(=O)N[C@H]3N=C(c4ccccc4)c4cccc(F)c4NC3=O)c(nn2C1)-c1ccccc1F |t:12| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | n/a | n/a | 36 | n/a | n/a | n/a | n/a |
Celgene Quanticel Research, Inc.
US Patent
| |
US Patent US9604961 (2017)
BindingDB Entry DOI: 10.7270/Q23B648H |
More data for this
Ligand-Target Pair | |
Nucleoprotein
(Human respiratory syncytial virus A (strain A2)) | BDBM614438
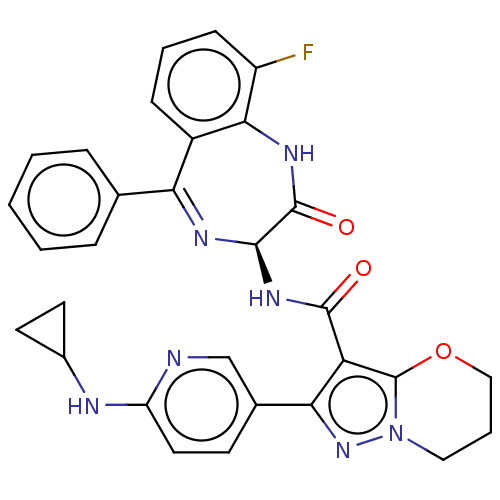
(2-[6-(Cyclopropylamino)pyridin-3-yl]-N-[(3S)-9-flu...)Show SMILES Fc1cccc2c1NC(=O)[C@@H](NC(=O)c1c3OCCCn3nc1-c1ccc(NC3CC3)nc1)N=C2c1ccccc1 |c:38| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | n/a | n/a | 30 | n/a | n/a | n/a | n/a |
Celgene Quanticel Research, Inc.
US Patent
| |
US Patent US9604961 (2017)
BindingDB Entry DOI: 10.7270/Q23B648H |
More data for this
Ligand-Target Pair | |
Nucleoprotein
(Human respiratory syncytial virus A (strain A2)) | BDBM614497
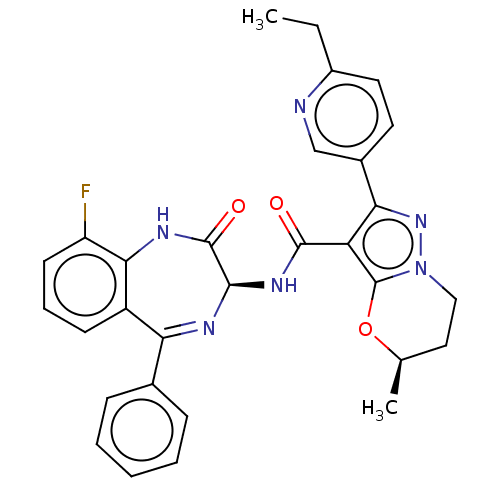
((5R)-2-(6- Ethylpyridin-3-yl)-5- methyl-N-[(3S)-9-...)Show SMILES CCc1ccc(cn1)-c1nn2CC[C@@H](C)Oc2c1C(=O)N[C@H]1N=C(c2ccccc2)c2cccc(F)c2NC1=O |t:25| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | n/a | n/a | 39 | n/a | n/a | n/a | n/a |
Celgene Quanticel Research, Inc.
US Patent
| |
US Patent US9604961 (2017)
BindingDB Entry DOI: 10.7270/Q23B648H |
More data for this
Ligand-Target Pair | |
Nucleoprotein
(Human respiratory syncytial virus A (strain A2)) | BDBM614498
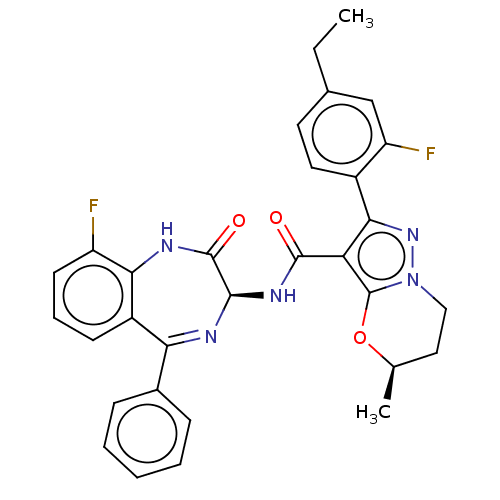
((5R)-2-(4-Ethyl-2- fluorophenyl)-5- methyl-N-[(3S)...)Show SMILES CCc1ccc(-c2nn3CC[C@@H](C)Oc3c2C(=O)N[C@H]2N=C(c3ccccc3)c3cccc(F)c3NC2=O)c(F)c1 |t:22| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | n/a | n/a | 158 | n/a | n/a | n/a | n/a |
Celgene Quanticel Research, Inc.
US Patent
| |
US Patent US9604961 (2017)
BindingDB Entry DOI: 10.7270/Q23B648H |
More data for this
Ligand-Target Pair | |
Nucleoprotein
(Human respiratory syncytial virus A (strain A2)) | BDBM614499
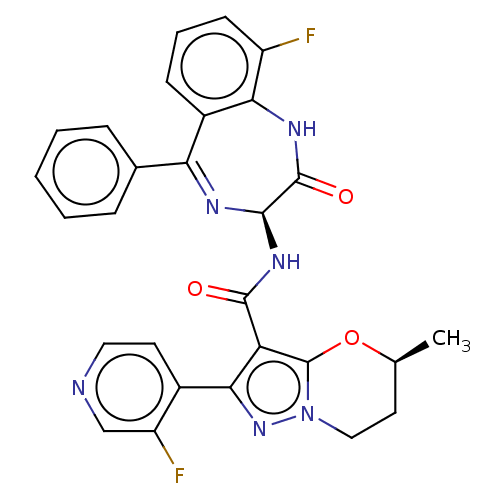
((5S)-N-[(3S)-9- fluoro-2-oxo-5- phenyl-1,3-dihydro...)Show SMILES C[C@H]1CCn2nc(c(C(=O)N[C@H]3N=C(c4ccccc4)c4cccc(F)c4NC3=O)c2O1)-c1ccncc1F |t:12| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | n/a | n/a | 93 | n/a | n/a | n/a | n/a |
Celgene Quanticel Research, Inc.
US Patent
| |
US Patent US9604961 (2017)
BindingDB Entry DOI: 10.7270/Q23B648H |
More data for this
Ligand-Target Pair | |
Nucleoprotein
(Human respiratory syncytial virus A (strain A2)) | BDBM614500
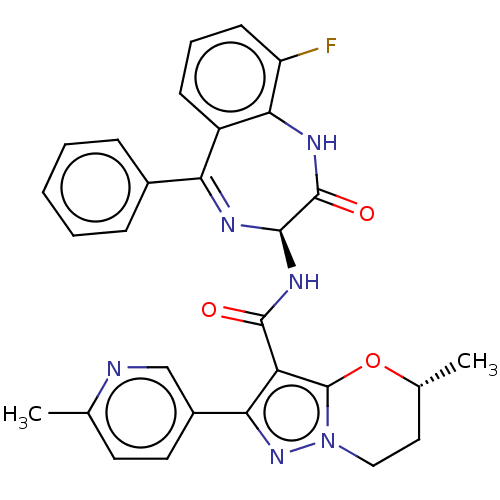
((5R)-5-Methyl-2-(6-methylpyridin-3-yl)-N-[(3S)-9-f...)Show SMILES C[C@@H]1CCn2nc(c(C(=O)N[C@H]3N=C(c4ccccc4)c4cccc(F)c4NC3=O)c2O1)-c1ccc(C)nc1 |t:12| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | n/a | n/a | 159 | n/a | n/a | n/a | n/a |
Celgene Quanticel Research, Inc.
US Patent
| |
US Patent US9604961 (2017)
BindingDB Entry DOI: 10.7270/Q23B648H |
More data for this
Ligand-Target Pair | |
Nucleoprotein
(Human respiratory syncytial virus A (strain A2)) | BDBM614501
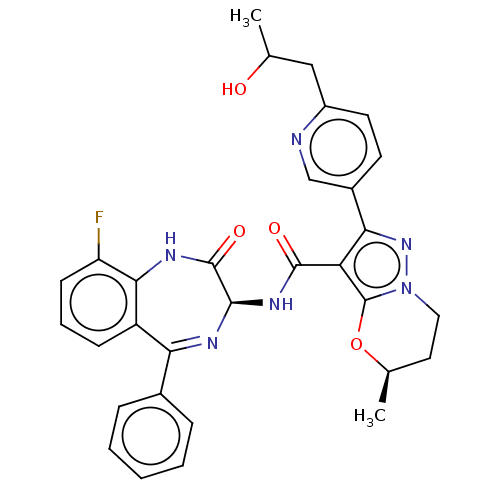
((5R)-2-[6-(2- Hydroxypropyl)pyridin- 3-yl]-5-methy...)Show SMILES CC(O)Cc1ccc(cn1)-c1nn2CC[C@@H](C)Oc2c1C(=O)N[C@H]1N=C(c2ccccc2)c2cccc(F)c2NC1=O |t:27| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | n/a | n/a | 217 | n/a | n/a | n/a | n/a |
Celgene Quanticel Research, Inc.
US Patent
| |
US Patent US9604961 (2017)
BindingDB Entry DOI: 10.7270/Q23B648H |
More data for this
Ligand-Target Pair | |
Nucleoprotein
(Human respiratory syncytial virus A (strain A2)) | BDBM614502
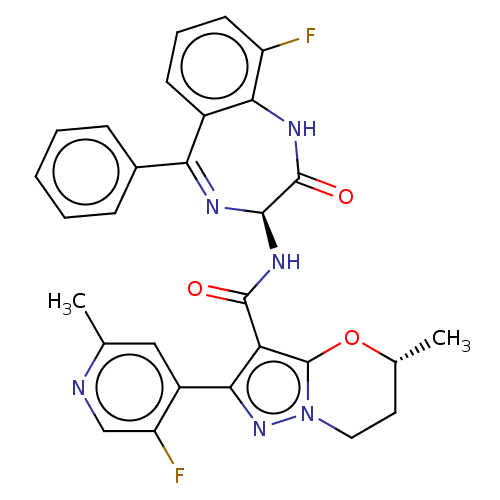
((5R)-2-(5-Fluoro-2- methylpyridin-4-yl)-N- [(3S)-9...)Show SMILES C[C@@H]1CCn2nc(c(C(=O)N[C@H]3N=C(c4ccccc4)c4cccc(F)c4NC3=O)c2O1)-c1cc(C)ncc1F |t:12| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | n/a | n/a | 115 | n/a | n/a | n/a | n/a |
Celgene Quanticel Research, Inc.
US Patent
| |
US Patent US9604961 (2017)
BindingDB Entry DOI: 10.7270/Q23B648H |
More data for this
Ligand-Target Pair | |
Nucleoprotein
(Human respiratory syncytial virus A (strain A2)) | BDBM614503
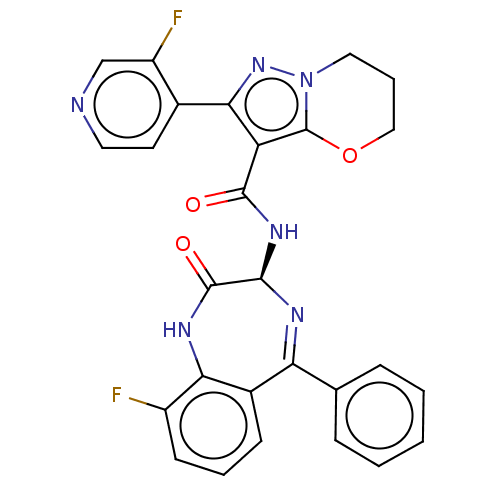
(N-[(3S)-9-fluoro-2- oxo-5-phenyl-1,3- dihydro-1,4-...)Show SMILES Fc1cccc2c1NC(=O)[C@@H](NC(=O)c1c(nn3CCCOc13)-c1ccncc1F)N=C2c1ccccc1 |c:34| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | n/a | n/a | 64 | n/a | n/a | n/a | n/a |
Celgene Quanticel Research, Inc.
US Patent
| |
US Patent US9604961 (2017)
BindingDB Entry DOI: 10.7270/Q23B648H |
More data for this
Ligand-Target Pair | |
Nucleoprotein
(Human respiratory syncytial virus A (strain A2)) | BDBM614513
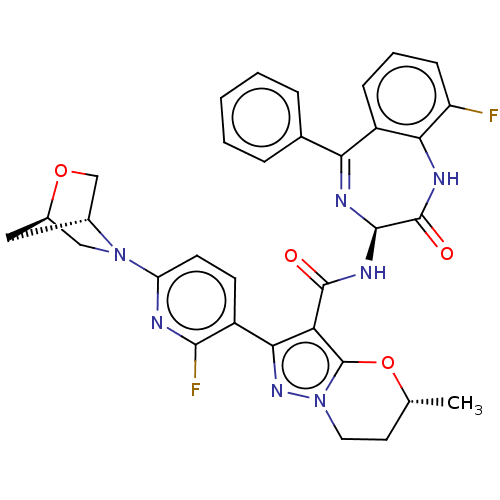
((5R)-2-[2-Fluoro-6-[(1R,4R)-2-oxa-5-azabicyclo[2.2...)Show SMILES C[C@@H]1CCn2nc(c(C(=O)N[C@H]3N=C(c4ccccc4)c4cccc(F)c4NC3=O)c2O1)-c1ccc(nc1F)N1C[C@H]2C[C@@H]1CO2 |t:12| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | n/a | n/a | 20 | n/a | n/a | n/a | n/a |
Celgene Quanticel Research, Inc.
US Patent
| |
US Patent US9604961 (2017)
BindingDB Entry DOI: 10.7270/Q23B648H |
More data for this
Ligand-Target Pair | |
Nucleoprotein
(Human respiratory syncytial virus A (strain A2)) | BDBM614514
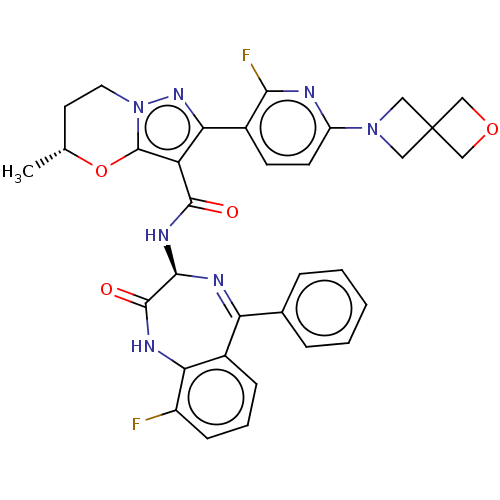
((5R)-2-[2-Fluoro-6-(2-oxa-6-azaspiro[3.3]heptan-6-...)Show SMILES C[C@@H]1CCn2nc(c(C(=O)N[C@H]3N=C(c4ccccc4)c4cccc(F)c4NC3=O)c2O1)-c1ccc(nc1F)N1CC2(COC2)C1 |r,t:12| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | n/a | n/a | 239 | n/a | n/a | n/a | n/a |
Celgene Quanticel Research, Inc.
US Patent
| |
US Patent US9604961 (2017)
BindingDB Entry DOI: 10.7270/Q23B648H |
More data for this
Ligand-Target Pair | |
Nucleoprotein
(Human respiratory syncytial virus A (strain A2)) | BDBM614515
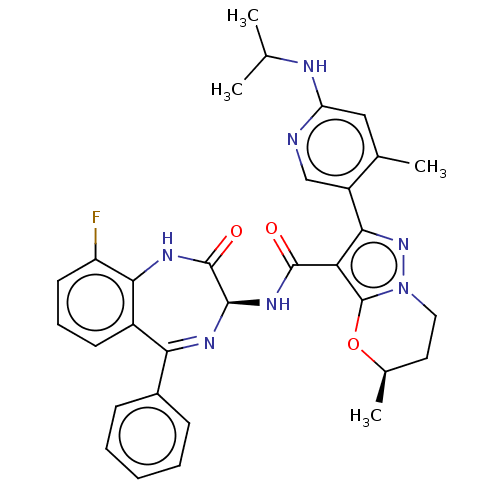
((5R)-5-Methyl-2-[4-methyl-6-(propan-2-ylamino)pyri...)Show SMILES CC(C)Nc1cc(C)c(cn1)-c1nn2CC[C@@H](C)Oc2c1C(=O)N[C@H]1N=C(c2ccccc2)c2cccc(F)c2NC1=O |t:28| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | n/a | n/a | 131 | n/a | n/a | n/a | n/a |
Celgene Quanticel Research, Inc.
US Patent
| |
US Patent US9604961 (2017)
BindingDB Entry DOI: 10.7270/Q23B648H |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data