 Found 120 hits with Last Name = 'bryant' and Initial = 'jw'
Found 120 hits with Last Name = 'bryant' and Initial = 'jw' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Endothelin-1 receptor
(Homo sapiens (Human)) | BDBM50077930
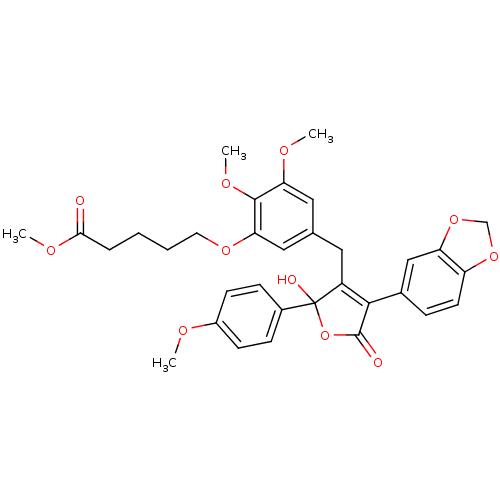
(5-{5-[4-Benzo[1,3]dioxol-5-yl-2-hydroxy-2-(4-metho...)Show SMILES COC(=O)CCCCOc1cc(CC2=C(C(=O)OC2(O)c2ccc(OC)cc2)c2ccc3OCOc3c2)cc(OC)c1OC |t:13| Show InChI InChI=1S/C33H34O11/c1-37-23-11-9-22(10-12-23)33(36)24(30(32(35)44-33)21-8-13-25-26(18-21)43-19-42-25)15-20-16-27(38-2)31(40-4)28(17-20)41-14-6-5-7-29(34)39-3/h8-13,16-18,36H,5-7,14-15,19H2,1-4H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Inhibition of [125I]-ET-1 binding to recombinant human Endothelin A receptor. |
J Med Chem 42: 2162-8 (1999)
Article DOI: 10.1021/jm980504w
BindingDB Entry DOI: 10.7270/Q2V12404 |
More data for this
Ligand-Target Pair | |
Endothelin-1 receptor
(Homo sapiens (Human)) | BDBM50077933
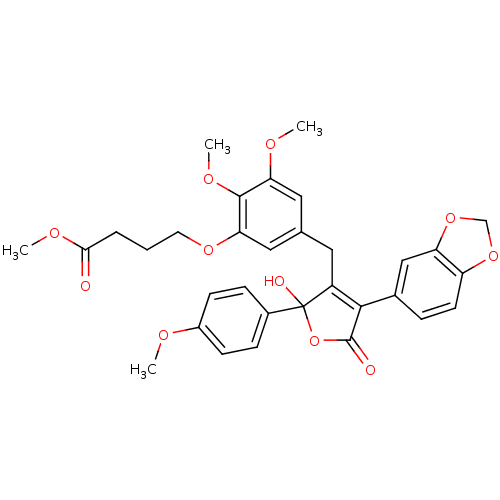
(4-{5-[4-Benzo[1,3]dioxol-5-yl-2-hydroxy-2-(4-metho...)Show SMILES COC(=O)CCCOc1cc(CC2=C(C(=O)OC2(O)c2ccc(OC)cc2)c2ccc3OCOc3c2)cc(OC)c1OC |t:12| Show InChI InChI=1S/C32H32O11/c1-36-22-10-8-21(9-11-22)32(35)23(29(31(34)43-32)20-7-12-24-25(17-20)42-18-41-24)14-19-15-26(37-2)30(39-4)27(16-19)40-13-5-6-28(33)38-3/h7-12,15-17,35H,5-6,13-14,18H2,1-4H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Inhibition of [125I]-ET-1 binding to recombinant human Endothelin A receptor. |
J Med Chem 42: 2162-8 (1999)
Article DOI: 10.1021/jm980504w
BindingDB Entry DOI: 10.7270/Q2V12404 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50328726
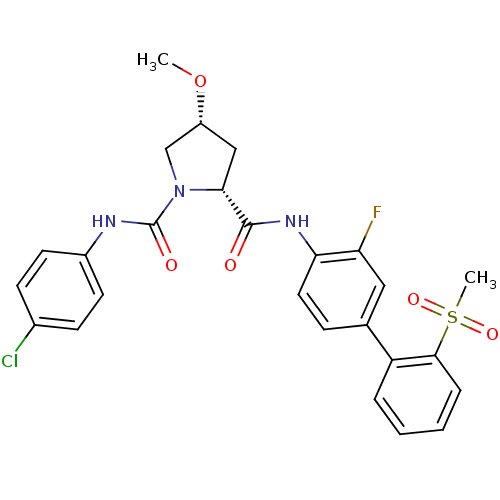
((2R,4R)-N1-(4-chlorophenyl)-N2-(3-fluoro-2'-(methy...)Show SMILES CO[C@@H]1C[C@@H](N(C1)C(=O)Nc1ccc(Cl)cc1)C(=O)Nc1ccc(cc1F)-c1ccccc1S(C)(=O)=O |r| Show InChI InChI=1S/C26H25ClFN3O5S/c1-36-19-14-23(31(15-19)26(33)29-18-10-8-17(27)9-11-18)25(32)30-22-12-7-16(13-21(22)28)20-5-3-4-6-24(20)37(2,34)35/h3-13,19,23H,14-15H2,1-2H3,(H,29,33)(H,30,32)/t19-,23-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development,
| Assay Description
FXa inhibition were determined by using an inhibition assay. |
Chem Biol Drug Des 69: 444-50 (2007)
Article DOI: 10.1111/j.1747-0285.2007.00520.x
BindingDB Entry DOI: 10.7270/Q2PZ5799 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Endothelin-1 receptor
(Homo sapiens (Human)) | BDBM50077934
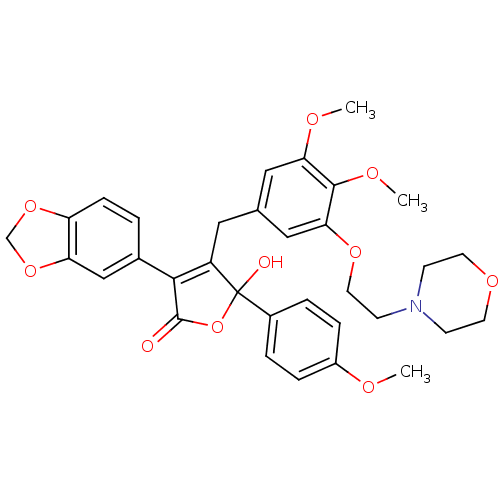
(3-Benzo[1,3]dioxol-5-yl-4-[3,4-dimethoxy-5-(2-morp...)Show SMILES COc1ccc(cc1)C1(O)OC(=O)C(=C1Cc1cc(OC)c(OC)c(OCCN2CCOCC2)c1)c1ccc2OCOc2c1 |c:14| Show InChI InChI=1S/C33H35NO10/c1-37-24-7-5-23(6-8-24)33(36)25(30(32(35)44-33)22-4-9-26-27(19-22)43-20-42-26)16-21-17-28(38-2)31(39-3)29(18-21)41-15-12-34-10-13-40-14-11-34/h4-9,17-19,36H,10-16,20H2,1-3H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Inhibition of [125I]-ET-1 binding to recombinant human Endothelin A receptor. |
J Med Chem 42: 2162-8 (1999)
Article DOI: 10.1021/jm980504w
BindingDB Entry DOI: 10.7270/Q2V12404 |
More data for this
Ligand-Target Pair | |
Endothelin-1 receptor
(Homo sapiens (Human)) | BDBM50077935
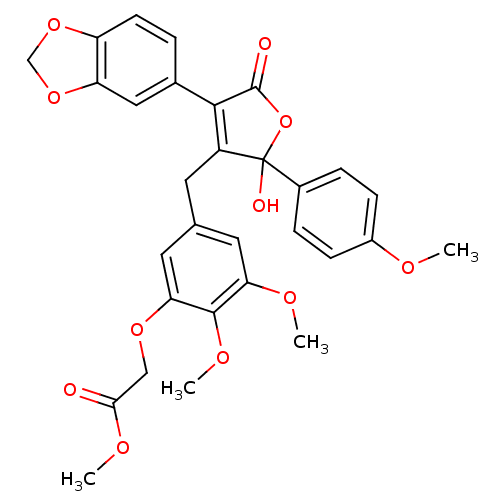
(CHEMBL308646 | {5-[4-Benzo[1,3]dioxol-5-yl-2-hydro...)Show SMILES COC(=O)COc1cc(CC2=C(C(=O)OC2(O)c2ccc(OC)cc2)c2ccc3OCOc3c2)cc(OC)c1OC |t:10| Show InChI InChI=1S/C30H28O11/c1-34-20-8-6-19(7-9-20)30(33)21(27(29(32)41-30)18-5-10-22-23(14-18)40-16-39-22)11-17-12-24(35-2)28(37-4)25(13-17)38-15-26(31)36-3/h5-10,12-14,33H,11,15-16H2,1-4H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Inhibition of [125I]-ET-1 binding to recombinant human Endothelin A receptor. |
J Med Chem 42: 2162-8 (1999)
Article DOI: 10.1021/jm980504w
BindingDB Entry DOI: 10.7270/Q2V12404 |
More data for this
Ligand-Target Pair | |
Endothelin-1 receptor
(Homo sapiens (Human)) | BDBM50077936
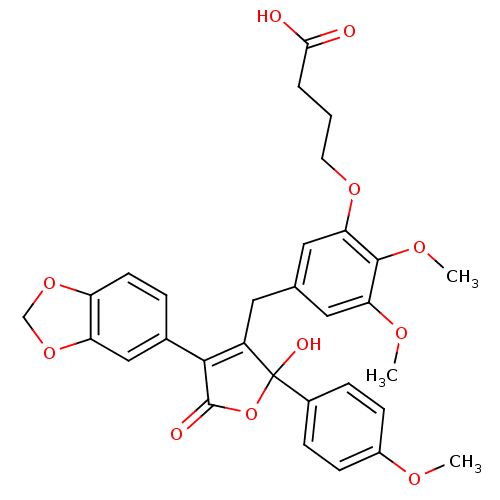
(4-{5-[4-Benzo[1,3]dioxol-5-yl-2-hydroxy-2-(4-metho...)Show SMILES COc1ccc(cc1)C1(O)OC(=O)C(=C1Cc1cc(OC)c(OC)c(OCCCC(O)=O)c1)c1ccc2OCOc2c1 |c:14| Show InChI InChI=1S/C31H30O11/c1-36-21-9-7-20(8-10-21)31(35)22(28(30(34)42-31)19-6-11-23-24(16-19)41-17-40-23)13-18-14-25(37-2)29(38-3)26(15-18)39-12-4-5-27(32)33/h6-11,14-16,35H,4-5,12-13,17H2,1-3H3,(H,32,33) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.290 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Inhibition of [125I]-ET-1 binding to recombinant human Endothelin A receptor. |
J Med Chem 42: 2162-8 (1999)
Article DOI: 10.1021/jm980504w
BindingDB Entry DOI: 10.7270/Q2V12404 |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM17967
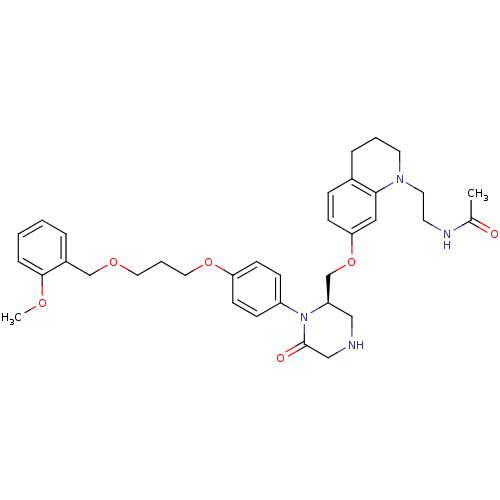
(CHEMBL411885 | Ketopiperazine-based compound, 16 |...)Show SMILES COc1ccccc1COCCCOc1ccc(cc1)N1[C@@H](COc2ccc3CCCN(CCNC(C)=O)c3c2)CNCC1=O |r| Show InChI InChI=1S/C35H44N4O6/c1-26(40)37-16-18-38-17-5-8-27-10-13-32(21-33(27)38)45-25-30-22-36-23-35(41)39(30)29-11-14-31(15-12-29)44-20-6-19-43-24-28-7-3-4-9-34(28)42-2/h3-4,7,9-15,21,30,36H,5-6,8,16-20,22-25H2,1-2H3,(H,37,40)/t30-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Pfizer
| Assay Description
The renin assay utilized a tandem Green Flourescent Protein (tGFP) substrate that was hydrolyzed by human rennin. The tGFP substrate contained a nine... |
Bioorg Med Chem 13: 2657-64 (2005)
Article DOI: 10.1016/j.bmc.2005.01.048
BindingDB Entry DOI: 10.7270/Q2KP80D6 |
More data for this
Ligand-Target Pair | |
Endothelin-1 receptor
(Homo sapiens (Human)) | BDBM50034267
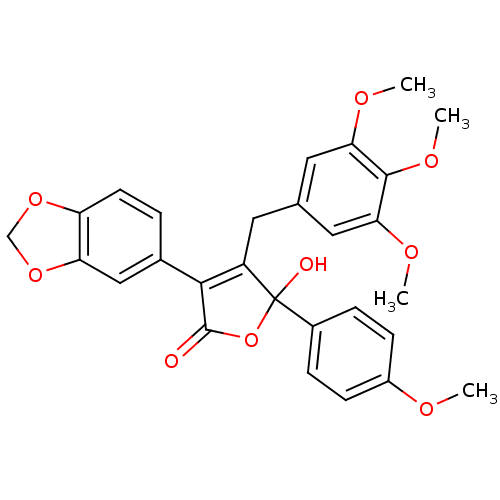
(3-(benzo[d][1,3]dioxol-5-yl)-5-hydroxy-5-(4-methox...)Show SMILES COc1ccc(cc1)C1(O)OC(=O)C(=C1Cc1cc(OC)c(OC)c(OC)c1)c1ccc2OCOc2c1 |c:14| Show InChI InChI=1S/C28H26O9/c1-31-19-8-6-18(7-9-19)28(30)20(11-16-12-23(32-2)26(34-4)24(13-16)33-3)25(27(29)37-28)17-5-10-21-22(14-17)36-15-35-21/h5-10,12-14,30H,11,15H2,1-4H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Inhibition of [125I]-ET-1 binding to recombinant human Endothelin A receptor. |
J Med Chem 42: 2162-8 (1999)
Article DOI: 10.1021/jm980504w
BindingDB Entry DOI: 10.7270/Q2V12404 |
More data for this
Ligand-Target Pair | |
Endothelin-1 receptor
(Homo sapiens (Human)) | BDBM50077946
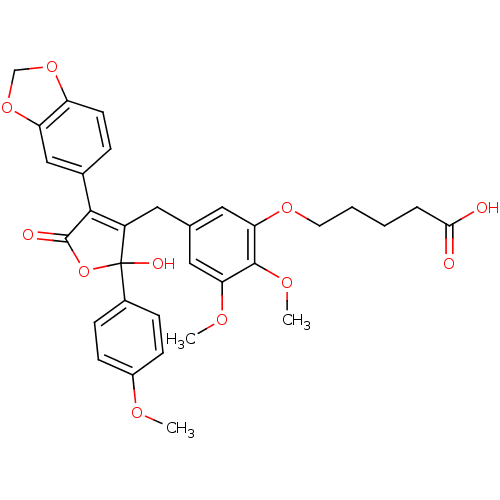
(5-{5-[4-Benzo[1,3]dioxol-5-yl-2-hydroxy-2-(4-metho...)Show SMILES COc1ccc(cc1)C1(O)OC(=O)C(=C1Cc1cc(OC)c(OC)c(OCCCCC(O)=O)c1)c1ccc2OCOc2c1 |c:14| Show InChI InChI=1S/C32H32O11/c1-37-22-10-8-21(9-11-22)32(36)23(29(31(35)43-32)20-7-12-24-25(17-20)42-18-41-24)14-19-15-26(38-2)30(39-3)27(16-19)40-13-5-4-6-28(33)34/h7-12,15-17,36H,4-6,13-14,18H2,1-3H3,(H,33,34) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Inhibition of [125I]-ET-1 binding to recombinant human Endothelin A receptor. |
J Med Chem 42: 2162-8 (1999)
Article DOI: 10.1021/jm980504w
BindingDB Entry DOI: 10.7270/Q2V12404 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50328728
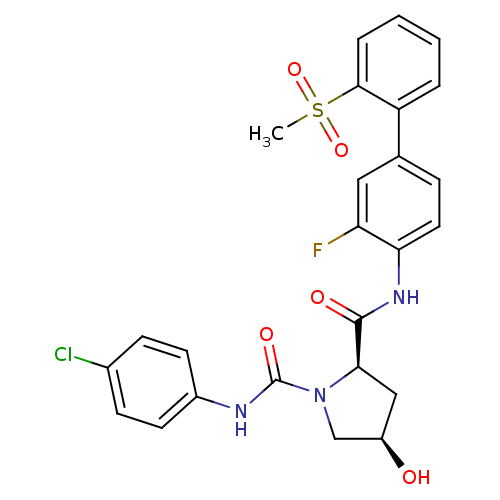
((2R,4R)-N1-(4-chlorophenyl)-N2-(3-fluoro-2'-(methy...)Show SMILES CS(=O)(=O)c1ccccc1-c1ccc(NC(=O)[C@H]2C[C@@H](O)CN2C(=O)Nc2ccc(Cl)cc2)c(F)c1 |r| Show InChI InChI=1S/C25H23ClFN3O5S/c1-36(34,35)23-5-3-2-4-19(23)15-6-11-21(20(27)12-15)29-24(32)22-13-18(31)14-30(22)25(33)28-17-9-7-16(26)8-10-17/h2-12,18,22,31H,13-14H2,1H3,(H,28,33)(H,29,32)/t18-,22-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.380 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development,
| Assay Description
FXa inhibition were determined by using an inhibition assay. |
Chem Biol Drug Des 69: 444-50 (2007)
Article DOI: 10.1111/j.1747-0285.2007.00520.x
BindingDB Entry DOI: 10.7270/Q2PZ5799 |
More data for this
Ligand-Target Pair | |
Endothelin-1 receptor
(Homo sapiens (Human)) | BDBM50077944
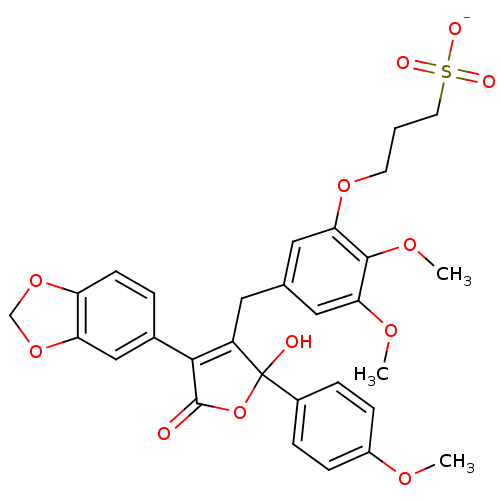
(CHEMBL408055 | Sodium; 3-{5-[4-benzo[1,3]dioxol-5-...)Show SMILES COc1ccc(cc1)C1(O)OC(=O)C(=C1Cc1cc(OC)c(OC)c(OCCCS([O-])(=O)=O)c1)c1ccc2OCOc2c1 |c:14| Show InChI InChI=1S/C30H30O12S/c1-36-21-8-6-20(7-9-21)30(32)22(27(29(31)42-30)19-5-10-23-24(16-19)41-17-40-23)13-18-14-25(37-2)28(38-3)26(15-18)39-11-4-12-43(33,34)35/h5-10,14-16,32H,4,11-13,17H2,1-3H3,(H,33,34,35)/p-1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.380 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Inhibition of [125I]-ET-1 binding to recombinant human Endothelin A receptor. |
J Med Chem 42: 2162-8 (1999)
Article DOI: 10.1021/jm980504w
BindingDB Entry DOI: 10.7270/Q2V12404 |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM17967

(CHEMBL411885 | Ketopiperazine-based compound, 16 |...)Show SMILES COc1ccccc1COCCCOc1ccc(cc1)N1[C@@H](COc2ccc3CCCN(CCNC(C)=O)c3c2)CNCC1=O |r| Show InChI InChI=1S/C35H44N4O6/c1-26(40)37-16-18-38-17-5-8-27-10-13-32(21-33(27)38)45-25-30-22-36-23-35(41)39(30)29-11-14-31(15-12-29)44-20-6-19-43-24-28-7-3-4-9-34(28)42-2/h3-4,7,9-15,21,30,36H,5-6,8,16-20,22-25H2,1-2H3,(H,37,40)/t30-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against renin in fluorescent tGFP assay |
Bioorg Med Chem Lett 15: 4713-6 (2005)
Article DOI: 10.1016/j.bmcl.2005.07.063
BindingDB Entry DOI: 10.7270/Q2SF2VP9 |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM17965
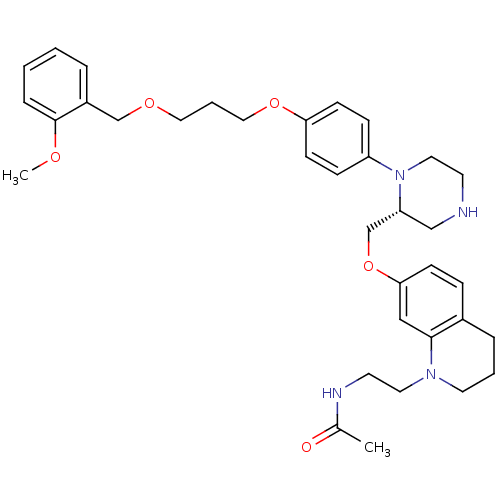
(N-[2-(7-{[(2R)-1-(4-{3-[(2-methoxyphenyl)methoxy]p...)Show SMILES COc1ccccc1COCCCOc1ccc(cc1)N1CCNC[C@@H]1COc1ccc2CCCN(CCNC(C)=O)c2c1 |r| Show InChI InChI=1S/C35H46N4O5/c1-27(40)37-17-19-38-18-5-8-28-10-13-33(23-34(28)38)44-26-31-24-36-16-20-39(31)30-11-14-32(15-12-30)43-22-6-21-42-25-29-7-3-4-9-35(29)41-2/h3-4,7,9-15,23,31,36H,5-6,8,16-22,24-26H2,1-2H3,(H,37,40)/t31-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Pfizer
| Assay Description
The renin assay utilized a tandem Green Flourescent Protein (tGFP) substrate that was hydrolyzed by human rennin. The tGFP substrate contained a nine... |
Bioorg Med Chem 13: 2657-64 (2005)
Article DOI: 10.1016/j.bmc.2005.01.048
BindingDB Entry DOI: 10.7270/Q2KP80D6 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Endothelin-1 receptor
(Homo sapiens (Human)) | BDBM50077941
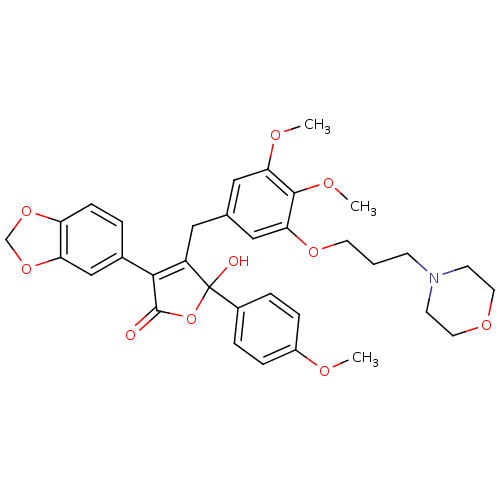
(3-Benzo[1,3]dioxol-5-yl-4-[3,4-dimethoxy-5-(3-morp...)Show SMILES COc1ccc(cc1)C1(O)OC(=O)C(=C1Cc1cc(OC)c(OC)c(OCCCN2CCOCC2)c1)c1ccc2OCOc2c1 |c:14| Show InChI InChI=1S/C34H37NO10/c1-38-25-8-6-24(7-9-25)34(37)26(31(33(36)45-34)23-5-10-27-28(20-23)44-21-43-27)17-22-18-29(39-2)32(40-3)30(19-22)42-14-4-11-35-12-15-41-16-13-35/h5-10,18-20,37H,4,11-17,21H2,1-3H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Inhibition of [125I]-ET-1 binding to recombinant human Endothelin A receptor. |
J Med Chem 42: 2162-8 (1999)
Article DOI: 10.1021/jm980504w
BindingDB Entry DOI: 10.7270/Q2V12404 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM81692
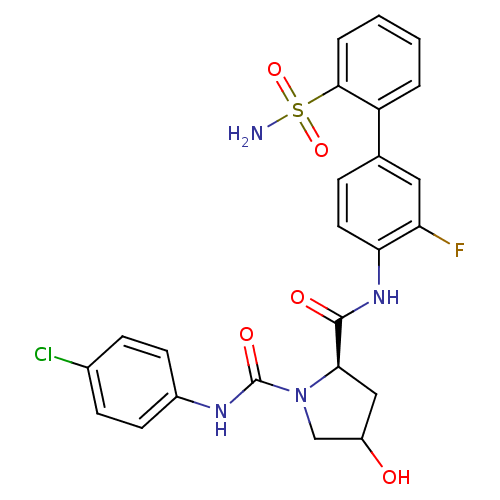
(4-Substituted Pyrrolidine Ring, 14)Show SMILES NS(=O)(=O)c1ccccc1-c1ccc(NC(=O)[C@H]2CC(O)CN2C(=O)Nc2ccc(Cl)cc2)c(F)c1 |r| Show InChI InChI=1S/C24H22ClFN4O5S/c25-15-6-8-16(9-7-15)28-24(33)30-13-17(31)12-21(30)23(32)29-20-10-5-14(11-19(20)26)18-3-1-2-4-22(18)36(27,34)35/h1-11,17,21,31H,12-13H2,(H,28,33)(H,29,32)(H2,27,34,35)/t17?,21-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.75 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development,
| Assay Description
FXa inhibition were determined by using an inhibition assay. |
Chem Biol Drug Des 69: 444-50 (2007)
Article DOI: 10.1111/j.1747-0285.2007.00520.x
BindingDB Entry DOI: 10.7270/Q2PZ5799 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM81698
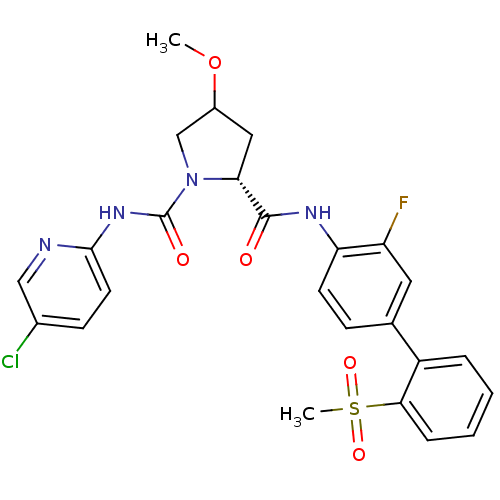
(4-Substituted Pyrrolidine Ring, 35)Show SMILES COC1C[C@@H](N(C1)C(=O)Nc1ccc(Cl)cn1)C(=O)Nc1ccc(cc1F)-c1ccccc1S(C)(=O)=O |r| Show InChI InChI=1S/C25H24ClFN4O5S/c1-36-17-12-21(31(14-17)25(33)30-23-10-8-16(26)13-28-23)24(32)29-20-9-7-15(11-19(20)27)18-5-3-4-6-22(18)37(2,34)35/h3-11,13,17,21H,12,14H2,1-2H3,(H,29,32)(H,28,30,33)/t17?,21-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development,
| Assay Description
FXa inhibition were determined by using an inhibition assay. |
Chem Biol Drug Des 69: 444-50 (2007)
Article DOI: 10.1111/j.1747-0285.2007.00520.x
BindingDB Entry DOI: 10.7270/Q2PZ5799 |
More data for this
Ligand-Target Pair | |
Endothelin-1 receptor
(Homo sapiens (Human)) | BDBM50077943
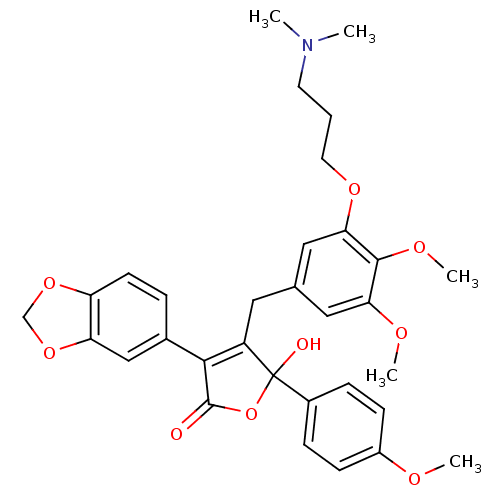
(3-Benzo[1,3]dioxol-5-yl-4-[3-(3-dimethylamino-prop...)Show SMILES COc1ccc(cc1)C1(O)OC(=O)C(=C1Cc1cc(OC)c(OC)c(OCCCN(C)C)c1)c1ccc2OCOc2c1 |c:14| Show InChI InChI=1S/C32H35NO9/c1-33(2)13-6-14-39-28-17-20(16-27(37-4)30(28)38-5)15-24-29(21-7-12-25-26(18-21)41-19-40-25)31(34)42-32(24,35)22-8-10-23(36-3)11-9-22/h7-12,16-18,35H,6,13-15,19H2,1-5H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Inhibition of [125I]-ET-1 binding to recombinant human Endothelin A receptor. |
J Med Chem 42: 2162-8 (1999)
Article DOI: 10.1021/jm980504w
BindingDB Entry DOI: 10.7270/Q2V12404 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM81693
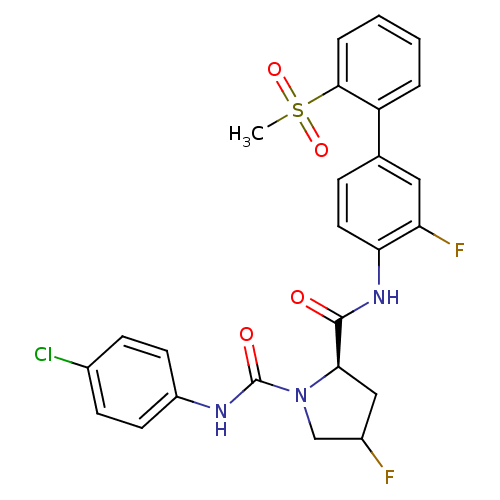
(4-Substituted Pyrrolidine Ring, 16 | 4-Substituted...)Show SMILES CS(=O)(=O)c1ccccc1-c1ccc(NC(=O)[C@H]2CC(F)CN2C(=O)Nc2ccc(Cl)cc2)c(F)c1 |r| Show InChI InChI=1S/C25H22ClF2N3O4S/c1-36(34,35)23-5-3-2-4-19(23)15-6-11-21(20(28)12-15)30-24(32)22-13-17(27)14-31(22)25(33)29-18-9-7-16(26)8-10-18/h2-12,17,22H,13-14H2,1H3,(H,29,33)(H,30,32)/t17?,22-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development,
| Assay Description
FXa inhibition were determined by using an inhibition assay. |
Chem Biol Drug Des 69: 444-50 (2007)
Article DOI: 10.1111/j.1747-0285.2007.00520.x
BindingDB Entry DOI: 10.7270/Q2PZ5799 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50328726

((2R,4R)-N1-(4-chlorophenyl)-N2-(3-fluoro-2'-(methy...)Show SMILES CO[C@@H]1C[C@@H](N(C1)C(=O)Nc1ccc(Cl)cc1)C(=O)Nc1ccc(cc1F)-c1ccccc1S(C)(=O)=O |r| Show InChI InChI=1S/C26H25ClFN3O5S/c1-36-19-14-23(31(15-19)26(33)29-18-10-8-17(27)9-11-18)25(32)30-22-12-7-16(13-21(22)28)20-5-3-4-6-24(20)37(2,34)35/h3-13,19,23H,14-15H2,1-2H3,(H,29,33)(H,30,32)/t19-,23-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development,
| Assay Description
FXa inhibition were determined by using an inhibition assay. |
Chem Biol Drug Des 69: 444-50 (2007)
Article DOI: 10.1111/j.1747-0285.2007.00520.x
BindingDB Entry DOI: 10.7270/Q2PZ5799 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Endothelin-1 receptor
(Homo sapiens (Human)) | BDBM50077947
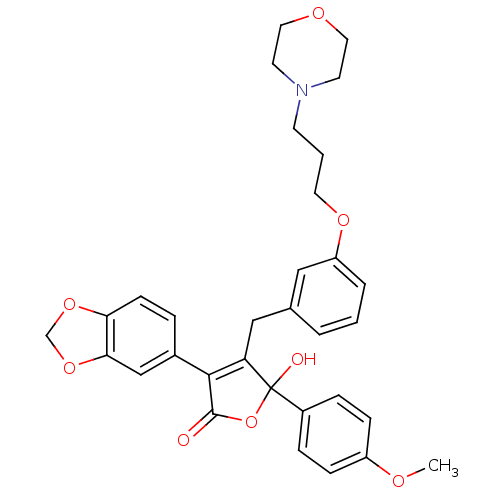
(3-Benzo[1,3]dioxol-5-yl-5-hydroxy-5-(4-methoxy-phe...)Show SMILES COc1ccc(cc1)C1(O)OC(=O)C(=C1Cc1cccc(OCCCN2CCOCC2)c1)c1ccc2OCOc2c1 |c:14| Show InChI InChI=1S/C32H33NO8/c1-36-25-9-7-24(8-10-25)32(35)27(30(31(34)41-32)23-6-11-28-29(20-23)40-21-39-28)19-22-4-2-5-26(18-22)38-15-3-12-33-13-16-37-17-14-33/h2,4-11,18,20,35H,3,12-17,19,21H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Inhibition of [125I]-ET-1 binding to recombinant human Endothelin A receptor. |
J Med Chem 42: 2162-8 (1999)
Article DOI: 10.1021/jm980504w
BindingDB Entry DOI: 10.7270/Q2V12404 |
More data for this
Ligand-Target Pair | |
Endothelin-1 receptor
(Homo sapiens (Human)) | BDBM50077938
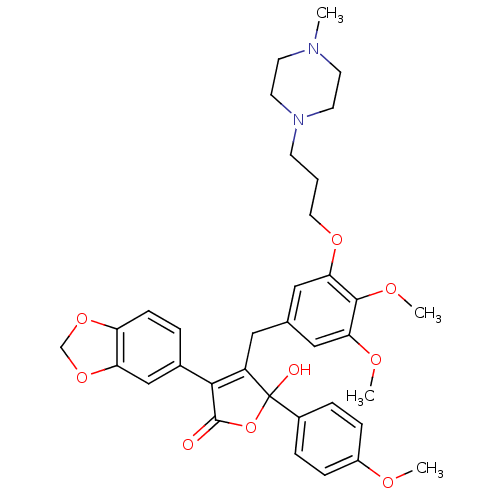
(3-Benzo[1,3]dioxol-5-yl-4-{3,4-dimethoxy-5-[3-(4-m...)Show SMILES COc1ccc(cc1)C1(O)OC(=O)C(=C1Cc1cc(OC)c(OC)c(OCCCN2CCN(C)CC2)c1)c1ccc2OCOc2c1 |c:14| Show InChI InChI=1S/C35H40N2O9/c1-36-13-15-37(16-14-36)12-5-17-43-31-20-23(19-30(41-3)33(31)42-4)18-27-32(24-6-11-28-29(21-24)45-22-44-28)34(38)46-35(27,39)25-7-9-26(40-2)10-8-25/h6-11,19-21,39H,5,12-18,22H2,1-4H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Inhibition of [125I]-ET-1 binding to recombinant human Endothelin A receptor. |
J Med Chem 42: 2162-8 (1999)
Article DOI: 10.1021/jm980504w
BindingDB Entry DOI: 10.7270/Q2V12404 |
More data for this
Ligand-Target Pair | |
Endothelin-1 receptor
(Homo sapiens (Human)) | BDBM50077945

(3-Benzo[1,3]dioxol-5-yl-4-[3-(2-dimethylamino-etho...)Show SMILES COc1ccc(cc1)C1(O)OC(=O)C(=C1Cc1cc(OC)c(OC)c(OCCN(C)C)c1)c1ccc2OCOc2c1 |c:14| Show InChI InChI=1S/C31H33NO9/c1-32(2)12-13-38-27-16-19(15-26(36-4)29(27)37-5)14-23-28(20-6-11-24-25(17-20)40-18-39-24)30(33)41-31(23,34)21-7-9-22(35-3)10-8-21/h6-11,15-17,34H,12-14,18H2,1-5H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Inhibition of [125I]-ET-1 binding to recombinant human Endothelin A receptor. |
J Med Chem 42: 2162-8 (1999)
Article DOI: 10.1021/jm980504w
BindingDB Entry DOI: 10.7270/Q2V12404 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM81695
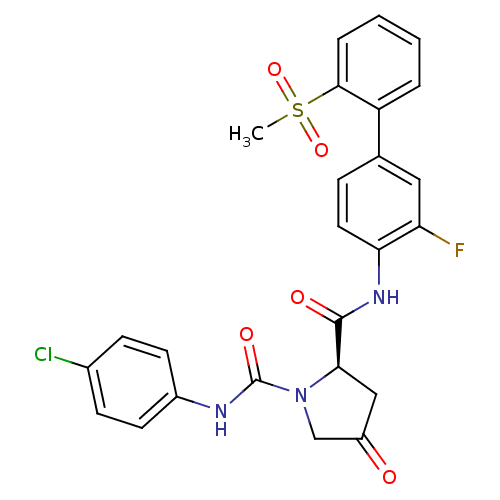
(4-Substituted Pyrrolidine Ring, 20)Show SMILES CS(=O)(=O)c1ccccc1-c1ccc(NC(=O)[C@H]2CC(=O)CN2C(=O)Nc2ccc(Cl)cc2)c(F)c1 |r| Show InChI InChI=1S/C25H21ClFN3O5S/c1-36(34,35)23-5-3-2-4-19(23)15-6-11-21(20(27)12-15)29-24(32)22-13-18(31)14-30(22)25(33)28-17-9-7-16(26)8-10-17/h2-12,22H,13-14H2,1H3,(H,28,33)(H,29,32)/t22-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development,
| Assay Description
FXa inhibition were determined by using an inhibition assay. |
Chem Biol Drug Des 69: 444-50 (2007)
Article DOI: 10.1111/j.1747-0285.2007.00520.x
BindingDB Entry DOI: 10.7270/Q2PZ5799 |
More data for this
Ligand-Target Pair | |
Endothelin-1 receptor
(Homo sapiens (Human)) | BDBM50077942
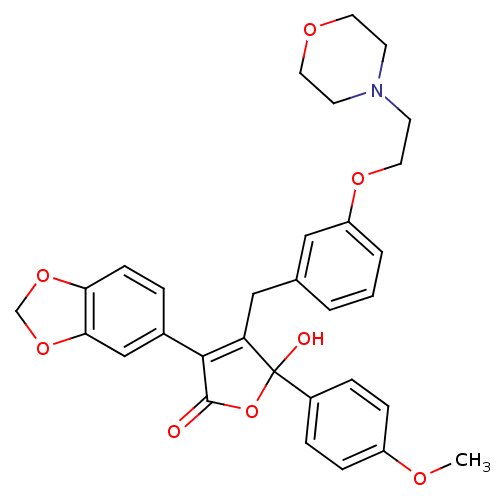
(3-Benzo[1,3]dioxol-5-yl-5-hydroxy-5-(4-methoxy-phe...)Show SMILES COc1ccc(cc1)C1(O)OC(=O)C(=C1Cc1cccc(OCCN2CCOCC2)c1)c1ccc2OCOc2c1 |c:14| Show InChI InChI=1S/C31H31NO8/c1-35-24-8-6-23(7-9-24)31(34)26(29(30(33)40-31)22-5-10-27-28(19-22)39-20-38-27)18-21-3-2-4-25(17-21)37-16-13-32-11-14-36-15-12-32/h2-10,17,19,34H,11-16,18,20H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Inhibition of [125I]-ET-1 binding to recombinant human Endothelin A receptor. |
J Med Chem 42: 2162-8 (1999)
Article DOI: 10.1021/jm980504w
BindingDB Entry DOI: 10.7270/Q2V12404 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM81697
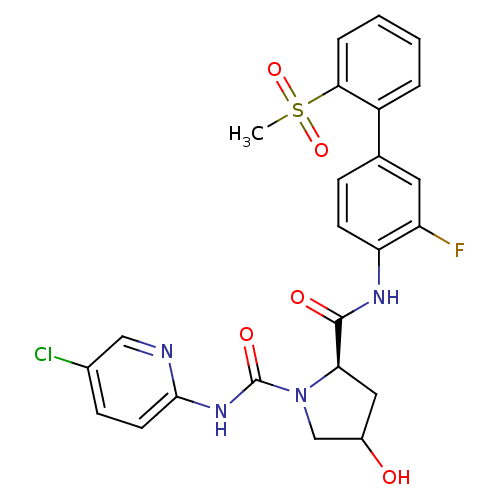
(4-Substituted Pyrrolidine Ring, 34)Show SMILES CS(=O)(=O)c1ccccc1-c1ccc(NC(=O)[C@H]2CC(O)CN2C(=O)Nc2ccc(Cl)cn2)c(F)c1 |r| Show InChI InChI=1S/C24H22ClFN4O5S/c1-36(34,35)21-5-3-2-4-17(21)14-6-8-19(18(26)10-14)28-23(32)20-11-16(31)13-30(20)24(33)29-22-9-7-15(25)12-27-22/h2-10,12,16,20,31H,11,13H2,1H3,(H,28,32)(H,27,29,33)/t16?,20-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development,
| Assay Description
FXa inhibition were determined by using an inhibition assay. |
Chem Biol Drug Des 69: 444-50 (2007)
Article DOI: 10.1111/j.1747-0285.2007.00520.x
BindingDB Entry DOI: 10.7270/Q2PZ5799 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM81696

(4-Substituted Pyrrolidine Ring, 21)Show SMILES CS(=O)(=O)c1ccccc1-c1ccc(NC(=O)[C@H]2CC(F)(F)CN2C(=O)Nc2ccc(Cl)cc2)c(F)c1 |r| Show InChI InChI=1S/C25H21ClF3N3O4S/c1-37(35,36)22-5-3-2-4-18(22)15-6-11-20(19(27)12-15)31-23(33)21-13-25(28,29)14-32(21)24(34)30-17-9-7-16(26)8-10-17/h2-12,21H,13-14H2,1H3,(H,30,34)(H,31,33)/t21-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development,
| Assay Description
FXa inhibition were determined by using an inhibition assay. |
Chem Biol Drug Des 69: 444-50 (2007)
Article DOI: 10.1111/j.1747-0285.2007.00520.x
BindingDB Entry DOI: 10.7270/Q2PZ5799 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50328728

((2R,4R)-N1-(4-chlorophenyl)-N2-(3-fluoro-2'-(methy...)Show SMILES CS(=O)(=O)c1ccccc1-c1ccc(NC(=O)[C@H]2C[C@@H](O)CN2C(=O)Nc2ccc(Cl)cc2)c(F)c1 |r| Show InChI InChI=1S/C25H23ClFN3O5S/c1-36(34,35)23-5-3-2-4-19(23)15-6-11-21(20(27)12-15)29-24(32)22-13-18(31)14-30(22)25(33)28-17-9-7-16(26)8-10-17/h2-12,18,22,31H,13-14H2,1H3,(H,28,33)(H,29,32)/t18-,22-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development,
| Assay Description
FXa inhibition were determined by using an inhibition assay. |
Chem Biol Drug Des 69: 444-50 (2007)
Article DOI: 10.1111/j.1747-0285.2007.00520.x
BindingDB Entry DOI: 10.7270/Q2PZ5799 |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM18031
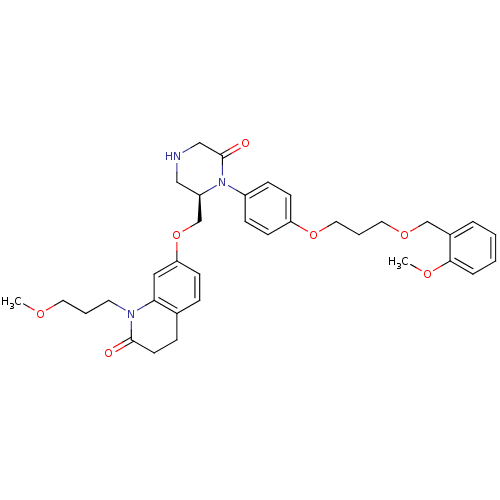
(CHEMBL193816 | Ketopiperazine-based inhibitor, 11)Show SMILES COCCCN1C(=O)CCc2ccc(OC[C@H]3CNCC(=O)N3c3ccc(OCCCOCc4ccccc4OC)cc3)cc12 |r| Show InChI InChI=1S/C35H43N3O7/c1-41-18-5-17-37-32-21-31(13-9-26(32)10-16-34(37)39)45-25-29-22-36-23-35(40)38(29)28-11-14-30(15-12-28)44-20-6-19-43-24-27-7-3-4-8-33(27)42-2/h3-4,7-9,11-15,21,29,36H,5-6,10,16-20,22-25H2,1-2H3/t29-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Concentration required to inhibit renin activity by 50% |
Bioorg Med Chem Lett 15: 2371-4 (2005)
Article DOI: 10.1016/j.bmcl.2005.02.085
BindingDB Entry DOI: 10.7270/Q2PV6JW0 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM81693

(4-Substituted Pyrrolidine Ring, 16 | 4-Substituted...)Show SMILES CS(=O)(=O)c1ccccc1-c1ccc(NC(=O)[C@H]2CC(F)CN2C(=O)Nc2ccc(Cl)cc2)c(F)c1 |r| Show InChI InChI=1S/C25H22ClF2N3O4S/c1-36(34,35)23-5-3-2-4-19(23)15-6-11-21(20(28)12-15)30-24(32)22-13-17(27)14-31(22)25(33)29-18-9-7-16(26)8-10-18/h2-12,17,22H,13-14H2,1H3,(H,29,33)(H,30,32)/t17?,22-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development,
| Assay Description
FXa inhibition were determined by using an inhibition assay. |
Chem Biol Drug Des 69: 444-50 (2007)
Article DOI: 10.1111/j.1747-0285.2007.00520.x
BindingDB Entry DOI: 10.7270/Q2PZ5799 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM81699
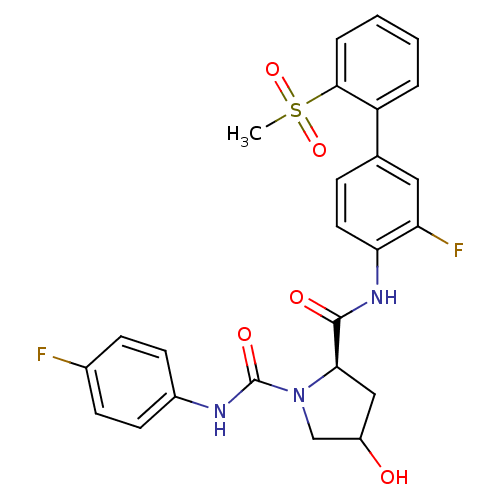
(4-Substituted Pyrrolidine Ring, 36)Show SMILES CS(=O)(=O)c1ccccc1-c1ccc(NC(=O)[C@H]2CC(O)CN2C(=O)Nc2ccc(F)cc2)c(F)c1 |r| Show InChI InChI=1S/C25H23F2N3O5S/c1-36(34,35)23-5-3-2-4-19(23)15-6-11-21(20(27)12-15)29-24(32)22-13-18(31)14-30(22)25(33)28-17-9-7-16(26)8-10-17/h2-12,18,22,31H,13-14H2,1H3,(H,28,33)(H,29,32)/t18?,22-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development,
| Assay Description
FXa inhibition were determined by using an inhibition assay. |
Chem Biol Drug Des 69: 444-50 (2007)
Article DOI: 10.1111/j.1747-0285.2007.00520.x
BindingDB Entry DOI: 10.7270/Q2PZ5799 |
More data for this
Ligand-Target Pair | |
Endothelin-1 receptor
(Homo sapiens (Human)) | BDBM50077931

(CHEMBL70017 | {5-[4-Benzo[1,3]dioxol-5-yl-2-hydrox...)Show SMILES COc1ccc(cc1)C1(O)OC(=O)C(=C1Cc1cc(OC)c(OC)c(OCC(O)=O)c1)c1ccc2OCOc2c1 |c:14| Show InChI InChI=1S/C29H26O11/c1-34-19-7-5-18(6-8-19)29(33)20(26(28(32)40-29)17-4-9-21-22(13-17)39-15-38-21)10-16-11-23(35-2)27(36-3)24(12-16)37-14-25(30)31/h4-9,11-13,33H,10,14-15H2,1-3H3,(H,30,31) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 8.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Inhibition of [125I]-ET-1 binding to recombinant human Endothelin A receptor. |
J Med Chem 42: 2162-8 (1999)
Article DOI: 10.1021/jm980504w
BindingDB Entry DOI: 10.7270/Q2V12404 |
More data for this
Ligand-Target Pair | |
Endothelin-1 receptor
(Homo sapiens (Human)) | BDBM50077937
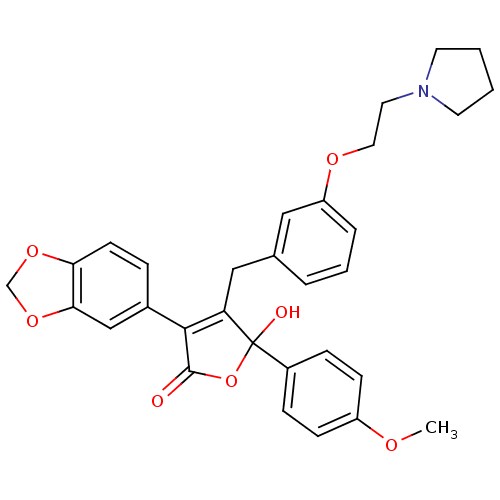
(3-Benzo[1,3]dioxol-5-yl-5-hydroxy-5-(4-methoxy-phe...)Show SMILES COc1ccc(cc1)C1(O)OC(=O)C(=C1Cc1cccc(OCCN2CCCC2)c1)c1ccc2OCOc2c1 |c:14| Show InChI InChI=1S/C31H31NO7/c1-35-24-10-8-23(9-11-24)31(34)26(29(30(33)39-31)22-7-12-27-28(19-22)38-20-37-27)18-21-5-4-6-25(17-21)36-16-15-32-13-2-3-14-32/h4-12,17,19,34H,2-3,13-16,18,20H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Inhibition of [125I]-ET-1 binding to recombinant human Endothelin A receptor. |
J Med Chem 42: 2162-8 (1999)
Article DOI: 10.1021/jm980504w
BindingDB Entry DOI: 10.7270/Q2V12404 |
More data for this
Ligand-Target Pair | |
Endothelin-1 receptor
(Homo sapiens (Human)) | BDBM50077939
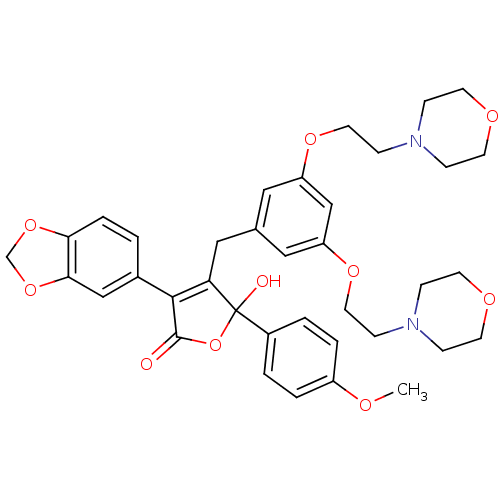
(3-Benzo[1,3]dioxol-5-yl-4-[3,5-bis-(2-morpholin-4-...)Show SMILES COc1ccc(cc1)C1(O)OC(=O)C(=C1Cc1cc(OCCN2CCOCC2)cc(OCCN2CCOCC2)c1)c1ccc2OCOc2c1 |c:14| Show InChI InChI=1S/C37H42N2O10/c1-42-29-5-3-28(4-6-29)37(41)32(35(36(40)49-37)27-2-7-33-34(23-27)48-25-47-33)22-26-20-30(45-18-12-38-8-14-43-15-9-38)24-31(21-26)46-19-13-39-10-16-44-17-11-39/h2-7,20-21,23-24,41H,8-19,22,25H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >10 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Inhibition of [125I]-ET-1 binding to recombinant human Endothelin A receptor. |
J Med Chem 42: 2162-8 (1999)
Article DOI: 10.1021/jm980504w
BindingDB Entry DOI: 10.7270/Q2V12404 |
More data for this
Ligand-Target Pair | |
Endothelin-1 receptor
(Homo sapiens (Human)) | BDBM50077928
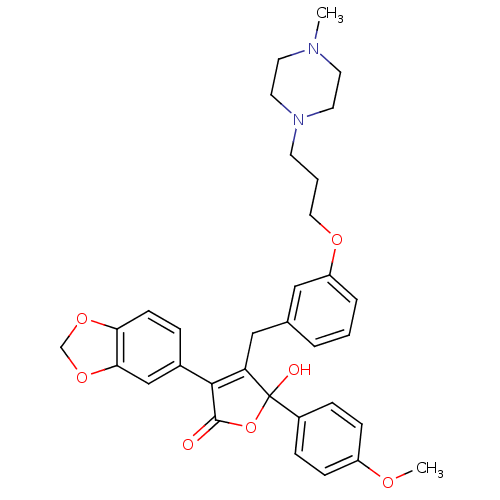
(3-Benzo[1,3]dioxol-5-yl-5-hydroxy-5-(4-methoxy-phe...)Show SMILES COc1ccc(cc1)C1(O)OC(=O)C(=C1Cc1cccc(OCCCN2CCN(C)CC2)c1)c1ccc2OCOc2c1 |c:14| Show InChI InChI=1S/C33H36N2O7/c1-34-14-16-35(17-15-34)13-4-18-39-27-6-3-5-23(19-27)20-28-31(24-7-12-29-30(21-24)41-22-40-29)32(36)42-33(28,37)25-8-10-26(38-2)11-9-25/h3,5-12,19,21,37H,4,13-18,20,22H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Inhibition of [125I]-ET-1 binding to recombinant human Endothelin A receptor. |
J Med Chem 42: 2162-8 (1999)
Article DOI: 10.1021/jm980504w
BindingDB Entry DOI: 10.7270/Q2V12404 |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50165800

(7-((S)-1-{4-[3-(2-Methoxy-benzyloxy)-propoxy]-phen...)Show SMILES COCCCN1C(=O)CCc2ccc(OC[C@@H]3CNCC(=O)N3c3ccc(OCCCOCc4ccccc4OC)cc3)cc12 Show InChI InChI=1S/C35H43N3O7/c1-41-18-5-17-37-32-21-31(13-9-26(32)10-16-34(37)39)45-25-29-22-36-23-35(40)38(29)28-11-14-30(15-12-28)44-20-6-19-43-24-27-7-3-4-8-33(27)42-2/h3-4,7-9,11-15,21,29,36H,5-6,10,16-20,22-25H2,1-2H3/t29-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Concentration required to inhibit renin activity by 50% |
Bioorg Med Chem Lett 15: 2371-4 (2005)
Article DOI: 10.1016/j.bmcl.2005.02.085
BindingDB Entry DOI: 10.7270/Q2PV6JW0 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50328725
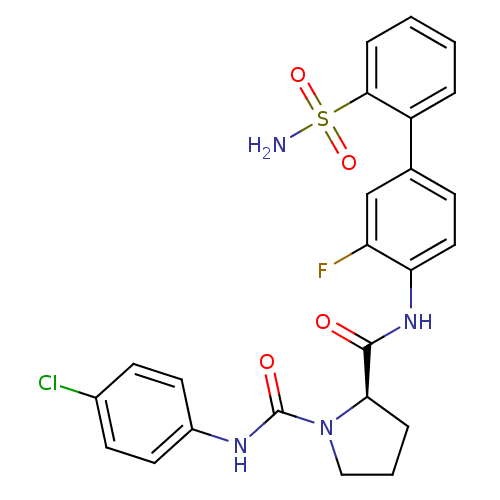
((R)-N1-(4-chlorophenyl)-N2-(3-fluoro-2'-sulfamoylb...)Show SMILES NS(=O)(=O)c1ccccc1-c1ccc(NC(=O)[C@H]2CCCN2C(=O)Nc2ccc(Cl)cc2)c(F)c1 |r| Show InChI InChI=1S/C24H22ClFN4O4S/c25-16-8-10-17(11-9-16)28-24(32)30-13-3-5-21(30)23(31)29-20-12-7-15(14-19(20)26)18-4-1-2-6-22(18)35(27,33)34/h1-2,4,6-12,14,21H,3,5,13H2,(H,28,32)(H,29,31)(H2,27,33,34)/t21-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development,
| Assay Description
FXa inhibition were determined by using an inhibition assay. |
Chem Biol Drug Des 69: 444-50 (2007)
Article DOI: 10.1111/j.1747-0285.2007.00520.x
BindingDB Entry DOI: 10.7270/Q2PZ5799 |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50165796
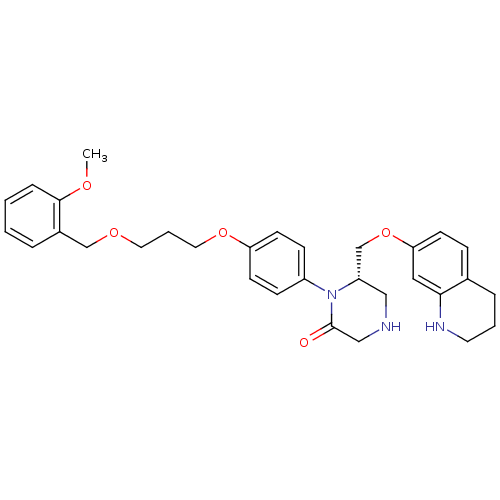
((S)-1-{4-[3-(2-Methoxy-benzyloxy)-propoxy]-phenyl}...)Show SMILES COc1ccccc1COCCCOc1ccc(cc1)N1[C@H](COc2ccc3CCCNc3c2)CNCC1=O Show InChI InChI=1S/C31H37N3O5/c1-36-30-8-3-2-6-24(30)21-37-16-5-17-38-27-13-10-25(11-14-27)34-26(19-32-20-31(34)35)22-39-28-12-9-23-7-4-15-33-29(23)18-28/h2-3,6,8-14,18,26,32-33H,4-5,7,15-17,19-22H2,1H3/t26-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Concentration required to inhibit renin activity by 50% |
Bioorg Med Chem Lett 15: 2371-4 (2005)
Article DOI: 10.1016/j.bmcl.2005.02.085
BindingDB Entry DOI: 10.7270/Q2PV6JW0 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Endothelin-1 receptor
(Homo sapiens (Human)) | BDBM50077929
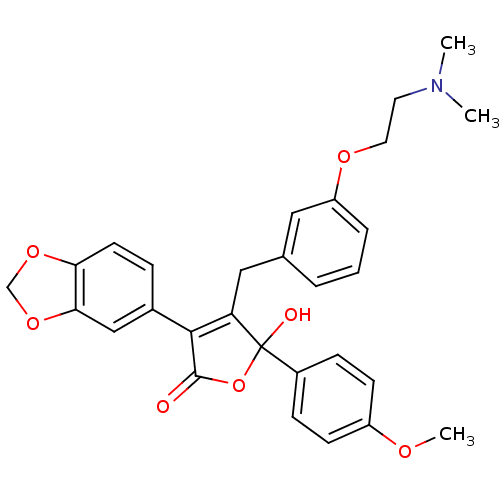
(3-Benzo[1,3]dioxol-5-yl-4-[3-(2-dimethylamino-etho...)Show SMILES COc1ccc(cc1)C1(O)OC(=O)C(=C1Cc1cccc(OCCN(C)C)c1)c1ccc2OCOc2c1 |c:14| Show InChI InChI=1S/C29H29NO7/c1-30(2)13-14-34-23-6-4-5-19(15-23)16-24-27(20-7-12-25-26(17-20)36-18-35-25)28(31)37-29(24,32)21-8-10-22(33-3)11-9-21/h4-12,15,17,32H,13-14,16,18H2,1-3H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Inhibition of [125I]-ET-1 binding to recombinant human Endothelin A receptor. |
J Med Chem 42: 2162-8 (1999)
Article DOI: 10.1021/jm980504w
BindingDB Entry DOI: 10.7270/Q2V12404 |
More data for this
Ligand-Target Pair | |
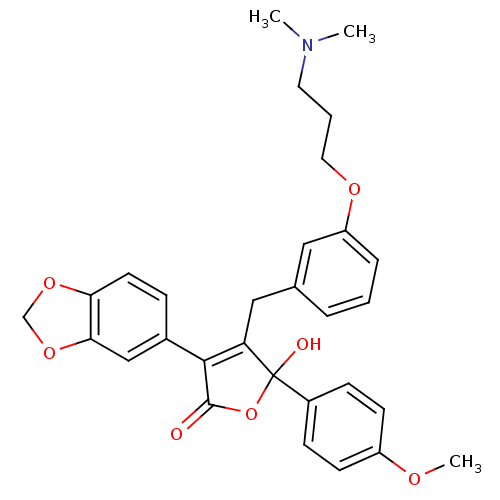
Endothelin-1 receptor
(Homo sapiens (Human)) | BDBM50077940
(3-Benzo[1,3]dioxol-5-yl-4-[3-(3-dimethylamino-prop...)Show SMILES COc1ccc(cc1)C1(O)OC(=O)C(=C1Cc1cccc(OCCCN(C)C)c1)c1ccc2OCOc2c1 |c:14| Show InChI InChI=1S/C30H31NO7/c1-31(2)14-5-15-35-24-7-4-6-20(16-24)17-25-28(21-8-13-26-27(18-21)37-19-36-26)29(32)38-30(25,33)22-9-11-23(34-3)12-10-22/h4,6-13,16,18,33H,5,14-15,17,19H2,1-3H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Inhibition of [125I]-ET-1 binding to recombinant human Endothelin A receptor. |
J Med Chem 42: 2162-8 (1999)
Article DOI: 10.1021/jm980504w
BindingDB Entry DOI: 10.7270/Q2V12404 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM81681
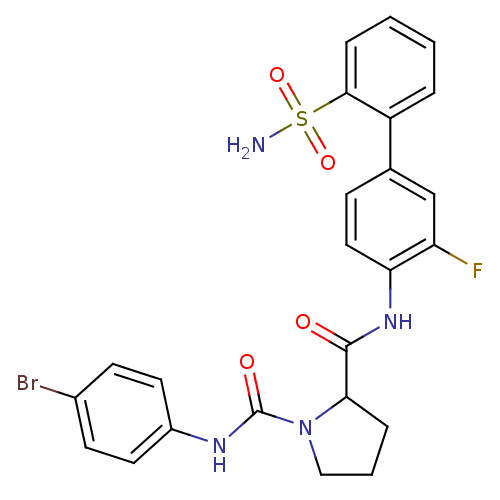
(P1 Phenyl Ring, 22)Show SMILES NS(=O)(=O)c1ccccc1-c1ccc(NC(=O)C2CCCN2C(=O)Nc2ccc(Br)cc2)c(F)c1 Show InChI InChI=1S/C24H22BrFN4O4S/c25-16-8-10-17(11-9-16)28-24(32)30-13-3-5-21(30)23(31)29-20-12-7-15(14-19(20)26)18-4-1-2-6-22(18)35(27,33)34/h1-2,4,6-12,14,21H,3,5,13H2,(H,28,32)(H,29,31)(H2,27,33,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development,
| Assay Description
FXa inhibition were determined by using an inhibition assay. |
Chem Biol Drug Des 69: 444-50 (2007)
Article DOI: 10.1111/j.1747-0285.2007.00520.x
BindingDB Entry DOI: 10.7270/Q2PZ5799 |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50165802
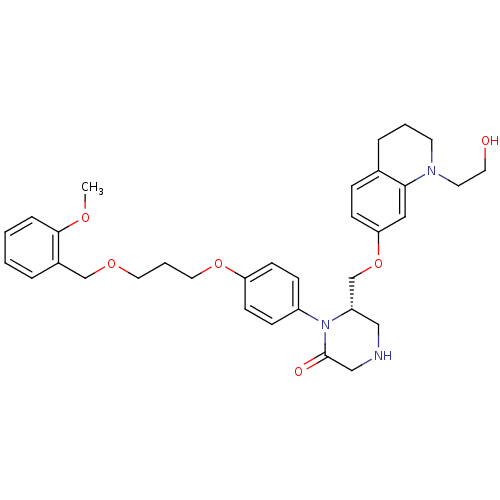
((S)-6-[1-(2-Hydroxy-ethyl)-1,2,3,4-tetrahydro-quin...)Show SMILES COc1ccccc1COCCCOc1ccc(cc1)N1[C@H](COc2ccc3CCCN(CCO)c3c2)CNCC1=O Show InChI InChI=1S/C33H41N3O6/c1-39-32-8-3-2-6-26(32)23-40-18-5-19-41-29-13-10-27(11-14-29)36-28(21-34-22-33(36)38)24-42-30-12-9-25-7-4-15-35(16-17-37)31(25)20-30/h2-3,6,8-14,20,28,34,37H,4-5,7,15-19,21-24H2,1H3/t28-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Concentration required to inhibit renin activity by 50% |
Bioorg Med Chem Lett 15: 2371-4 (2005)
Article DOI: 10.1016/j.bmcl.2005.02.085
BindingDB Entry DOI: 10.7270/Q2PV6JW0 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Renin
(Homo sapiens (Human)) | BDBM50165804
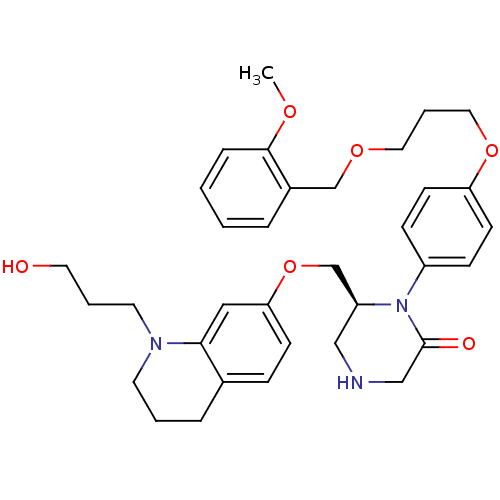
((S)-6-[1-(3-Hydroxy-propyl)-1,2,3,4-tetrahydro-qui...)Show SMILES COc1ccccc1COCCCOc1ccc(cc1)N1[C@H](COc2ccc3CCCN(CCCO)c3c2)CNCC1=O Show InChI InChI=1S/C34H43N3O6/c1-40-33-9-3-2-7-27(33)24-41-19-6-20-42-30-14-11-28(12-15-30)37-29(22-35-23-34(37)39)25-43-31-13-10-26-8-4-16-36(17-5-18-38)32(26)21-31/h2-3,7,9-15,21,29,35,38H,4-6,8,16-20,22-25H2,1H3/t29-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Concentration required to inhibit renin activity by 50% |
Bioorg Med Chem Lett 15: 2371-4 (2005)
Article DOI: 10.1016/j.bmcl.2005.02.085
BindingDB Entry DOI: 10.7270/Q2PV6JW0 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50328725

((R)-N1-(4-chlorophenyl)-N2-(3-fluoro-2'-sulfamoylb...)Show SMILES NS(=O)(=O)c1ccccc1-c1ccc(NC(=O)[C@H]2CCCN2C(=O)Nc2ccc(Cl)cc2)c(F)c1 |r| Show InChI InChI=1S/C24H22ClFN4O4S/c25-16-8-10-17(11-9-16)28-24(32)30-13-3-5-21(30)23(31)29-20-12-7-15(14-19(20)26)18-4-1-2-6-22(18)35(27,33)34/h1-2,4,6-12,14,21H,3,5,13H2,(H,28,32)(H,29,31)(H2,27,33,34)/t21-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development,
| Assay Description
FXa inhibition were determined by using an inhibition assay. |
Chem Biol Drug Des 69: 444-50 (2007)
Article DOI: 10.1111/j.1747-0285.2007.00520.x
BindingDB Entry DOI: 10.7270/Q2PZ5799 |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM18029
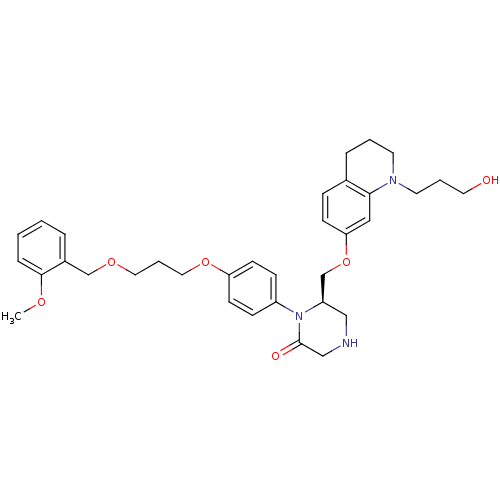
((6R)-6-({[1-(3-hydroxypropyl)-1,2,3,4-tetrahydroqu...)Show SMILES COc1ccccc1COCCCOc1ccc(cc1)N1[C@@H](COc2ccc3CCCN(CCCO)c3c2)CNCC1=O |r| Show InChI InChI=1S/C34H43N3O6/c1-40-33-9-3-2-7-27(33)24-41-19-6-20-42-30-14-11-28(12-15-30)37-29(22-35-23-34(37)39)25-43-31-13-10-26-8-4-16-36(17-5-18-38)32(26)21-31/h2-3,7,9-15,21,29,35,38H,4-6,8,16-20,22-25H2,1H3/t29-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Concentration required to inhibit renin activity by 50% |
Bioorg Med Chem Lett 15: 2371-4 (2005)
Article DOI: 10.1016/j.bmcl.2005.02.085
BindingDB Entry DOI: 10.7270/Q2PV6JW0 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Renin
(Homo sapiens (Human)) | BDBM17966
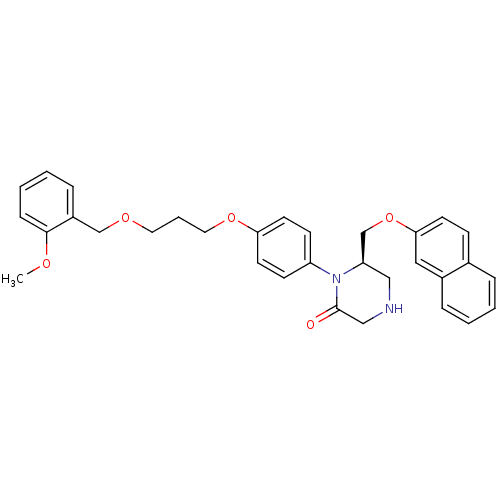
((6R)-1-(4-{3-[(2-methoxyphenyl)methoxy]propoxy}phe...)Show SMILES COc1ccccc1COCCCOc1ccc(cc1)N1[C@@H](COc2ccc3ccccc3c2)CNCC1=O |r| Show InChI InChI=1S/C32H34N2O5/c1-36-31-10-5-4-9-26(31)22-37-17-6-18-38-29-15-12-27(13-16-29)34-28(20-33-21-32(34)35)23-39-30-14-11-24-7-2-3-8-25(24)19-30/h2-5,7-16,19,28,33H,6,17-18,20-23H2,1H3/t28-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 54 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Pfizer
| Assay Description
The renin assay utilized a tandem Green Flourescent Protein (tGFP) substrate that was hydrolyzed by human rennin. The tGFP substrate contained a nine... |
Bioorg Med Chem 13: 2657-64 (2005)
Article DOI: 10.1016/j.bmc.2005.01.048
BindingDB Entry DOI: 10.7270/Q2KP80D6 |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM17966

((6R)-1-(4-{3-[(2-methoxyphenyl)methoxy]propoxy}phe...)Show SMILES COc1ccccc1COCCCOc1ccc(cc1)N1[C@@H](COc2ccc3ccccc3c2)CNCC1=O |r| Show InChI InChI=1S/C32H34N2O5/c1-36-31-10-5-4-9-26(31)22-37-17-6-18-38-29-15-12-27(13-16-29)34-28(20-33-21-32(34)35)23-39-30-14-11-24-7-2-3-8-25(24)19-30/h2-5,7-16,19,28,33H,6,17-18,20-23H2,1H3/t28-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 54 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Concentration required to inhibit renin activity by 50% |
Bioorg Med Chem Lett 15: 2371-4 (2005)
Article DOI: 10.1016/j.bmcl.2005.02.085
BindingDB Entry DOI: 10.7270/Q2PV6JW0 |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50165803
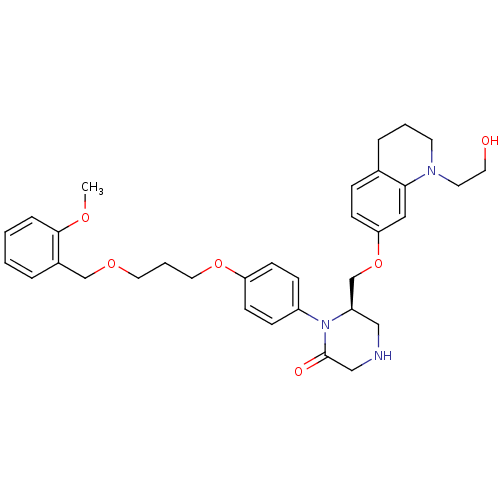
((R)-6-[1-(2-Hydroxy-ethyl)-1,2,3,4-tetrahydro-quin...)Show SMILES COc1ccccc1COCCCOc1ccc(cc1)N1[C@@H](COc2ccc3CCCN(CCO)c3c2)CNCC1=O Show InChI InChI=1S/C33H41N3O6/c1-39-32-8-3-2-6-26(32)23-40-18-5-19-41-29-13-10-27(11-14-29)36-28(21-34-22-33(36)38)24-42-30-12-9-25-7-4-15-35(16-17-37)31(25)20-30/h2-3,6,8-14,20,28,34,37H,4-5,7,15-19,21-24H2,1H3/t28-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 64 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Concentration required to inhibit renin activity by 50% |
Bioorg Med Chem Lett 15: 2371-4 (2005)
Article DOI: 10.1016/j.bmcl.2005.02.085
BindingDB Entry DOI: 10.7270/Q2PV6JW0 |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50165798
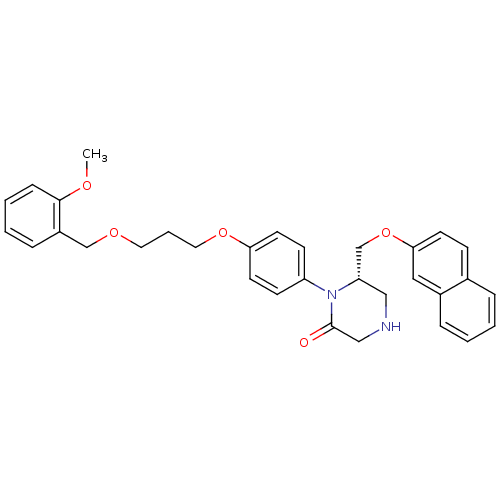
((S)-1-{4-[3-(2-Methoxy-benzyloxy)-propoxy]-phenyl}...)Show SMILES COc1ccccc1COCCCOc1ccc(cc1)N1[C@H](COc2ccc3ccccc3c2)CNCC1=O Show InChI InChI=1S/C32H34N2O5/c1-36-31-10-5-4-9-26(31)22-37-17-6-18-38-29-15-12-27(13-16-29)34-28(20-33-21-32(34)35)23-39-30-14-11-24-7-2-3-8-25(24)19-30/h2-5,7-16,19,28,33H,6,17-18,20-23H2,1H3/t28-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 66 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Concentration required to inhibit renin activity by 50% |
Bioorg Med Chem Lett 15: 2371-4 (2005)
Article DOI: 10.1016/j.bmcl.2005.02.085
BindingDB Entry DOI: 10.7270/Q2PV6JW0 |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50165799

((R)-1-{4-[3-(2-Methoxy-benzyloxy)-propoxy]-phenyl}...)Show SMILES COc1ccccc1COCCCOc1ccc(cc1)N1[C@@H](COc2ccc3CCCNc3c2)CNCC1=O Show InChI InChI=1S/C31H37N3O5/c1-36-30-8-3-2-6-24(30)21-37-16-5-17-38-27-13-10-25(11-14-27)34-26(19-32-20-31(34)35)22-39-28-12-9-23-7-4-15-33-29(23)18-28/h2-3,6,8-14,18,26,32-33H,4-5,7,15-17,19-22H2,1H3/t26-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Concentration required to inhibit renin activity by 50% |
Bioorg Med Chem Lett 15: 2371-4 (2005)
Article DOI: 10.1016/j.bmcl.2005.02.085
BindingDB Entry DOI: 10.7270/Q2PV6JW0 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Renin
(Homo sapiens (Human)) | BDBM50173065
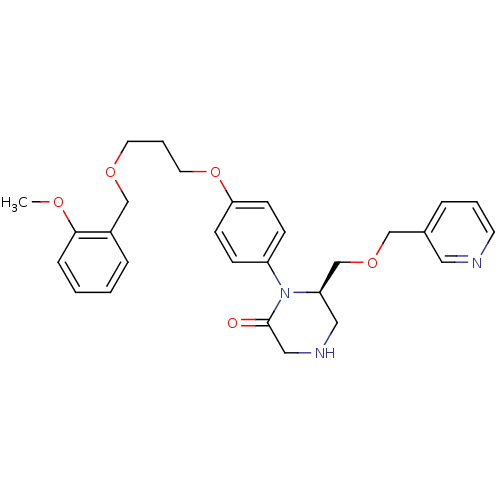
((R)-1-{4-[3-(2-Methoxy-benzyloxy)-propoxy]-phenyl}...)Show SMILES COc1ccccc1COCCCOc1ccc(cc1)N1[C@@H](COCc2cccnc2)CNCC1=O Show InChI InChI=1S/C28H33N3O5/c1-33-27-8-3-2-7-23(27)20-34-14-5-15-36-26-11-9-24(10-12-26)31-25(17-30-18-28(31)32)21-35-19-22-6-4-13-29-16-22/h2-4,6-13,16,25,30H,5,14-15,17-21H2,1H3/t25-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against renin in fluorescent tGFP assay |
Bioorg Med Chem Lett 15: 4713-6 (2005)
Article DOI: 10.1016/j.bmcl.2005.07.063
BindingDB Entry DOI: 10.7270/Q2SF2VP9 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data