

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| cAMP-specific 3',5'-cyclic phosphodiesterase 4D (Homo sapiens (Human)) | BDBM50085135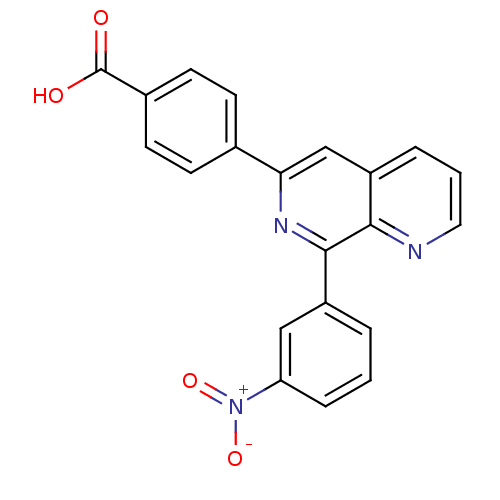 (4-(8-(3-nitrophenyl)-1,7-naphthyridin-6-yl)benzoic...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG Curated by ChEMBL | Assay Description Inhibitory activity against Phosphodiesterase 4D (PDE4D) from human source expressed in Saccharomyces cerevisiae | J Med Chem 43: 675-82 (2000) BindingDB Entry DOI: 10.7270/Q2N58N3G | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4D (Homo sapiens (Human)) | BDBM50085136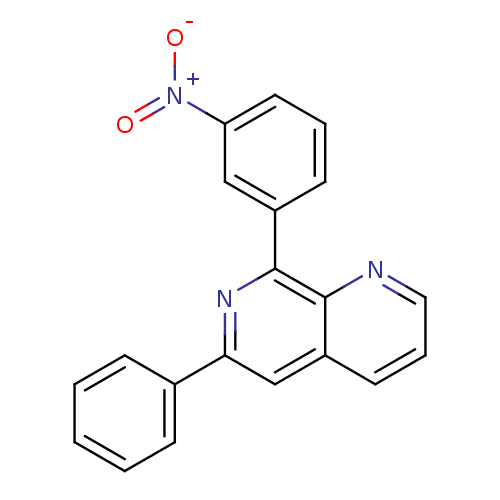 (8-(3-Nitro-phenyl)-6-phenyl-[1,7]naphthyridine | C...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG Curated by ChEMBL | Assay Description Inhibitory activity against Phosphodiesterase 4D (PDE4D) from human source expressed in Saccharomyces cerevisiae | J Med Chem 43: 675-82 (2000) BindingDB Entry DOI: 10.7270/Q2N58N3G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4D (Homo sapiens (Human)) | BDBM50085138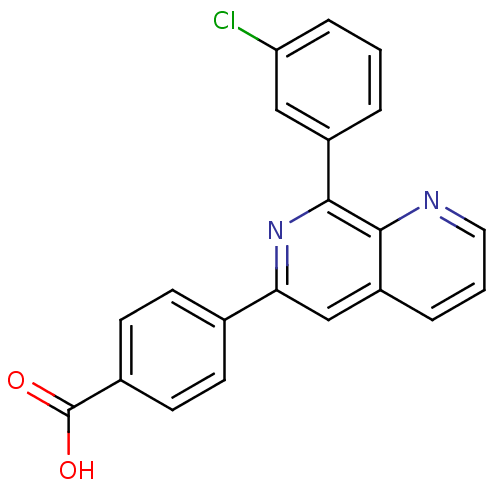 (4-[8-(3-Chloro-phenyl)-[1,7]naphthyridin-6-yl]-ben...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG Curated by ChEMBL | Assay Description Inhibitory activity against Phosphodiesterase 4D (PDE4D) from human source expressed in Saccharomyces cerevisiae | J Med Chem 43: 675-82 (2000) BindingDB Entry DOI: 10.7270/Q2N58N3G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4D (Homo sapiens (Human)) | BDBM50085146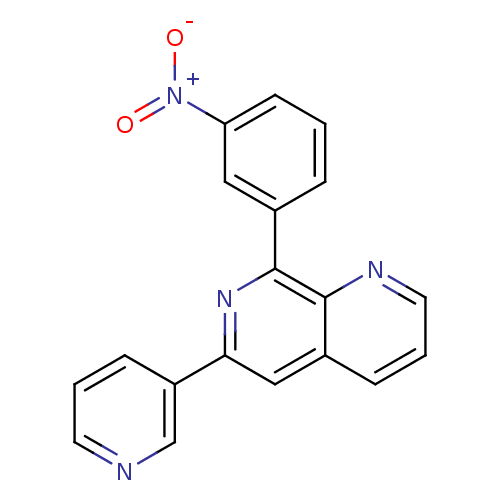 (8-(3-Nitro-phenyl)-6-pyridin-3-yl-[1,7]naphthyridi...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG Curated by ChEMBL | Assay Description Inhibitory activity against Phosphodiesterase 4D (PDE4D) from human source expressed in Saccharomyces cerevisiae | J Med Chem 43: 675-82 (2000) BindingDB Entry DOI: 10.7270/Q2N58N3G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4D (Homo sapiens (Human)) | BDBM50085139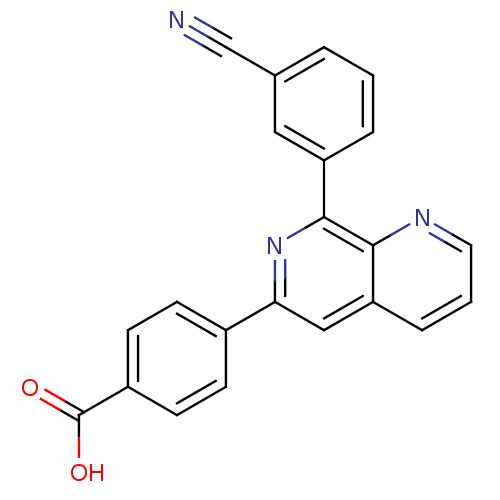 (4-[8-(3-Cyano-phenyl)-[1,7]naphthyridin-6-yl]-benz...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG Curated by ChEMBL | Assay Description Inhibitory activity against Phosphodiesterase 4D (PDE4D) from human source expressed in Saccharomyces cerevisiae | J Med Chem 43: 675-82 (2000) BindingDB Entry DOI: 10.7270/Q2N58N3G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4B (RAT) | BDBM14361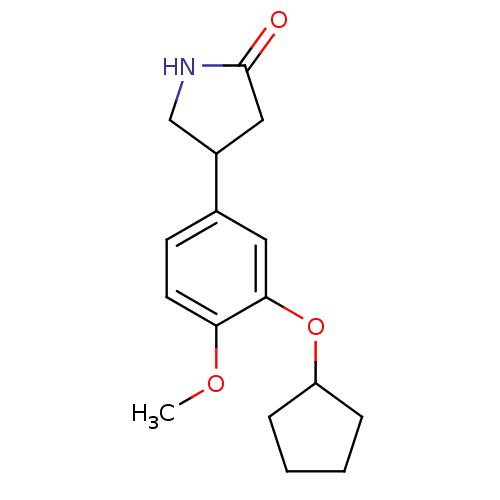 ((R,S)-Rolipram | 4-(3-cyclopentyloxy-4-methoxy-phe...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | n/a | n/a | 5.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Horsham Research Center Curated by ChEMBL | Assay Description Inhibition of phosphodiesterase (PDE) 4B | Bioorg Med Chem Lett 8: 3229-34 (1999) BindingDB Entry DOI: 10.7270/Q2ZS2Z11 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4A (Homo sapiens (Human)) | BDBM14361 ((R,S)-Rolipram | 4-(3-cyclopentyloxy-4-methoxy-phe...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 5.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Horsham Research Center Curated by ChEMBL | Assay Description Inhibition of phosphodiesterase (PDE) 4A | Bioorg Med Chem Lett 8: 3229-34 (1999) BindingDB Entry DOI: 10.7270/Q2ZS2Z11 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4D (Homo sapiens (Human)) | BDBM14361 ((R,S)-Rolipram | 4-(3-cyclopentyloxy-4-methoxy-phe...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | n/a | n/a | 6.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Horsham Research Center Curated by ChEMBL | Assay Description Inhibition of phosphodiesterase (PDE) 4D | Bioorg Med Chem Lett 8: 3229-34 (1999) BindingDB Entry DOI: 10.7270/Q2ZS2Z11 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4D (Homo sapiens (Human)) | BDBM50085140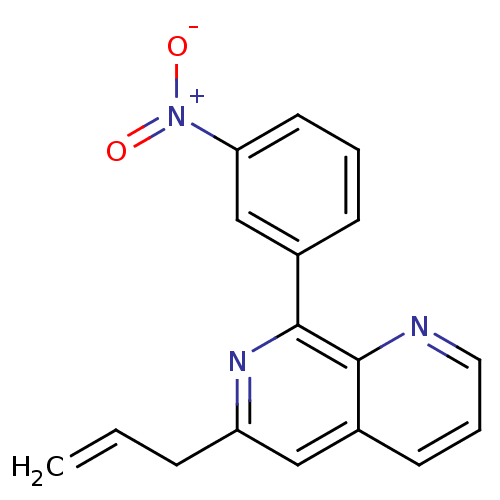 (6-Allyl-8-(3-nitro-phenyl)-[1,7]naphthyridine | CH...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG Curated by ChEMBL | Assay Description Inhibitory activity against Phosphodiesterase 4D (PDE4D) from human source expressed in Saccharomyces cerevisiae | J Med Chem 43: 675-82 (2000) BindingDB Entry DOI: 10.7270/Q2N58N3G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4B (RAT) | BDBM50450772 (CHEMBL322092) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 7.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Horsham Research Center Curated by ChEMBL | Assay Description Inhibition of phosphodiesterase (PDE) 4B | Bioorg Med Chem Lett 8: 3229-34 (1999) BindingDB Entry DOI: 10.7270/Q2ZS2Z11 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4A (Homo sapiens (Human)) | BDBM50450772 (CHEMBL322092) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Horsham Research Center Curated by ChEMBL | Assay Description Inhibition of phosphodiesterase (PDE) 4A | Bioorg Med Chem Lett 8: 3229-34 (1999) BindingDB Entry DOI: 10.7270/Q2ZS2Z11 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4D (Homo sapiens (Human)) | BDBM50450772 (CHEMBL322092) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Horsham Research Center Curated by ChEMBL | Assay Description Inhibition of phosphodiesterase (PDE) 4D | Bioorg Med Chem Lett 8: 3229-34 (1999) BindingDB Entry DOI: 10.7270/Q2ZS2Z11 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4D (Homo sapiens (Human)) | BDBM50450770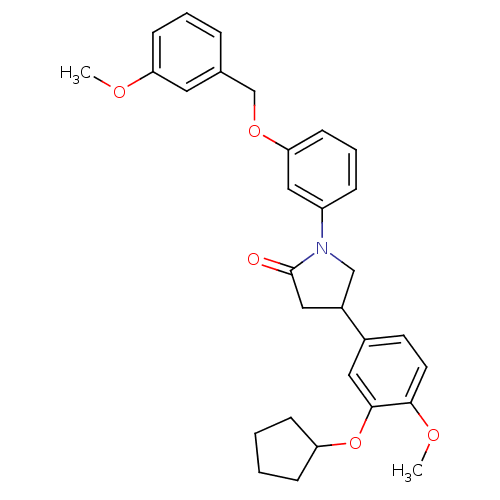 (CHEMBL320307) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 7.94 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Horsham Research Center Curated by ChEMBL | Assay Description Inhibition of phosphodiesterase (PDE) 4D | Bioorg Med Chem Lett 8: 3229-34 (1999) BindingDB Entry DOI: 10.7270/Q2ZS2Z11 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4D (Homo sapiens (Human)) | BDBM50085137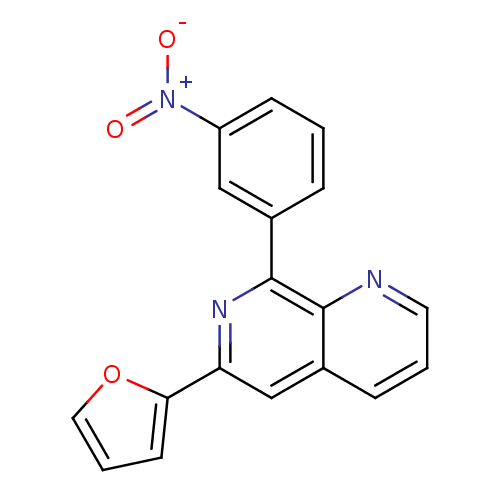 (6-Furan-2-yl-8-(3-nitro-phenyl)-[1,7]naphthyridine...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG Curated by ChEMBL | Assay Description Inhibitory activity against Phosphodiesterase 4D (PDE4D) from human source expressed in Saccharomyces cerevisiae | J Med Chem 43: 675-82 (2000) BindingDB Entry DOI: 10.7270/Q2N58N3G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4A (Homo sapiens (Human)) | BDBM50450770 (CHEMBL320307) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 8.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Horsham Research Center Curated by ChEMBL | Assay Description Inhibition of phosphodiesterase (PDE) 4A | Bioorg Med Chem Lett 8: 3229-34 (1999) BindingDB Entry DOI: 10.7270/Q2ZS2Z11 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4B (RAT) | BDBM50450771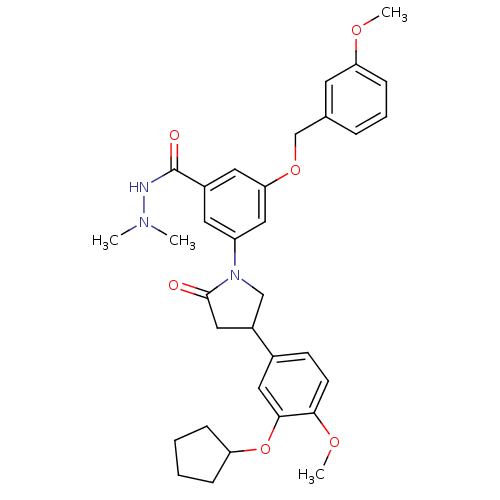 (CHEMBL326655) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 8.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Horsham Research Center Curated by ChEMBL | Assay Description Inhibition of phosphodiesterase (PDE) 4B | Bioorg Med Chem Lett 8: 3229-34 (1999) BindingDB Entry DOI: 10.7270/Q2ZS2Z11 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4A (Homo sapiens (Human)) | BDBM50450771 (CHEMBL326655) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 8.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Horsham Research Center Curated by ChEMBL | Assay Description Inhibition of phosphodiesterase (PDE) 4A | Bioorg Med Chem Lett 8: 3229-34 (1999) BindingDB Entry DOI: 10.7270/Q2ZS2Z11 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4B (RAT) | BDBM50450770 (CHEMBL320307) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 8.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Horsham Research Center Curated by ChEMBL | Assay Description Inhibition of phosphodiesterase (PDE) 4B | Bioorg Med Chem Lett 8: 3229-34 (1999) BindingDB Entry DOI: 10.7270/Q2ZS2Z11 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4D (Homo sapiens (Human)) | BDBM50450771 (CHEMBL326655) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 8.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Horsham Research Center Curated by ChEMBL | Assay Description Inhibition of phosphodiesterase (PDE) 4D | Bioorg Med Chem Lett 8: 3229-34 (1999) BindingDB Entry DOI: 10.7270/Q2ZS2Z11 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4D (Homo sapiens (Human)) | BDBM50085141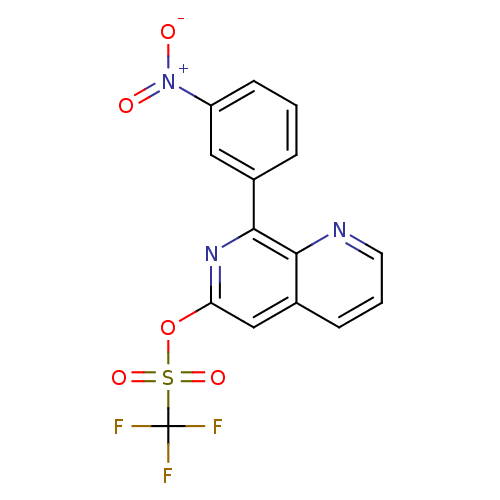 (CHEMBL351900 | Trifluoro-methanesulfonic acid 8-(3...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG Curated by ChEMBL | Assay Description Inhibitory activity against Phosphodiesterase 4D (PDE4D) from human source expressed in Saccharomyces cerevisiae | J Med Chem 43: 675-82 (2000) BindingDB Entry DOI: 10.7270/Q2N58N3G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4B (RAT) | BDBM50085138 (4-[8-(3-Chloro-phenyl)-[1,7]naphthyridin-6-yl]-ben...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG Curated by ChEMBL | Assay Description Inhibitory activity against Phosphodiesterase 4B (PDE4B) from rat source expressed in Saccharomyces cerevisiae | J Med Chem 43: 675-82 (2000) BindingDB Entry DOI: 10.7270/Q2N58N3G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4D (Homo sapiens (Human)) | BDBM50085145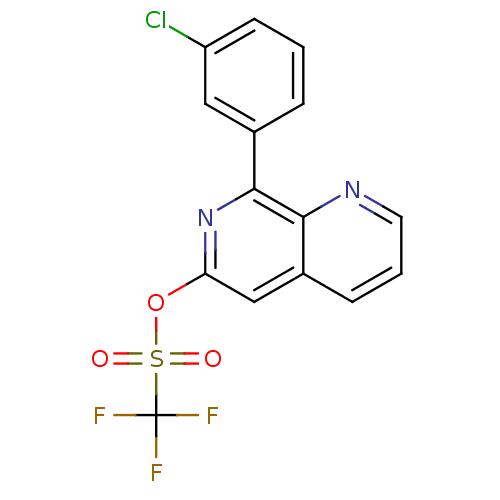 (CHEMBL161714 | Trifluoro-methanesulfonic acid 8-(3...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 48 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG Curated by ChEMBL | Assay Description Inhibitory activity against Phosphodiesterase 4D (PDE4D) from human source expressed in Saccharomyces cerevisiae | J Med Chem 43: 675-82 (2000) BindingDB Entry DOI: 10.7270/Q2N58N3G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4B (RAT) | BDBM50085135 (4-(8-(3-nitrophenyl)-1,7-naphthyridin-6-yl)benzoic...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | n/a | n/a | 49 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG Curated by ChEMBL | Assay Description Inhibitory activity against Phosphodiesterase 4B (PDE4B) from rat source expressed in Saccharomyces cerevisiae | J Med Chem 43: 675-82 (2000) BindingDB Entry DOI: 10.7270/Q2N58N3G | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4A (Homo sapiens (Human)) | BDBM50085138 (4-[8-(3-Chloro-phenyl)-[1,7]naphthyridin-6-yl]-ben...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 59 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG Curated by ChEMBL | Assay Description Inhibitory activity against Phosphodiesterase 4A (PDE4A) from human source expressed in Saccharomyces cerevisiae | J Med Chem 43: 675-82 (2000) BindingDB Entry DOI: 10.7270/Q2N58N3G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4C (Homo sapiens (Human)) | BDBM50085135 (4-(8-(3-nitrophenyl)-1,7-naphthyridin-6-yl)benzoic...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | n/a | n/a | 68 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG Curated by ChEMBL | Assay Description Inhibitory activity against Phosphodiesterase 4C (PDE4C) from human source expressed in Saccharomyces cerevisiae | J Med Chem 43: 675-82 (2000) BindingDB Entry DOI: 10.7270/Q2N58N3G | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4D (Homo sapiens (Human)) | BDBM50085147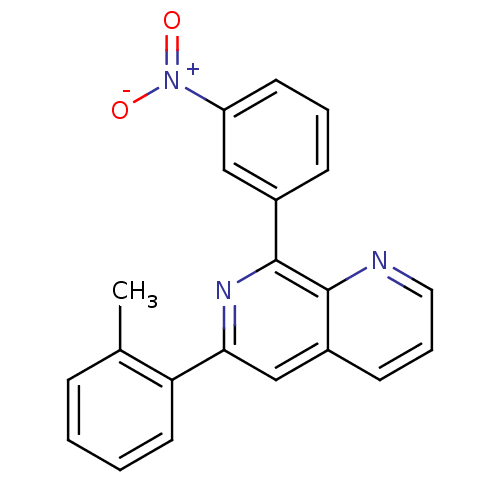 (8-(3-Nitro-phenyl)-6-o-tolyl-[1,7]naphthyridine | ...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG Curated by ChEMBL | Assay Description Inhibitory activity against Phosphodiesterase 4D (PDE4D) from human source expressed in Saccharomyces cerevisiae | J Med Chem 43: 675-82 (2000) BindingDB Entry DOI: 10.7270/Q2N58N3G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4D (Homo sapiens (Human)) | BDBM50085143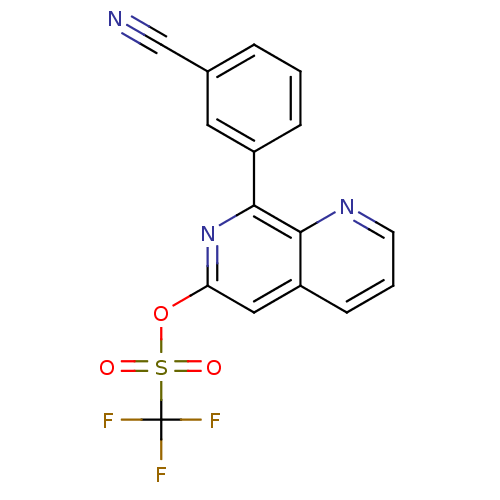 (CHEMBL158286 | Trifluoro-methanesulfonic acid 8-(3...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 72 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG Curated by ChEMBL | Assay Description Inhibitory activity against Phosphodiesterase 4D (PDE4D) from human source expressed in Saccharomyces cerevisiae | J Med Chem 43: 675-82 (2000) BindingDB Entry DOI: 10.7270/Q2N58N3G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4D (Homo sapiens (Human)) | BDBM50346088 ((1r,4r)-4-cyano-4-(3-(cyclopentyloxy)-4-methoxyphe...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | n/a | n/a | 79 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG Curated by ChEMBL | Assay Description Inhibitory activity against Phosphodiesterase 4D (PDE4D) from human source expressed in Saccharomyces cerevisiae | J Med Chem 43: 675-82 (2000) BindingDB Entry DOI: 10.7270/Q2N58N3G | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4B (RAT) | BDBM50085146 (8-(3-Nitro-phenyl)-6-pyridin-3-yl-[1,7]naphthyridi...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 81 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG Curated by ChEMBL | Assay Description Inhibitory activity against Phosphodiesterase 4B (PDE4B) from rat source expressed in Saccharomyces cerevisiae | J Med Chem 43: 675-82 (2000) BindingDB Entry DOI: 10.7270/Q2N58N3G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4B (RAT) | BDBM50085139 (4-[8-(3-Cyano-phenyl)-[1,7]naphthyridin-6-yl]-benz...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | n/a | n/a | 85 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG Curated by ChEMBL | Assay Description Inhibitory activity against Phosphodiesterase 4B (PDE4B) from rat source expressed in Saccharomyces cerevisiae | J Med Chem 43: 675-82 (2000) BindingDB Entry DOI: 10.7270/Q2N58N3G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4A (Homo sapiens (Human)) | BDBM50085135 (4-(8-(3-nitrophenyl)-1,7-naphthyridin-6-yl)benzoic...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB PubMed | n/a | n/a | 88 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG Curated by ChEMBL | Assay Description Inhibitory activity against Phosphodiesterase 4A (PDE4A) from human source expressed in Saccharomyces cerevisiae | J Med Chem 43: 675-82 (2000) BindingDB Entry DOI: 10.7270/Q2N58N3G | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4B (RAT) | BDBM50085136 (8-(3-Nitro-phenyl)-6-phenyl-[1,7]naphthyridine | C...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG Curated by ChEMBL | Assay Description Inhibitory activity against Phosphodiesterase 4B (PDE4B) from rat source expressed in Saccharomyces cerevisiae | J Med Chem 43: 675-82 (2000) BindingDB Entry DOI: 10.7270/Q2N58N3G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4A (Homo sapiens (Human)) | BDBM50085146 (8-(3-Nitro-phenyl)-6-pyridin-3-yl-[1,7]naphthyridi...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG Curated by ChEMBL | Assay Description Inhibitory activity against Phosphodiesterase 4A (PDE4A) from human source expressed in Saccharomyces cerevisiae | J Med Chem 43: 675-82 (2000) BindingDB Entry DOI: 10.7270/Q2N58N3G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4A (Homo sapiens (Human)) | BDBM50085139 (4-[8-(3-Cyano-phenyl)-[1,7]naphthyridin-6-yl]-benz...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG Curated by ChEMBL | Assay Description Inhibitory activity against Phosphodiesterase 4A (PDE4A) from human source expressed in Saccharomyces cerevisiae | J Med Chem 43: 675-82 (2000) BindingDB Entry DOI: 10.7270/Q2N58N3G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4B (RAT) | BDBM50085140 (6-Allyl-8-(3-nitro-phenyl)-[1,7]naphthyridine | CH...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG Curated by ChEMBL | Assay Description Inhibitory activity against Phosphodiesterase 4B (PDE4B) from rat source expressed in Saccharomyces cerevisiae | J Med Chem 43: 675-82 (2000) BindingDB Entry DOI: 10.7270/Q2N58N3G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4C (Homo sapiens (Human)) | BDBM50085138 (4-[8-(3-Chloro-phenyl)-[1,7]naphthyridin-6-yl]-ben...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG Curated by ChEMBL | Assay Description Inhibitory activity against Phosphodiesterase 4C (PDE4C) from human source expressed in Saccharomyces cerevisiae | J Med Chem 43: 675-82 (2000) BindingDB Entry DOI: 10.7270/Q2N58N3G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4A (Homo sapiens (Human)) | BDBM50085140 (6-Allyl-8-(3-nitro-phenyl)-[1,7]naphthyridine | CH...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 279 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG Curated by ChEMBL | Assay Description Inhibitory activity against Phosphodiesterase 4A (PDE4A) from human source expressed in Saccharomyces cerevisiae | J Med Chem 43: 675-82 (2000) BindingDB Entry DOI: 10.7270/Q2N58N3G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4B (RAT) | BDBM50085141 (CHEMBL351900 | Trifluoro-methanesulfonic acid 8-(3...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | n/a | n/a | 310 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG Curated by ChEMBL | Assay Description Inhibitory activity against Phosphodiesterase 4B (PDE4B) from rat source expressed in Saccharomyces cerevisiae | J Med Chem 43: 675-82 (2000) BindingDB Entry DOI: 10.7270/Q2N58N3G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4B (RAT) | BDBM50346088 ((1r,4r)-4-cyano-4-(3-(cyclopentyloxy)-4-methoxyphe...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | n/a | n/a | 310 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG Curated by ChEMBL | Assay Description Inhibitory activity against Phosphodiesterase 4B (PDE4B) from rat source expressed in Saccharomyces cerevisiae | J Med Chem 43: 675-82 (2000) BindingDB Entry DOI: 10.7270/Q2N58N3G | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4B (RAT) | BDBM50085147 (8-(3-Nitro-phenyl)-6-o-tolyl-[1,7]naphthyridine | ...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 340 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG Curated by ChEMBL | Assay Description Inhibitory activity against Phosphodiesterase 4B (PDE4B) from rat source expressed in Saccharomyces cerevisiae | J Med Chem 43: 675-82 (2000) BindingDB Entry DOI: 10.7270/Q2N58N3G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4A (Homo sapiens (Human)) | BDBM50085136 (8-(3-Nitro-phenyl)-6-phenyl-[1,7]naphthyridine | C...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 350 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG Curated by ChEMBL | Assay Description Inhibitory activity against Phosphodiesterase 4A (PDE4A) from human source expressed in Saccharomyces cerevisiae | J Med Chem 43: 675-82 (2000) BindingDB Entry DOI: 10.7270/Q2N58N3G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4D (Homo sapiens (Human)) | BDBM14361 ((R,S)-Rolipram | 4-(3-cyclopentyloxy-4-methoxy-phe...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | n/a | n/a | 390 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG Curated by ChEMBL | Assay Description Inhibitory activity against Phosphodiesterase 4D (PDE4D) from human source expressed in Saccharomyces cerevisiae | J Med Chem 43: 675-82 (2000) BindingDB Entry DOI: 10.7270/Q2N58N3G | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4A (Homo sapiens (Human)) | BDBM50346088 ((1r,4r)-4-cyano-4-(3-(cyclopentyloxy)-4-methoxyphe...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 410 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG Curated by ChEMBL | Assay Description Inhibitory activity against Phosphodiesterase 4A (PDE4A) from human source expressed in Saccharomyces cerevisiae | J Med Chem 43: 675-82 (2000) BindingDB Entry DOI: 10.7270/Q2N58N3G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4B (RAT) | BDBM50085143 (CHEMBL158286 | Trifluoro-methanesulfonic acid 8-(3...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 420 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG Curated by ChEMBL | Assay Description Inhibitory activity against Phosphodiesterase 4B (PDE4B) from rat source expressed in Saccharomyces cerevisiae | J Med Chem 43: 675-82 (2000) BindingDB Entry DOI: 10.7270/Q2N58N3G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4B (RAT) | BDBM50085145 (CHEMBL161714 | Trifluoro-methanesulfonic acid 8-(3...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 460 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG Curated by ChEMBL | Assay Description Inhibitory activity against Phosphodiesterase 4B (PDE4B) from rat source expressed in Saccharomyces cerevisiae | J Med Chem 43: 675-82 (2000) BindingDB Entry DOI: 10.7270/Q2N58N3G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4B (RAT) | BDBM50085137 (6-Furan-2-yl-8-(3-nitro-phenyl)-[1,7]naphthyridine...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 510 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG Curated by ChEMBL | Assay Description Inhibitory activity against Phosphodiesterase 4B (PDE4B) from rat source expressed in Saccharomyces cerevisiae | J Med Chem 43: 675-82 (2000) BindingDB Entry DOI: 10.7270/Q2N58N3G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4C (Homo sapiens (Human)) | BDBM50085139 (4-[8-(3-Cyano-phenyl)-[1,7]naphthyridin-6-yl]-benz...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | n/a | n/a | 570 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG Curated by ChEMBL | Assay Description Inhibitory activity against Phosphodiesterase 4C (PDE4C) from human source expressed in Saccharomyces cerevisiae | J Med Chem 43: 675-82 (2000) BindingDB Entry DOI: 10.7270/Q2N58N3G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4C (Homo sapiens (Human)) | BDBM50085146 (8-(3-Nitro-phenyl)-6-pyridin-3-yl-[1,7]naphthyridi...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 610 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG Curated by ChEMBL | Assay Description Inhibitory activity against Phosphodiesterase 4C (PDE4C) from human source expressed in Saccharomyces cerevisiae | J Med Chem 43: 675-82 (2000) BindingDB Entry DOI: 10.7270/Q2N58N3G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4A (Homo sapiens (Human)) | BDBM50085143 (CHEMBL158286 | Trifluoro-methanesulfonic acid 8-(3...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 770 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG Curated by ChEMBL | Assay Description Inhibitory activity against Phosphodiesterase 4A (PDE4A) from human source expressed in Saccharomyces cerevisiae | J Med Chem 43: 675-82 (2000) BindingDB Entry DOI: 10.7270/Q2N58N3G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4C (Homo sapiens (Human)) | BDBM50346088 ((1r,4r)-4-cyano-4-(3-(cyclopentyloxy)-4-methoxyphe...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | n/a | n/a | 840 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG Curated by ChEMBL | Assay Description Inhibitory activity against Phosphodiesterase 4C (PDE4C) from human source expressed in Saccharomyces cerevisiae | J Med Chem 43: 675-82 (2000) BindingDB Entry DOI: 10.7270/Q2N58N3G | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Displayed 1 to 50 (of 80 total ) | Next | Last >> |