

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Protein kinase C theta type (Homo sapiens (Human)) | BDBM35129 (5-vinyl-3-pyridinecarbonitrile, 14b) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.880 | n/a | n/a | n/a | n/a | 7.2 | 22 |
Wyeth Research | Assay Description All IC50s were measured by using a modified IMAP protocol from Molecular Devices. The kinase reaction was carried out in a Corning Costar 384 well pl... | Bioorg Med Chem 17: 7933-48 (2009) Article DOI: 10.1016/j.bmc.2009.10.020 BindingDB Entry DOI: 10.7270/Q2M043RT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM50431385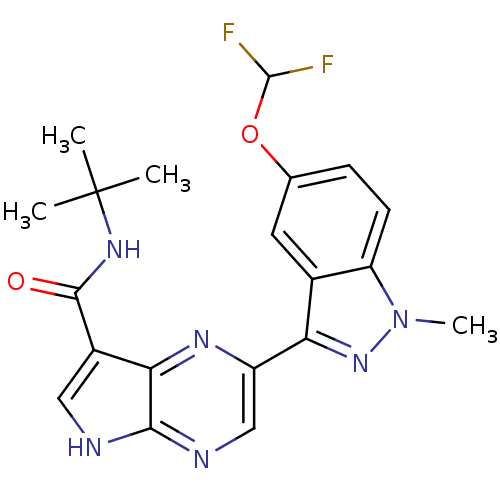 (CHEMBL2346686) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant truncated SYK (360 to 365 amino acid residues) using N-terminally biotinylated EPEGDYEEVLE peptide as substrate asses... | J Med Chem 56: 1677-92 (2013) Article DOI: 10.1021/jm301720p BindingDB Entry DOI: 10.7270/Q22N53M0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM50400047 (BIIB-057 | CHEMBL2177736 | US9579320, Example 87) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant truncated SYK (360 to 365 amino acid residues) using N-terminally biotinylated EPEGDYEEVLE peptide as substrate asses... | J Med Chem 56: 1677-92 (2013) Article DOI: 10.1021/jm301720p BindingDB Entry DOI: 10.7270/Q22N53M0 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Protein kinase C theta type (Homo sapiens (Human)) | BDBM35141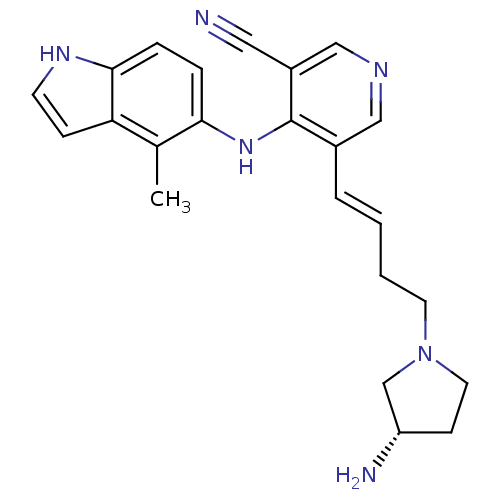 (5-vinyl-3-pyridinecarbonitrile, 23) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | 7.2 | 22 |
Wyeth Research | Assay Description All IC50s were measured by using a modified IMAP protocol from Molecular Devices. The kinase reaction was carried out in a Corning Costar 384 well pl... | Bioorg Med Chem 17: 7933-48 (2009) Article DOI: 10.1016/j.bmc.2009.10.020 BindingDB Entry DOI: 10.7270/Q2M043RT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C theta type (Homo sapiens (Human)) | BDBM35137 (5-vinyl-3-pyridinecarbonitrile, 19) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | 7.2 | 22 |
Wyeth Research | Assay Description All IC50s were measured by using a modified IMAP protocol from Molecular Devices. The kinase reaction was carried out in a Corning Costar 384 well pl... | Bioorg Med Chem 17: 7933-48 (2009) Article DOI: 10.1016/j.bmc.2009.10.020 BindingDB Entry DOI: 10.7270/Q2M043RT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM50431383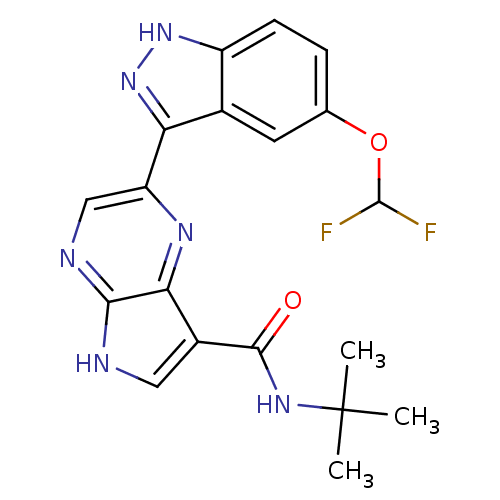 (CHEMBL2347405) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant truncated SYK (360 to 365 amino acid residues) using N-terminally biotinylated EPEGDYEEVLE peptide as substrate asses... | J Med Chem 56: 1677-92 (2013) Article DOI: 10.1021/jm301720p BindingDB Entry DOI: 10.7270/Q22N53M0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM50431372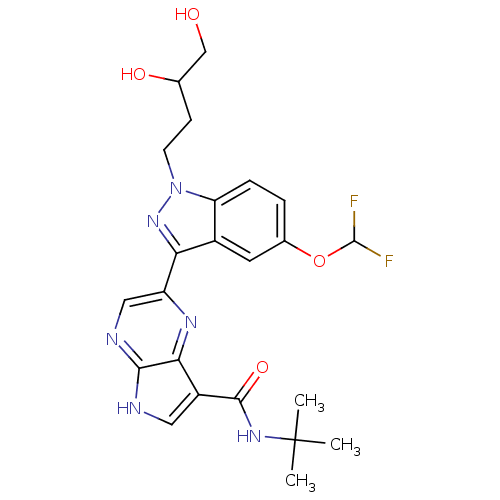 (CHEMBL2347988) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant truncated SYK (360 to 365 amino acid residues) using N-terminally biotinylated EPEGDYEEVLE peptide as substrate asses... | J Med Chem 56: 1677-92 (2013) Article DOI: 10.1021/jm301720p BindingDB Entry DOI: 10.7270/Q22N53M0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK [360-635] (Homo sapiens (Human)) | BDBM295272 (6-(1-aminomethyl-3- methyl-butylamino)- 4-(6-isopr...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | 7.5 | n/a |
HOFFMANN-LA ROCHE INC. US Patent | Assay Description SYK kinase assay is a standard kinase assay adapted to a 96 well plate format. This assay is performed in 96-well format for IC50 determination with ... | US Patent US10112928 (2018) BindingDB Entry DOI: 10.7270/Q2Z03B68 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C theta type (Homo sapiens (Human)) | BDBM35123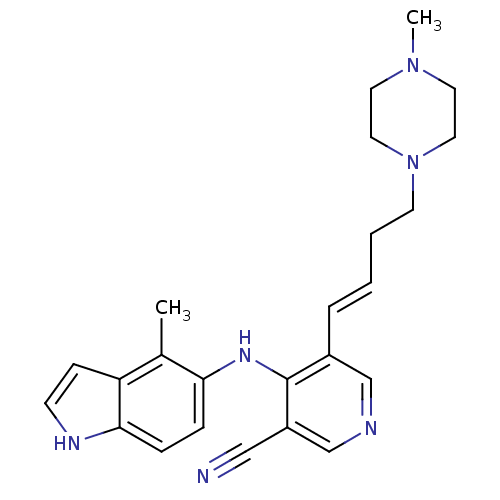 (5-vinyl-3-pyridinecarbonitrile, 12b) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | 7.2 | 22 |
Wyeth Research | Assay Description All IC50s were measured by using a modified IMAP protocol from Molecular Devices. The kinase reaction was carried out in a Corning Costar 384 well pl... | Bioorg Med Chem 17: 7933-48 (2009) Article DOI: 10.1016/j.bmc.2009.10.020 BindingDB Entry DOI: 10.7270/Q2M043RT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C delta type (Homo sapiens (Human)) | BDBM35123 (5-vinyl-3-pyridinecarbonitrile, 12b) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research | Assay Description All IC50s were measured by using a modified IMAP protocol from Molecular Devices. The kinase reaction was carried out in a Corning Costar 384 well pl... | Bioorg Med Chem 17: 7933-48 (2009) Article DOI: 10.1016/j.bmc.2009.10.020 BindingDB Entry DOI: 10.7270/Q2M043RT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM50007086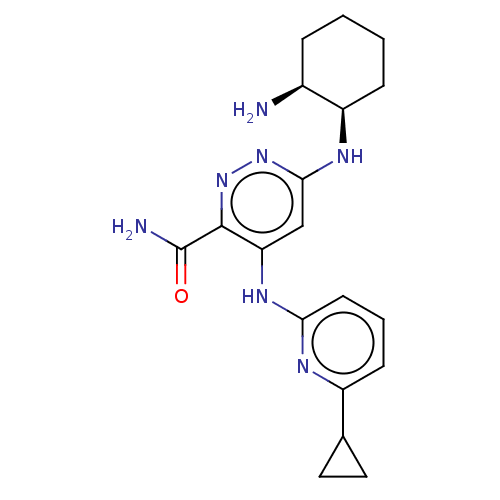 (CHEMBL3237558) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. Curated by ChEMBL | Assay Description Inhibition of human SYK (360 to 635) using biotin-EPEGDYEEVLE as substrate preincubated for 10 mins followed by substrate/ATP/[33Pgamma]ATP addition ... | J Med Chem 57: 2683-91 (2014) Article DOI: 10.1021/jm401982j BindingDB Entry DOI: 10.7270/Q2HT2QTR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C theta type (Homo sapiens (Human)) | BDBM35127 (5-vinyl-3-pyridinecarbonitrile, 13c) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | 7.2 | 22 |
Wyeth Research | Assay Description All IC50s were measured by using a modified IMAP protocol from Molecular Devices. The kinase reaction was carried out in a Corning Costar 384 well pl... | Bioorg Med Chem 17: 7933-48 (2009) Article DOI: 10.1016/j.bmc.2009.10.020 BindingDB Entry DOI: 10.7270/Q2M043RT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM50431373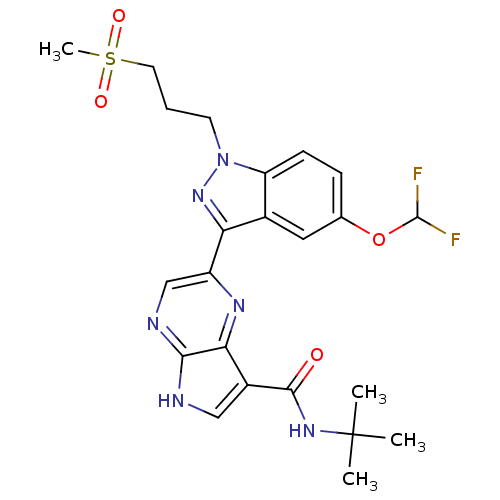 (CHEMBL2347420) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant truncated SYK (360 to 365 amino acid residues) using N-terminally biotinylated EPEGDYEEVLE peptide as substrate asses... | J Med Chem 56: 1677-92 (2013) Article DOI: 10.1021/jm301720p BindingDB Entry DOI: 10.7270/Q22N53M0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM50431382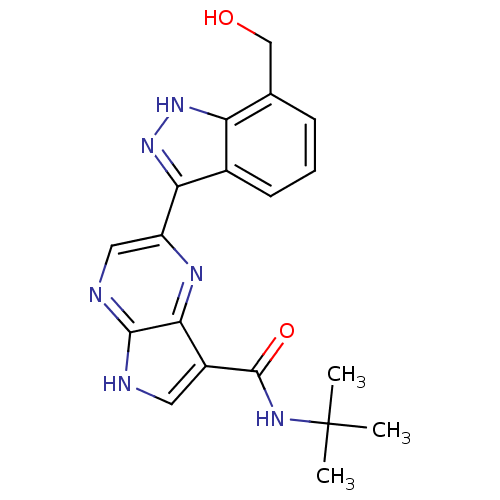 (CHEMBL2347406) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant truncated SYK (360 to 365 amino acid residues) using N-terminally biotinylated EPEGDYEEVLE peptide as substrate asses... | J Med Chem 56: 1677-92 (2013) Article DOI: 10.1021/jm301720p BindingDB Entry DOI: 10.7270/Q22N53M0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM50431375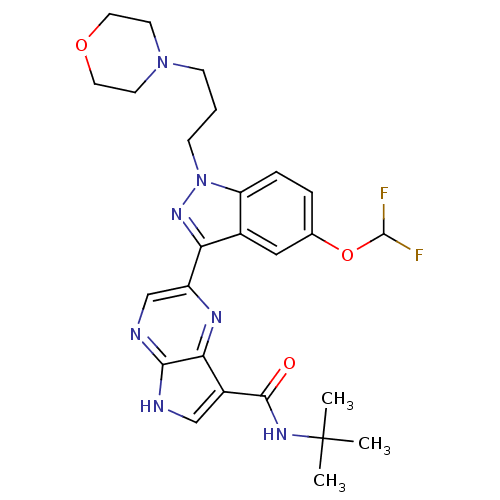 (CHEMBL2347418) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant truncated SYK (360 to 365 amino acid residues) using N-terminally biotinylated EPEGDYEEVLE peptide as substrate asses... | J Med Chem 56: 1677-92 (2013) Article DOI: 10.1021/jm301720p BindingDB Entry DOI: 10.7270/Q22N53M0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM50007105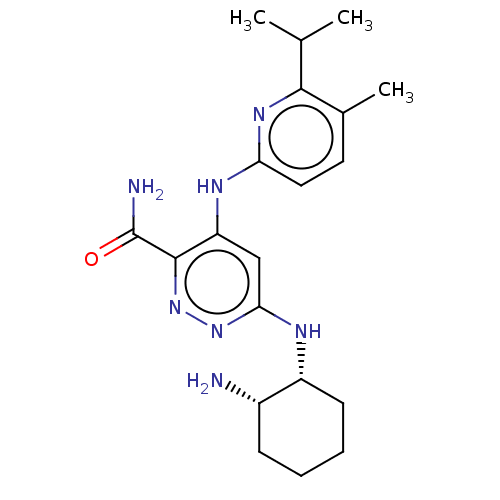 (CHEMBL3237573) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. Curated by ChEMBL | Assay Description Inhibition of human SYK (360 to 635) using biotin-EPEGDYEEVLE as substrate preincubated for 10 mins followed by substrate/ATP/[33Pgamma]ATP addition ... | J Med Chem 57: 2683-91 (2014) Article DOI: 10.1021/jm401982j BindingDB Entry DOI: 10.7270/Q2HT2QTR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM50007039 (CHEMBL3237556) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. Curated by ChEMBL | Assay Description Inhibition of human SYK (360 to 635) using biotin-EPEGDYEEVLE as substrate preincubated for 10 mins followed by substrate/ATP/[33Pgamma]ATP addition ... | J Med Chem 57: 2683-91 (2014) Article DOI: 10.1021/jm401982j BindingDB Entry DOI: 10.7270/Q2HT2QTR | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM50007186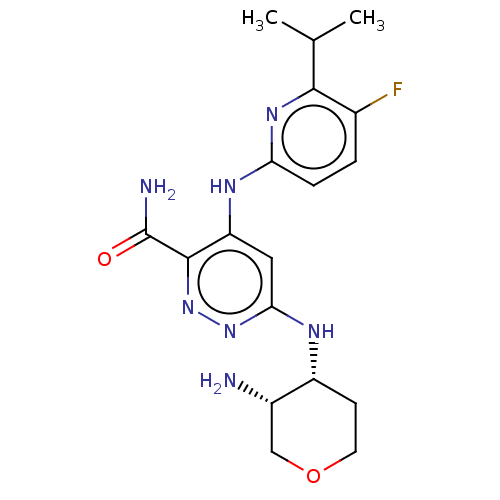 (CHEMBL3237580) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. Curated by ChEMBL | Assay Description Inhibition of human SYK (360 to 635) using biotin-EPEGDYEEVLE as substrate preincubated for 10 mins followed by substrate/ATP/[33Pgamma]ATP addition ... | J Med Chem 57: 2683-91 (2014) Article DOI: 10.1021/jm401982j BindingDB Entry DOI: 10.7270/Q2HT2QTR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C delta type (Homo sapiens (Human)) | BDBM35155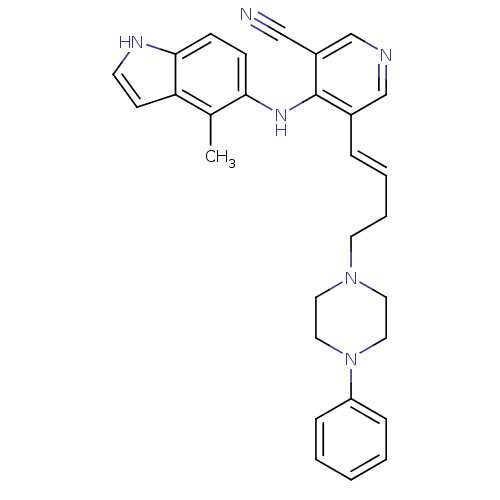 (5-vinyl-3-pyridinecarbonitrile, 37) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research | Assay Description All IC50s were measured by using a modified IMAP protocol from Molecular Devices. The kinase reaction was carried out in a Corning Costar 384 well pl... | Bioorg Med Chem 17: 7933-48 (2009) Article DOI: 10.1016/j.bmc.2009.10.020 BindingDB Entry DOI: 10.7270/Q2M043RT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C theta type (Homo sapiens (Human)) | BDBM35140 (5-vinyl-3-pyridinecarbonitrile, 22) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | 7.2 | 22 |
Wyeth Research | Assay Description All IC50s were measured by using a modified IMAP protocol from Molecular Devices. The kinase reaction was carried out in a Corning Costar 384 well pl... | Bioorg Med Chem 17: 7933-48 (2009) Article DOI: 10.1016/j.bmc.2009.10.020 BindingDB Entry DOI: 10.7270/Q2M043RT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C theta type (Homo sapiens (Human)) | BDBM50302529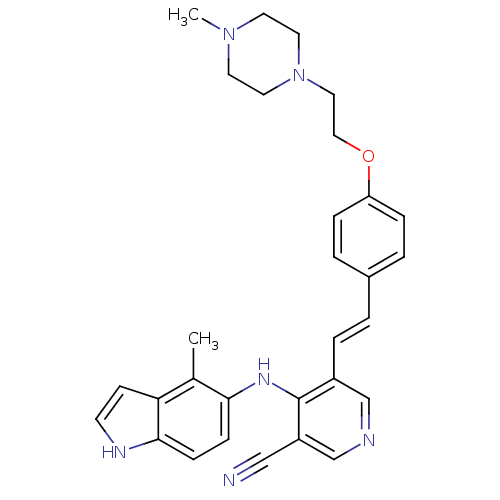 (4-(4-methyl-1H-indol-5-ylamino)-5-(4-(2-(4-methylp...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Inhibition of human PKCtheta by IMAP kinase assay | Bioorg Med Chem Lett 19: 5829-32 (2009) Article DOI: 10.1016/j.bmcl.2009.08.086 BindingDB Entry DOI: 10.7270/Q2ZS2WKC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C theta type (Homo sapiens (Human)) | BDBM35154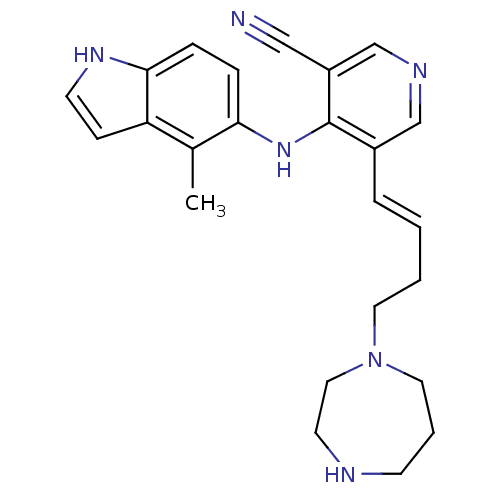 (5-vinyl-3-pyridinecarbonitrile, 36) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research | Assay Description All IC50s were measured by using a modified IMAP protocol from Molecular Devices. The kinase reaction was carried out in a Corning Costar 384 well pl... | Bioorg Med Chem 17: 7933-48 (2009) Article DOI: 10.1016/j.bmc.2009.10.020 BindingDB Entry DOI: 10.7270/Q2M043RT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C theta type (Homo sapiens (Human)) | BDBM35128 (5-vinyl-3-pyridinecarbonitrile, 14a) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | 7.2 | 22 |
Wyeth Research | Assay Description All IC50s were measured by using a modified IMAP protocol from Molecular Devices. The kinase reaction was carried out in a Corning Costar 384 well pl... | Bioorg Med Chem 17: 7933-48 (2009) Article DOI: 10.1016/j.bmc.2009.10.020 BindingDB Entry DOI: 10.7270/Q2M043RT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM50007088 (CHEMBL3237559) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. Curated by ChEMBL | Assay Description Inhibition of human SYK (360 to 635) using biotin-EPEGDYEEVLE as substrate preincubated for 10 mins followed by substrate/ATP/[33Pgamma]ATP addition ... | J Med Chem 57: 2683-91 (2014) Article DOI: 10.1021/jm401982j BindingDB Entry DOI: 10.7270/Q2HT2QTR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM50007094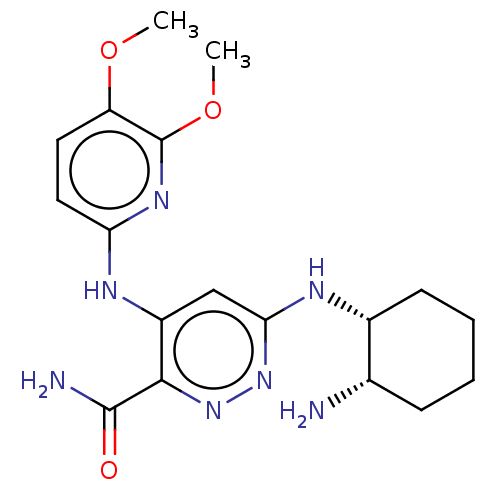 (CHEMBL3237565) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. Curated by ChEMBL | Assay Description Inhibition of human SYK (360 to 635) using biotin-EPEGDYEEVLE as substrate preincubated for 10 mins followed by substrate/ATP/[33Pgamma]ATP addition ... | J Med Chem 57: 2683-91 (2014) Article DOI: 10.1021/jm401982j BindingDB Entry DOI: 10.7270/Q2HT2QTR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM50431367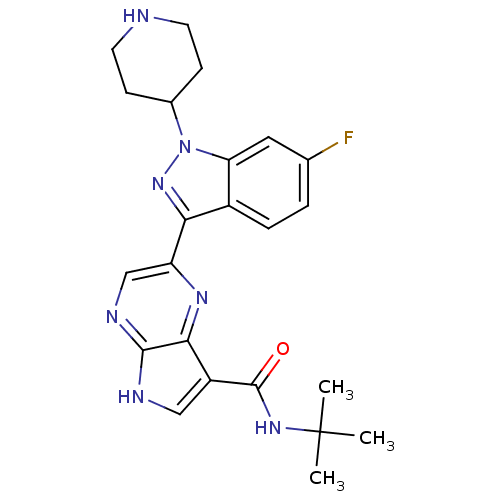 (CHEMBL2347994) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant truncated SYK (360 to 365 amino acid residues) using N-terminally biotinylated EPEGDYEEVLE peptide as substrate asses... | J Med Chem 56: 1677-92 (2013) Article DOI: 10.1021/jm301720p BindingDB Entry DOI: 10.7270/Q22N53M0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C theta type (Homo sapiens (Human)) | BDBM35126 (5-vinyl-3-pyridinecarbonitrile, 13b) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | 7.2 | 22 |
Wyeth Research | Assay Description All IC50s were measured by using a modified IMAP protocol from Molecular Devices. The kinase reaction was carried out in a Corning Costar 384 well pl... | Bioorg Med Chem 17: 7933-48 (2009) Article DOI: 10.1016/j.bmc.2009.10.020 BindingDB Entry DOI: 10.7270/Q2M043RT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM50431389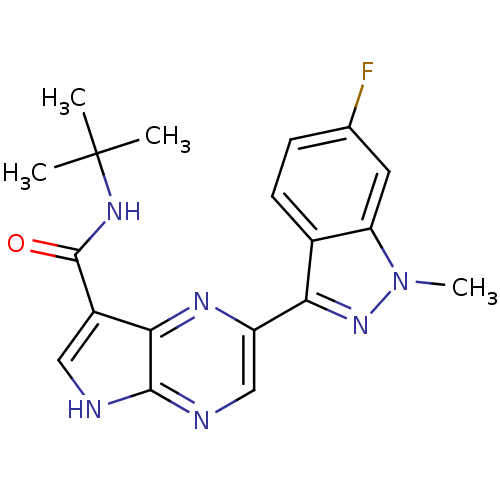 (CHEMBL2348854) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant truncated SYK (360 to 365 amino acid residues) using N-terminally biotinylated EPEGDYEEVLE peptide as substrate asses... | J Med Chem 56: 1677-92 (2013) Article DOI: 10.1021/jm301720p BindingDB Entry DOI: 10.7270/Q22N53M0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM50431376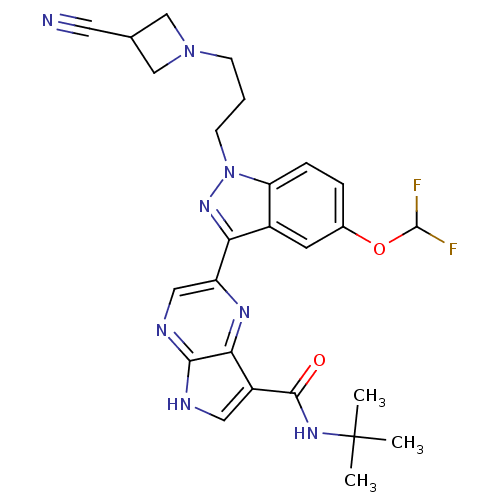 (CHEMBL2347417) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant truncated SYK (360 to 365 amino acid residues) using N-terminally biotinylated EPEGDYEEVLE peptide as substrate asses... | J Med Chem 56: 1677-92 (2013) Article DOI: 10.1021/jm301720p BindingDB Entry DOI: 10.7270/Q22N53M0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM50431379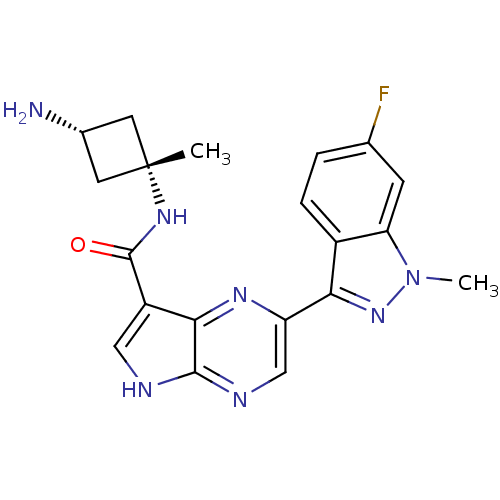 (CHEMBL2347413) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant truncated SYK (360 to 365 amino acid residues) using N-terminally biotinylated EPEGDYEEVLE peptide as substrate asses... | J Med Chem 56: 1677-92 (2013) Article DOI: 10.1021/jm301720p BindingDB Entry DOI: 10.7270/Q22N53M0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C theta type (Homo sapiens (Human)) | BDBM50302531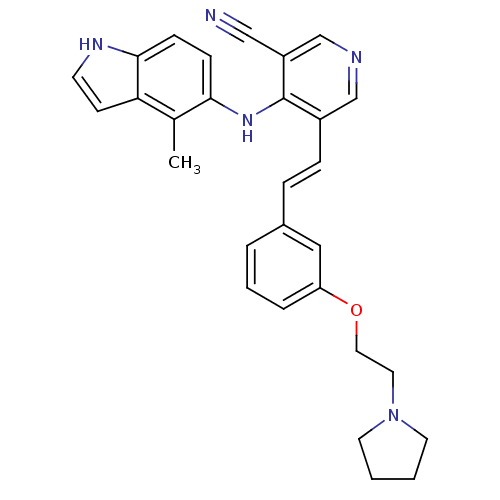 (4-(4-methyl-1H-indol-5-ylamino)-5-(3-(2-(pyrrolidi...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Inhibition of human PKCtheta by IMAP kinase assay | Bioorg Med Chem Lett 19: 5829-32 (2009) Article DOI: 10.1016/j.bmcl.2009.08.086 BindingDB Entry DOI: 10.7270/Q2ZS2WKC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C theta type (Homo sapiens (Human)) | BDBM50302507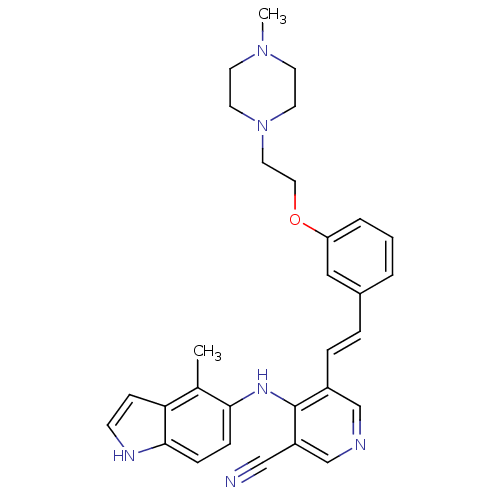 (4-(4-methyl-1H-indol-5-ylamino)-5-(3-(2-(4-methylp...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Inhibition of human PKCtheta by IMAP kinase assay | Bioorg Med Chem Lett 19: 5829-32 (2009) Article DOI: 10.1016/j.bmcl.2009.08.086 BindingDB Entry DOI: 10.7270/Q2ZS2WKC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C theta type (Homo sapiens (Human)) | BDBM35135 (5-vinyl-3-pyridinecarbonitrile, 17) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.90 | n/a | n/a | n/a | n/a | 7.2 | 22 |
Wyeth Research | Assay Description All IC50s were measured by using a modified IMAP protocol from Molecular Devices. The kinase reaction was carried out in a Corning Costar 384 well pl... | Bioorg Med Chem 17: 7933-48 (2009) Article DOI: 10.1016/j.bmc.2009.10.020 BindingDB Entry DOI: 10.7270/Q2M043RT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM50431370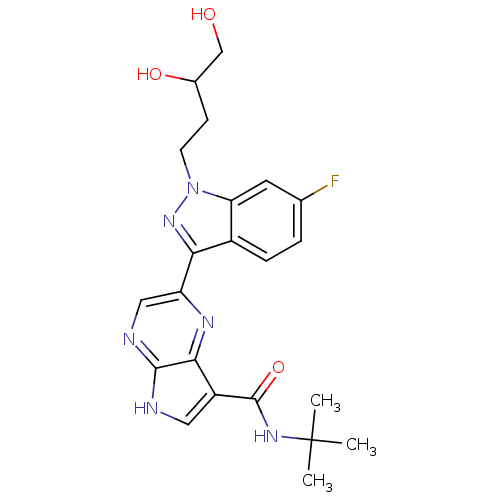 (CHEMBL2347991) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant truncated SYK (360 to 365 amino acid residues) using N-terminally biotinylated EPEGDYEEVLE peptide as substrate asses... | J Med Chem 56: 1677-92 (2013) Article DOI: 10.1021/jm301720p BindingDB Entry DOI: 10.7270/Q22N53M0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM50431384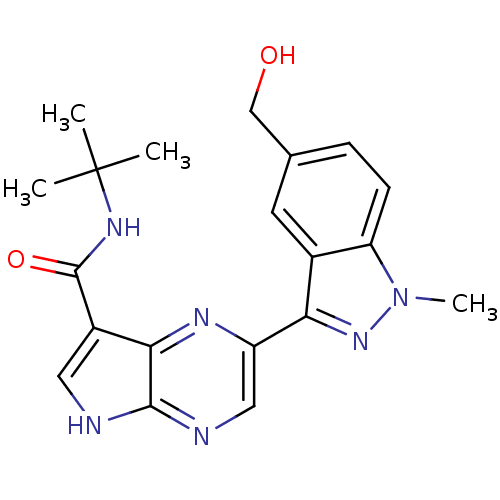 (CHEMBL2348858) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant truncated SYK (360 to 365 amino acid residues) using N-terminally biotinylated EPEGDYEEVLE peptide as substrate asses... | J Med Chem 56: 1677-92 (2013) Article DOI: 10.1021/jm301720p BindingDB Entry DOI: 10.7270/Q22N53M0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM50431395 (CHEMBL2347415) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant truncated SYK (360 to 365 amino acid residues) using N-terminally biotinylated EPEGDYEEVLE peptide as substrate asses... | J Med Chem 56: 1677-92 (2013) Article DOI: 10.1021/jm301720p BindingDB Entry DOI: 10.7270/Q22N53M0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM50431371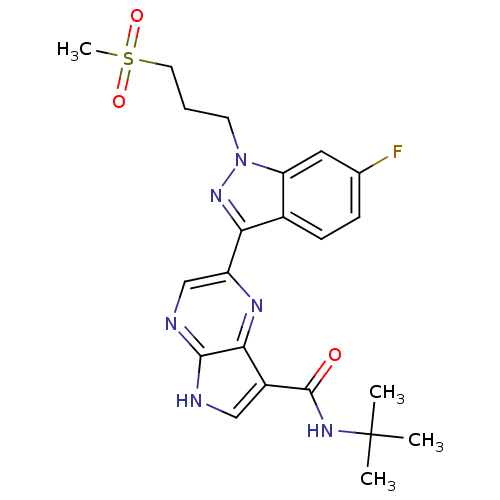 (CHEMBL2347989) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant truncated SYK (360 to 365 amino acid residues) using N-terminally biotinylated EPEGDYEEVLE peptide as substrate asses... | J Med Chem 56: 1677-92 (2013) Article DOI: 10.1021/jm301720p BindingDB Entry DOI: 10.7270/Q22N53M0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM50007068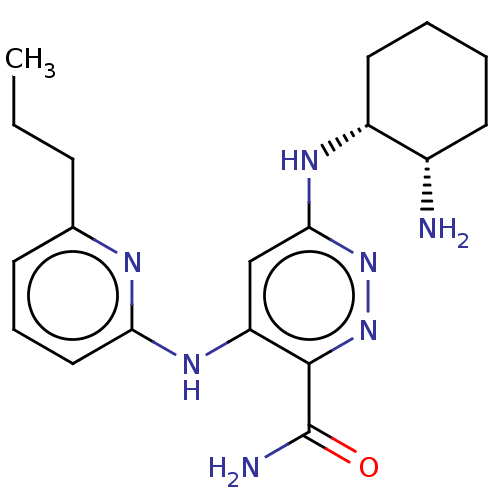 (CHEMBL3237557) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. Curated by ChEMBL | Assay Description Inhibition of human SYK (360 to 635) using biotin-EPEGDYEEVLE as substrate preincubated for 10 mins followed by substrate/ATP/[33Pgamma]ATP addition ... | J Med Chem 57: 2683-91 (2014) Article DOI: 10.1021/jm401982j BindingDB Entry DOI: 10.7270/Q2HT2QTR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM50007090 (CHEMBL3237561) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. Curated by ChEMBL | Assay Description Inhibition of human SYK (360 to 635) using biotin-EPEGDYEEVLE as substrate preincubated for 10 mins followed by substrate/ATP/[33Pgamma]ATP addition ... | J Med Chem 57: 2683-91 (2014) Article DOI: 10.1021/jm401982j BindingDB Entry DOI: 10.7270/Q2HT2QTR | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM50007092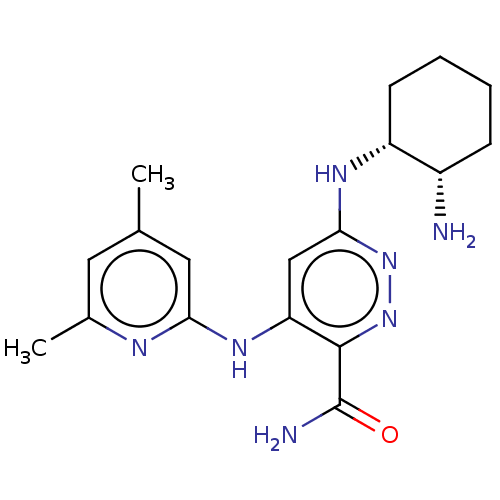 (CHEMBL3237563) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. Curated by ChEMBL | Assay Description Inhibition of human SYK (360 to 635) using biotin-EPEGDYEEVLE as substrate preincubated for 10 mins followed by substrate/ATP/[33Pgamma]ATP addition ... | J Med Chem 57: 2683-91 (2014) Article DOI: 10.1021/jm401982j BindingDB Entry DOI: 10.7270/Q2HT2QTR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM50007106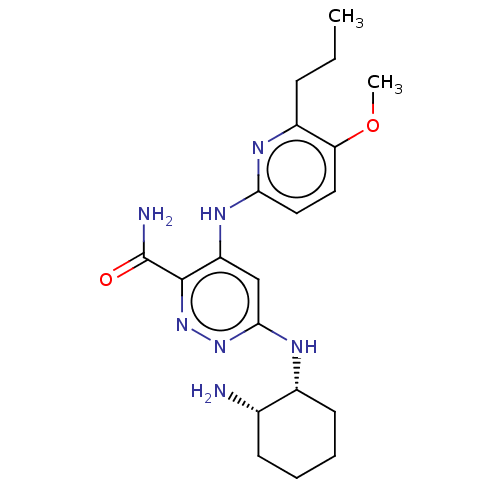 (CHEMBL3237574) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. Curated by ChEMBL | Assay Description Inhibition of human SYK (360 to 635) using biotin-EPEGDYEEVLE as substrate preincubated for 10 mins followed by substrate/ATP/[33Pgamma]ATP addition ... | J Med Chem 57: 2683-91 (2014) Article DOI: 10.1021/jm401982j BindingDB Entry DOI: 10.7270/Q2HT2QTR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C theta type (Homo sapiens (Human)) | BDBM35130 (5-vinyl-3-pyridinecarbonitrile, 14c) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.20 | n/a | n/a | n/a | n/a | 7.2 | 22 |
Wyeth Research | Assay Description All IC50s were measured by using a modified IMAP protocol from Molecular Devices. The kinase reaction was carried out in a Corning Costar 384 well pl... | Bioorg Med Chem 17: 7933-48 (2009) Article DOI: 10.1016/j.bmc.2009.10.020 BindingDB Entry DOI: 10.7270/Q2M043RT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C theta type (Homo sapiens (Human)) | BDBM35165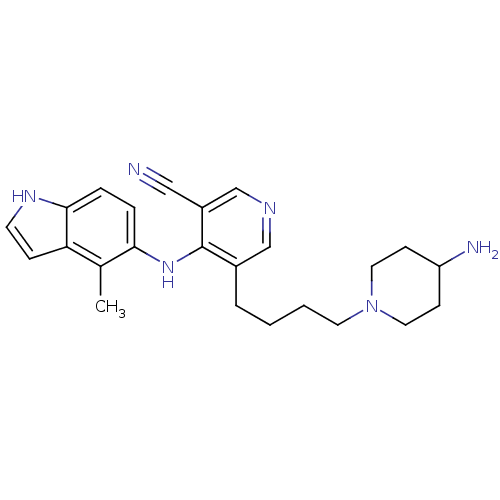 (3-pyridinecarbonitrile, 48) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research | Assay Description All IC50s were measured by using a modified IMAP protocol from Molecular Devices. The kinase reaction was carried out in a Corning Costar 384 well pl... | Bioorg Med Chem 17: 7933-48 (2009) Article DOI: 10.1016/j.bmc.2009.10.020 BindingDB Entry DOI: 10.7270/Q2M043RT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C theta type (Homo sapiens (Human)) | BDBM35136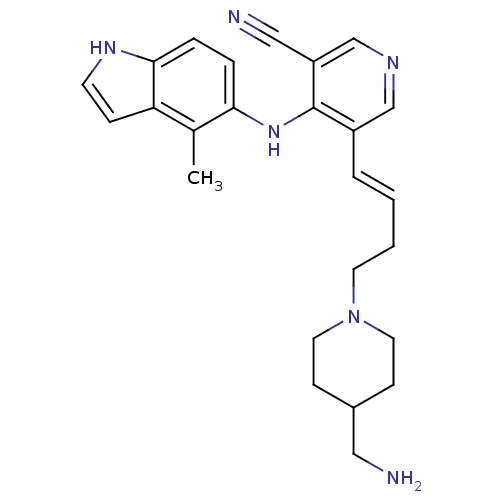 (5-vinyl-3-pyridinecarbonitrile, 18) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.70 | n/a | n/a | n/a | n/a | 7.2 | 22 |
Wyeth Research | Assay Description All IC50s were measured by using a modified IMAP protocol from Molecular Devices. The kinase reaction was carried out in a Corning Costar 384 well pl... | Bioorg Med Chem 17: 7933-48 (2009) Article DOI: 10.1016/j.bmc.2009.10.020 BindingDB Entry DOI: 10.7270/Q2M043RT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C delta type (Homo sapiens (Human)) | BDBM35129 (5-vinyl-3-pyridinecarbonitrile, 14b) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research | Assay Description All IC50s were measured by using a modified IMAP protocol from Molecular Devices. The kinase reaction was carried out in a Corning Costar 384 well pl... | Bioorg Med Chem 17: 7933-48 (2009) Article DOI: 10.1016/j.bmc.2009.10.020 BindingDB Entry DOI: 10.7270/Q2M043RT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM50431387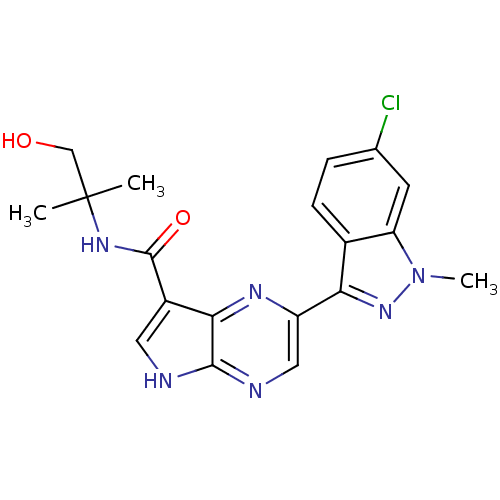 (CHEMBL2348856) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant truncated SYK (360 to 365 amino acid residues) using N-terminally biotinylated EPEGDYEEVLE peptide as substrate asses... | J Med Chem 56: 1677-92 (2013) Article DOI: 10.1021/jm301720p BindingDB Entry DOI: 10.7270/Q22N53M0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C theta type (Homo sapiens (Human)) | BDBM35132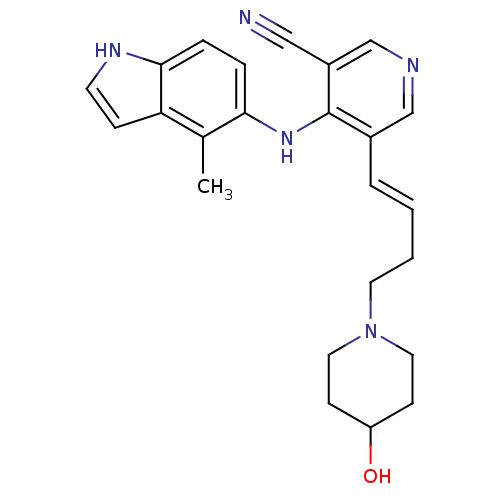 (5-vinyl-3-pyridinecarbonitrile, 15b) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | 7.2 | 22 |
Wyeth Research | Assay Description All IC50s were measured by using a modified IMAP protocol from Molecular Devices. The kinase reaction was carried out in a Corning Costar 384 well pl... | Bioorg Med Chem 17: 7933-48 (2009) Article DOI: 10.1016/j.bmc.2009.10.020 BindingDB Entry DOI: 10.7270/Q2M043RT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK [360-635] (Homo sapiens (Human)) | BDBM50007104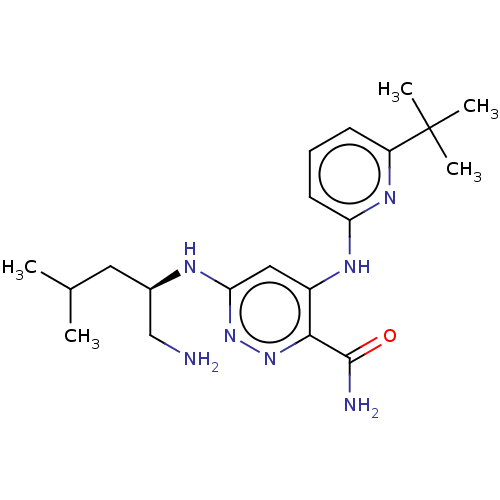 (CHEMBL3237572 | US10112928, Compound I-17) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 6 | n/a | n/a | n/a | n/a | 7.5 | n/a |
HOFFMANN-LA ROCHE INC. US Patent | Assay Description SYK kinase assay is a standard kinase assay adapted to a 96 well plate format. This assay is performed in 96-well format for IC50 determination with ... | US Patent US10112928 (2018) BindingDB Entry DOI: 10.7270/Q2Z03B68 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK [360-635] (Homo sapiens (Human)) | BDBM295270 (6-(1-amino-4- methylpentan-2- ylamino)-4-(6-tert- ...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 6 | n/a | n/a | n/a | n/a | 7.5 | n/a |
HOFFMANN-LA ROCHE INC. US Patent | Assay Description SYK kinase assay is a standard kinase assay adapted to a 96 well plate format. This assay is performed in 96-well format for IC50 determination with ... | US Patent US10112928 (2018) BindingDB Entry DOI: 10.7270/Q2Z03B68 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM50007104 (CHEMBL3237572 | US10112928, Compound I-17) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. Curated by ChEMBL | Assay Description Inhibition of human SYK (360 to 635) using biotin-EPEGDYEEVLE as substrate preincubated for 10 mins followed by substrate/ATP/[33Pgamma]ATP addition ... | J Med Chem 57: 2683-91 (2014) Article DOI: 10.1021/jm401982j BindingDB Entry DOI: 10.7270/Q2HT2QTR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 444 total ) | Next | Last >> |