

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Retinoic acid receptor RXR-alpha (Homo sapiens (Human)) | BDBM50143826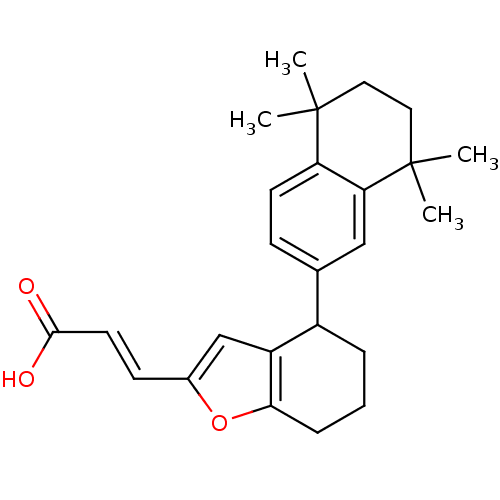 ((E)-3-[4-(5,5,8,8-Tetramethyl-5,6,7,8-tetrahydro-n...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Binding affinity for retinoid X receptor alpha (RXRalpha), using 9-cis-[3H]-retinoic acid | J Med Chem 47: 2010-29 (2004) Article DOI: 10.1021/jm030565g BindingDB Entry DOI: 10.7270/Q2KH0MRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor RXR-alpha (Homo sapiens (Human)) | BDBM50143826 ((E)-3-[4-(5,5,8,8-Tetramethyl-5,6,7,8-tetrahydro-n...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Binding affinity for retinoid X receptor alpha (RXRalpha), using 9-cis-[3H]-retinoic acid | J Med Chem 47: 2010-29 (2004) Article DOI: 10.1021/jm030565g BindingDB Entry DOI: 10.7270/Q2KH0MRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor RXR-alpha (Homo sapiens (Human)) | BDBM50143826 ((E)-3-[4-(5,5,8,8-Tetramethyl-5,6,7,8-tetrahydro-n...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Binding affinity for retinoid X receptor alpha (RXRalpha), using 9-cis-[3H]-retinoic acid | J Med Chem 47: 2010-29 (2004) Article DOI: 10.1021/jm030565g BindingDB Entry DOI: 10.7270/Q2KH0MRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor RXR-alpha (Homo sapiens (Human)) | BDBM50143827 ((E)-3-[4-(3-Methoxy-5,5,8,8-tetramethyl-5,6,7,8-te...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Binding affinity for retinoid X receptor alpha (RXRalpha), using 9-cis-[3H]-retinoic acid | J Med Chem 47: 2010-29 (2004) Article DOI: 10.1021/jm030565g BindingDB Entry DOI: 10.7270/Q2KH0MRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor RXR-alpha (Homo sapiens (Human)) | BDBM50143825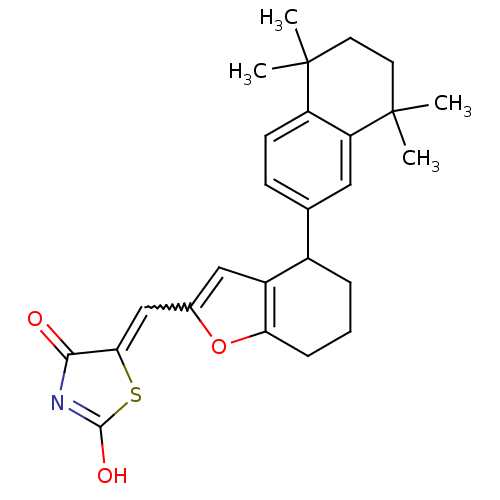 (5-[1-[4-(5,5,8,8-Tetramethyl-5,6,7,8-tetrahydro-na...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Binding affinity for retinoid X receptor alpha (RXRalpha), using 9-cis-[3H]-retinoic acid | J Med Chem 47: 2010-29 (2004) Article DOI: 10.1021/jm030565g BindingDB Entry DOI: 10.7270/Q2KH0MRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor RXR-alpha (Homo sapiens (Human)) | BDBM50143832 ((E)-3-[4-(3,5,5,8,8-Pentamethyl-5,6,7,8-tetrahydro...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Binding affinity for retinoid X receptor alpha (RXRalpha), using 9-cis-[3H]-retinoic acid | J Med Chem 47: 2010-29 (2004) Article DOI: 10.1021/jm030565g BindingDB Entry DOI: 10.7270/Q2KH0MRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor RXR-alpha (Homo sapiens (Human)) | BDBM50143833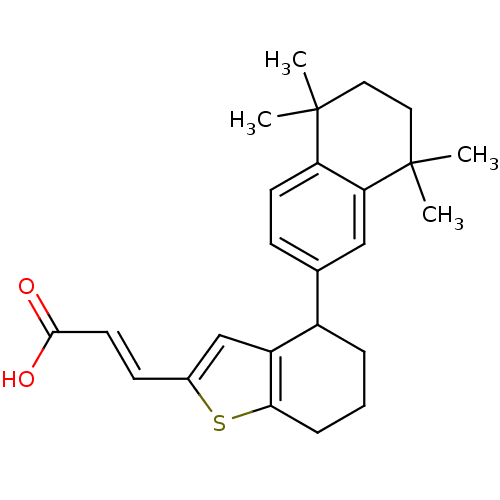 ((E)-3-[4-(5,5,8,8-Tetramethyl-5,6,7,8-tetrahydro-n...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Binding affinity for retinoid X receptor alpha (RXRalpha), using 9-cis-[3H]-retinoic acid | J Med Chem 47: 2010-29 (2004) Article DOI: 10.1021/jm030565g BindingDB Entry DOI: 10.7270/Q2KH0MRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor RXR-alpha (Homo sapiens (Human)) | BDBM50409928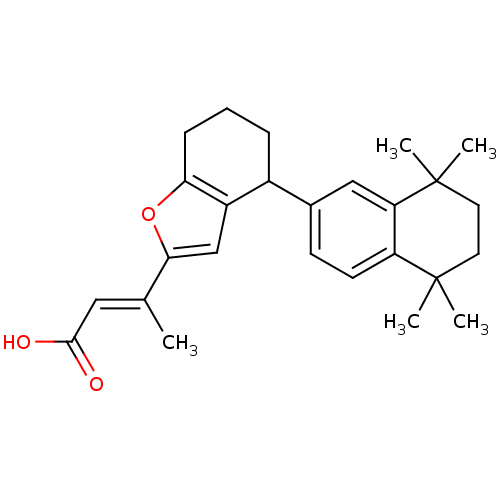 (CHEMBL2113737) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 63 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Binding affinity for retinoid X receptor alpha (RXRalpha), using 9-cis-[3H]-retinoic acid | J Med Chem 47: 2010-29 (2004) Article DOI: 10.1021/jm030565g BindingDB Entry DOI: 10.7270/Q2KH0MRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor RXR-alpha (Homo sapiens (Human)) | BDBM50409929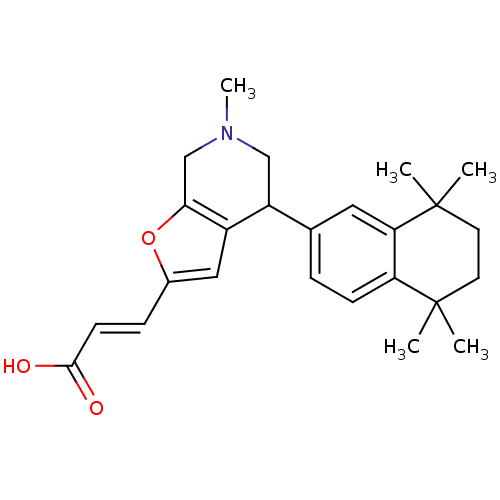 (CHEMBL2113736) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 79 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Binding affinity for retinoid X receptor alpha (RXRalpha), using 9-cis-[3H]-retinoic acid | J Med Chem 47: 2010-29 (2004) Article DOI: 10.1021/jm030565g BindingDB Entry DOI: 10.7270/Q2KH0MRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor RXR-alpha (Homo sapiens (Human)) | BDBM50143826 ((E)-3-[4-(5,5,8,8-Tetramethyl-5,6,7,8-tetrahydro-n...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Binding affinity for retinoid X receptor alpha (RXRalpha), using 9-cis-[3H]-retinoic acid | J Med Chem 47: 2010-29 (2004) Article DOI: 10.1021/jm030565g BindingDB Entry DOI: 10.7270/Q2KH0MRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor alpha (Homo sapiens (Human)) | BDBM50143824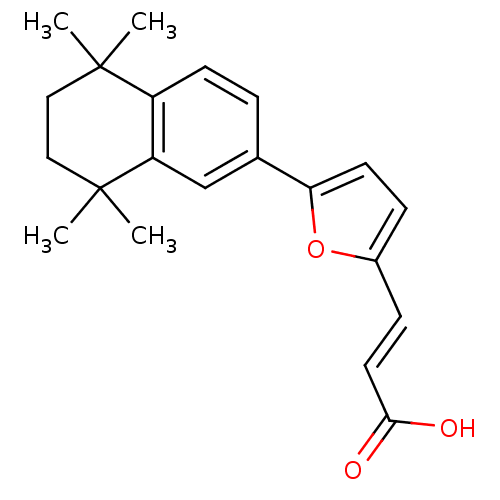 ((E)-3-[5-(5,5,8,8-Tetramethyl-5,6,7,8-tetrahydro-n...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 93 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Binding affinity for retinoic acid receptor alpha (RARalpha), using 9-cis-[3H]-retinoic acid | J Med Chem 47: 2010-29 (2004) Article DOI: 10.1021/jm030565g BindingDB Entry DOI: 10.7270/Q2KH0MRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor RXR-alpha (Homo sapiens (Human)) | BDBM50143821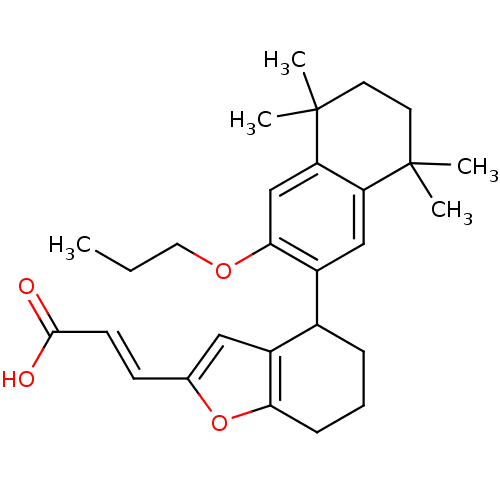 ((E)-3-[4-(5,5,8,8-Tetramethyl-3-propoxy-5,6,7,8-te...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Binding affinity for retinoid X receptor alpha (RXRalpha), using 9-cis-[3H]-retinoic acid | J Med Chem 47: 2010-29 (2004) Article DOI: 10.1021/jm030565g BindingDB Entry DOI: 10.7270/Q2KH0MRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor RXR-alpha (Homo sapiens (Human)) | BDBM50143835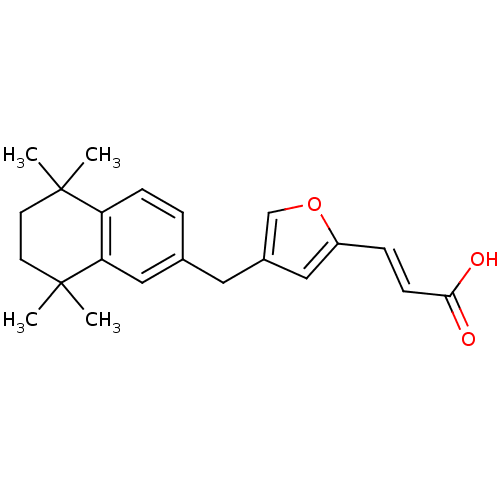 ((E)-3-[4-(5,5,8,8-Tetramethyl-5,6,7,8-tetrahydro-n...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 138 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Binding affinity for retinoid X receptor alpha (RXRalpha), using 9-cis-[3H]-retinoic acid | J Med Chem 47: 2010-29 (2004) Article DOI: 10.1021/jm030565g BindingDB Entry DOI: 10.7270/Q2KH0MRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor RXR-alpha (Homo sapiens (Human)) | BDBM50143831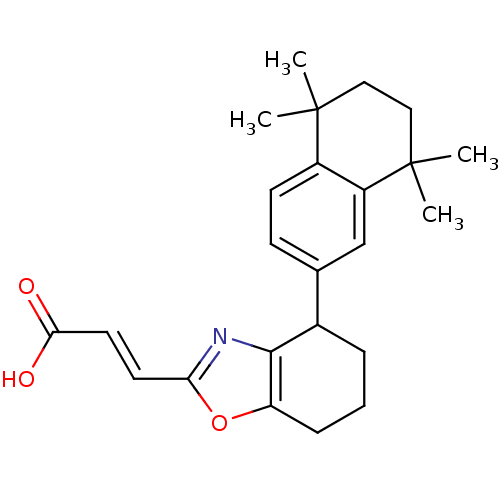 ((E)-3-[4-(5,5,8,8-Tetramethyl-5,6,7,8-tetrahydro-n...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 158 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Binding affinity for retinoid X receptor alpha (RXRalpha), using 9-cis-[3H]-retinoic acid | J Med Chem 47: 2010-29 (2004) Article DOI: 10.1021/jm030565g BindingDB Entry DOI: 10.7270/Q2KH0MRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor RXR-alpha (Homo sapiens (Human)) | BDBM50143823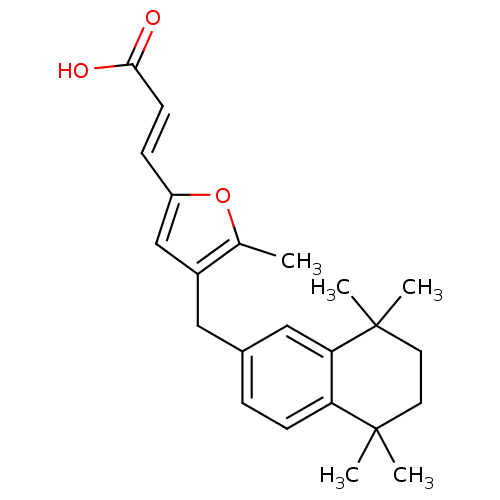 ((E)-3-[5-Methyl-4-(5,5,8,8-tetramethyl-5,6,7,8-tet...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 158 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Binding affinity for retinoid X receptor alpha (RXRalpha), using 9-cis-[3H]-retinoic acid | J Med Chem 47: 2010-29 (2004) Article DOI: 10.1021/jm030565g BindingDB Entry DOI: 10.7270/Q2KH0MRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor RXR-alpha (Homo sapiens (Human)) | BDBM50143828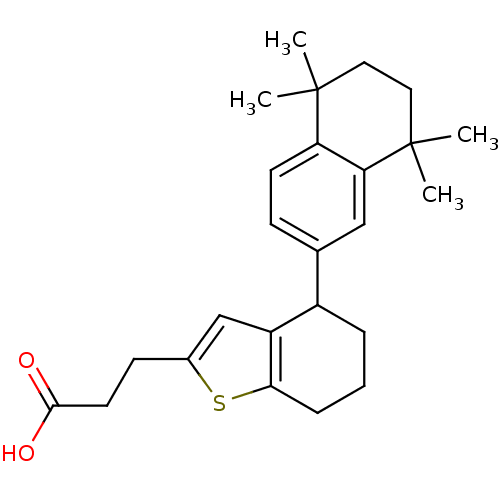 (3-[4-(5,5,8,8-Tetramethyl-5,6,7,8-tetrahydro-napht...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Binding affinity for retinoid X receptor alpha (RXRalpha), using 9-cis-[3H]-retinoic acid | J Med Chem 47: 2010-29 (2004) Article DOI: 10.1021/jm030565g BindingDB Entry DOI: 10.7270/Q2KH0MRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor alpha (Homo sapiens (Human)) | BDBM50143832 ((E)-3-[4-(3,5,5,8,8-Pentamethyl-5,6,7,8-tetrahydro...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Binding affinity for retinoic acid receptor alpha (RARalpha), using 9-cis-[3H]-retinoic acid | J Med Chem 47: 2010-29 (2004) Article DOI: 10.1021/jm030565g BindingDB Entry DOI: 10.7270/Q2KH0MRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor alpha (Homo sapiens (Human)) | BDBM50143835 ((E)-3-[4-(5,5,8,8-Tetramethyl-5,6,7,8-tetrahydro-n...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Binding affinity for retinoic acid receptor alpha (RARalpha), using 9-cis-[3H]-retinoic acid | J Med Chem 47: 2010-29 (2004) Article DOI: 10.1021/jm030565g BindingDB Entry DOI: 10.7270/Q2KH0MRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor alpha (Homo sapiens (Human)) | BDBM50143831 ((E)-3-[4-(5,5,8,8-Tetramethyl-5,6,7,8-tetrahydro-n...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Binding affinity for retinoic acid receptor alpha (RARalpha), using 9-cis-[3H]-retinoic acid | J Med Chem 47: 2010-29 (2004) Article DOI: 10.1021/jm030565g BindingDB Entry DOI: 10.7270/Q2KH0MRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor alpha (Homo sapiens (Human)) | BDBM50143830 (4-(5,5,8,8-Tetramethyl-5,6,7,8-tetrahydro-naphthal...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Binding affinity for retinoic acid receptor alpha (RARalpha), using 9-cis-[3H]-retinoic acid | J Med Chem 47: 2010-29 (2004) Article DOI: 10.1021/jm030565g BindingDB Entry DOI: 10.7270/Q2KH0MRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor alpha (Homo sapiens (Human)) | BDBM50143825 (5-[1-[4-(5,5,8,8-Tetramethyl-5,6,7,8-tetrahydro-na...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Binding affinity for retinoic acid receptor alpha (RARalpha), using 9-cis-[3H]-retinoic acid | J Med Chem 47: 2010-29 (2004) Article DOI: 10.1021/jm030565g BindingDB Entry DOI: 10.7270/Q2KH0MRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor alpha (Homo sapiens (Human)) | BDBM50143833 ((E)-3-[4-(5,5,8,8-Tetramethyl-5,6,7,8-tetrahydro-n...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Binding affinity for retinoic acid receptor alpha (RARalpha), using 9-cis-[3H]-retinoic acid | J Med Chem 47: 2010-29 (2004) Article DOI: 10.1021/jm030565g BindingDB Entry DOI: 10.7270/Q2KH0MRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor alpha (Homo sapiens (Human)) | BDBM50143828 (3-[4-(5,5,8,8-Tetramethyl-5,6,7,8-tetrahydro-napht...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Binding affinity for retinoic acid receptor alpha (RARalpha), using 9-cis-[3H]-retinoic acid | J Med Chem 47: 2010-29 (2004) Article DOI: 10.1021/jm030565g BindingDB Entry DOI: 10.7270/Q2KH0MRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor RXR-alpha (Homo sapiens (Human)) | BDBM50143824 ((E)-3-[5-(5,5,8,8-Tetramethyl-5,6,7,8-tetrahydro-n...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Binding affinity for retinoid X receptor alpha (RXRalpha), using 9-cis-[3H]-retinoic acid | J Med Chem 47: 2010-29 (2004) Article DOI: 10.1021/jm030565g BindingDB Entry DOI: 10.7270/Q2KH0MRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor alpha (Homo sapiens (Human)) | BDBM50143827 ((E)-3-[4-(3-Methoxy-5,5,8,8-tetramethyl-5,6,7,8-te...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Binding affinity for retinoic acid receptor alpha (RARalpha), using 9-cis-[3H]-retinoic acid | J Med Chem 47: 2010-29 (2004) Article DOI: 10.1021/jm030565g BindingDB Entry DOI: 10.7270/Q2KH0MRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor alpha (Homo sapiens (Human)) | BDBM50143823 ((E)-3-[5-Methyl-4-(5,5,8,8-tetramethyl-5,6,7,8-tet...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Binding affinity for retinoic acid receptor alpha (RARalpha), using 9-cis-[3H]-retinoic acid | J Med Chem 47: 2010-29 (2004) Article DOI: 10.1021/jm030565g BindingDB Entry DOI: 10.7270/Q2KH0MRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor alpha (Homo sapiens (Human)) | BDBM50409928 (CHEMBL2113737) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Binding affinity for retinoic acid receptor alpha (RARalpha), using 9-cis-[3H]-retinoic acid | J Med Chem 47: 2010-29 (2004) Article DOI: 10.1021/jm030565g BindingDB Entry DOI: 10.7270/Q2KH0MRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor alpha (Homo sapiens (Human)) | BDBM50143821 ((E)-3-[4-(5,5,8,8-Tetramethyl-3-propoxy-5,6,7,8-te...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Binding affinity for retinoic acid receptor alpha (RARalpha), using 9-cis-[3H]-retinoic acid | J Med Chem 47: 2010-29 (2004) Article DOI: 10.1021/jm030565g BindingDB Entry DOI: 10.7270/Q2KH0MRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor alpha (Homo sapiens (Human)) | BDBM50143826 ((E)-3-[4-(5,5,8,8-Tetramethyl-5,6,7,8-tetrahydro-n...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Binding affinity for retinoic acid receptor alpha (RARalpha), using 9-cis-[3H]-retinoic acid | J Med Chem 47: 2010-29 (2004) Article DOI: 10.1021/jm030565g BindingDB Entry DOI: 10.7270/Q2KH0MRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor alpha (Homo sapiens (Human)) | BDBM50409929 (CHEMBL2113736) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Binding affinity for retinoic acid receptor alpha (RARalpha), using 9-cis-[3H]-retinoic acid | J Med Chem 47: 2010-29 (2004) Article DOI: 10.1021/jm030565g BindingDB Entry DOI: 10.7270/Q2KH0MRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor RXR-alpha (Homo sapiens (Human)) | BDBM50143834 (5-[1-[5-(3-Bromo-phenyl)-furan-2-yl]-meth-(Z)-ylid...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Binding affinity for retinoid X receptor alpha (RXRalpha), using 9-cis-[3H]-retinoic acid | J Med Chem 47: 2010-29 (2004) Article DOI: 10.1021/jm030565g BindingDB Entry DOI: 10.7270/Q2KH0MRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor RXR-alpha (Homo sapiens (Human)) | BDBM50143830 (4-(5,5,8,8-Tetramethyl-5,6,7,8-tetrahydro-naphthal...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | >3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Binding affinity for retinoid X receptor alpha (RXRalpha), using 9-cis-[3H]-retinoic acid | J Med Chem 47: 2010-29 (2004) Article DOI: 10.1021/jm030565g BindingDB Entry DOI: 10.7270/Q2KH0MRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor RXR-alpha (Homo sapiens (Human)) | BDBM50143836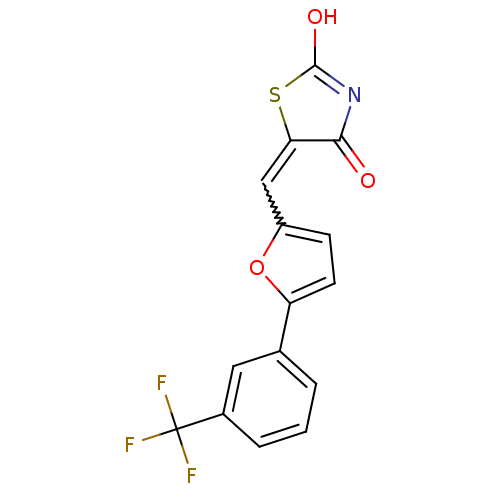 (5-[1-[5-(3-Trifluoromethyl-phenyl)-furan-2-yl]-met...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Binding affinity for retinoid X receptor alpha (RXRalpha), using 9-cis-[3H]-retinoic acid | J Med Chem 47: 2010-29 (2004) Article DOI: 10.1021/jm030565g BindingDB Entry DOI: 10.7270/Q2KH0MRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50412519 (CHEMBL455662) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.16 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Binding affinity to glucocorticoid receptor | Bioorg Med Chem Lett 18: 6097-9 (2008) Article DOI: 10.1016/j.bmcl.2008.10.021 BindingDB Entry DOI: 10.7270/Q2668FDB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor alpha (Homo sapiens (Human)) | BDBM50143821 ((E)-3-[4-(5,5,8,8-Tetramethyl-3-propoxy-5,6,7,8-te...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Inhibitory concentration for lipogenesis induced by retinoic acid receptor alpha in C3H10T1/2 clone 8 fibroblast cells | J Med Chem 47: 2010-29 (2004) Article DOI: 10.1021/jm030565g BindingDB Entry DOI: 10.7270/Q2KH0MRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM20164 ((1S,2R,5S,10S,11S,14R,15R)-14-[(2R)-4-[(2S)-3,3-di...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 460 | n/a | n/a | 7.5 | 22 |
Dartmouth College | Assay Description The LXR LiSA measures the ligand-dependent recruitment of a 25 amino acid fragment of the steroid receptor coactivator 1 (SRC1) to the ligand-binding... | J Med Chem 44: 886-97 (2001) Article DOI: 10.1021/jm0004749 BindingDB Entry DOI: 10.7270/Q2Z899P5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM20174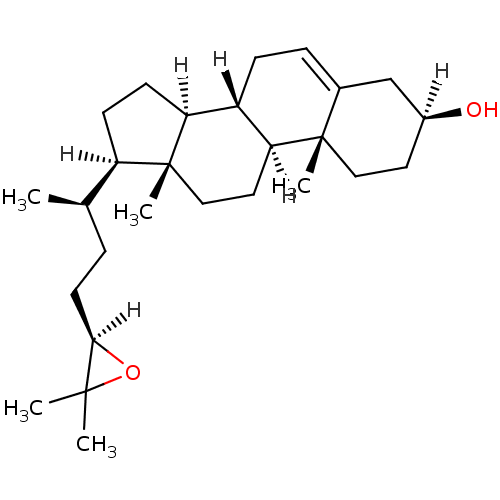 ((1S,2R,5S,10S,11S,14R,15R)-14-[(2R)-4-[(2R)-3,3-di...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 670 | n/a | n/a | 7.5 | 22 |
Dartmouth College | Assay Description The LXR LiSA measures the ligand-dependent recruitment of a 25 amino acid fragment of the steroid receptor coactivator 1 (SRC1) to the ligand-binding... | J Med Chem 44: 886-97 (2001) Article DOI: 10.1021/jm0004749 BindingDB Entry DOI: 10.7270/Q2Z899P5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM20175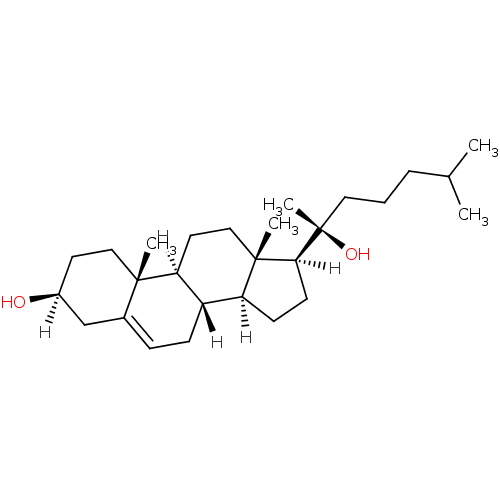 ((1S,2R,5S,10S,11S,14S,15S)-14-[(2S)-2-hydroxy-6-me...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 470 | n/a | n/a | 7.5 | 22 |
Dartmouth College | Assay Description The LXR LiSA measures the ligand-dependent recruitment of a 25 amino acid fragment of the steroid receptor coactivator 1 (SRC1) to the ligand-binding... | J Med Chem 44: 886-97 (2001) Article DOI: 10.1021/jm0004749 BindingDB Entry DOI: 10.7270/Q2Z899P5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM20176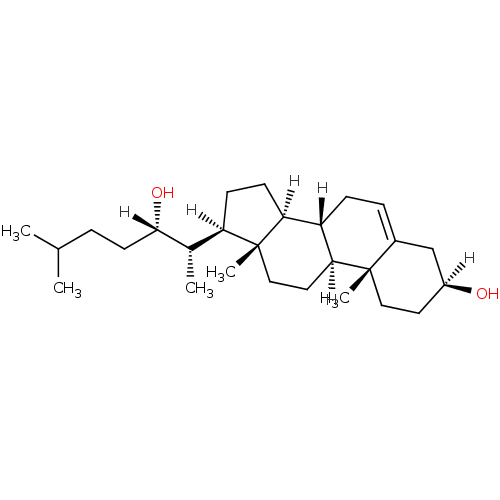 ((1S,2R,5S,10S,11S,14R,15S)-14-[(2S,3S)-3-hydroxy-6...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Dartmouth College | Assay Description The LXR LiSA measures the ligand-dependent recruitment of a 25 amino acid fragment of the steroid receptor coactivator 1 (SRC1) to the ligand-binding... | J Med Chem 44: 886-97 (2001) Article DOI: 10.1021/jm0004749 BindingDB Entry DOI: 10.7270/Q2Z899P5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM20177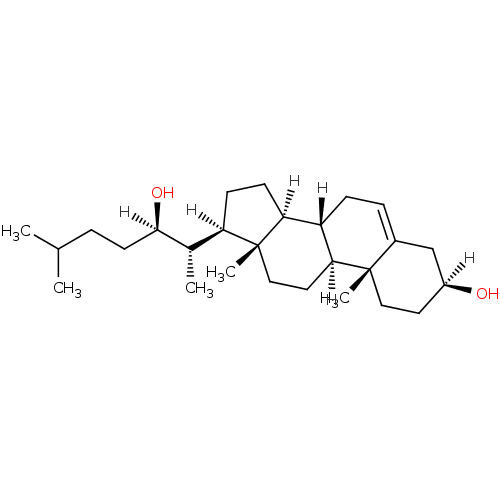 ((1S,2R,5S,10S,11S,14R,15S)-14-[(2S,3R)-3-hydroxy-6...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 325 | n/a | n/a | 7.5 | 22 |
Dartmouth College | Assay Description The LXR LiSA measures the ligand-dependent recruitment of a 25 amino acid fragment of the steroid receptor coactivator 1 (SRC1) to the ligand-binding... | J Med Chem 44: 886-97 (2001) Article DOI: 10.1021/jm0004749 BindingDB Entry DOI: 10.7270/Q2Z899P5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM20178 ((1S,2R,5S,10S,11S,14R,15R)-14-[(2R,4S)-4-hydroxy-6...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 5.90E+3 | n/a | n/a | 7.5 | 22 |
Dartmouth College | Assay Description The LXR LiSA measures the ligand-dependent recruitment of a 25 amino acid fragment of the steroid receptor coactivator 1 (SRC1) to the ligand-binding... | J Med Chem 44: 886-97 (2001) Article DOI: 10.1021/jm0004749 BindingDB Entry DOI: 10.7270/Q2Z899P5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM20179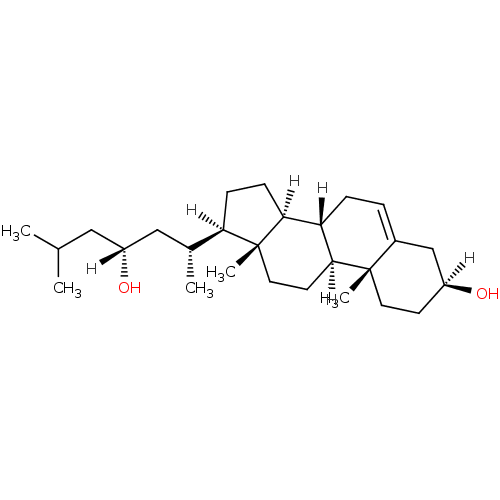 ((1S,2R,5S,10S,11S,14R,15R)-14-[(2R,4R)-4-hydroxy-6...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Dartmouth College | Assay Description The LXR LiSA measures the ligand-dependent recruitment of a 25 amino acid fragment of the steroid receptor coactivator 1 (SRC1) to the ligand-binding... | J Med Chem 44: 886-97 (2001) Article DOI: 10.1021/jm0004749 BindingDB Entry DOI: 10.7270/Q2Z899P5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM20180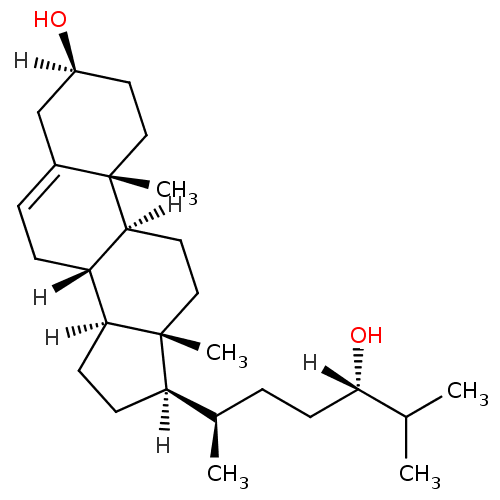 ((1S,2R,5S,10S,11S,14R,15R)-14-[(2R,5S)-5-hydroxy-6...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 130 | n/a | n/a | 7.5 | 22 |
Dartmouth College | Assay Description The LXR LiSA measures the ligand-dependent recruitment of a 25 amino acid fragment of the steroid receptor coactivator 1 (SRC1) to the ligand-binding... | J Med Chem 44: 886-97 (2001) Article DOI: 10.1021/jm0004749 BindingDB Entry DOI: 10.7270/Q2Z899P5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM20181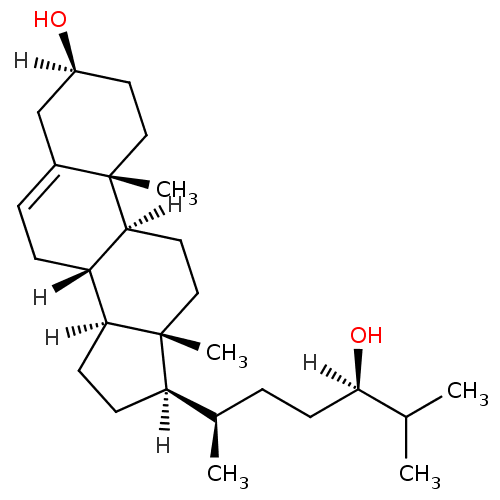 ((1S,2R,5S,10S,11S,14R,15R)-14-[(2R,5R)-5-hydroxy-6...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 220 | n/a | n/a | 7.5 | 22 |
Dartmouth College | Assay Description The LXR LiSA measures the ligand-dependent recruitment of a 25 amino acid fragment of the steroid receptor coactivator 1 (SRC1) to the ligand-binding... | J Med Chem 44: 886-97 (2001) Article DOI: 10.1021/jm0004749 BindingDB Entry DOI: 10.7270/Q2Z899P5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM20182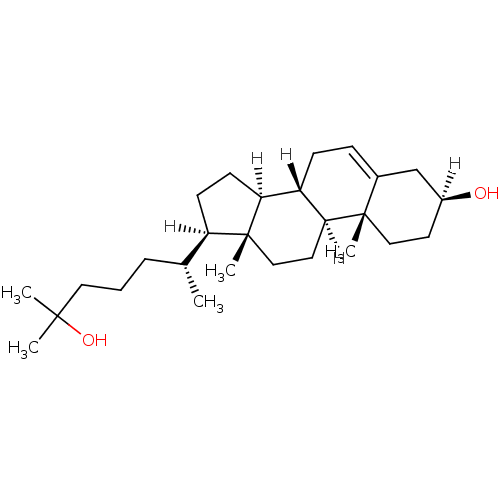 ((1S,2R,5S,10S,11S,14R,15R)-14-[(2R)-6-hydroxy-6-me...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.16E+3 | n/a | n/a | 7.5 | 22 |
Dartmouth College | Assay Description The LXR LiSA measures the ligand-dependent recruitment of a 25 amino acid fragment of the steroid receptor coactivator 1 (SRC1) to the ligand-binding... | J Med Chem 44: 886-97 (2001) Article DOI: 10.1021/jm0004749 BindingDB Entry DOI: 10.7270/Q2Z899P5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM20183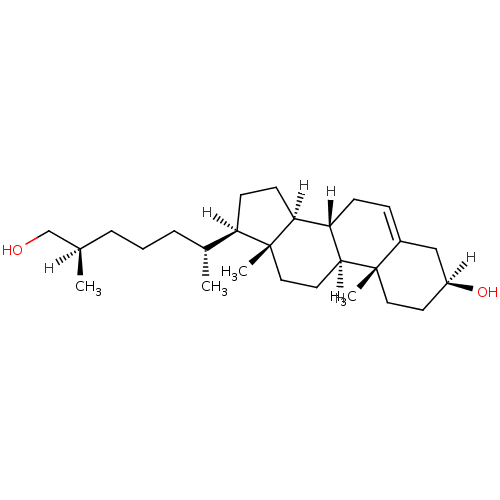 ((1S,2R,5S,10S,11S,14R,15R)-14-[(2R,6R)-7-hydroxy-6...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 250 | n/a | n/a | 7.5 | 22 |
Dartmouth College | Assay Description The LXR LiSA measures the ligand-dependent recruitment of a 25 amino acid fragment of the steroid receptor coactivator 1 (SRC1) to the ligand-binding... | J Med Chem 44: 886-97 (2001) Article DOI: 10.1021/jm0004749 BindingDB Entry DOI: 10.7270/Q2Z899P5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM20184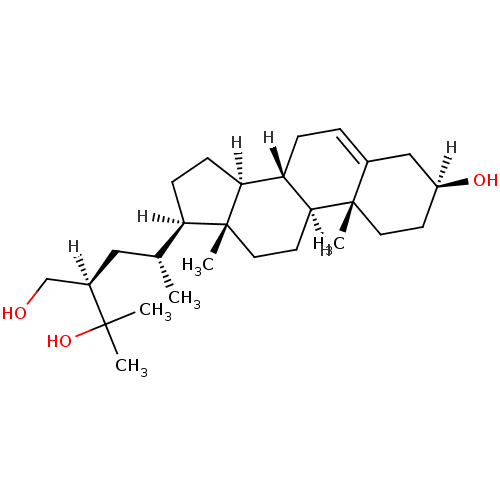 ((2R)-2-[(2R)-2-[(1S,2R,5S,10S,11S,14R,15R)-5-hydro...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Dartmouth College | Assay Description The LXR LiSA measures the ligand-dependent recruitment of a 25 amino acid fragment of the steroid receptor coactivator 1 (SRC1) to the ligand-binding... | J Med Chem 44: 886-97 (2001) Article DOI: 10.1021/jm0004749 BindingDB Entry DOI: 10.7270/Q2Z899P5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM20185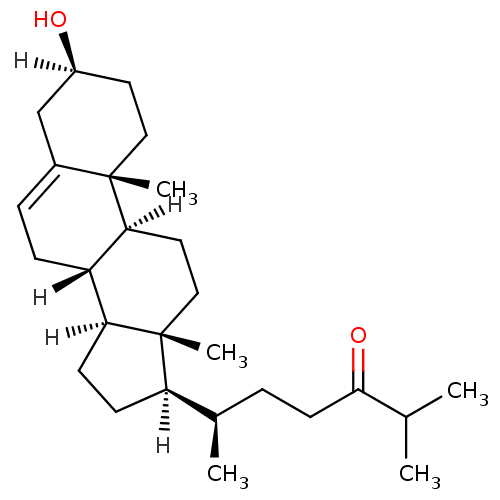 ((6R)-6-[(1S,2R,5S,10S,11S,14R,15R)-5-hydroxy-2,15-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 180 | n/a | n/a | 7.5 | 22 |
Dartmouth College | Assay Description The LXR LiSA measures the ligand-dependent recruitment of a 25 amino acid fragment of the steroid receptor coactivator 1 (SRC1) to the ligand-binding... | J Med Chem 44: 886-97 (2001) Article DOI: 10.1021/jm0004749 BindingDB Entry DOI: 10.7270/Q2Z899P5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM20186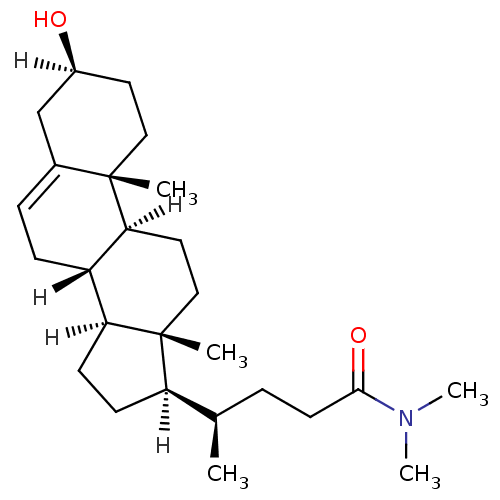 ((4R)-4-[(1S,2R,5S,10S,11S,14R,15R)-5-hydroxy-2,15-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 170 | n/a | n/a | 7.5 | 22 |
Dartmouth College | Assay Description The LXR LiSA measures the ligand-dependent recruitment of a 25 amino acid fragment of the steroid receptor coactivator 1 (SRC1) to the ligand-binding... | J Med Chem 44: 886-97 (2001) Article DOI: 10.1021/jm0004749 BindingDB Entry DOI: 10.7270/Q2Z899P5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM20187 ((4R)-4-[(1S,2R,5S,10S,11S,14R,15R)-5-hydroxy-2,15-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 720 | n/a | n/a | 7.5 | 22 |
Dartmouth College | Assay Description The LXR LiSA measures the ligand-dependent recruitment of a 25 amino acid fragment of the steroid receptor coactivator 1 (SRC1) to the ligand-binding... | J Med Chem 44: 886-97 (2001) Article DOI: 10.1021/jm0004749 BindingDB Entry DOI: 10.7270/Q2Z899P5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 107 total ) | Next | Last >> |