 Found 44 hits with Last Name = 'bourne' and Initial = 'g'
Found 44 hits with Last Name = 'bourne' and Initial = 'g' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Cholecystokinin receptor type A
(RAT) | BDBM50005463

((R)-1H-Indole-2-carboxylic acid (1-methyl-2-oxo-5-...)Show SMILES CN1c2ccccc2C(=N[C@H](NC(=O)c2cc3ccccc3[nH]2)C1=O)c1ccccc1 |r,c:9| Show InChI InChI=1S/C25H20N4O2/c1-29-21-14-8-6-12-18(21)22(16-9-3-2-4-10-16)27-23(25(29)31)28-24(30)20-15-17-11-5-7-13-19(17)26-20/h2-15,23,26H,1H3,(H,28,30)/t23-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of [125I]- Bolton-Hunter CCK-26-33 binding to Cholecystokinin type A receptor of rat pancreas |
Bioorg Med Chem Lett 3: 889-894 (1993)
Article DOI: 10.1016/S0960-894X(00)80687-3
BindingDB Entry DOI: 10.7270/Q28G8M63 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(MOUSE) | BDBM21147

((3S)-3-[(2S)-2-[(2S)-2-{2-[(2S)-2-[(2S)-2-[(3S)-3-...)Show SMILES CSCC[C@H](NC(=O)[C@H](Cc1ccc(OS(O)(=O)=O)cc1)NC(=O)[C@@H](N)CC(O)=O)C(=O)NCC(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CCSC)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccccc1)C(N)=O Show InChI InChI=1S/C49H62N10O16S3/c1-76-18-16-34(55-47(69)37(58-44(66)32(50)23-41(61)62)21-28-12-14-30(15-13-28)75-78(72,73)74)45(67)53-26-40(60)54-38(22-29-25-52-33-11-7-6-10-31(29)33)48(70)56-35(17-19-77-2)46(68)59-39(24-42(63)64)49(71)57-36(43(51)65)20-27-8-4-3-5-9-27/h3-15,25,32,34-39,52H,16-24,26,50H2,1-2H3,(H2,51,65)(H,53,67)(H,54,60)(H,55,69)(H,56,70)(H,57,71)(H,58,66)(H,59,68)(H,61,62)(H,63,64)(H,72,73,74)/t32-,34-,35-,36-,37-,38-,39-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of [125I]- Bolton-Hunter CCK-26-33 binding to Cholecystokinin type B receptor of mouse cerebral cortex |
Bioorg Med Chem Lett 3: 889-894 (1993)
Article DOI: 10.1016/S0960-894X(00)80687-3
BindingDB Entry DOI: 10.7270/Q28G8M63 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(RAT) | BDBM21147

((3S)-3-[(2S)-2-[(2S)-2-{2-[(2S)-2-[(2S)-2-[(3S)-3-...)Show SMILES CSCC[C@H](NC(=O)[C@H](Cc1ccc(OS(O)(=O)=O)cc1)NC(=O)[C@@H](N)CC(O)=O)C(=O)NCC(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CCSC)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccccc1)C(N)=O Show InChI InChI=1S/C49H62N10O16S3/c1-76-18-16-34(55-47(69)37(58-44(66)32(50)23-41(61)62)21-28-12-14-30(15-13-28)75-78(72,73)74)45(67)53-26-40(60)54-38(22-29-25-52-33-11-7-6-10-31(29)33)48(70)56-35(17-19-77-2)46(68)59-39(24-42(63)64)49(71)57-36(43(51)65)20-27-8-4-3-5-9-27/h3-15,25,32,34-39,52H,16-24,26,50H2,1-2H3,(H2,51,65)(H,53,67)(H,54,60)(H,55,69)(H,56,70)(H,57,71)(H,58,66)(H,59,68)(H,61,62)(H,63,64)(H,72,73,74)/t32-,34-,35-,36-,37-,38-,39-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of [125I]- Bolton-Hunter CCK-26-33 binding to Cholecystokinin type A receptor of rat pancreas |
Bioorg Med Chem Lett 3: 889-894 (1993)
Article DOI: 10.1016/S0960-894X(00)80687-3
BindingDB Entry DOI: 10.7270/Q28G8M63 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(RAT) | BDBM50007916
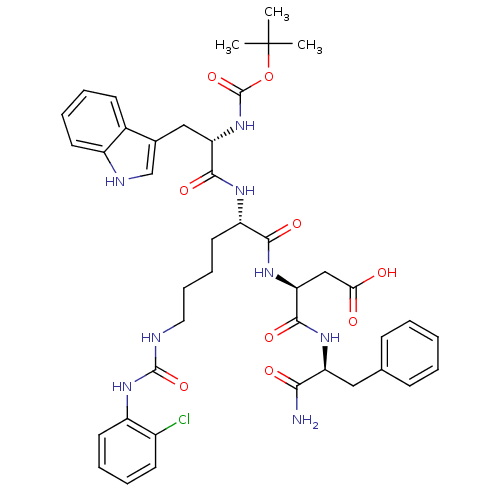
(3-{1-[1-aminobutyl oxycarbonylamino-2-(1H-3-indoly...)Show SMILES CC(C)(C)OC(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CCCCNC(=O)Nc1ccccc1Cl)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccccc1)C(N)=O Show InChI InChI=1S/C42H51ClN8O9/c1-42(2,3)60-41(59)51-33(22-26-24-46-29-17-9-7-15-27(26)29)38(56)47-31(19-11-12-20-45-40(58)50-30-18-10-8-16-28(30)43)37(55)49-34(23-35(52)53)39(57)48-32(36(44)54)21-25-13-5-4-6-14-25/h4-10,13-18,24,31-34,46H,11-12,19-23H2,1-3H3,(H2,44,54)(H,47,56)(H,48,57)(H,49,55)(H,51,59)(H,52,53)(H2,45,50,58)/t31-,32-,33-,34-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of [125I]- Bolton-Hunter CCK-26-33 binding to Cholecystokinin type A receptor of rat pancreas |
Bioorg Med Chem Lett 3: 889-894 (1993)
Article DOI: 10.1016/S0960-894X(00)80687-3
BindingDB Entry DOI: 10.7270/Q28G8M63 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(MOUSE) | BDBM50005463

((R)-1H-Indole-2-carboxylic acid (1-methyl-2-oxo-5-...)Show SMILES CN1c2ccccc2C(=N[C@H](NC(=O)c2cc3ccccc3[nH]2)C1=O)c1ccccc1 |r,c:9| Show InChI InChI=1S/C25H20N4O2/c1-29-21-14-8-6-12-18(21)22(16-9-3-2-4-10-16)27-23(25(29)31)28-24(30)20-15-17-11-5-7-13-19(17)26-20/h2-15,23,26H,1H3,(H,28,30)/t23-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
| n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of [125I]- Bolton-Hunter CCK-26-33 binding to Cholecystokinin type B receptor of mouse cerebral cortex |
Bioorg Med Chem Lett 3: 889-894 (1993)
Article DOI: 10.1016/S0960-894X(00)80687-3
BindingDB Entry DOI: 10.7270/Q28G8M63 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(RAT) | BDBM50005459
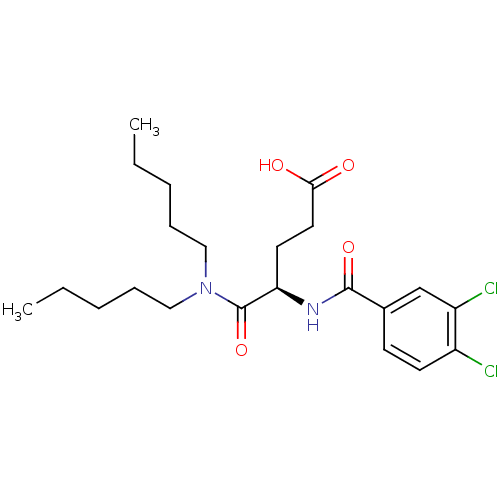
(4-(3,4-Dichloro-benzoylamino)-4-dipentylcarbamoyl-...)Show SMILES CCCCCN(CCCCC)C(=O)[C@@H](CCC(O)=O)NC(=O)c1ccc(Cl)c(Cl)c1 Show InChI InChI=1S/C22H32Cl2N2O4/c1-3-5-7-13-26(14-8-6-4-2)22(30)19(11-12-20(27)28)25-21(29)16-9-10-17(23)18(24)15-16/h9-10,15,19H,3-8,11-14H2,1-2H3,(H,25,29)(H,27,28)/t19-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of [125I]- Bolton-Hunter CCK-26-33 binding to Cholecystokinin type A receptor of rat pancreas |
Bioorg Med Chem Lett 3: 889-894 (1993)
Article DOI: 10.1016/S0960-894X(00)80687-3
BindingDB Entry DOI: 10.7270/Q28G8M63 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(RAT) | BDBM50281743
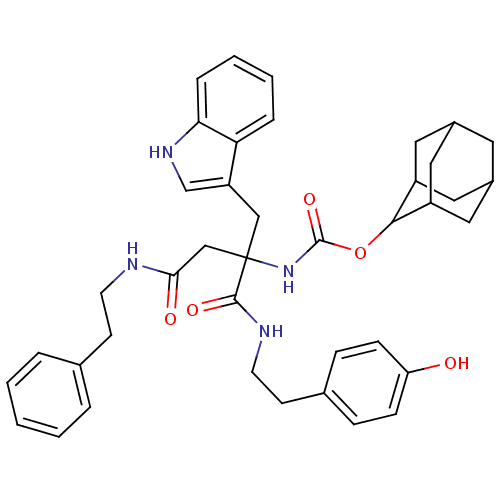
(CHEMBL169710 | [1-[2-(4-Hydroxy-phenyl)-ethylcarba...)Show SMILES Oc1ccc(CCNC(=O)C(CC(=O)NCCc2ccccc2)(Cc2c[nH]c3ccccc23)NC(=O)OC2C3CC4CC(C3)CC2C4)cc1 |TLB:36:37:39:42.43.41,THB:44:45:39:42.43.41,44:42:39:37.45.46,41:40:37:42.44.43,41:42:37:40.39.46,(9.63,-1.36,;9.64,-2.9,;10.99,-3.65,;11,-5.2,;9.68,-5.97,;9.69,-7.51,;8.35,-8.31,;8.38,-9.85,;7.04,-10.64,;5.7,-9.87,;7.06,-12.19,;8.4,-11.42,;9.73,-12.19,;9.73,-13.73,;11.07,-11.42,;12.4,-12.19,;13.75,-11.42,;15.08,-12.18,;16.42,-11.42,;17.74,-12.18,;17.75,-13.71,;16.41,-14.48,;15.08,-13.71,;5.73,-11.42,;4.4,-12.19,;4.22,-13.72,;2.72,-14.05,;1.95,-12.7,;.45,-12.4,;-.04,-10.94,;.99,-9.8,;2.5,-10.11,;2.98,-11.57,;7.06,-13.73,;7.06,-15.27,;5.73,-16.05,;8.4,-16.05,;9.94,-16.04,;10.79,-14.76,;12.33,-14.25,;13.19,-15.5,;14.68,-15.97,;12.98,-16.37,;12.14,-15.24,;12.16,-17.63,;10.79,-17.28,;12.33,-16.82,;8.33,-5.22,;8.31,-3.69,)| Show InChI InChI=1S/C40H46N4O5/c45-33-12-10-27(11-13-33)15-17-42-38(47)40(23-32-25-43-35-9-5-4-8-34(32)35,24-36(46)41-16-14-26-6-2-1-3-7-26)44-39(48)49-37-30-19-28-18-29(21-30)22-31(37)20-28/h1-13,25,28-31,37,43,45H,14-24H2,(H,41,46)(H,42,47)(H,44,48) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 57 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of [125I]- Bolton-Hunter CCK-26-33 binding to Cholecystokinin type A receptor of rat pancreas |
Bioorg Med Chem Lett 3: 889-894 (1993)
Article DOI: 10.1016/S0960-894X(00)80687-3
BindingDB Entry DOI: 10.7270/Q28G8M63 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(RAT) | BDBM50089109
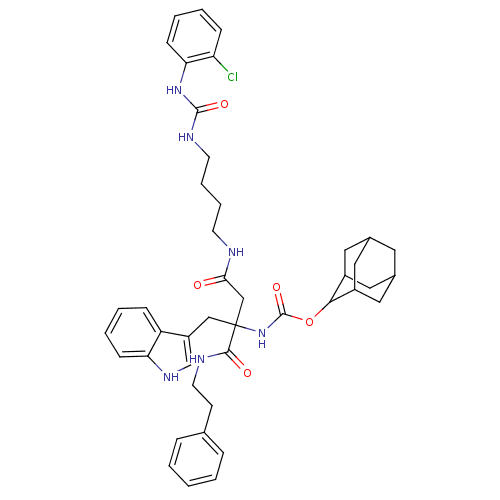
(CHEMBL278780 | [2-{4-[3-(2-Chloro-phenyl)-ureido]-...)Show SMILES Clc1ccccc1NC(=O)NCCCCNC(=O)CC(Cc1c[nH]c2ccccc12)(NC(=O)OC1C2CC3CC(C2)CC1C3)C(=O)NCCc1ccccc1 |TLB:40:35:43:39.41.38,40:39:34.35.36:43,THB:38:37:34:39.40.41,38:39:34:37.36.43,33:34:43:39.41.38,(16.48,3.44,;15.71,4.78,;16.5,6.1,;15.71,7.43,;14.15,7.43,;13.4,6.1,;14.15,4.78,;13.39,3.45,;11.83,3.45,;11.06,4.78,;11.06,2.11,;9.51,2.11,;8.73,.78,;7.2,.78,;6.42,-.55,;4.87,-.55,;3.3,-.9,;2.31,.04,;2.59,-2.41,;3.08,-4.15,;2.79,-5.76,;3.61,-7.11,;2.8,-8.42,;3.82,-9.57,;5.23,-8.99,;6.62,-9.63,;7.86,-8.76,;7.75,-7.24,;6.35,-6.58,;5.1,-7.45,;1.8,-3.33,;.42,-4.02,;.34,-5.56,;-.87,-3.18,;-2.27,-3.89,;-3.58,-3.13,;-4.77,-4.23,;-4.95,-5.81,;-6.51,-6.05,;-5.16,-4.92,;-4.98,-3.46,;-3.9,-5.58,;-2.46,-5.4,;-3.62,-6.53,;4.48,-3.46,;4.56,-1.94,;5.78,-4.31,;7.14,-3.62,;8.45,-4.46,;9.83,-3.76,;11.14,-4.59,;12.52,-3.89,;12.58,-2.36,;11.29,-1.52,;9.91,-2.23,)| Show InChI InChI=1S/C43H51ClN6O5/c44-35-13-5-7-15-37(35)49-41(53)47-18-9-8-17-45-38(51)26-43(25-33-27-48-36-14-6-4-12-34(33)36,40(52)46-19-16-28-10-2-1-3-11-28)50-42(54)55-39-31-21-29-20-30(23-31)24-32(39)22-29/h1-7,10-15,27,29-32,39,48H,8-9,16-26H2,(H,45,51)(H,46,52)(H,50,54)(H2,47,49,53) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 75 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of [125I]- Bolton-Hunter CCK-26-33 binding to Cholecystokinin type A receptor of rat pancreas |
Bioorg Med Chem Lett 3: 889-894 (1993)
Article DOI: 10.1016/S0960-894X(00)80687-3
BindingDB Entry DOI: 10.7270/Q28G8M63 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(RAT) | BDBM50281741
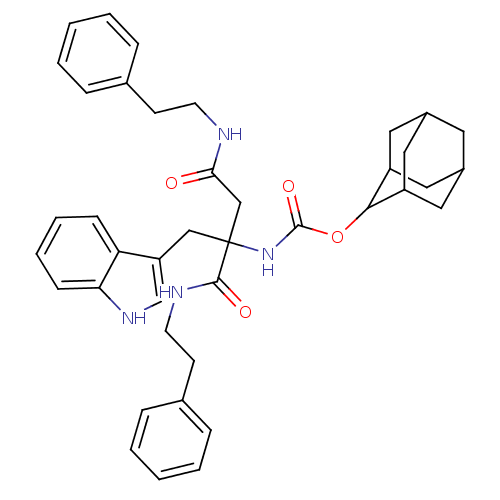
(CHEMBL355275 | [1-(1H-Indol-3-ylmethyl)-1,2-bis-ph...)Show SMILES O=C(CC(Cc1c[nH]c2ccccc12)(NC(=O)OC1C2CC3CC(C2)CC1C3)C(=O)NCCc1ccccc1)NCCc1ccccc1 |TLB:17:18:20:23.24.22,THB:25:26:20:23.24.22,25:23:20:18.26.27,22:21:18:23.25.24,22:23:18:21.20.27,(12.05,-14.95,;12.05,-13.41,;10.72,-12.63,;9.38,-13.41,;8.04,-12.63,;6.71,-13.41,;6.53,-14.95,;5.03,-15.28,;4.26,-13.94,;2.77,-13.62,;2.28,-12.17,;3.3,-11.02,;4.82,-11.34,;5.29,-12.8,;9.38,-14.95,;9.38,-16.49,;8.04,-17.26,;10.72,-17.26,;12.25,-17.26,;13.1,-15.99,;14.64,-15.48,;15.51,-16.73,;17,-17.19,;15.3,-17.61,;14.46,-16.47,;14.48,-18.85,;13.1,-18.5,;14.64,-18.05,;9.36,-11.86,;8.02,-11.09,;10.69,-11.07,;10.67,-9.52,;12,-8.74,;11.99,-7.2,;10.65,-6.45,;10.62,-4.92,;11.96,-4.12,;13.3,-4.89,;13.31,-6.41,;13.38,-12.63,;14.71,-13.41,;16.06,-12.63,;17.4,-13.41,;17.4,-14.95,;18.73,-15.72,;20.07,-14.95,;20.06,-13.41,;18.73,-12.63,)| Show InChI InChI=1S/C40H46N4O4/c45-36(41-17-15-27-9-3-1-4-10-27)25-40(24-33-26-43-35-14-8-7-13-34(33)35,38(46)42-18-16-28-11-5-2-6-12-28)44-39(47)48-37-31-20-29-19-30(22-31)23-32(37)21-29/h1-14,26,29-32,37,43H,15-25H2,(H,41,45)(H,42,46)(H,44,47) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of [125I]- Bolton-Hunter CCK-26-33 binding to Cholecystokinin type A receptor of rat pancreas |
Bioorg Med Chem Lett 3: 889-894 (1993)
Article DOI: 10.1016/S0960-894X(00)80687-3
BindingDB Entry DOI: 10.7270/Q28G8M63 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(RAT) | BDBM50281740
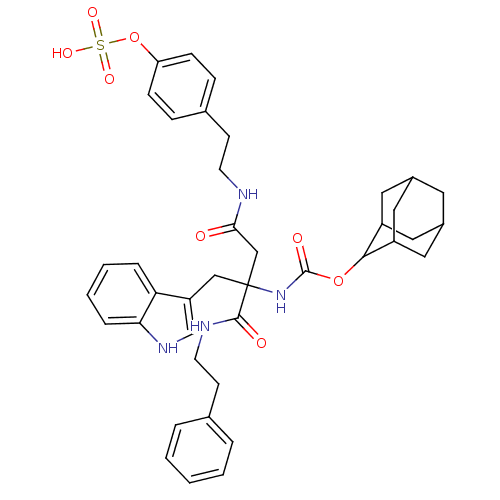
(CHEMBL355052 | {1-(1H-Indol-3-ylmethyl)-1-phenethy...)Show SMILES OS(=O)(=O)Oc1ccc(CCNC(=O)CC(Cc2c[nH]c3ccccc23)(NC(=O)OC2C3CC4CC(C3)CC2C4)C(=O)NCCc2ccccc2)cc1 |TLB:32:33:37:30.31.36,29:30:37:33.39.34,THB:32:31:37:33.39.34,34:35:30:33.32.39,34:33:30:35.37.36,(19.93,-18.54,;19.94,-17,;21.49,-17,;18.4,-16.98,;19.94,-15.46,;18.6,-14.68,;18.6,-13.15,;17.26,-12.39,;15.93,-13.15,;14.6,-12.39,;13.26,-13.16,;11.92,-12.39,;10.58,-13.16,;10.58,-14.71,;9.24,-12.39,;7.91,-13.16,;6.58,-12.39,;5.25,-13.16,;5.07,-14.69,;3.56,-15.02,;2.79,-13.69,;1.29,-13.37,;.82,-11.91,;1.85,-10.76,;3.35,-11.09,;3.82,-12.54,;7.91,-14.71,;7.91,-16.25,;6.58,-17.02,;9.24,-17.02,;10.79,-17.01,;11.63,-15.74,;13.17,-15.23,;14.05,-16.47,;15.53,-16.94,;13.83,-17.36,;12.98,-16.21,;13.02,-18.6,;11.64,-18.26,;13.19,-17.8,;7.9,-11.61,;6.55,-10.85,;9.22,-10.83,;9.21,-9.28,;10.55,-8.5,;10.53,-6.95,;9.17,-6.2,;9.17,-4.66,;10.5,-3.87,;11.84,-4.64,;11.85,-6.17,;15.93,-14.69,;17.26,-15.46,)| Show InChI InChI=1S/C40H46N4O8S/c45-36(41-16-14-27-10-12-33(13-11-27)52-53(48,49)50)24-40(23-32-25-43-35-9-5-4-8-34(32)35,38(46)42-17-15-26-6-2-1-3-7-26)44-39(47)51-37-30-19-28-18-29(21-30)22-31(37)20-28/h1-13,25,28-31,37,43H,14-24H2,(H,41,45)(H,42,46)(H,44,47)(H,48,49,50) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of [125I]- Bolton-Hunter CCK-26-33 binding to Cholecystokinin type A receptor of rat pancreas |
Bioorg Med Chem Lett 3: 889-894 (1993)
Article DOI: 10.1016/S0960-894X(00)80687-3
BindingDB Entry DOI: 10.7270/Q28G8M63 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(RAT) | BDBM50281738
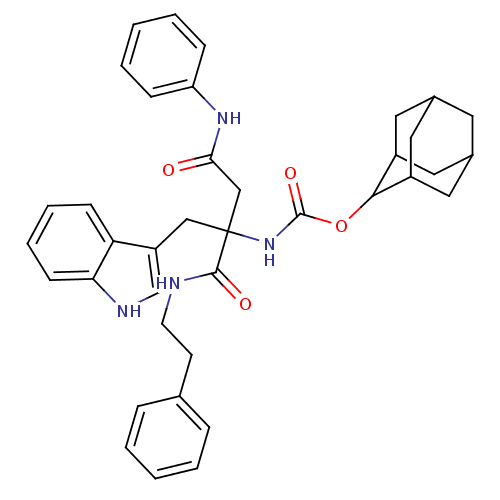
(CHEMBL170129 | [1-(1H-Indol-3-ylmethyl)-1-phenethy...)Show SMILES O=C(CC(Cc1c[nH]c2ccccc12)(NC(=O)OC1C2CC3CC(C2)CC1C3)C(=O)NCCc1ccccc1)Nc1ccccc1 |TLB:20:21:25:18.19.24,17:18:25:21.27.22,THB:20:19:25:21.27.22,22:21:18:23.25.24,22:23:18:21.20.27,(10.64,-10.93,;10.64,-9.38,;9.31,-8.61,;7.96,-9.38,;6.62,-8.61,;5.29,-9.38,;5.13,-10.93,;3.62,-11.25,;2.85,-9.92,;1.36,-9.59,;.87,-8.14,;1.9,-7,;3.4,-7.33,;3.87,-8.78,;7.96,-10.93,;7.96,-12.47,;6.62,-13.24,;9.29,-13.24,;10.83,-13.24,;11.68,-11.98,;13.23,-11.44,;14.1,-12.7,;15.58,-13.17,;13.87,-13.59,;13.05,-12.45,;13.08,-14.83,;11.69,-14.48,;13.23,-14.04,;7.96,-7.84,;6.6,-7.07,;9.28,-7.05,;9.27,-5.5,;10.6,-4.73,;10.58,-3.19,;9.24,-2.44,;9.22,-.9,;10.55,-.1,;11.89,-.87,;11.91,-2.39,;11.98,-8.61,;13.31,-9.38,;14.64,-8.61,;15.98,-9.38,;15.98,-10.93,;14.64,-11.7,;13.31,-10.93,)| Show InChI InChI=1S/C38H42N4O4/c43-34(41-31-11-5-2-6-12-31)23-38(22-30-24-40-33-14-8-7-13-32(30)33,36(44)39-16-15-25-9-3-1-4-10-25)42-37(45)46-35-28-18-26-17-27(20-28)21-29(35)19-26/h1-14,24,26-29,35,40H,15-23H2,(H,39,44)(H,41,43)(H,42,45) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 230 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of [125I]- Bolton-Hunter CCK-26-33 binding to Cholecystokinin type A receptor of rat pancreas |
Bioorg Med Chem Lett 3: 889-894 (1993)
Article DOI: 10.1016/S0960-894X(00)80687-3
BindingDB Entry DOI: 10.7270/Q28G8M63 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(MOUSE) | BDBM50281739
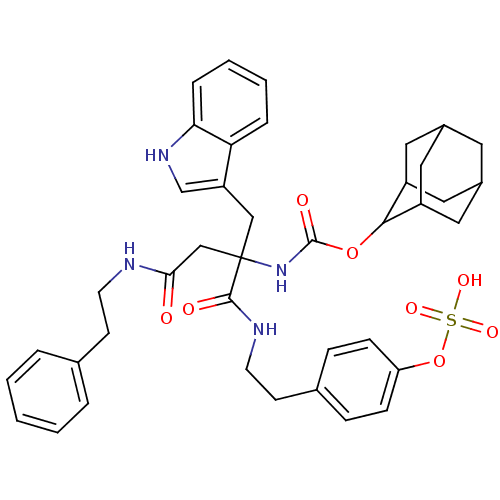
(CHEMBL171375 | {1-(1H-Indol-3-ylmethyl)-2-phenethy...)Show SMILES OS(=O)(=O)Oc1ccc(CCNC(=O)C(CC(=O)NCCc2ccccc2)(Cc2c[nH]c3ccccc23)NC(=O)OC2C3CC4CC(C3)CC2C4)cc1 |TLB:43:44:48:41.42.47,40:41:48:44.50.45,THB:43:42:48:44.50.45,45:46:41:44.43.50,45:44:41:46.48.47,(14.75,-3.3,;13.26,-3.7,;12.65,-2.28,;13.87,-5.12,;11.94,-4.49,;11.95,-6.03,;10.62,-6.83,;10.64,-8.35,;11.99,-9.1,;12,-10.64,;10.67,-11.44,;10.69,-12.98,;9.36,-13.77,;8.02,-13,;9.37,-15.32,;10.71,-14.55,;12.05,-15.32,;12.05,-16.86,;13.38,-14.55,;14.71,-15.32,;16.06,-14.55,;17.39,-15.31,;18.73,-14.55,;20.06,-15.31,;20.06,-16.84,;18.72,-17.61,;17.39,-16.84,;8.04,-14.55,;6.71,-15.32,;6.53,-16.85,;5.03,-17.18,;4.26,-15.83,;2.77,-15.53,;2.28,-14.07,;3.3,-12.93,;4.82,-13.24,;5.29,-14.7,;9.37,-16.86,;9.37,-18.4,;8.03,-19.18,;10.71,-19.18,;12.25,-19.17,;13.09,-17.89,;14.64,-17.38,;15.51,-18.63,;16.99,-19.1,;15.29,-19.5,;14.45,-18.37,;14.48,-20.76,;13.1,-20.41,;14.64,-19.95,;13.31,-8.33,;13.3,-6.79,)| Show InChI InChI=1S/C40H46N4O8S/c45-36(41-16-14-26-6-2-1-3-7-26)24-40(23-32-25-43-35-9-5-4-8-34(32)35,38(46)42-17-15-27-10-12-33(13-11-27)52-53(48,49)50)44-39(47)51-37-30-19-28-18-29(21-30)22-31(37)20-28/h1-13,25,28-31,37,43H,14-24H2,(H,41,45)(H,42,46)(H,44,47)(H,48,49,50) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 330 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of [125I]- Bolton-Hunter CCK-26-33 binding to Cholecystokinin type B receptor of mouse cerebral cortex |
Bioorg Med Chem Lett 3: 889-894 (1993)
Article DOI: 10.1016/S0960-894X(00)80687-3
BindingDB Entry DOI: 10.7270/Q28G8M63 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(MOUSE) | BDBM50281743

(CHEMBL169710 | [1-[2-(4-Hydroxy-phenyl)-ethylcarba...)Show SMILES Oc1ccc(CCNC(=O)C(CC(=O)NCCc2ccccc2)(Cc2c[nH]c3ccccc23)NC(=O)OC2C3CC4CC(C3)CC2C4)cc1 |TLB:36:37:39:42.43.41,THB:44:45:39:42.43.41,44:42:39:37.45.46,41:40:37:42.44.43,41:42:37:40.39.46,(9.63,-1.36,;9.64,-2.9,;10.99,-3.65,;11,-5.2,;9.68,-5.97,;9.69,-7.51,;8.35,-8.31,;8.38,-9.85,;7.04,-10.64,;5.7,-9.87,;7.06,-12.19,;8.4,-11.42,;9.73,-12.19,;9.73,-13.73,;11.07,-11.42,;12.4,-12.19,;13.75,-11.42,;15.08,-12.18,;16.42,-11.42,;17.74,-12.18,;17.75,-13.71,;16.41,-14.48,;15.08,-13.71,;5.73,-11.42,;4.4,-12.19,;4.22,-13.72,;2.72,-14.05,;1.95,-12.7,;.45,-12.4,;-.04,-10.94,;.99,-9.8,;2.5,-10.11,;2.98,-11.57,;7.06,-13.73,;7.06,-15.27,;5.73,-16.05,;8.4,-16.05,;9.94,-16.04,;10.79,-14.76,;12.33,-14.25,;13.19,-15.5,;14.68,-15.97,;12.98,-16.37,;12.14,-15.24,;12.16,-17.63,;10.79,-17.28,;12.33,-16.82,;8.33,-5.22,;8.31,-3.69,)| Show InChI InChI=1S/C40H46N4O5/c45-33-12-10-27(11-13-33)15-17-42-38(47)40(23-32-25-43-35-9-5-4-8-34(32)35,24-36(46)41-16-14-26-6-2-1-3-7-26)44-39(48)49-37-30-19-28-18-29(21-30)22-31(37)20-28/h1-13,25,28-31,37,43,45H,14-24H2,(H,41,46)(H,42,47)(H,44,48) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 540 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of [125I]- Bolton-Hunter CCK-26-33 binding to Cholecystokinin type B receptor of mouse cerebral cortex |
Bioorg Med Chem Lett 3: 889-894 (1993)
Article DOI: 10.1016/S0960-894X(00)80687-3
BindingDB Entry DOI: 10.7270/Q28G8M63 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(MOUSE) | BDBM50281740

(CHEMBL355052 | {1-(1H-Indol-3-ylmethyl)-1-phenethy...)Show SMILES OS(=O)(=O)Oc1ccc(CCNC(=O)CC(Cc2c[nH]c3ccccc23)(NC(=O)OC2C3CC4CC(C3)CC2C4)C(=O)NCCc2ccccc2)cc1 |TLB:32:33:37:30.31.36,29:30:37:33.39.34,THB:32:31:37:33.39.34,34:35:30:33.32.39,34:33:30:35.37.36,(19.93,-18.54,;19.94,-17,;21.49,-17,;18.4,-16.98,;19.94,-15.46,;18.6,-14.68,;18.6,-13.15,;17.26,-12.39,;15.93,-13.15,;14.6,-12.39,;13.26,-13.16,;11.92,-12.39,;10.58,-13.16,;10.58,-14.71,;9.24,-12.39,;7.91,-13.16,;6.58,-12.39,;5.25,-13.16,;5.07,-14.69,;3.56,-15.02,;2.79,-13.69,;1.29,-13.37,;.82,-11.91,;1.85,-10.76,;3.35,-11.09,;3.82,-12.54,;7.91,-14.71,;7.91,-16.25,;6.58,-17.02,;9.24,-17.02,;10.79,-17.01,;11.63,-15.74,;13.17,-15.23,;14.05,-16.47,;15.53,-16.94,;13.83,-17.36,;12.98,-16.21,;13.02,-18.6,;11.64,-18.26,;13.19,-17.8,;7.9,-11.61,;6.55,-10.85,;9.22,-10.83,;9.21,-9.28,;10.55,-8.5,;10.53,-6.95,;9.17,-6.2,;9.17,-4.66,;10.5,-3.87,;11.84,-4.64,;11.85,-6.17,;15.93,-14.69,;17.26,-15.46,)| Show InChI InChI=1S/C40H46N4O8S/c45-36(41-16-14-27-10-12-33(13-11-27)52-53(48,49)50)24-40(23-32-25-43-35-9-5-4-8-34(32)35,38(46)42-17-15-26-6-2-1-3-7-26)44-39(47)51-37-30-19-28-18-29(21-30)22-31(37)20-28/h1-13,25,28-31,37,43H,14-24H2,(H,41,45)(H,42,46)(H,44,47)(H,48,49,50) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 550 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of [125I]- Bolton-Hunter CCK-26-33 binding to Cholecystokinin type B receptor of mouse cerebral cortex |
Bioorg Med Chem Lett 3: 889-894 (1993)
Article DOI: 10.1016/S0960-894X(00)80687-3
BindingDB Entry DOI: 10.7270/Q28G8M63 |
More data for this
Ligand-Target Pair | |
Hematopoietic prostaglandin D synthase
(Homo sapiens (Human)) | BDBM50324491
(CHEMBL1215159 | N-Benzhydryl-5-(3-hydroxyphenyl)th...)Show SMILES Oc1cccc(c1)-c1ccc(s1)C(=O)NC(c1ccccc1)c1ccccc1 Show InChI InChI=1S/C24H19NO2S/c26-20-13-7-12-19(16-20)21-14-15-22(28-21)24(27)25-23(17-8-3-1-4-9-17)18-10-5-2-6-11-18/h1-16,23,26H,(H,25,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Inhibition of human H-PGDS expressed in Escherichia coli BL21 DE2 by enzyme immuno assay |
J Med Chem 53: 5536-48 (2010)
Article DOI: 10.1021/jm100194a
BindingDB Entry DOI: 10.7270/Q21J99Z4 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(RAT) | BDBM50281745
(3-(Adamantan-2-yloxycarbonylamino)-3-(1H-indol-3-y...)Show SMILES O=C(CC(Cc1c[nH]c2ccccc12)(NC(=O)OC1C2CC3CC(C2)CC1C3)C(=O)NCCc1ccccc1)OCc1ccccc1 |TLB:17:18:20:23.24.22,THB:25:26:20:23.24.22,25:23:20:18.26.27,22:21:18:23.25.24,22:23:18:21.20.27,(12.03,-16.84,;12.03,-15.3,;10.7,-14.53,;9.36,-15.3,;8.03,-14.53,;6.7,-15.3,;6.52,-16.84,;5.03,-17.16,;4.26,-15.82,;2.76,-15.51,;2.27,-14.06,;3.3,-12.91,;4.81,-13.22,;5.29,-14.69,;9.36,-16.84,;9.36,-18.39,;8.02,-19.16,;10.7,-19.16,;12.24,-19.15,;13.08,-17.87,;14.63,-17.36,;15.49,-18.61,;16.98,-19.08,;15.27,-19.48,;14.44,-18.35,;14.46,-20.74,;13.08,-20.39,;14.63,-19.93,;9.35,-13.75,;8.01,-12.99,;10.68,-12.97,;10.66,-11.43,;11.99,-10.63,;11.98,-9.09,;13.29,-8.32,;13.29,-6.78,;11.94,-6.02,;10.61,-6.82,;10.63,-8.34,;13.36,-14.53,;14.7,-15.3,;16.04,-14.53,;17.37,-15.3,;18.7,-14.53,;18.7,-12.99,;17.36,-12.22,;16.03,-12.99,)| Show InChI InChI=1S/C39H43N3O5/c43-35(46-25-27-11-5-2-6-12-27)23-39(22-32-24-41-34-14-8-7-13-33(32)34,37(44)40-16-15-26-9-3-1-4-10-26)42-38(45)47-36-30-18-28-17-29(20-30)21-31(36)19-28/h1-14,24,28-31,36,41H,15-23,25H2,(H,40,44)(H,42,45) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of [125I]- Bolton-Hunter CCK-26-33 binding to Cholecystokinin type A receptor of rat pancreas |
Bioorg Med Chem Lett 3: 889-894 (1993)
Article DOI: 10.1016/S0960-894X(00)80687-3
BindingDB Entry DOI: 10.7270/Q28G8M63 |
More data for this
Ligand-Target Pair | |
Hematopoietic prostaglandin D synthase
(Homo sapiens (Human)) | BDBM21625
(2-phenyl-5-(1H-pyrazol-3-yl)-1,3-thiazole | 2-phen...)Show InChI InChI=1S/C12H9N3S/c1-2-4-9(5-3-1)12-13-8-11(16-12)10-6-7-14-15-10/h1-8H,(H,14,15) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Inhibition of human H-PGDS expressed in Escherichia coli BL21 DE2 by enzyme immuno assay |
J Med Chem 53: 5536-48 (2010)
Article DOI: 10.1021/jm100194a
BindingDB Entry DOI: 10.7270/Q21J99Z4 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Gastrin/cholecystokinin type B receptor
(MOUSE) | BDBM50281738

(CHEMBL170129 | [1-(1H-Indol-3-ylmethyl)-1-phenethy...)Show SMILES O=C(CC(Cc1c[nH]c2ccccc12)(NC(=O)OC1C2CC3CC(C2)CC1C3)C(=O)NCCc1ccccc1)Nc1ccccc1 |TLB:20:21:25:18.19.24,17:18:25:21.27.22,THB:20:19:25:21.27.22,22:21:18:23.25.24,22:23:18:21.20.27,(10.64,-10.93,;10.64,-9.38,;9.31,-8.61,;7.96,-9.38,;6.62,-8.61,;5.29,-9.38,;5.13,-10.93,;3.62,-11.25,;2.85,-9.92,;1.36,-9.59,;.87,-8.14,;1.9,-7,;3.4,-7.33,;3.87,-8.78,;7.96,-10.93,;7.96,-12.47,;6.62,-13.24,;9.29,-13.24,;10.83,-13.24,;11.68,-11.98,;13.23,-11.44,;14.1,-12.7,;15.58,-13.17,;13.87,-13.59,;13.05,-12.45,;13.08,-14.83,;11.69,-14.48,;13.23,-14.04,;7.96,-7.84,;6.6,-7.07,;9.28,-7.05,;9.27,-5.5,;10.6,-4.73,;10.58,-3.19,;9.24,-2.44,;9.22,-.9,;10.55,-.1,;11.89,-.87,;11.91,-2.39,;11.98,-8.61,;13.31,-9.38,;14.64,-8.61,;15.98,-9.38,;15.98,-10.93,;14.64,-11.7,;13.31,-10.93,)| Show InChI InChI=1S/C38H42N4O4/c43-34(41-31-11-5-2-6-12-31)23-38(22-30-24-40-33-14-8-7-13-32(30)33,36(44)39-16-15-25-9-3-1-4-10-25)42-37(45)46-35-28-18-26-17-27(20-28)21-29(35)19-26/h1-14,24,26-29,35,40H,15-23H2,(H,39,44)(H,41,43)(H,42,45) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 810 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of [125I]- Bolton-Hunter CCK-26-33 binding to Cholecystokinin type B receptor of mouse cerebral cortex |
Bioorg Med Chem Lett 3: 889-894 (1993)
Article DOI: 10.1016/S0960-894X(00)80687-3
BindingDB Entry DOI: 10.7270/Q28G8M63 |
More data for this
Ligand-Target Pair | |
Hematopoietic prostaglandin D synthase
(Homo sapiens (Human)) | BDBM50324482

(CHEMBL1215370 | N-(1-Amino-3-(1H-indol-3-yl)-1-oxo...)Show SMILES NC(=O)C(Cc1c[nH]c2ccccc12)NC(=O)c1ccc(cc1)-c1cccc(O)c1 Show InChI InChI=1S/C24H21N3O3/c25-23(29)22(13-18-14-26-21-7-2-1-6-20(18)21)27-24(30)16-10-8-15(9-11-16)17-4-3-5-19(28)12-17/h1-12,14,22,26,28H,13H2,(H2,25,29)(H,27,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Inhibition of human H-PGDS expressed in Escherichia coli BL21 DE2 by enzyme immuno assay |
J Med Chem 53: 5536-48 (2010)
Article DOI: 10.1021/jm100194a
BindingDB Entry DOI: 10.7270/Q21J99Z4 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(RAT) | BDBM50281739

(CHEMBL171375 | {1-(1H-Indol-3-ylmethyl)-2-phenethy...)Show SMILES OS(=O)(=O)Oc1ccc(CCNC(=O)C(CC(=O)NCCc2ccccc2)(Cc2c[nH]c3ccccc23)NC(=O)OC2C3CC4CC(C3)CC2C4)cc1 |TLB:43:44:48:41.42.47,40:41:48:44.50.45,THB:43:42:48:44.50.45,45:46:41:44.43.50,45:44:41:46.48.47,(14.75,-3.3,;13.26,-3.7,;12.65,-2.28,;13.87,-5.12,;11.94,-4.49,;11.95,-6.03,;10.62,-6.83,;10.64,-8.35,;11.99,-9.1,;12,-10.64,;10.67,-11.44,;10.69,-12.98,;9.36,-13.77,;8.02,-13,;9.37,-15.32,;10.71,-14.55,;12.05,-15.32,;12.05,-16.86,;13.38,-14.55,;14.71,-15.32,;16.06,-14.55,;17.39,-15.31,;18.73,-14.55,;20.06,-15.31,;20.06,-16.84,;18.72,-17.61,;17.39,-16.84,;8.04,-14.55,;6.71,-15.32,;6.53,-16.85,;5.03,-17.18,;4.26,-15.83,;2.77,-15.53,;2.28,-14.07,;3.3,-12.93,;4.82,-13.24,;5.29,-14.7,;9.37,-16.86,;9.37,-18.4,;8.03,-19.18,;10.71,-19.18,;12.25,-19.17,;13.09,-17.89,;14.64,-17.38,;15.51,-18.63,;16.99,-19.1,;15.29,-19.5,;14.45,-18.37,;14.48,-20.76,;13.1,-20.41,;14.64,-19.95,;13.31,-8.33,;13.3,-6.79,)| Show InChI InChI=1S/C40H46N4O8S/c45-36(41-16-14-26-6-2-1-3-7-26)24-40(23-32-25-43-35-9-5-4-8-34(32)35,38(46)42-17-15-27-10-12-33(13-11-27)52-53(48,49)50)44-39(47)51-37-30-19-28-18-29(21-30)22-31(37)20-28/h1-13,25,28-31,37,43H,14-24H2,(H,41,45)(H,42,46)(H,44,47)(H,48,49,50) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of [125I]- Bolton-Hunter CCK-26-33 binding to Cholecystokinin type A receptor of rat pancreas |
Bioorg Med Chem Lett 3: 889-894 (1993)
Article DOI: 10.1016/S0960-894X(00)80687-3
BindingDB Entry DOI: 10.7270/Q28G8M63 |
More data for this
Ligand-Target Pair | |
Hematopoietic prostaglandin D synthase
(Homo sapiens (Human)) | BDBM50324480
(CHEMBL1215516 | N-(1-Amino-1-oxo-3-phenylpropan-2-...)Show InChI InChI=1S/C19H17N3O2S/c20-18(23)16(11-13-5-2-1-3-6-13)22-19(24)14-8-9-15(21-12-14)17-7-4-10-25-17/h1-10,12,16H,11H2,(H2,20,23)(H,22,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Inhibition of human H-PGDS expressed in Escherichia coli BL21 DE2 by enzyme immuno assay |
J Med Chem 53: 5536-48 (2010)
Article DOI: 10.1021/jm100194a
BindingDB Entry DOI: 10.7270/Q21J99Z4 |
More data for this
Ligand-Target Pair | |
Hematopoietic prostaglandin D synthase
(Homo sapiens (Human)) | BDBM50324485
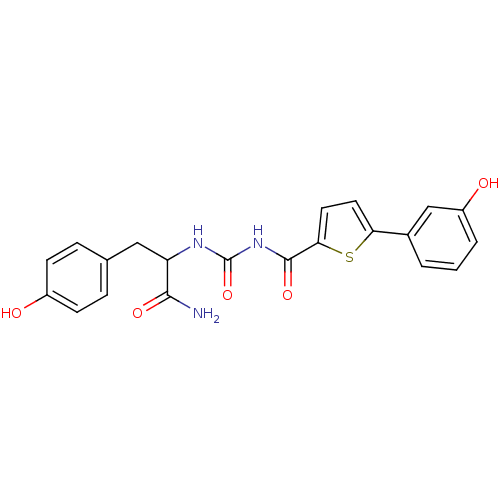
(CHEMBL1215297 | N-(1-Amino-3-(4-hydroxyphenyl)-1-o...)Show SMILES NC(=O)C(Cc1ccc(O)cc1)NC(=O)NC(=O)c1ccc(s1)-c1cccc(O)c1 Show InChI InChI=1S/C21H19N3O5S/c22-19(27)16(10-12-4-6-14(25)7-5-12)23-21(29)24-20(28)18-9-8-17(30-18)13-2-1-3-15(26)11-13/h1-9,11,16,25-26H,10H2,(H2,22,27)(H2,23,24,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Inhibition of human H-PGDS expressed in Escherichia coli BL21 DE2 by enzyme immuno assay |
J Med Chem 53: 5536-48 (2010)
Article DOI: 10.1021/jm100194a
BindingDB Entry DOI: 10.7270/Q21J99Z4 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(MOUSE) | BDBM50281742
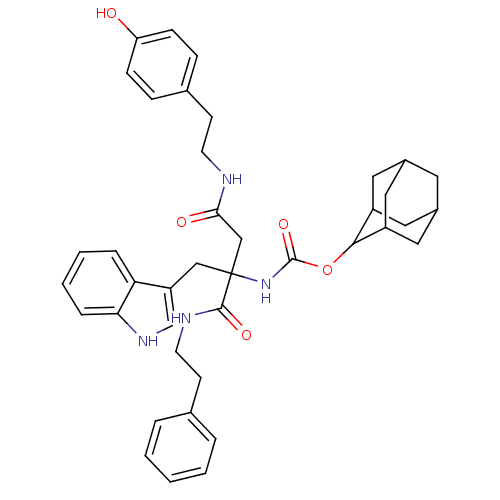
(CHEMBL434113 | [2-[2-(4-Hydroxy-phenyl)-ethylcarba...)Show SMILES Oc1ccc(CCNC(=O)CC(Cc2c[nH]c3ccccc23)(NC(=O)OC2C3CC4CC(C3)CC2C4)C(=O)NCCc2ccccc2)cc1 |TLB:25:26:28:31.32.30,THB:33:34:28:31.32.30,33:31:28:26.34.35,30:29:26:31.33.32,30:31:26:29.28.35,(20.72,-15.7,;19.37,-14.93,;18.04,-15.71,;16.7,-14.94,;16.7,-13.41,;15.37,-12.63,;14.03,-13.41,;12.7,-12.63,;11.35,-13.41,;11.35,-14.95,;10.02,-12.63,;8.68,-13.41,;7.35,-12.63,;6.02,-13.41,;5.85,-14.95,;4.33,-15.28,;3.56,-13.94,;2.07,-13.62,;1.59,-12.17,;2.62,-11.02,;4.12,-11.34,;4.59,-12.8,;8.68,-14.95,;8.68,-16.49,;7.35,-17.26,;10.02,-17.26,;11.56,-17.26,;12.4,-15.99,;13.96,-15.48,;14.82,-16.73,;16.3,-17.19,;14.6,-17.61,;13.77,-16.47,;13.8,-18.85,;12.42,-18.5,;13.96,-18.05,;8.67,-11.86,;7.33,-11.09,;9.99,-11.07,;9.98,-9.52,;11.32,-8.74,;11.3,-7.2,;9.95,-6.45,;9.94,-4.92,;11.27,-4.12,;12.61,-4.89,;12.63,-6.41,;18.04,-12.63,;19.37,-13.41,)| Show InChI InChI=1S/C40H46N4O5/c45-33-12-10-27(11-13-33)14-16-41-36(46)24-40(23-32-25-43-35-9-5-4-8-34(32)35,38(47)42-17-15-26-6-2-1-3-7-26)44-39(48)49-37-30-19-28-18-29(21-30)22-31(37)20-28/h1-13,25,28-31,37,43,45H,14-24H2,(H,41,46)(H,42,47)(H,44,48) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of [125I]- Bolton-Hunter CCK-26-33 binding to Cholecystokinin type B receptor of mouse cerebral cortex |
Bioorg Med Chem Lett 3: 889-894 (1993)
Article DOI: 10.1016/S0960-894X(00)80687-3
BindingDB Entry DOI: 10.7270/Q28G8M63 |
More data for this
Ligand-Target Pair | |
Hematopoietic prostaglandin D synthase
(Homo sapiens (Human)) | BDBM21624
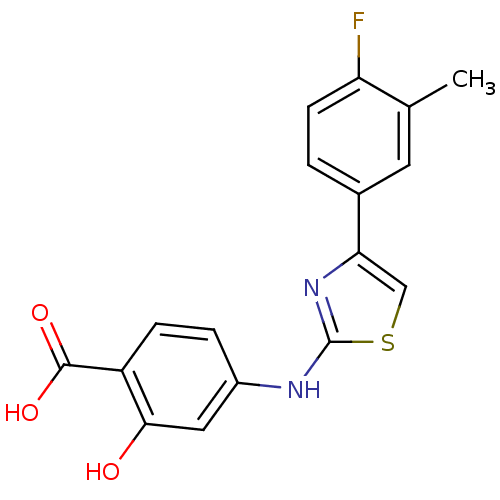
(4-{[4-(4-fluoro-3-methylphenyl)-1,3-thiazol-2-yl]a...)Show InChI InChI=1S/C17H13FN2O3S/c1-9-6-10(2-5-13(9)18)14-8-24-17(20-14)19-11-3-4-12(16(22)23)15(21)7-11/h2-8,21H,1H3,(H,19,20)(H,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
| MMDB
PDB
Article
PubMed
| n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Inhibition of human H-PGDS expressed in Escherichia coli BL21 DE2 by enzyme immuno assay |
J Med Chem 53: 5536-48 (2010)
Article DOI: 10.1021/jm100194a
BindingDB Entry DOI: 10.7270/Q21J99Z4 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cholecystokinin receptor type A
(RAT) | BDBM50281746
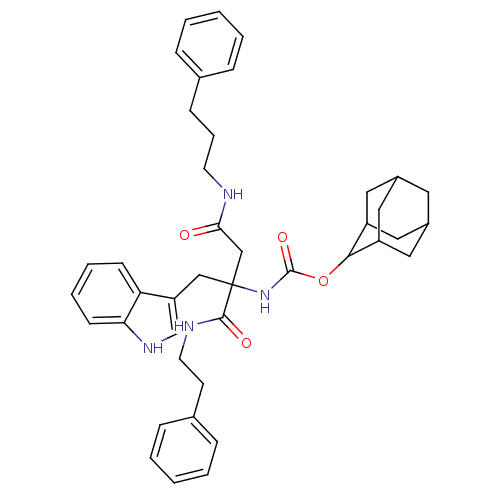
(CHEMBL352817 | [1-(1H-Indol-3-ylmethyl)-1-phenethy...)Show SMILES O=C(CC(Cc1c[nH]c2ccccc12)(NC(=O)OC1C2CC3CC(C2)CC1C3)C(=O)NCCc1ccccc1)NCCCc1ccccc1 |TLB:17:18:20:23.24.22,THB:25:26:20:23.24.22,25:23:20:18.26.27,22:21:18:23.25.24,22:23:18:21.20.27,(11.37,-14.95,;11.37,-13.41,;10.04,-12.63,;8.71,-13.41,;7.37,-12.63,;6.04,-13.41,;5.87,-14.95,;4.36,-15.28,;3.59,-13.94,;2.09,-13.62,;1.61,-12.17,;2.64,-11.02,;4.15,-11.34,;4.61,-12.8,;8.71,-14.95,;8.71,-16.49,;7.37,-17.26,;10.04,-17.26,;11.58,-17.26,;12.42,-15.99,;13.97,-15.48,;14.85,-16.73,;16.33,-17.19,;14.62,-17.61,;13.78,-16.47,;13.82,-18.85,;12.44,-18.5,;13.98,-18.05,;8.7,-11.86,;7.35,-11.09,;10.02,-11.07,;10.01,-9.52,;11.34,-8.74,;11.32,-7.2,;12.65,-6.41,;12.63,-4.89,;11.29,-4.12,;9.96,-4.92,;9.97,-6.45,;12.72,-12.63,;14.05,-13.41,;15.39,-12.63,;16.73,-13.41,;18.06,-12.63,;19.39,-13.41,;20.72,-12.65,;20.72,-11.09,;19.39,-10.32,;18.06,-11.11,)| Show InChI InChI=1S/C41H48N4O4/c46-37(42-18-9-14-28-10-3-1-4-11-28)26-41(25-34-27-44-36-16-8-7-15-35(34)36,39(47)43-19-17-29-12-5-2-6-13-29)45-40(48)49-38-32-21-30-20-31(23-32)24-33(38)22-30/h1-8,10-13,15-16,27,30-33,38,44H,9,14,17-26H2,(H,42,46)(H,43,47)(H,45,48) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of [125I]- Bolton-Hunter CCK-26-33 binding to Cholecystokinin type A receptor of rat pancreas |
Bioorg Med Chem Lett 3: 889-894 (1993)
Article DOI: 10.1016/S0960-894X(00)80687-3
BindingDB Entry DOI: 10.7270/Q28G8M63 |
More data for this
Ligand-Target Pair | |
Hematopoietic prostaglandin D synthase
(Homo sapiens (Human)) | BDBM50324490
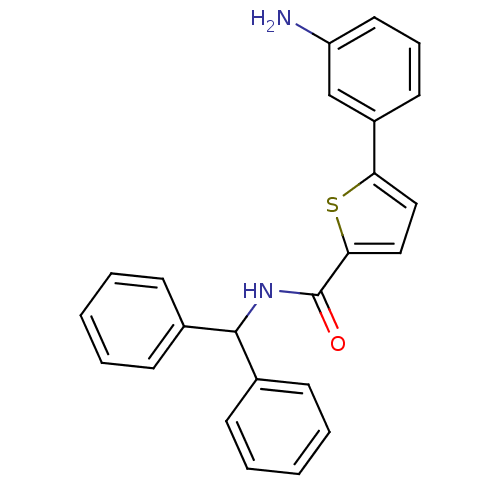
(5-(3-Aminophenyl)-N-benzhydrylthiophene-2-carboxam...)Show SMILES Nc1cccc(c1)-c1ccc(s1)C(=O)NC(c1ccccc1)c1ccccc1 Show InChI InChI=1S/C24H20N2OS/c25-20-13-7-12-19(16-20)21-14-15-22(28-21)24(27)26-23(17-8-3-1-4-9-17)18-10-5-2-6-11-18/h1-16,23H,25H2,(H,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Inhibition of human H-PGDS expressed in Escherichia coli BL21 DE2 by enzyme immuno assay |
J Med Chem 53: 5536-48 (2010)
Article DOI: 10.1021/jm100194a
BindingDB Entry DOI: 10.7270/Q21J99Z4 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(MOUSE) | BDBM50007916

(3-{1-[1-aminobutyl oxycarbonylamino-2-(1H-3-indoly...)Show SMILES CC(C)(C)OC(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CCCCNC(=O)Nc1ccccc1Cl)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccccc1)C(N)=O Show InChI InChI=1S/C42H51ClN8O9/c1-42(2,3)60-41(59)51-33(22-26-24-46-29-17-9-7-15-27(26)29)38(56)47-31(19-11-12-20-45-40(58)50-30-18-10-8-16-28(30)43)37(55)49-34(23-35(52)53)39(57)48-32(36(44)54)21-25-13-5-4-6-14-25/h4-10,13-18,24,31-34,46H,11-12,19-23H2,1-3H3,(H2,44,54)(H,47,56)(H,48,57)(H,49,55)(H,51,59)(H,52,53)(H2,45,50,58)/t31-,32-,33-,34-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of [125I]- Bolton-Hunter CCK-26-33 binding to Cholecystokinin type B receptor of mouse cerebral cortex |
Bioorg Med Chem Lett 3: 889-894 (1993)
Article DOI: 10.1016/S0960-894X(00)80687-3
BindingDB Entry DOI: 10.7270/Q28G8M63 |
More data for this
Ligand-Target Pair | |
Hematopoietic prostaglandin D synthase
(Homo sapiens (Human)) | BDBM50324484

(CHEMBL1215298 | N-(1-Amino-3-(1H-indol-3-yl)-1-oxo...)Show SMILES NC(=O)C(Cc1c[nH]c2ccccc12)NC(=O)c1ccc(s1)-c1cccc(O)c1 Show InChI InChI=1S/C22H19N3O3S/c23-21(27)18(11-14-12-24-17-7-2-1-6-16(14)17)25-22(28)20-9-8-19(29-20)13-4-3-5-15(26)10-13/h1-10,12,18,24,26H,11H2,(H2,23,27)(H,25,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Inhibition of human H-PGDS expressed in Escherichia coli BL21 DE2 by enzyme immuno assay |
J Med Chem 53: 5536-48 (2010)
Article DOI: 10.1021/jm100194a
BindingDB Entry DOI: 10.7270/Q21J99Z4 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(MOUSE) | BDBM50281741

(CHEMBL355275 | [1-(1H-Indol-3-ylmethyl)-1,2-bis-ph...)Show SMILES O=C(CC(Cc1c[nH]c2ccccc12)(NC(=O)OC1C2CC3CC(C2)CC1C3)C(=O)NCCc1ccccc1)NCCc1ccccc1 |TLB:17:18:20:23.24.22,THB:25:26:20:23.24.22,25:23:20:18.26.27,22:21:18:23.25.24,22:23:18:21.20.27,(12.05,-14.95,;12.05,-13.41,;10.72,-12.63,;9.38,-13.41,;8.04,-12.63,;6.71,-13.41,;6.53,-14.95,;5.03,-15.28,;4.26,-13.94,;2.77,-13.62,;2.28,-12.17,;3.3,-11.02,;4.82,-11.34,;5.29,-12.8,;9.38,-14.95,;9.38,-16.49,;8.04,-17.26,;10.72,-17.26,;12.25,-17.26,;13.1,-15.99,;14.64,-15.48,;15.51,-16.73,;17,-17.19,;15.3,-17.61,;14.46,-16.47,;14.48,-18.85,;13.1,-18.5,;14.64,-18.05,;9.36,-11.86,;8.02,-11.09,;10.69,-11.07,;10.67,-9.52,;12,-8.74,;11.99,-7.2,;10.65,-6.45,;10.62,-4.92,;11.96,-4.12,;13.3,-4.89,;13.31,-6.41,;13.38,-12.63,;14.71,-13.41,;16.06,-12.63,;17.4,-13.41,;17.4,-14.95,;18.73,-15.72,;20.07,-14.95,;20.06,-13.41,;18.73,-12.63,)| Show InChI InChI=1S/C40H46N4O4/c45-36(41-17-15-27-9-3-1-4-10-27)25-40(24-33-26-43-35-14-8-7-13-34(33)35,38(46)42-18-16-28-11-5-2-6-12-28)44-39(47)48-37-31-20-29-19-30(22-31)23-32(37)21-29/h1-14,26,29-32,37,43H,15-25H2,(H,41,45)(H,42,46)(H,44,47) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of [125I]- Bolton-Hunter CCK-26-33 binding to Cholecystokinin type B receptor of mouse cerebral cortex |
Bioorg Med Chem Lett 3: 889-894 (1993)
Article DOI: 10.1016/S0960-894X(00)80687-3
BindingDB Entry DOI: 10.7270/Q28G8M63 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(RAT) | BDBM50281744
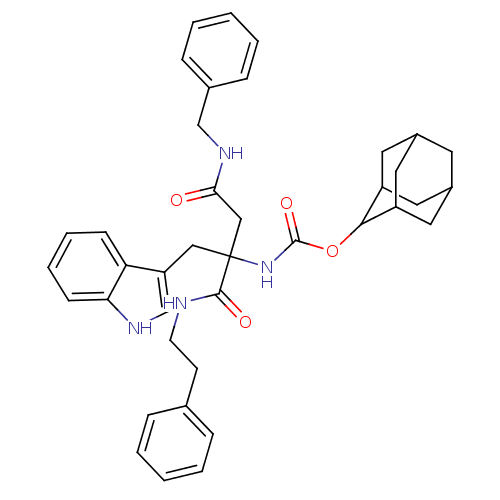
(CHEMBL171562 | [2-Benzylcarbamoyl-1-(1H-indol-3-yl...)Show SMILES O=C(CC(Cc1c[nH]c2ccccc12)(NC(=O)OC1C2CC3CC(C2)CC1C3)C(=O)NCCc1ccccc1)NCc1ccccc1 |TLB:17:18:20:23.24.22,THB:25:26:20:23.24.22,25:23:20:18.26.27,22:21:18:23.25.24,22:23:18:21.20.27,(8.74,-8.37,;8.74,-6.83,;7.41,-6.06,;6.06,-6.83,;4.73,-6.06,;3.4,-6.83,;3.23,-8.36,;1.73,-8.68,;.96,-7.35,;-.54,-7.04,;-1.02,-5.57,;.01,-4.43,;1.5,-4.75,;1.99,-6.2,;6.06,-8.37,;6.06,-9.91,;4.73,-10.69,;7.41,-10.69,;8.94,-10.67,;9.8,-9.41,;11.34,-8.89,;12.21,-10.13,;13.69,-10.6,;11.99,-11.02,;11.15,-9.87,;11.18,-12.26,;9.8,-11.92,;11.35,-11.46,;6.06,-5.27,;4.71,-4.51,;7.39,-4.49,;7.37,-2.95,;8.71,-2.16,;8.68,-.62,;10.02,.16,;9.99,1.7,;8.66,2.47,;7.33,1.68,;7.34,.13,;10.09,-6.06,;11.42,-6.83,;12.75,-6.06,;14.08,-6.83,;15.42,-6.06,;15.43,-4.51,;14.08,-3.74,;12.75,-4.52,)| Show InChI InChI=1S/C39H44N4O4/c44-35(42-24-27-11-5-2-6-12-27)23-39(22-32-25-41-34-14-8-7-13-33(32)34,37(45)40-16-15-26-9-3-1-4-10-26)43-38(46)47-36-30-18-28-17-29(20-30)21-31(36)19-28/h1-14,25,28-31,36,41H,15-24H2,(H,40,45)(H,42,44)(H,43,46) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 2.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of [125I]- Bolton-Hunter CCK-26-33 binding to Cholecystokinin type A receptor of rat pancreas |
Bioorg Med Chem Lett 3: 889-894 (1993)
Article DOI: 10.1016/S0960-894X(00)80687-3
BindingDB Entry DOI: 10.7270/Q28G8M63 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(MOUSE) | BDBM50005459

(4-(3,4-Dichloro-benzoylamino)-4-dipentylcarbamoyl-...)Show SMILES CCCCCN(CCCCC)C(=O)[C@@H](CCC(O)=O)NC(=O)c1ccc(Cl)c(Cl)c1 Show InChI InChI=1S/C22H32Cl2N2O4/c1-3-5-7-13-26(14-8-6-4-2)22(30)19(11-12-20(27)28)25-21(29)16-9-10-17(23)18(24)15-16/h9-10,15,19H,3-8,11-14H2,1-2H3,(H,25,29)(H,27,28)/t19-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of [125I]- Bolton-Hunter CCK-26-33 binding to Cholecystokinin type B receptor of mouse cerebral cortex |
Bioorg Med Chem Lett 3: 889-894 (1993)
Article DOI: 10.1016/S0960-894X(00)80687-3
BindingDB Entry DOI: 10.7270/Q28G8M63 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(RAT) | BDBM50281742

(CHEMBL434113 | [2-[2-(4-Hydroxy-phenyl)-ethylcarba...)Show SMILES Oc1ccc(CCNC(=O)CC(Cc2c[nH]c3ccccc23)(NC(=O)OC2C3CC4CC(C3)CC2C4)C(=O)NCCc2ccccc2)cc1 |TLB:25:26:28:31.32.30,THB:33:34:28:31.32.30,33:31:28:26.34.35,30:29:26:31.33.32,30:31:26:29.28.35,(20.72,-15.7,;19.37,-14.93,;18.04,-15.71,;16.7,-14.94,;16.7,-13.41,;15.37,-12.63,;14.03,-13.41,;12.7,-12.63,;11.35,-13.41,;11.35,-14.95,;10.02,-12.63,;8.68,-13.41,;7.35,-12.63,;6.02,-13.41,;5.85,-14.95,;4.33,-15.28,;3.56,-13.94,;2.07,-13.62,;1.59,-12.17,;2.62,-11.02,;4.12,-11.34,;4.59,-12.8,;8.68,-14.95,;8.68,-16.49,;7.35,-17.26,;10.02,-17.26,;11.56,-17.26,;12.4,-15.99,;13.96,-15.48,;14.82,-16.73,;16.3,-17.19,;14.6,-17.61,;13.77,-16.47,;13.8,-18.85,;12.42,-18.5,;13.96,-18.05,;8.67,-11.86,;7.33,-11.09,;9.99,-11.07,;9.98,-9.52,;11.32,-8.74,;11.3,-7.2,;9.95,-6.45,;9.94,-4.92,;11.27,-4.12,;12.61,-4.89,;12.63,-6.41,;18.04,-12.63,;19.37,-13.41,)| Show InChI InChI=1S/C40H46N4O5/c45-33-12-10-27(11-13-33)14-16-41-36(46)24-40(23-32-25-43-35-9-5-4-8-34(32)35,38(47)42-17-15-26-6-2-1-3-7-26)44-39(48)49-37-30-19-28-18-29(21-30)22-31(37)20-28/h1-13,25,28-31,37,43,45H,14-24H2,(H,41,46)(H,42,47)(H,44,48) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 3.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of [125I]- Bolton-Hunter CCK-26-33 binding to Cholecystokinin type A receptor of rat pancreas |
Bioorg Med Chem Lett 3: 889-894 (1993)
Article DOI: 10.1016/S0960-894X(00)80687-3
BindingDB Entry DOI: 10.7270/Q28G8M63 |
More data for this
Ligand-Target Pair | |
Hematopoietic prostaglandin D synthase
(Homo sapiens (Human)) | BDBM50324486
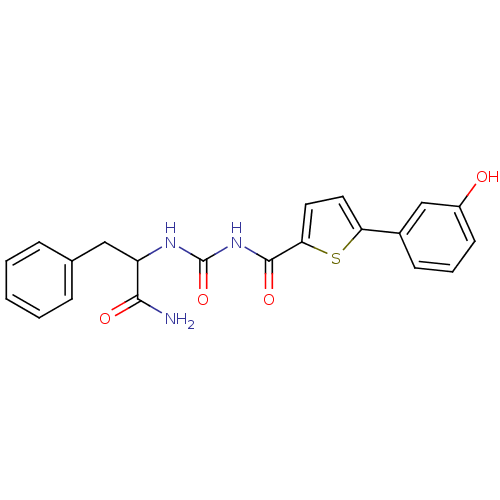
(CHEMBL1215296 | N-(1-Amino-1-oxo-3-phenylpropan-2-...)Show SMILES NC(=O)C(Cc1ccccc1)NC(=O)NC(=O)c1ccc(s1)-c1cccc(O)c1 Show InChI InChI=1S/C21H19N3O4S/c22-19(26)16(11-13-5-2-1-3-6-13)23-21(28)24-20(27)18-10-9-17(29-18)14-7-4-8-15(25)12-14/h1-10,12,16,25H,11H2,(H2,22,26)(H2,23,24,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Inhibition of human H-PGDS expressed in Escherichia coli BL21 DE2 by enzyme immuno assay |
J Med Chem 53: 5536-48 (2010)
Article DOI: 10.1021/jm100194a
BindingDB Entry DOI: 10.7270/Q21J99Z4 |
More data for this
Ligand-Target Pair | |
Hematopoietic prostaglandin D synthase
(Homo sapiens (Human)) | BDBM50300128

(1-(3-(1H-tetrazol-5-yl)propyl)-4-(benzhydryloxy)pi...)Show SMILES C(CN1CCC(CC1)OC(c1ccccc1)c1ccccc1)Cc1nnn[nH]1 Show InChI InChI=1S/C22H27N5O/c1-3-8-18(9-4-1)22(19-10-5-2-6-11-19)28-20-13-16-27(17-14-20)15-7-12-21-23-25-26-24-21/h1-6,8-11,20,22H,7,12-17H2,(H,23,24,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
| MMDB
PDB
Article
PubMed
| n/a | n/a | 3.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Inhibition of human H-PGDS expressed in Escherichia coli BL21 DE2 by enzyme immuno assay |
J Med Chem 53: 5536-48 (2010)
Article DOI: 10.1021/jm100194a
BindingDB Entry DOI: 10.7270/Q21J99Z4 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Hematopoietic prostaglandin D synthase
(Homo sapiens (Human)) | BDBM50324483
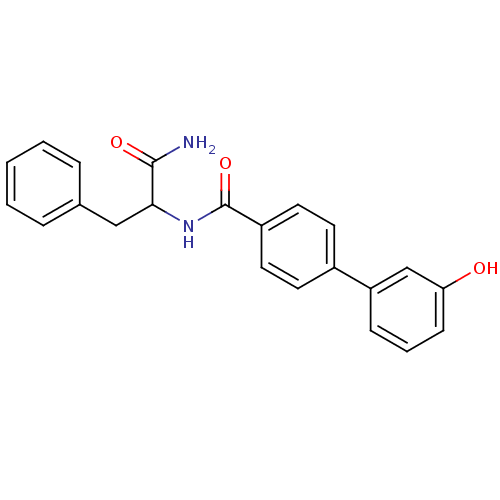
(CHEMBL1215369 | N-(1-amino-1-oxo-3-phenylpropan-2-...)Show SMILES NC(=O)C(Cc1ccccc1)NC(=O)c1ccc(cc1)-c1cccc(O)c1 Show InChI InChI=1S/C22H20N2O3/c23-21(26)20(13-15-5-2-1-3-6-15)24-22(27)17-11-9-16(10-12-17)18-7-4-8-19(25)14-18/h1-12,14,20,25H,13H2,(H2,23,26)(H,24,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Inhibition of human H-PGDS expressed in Escherichia coli BL21 DE2 by enzyme immuno assay |
J Med Chem 53: 5536-48 (2010)
Article DOI: 10.1021/jm100194a
BindingDB Entry DOI: 10.7270/Q21J99Z4 |
More data for this
Ligand-Target Pair | |
Hematopoietic prostaglandin D synthase
(Homo sapiens (Human)) | BDBM50324487
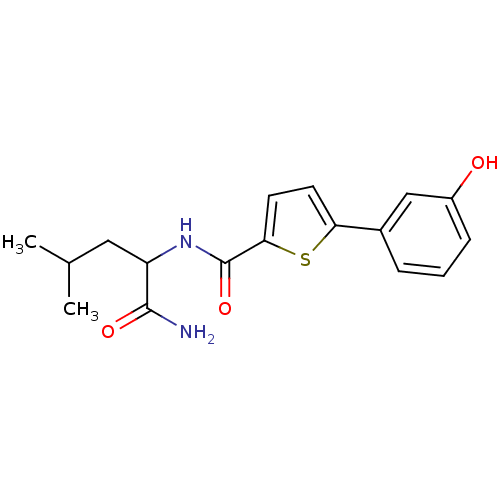
(CHEMBL1215295 | N-(1-Amino-4-methyl-1-oxopentan-2-...)Show InChI InChI=1S/C17H20N2O3S/c1-10(2)8-13(16(18)21)19-17(22)15-7-6-14(23-15)11-4-3-5-12(20)9-11/h3-7,9-10,13,20H,8H2,1-2H3,(H2,18,21)(H,19,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Inhibition of human H-PGDS expressed in Escherichia coli BL21 DE2 by enzyme immuno assay |
J Med Chem 53: 5536-48 (2010)
Article DOI: 10.1021/jm100194a
BindingDB Entry DOI: 10.7270/Q21J99Z4 |
More data for this
Ligand-Target Pair | |
Hematopoietic prostaglandin D synthase
(Homo sapiens (Human)) | BDBM50324488
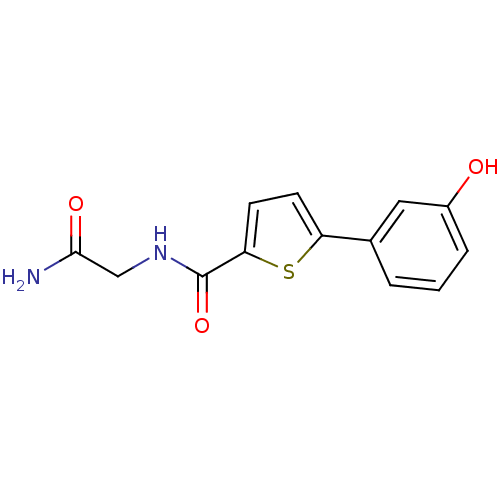
(CHEMBL1215294 | N-(2-Amino-2-oxoethyl)-5-(3-hydrox...)Show InChI InChI=1S/C13H12N2O3S/c14-12(17)7-15-13(18)11-5-4-10(19-11)8-2-1-3-9(16)6-8/h1-6,16H,7H2,(H2,14,17)(H,15,18) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Inhibition of human H-PGDS expressed in Escherichia coli BL21 DE2 by enzyme immuno assay |
J Med Chem 53: 5536-48 (2010)
Article DOI: 10.1021/jm100194a
BindingDB Entry DOI: 10.7270/Q21J99Z4 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(MOUSE) | BDBM50281744

(CHEMBL171562 | [2-Benzylcarbamoyl-1-(1H-indol-3-yl...)Show SMILES O=C(CC(Cc1c[nH]c2ccccc12)(NC(=O)OC1C2CC3CC(C2)CC1C3)C(=O)NCCc1ccccc1)NCc1ccccc1 |TLB:17:18:20:23.24.22,THB:25:26:20:23.24.22,25:23:20:18.26.27,22:21:18:23.25.24,22:23:18:21.20.27,(8.74,-8.37,;8.74,-6.83,;7.41,-6.06,;6.06,-6.83,;4.73,-6.06,;3.4,-6.83,;3.23,-8.36,;1.73,-8.68,;.96,-7.35,;-.54,-7.04,;-1.02,-5.57,;.01,-4.43,;1.5,-4.75,;1.99,-6.2,;6.06,-8.37,;6.06,-9.91,;4.73,-10.69,;7.41,-10.69,;8.94,-10.67,;9.8,-9.41,;11.34,-8.89,;12.21,-10.13,;13.69,-10.6,;11.99,-11.02,;11.15,-9.87,;11.18,-12.26,;9.8,-11.92,;11.35,-11.46,;6.06,-5.27,;4.71,-4.51,;7.39,-4.49,;7.37,-2.95,;8.71,-2.16,;8.68,-.62,;10.02,.16,;9.99,1.7,;8.66,2.47,;7.33,1.68,;7.34,.13,;10.09,-6.06,;11.42,-6.83,;12.75,-6.06,;14.08,-6.83,;15.42,-6.06,;15.43,-4.51,;14.08,-3.74,;12.75,-4.52,)| Show InChI InChI=1S/C39H44N4O4/c44-35(42-24-27-11-5-2-6-12-27)23-39(22-32-25-41-34-14-8-7-13-33(32)34,37(45)40-16-15-26-9-3-1-4-10-26)43-38(46)47-36-30-18-28-17-29(20-30)21-31(36)19-28/h1-14,25,28-31,36,41H,15-24H2,(H,40,45)(H,42,44)(H,43,46) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 8.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of [125I]- Bolton-Hunter CCK-26-33 binding to Cholecystokinin type B receptor of mouse cerebral cortex |
Bioorg Med Chem Lett 3: 889-894 (1993)
Article DOI: 10.1016/S0960-894X(00)80687-3
BindingDB Entry DOI: 10.7270/Q28G8M63 |
More data for this
Ligand-Target Pair | |
Hematopoietic prostaglandin D synthase
(Homo sapiens (Human)) | BDBM50324489
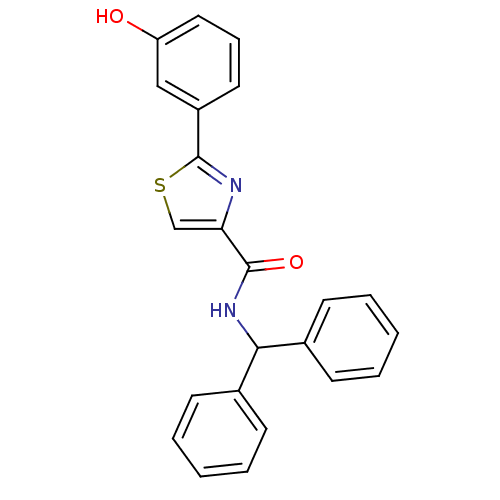
(CHEMBL1215162 | N-benzhydryl-2-(3-hydroxyphenyl)th...)Show SMILES Oc1cccc(c1)-c1nc(cs1)C(=O)NC(c1ccccc1)c1ccccc1 Show InChI InChI=1S/C23H18N2O2S/c26-19-13-7-12-18(14-19)23-24-20(15-28-23)22(27)25-21(16-8-3-1-4-9-16)17-10-5-2-6-11-17/h1-15,21,26H,(H,25,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.08E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Inhibition of human H-PGDS expressed in Escherichia coli BL21 DE2 by enzyme immuno assay |
J Med Chem 53: 5536-48 (2010)
Article DOI: 10.1021/jm100194a
BindingDB Entry DOI: 10.7270/Q21J99Z4 |
More data for this
Ligand-Target Pair | |
Hematopoietic prostaglandin D synthase
(Homo sapiens (Human)) | BDBM50300127
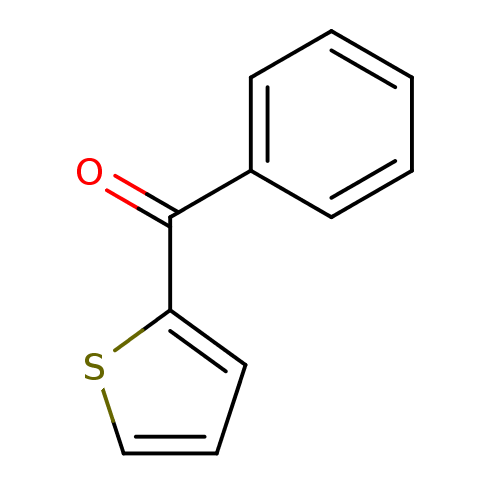
(CHEMBL567059 | NSC-4502 | phenyl(thiophen-2-yl)met...)Show InChI InChI=1S/C11H8OS/c12-11(10-7-4-8-13-10)9-5-2-1-3-6-9/h1-8H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 1.28E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Inhibition of human H-PGDS expressed in Escherichia coli BL21 DE2 by enzyme immuno assay |
J Med Chem 53: 5536-48 (2010)
Article DOI: 10.1021/jm100194a
BindingDB Entry DOI: 10.7270/Q21J99Z4 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(MOUSE) | BDBM50281746

(CHEMBL352817 | [1-(1H-Indol-3-ylmethyl)-1-phenethy...)Show SMILES O=C(CC(Cc1c[nH]c2ccccc12)(NC(=O)OC1C2CC3CC(C2)CC1C3)C(=O)NCCc1ccccc1)NCCCc1ccccc1 |TLB:17:18:20:23.24.22,THB:25:26:20:23.24.22,25:23:20:18.26.27,22:21:18:23.25.24,22:23:18:21.20.27,(11.37,-14.95,;11.37,-13.41,;10.04,-12.63,;8.71,-13.41,;7.37,-12.63,;6.04,-13.41,;5.87,-14.95,;4.36,-15.28,;3.59,-13.94,;2.09,-13.62,;1.61,-12.17,;2.64,-11.02,;4.15,-11.34,;4.61,-12.8,;8.71,-14.95,;8.71,-16.49,;7.37,-17.26,;10.04,-17.26,;11.58,-17.26,;12.42,-15.99,;13.97,-15.48,;14.85,-16.73,;16.33,-17.19,;14.62,-17.61,;13.78,-16.47,;13.82,-18.85,;12.44,-18.5,;13.98,-18.05,;8.7,-11.86,;7.35,-11.09,;10.02,-11.07,;10.01,-9.52,;11.34,-8.74,;11.32,-7.2,;12.65,-6.41,;12.63,-4.89,;11.29,-4.12,;9.96,-4.92,;9.97,-6.45,;12.72,-12.63,;14.05,-13.41,;15.39,-12.63,;16.73,-13.41,;18.06,-12.63,;19.39,-13.41,;20.72,-12.65,;20.72,-11.09,;19.39,-10.32,;18.06,-11.11,)| Show InChI InChI=1S/C41H48N4O4/c46-37(42-18-9-14-28-10-3-1-4-11-28)26-41(25-34-27-44-36-16-8-7-15-35(34)36,39(47)43-19-17-29-12-5-2-6-13-29)45-40(48)49-38-32-21-30-20-31(23-32)24-33(38)22-30/h1-8,10-13,15-16,27,30-33,38,44H,9,14,17-26H2,(H,42,46)(H,43,47)(H,45,48) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 1.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of [125I]- Bolton-Hunter CCK-26-33 binding to Cholecystokinin type B receptor of mouse cerebral cortex |
Bioorg Med Chem Lett 3: 889-894 (1993)
Article DOI: 10.1016/S0960-894X(00)80687-3
BindingDB Entry DOI: 10.7270/Q28G8M63 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(MOUSE) | BDBM50089109

(CHEMBL278780 | [2-{4-[3-(2-Chloro-phenyl)-ureido]-...)Show SMILES Clc1ccccc1NC(=O)NCCCCNC(=O)CC(Cc1c[nH]c2ccccc12)(NC(=O)OC1C2CC3CC(C2)CC1C3)C(=O)NCCc1ccccc1 |TLB:40:35:43:39.41.38,40:39:34.35.36:43,THB:38:37:34:39.40.41,38:39:34:37.36.43,33:34:43:39.41.38,(16.48,3.44,;15.71,4.78,;16.5,6.1,;15.71,7.43,;14.15,7.43,;13.4,6.1,;14.15,4.78,;13.39,3.45,;11.83,3.45,;11.06,4.78,;11.06,2.11,;9.51,2.11,;8.73,.78,;7.2,.78,;6.42,-.55,;4.87,-.55,;3.3,-.9,;2.31,.04,;2.59,-2.41,;3.08,-4.15,;2.79,-5.76,;3.61,-7.11,;2.8,-8.42,;3.82,-9.57,;5.23,-8.99,;6.62,-9.63,;7.86,-8.76,;7.75,-7.24,;6.35,-6.58,;5.1,-7.45,;1.8,-3.33,;.42,-4.02,;.34,-5.56,;-.87,-3.18,;-2.27,-3.89,;-3.58,-3.13,;-4.77,-4.23,;-4.95,-5.81,;-6.51,-6.05,;-5.16,-4.92,;-4.98,-3.46,;-3.9,-5.58,;-2.46,-5.4,;-3.62,-6.53,;4.48,-3.46,;4.56,-1.94,;5.78,-4.31,;7.14,-3.62,;8.45,-4.46,;9.83,-3.76,;11.14,-4.59,;12.52,-3.89,;12.58,-2.36,;11.29,-1.52,;9.91,-2.23,)| Show InChI InChI=1S/C43H51ClN6O5/c44-35-13-5-7-15-37(35)49-41(53)47-18-9-8-17-45-38(51)26-43(25-33-27-48-36-14-6-4-12-34(33)36,40(52)46-19-16-28-10-2-1-3-11-28)50-42(54)55-39-31-21-29-20-30(23-31)24-32(39)22-29/h1-7,10-15,27,29-32,39,48H,8-9,16-26H2,(H,45,51)(H,46,52)(H,50,54)(H2,47,49,53) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 1.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of [125I]- Bolton-Hunter CCK-26-33 binding to Cholecystokinin type B receptor of mouse cerebral cortex |
Bioorg Med Chem Lett 3: 889-894 (1993)
Article DOI: 10.1016/S0960-894X(00)80687-3
BindingDB Entry DOI: 10.7270/Q28G8M63 |
More data for this
Ligand-Target Pair | |
Hematopoietic prostaglandin D synthase
(Homo sapiens (Human)) | BDBM50324479
(5-amino-4-(3'-hydroxy-[1,1'-biphenyl]-4-ylcarboxam...)Show SMILES NC(=O)C(CCC(O)=O)NC(=O)c1cccc(c1)-c1cccc(O)c1 Show InChI InChI=1S/C18H18N2O5/c19-17(24)15(7-8-16(22)23)20-18(25)13-5-1-3-11(9-13)12-4-2-6-14(21)10-12/h1-6,9-10,15,21H,7-8H2,(H2,19,24)(H,20,25)(H,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Inhibition of human H-PGDS expressed in Escherichia coli BL21 DE2 by enzyme immuno assay |
J Med Chem 53: 5536-48 (2010)
Article DOI: 10.1021/jm100194a
BindingDB Entry DOI: 10.7270/Q21J99Z4 |
More data for this
Ligand-Target Pair | |
Hematopoietic prostaglandin D synthase
(Homo sapiens (Human)) | BDBM50324481

(CHEMBL1215446 | N-(1-amino-1-oxo-3-phenylpropan-2-...)Show SMILES NC(=O)C(Cc1ccccc1)NC(=O)c1ccc(cc1O)-c1cccc(O)c1 Show InChI InChI=1S/C22H20N2O4/c23-21(27)19(11-14-5-2-1-3-6-14)24-22(28)18-10-9-16(13-20(18)26)15-7-4-8-17(25)12-15/h1-10,12-13,19,25-26H,11H2,(H2,23,27)(H,24,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.48E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Inhibition of human H-PGDS expressed in Escherichia coli BL21 DE2 by enzyme immuno assay |
J Med Chem 53: 5536-48 (2010)
Article DOI: 10.1021/jm100194a
BindingDB Entry DOI: 10.7270/Q21J99Z4 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data