

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50480930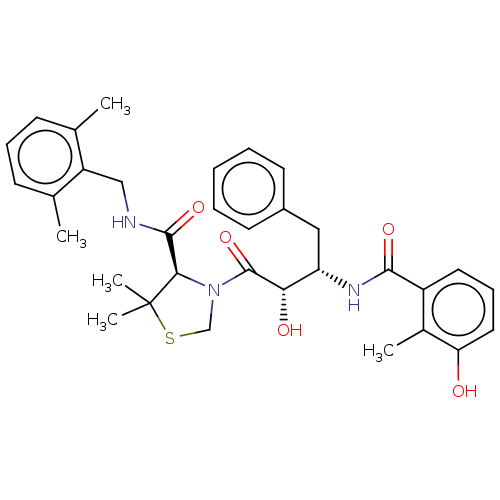 (CHEMBL584130 | KNI-814) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.00240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of HIV1 recombinant protease after 15 mins by fluorescence assay | J Med Chem 52: 7604-17 (2009) Article DOI: 10.1021/jm9005115 BindingDB Entry DOI: 10.7270/Q2FR00F2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM580 ((4R)-3-[(2S,3S)-2-hydroxy-3-[(3-hydroxy-2-methylph...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | MCE MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.0310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of HIV1 recombinant protease after 15 mins by fluorescence assay | J Med Chem 52: 7604-17 (2009) Article DOI: 10.1021/jm9005115 BindingDB Entry DOI: 10.7270/Q2FR00F2 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Neuraminidase (Influenza B virus (B/Lee/40)) | BDBM50330326 ((4S,5R,6R)-5-Acetylamino-4-guanidino-6-((1R,3R)-1,...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of influenza B/Memphis/3/89 neuraminidase | Bioorg Med Chem Lett 18: 1692-5 (2008) Article DOI: 10.1016/j.bmcl.2008.01.048 BindingDB Entry DOI: 10.7270/Q2WW7JHN | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Neuraminidase (Influenza B virus (B/Lee/40)) | BDBM50371257 (A-315675 | CHEMBL473062) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of influenza B/Memphis/3/89 neuraminidase | Bioorg Med Chem Lett 18: 1692-5 (2008) Article DOI: 10.1016/j.bmcl.2008.01.048 BindingDB Entry DOI: 10.7270/Q2WW7JHN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50480931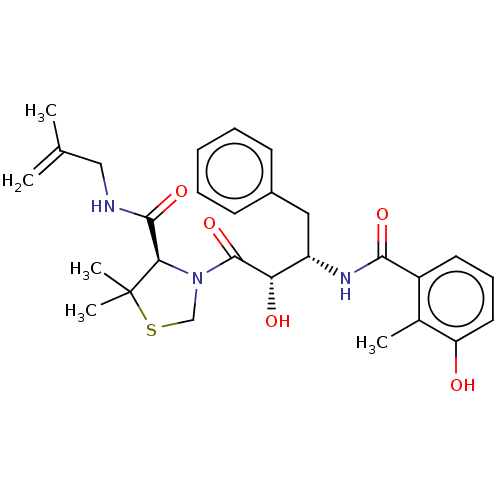 (CHEMBL575512 | KNI-1614) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of HIV1 recombinant protease after 15 mins by fluorescence assay | J Med Chem 52: 7604-17 (2009) Article DOI: 10.1021/jm9005115 BindingDB Entry DOI: 10.7270/Q2FR00F2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza B virus (B/Lee/40)) | BDBM50233737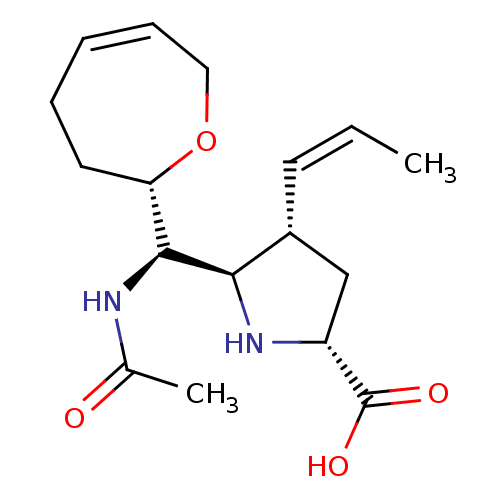 ((2R,4S,5R)-5-((R)-acetamido((S,Z)-2,3,4,7-tetrahyd...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of influenza B/Memphis/3/89 neuraminidase | Bioorg Med Chem Lett 18: 1692-5 (2008) Article DOI: 10.1016/j.bmcl.2008.01.048 BindingDB Entry DOI: 10.7270/Q2WW7JHN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza B virus (B/Lee/40)) | BDBM50233728 ((2R,4S,5R)-5-((1R,2S)-1-acetamido-2-methoxybut-3-e...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of influenza B/Memphis/3/89 neuraminidase | Bioorg Med Chem Lett 18: 1692-5 (2008) Article DOI: 10.1016/j.bmcl.2008.01.048 BindingDB Entry DOI: 10.7270/Q2WW7JHN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza B virus (B/Lee/40)) | BDBM50233741 ((2R,4S,5R)-5-((1R,2S)-1-acetamido-2-methoxypent-4-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.670 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of influenza B/Memphis/3/89 neuraminidase | Bioorg Med Chem Lett 18: 1692-5 (2008) Article DOI: 10.1016/j.bmcl.2008.01.048 BindingDB Entry DOI: 10.7270/Q2WW7JHN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50480929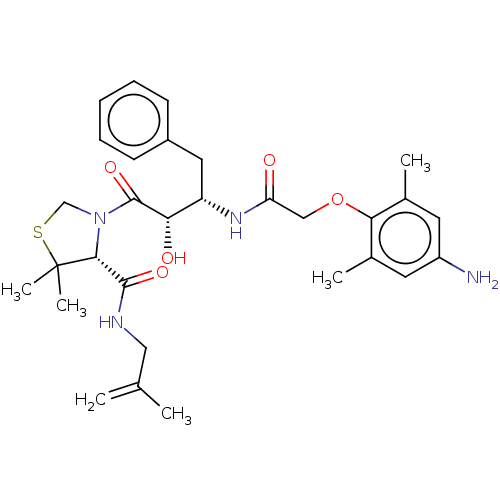 (CHEMBL573975 | KNI-1689) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | 0.830 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of HIV1 recombinant protease after 15 mins by fluorescence assay | J Med Chem 52: 7604-17 (2009) Article DOI: 10.1021/jm9005115 BindingDB Entry DOI: 10.7270/Q2FR00F2 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Transporter (Rattus norvegicus) | BDBM50198230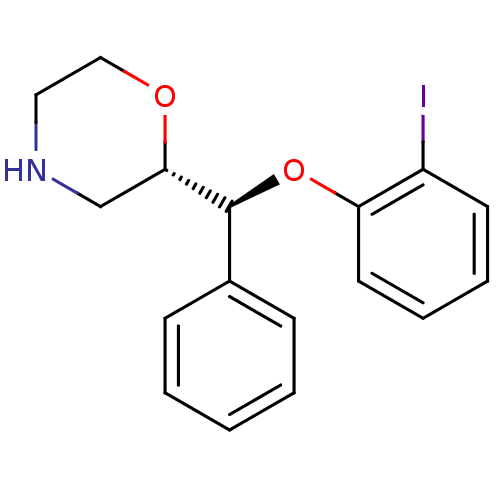 ((S)-2-((S)-(2-iodophenoxy)(phenyl)methyl)morpholin...) | Reactome pathway KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.840 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Degenerative Diseases Curated by ChEMBL | Assay Description Displacement of [3H]nisoxetine from NET in rat brain membranes | Bioorg Med Chem Lett 17: 533-7 (2007) Article DOI: 10.1016/j.bmcl.2006.10.018 BindingDB Entry DOI: 10.7270/Q2WS8SX8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza B virus (B/Lee/40)) | BDBM4994 ((3R,4R,5S)-5-amino-4-acetamido-3-(pentan-3-yloxy)c...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of influenza B/Memphis/3/89 neuraminidase | Bioorg Med Chem Lett 18: 1692-5 (2008) Article DOI: 10.1016/j.bmcl.2008.01.048 BindingDB Entry DOI: 10.7270/Q2WW7JHN | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Neuraminidase (Influenza B virus (B/Lee/40)) | BDBM50233723 ((2R,4S,5R)-5-((1R,2R)-1-acetamido-2-hydroxy-2-meth...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of influenza B/Memphis/3/89 neuraminidase | Bioorg Med Chem Lett 18: 1692-5 (2008) Article DOI: 10.1016/j.bmcl.2008.01.048 BindingDB Entry DOI: 10.7270/Q2WW7JHN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza B virus (B/Lee/40)) | BDBM50233724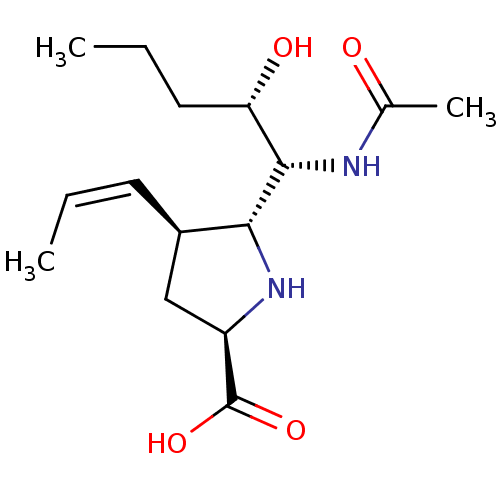 ((2R,4S,5R)-5-((1R,2S)-1-acetamido-2-hydroxypentyl)...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of influenza B/Memphis/3/89 neuraminidase | Bioorg Med Chem Lett 18: 1692-5 (2008) Article DOI: 10.1016/j.bmcl.2008.01.048 BindingDB Entry DOI: 10.7270/Q2WW7JHN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza B virus (B/Lee/40)) | BDBM50233732 ((2R,4S,5R)-5-((1R,2S)-1-acetamido-2-hydroxypent-4-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of influenza B/Memphis/3/89 neuraminidase | Bioorg Med Chem Lett 18: 1692-5 (2008) Article DOI: 10.1016/j.bmcl.2008.01.048 BindingDB Entry DOI: 10.7270/Q2WW7JHN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza B virus (B/Lee/40)) | BDBM50233742 ((2R,4S,5R)-5-((1R,2S)-1-acetamido-2-hydroxybut-3-e...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of influenza B/Memphis/3/89 neuraminidase | Bioorg Med Chem Lett 18: 1692-5 (2008) Article DOI: 10.1016/j.bmcl.2008.01.048 BindingDB Entry DOI: 10.7270/Q2WW7JHN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transporter (Rattus norvegicus) | BDBM50198226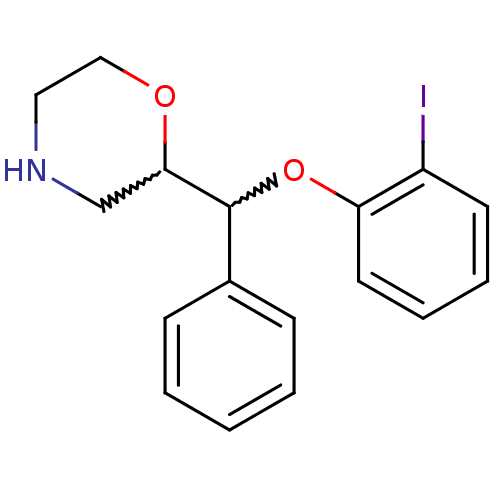 (2-((2-iodophenoxy)(phenyl)methyl)morpholine | CHEM...) | Reactome pathway KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.47 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Degenerative Diseases Curated by ChEMBL | Assay Description Displacement of [3H]nisoxetine from NET in rat brain membranes | Bioorg Med Chem Lett 17: 533-7 (2007) Article DOI: 10.1016/j.bmcl.2006.10.018 BindingDB Entry DOI: 10.7270/Q2WS8SX8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza B virus (B/Lee/40)) | BDBM50233744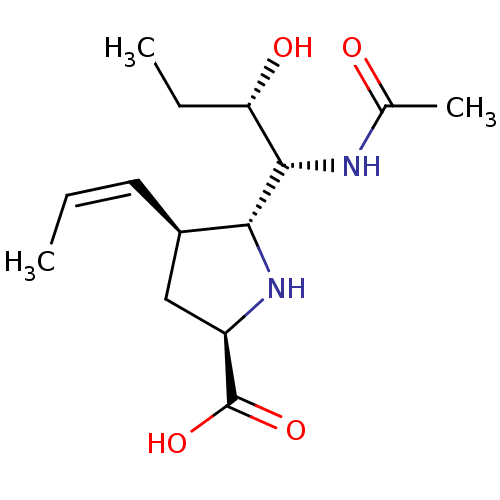 ((2R,4S,5R)-5-((1R,2S)-1-acetamido-2-hydroxybutyl)-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of influenza B/Memphis/3/89 neuraminidase | Bioorg Med Chem Lett 18: 1692-5 (2008) Article DOI: 10.1016/j.bmcl.2008.01.048 BindingDB Entry DOI: 10.7270/Q2WW7JHN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza B virus (B/Lee/40)) | BDBM50233726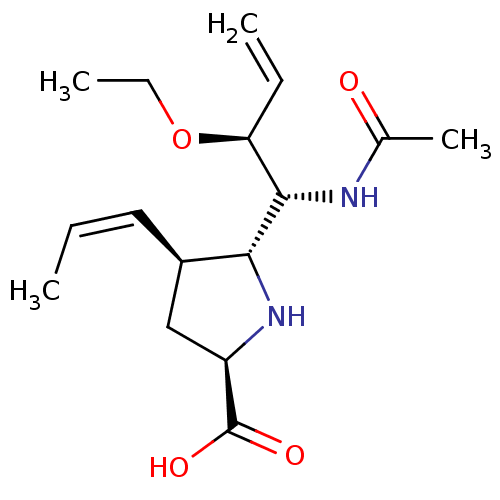 ((2R,4S,5R)-5-((1R,2S)-1-acetamido-2-ethoxybut-3-en...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of influenza B/Memphis/3/89 neuraminidase | Bioorg Med Chem Lett 18: 1692-5 (2008) Article DOI: 10.1016/j.bmcl.2008.01.048 BindingDB Entry DOI: 10.7270/Q2WW7JHN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza B virus (B/Lee/40)) | BDBM50233725 ((2R,4S,5R)-5-((1R,2S)-1-acetamido-2-hydroxypropyl)...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of influenza B/Memphis/3/89 neuraminidase | Bioorg Med Chem Lett 18: 1692-5 (2008) Article DOI: 10.1016/j.bmcl.2008.01.048 BindingDB Entry DOI: 10.7270/Q2WW7JHN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza B virus (B/Lee/40)) | BDBM50233727 ((2R,4S,5R)-5-((1R,2S)-1-acetamido-2-(allyloxy)but-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 9.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of influenza B/Memphis/3/89 neuraminidase | Bioorg Med Chem Lett 18: 1692-5 (2008) Article DOI: 10.1016/j.bmcl.2008.01.048 BindingDB Entry DOI: 10.7270/Q2WW7JHN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza B virus (B/Lee/40)) | BDBM50233722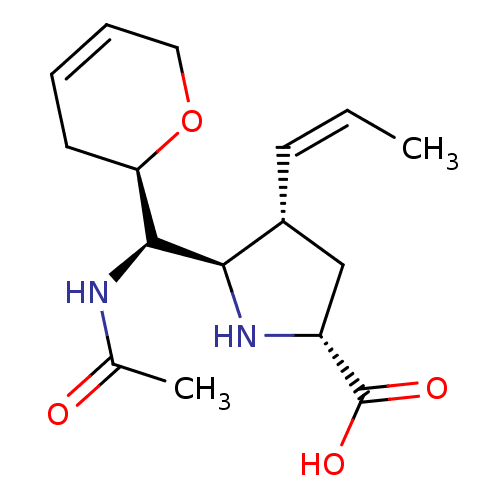 ((2R,4S,5R)-5-((R)-acetamido((R)-3,6-dihydro-2H-pyr...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 10.3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of influenza B/Memphis/3/89 neuraminidase | Bioorg Med Chem Lett 18: 1692-5 (2008) Article DOI: 10.1016/j.bmcl.2008.01.048 BindingDB Entry DOI: 10.7270/Q2WW7JHN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza B virus (B/Lee/40)) | BDBM50233733 ((2R,4S,5R)-5-((1R,2S)-1-acetamido-2-ethoxypent-4-e...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 10.6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of influenza B/Memphis/3/89 neuraminidase | Bioorg Med Chem Lett 18: 1692-5 (2008) Article DOI: 10.1016/j.bmcl.2008.01.048 BindingDB Entry DOI: 10.7270/Q2WW7JHN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza B virus (B/Lee/40)) | BDBM50233740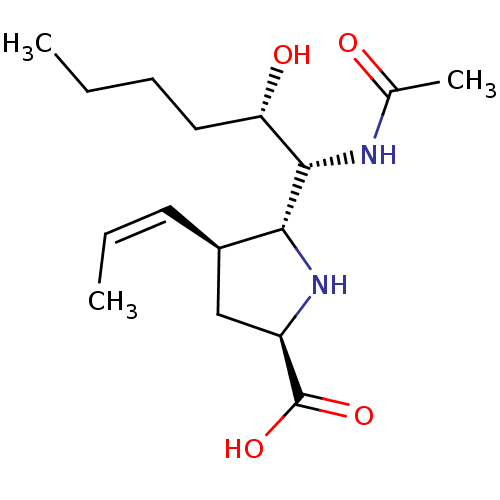 ((2R,4S,5R)-5-((1R,2S)-1-acetamido-2-hydroxyhexyl)-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of influenza B/Memphis/3/89 neuraminidase | Bioorg Med Chem Lett 18: 1692-5 (2008) Article DOI: 10.1016/j.bmcl.2008.01.048 BindingDB Entry DOI: 10.7270/Q2WW7JHN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent dopamine transporter (Rattus norvegicus (rat)) | BDBM50198231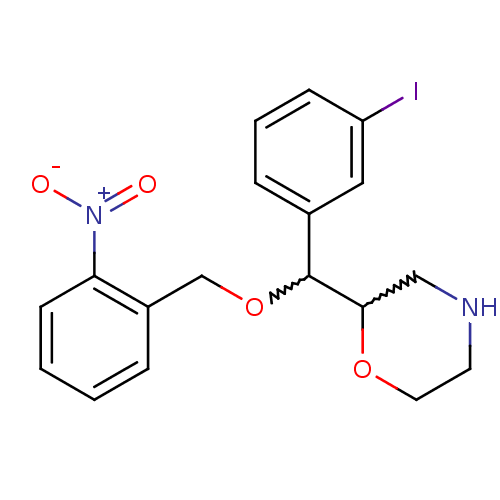 (2-((2-nitrobenzyloxy)(3-iodophenyl)methyl)morpholi...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 14.2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Degenerative Diseases Curated by ChEMBL | Assay Description Displacement of [3H]GBR from DAT in rat brain membranes | Bioorg Med Chem Lett 17: 533-7 (2007) Article DOI: 10.1016/j.bmcl.2006.10.018 BindingDB Entry DOI: 10.7270/Q2WS8SX8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transporter (Rattus norvegicus) | BDBM50198233 (2-((3-iodophenyl)(2-methoxyphenoxy)methyl)morpholi...) | Reactome pathway KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 15.4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Degenerative Diseases Curated by ChEMBL | Assay Description Displacement of [3H]nisoxetine from NET in rat brain membranes | Bioorg Med Chem Lett 17: 533-7 (2007) Article DOI: 10.1016/j.bmcl.2006.10.018 BindingDB Entry DOI: 10.7270/Q2WS8SX8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza B virus (B/Lee/40)) | BDBM50233743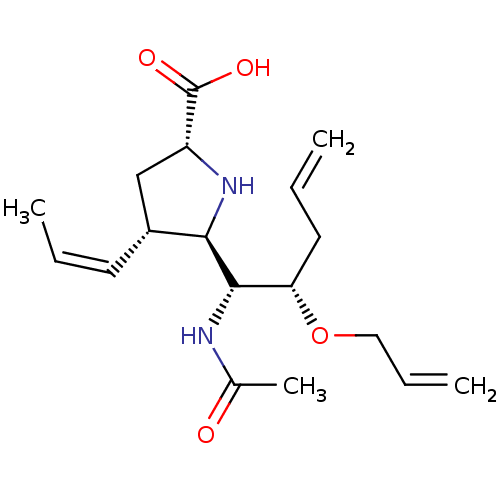 ((2R,4S,5R)-5-((1R,2S)-1-acetamido-2-(allyloxy)pent...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 17.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of influenza B/Memphis/3/89 neuraminidase | Bioorg Med Chem Lett 18: 1692-5 (2008) Article DOI: 10.1016/j.bmcl.2008.01.048 BindingDB Entry DOI: 10.7270/Q2WW7JHN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus (A/Tokyo/3/67(H2N2))) | BDBM5202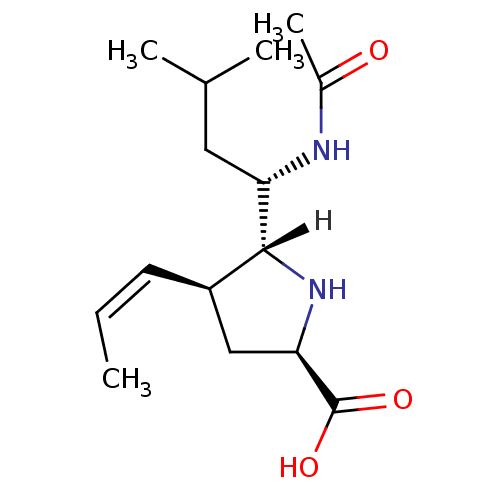 ((+/-)-(2R,4S,5R,1 S)-5-(1 -Acetylamino-3 -methyl-b...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories | Assay Description A fluorogenic assay was used to measure influenza virus neuraminidase activity. The substrate, 4-methylumbelliferyl-N-acetylneuraminic acid, is cleav... | J Med Chem 48: 3980-90 (2005) Article DOI: 10.1021/jm049276y BindingDB Entry DOI: 10.7270/Q2KP80B9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza B virus (B/Lee/40)) | BDBM50233731 ((2R,4S,5R)-5-((1R,2R)-1-acetamido-2-hydroxybut-3-e...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of influenza B/Memphis/3/89 neuraminidase | Bioorg Med Chem Lett 18: 1692-5 (2008) Article DOI: 10.1016/j.bmcl.2008.01.048 BindingDB Entry DOI: 10.7270/Q2WW7JHN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza B virus (B/Lee/40)) | BDBM50233721 ((2R,4S,5R)-5-((R)-acetamido((S)-3,6-dihydro-2H-pyr...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 24.2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of influenza B/Memphis/3/89 neuraminidase | Bioorg Med Chem Lett 18: 1692-5 (2008) Article DOI: 10.1016/j.bmcl.2008.01.048 BindingDB Entry DOI: 10.7270/Q2WW7JHN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transporter (Rattus norvegicus) | BDBM50198232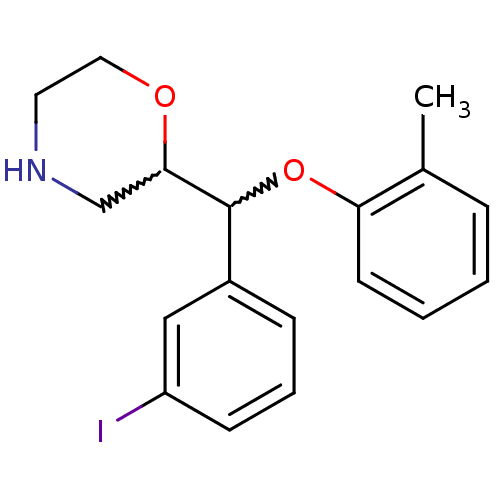 (2-((3-iodophenyl)(o-tolyloxy)methyl)morpholine | C...) | Reactome pathway KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 27.6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Degenerative Diseases Curated by ChEMBL | Assay Description Displacement of [3H]nisoxetine from NET in rat brain membranes | Bioorg Med Chem Lett 17: 533-7 (2007) Article DOI: 10.1016/j.bmcl.2006.10.018 BindingDB Entry DOI: 10.7270/Q2WS8SX8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza B virus (B/Lee/40)) | BDBM50233730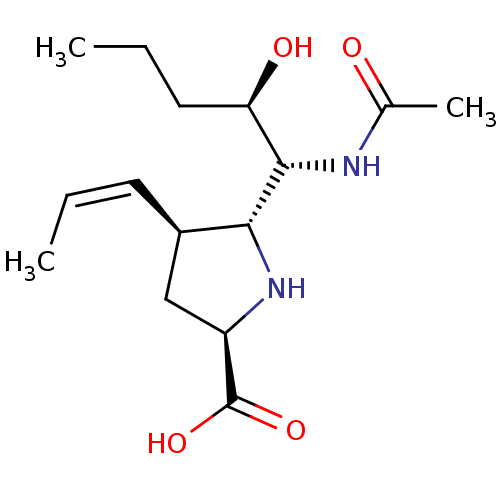 ((2R,4S,5R)-5-((1R,2R)-1-acetamido-2-hydroxypentyl)...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of influenza B/Memphis/3/89 neuraminidase | Bioorg Med Chem Lett 18: 1692-5 (2008) Article DOI: 10.1016/j.bmcl.2008.01.048 BindingDB Entry DOI: 10.7270/Q2WW7JHN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transporter (Rattus norvegicus) | BDBM50198227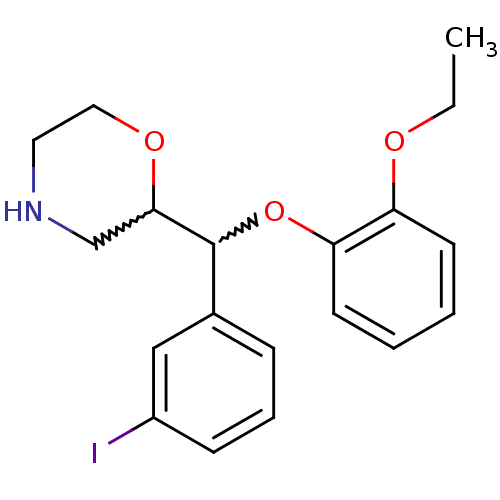 (2-((2-ethoxyphenoxy)(3-iodophenyl)methyl)morpholin...) | Reactome pathway KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 28.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Degenerative Diseases Curated by ChEMBL | Assay Description Displacement of [3H]nisoxetine from NET in rat brain membranes | Bioorg Med Chem Lett 17: 533-7 (2007) Article DOI: 10.1016/j.bmcl.2006.10.018 BindingDB Entry DOI: 10.7270/Q2WS8SX8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza B virus (B/Lee/40)) | BDBM50233738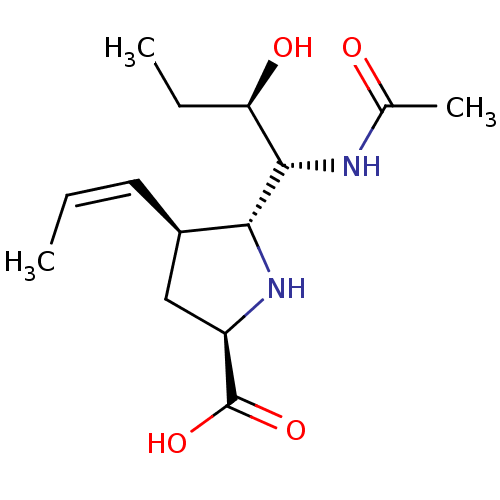 ((2R,4S,5R)-5-((1R,2R)-1-acetamido-2-hydroxybutyl)-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of influenza B/Memphis/3/89 neuraminidase | Bioorg Med Chem Lett 18: 1692-5 (2008) Article DOI: 10.1016/j.bmcl.2008.01.048 BindingDB Entry DOI: 10.7270/Q2WW7JHN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza B virus (B/Memphis/3/93)) | BDBM5202 ((+/-)-(2R,4S,5R,1 S)-5-(1 -Acetylamino-3 -methyl-b...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories | Assay Description A fluorogenic assay was used to measure influenza virus neuraminidase activity. The substrate, 4-methylumbelliferyl-N-acetylneuraminic acid, is cleav... | J Med Chem 48: 3980-90 (2005) Article DOI: 10.1021/jm049276y BindingDB Entry DOI: 10.7270/Q2KP80B9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza B virus (B/Lee/40)) | BDBM5202 ((+/-)-(2R,4S,5R,1 S)-5-(1 -Acetylamino-3 -methyl-b...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of influenza B/Memphis/3/89 neuraminidase | Bioorg Med Chem Lett 18: 1692-5 (2008) Article DOI: 10.1016/j.bmcl.2008.01.048 BindingDB Entry DOI: 10.7270/Q2WW7JHN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza B virus (B/Lee/40)) | BDBM50233735 ((2R,4S,5R)-5-((1R,2R)-1-acetamido-2-methoxypent-4-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 38 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of influenza B/Memphis/3/89 neuraminidase | Bioorg Med Chem Lett 18: 1692-5 (2008) Article DOI: 10.1016/j.bmcl.2008.01.048 BindingDB Entry DOI: 10.7270/Q2WW7JHN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50198230 ((S)-2-((S)-(2-iodophenoxy)(phenyl)methyl)morpholin...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 40.4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Degenerative Diseases Curated by ChEMBL | Assay Description Displacement of [3H]paroxetine from SERT in brain membranes | Bioorg Med Chem Lett 17: 533-7 (2007) Article DOI: 10.1016/j.bmcl.2006.10.018 BindingDB Entry DOI: 10.7270/Q2WS8SX8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus (A/Tokyo/3/67(H2N2))) | BDBM5200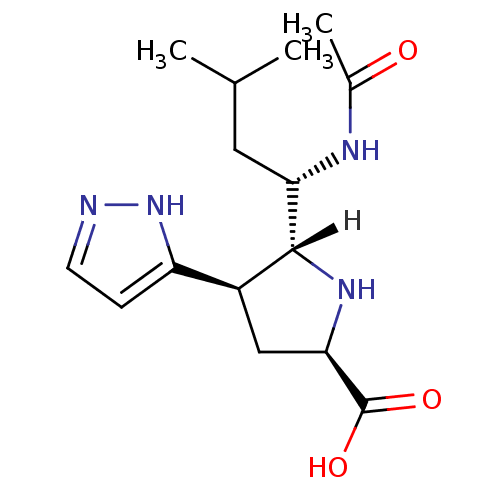 ((+/-)-(2R,4R,5R,1 S)-5-(1 -Acetylamino-3 -methylbu...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 45 | -41.5 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Abbott Laboratories | Assay Description A fluorogenic assay was used to measure influenza virus neuraminidase activity. The substrate, 4-methylumbelliferyl-N-acetylneuraminic acid, is cleav... | J Med Chem 48: 3980-90 (2005) Article DOI: 10.1021/jm049276y BindingDB Entry DOI: 10.7270/Q2KP80B9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza B virus (B/Lee/40)) | BDBM50233736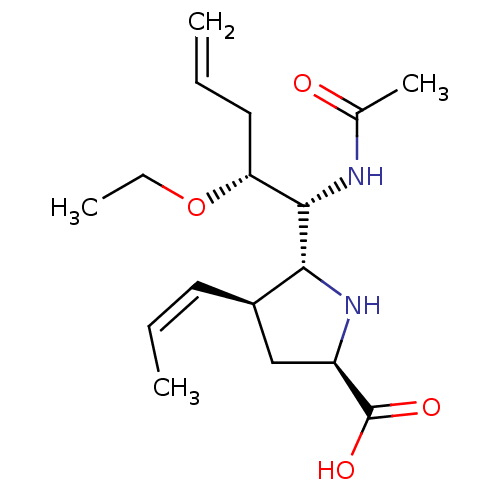 ((2R,4S,5R)-5-((1R,2R)-1-acetamido-2-ethoxypent-4-e...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 46 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of influenza B/Memphis/3/89 neuraminidase | Bioorg Med Chem Lett 18: 1692-5 (2008) Article DOI: 10.1016/j.bmcl.2008.01.048 BindingDB Entry DOI: 10.7270/Q2WW7JHN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza B virus (B/Lee/40)) | BDBM50233729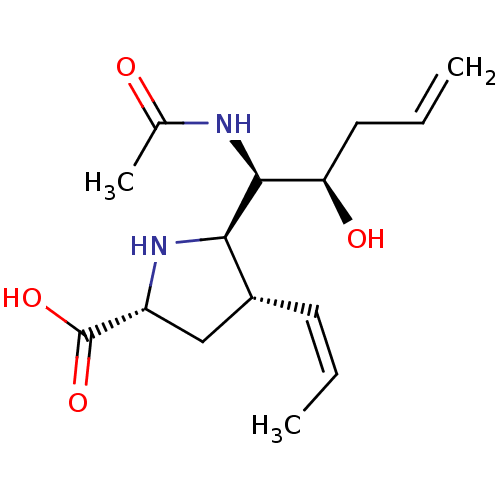 ((2R,4S,5R)-5-((1R,2R)-1-acetamido-2-hydroxypent-4-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 49 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of influenza B/Memphis/3/89 neuraminidase | Bioorg Med Chem Lett 18: 1692-5 (2008) Article DOI: 10.1016/j.bmcl.2008.01.048 BindingDB Entry DOI: 10.7270/Q2WW7JHN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza B virus (B/Memphis/3/93)) | BDBM5200 ((+/-)-(2R,4R,5R,1 S)-5-(1 -Acetylamino-3 -methylbu...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 57 | -40.9 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Abbott Laboratories | Assay Description A fluorogenic assay was used to measure influenza virus neuraminidase activity. The substrate, 4-methylumbelliferyl-N-acetylneuraminic acid, is cleav... | J Med Chem 48: 3980-90 (2005) Article DOI: 10.1021/jm049276y BindingDB Entry DOI: 10.7270/Q2KP80B9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transporter (Rattus norvegicus) | BDBM50198231 (2-((2-nitrobenzyloxy)(3-iodophenyl)methyl)morpholi...) | Reactome pathway KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 70.4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Degenerative Diseases Curated by ChEMBL | Assay Description Displacement of [3H]nisoxetine from NET in rat brain membranes | Bioorg Med Chem Lett 17: 533-7 (2007) Article DOI: 10.1016/j.bmcl.2006.10.018 BindingDB Entry DOI: 10.7270/Q2WS8SX8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transporter (Rattus norvegicus) | BDBM50198234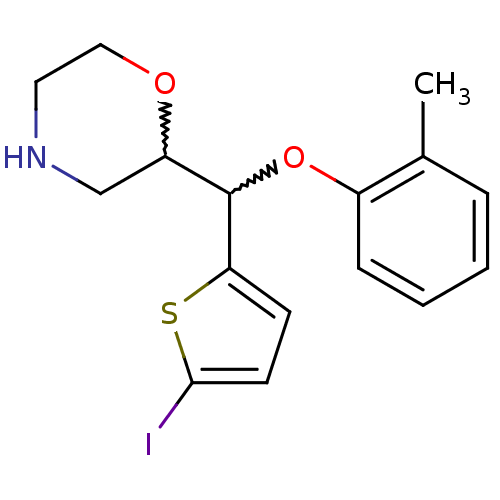 (2-((5-iodothiophen-2-yl)(o-tolyloxy)methyl)morphol...) | Reactome pathway KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 71.9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Degenerative Diseases Curated by ChEMBL | Assay Description Displacement of [3H]nisoxetine from NET in rat brain membranes | Bioorg Med Chem Lett 17: 533-7 (2007) Article DOI: 10.1016/j.bmcl.2006.10.018 BindingDB Entry DOI: 10.7270/Q2WS8SX8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transporter (Rattus norvegicus) | BDBM50198225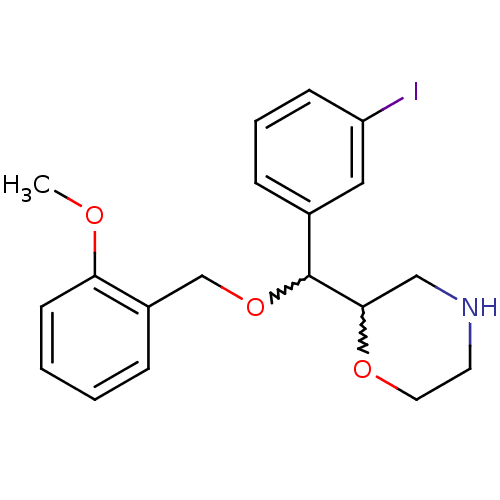 (2-((2-methoxybenzyloxy)(3-iodophenyl)methyl)morpho...) | Reactome pathway KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 73.8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Degenerative Diseases Curated by ChEMBL | Assay Description Displacement of [3H]nisoxetine from NET in rat brain membranes | Bioorg Med Chem Lett 17: 533-7 (2007) Article DOI: 10.1016/j.bmcl.2006.10.018 BindingDB Entry DOI: 10.7270/Q2WS8SX8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus (A/Tokyo/3/67(H2N2))) | BDBM5207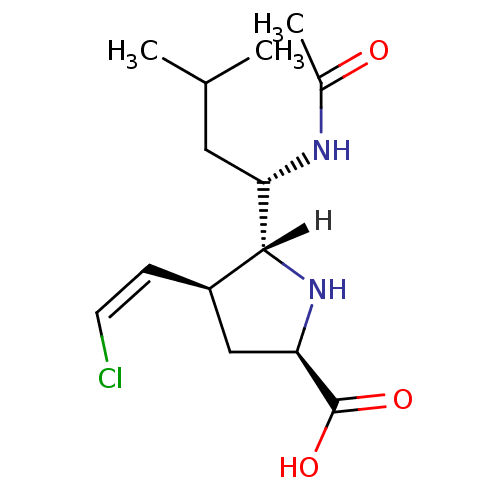 ((+/-)-(2R,4S,5R,1 S)-5-(1 -Acetylamino-3 -methyl-b...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 79 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories | Assay Description A fluorogenic assay was used to measure influenza virus neuraminidase activity. The substrate, 4-methylumbelliferyl-N-acetylneuraminic acid, is cleav... | J Med Chem 48: 3980-90 (2005) Article DOI: 10.1021/jm049276y BindingDB Entry DOI: 10.7270/Q2KP80B9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transporter (Rattus norvegicus) | BDBM50198228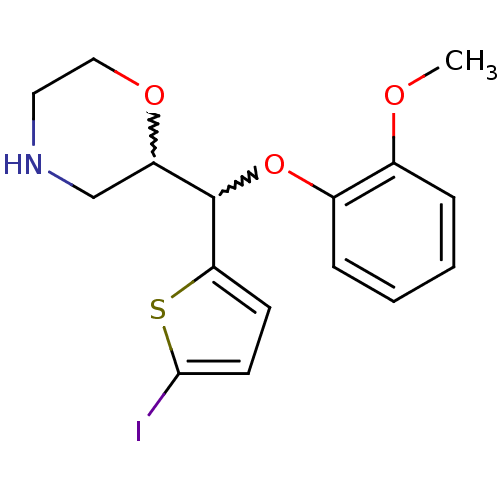 (2-((5-iodothiophen-2-yl)(2-methoxyphenoxy)methyl)m...) | Reactome pathway KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 79.6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Degenerative Diseases Curated by ChEMBL | Assay Description Displacement of [3H]nisoxetine from NET in rat brain membranes | Bioorg Med Chem Lett 17: 533-7 (2007) Article DOI: 10.1016/j.bmcl.2006.10.018 BindingDB Entry DOI: 10.7270/Q2WS8SX8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza B virus (B/Lee/40)) | BDBM50233745 ((2R,4S,5R)-5-((1R,2R)-1-acetamido-2-hydroxypropyl)...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 85 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of influenza B/Memphis/3/89 neuraminidase | Bioorg Med Chem Lett 18: 1692-5 (2008) Article DOI: 10.1016/j.bmcl.2008.01.048 BindingDB Entry DOI: 10.7270/Q2WW7JHN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza B virus (B/Lee/40)) | BDBM50233739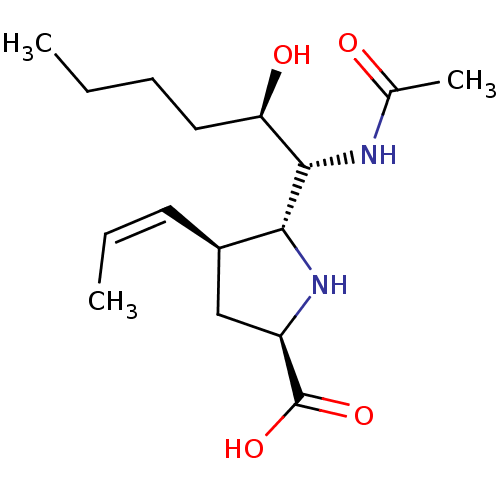 ((2R,4S,5R)-5-((1R,2R)-1-acetamido-2-hydroxyhexyl)-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 86 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of influenza B/Memphis/3/89 neuraminidase | Bioorg Med Chem Lett 18: 1692-5 (2008) Article DOI: 10.1016/j.bmcl.2008.01.048 BindingDB Entry DOI: 10.7270/Q2WW7JHN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transporter (Rattus norvegicus) | BDBM50198229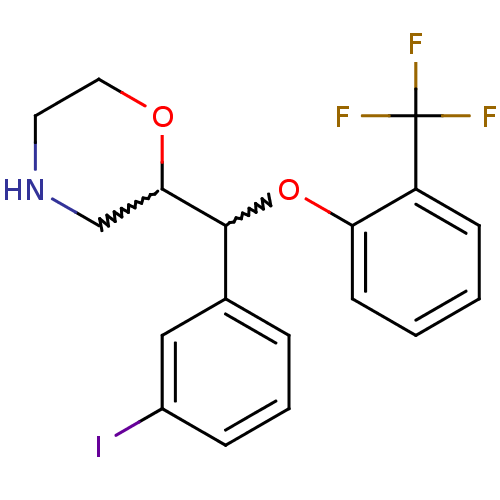 (2-((3-iodophenyl)(2-(trifluoromethyl)phenoxy)methy...) | Reactome pathway KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 101 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Degenerative Diseases Curated by ChEMBL | Assay Description Displacement of [3H]nisoxetine from NET in rat brain membranes | Bioorg Med Chem Lett 17: 533-7 (2007) Article DOI: 10.1016/j.bmcl.2006.10.018 BindingDB Entry DOI: 10.7270/Q2WS8SX8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza B virus (B/Lee/40)) | BDBM50233734 ((2R,4S,5R)-5-((1R,2R)-1-acetamido-2-(allyloxy)pent...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of influenza B/Memphis/3/89 neuraminidase | Bioorg Med Chem Lett 18: 1692-5 (2008) Article DOI: 10.1016/j.bmcl.2008.01.048 BindingDB Entry DOI: 10.7270/Q2WW7JHN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 149 total ) | Next | Last >> |