

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Renin (Homo sapiens (Human)) | BDBM50077669 ((S)-2-Benzyl-N-[(S)-1-((1S,2R,3S)-1-cyclohexylmeth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB PubMed | n/a | n/a | 0.0250 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd Curated by ChEMBL | Assay Description In vitro inhibitory activity against purified recombinant human renin | Bioorg Med Chem Lett 9: 1397-402 (1999) BindingDB Entry DOI: 10.7270/Q24F1PWN | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50077678 ((3R,4R)-3-(1,4-Dimethoxy-naphthalen-2-ylmethoxy)-4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd Curated by ChEMBL | Assay Description In vitro inhibitory activity against purified recombinant human renin | Bioorg Med Chem Lett 9: 1397-402 (1999) BindingDB Entry DOI: 10.7270/Q24F1PWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50077678 ((3R,4R)-3-(1,4-Dimethoxy-naphthalen-2-ylmethoxy)-4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.0870 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against purified recombinant human renin | Bioorg Med Chem Lett 9: 1403-8 (1999) BindingDB Entry DOI: 10.7270/Q20P0Z6S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50000558 (CHEMBL437472 | ET-1 | Endothelin -1 | Endothelin 1...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Receptor binding affinity was determined against [125I]ET1 with recombinant human ETA receptor, expressed in baculovirus-infected Sf9 cells | Bioorg Med Chem Lett 7: 2223-2228 (1997) Article DOI: 10.1016/S0960-894X(97)00400-9 BindingDB Entry DOI: 10.7270/Q2WQ049M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin receptor type B (Homo sapiens (Human)) | BDBM50000558 (CHEMBL437472 | ET-1 | Endothelin -1 | Endothelin 1...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Receptor binding affinity was determined against [125I]ET1 with membranes prepared from human placenta for ETB receptor | Bioorg Med Chem Lett 7: 2223-2228 (1997) Article DOI: 10.1016/S0960-894X(97)00400-9 BindingDB Entry DOI: 10.7270/Q2WQ049M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50290050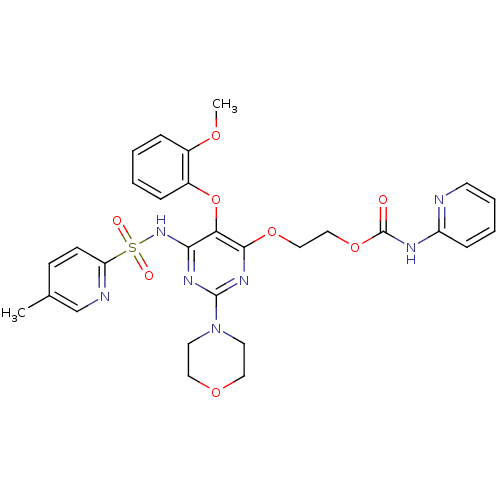 (CHEMBL70385 | Pyridin-2-yl-carbamic acid 2-[5-(2-m...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Receptor binding affinity was determined against [125I]ET1 with recombinant human ETA receptor, expressed in baculovirus-infected Sf9 cells | Bioorg Med Chem Lett 7: 2223-2228 (1997) Article DOI: 10.1016/S0960-894X(97)00400-9 BindingDB Entry DOI: 10.7270/Q2WQ049M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50290043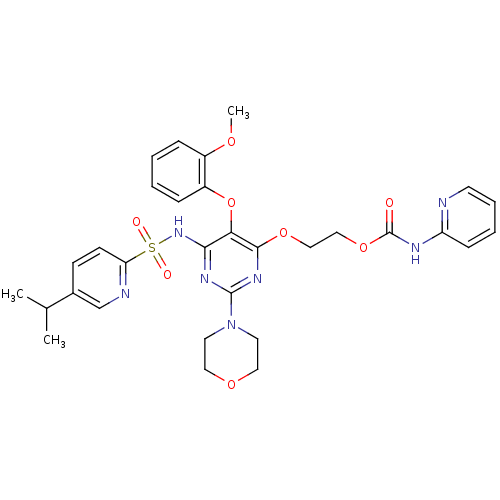 (CHEMBL72037 | Pyridin-2-yl-carbamic acid 2-[6-(5-i...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Receptor binding affinity was determined against [125I]ET1 with recombinant human ETA receptor, expressed in baculovirus-infected Sf9 cells | Bioorg Med Chem Lett 7: 2223-2228 (1997) Article DOI: 10.1016/S0960-894X(97)00400-9 BindingDB Entry DOI: 10.7270/Q2WQ049M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50000558 (CHEMBL437472 | ET-1 | Endothelin -1 | Endothelin 1...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Evaluation for functional inhibitory potency prevention of ET-1 induced constriction of rat aortic rings (ETA receptors) | Bioorg Med Chem Lett 7: 2223-2228 (1997) Article DOI: 10.1016/S0960-894X(97)00400-9 BindingDB Entry DOI: 10.7270/Q2WQ049M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50077702 (4-[(3S,4R,5R)-4-[4-(3-Benzyloxy-propoxy)-phenyl]-5...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against purified recombinant human renin | Bioorg Med Chem Lett 9: 1403-8 (1999) BindingDB Entry DOI: 10.7270/Q20P0Z6S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50077693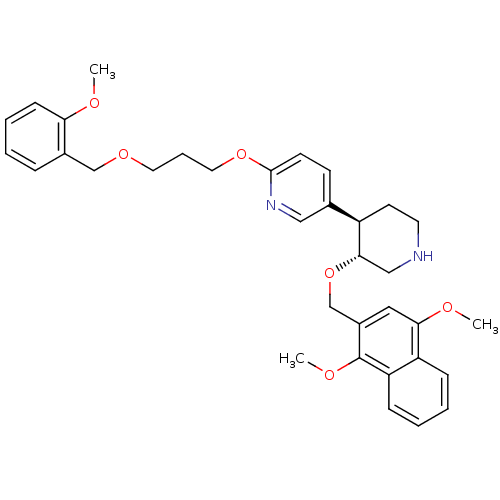 ((3'R,4'R)-3'-(1,4-Dimethoxy-naphthalen-2-ylmethoxy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against purified recombinant human renin | Bioorg Med Chem Lett 9: 1403-8 (1999) BindingDB Entry DOI: 10.7270/Q20P0Z6S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50077699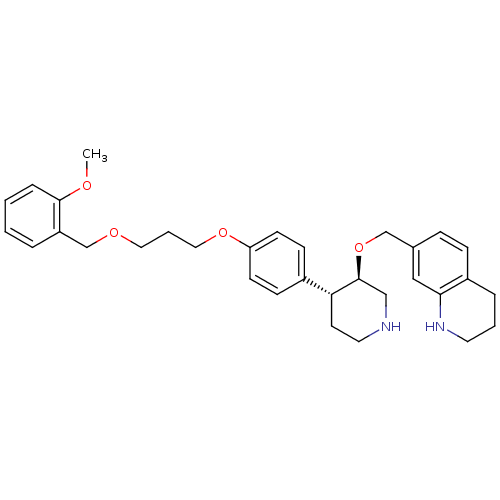 (7-((3R,4R)-4-{4-[3-(2-Methoxy-benzyloxy)-propoxy]-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.670 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against human plasma renin | Bioorg Med Chem Lett 9: 1403-8 (1999) BindingDB Entry DOI: 10.7270/Q20P0Z6S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50290043 (CHEMBL72037 | Pyridin-2-yl-carbamic acid 2-[6-(5-i...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Evaluation for functional inhibitory potency prevention of ET-1 induced constriction of rat aortic rings (ETA receptors) | Bioorg Med Chem Lett 7: 2223-2228 (1997) Article DOI: 10.1016/S0960-894X(97)00400-9 BindingDB Entry DOI: 10.7270/Q2WQ049M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50077692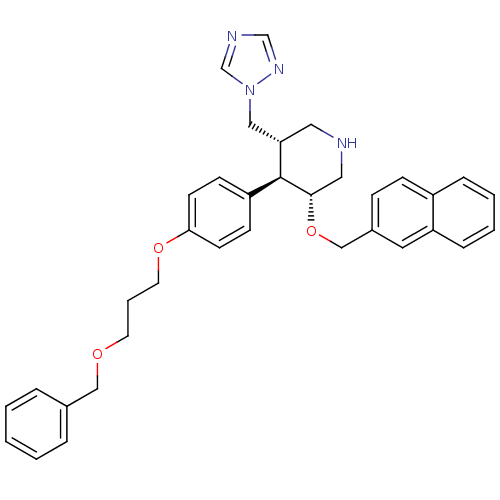 ((3R,4R,5S)-4-[4-(3-Benzyloxy-propoxy)-phenyl]-3-(n...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | n/a | n/a | 0.920 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against purified recombinant human renin | Bioorg Med Chem Lett 9: 1403-8 (1999) BindingDB Entry DOI: 10.7270/Q20P0Z6S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50290047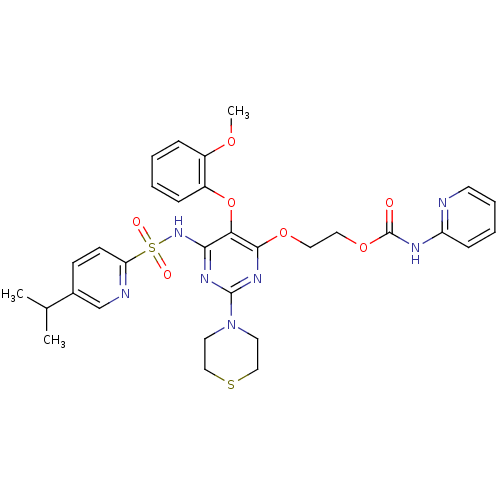 (CHEMBL308088 | Pyridin-2-yl-carbamic acid 2-[6-(5-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Receptor binding affinity was determined against [125I]ET1 with recombinant human ETA receptor, expressed in baculovirus-infected Sf9 cells | Bioorg Med Chem Lett 7: 2223-2228 (1997) Article DOI: 10.1016/S0960-894X(97)00400-9 BindingDB Entry DOI: 10.7270/Q2WQ049M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50077688 ((3R,4R)-4-{4-[3-(2-Methoxy-benzyloxy)-propoxy]-phe...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd Curated by ChEMBL | Assay Description In vitro inhibitory activity against purified recombinant human renin | Bioorg Med Chem Lett 9: 1397-402 (1999) BindingDB Entry DOI: 10.7270/Q24F1PWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50077703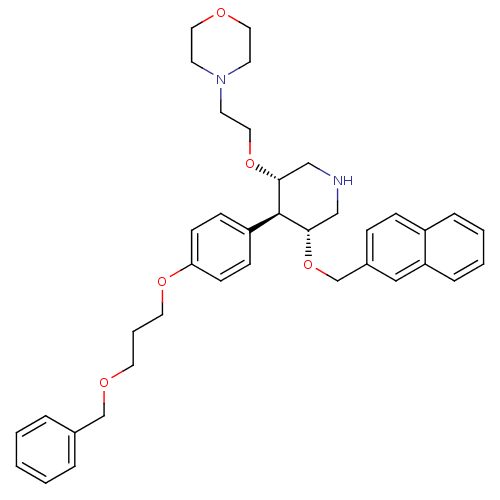 (4-{2-[(3S,4R,5R)-4-[4-(3-Benzyloxy-propoxy)-phenyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against purified recombinant human renin | Bioorg Med Chem Lett 9: 1403-8 (1999) BindingDB Entry DOI: 10.7270/Q20P0Z6S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50077704 (CHEMBL31880 | Sulfuric acid mono-[(3S,4R,5R)-4-[4-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem | PubMed | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against human plasma renin | Bioorg Med Chem Lett 9: 1403-8 (1999) BindingDB Entry DOI: 10.7270/Q20P0Z6S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50290059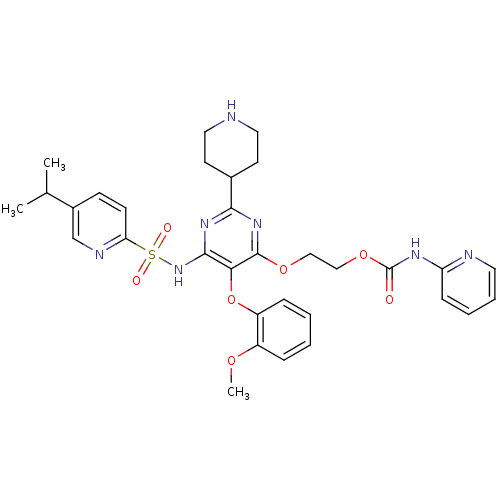 (CHEMBL70895 | Pyridin-2-yl-carbamic acid 2-[6-(5-i...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Receptor binding affinity was determined against [125I]ET1 with recombinant human ETA receptor, expressed in baculovirus-infected Sf9 cells | Bioorg Med Chem Lett 7: 2223-2228 (1997) Article DOI: 10.1016/S0960-894X(97)00400-9 BindingDB Entry DOI: 10.7270/Q2WQ049M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50077709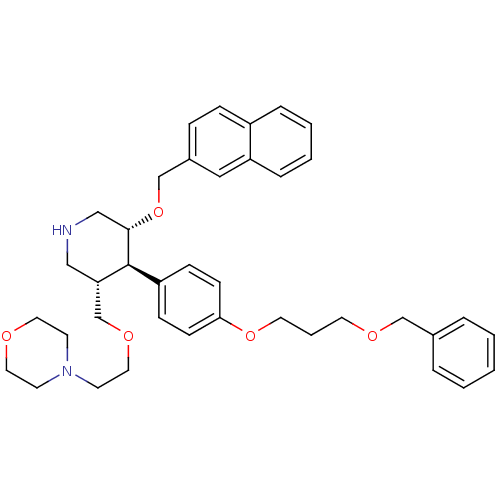 (4-{2-[(3S,4R,5R)-4-[4-(3-Benzyloxy-propoxy)-phenyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against purified recombinant human renin | Bioorg Med Chem Lett 9: 1403-8 (1999) BindingDB Entry DOI: 10.7270/Q20P0Z6S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50290060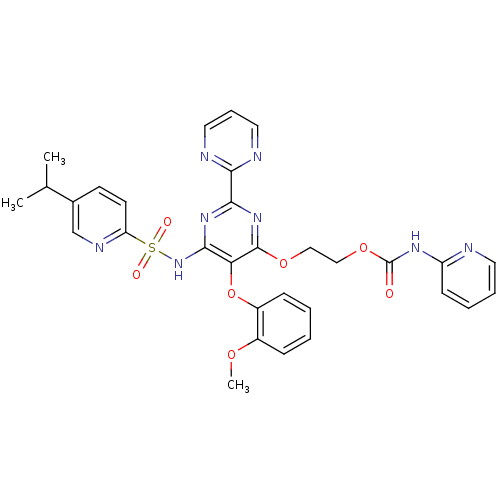 (CHEMBL70293 | Pyridin-2-yl-carbamic acid 2-[6-(5-i...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Receptor binding affinity was determined against [125I]ET1 with recombinant human ETA receptor, expressed in baculovirus-infected Sf9 cells | Bioorg Med Chem Lett 7: 2223-2228 (1997) Article DOI: 10.1016/S0960-894X(97)00400-9 BindingDB Entry DOI: 10.7270/Q2WQ049M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50290057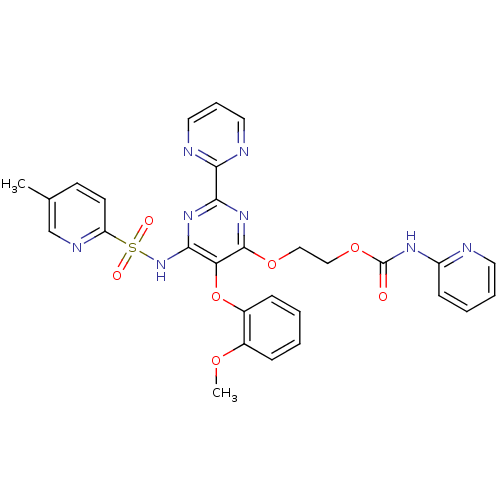 (CHEMBL70303 | Pyridin-2-yl-carbamic acid 2-[5-(2-m...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Receptor binding affinity was determined against [125I]ET1 with recombinant human ETA receptor, expressed in baculovirus-infected Sf9 cells | Bioorg Med Chem Lett 7: 2223-2228 (1997) Article DOI: 10.1016/S0960-894X(97)00400-9 BindingDB Entry DOI: 10.7270/Q2WQ049M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50077695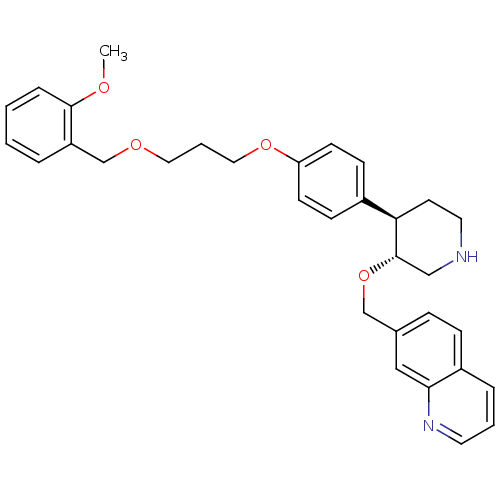 (7-((3R,4R)-4-{4-[3-(2-Methoxy-benzyloxy)-propoxy]-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against purified recombinant human renin | Bioorg Med Chem Lett 9: 1403-8 (1999) BindingDB Entry DOI: 10.7270/Q20P0Z6S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50077689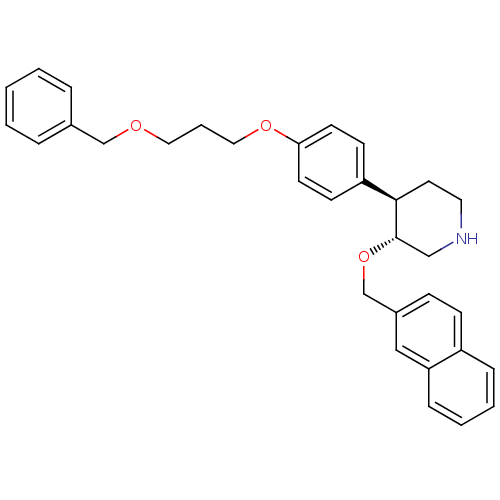 ((3R,4R)-4-[4-(3-Benzyloxy-propoxy)-phenyl]-3-(naph...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against purified recombinant human renin | Bioorg Med Chem Lett 9: 1403-8 (1999) BindingDB Entry DOI: 10.7270/Q20P0Z6S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin receptor type B (Homo sapiens (Human)) | BDBM50290043 (CHEMBL72037 | Pyridin-2-yl-carbamic acid 2-[6-(5-i...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Receptor binding affinity was determined against [125I]ET1 with membranes prepared from human placenta for ETB receptor | Bioorg Med Chem Lett 7: 2223-2228 (1997) Article DOI: 10.1016/S0960-894X(97)00400-9 BindingDB Entry DOI: 10.7270/Q2WQ049M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50077694 (7-{(3R,4R)-4-[4-(3-Benzyloxy-propoxy)-phenyl]-pipe...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5.10 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against purified recombinant human renin | Bioorg Med Chem Lett 9: 1403-8 (1999) BindingDB Entry DOI: 10.7270/Q20P0Z6S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50290044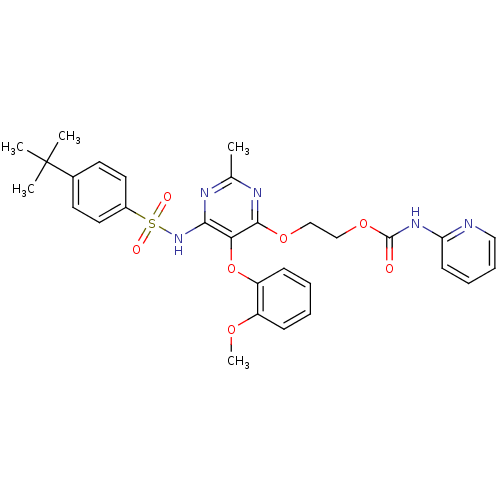 (CHEMBL70800 | Pyridin-2-yl-carbamic acid 2-[6-(4-t...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Receptor binding affinity was determined against [125I]ET1 with recombinant human ETA receptor, expressed in baculovirus-infected Sf9 cells | Bioorg Med Chem Lett 7: 2223-2228 (1997) Article DOI: 10.1016/S0960-894X(97)00400-9 BindingDB Entry DOI: 10.7270/Q2WQ049M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50290041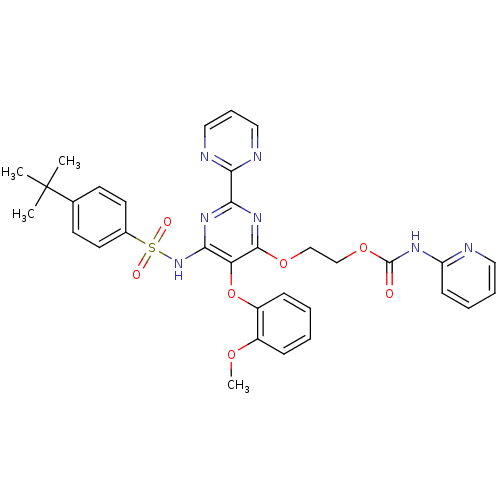 (CHEMBL70203 | Pyridin-2-yl-carbamic acid 2-[6-(4-t...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Receptor binding affinity was determined against [125I]ET1 with recombinant human ETA receptor, expressed in baculovirus-infected Sf9 cells | Bioorg Med Chem Lett 7: 2223-2228 (1997) Article DOI: 10.1016/S0960-894X(97)00400-9 BindingDB Entry DOI: 10.7270/Q2WQ049M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50077705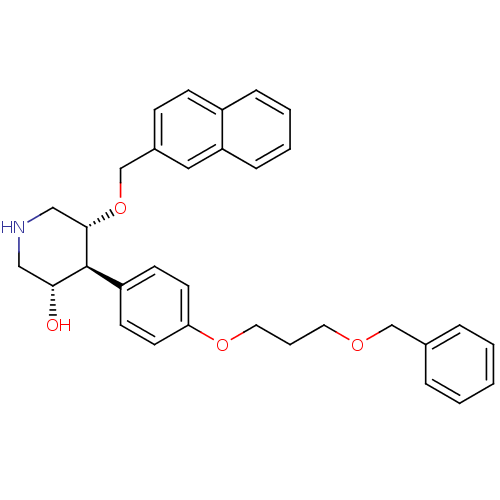 ((3S,4S,5R)-4-[4-(3-Benzyloxy-propoxy)-phenyl]-5-(n...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 6.40 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against purified recombinant human renin | Bioorg Med Chem Lett 9: 1403-8 (1999) BindingDB Entry DOI: 10.7270/Q20P0Z6S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50290049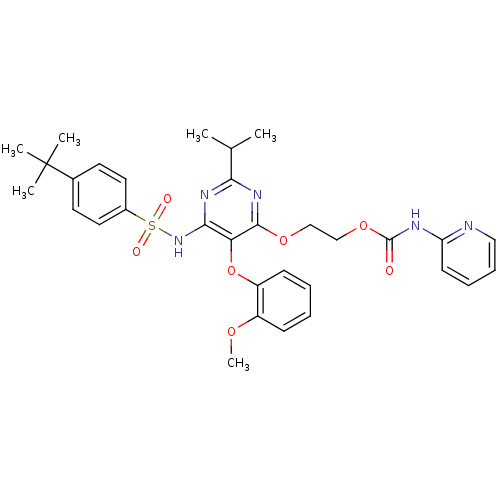 (CHEMBL71070 | Pyridin-2-yl-carbamic acid 2-[6-(4-t...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Receptor binding affinity was determined against [125I]ET1 with recombinant human ETA receptor, expressed in baculovirus-infected Sf9 cells | Bioorg Med Chem Lett 7: 2223-2228 (1997) Article DOI: 10.1016/S0960-894X(97)00400-9 BindingDB Entry DOI: 10.7270/Q2WQ049M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50290042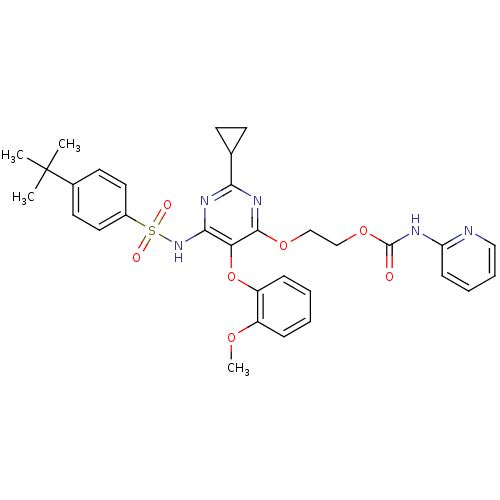 (CHEMBL70487 | Pyridin-2-yl-carbamic acid 2-[6-(4-t...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Receptor binding affinity was determined against [125I]ET1 with recombinant human ETA receptor, expressed in baculovirus-infected Sf9 cells | Bioorg Med Chem Lett 7: 2223-2228 (1997) Article DOI: 10.1016/S0960-894X(97)00400-9 BindingDB Entry DOI: 10.7270/Q2WQ049M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50077697 (7-((3R,4R)-4-{4-[3-(2-Methoxy-benzyloxy)-propoxy]-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against purified recombinant human renin | Bioorg Med Chem Lett 9: 1403-8 (1999) BindingDB Entry DOI: 10.7270/Q20P0Z6S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50077689 ((3R,4R)-4-[4-(3-Benzyloxy-propoxy)-phenyl]-3-(naph...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd Curated by ChEMBL | Assay Description In vitro inhibitory activity against purified recombinant human renin | Bioorg Med Chem Lett 9: 1397-402 (1999) BindingDB Entry DOI: 10.7270/Q24F1PWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50290055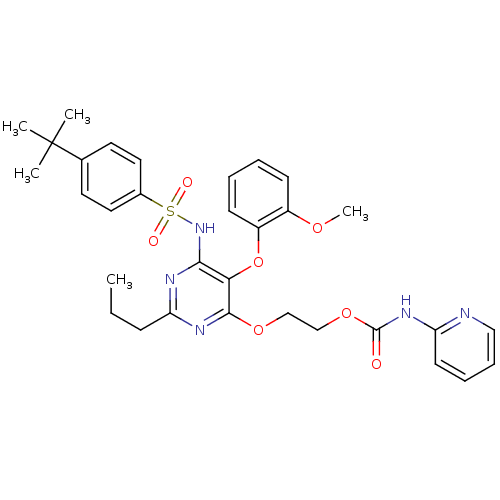 (CHEMBL303428 | Pyridin-2-yl-carbamic acid 2-[6-(4-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Receptor binding affinity was determined against [125I]ET1 with recombinant human ETA receptor, expressed in baculovirus-infected Sf9 cells | Bioorg Med Chem Lett 7: 2223-2228 (1997) Article DOI: 10.1016/S0960-894X(97)00400-9 BindingDB Entry DOI: 10.7270/Q2WQ049M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50077707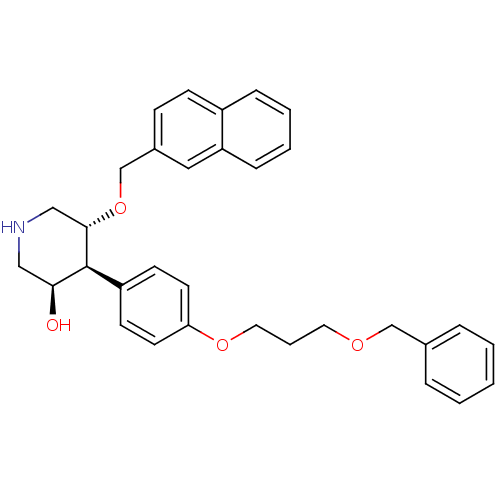 ((3R,4S,5R)-4-[4-(3-Benzyloxy-propoxy)-phenyl]-5-(n...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against purified recombinant human renin | Bioorg Med Chem Lett 9: 1403-8 (1999) BindingDB Entry DOI: 10.7270/Q20P0Z6S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50077698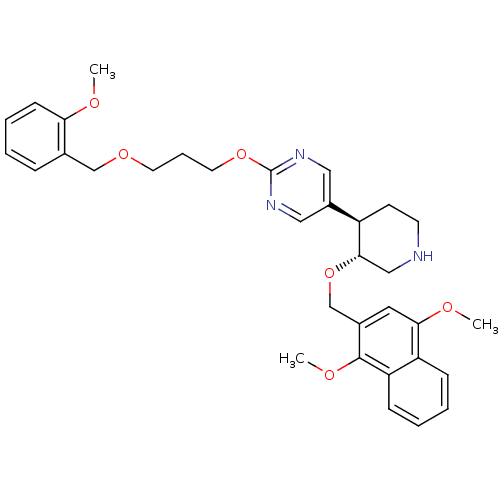 (5-[(3R,4R)-3-(1,4-Dimethoxy-naphthalen-2-ylmethoxy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against purified recombinant human renin | Bioorg Med Chem Lett 9: 1403-8 (1999) BindingDB Entry DOI: 10.7270/Q20P0Z6S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50077678 ((3R,4R)-3-(1,4-Dimethoxy-naphthalen-2-ylmethoxy)-4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against human plasma renin | Bioorg Med Chem Lett 9: 1403-8 (1999) BindingDB Entry DOI: 10.7270/Q20P0Z6S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50077706 (7-{(3R,4R)-4-[4-(3-Benzyloxy-propoxy)-phenyl]-pipe...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against purified recombinant human renin | Bioorg Med Chem Lett 9: 1403-8 (1999) BindingDB Entry DOI: 10.7270/Q20P0Z6S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50077696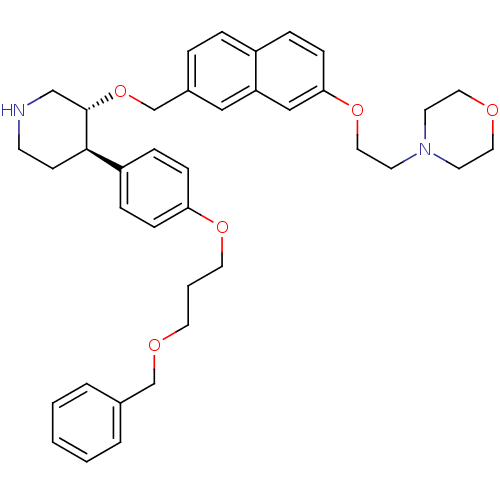 (4-[2-(7-{(3R,4R)-4-[4-(3-Benzyloxy-propoxy)-phenyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against human plasma renin | Bioorg Med Chem Lett 9: 1403-8 (1999) BindingDB Entry DOI: 10.7270/Q20P0Z6S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin receptor type B (Homo sapiens (Human)) | BDBM50290047 (CHEMBL308088 | Pyridin-2-yl-carbamic acid 2-[6-(5-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Receptor binding affinity was determined against [125I]ET1 with membranes prepared from human placenta for ETB receptor | Bioorg Med Chem Lett 7: 2223-2228 (1997) Article DOI: 10.1016/S0960-894X(97)00400-9 BindingDB Entry DOI: 10.7270/Q2WQ049M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50290051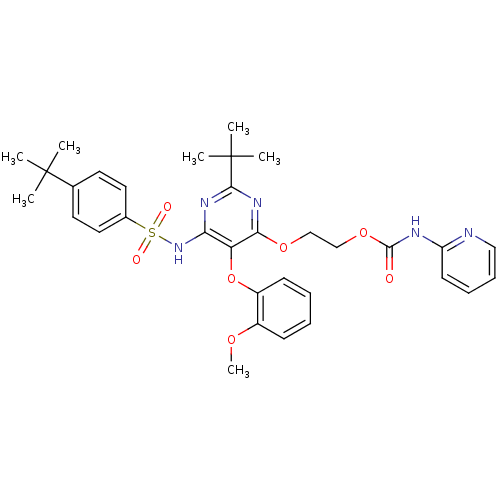 (CHEMBL303649 | Pyridin-2-yl-carbamic acid 2-[2-ter...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Receptor binding affinity was determined against [125I]ET1 with recombinant human ETA receptor, expressed in baculovirus-infected Sf9 cells | Bioorg Med Chem Lett 7: 2223-2228 (1997) Article DOI: 10.1016/S0960-894X(97)00400-9 BindingDB Entry DOI: 10.7270/Q2WQ049M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50290053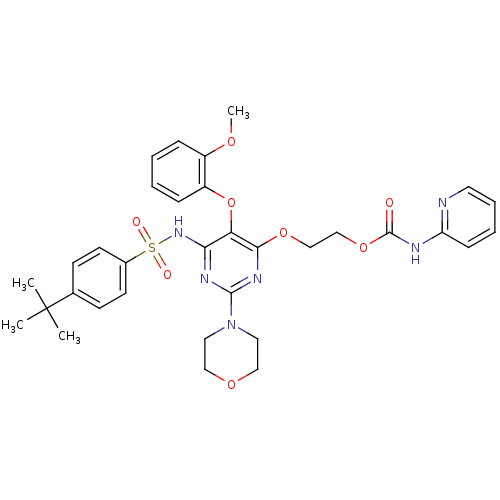 (CHEMBL70339 | Pyridin-2-yl-carbamic acid 2-[6-(4-t...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Receptor binding affinity was determined against [125I]ET1 with recombinant human ETA receptor, expressed in baculovirus-infected Sf9 cells | Bioorg Med Chem Lett 7: 2223-2228 (1997) Article DOI: 10.1016/S0960-894X(97)00400-9 BindingDB Entry DOI: 10.7270/Q2WQ049M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin receptor type B (Homo sapiens (Human)) | BDBM50290053 (CHEMBL70339 | Pyridin-2-yl-carbamic acid 2-[6-(4-t...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Receptor binding affinity was determined against [125I]ET1 with membranes prepared from human placenta for ETB receptor | Bioorg Med Chem Lett 7: 2223-2228 (1997) Article DOI: 10.1016/S0960-894X(97)00400-9 BindingDB Entry DOI: 10.7270/Q2WQ049M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50077708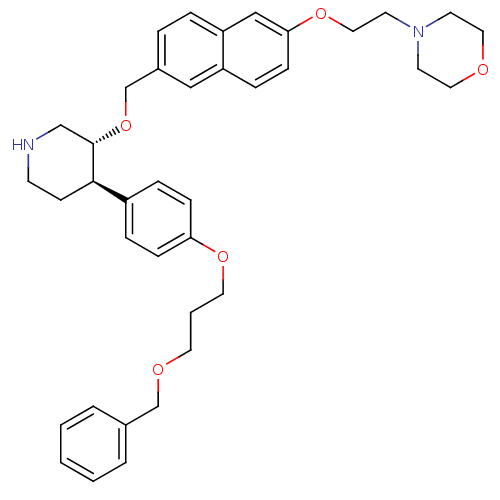 (4-[2-(6-{(3R,4R)-4-[4-(3-Benzyloxy-propoxy)-phenyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against human plasma renin | Bioorg Med Chem Lett 9: 1403-8 (1999) BindingDB Entry DOI: 10.7270/Q20P0Z6S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin receptor type B (Homo sapiens (Human)) | BDBM50290050 (CHEMBL70385 | Pyridin-2-yl-carbamic acid 2-[5-(2-m...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Receptor binding affinity was determined against [125I]ET1 with membranes prepared from human placenta for ETB receptor | Bioorg Med Chem Lett 7: 2223-2228 (1997) Article DOI: 10.1016/S0960-894X(97)00400-9 BindingDB Entry DOI: 10.7270/Q2WQ049M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin receptor type B (Homo sapiens (Human)) | BDBM50290059 (CHEMBL70895 | Pyridin-2-yl-carbamic acid 2-[6-(5-i...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Receptor binding affinity was determined against [125I]ET1 with membranes prepared from human placenta for ETB receptor | Bioorg Med Chem Lett 7: 2223-2228 (1997) Article DOI: 10.1016/S0960-894X(97)00400-9 BindingDB Entry DOI: 10.7270/Q2WQ049M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50077682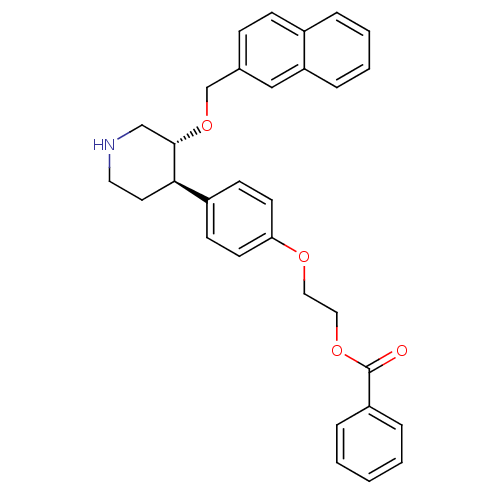 (Benzoic acid 2-{4-[(3R,4R)-3-(naphthalen-2-ylmetho...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd Curated by ChEMBL | Assay Description In vitro inhibitory activity against purified recombinant human renin | Bioorg Med Chem Lett 9: 1397-402 (1999) BindingDB Entry DOI: 10.7270/Q24F1PWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50290061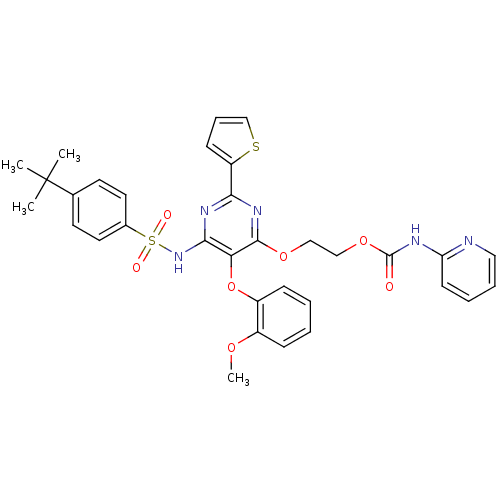 (CHEMBL70365 | Pyridin-2-yl-carbamic acid 2-[6-(4-t...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Receptor binding affinity was determined against [125I]ET1 with recombinant human ETA receptor, expressed in baculovirus-infected Sf9 cells | Bioorg Med Chem Lett 7: 2223-2228 (1997) Article DOI: 10.1016/S0960-894X(97)00400-9 BindingDB Entry DOI: 10.7270/Q2WQ049M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50077699 (7-((3R,4R)-4-{4-[3-(2-Methoxy-benzyloxy)-propoxy]-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against human plasma renin | Bioorg Med Chem Lett 9: 1403-8 (1999) BindingDB Entry DOI: 10.7270/Q20P0Z6S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50077671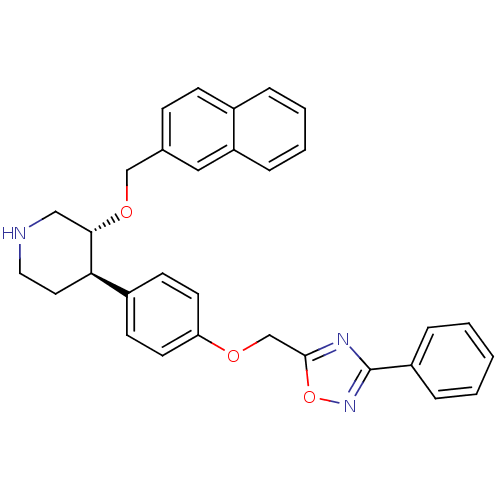 ((3R,4R)-3-(Naphthalen-2-ylmethoxy)-4-[4-(3-phenyl-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd Curated by ChEMBL | Assay Description In vitro inhibitory activity against purified recombinant human renin | Bioorg Med Chem Lett 9: 1397-402 (1999) BindingDB Entry DOI: 10.7270/Q24F1PWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin receptor type B (Homo sapiens (Human)) | BDBM50290041 (CHEMBL70203 | Pyridin-2-yl-carbamic acid 2-[6-(4-t...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 43 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Receptor binding affinity was determined against [125I]ET1 with membranes prepared from human placenta for ETB receptor | Bioorg Med Chem Lett 7: 2223-2228 (1997) Article DOI: 10.1016/S0960-894X(97)00400-9 BindingDB Entry DOI: 10.7270/Q2WQ049M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 115 total ) | Next | Last >> |