

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Beta-secretase 1 [1-453] (Homo sapiens (Human)) | BDBM16047 ((4S)-4-[(2S)-2-[(2R,4S,5S)-5-[(2S)-2-[(2S)-2-[(4S)...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | 4.5 | 22 |
Universite de Montreal at Succursale Centre-Ville | Assay Description Inhibition assays were done in black 96-well plates by adding test compounds to the respective enzyme in assay buffer. Plates were incubated at room ... | J Med Chem 48: 5175-90 (2005) Article DOI: 10.1021/jm050142+ BindingDB Entry DOI: 10.7270/Q2WM1BPC | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cathepsin D (Homo sapiens (Human)) | BDBM16047 ((4S)-4-[(2S)-2-[(2R,4S,5S)-5-[(2S)-2-[(2S)-2-[(4S)...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | 25 |
Universite de Montreal at Succursale Centre-Ville | Assay Description Inhibition assays were done in black 96-well plates by adding test compounds to the respective enzyme in assay buffer. Plates were incubated at room ... | J Med Chem 48: 5175-90 (2005) Article DOI: 10.1021/jm050142+ BindingDB Entry DOI: 10.7270/Q2WM1BPC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 [1-453] (Homo sapiens (Human)) | BDBM16060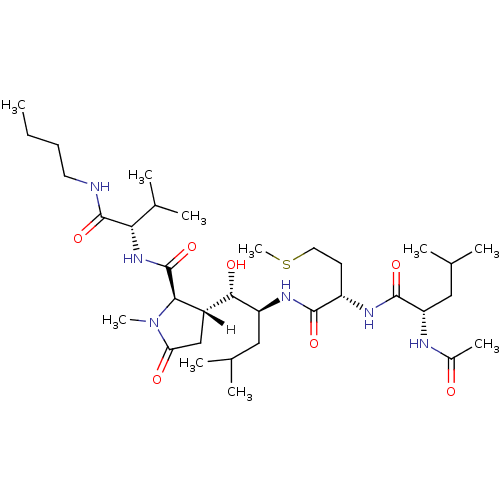 ((2R,3R)-3-[(1S,2S)-2-[[(2S)-2-[[(2S)-2-acetamido-4...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | <10 | n/a | n/a | n/a | n/a | 4.5 | 22 |
Universite de Montreal at Succursale Centre-Ville | Assay Description Inhibition assays were done in black 96-well plates by adding test compounds to the respective enzyme in assay buffer. Plates were incubated at room ... | J Med Chem 48: 5175-90 (2005) Article DOI: 10.1021/jm050142+ BindingDB Entry DOI: 10.7270/Q2WM1BPC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin D (Homo sapiens (Human)) | BDBM16055 ((2S)-N-[(1S)-1-{[(1S,2S)-1-[(1R,2R)-2-{[(1S)-1-(bu...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | 25 |
Universite de Montreal at Succursale Centre-Ville | Assay Description Inhibition assays were done in black 96-well plates by adding test compounds to the respective enzyme in assay buffer. Plates were incubated at room ... | J Med Chem 48: 5175-90 (2005) Article DOI: 10.1021/jm050142+ BindingDB Entry DOI: 10.7270/Q2WM1BPC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin D (Homo sapiens (Human)) | BDBM16049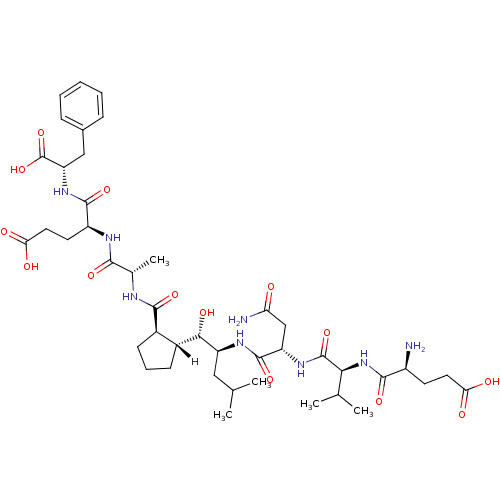 ((4S)-4-[(2S)-2-{[(1R,2R)-2-[(1S,2S)-2-[(2S)-2-[(2S...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | 25 |
Universite de Montreal at Succursale Centre-Ville | Assay Description Inhibition assays were done in black 96-well plates by adding test compounds to the respective enzyme in assay buffer. Plates were incubated at room ... | J Med Chem 48: 5175-90 (2005) Article DOI: 10.1021/jm050142+ BindingDB Entry DOI: 10.7270/Q2WM1BPC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin D (Homo sapiens (Human)) | BDBM16054 ((2S)-N-[(1S)-1-{[(1S,2S)-1-[(1R,2R)-2-{[(1S)-1-(bu...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | 25 |
Universite de Montreal at Succursale Centre-Ville | Assay Description Inhibition assays were done in black 96-well plates by adding test compounds to the respective enzyme in assay buffer. Plates were incubated at room ... | J Med Chem 48: 5175-90 (2005) Article DOI: 10.1021/jm050142+ BindingDB Entry DOI: 10.7270/Q2WM1BPC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 [1-453] (Homo sapiens (Human)) | BDBM16057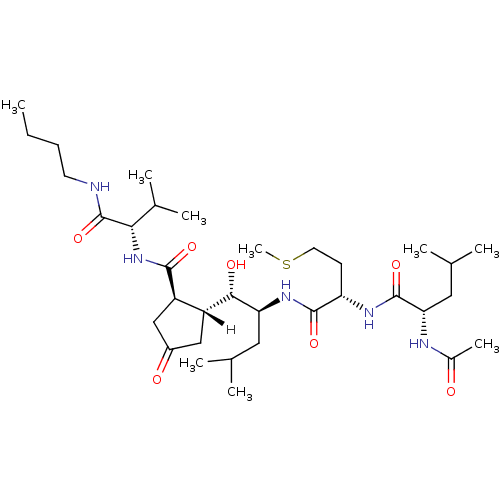 ((2S)-N-[(1S)-1-{[(1S,2S)-1-[(1R,2R)-2-{[(1S)-1-(bu...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | 4.5 | 22 |
Universite de Montreal at Succursale Centre-Ville | Assay Description Inhibition assays were done in black 96-well plates by adding test compounds to the respective enzyme in assay buffer. Plates were incubated at room ... | J Med Chem 48: 5175-90 (2005) Article DOI: 10.1021/jm050142+ BindingDB Entry DOI: 10.7270/Q2WM1BPC | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Beta-secretase 1 [1-453] (Homo sapiens (Human)) | BDBM16048 ((4S)-4-amino-4-{[(1S)-1-{[(1S)-2-carbamoyl-1-{[(1R...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | 4.5 | 22 |
Universite de Montreal at Succursale Centre-Ville | Assay Description Inhibition assays were done in black 96-well plates by adding test compounds to the respective enzyme in assay buffer. Plates were incubated at room ... | J Med Chem 48: 5175-90 (2005) Article DOI: 10.1021/jm050142+ BindingDB Entry DOI: 10.7270/Q2WM1BPC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mas-related G-protein coupled receptor member X1 (Homo sapiens (Human)) | BDBM50340753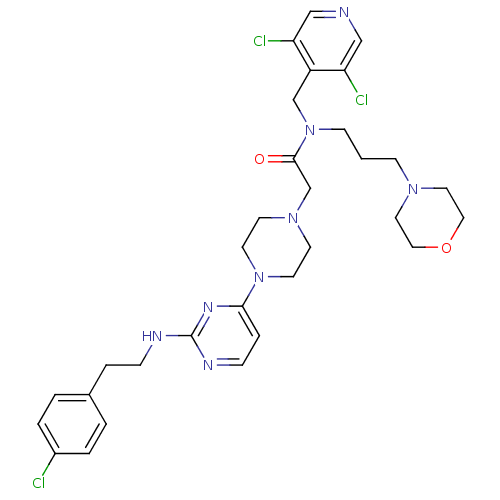 (2-(4-(2-(4-chlorophenethylamino)pyrimidin-4-yl)pip...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Montreal Curated by ChEMBL | Assay Description Antagonist activity at SNSR4 in human HEK293 cells by FLIPR assay | Bioorg Med Chem Lett 21: 2102-5 (2011) Article DOI: 10.1016/j.bmcl.2011.01.138 BindingDB Entry DOI: 10.7270/Q2D21XW7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50177433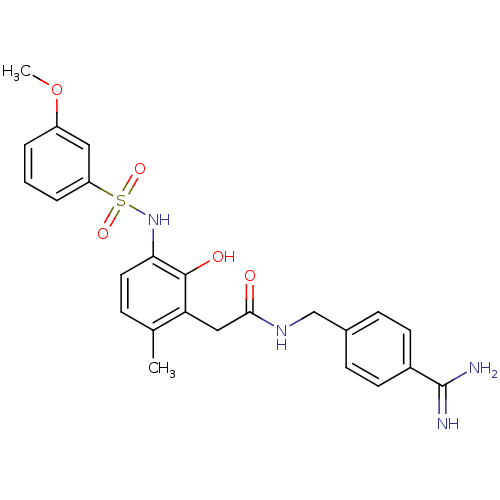 (CHEMBL535160 | N-(4-carbamimidoylbenzyl)-2-(2-hydr...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Montr£al Curated by ChEMBL | Assay Description Inhibitory activity against thrombin | Bioorg Med Chem Lett 16: 1032-6 (2006) Article DOI: 10.1016/j.bmcl.2005.10.082 BindingDB Entry DOI: 10.7270/Q2V125K5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 [1-453] (Homo sapiens (Human)) | BDBM16049 ((4S)-4-[(2S)-2-{[(1R,2R)-2-[(1S,2S)-2-[(2S)-2-[(2S...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | 4.5 | 22 |
Universite de Montreal at Succursale Centre-Ville | Assay Description Inhibition assays were done in black 96-well plates by adding test compounds to the respective enzyme in assay buffer. Plates were incubated at room ... | J Med Chem 48: 5175-90 (2005) Article DOI: 10.1021/jm050142+ BindingDB Entry DOI: 10.7270/Q2WM1BPC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50177436 (CHEMBL557787 | N-(4-carbamimidoyl-benzyl)-2-[2-hyd...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Montr£al Curated by ChEMBL | Assay Description Inhibitory activity against thrombin | Bioorg Med Chem Lett 16: 1032-6 (2006) Article DOI: 10.1016/j.bmcl.2005.10.082 BindingDB Entry DOI: 10.7270/Q2V125K5 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Mas-related G-protein coupled receptor member X1 (Homo sapiens (Human)) | BDBM50340743 (2-(4-(2-(4-chlorophenethylamino)pyrimidin-4-yl)pip...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Montreal Curated by ChEMBL | Assay Description Antagonist activity at SNSR4 in human HEK293 cells by FLIPR assay | Bioorg Med Chem Lett 21: 2102-5 (2011) Article DOI: 10.1016/j.bmcl.2011.01.138 BindingDB Entry DOI: 10.7270/Q2D21XW7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mas-related G-protein coupled receptor member X1 (Homo sapiens (Human)) | BDBM50340744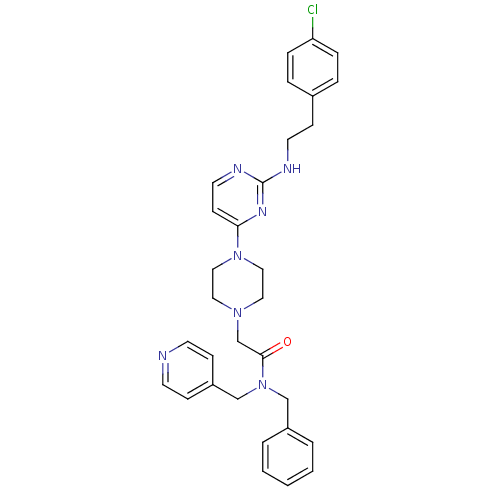 (CHEMBL1762699 | N-benzyl-2-(4-(2-(4-chlorophenethy...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Montreal Curated by ChEMBL | Assay Description Antagonist activity at SNSR4 in human HEK293 cells by FLIPR assay | Bioorg Med Chem Lett 21: 2102-5 (2011) Article DOI: 10.1016/j.bmcl.2011.01.138 BindingDB Entry DOI: 10.7270/Q2D21XW7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 3 (Homo sapiens (Human)) | BDBM50379718 (CHEMBL2011127) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Antagonist activity at human P2X3 receptor expressed in RLE cells assessed as inhibition of (alpha,beta)me-ATP-induced intracellular calcium level by... | Bioorg Med Chem Lett 22: 2565-71 (2012) Article DOI: 10.1016/j.bmcl.2012.01.124 BindingDB Entry DOI: 10.7270/Q29W0GGF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50235535 (CHEMBL559192 | N-(4-carbamimidoyl-benzyl)-2-[4-met...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Montr£al Curated by ChEMBL | Assay Description Inhibition of thrombin | Bioorg Med Chem Lett 18: 1972-6 (2008) Article DOI: 10.1016/j.bmcl.2008.01.122 BindingDB Entry DOI: 10.7270/Q2833SV0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 3 (Homo sapiens (Human)) | BDBM50379716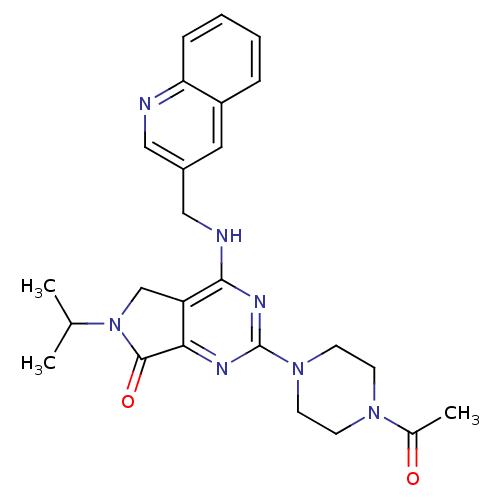 (CHEMBL2011125) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Antagonist activity at human P2X3 receptor expressed in RLE cells assessed as inhibition of (alpha,beta)me-ATP-induced intracellular calcium level by... | Bioorg Med Chem Lett 22: 2565-71 (2012) Article DOI: 10.1016/j.bmcl.2012.01.124 BindingDB Entry DOI: 10.7270/Q29W0GGF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 3 (Homo sapiens (Human)) | BDBM50379717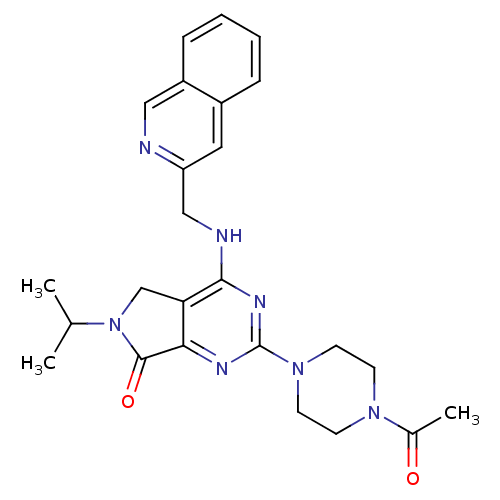 (CHEMBL2011126) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Antagonist activity at human P2X3 receptor expressed in RLE cells assessed as inhibition of (alpha,beta)me-ATP-induced intracellular calcium level by... | Bioorg Med Chem Lett 22: 2565-71 (2012) Article DOI: 10.1016/j.bmcl.2012.01.124 BindingDB Entry DOI: 10.7270/Q29W0GGF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mas-related G-protein coupled receptor member X1 (Homo sapiens (Human)) | BDBM50340745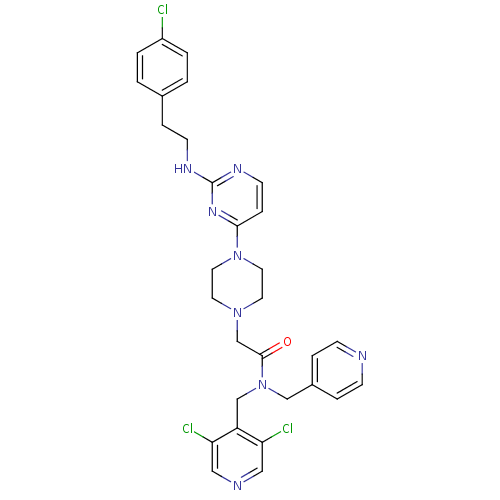 (2-(4-(2-(4-chlorophenethylamino)pyrimidin-4-yl)pip...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Montreal Curated by ChEMBL | Assay Description Antagonist activity at SNSR4 in human HEK293 cells by FLIPR assay | Bioorg Med Chem Lett 21: 2102-5 (2011) Article DOI: 10.1016/j.bmcl.2011.01.138 BindingDB Entry DOI: 10.7270/Q2D21XW7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50177425 (CHEMBL537420 | N-(4-carbamimidoylbenzyl)-2-(3-(2,5...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Montr£al Curated by ChEMBL | Assay Description Inhibitory activity against thrombin | Bioorg Med Chem Lett 16: 1032-6 (2006) Article DOI: 10.1016/j.bmcl.2005.10.082 BindingDB Entry DOI: 10.7270/Q2V125K5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 [1-453] (Homo sapiens (Human)) | BDBM16055 ((2S)-N-[(1S)-1-{[(1S,2S)-1-[(1R,2R)-2-{[(1S)-1-(bu...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | 4.5 | 22 |
Universite de Montreal at Succursale Centre-Ville | Assay Description Inhibition assays were done in black 96-well plates by adding test compounds to the respective enzyme in assay buffer. Plates were incubated at room ... | J Med Chem 48: 5175-90 (2005) Article DOI: 10.1021/jm050142+ BindingDB Entry DOI: 10.7270/Q2WM1BPC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin D (Homo sapiens (Human)) | BDBM16053 ((2S)-2-{[(1R,2R)-2-[(1S,2S)-2-[(2S)-2-[(2S)-2-acet...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | 25 |
Universite de Montreal at Succursale Centre-Ville | Assay Description Inhibition assays were done in black 96-well plates by adding test compounds to the respective enzyme in assay buffer. Plates were incubated at room ... | J Med Chem 48: 5175-90 (2005) Article DOI: 10.1021/jm050142+ BindingDB Entry DOI: 10.7270/Q2WM1BPC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 3 (Homo sapiens (Human)) | BDBM50379714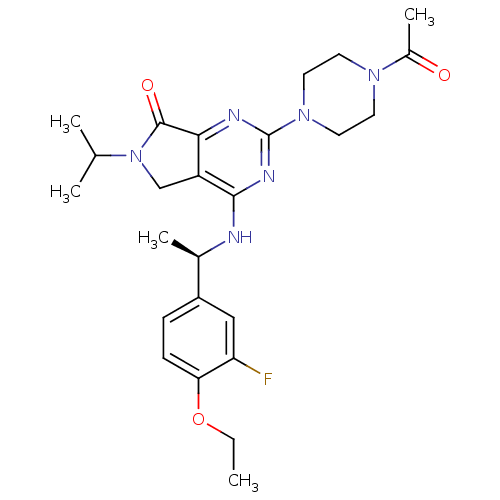 (CHEMBL2011123) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 38 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Antagonist activity at human P2X3 receptor expressed in RLE cells assessed as inhibition of (alpha,beta)me-ATP-induced intracellular calcium level by... | Bioorg Med Chem Lett 22: 2565-71 (2012) Article DOI: 10.1016/j.bmcl.2012.01.124 BindingDB Entry DOI: 10.7270/Q29W0GGF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 [1-453] (Homo sapiens (Human)) | BDBM16051 ((4S)-4-amino-4-{[(1S)-1-{[(1S)-2-carbamoyl-1-{[(1S...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | n/a | n/a | 39 | n/a | n/a | n/a | n/a | 4.5 | 22 |
Universite de Montreal at Succursale Centre-Ville | Assay Description Inhibition assays were done in black 96-well plates by adding test compounds to the respective enzyme in assay buffer. Plates were incubated at room ... | J Med Chem 48: 5175-90 (2005) Article DOI: 10.1021/jm050142+ BindingDB Entry DOI: 10.7270/Q2WM1BPC | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50177421 (CHEMBL558235 | N-(4-carbamimidoylbenzyl)-2-(3-(3,4...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 39 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Montr£al Curated by ChEMBL | Assay Description Inhibitory activity against thrombin | Bioorg Med Chem Lett 16: 1032-6 (2006) Article DOI: 10.1016/j.bmcl.2005.10.082 BindingDB Entry DOI: 10.7270/Q2V125K5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mas-related G-protein coupled receptor member X1 (Homo sapiens (Human)) | BDBM50340746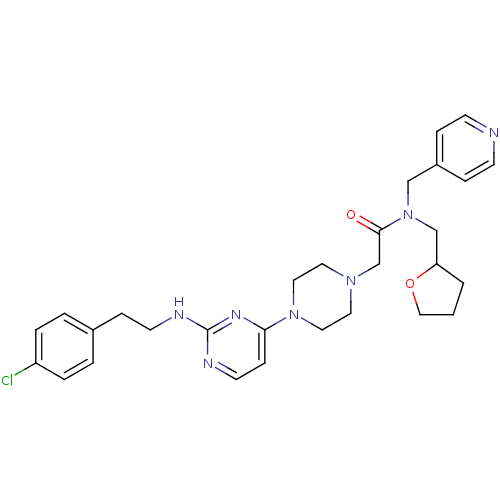 (2-(4-(2-(4-chlorophenethylamino)pyrimidin-4-yl)pip...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Montreal Curated by ChEMBL | Assay Description Antagonist activity at SNSR4 in human HEK293 cells by FLIPR assay | Bioorg Med Chem Lett 21: 2102-5 (2011) Article DOI: 10.1016/j.bmcl.2011.01.138 BindingDB Entry DOI: 10.7270/Q2D21XW7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 [1-453] (Homo sapiens (Human)) | BDBM16050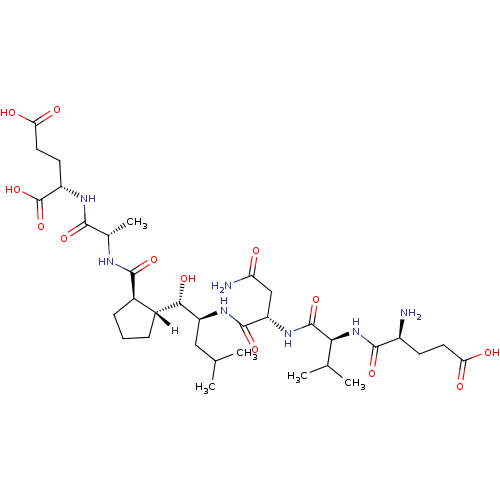 ((2S)-2-[(2S)-2-{[(1R,2R)-2-[(1S,2S)-2-[(2S)-2-[(2S...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | 4.5 | 22 |
Universite de Montreal at Succursale Centre-Ville | Assay Description Inhibition assays were done in black 96-well plates by adding test compounds to the respective enzyme in assay buffer. Plates were incubated at room ... | J Med Chem 48: 5175-90 (2005) Article DOI: 10.1021/jm050142+ BindingDB Entry DOI: 10.7270/Q2WM1BPC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50177419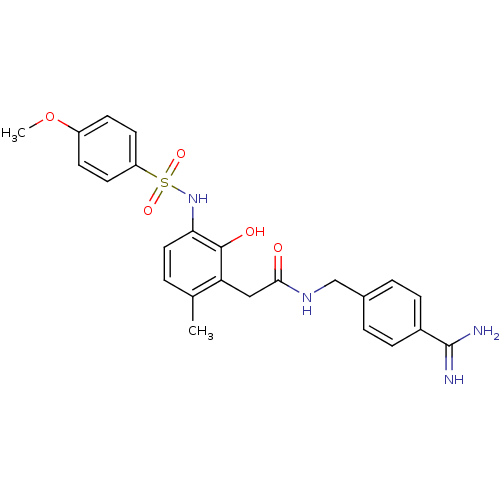 (CHEMBL557779 | N-(4-carbamimidoylbenzyl)-2-(2-hydr...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Montr£al Curated by ChEMBL | Assay Description Inhibitory activity against thrombin | Bioorg Med Chem Lett 16: 1032-6 (2006) Article DOI: 10.1016/j.bmcl.2005.10.082 BindingDB Entry DOI: 10.7270/Q2V125K5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin D (Homo sapiens (Human)) | BDBM16057 ((2S)-N-[(1S)-1-{[(1S,2S)-1-[(1R,2R)-2-{[(1S)-1-(bu...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 56 | n/a | n/a | n/a | n/a | n/a | 25 |
Universite de Montreal at Succursale Centre-Ville | Assay Description Inhibition assays were done in black 96-well plates by adding test compounds to the respective enzyme in assay buffer. Plates were incubated at room ... | J Med Chem 48: 5175-90 (2005) Article DOI: 10.1021/jm050142+ BindingDB Entry DOI: 10.7270/Q2WM1BPC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin D (Homo sapiens (Human)) | BDBM16056 ((1R,2R)-N-butyl-2-[(1S,2S)-2-[(2S)-2-[(2S)-2-aceta...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | 25 |
Universite de Montreal at Succursale Centre-Ville | Assay Description Inhibition assays were done in black 96-well plates by adding test compounds to the respective enzyme in assay buffer. Plates were incubated at room ... | J Med Chem 48: 5175-90 (2005) Article DOI: 10.1021/jm050142+ BindingDB Entry DOI: 10.7270/Q2WM1BPC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin D (Homo sapiens (Human)) | BDBM16050 ((2S)-2-[(2S)-2-{[(1R,2R)-2-[(1S,2S)-2-[(2S)-2-[(2S...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | 25 |
Universite de Montreal at Succursale Centre-Ville | Assay Description Inhibition assays were done in black 96-well plates by adding test compounds to the respective enzyme in assay buffer. Plates were incubated at room ... | J Med Chem 48: 5175-90 (2005) Article DOI: 10.1021/jm050142+ BindingDB Entry DOI: 10.7270/Q2WM1BPC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 3 (Homo sapiens (Human)) | BDBM50379707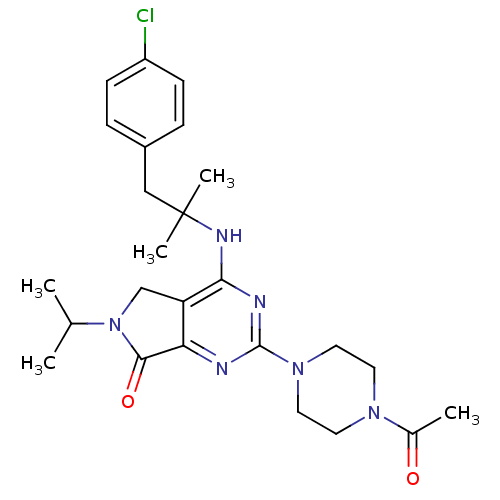 (CHEMBL2011112) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 61 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Antagonist activity at human P2X3 receptor expressed in RLE cells assessed as inhibition of (alpha,beta)me-ATP-induced intracellular calcium level by... | Bioorg Med Chem Lett 22: 2565-71 (2012) Article DOI: 10.1016/j.bmcl.2012.01.124 BindingDB Entry DOI: 10.7270/Q29W0GGF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 3 (Homo sapiens (Human)) | BDBM50379719 (CHEMBL2011118) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 64 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Antagonist activity at human P2X3 receptor expressed in RLE cells assessed as inhibition of (alpha,beta)me-ATP-induced intracellular calcium level by... | Bioorg Med Chem Lett 22: 2565-71 (2012) Article DOI: 10.1016/j.bmcl.2012.01.124 BindingDB Entry DOI: 10.7270/Q29W0GGF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 3 (Homo sapiens (Human)) | BDBM50379736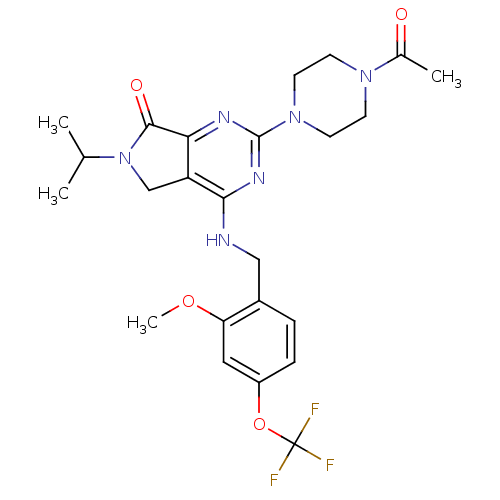 (CHEMBL2011116) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 67 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Antagonist activity at human P2X3 receptor expressed in RLE cells assessed as inhibition of (alpha,beta)me-ATP-induced intracellular calcium level by... | Bioorg Med Chem Lett 22: 2565-71 (2012) Article DOI: 10.1016/j.bmcl.2012.01.124 BindingDB Entry DOI: 10.7270/Q29W0GGF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 3 (Homo sapiens (Human)) | BDBM50379711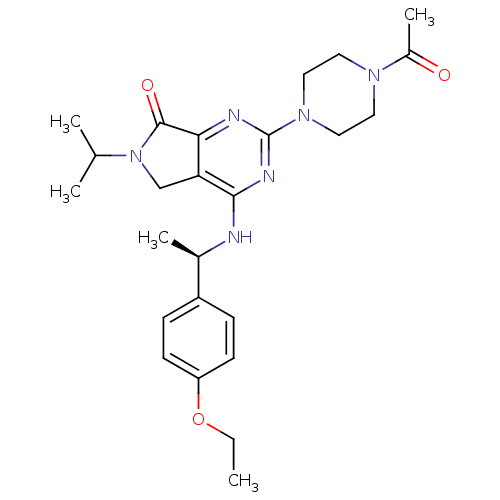 (CHEMBL2011120) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 69 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Antagonist activity at human P2X3 receptor expressed in RLE cells assessed as inhibition of (alpha,beta)me-ATP-induced intracellular calcium level by... | Bioorg Med Chem Lett 22: 2565-71 (2012) Article DOI: 10.1016/j.bmcl.2012.01.124 BindingDB Entry DOI: 10.7270/Q29W0GGF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin D (Homo sapiens (Human)) | BDBM16060 ((2R,3R)-3-[(1S,2S)-2-[[(2S)-2-[[(2S)-2-acetamido-4...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | 25 |
Universite de Montreal at Succursale Centre-Ville | Assay Description Inhibition assays were done in black 96-well plates by adding test compounds to the respective enzyme in assay buffer. Plates were incubated at room ... | J Med Chem 48: 5175-90 (2005) Article DOI: 10.1021/jm050142+ BindingDB Entry DOI: 10.7270/Q2WM1BPC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mas-related G-protein coupled receptor member X1 (Homo sapiens (Human)) | BDBM50340750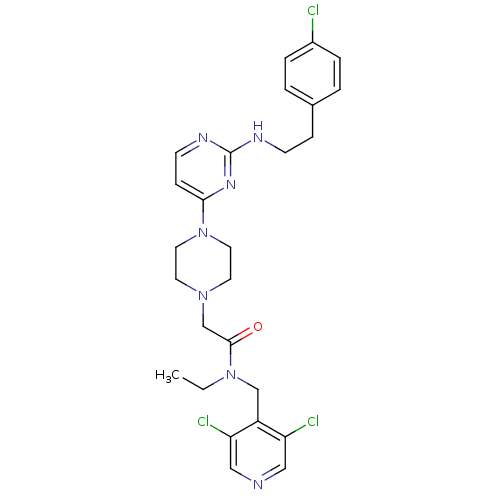 (2-(4-(2-(4-chlorophenethylamino)pyrimidin-4-yl)pip...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 77 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Montreal Curated by ChEMBL | Assay Description Antagonist activity at SNSR4 in human HEK293 cells by FLIPR assay | Bioorg Med Chem Lett 21: 2102-5 (2011) Article DOI: 10.1016/j.bmcl.2011.01.138 BindingDB Entry DOI: 10.7270/Q2D21XW7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50177430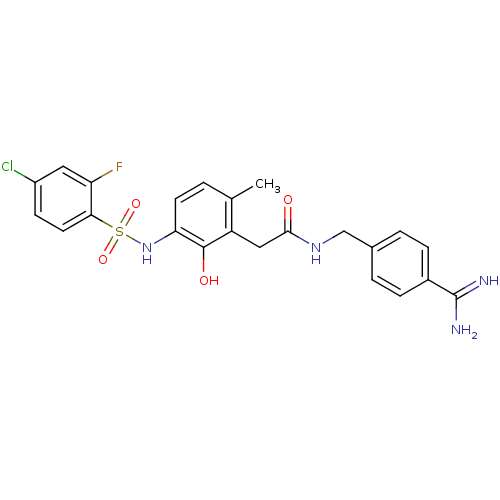 (CHEMBL541383 | N-(4-carbamimidoylbenzyl)-2-(3-(4-c...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 82 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Montr£al Curated by ChEMBL | Assay Description Inhibitory activity against thrombin | Bioorg Med Chem Lett 16: 1032-6 (2006) Article DOI: 10.1016/j.bmcl.2005.10.082 BindingDB Entry DOI: 10.7270/Q2V125K5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mas-related G-protein coupled receptor member X1 (Homo sapiens (Human)) | BDBM50340747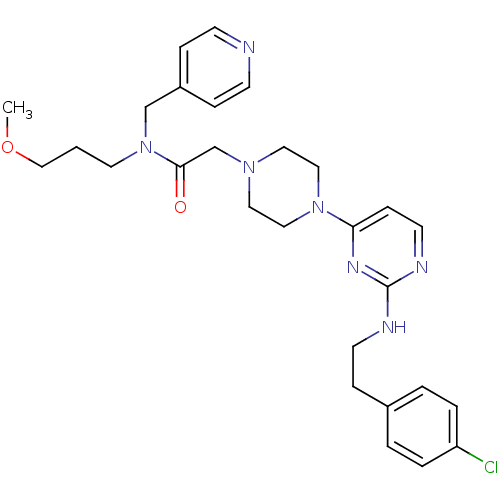 (2-(4-(2-(4-chlorophenethylamino)pyrimidin-4-yl)pip...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 88 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Montreal Curated by ChEMBL | Assay Description Antagonist activity at SNSR4 in human HEK293 cells by FLIPR assay | Bioorg Med Chem Lett 21: 2102-5 (2011) Article DOI: 10.1016/j.bmcl.2011.01.138 BindingDB Entry DOI: 10.7270/Q2D21XW7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin D (Homo sapiens (Human)) | BDBM16052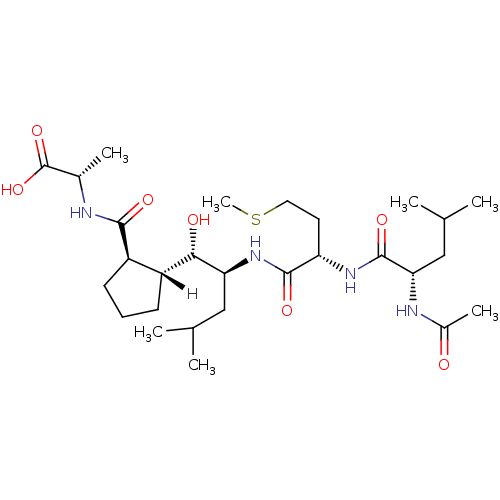 ((2S)-2-{[(1R,2R)-2-[(1S,2S)-2-[(2S)-2-[(2S)-2-acet...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | 25 |
Universite de Montreal at Succursale Centre-Ville | Assay Description Inhibition assays were done in black 96-well plates by adding test compounds to the respective enzyme in assay buffer. Plates were incubated at room ... | J Med Chem 48: 5175-90 (2005) Article DOI: 10.1021/jm050142+ BindingDB Entry DOI: 10.7270/Q2WM1BPC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 [49-170] (Homo sapiens (Human)) | BDBM449849 (5-[2-cyclobutyl-3-[2-(trifluoromethoxy)ethyl]benzi...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 91 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description To measure activity of bromodomain inhibitors, a His-epitope tagged BRD4 BD149-170 is purchased from BPS Bioscience. BRD4 binding and inhibition is a... | Citation and Details BindingDB Entry DOI: 10.7270/Q22V2K9P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50235527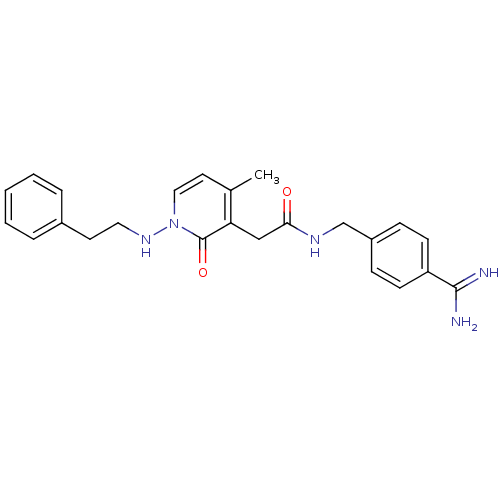 (CHEMBL535639 | N-(4-carbamimidoylbenzyl)-2-(4-meth...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 91 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Montr£al Curated by ChEMBL | Assay Description Inhibition of thrombin | Bioorg Med Chem Lett 18: 1972-6 (2008) Article DOI: 10.1016/j.bmcl.2008.01.122 BindingDB Entry DOI: 10.7270/Q2833SV0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM449849 (5-[2-cyclobutyl-3-[2-(trifluoromethoxy)ethyl]benzi...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 91 | n/a | n/a | n/a | n/a | n/a | n/a |
Neomed Institute US Patent | Assay Description To measure activity of bromodomain inhibitors, a His-epitope tagged BRD4 BD149-170 is purchased from BPS Bioscience. BRD4 binding and inhibition is a... | US Patent US10703740 (2020) BindingDB Entry DOI: 10.7270/Q2ST7SWV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50235519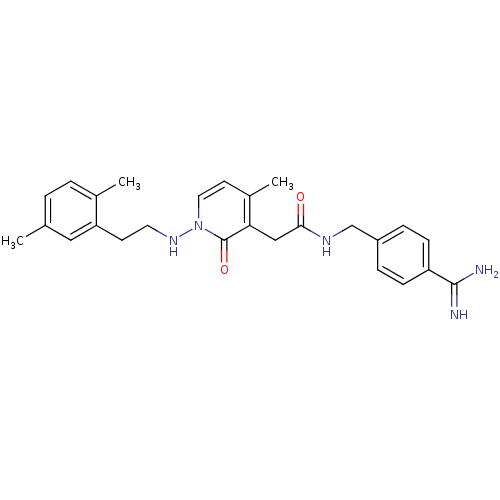 (2-(1-(2,5-dimethylphenethylamino)-4-methyl-2-oxo-1...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 92 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Montr£al Curated by ChEMBL | Assay Description Inhibition of thrombin | Bioorg Med Chem Lett 18: 1972-6 (2008) Article DOI: 10.1016/j.bmcl.2008.01.122 BindingDB Entry DOI: 10.7270/Q2833SV0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50177420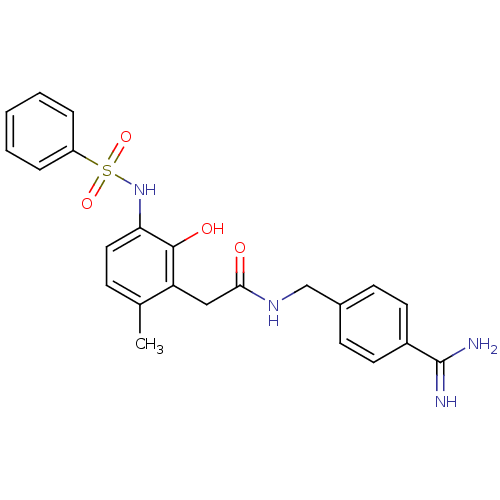 (CHEMBL534714 | N-(4-carbamimidoylbenzyl)-2-(2-hydr...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 97 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Montr£al Curated by ChEMBL | Assay Description Inhibitory activity against thrombin | Bioorg Med Chem Lett 16: 1032-6 (2006) Article DOI: 10.1016/j.bmcl.2005.10.082 BindingDB Entry DOI: 10.7270/Q2V125K5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM449854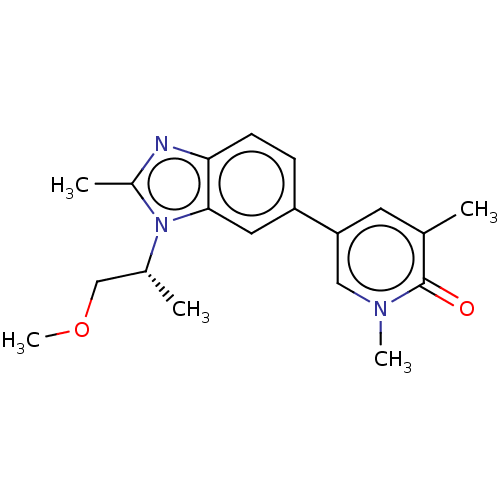 (5-[3-[(1R)-2-methoxy-1-methyl-ethyl]-2-methyl-benz...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 98 | n/a | n/a | n/a | n/a | n/a | n/a |
Neomed Institute US Patent | Assay Description To measure activity of bromodomain inhibitors, a His-epitope tagged BRD4 BD149-170 is purchased from BPS Bioscience. BRD4 binding and inhibition is a... | US Patent US10703740 (2020) BindingDB Entry DOI: 10.7270/Q2ST7SWV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 [49-170] (Homo sapiens (Human)) | BDBM449854 (5-[3-[(1R)-2-methoxy-1-methyl-ethyl]-2-methyl-benz...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 98 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description To measure activity of bromodomain inhibitors, a His-epitope tagged BRD4 BD149-170 is purchased from BPS Bioscience. BRD4 binding and inhibition is a... | Citation and Details BindingDB Entry DOI: 10.7270/Q22V2K9P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin D (Homo sapiens (Human)) | BDBM16048 ((4S)-4-amino-4-{[(1S)-1-{[(1S)-2-carbamoyl-1-{[(1R...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 98 | n/a | n/a | n/a | n/a | n/a | 25 |
Universite de Montreal at Succursale Centre-Ville | Assay Description Inhibition assays were done in black 96-well plates by adding test compounds to the respective enzyme in assay buffer. Plates were incubated at room ... | J Med Chem 48: 5175-90 (2005) Article DOI: 10.1021/jm050142+ BindingDB Entry DOI: 10.7270/Q2WM1BPC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 [49-170] (Homo sapiens (Human)) | BDBM449853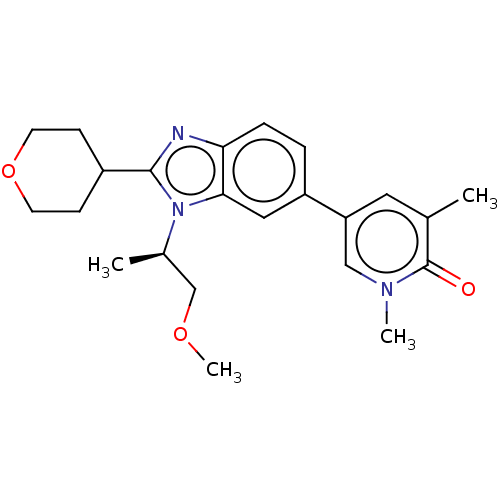 (5-[3-[(1R)-2-methoxy-1-methyl-ethyl]-2-tetrahydrop...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 99 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description To measure activity of bromodomain inhibitors, a His-epitope tagged BRD4 BD149-170 is purchased from BPS Bioscience. BRD4 binding and inhibition is a... | Citation and Details BindingDB Entry DOI: 10.7270/Q22V2K9P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM449853 (5-[3-[(1R)-2-methoxy-1-methyl-ethyl]-2-tetrahydrop...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 99 | n/a | n/a | n/a | n/a | n/a | n/a |
Neomed Institute US Patent | Assay Description To measure activity of bromodomain inhibitors, a His-epitope tagged BRD4 BD149-170 is purchased from BPS Bioscience. BRD4 binding and inhibition is a... | US Patent US10703740 (2020) BindingDB Entry DOI: 10.7270/Q2ST7SWV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 198 total ) | Next | Last >> |