 Found 14029 hits with Last Name = 'bu' and Initial = 'p'
Found 14029 hits with Last Name = 'bu' and Initial = 'p' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50176988
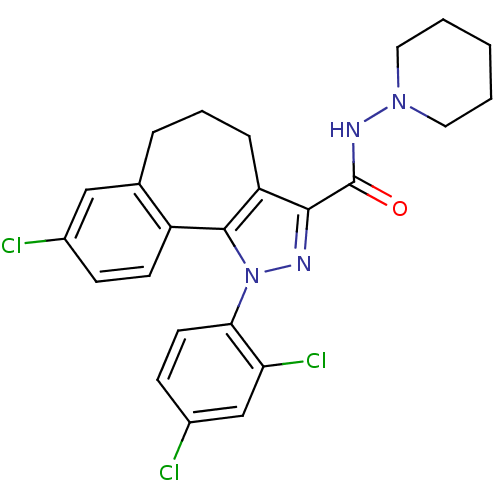
(8-Chloro-1-(2,4-dichloro-phenyl)-1,3a,4,5,6,10b-he...)Show SMILES Clc1ccc(c(Cl)c1)-n1nc(C(=O)NN2CCCCC2)c2CCCc3cc(Cl)ccc3-c12 Show InChI InChI=1S/C24H23Cl3N4O/c25-16-7-9-18-15(13-16)5-4-6-19-22(24(32)29-30-11-2-1-3-12-30)28-31(23(18)19)21-10-8-17(26)14-20(21)27/h7-10,13-14H,1-6,11-12H2,(H,29,32) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.000350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute
Curated by ChEMBL
| Assay Description
Binding affinity to CB1 receptor (unknown origin) |
J Med Chem 51: 3526-39 (2008)
Article DOI: 10.1021/jm8000778
BindingDB Entry DOI: 10.7270/Q29K4B0X |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4
(Rattus norvegicus (Rat)) | BDBM86812

(CAS_45263784 | NSC_45263784 | rac-2-(6-fluoro-5-(4...)Show SMILES [O-][N+](=O)c1ccc(cc1)-c1cc(cnc1F)C1CC2CCC1N2 |TLB:11:16:19.20:22| Show InChI InChI=1S/C17H16FN3O2/c18-17-15(10-1-4-13(5-2-10)21(22)23)7-11(9-19-17)14-8-12-3-6-16(14)20-12/h1-2,4-5,7,9,12,14,16,20H,3,6,8H2 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.00900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute
Curated by PDSP Ki Database
| |
Bioorg Med Chem 16: 746-54 (2008)
Article DOI: 10.1016/j.bmc.2007.10.027
BindingDB Entry DOI: 10.7270/Q2H130KC |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4
(Rattus norvegicus (Rat)) | BDBM50100717
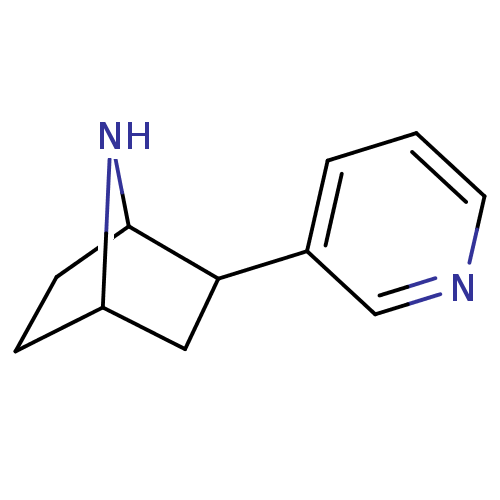
(2-(pyridin-3-yl)-7-aza-bicyclo[2.2.1]heptane | 2-P...)Show InChI InChI=1S/C11H14N2/c1-2-8(7-12-5-1)10-6-9-3-4-11(10)13-9/h1-2,5,7,9-11,13H,3-4,6H2 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute
Curated by PDSP Ki Database
| |
Bioorg Med Chem 16: 746-54 (2008)
Article DOI: 10.1016/j.bmc.2007.10.027
BindingDB Entry DOI: 10.7270/Q2H130KC |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4
(Rattus norvegicus (Rat)) | BDBM50049757

(()-2-(6-Chloro-pyridin-3-yl)-7-aza-bicyclo[2.2.1]h...)Show InChI InChI=1S/C11H13ClN2/c12-11-4-1-7(6-13-11)9-5-8-2-3-10(9)14-8/h1,4,6,8-10,14H,2-3,5H2 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.0260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute
Curated by PDSP Ki Database
| |
Bioorg Med Chem 16: 746-54 (2008)
Article DOI: 10.1016/j.bmc.2007.10.027
BindingDB Entry DOI: 10.7270/Q2H130KC |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4
(Rattus norvegicus (Rat)) | BDBM86815
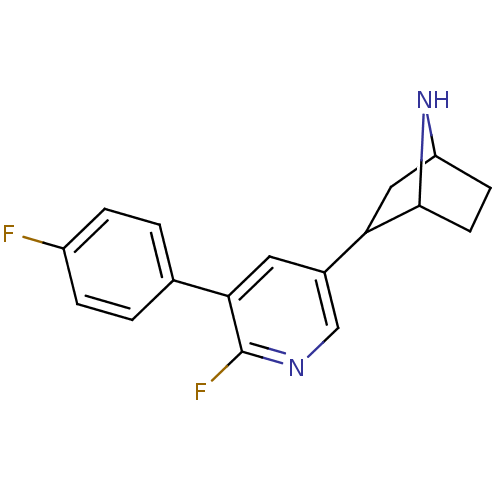
(CAS_45266019 | NSC_45266019 | rac-2-(6-fluoro-5-(4...)Show SMILES Fc1ccc(cc1)-c1cc(cnc1F)C1CC2CCC1N2 |TLB:9:14:17.18:20| Show InChI InChI=1S/C17H16F2N2/c18-12-3-1-10(2-4-12)15-7-11(9-20-17(15)19)14-8-13-5-6-16(14)21-13/h1-4,7,9,13-14,16,21H,5-6,8H2 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute
Curated by PDSP Ki Database
| |
Bioorg Med Chem 16: 746-54 (2008)
Article DOI: 10.1016/j.bmc.2007.10.027
BindingDB Entry DOI: 10.7270/Q2H130KC |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50085666

((2S,3S,4R,5R)-5-{6-(2,2-Diphenyl-ethylamino)-2-[2-...)Show SMILES CCNC(=O)[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(NCC(c3ccccc3)c3ccccc3)nc(NCCc3cnc[nH]3)nc12 Show InChI InChI=1S/C31H35N9O4/c1-2-33-29(43)26-24(41)25(42)30(44-26)40-18-37-23-27(38-31(39-28(23)40)34-14-13-21-15-32-17-36-21)35-16-22(19-9-5-3-6-10-19)20-11-7-4-8-12-20/h3-12,15,17-18,22,24-26,30,41-42H,2,13-14,16H2,1H3,(H,32,36)(H,33,43)(H2,34,35,38,39)/t24-,25+,26-,30+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre
Curated by ChEMBL
| Assay Description
In vitro inhibition of human neutrophil activation via Adenosine A2A receptor. |
Bioorg Med Chem Lett 10: 403-6 (2000)
BindingDB Entry DOI: 10.7270/Q2XK8G2K |
More data for this
Ligand-Target Pair | |
Thyroid hormone receptor beta
(Homo sapiens (Human)) | BDBM50063687
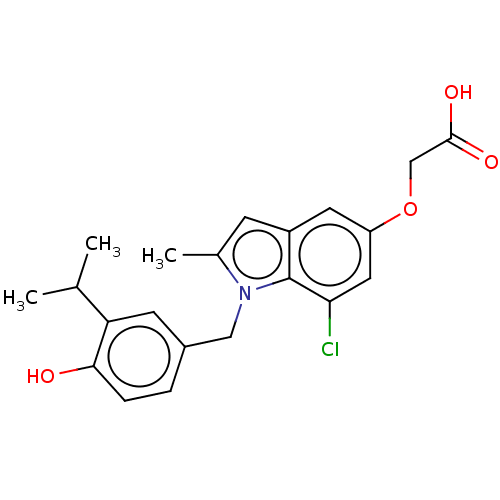
(CHEMBL3397339)Show SMILES CC(C)c1cc(Cn2c(C)cc3cc(OCC(O)=O)cc(Cl)c23)ccc1O Show InChI InChI=1S/C21H22ClNO4/c1-12(2)17-7-14(4-5-19(17)24)10-23-13(3)6-15-8-16(27-11-20(25)26)9-18(22)21(15)23/h4-9,12,24H,10-11H2,1-3H3,(H,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]-L-3,5,3'-triiodothyronine from human TRbeta expressed in human Hela cell lysate measured after overnight incubation by competi... |
Bioorg Med Chem Lett 25: 1377-80 (2015)
Article DOI: 10.1016/j.bmcl.2015.02.062
BindingDB Entry DOI: 10.7270/Q2PV6N2G |
More data for this
Ligand-Target Pair | |
Thyroid hormone receptor beta
(Homo sapiens (Human)) | BDBM50063687

(CHEMBL3397339)Show SMILES CC(C)c1cc(Cn2c(C)cc3cc(OCC(O)=O)cc(Cl)c23)ccc1O Show InChI InChI=1S/C21H22ClNO4/c1-12(2)17-7-14(4-5-19(17)24)10-23-13(3)6-15-8-16(27-11-20(25)26)9-18(22)21(15)23/h4-9,12,24H,10-11H2,1-3H3,(H,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]-L-3,5,3'-triiodothyronine from human TRbeta expressed in human Hela cell lysate measured after overnight incubation by competi... |
Bioorg Med Chem Lett 25: 1377-80 (2015)
Article DOI: 10.1016/j.bmcl.2015.02.062
BindingDB Entry DOI: 10.7270/Q2PV6N2G |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4
(Rattus norvegicus (Rat)) | BDBM86816

(CAS_45263769 | NSC_45263769 | rac-2-(5-(4-chloroph...)Show SMILES Fc1ncc(cc1-c1ccc(Cl)cc1)C1CC2CCC1N2 |TLB:4:14:17.18:20| Show InChI InChI=1S/C17H16ClFN2/c18-12-3-1-10(2-4-12)15-7-11(9-20-17(15)19)14-8-13-5-6-16(14)21-13/h1-4,7,9,13-14,16,21H,5-6,8H2 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0440 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute
Curated by PDSP Ki Database
| |
Bioorg Med Chem 16: 746-54 (2008)
Article DOI: 10.1016/j.bmc.2007.10.027
BindingDB Entry DOI: 10.7270/Q2H130KC |
More data for this
Ligand-Target Pair | |
Tubulin beta chain
(Sus scrofa) | BDBM50485941

(CHEMBL2181004)Show SMILES COc1ccc(CN[C@H]2CCc3cc(OC)c(OC)c(OC)c3-c3ccc(OC)c(=O)cc23)cc1 |r| Show InChI InChI=1S/C28H31NO6/c1-31-19-9-6-17(7-10-19)16-29-22-12-8-18-14-25(33-3)27(34-4)28(35-5)26(18)20-11-13-24(32-2)23(30)15-21(20)22/h6-7,9-11,13-15,22,29H,8,12,16H2,1-5H3/t22-/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bradford
Curated by ChEMBL
| Assay Description
Binding affinity to pig brain tubulin |
J Med Chem 55: 11062-6 (2012)
Article DOI: 10.1021/jm301151t
BindingDB Entry DOI: 10.7270/Q22V2K0N |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50085674
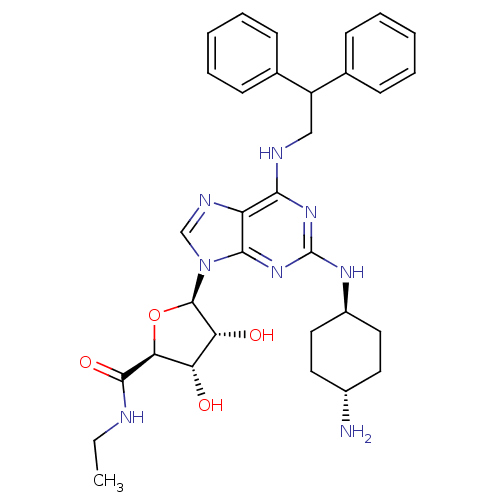
((2S,3S,4R,5R)-5-[2-(4-Amino-cyclohexylamino)-6-(2,...)Show SMILES CCNC(=O)[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(NCC(c3ccccc3)c3ccccc3)nc(N[C@H]3CC[C@H](N)CC3)nc12 |wU:7.12,5.4,35.37,wD:8.8,10.11,38.41,(-2.14,-10.42,;-1.05,-9.34,;-1.45,-7.85,;-.35,-6.77,;-.7,-5.26,;1.12,-7.24,;2.36,-6.34,;3.6,-7.24,;3.12,-8.72,;4.04,-9.95,;1.59,-8.72,;.69,-9.95,;3.38,-4.67,;4.3,-3.42,;3.38,-2.18,;1.92,-2.66,;.59,-1.89,;.57,-.35,;1.92,.42,;1.89,1.96,;3.23,2.73,;4.55,1.96,;5.89,2.73,;5.89,4.28,;4.53,5.03,;3.21,4.26,;.57,2.71,;.57,4.26,;-.77,5.01,;-2.1,4.24,;-2.09,2.7,;-.77,1.93,;-.75,-2.66,;-.75,-4.22,;-2.09,-4.98,;-2.87,-3.64,;-4.41,-3.66,;-5.2,-2.31,;-4.42,-.95,;-5.2,.37,;-2.87,-.95,;-2.09,-2.29,;.59,-4.98,;1.92,-4.2,)| Show InChI InChI=1S/C32H40N8O4/c1-2-34-30(43)27-25(41)26(42)31(44-27)40-18-36-24-28(38-32(39-29(24)40)37-22-15-13-21(33)14-16-22)35-17-23(19-9-5-3-6-10-19)20-11-7-4-8-12-20/h3-12,18,21-23,25-27,31,41-42H,2,13-17,33H2,1H3,(H,34,43)(H2,35,37,38,39)/t21-,22-,25-,26+,27-,31+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre
Curated by ChEMBL
| Assay Description
In vitro inhibition of human neutrophil activation via Adenosine A2A receptor. |
Bioorg Med Chem Lett 10: 403-6 (2000)
BindingDB Entry DOI: 10.7270/Q2XK8G2K |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4
(Rattus norvegicus (Rat)) | BDBM86810
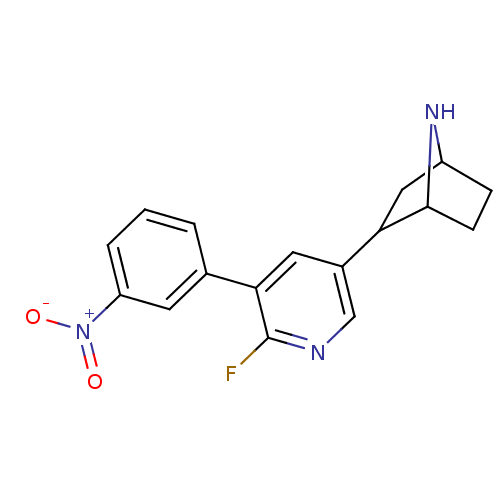
(CAS_45266065 | NSC_45266065 | rac-2-(6-fluoro-5-(3...)Show SMILES [O-][N+](=O)c1cccc(c1)-c1cc(cnc1F)C1CC2CCC1N2 |TLB:11:16:19.20:22| Show InChI InChI=1S/C17H16FN3O2/c18-17-15(10-2-1-3-13(6-10)21(22)23)7-11(9-19-17)14-8-12-4-5-16(14)20-12/h1-3,6-7,9,12,14,16,20H,4-5,8H2 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0530 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute
Curated by PDSP Ki Database
| |
Bioorg Med Chem 16: 746-54 (2008)
Article DOI: 10.1016/j.bmc.2007.10.027
BindingDB Entry DOI: 10.7270/Q2H130KC |
More data for this
Ligand-Target Pair | |
Tubulin beta chain
(Sus scrofa) | BDBM50485945

(CHEMBL2181003)Show SMILES COc1cc2CC[C@H](NCc3ccc(cc3)[N+]([O-])=O)c3cc(=O)c(OC)ccc3-c2c(OC)c1OC |r| Show InChI InChI=1S/C27H28N2O7/c1-33-23-12-10-19-20(14-22(23)30)21(28-15-16-5-8-18(9-6-16)29(31)32)11-7-17-13-24(34-2)26(35-3)27(36-4)25(17)19/h5-6,8-10,12-14,21,28H,7,11,15H2,1-4H3/t21-/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0585 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bradford
Curated by ChEMBL
| Assay Description
Binding affinity to pig brain tubulin |
J Med Chem 55: 11062-6 (2012)
Article DOI: 10.1021/jm301151t
BindingDB Entry DOI: 10.7270/Q22V2K0N |
More data for this
Ligand-Target Pair | |
Tubulin beta chain
(Sus scrofa) | BDBM50485942

(CHEMBL2181002)Show SMILES COc1cc2CC[C@H](NCc3cc(F)c(F)c(F)c3)c3cc(=O)c(OC)ccc3-c2c(OC)c1OC |r| Show InChI InChI=1S/C27H26F3NO5/c1-33-22-8-6-16-17(12-21(22)32)20(31-13-14-9-18(28)25(30)19(29)10-14)7-5-15-11-23(34-2)26(35-3)27(36-4)24(15)16/h6,8-12,20,31H,5,7,13H2,1-4H3/t20-/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0637 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bradford
Curated by ChEMBL
| Assay Description
Binding affinity to pig brain tubulin |
J Med Chem 55: 11062-6 (2012)
Article DOI: 10.1021/jm301151t
BindingDB Entry DOI: 10.7270/Q22V2K0N |
More data for this
Ligand-Target Pair | |
Thyroid hormone receptor beta
(Homo sapiens (Human)) | BDBM50063689
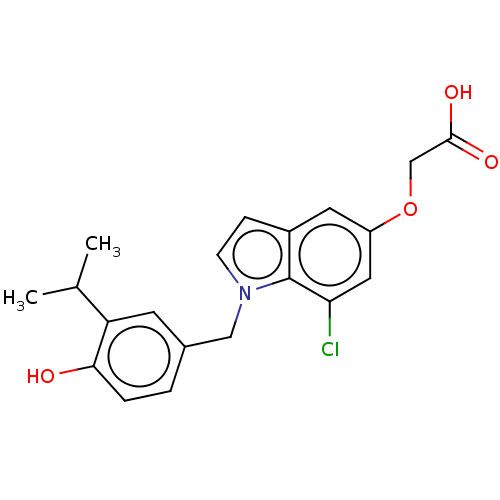
(CHEMBL3397337)Show SMILES CC(C)c1cc(Cn2ccc3cc(OCC(O)=O)cc(Cl)c23)ccc1O Show InChI InChI=1S/C20H20ClNO4/c1-12(2)16-7-13(3-4-18(16)23)10-22-6-5-14-8-15(26-11-19(24)25)9-17(21)20(14)22/h3-9,12,23H,10-11H2,1-2H3,(H,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]-L-3,5,3'-triiodothyronine from human TRbeta expressed in human Hela cell lysate measured after overnight incubation by competi... |
Bioorg Med Chem Lett 25: 1377-80 (2015)
Article DOI: 10.1016/j.bmcl.2015.02.062
BindingDB Entry DOI: 10.7270/Q2PV6N2G |
More data for this
Ligand-Target Pair | |
Thyroid hormone receptor beta
(Homo sapiens (Human)) | BDBM50063689

(CHEMBL3397337)Show SMILES CC(C)c1cc(Cn2ccc3cc(OCC(O)=O)cc(Cl)c23)ccc1O Show InChI InChI=1S/C20H20ClNO4/c1-12(2)16-7-13(3-4-18(16)23)10-22-6-5-14-8-15(26-11-19(24)25)9-17(21)20(14)22/h3-9,12,23H,10-11H2,1-2H3,(H,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]-L-3,5,3'-triiodothyronine from human TRbeta expressed in human Hela cell lysate measured after overnight incubation by competi... |
Bioorg Med Chem Lett 25: 1377-80 (2015)
Article DOI: 10.1016/j.bmcl.2015.02.062
BindingDB Entry DOI: 10.7270/Q2PV6N2G |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4
(Rattus norvegicus (Rat)) | BDBM86805
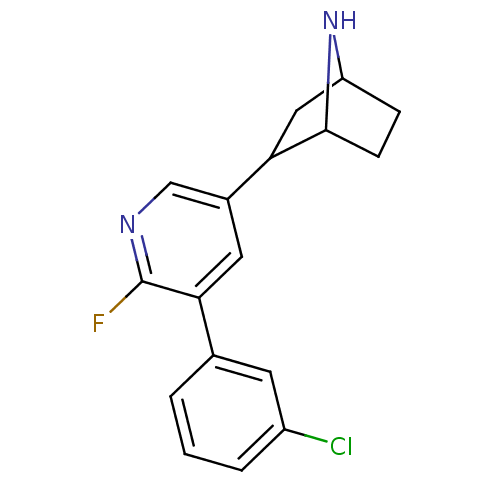
(CAS_45263788 | NSC_45263788 | US9150581, RTI-7527-...)Show SMILES Fc1ncc(cc1-c1cccc(Cl)c1)C1CC2CCC1N2 |TLB:4:14:17.18:20| Show InChI InChI=1S/C17H16ClFN2/c18-12-3-1-2-10(6-12)15-7-11(9-20-17(15)19)14-8-13-4-5-16(14)21-13/h1-3,6-7,9,13-14,16,21H,4-5,8H2 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0730 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute
Curated by PDSP Ki Database
| |
Bioorg Med Chem 16: 746-54 (2008)
Article DOI: 10.1016/j.bmc.2007.10.027
BindingDB Entry DOI: 10.7270/Q2H130KC |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50085671
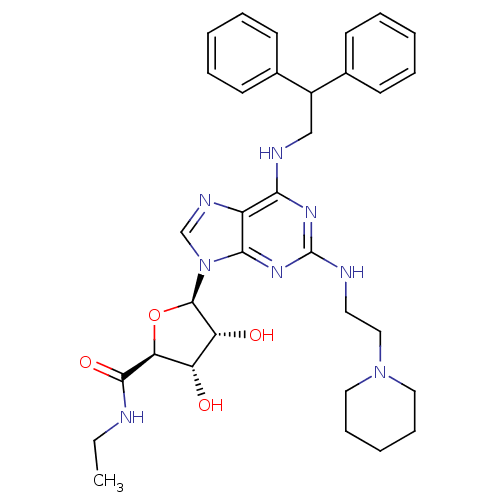
((2S,3S,4R,5R)-5-[6-(2,2-Diphenyl-ethylamino)-2-(2-...)Show SMILES CCNC(=O)[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(NCC(c3ccccc3)c3ccccc3)nc(NCCN3CCCCC3)nc12 Show InChI InChI=1S/C33H42N8O4/c1-2-34-31(44)28-26(42)27(43)32(45-28)41-21-37-25-29(38-33(39-30(25)41)35-16-19-40-17-10-5-11-18-40)36-20-24(22-12-6-3-7-13-22)23-14-8-4-9-15-23/h3-4,6-9,12-15,21,24,26-28,32,42-43H,2,5,10-11,16-20H2,1H3,(H,34,44)(H2,35,36,38,39)/t26-,27+,28-,32+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre
Curated by ChEMBL
| Assay Description
In vitro inhibition of human neutrophil activation via Adenosine A2A receptor. |
Bioorg Med Chem Lett 10: 403-6 (2000)
BindingDB Entry DOI: 10.7270/Q2XK8G2K |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4
(Rattus norvegicus (Rat)) | BDBM86819

(CAS_45263772 | NSC_45263772 | rac-2-(6-fluoro-5-(3...)Show SMILES Fc1cccc(c1)-c1cc(cnc1F)C1CC2CCC1N2 |TLB:9:14:17.18:20| Show InChI InChI=1S/C17H16F2N2/c18-12-3-1-2-10(6-12)15-7-11(9-20-17(15)19)14-8-13-4-5-16(14)21-13/h1-3,6-7,9,13-14,16,21H,4-5,8H2 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0870 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute
Curated by PDSP Ki Database
| |
Bioorg Med Chem 16: 746-54 (2008)
Article DOI: 10.1016/j.bmc.2007.10.027
BindingDB Entry DOI: 10.7270/Q2H130KC |
More data for this
Ligand-Target Pair | |
Thyroid hormone receptor beta
(Homo sapiens (Human)) | BDBM50063755
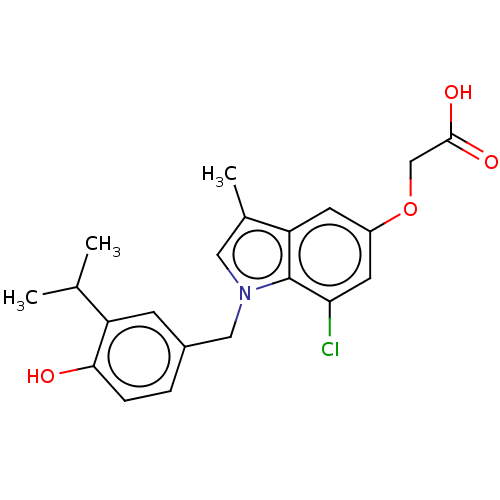
(CHEMBL3397341)Show SMILES CC(C)c1cc(Cn2cc(C)c3cc(OCC(O)=O)cc(Cl)c23)ccc1O Show InChI InChI=1S/C21H22ClNO4/c1-12(2)16-6-14(4-5-19(16)24)10-23-9-13(3)17-7-15(27-11-20(25)26)8-18(22)21(17)23/h4-9,12,24H,10-11H2,1-3H3,(H,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]-L-3,5,3'-triiodothyronine from human TRbeta expressed in human Hela cell lysate measured after overnight incubation by competi... |
Bioorg Med Chem Lett 25: 1377-80 (2015)
Article DOI: 10.1016/j.bmcl.2015.02.062
BindingDB Entry DOI: 10.7270/Q2PV6N2G |
More data for this
Ligand-Target Pair | |
Thyroid hormone receptor beta
(Homo sapiens (Human)) | BDBM50063755

(CHEMBL3397341)Show SMILES CC(C)c1cc(Cn2cc(C)c3cc(OCC(O)=O)cc(Cl)c23)ccc1O Show InChI InChI=1S/C21H22ClNO4/c1-12(2)16-6-14(4-5-19(16)24)10-23-9-13(3)17-7-15(27-11-20(25)26)8-18(22)21(17)23/h4-9,12,24H,10-11H2,1-3H3,(H,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]-L-3,5,3'-triiodothyronine from human TRbeta expressed in human Hela cell lysate measured after overnight incubation by competi... |
Bioorg Med Chem Lett 25: 1377-80 (2015)
Article DOI: 10.1016/j.bmcl.2015.02.062
BindingDB Entry DOI: 10.7270/Q2PV6N2G |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4
(Rattus norvegicus (Rat)) | BDBM86817
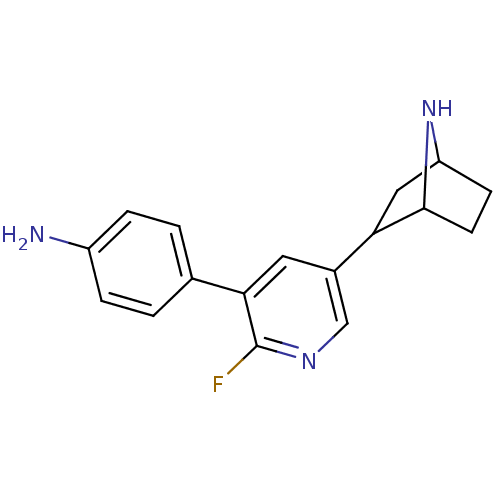
(CAS_45263779 | NSC_45263779 | rac-4-(5-(7-aza-bicy...)Show SMILES Nc1ccc(cc1)-c1cc(cnc1F)C1CC2CCC1N2 |TLB:9:14:17.18:20| Show InChI InChI=1S/C17H18FN3/c18-17-15(10-1-3-12(19)4-2-10)7-11(9-20-17)14-8-13-5-6-16(14)21-13/h1-4,7,9,13-14,16,21H,5-6,8,19H2 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0950 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute
Curated by PDSP Ki Database
| |
Bioorg Med Chem 16: 746-54 (2008)
Article DOI: 10.1016/j.bmc.2007.10.027
BindingDB Entry DOI: 10.7270/Q2H130KC |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50228471
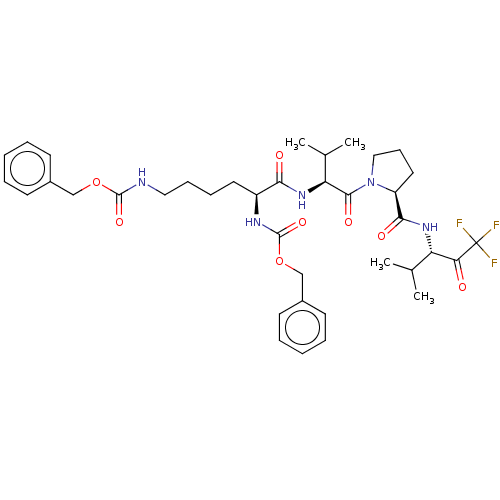
(CHEMBL131548)Show SMILES CC(C)[C@H](NC(=O)[C@H](CCCCNC(=O)OCc1ccccc1)NC(=O)OCc1ccccc1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](C(C)C)C(=O)C(F)(F)F Show InChI InChI=1S/C38H50F3N5O8/c1-24(2)30(32(47)38(39,40)41)44-34(49)29-19-13-21-46(29)35(50)31(25(3)4)45-33(48)28(43-37(52)54-23-27-16-9-6-10-17-27)18-11-12-20-42-36(51)53-22-26-14-7-5-8-15-26/h5-10,14-17,24-25,28-31H,11-13,18-23H2,1-4H3,(H,42,51)(H,43,52)(H,44,49)(H,45,48)/t28-,29-,30-,31-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| <0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merrell Dow Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity against human leukocyte Elastase |
J Med Chem 33: 394-407 (1990)
BindingDB Entry DOI: 10.7270/Q26D5RZ5 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50085668

((2S,3S,4R,5R)-5-[6-Amino-2-((1R,2R)-2-hydroxy-cycl...)Show SMILES CCNC(=O)[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)nc(N[C@@H]3CCC[C@H]3O)nc12 Show InChI InChI=1S/C17H25N7O5/c1-2-19-15(28)12-10(26)11(27)16(29-12)24-6-20-9-13(18)22-17(23-14(9)24)21-7-4-3-5-8(7)25/h6-8,10-12,16,25-27H,2-5H2,1H3,(H,19,28)(H3,18,21,22,23)/t7-,8-,10+,11-,12+,16-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre
Curated by ChEMBL
| Assay Description
In vitro inhibition of human neutrophil activation via Adenosine A2A receptor. |
Bioorg Med Chem Lett 10: 403-6 (2000)
BindingDB Entry DOI: 10.7270/Q2XK8G2K |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4
(Rattus norvegicus (Rat)) | BDBM86807
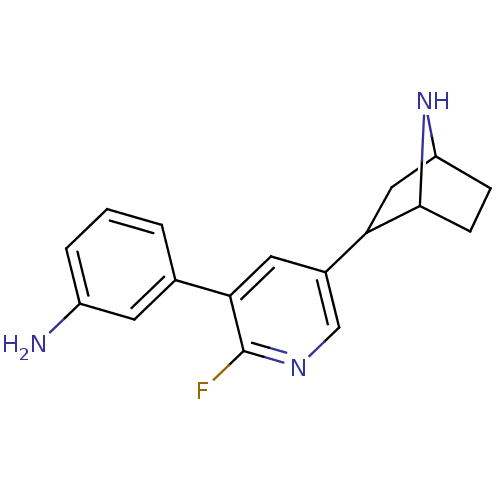
(CAS_45263775 | NSC_45263775 | rac-3-(5-(7-aza-bicy...)Show SMILES Nc1cccc(c1)-c1cc(cnc1F)C1CC2CCC1N2 |TLB:9:14:17.18:20| Show InChI InChI=1S/C17H18FN3/c18-17-15(10-2-1-3-12(19)6-10)7-11(9-20-17)14-8-13-4-5-16(14)21-13/h1-3,6-7,9,13-14,16,21H,4-5,8,19H2 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute
Curated by PDSP Ki Database
| |
Bioorg Med Chem 16: 746-54 (2008)
Article DOI: 10.1016/j.bmc.2007.10.027
BindingDB Entry DOI: 10.7270/Q2H130KC |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4
(Rattus norvegicus (Rat)) | BDBM86818

(CAS_45266054 | NSC_45266054 | rac-2-(6-fluoro-5-(3...)Show SMILES COc1cccc(c1)-c1cc(cnc1F)C1CC2CCC1N2 |THB:10:15:18.19:21| Show InChI InChI=1S/C18H19FN2O/c1-22-14-4-2-3-11(7-14)16-8-12(10-20-18(16)19)15-9-13-5-6-17(15)21-13/h2-4,7-8,10,13,15,17,21H,5-6,9H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute
Curated by PDSP Ki Database
| |
Bioorg Med Chem 16: 746-54 (2008)
Article DOI: 10.1016/j.bmc.2007.10.027
BindingDB Entry DOI: 10.7270/Q2H130KC |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4
(Rattus norvegicus (Rat)) | BDBM50166908

(5,8,14-triazatetracyclo[10.3.1.02,11.04,9]hexadeca...)Show InChI InChI=1S/C13H13N3/c1-2-16-13-5-11-9-3-8(6-14-7-9)10(11)4-12(13)15-1/h1-2,4-5,8-9,14H,3,6-7H2 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute
Curated by PDSP Ki Database
| |
Bioorg Med Chem 16: 746-54 (2008)
Article DOI: 10.1016/j.bmc.2007.10.027
BindingDB Entry DOI: 10.7270/Q2H130KC |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tubulin beta chain
(Sus scrofa) | BDBM50485950
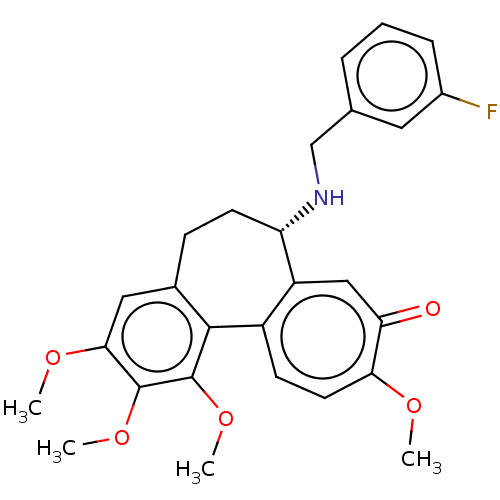
(CHEMBL2181009)Show SMILES COc1cc2CC[C@H](NCc3cccc(F)c3)c3cc(=O)c(OC)ccc3-c2c(OC)c1OC |r| Show InChI InChI=1S/C27H28FNO5/c1-31-23-11-9-19-20(14-22(23)30)21(29-15-16-6-5-7-18(28)12-16)10-8-17-13-24(32-2)26(33-3)27(34-4)25(17)19/h5-7,9,11-14,21,29H,8,10,15H2,1-4H3/t21-/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.122 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bradford
Curated by ChEMBL
| Assay Description
Binding affinity to pig brain tubulin |
J Med Chem 55: 11062-6 (2012)
Article DOI: 10.1021/jm301151t
BindingDB Entry DOI: 10.7270/Q22V2K0N |
More data for this
Ligand-Target Pair | |
Tubulin beta chain
(Sus scrofa) | BDBM50485944

(CHEMBL2181006)Show SMILES COc1cc2CC[C@H](NCc3ccc(Cl)cc3)c3cc(=O)c(OC)ccc3-c2c(OC)c1OC |r| Show InChI InChI=1S/C27H28ClNO5/c1-31-23-12-10-19-20(14-22(23)30)21(29-15-16-5-8-18(28)9-6-16)11-7-17-13-24(32-2)26(33-3)27(34-4)25(17)19/h5-6,8-10,12-14,21,29H,7,11,15H2,1-4H3/t21-/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.127 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bradford
Curated by ChEMBL
| Assay Description
Binding affinity to pig brain tubulin |
J Med Chem 55: 11062-6 (2012)
Article DOI: 10.1021/jm301151t
BindingDB Entry DOI: 10.7270/Q22V2K0N |
More data for this
Ligand-Target Pair | |
Tubulin beta chain
(Sus scrofa) | BDBM50485943
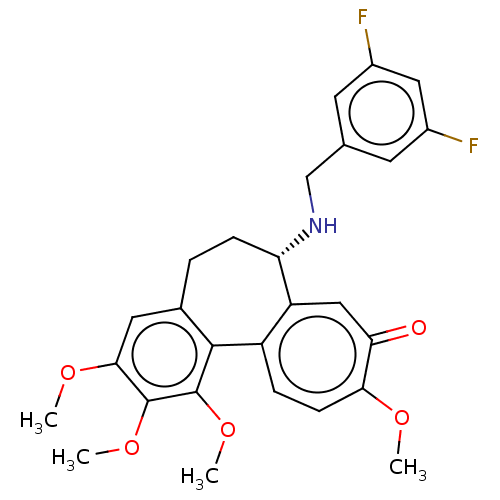
(CHEMBL2181001)Show SMILES COc1cc2CC[C@H](NCc3cc(F)cc(F)c3)c3cc(=O)c(OC)ccc3-c2c(OC)c1OC |r| Show InChI InChI=1S/C27H27F2NO5/c1-32-23-8-6-19-20(13-22(23)31)21(30-14-15-9-17(28)12-18(29)10-15)7-5-16-11-24(33-2)26(34-3)27(35-4)25(16)19/h6,8-13,21,30H,5,7,14H2,1-4H3/t21-/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.131 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bradford
Curated by ChEMBL
| Assay Description
Binding affinity to pig brain tubulin |
J Med Chem 55: 11062-6 (2012)
Article DOI: 10.1021/jm301151t
BindingDB Entry DOI: 10.7270/Q22V2K0N |
More data for this
Ligand-Target Pair | |
Thyroid hormone receptor beta
(Homo sapiens (Human)) | BDBM50063688
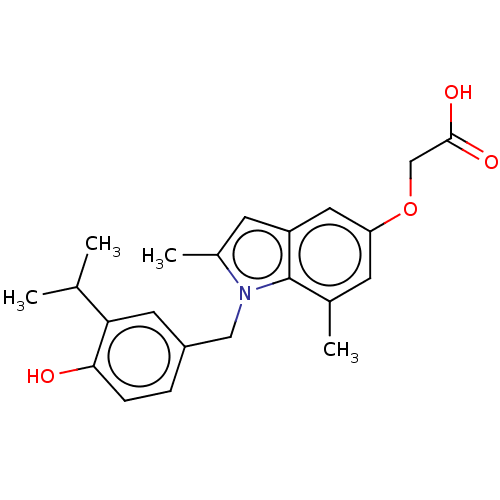
(CHEMBL3397338)Show SMILES CC(C)c1cc(Cn2c(C)cc3cc(OCC(O)=O)cc(C)c23)ccc1O Show InChI InChI=1S/C22H25NO4/c1-13(2)19-9-16(5-6-20(19)24)11-23-15(4)8-17-10-18(27-12-21(25)26)7-14(3)22(17)23/h5-10,13,24H,11-12H2,1-4H3,(H,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]-L-3,5,3'-triiodothyronine from human TRbeta expressed in human Hela cell lysate measured after overnight incubation by competi... |
Bioorg Med Chem Lett 25: 1377-80 (2015)
Article DOI: 10.1016/j.bmcl.2015.02.062
BindingDB Entry DOI: 10.7270/Q2PV6N2G |
More data for this
Ligand-Target Pair | |
Thyroid hormone receptor beta
(Homo sapiens (Human)) | BDBM50063688

(CHEMBL3397338)Show SMILES CC(C)c1cc(Cn2c(C)cc3cc(OCC(O)=O)cc(C)c23)ccc1O Show InChI InChI=1S/C22H25NO4/c1-13(2)19-9-16(5-6-20(19)24)11-23-15(4)8-17-10-18(27-12-21(25)26)7-14(3)22(17)23/h5-10,13,24H,11-12H2,1-4H3,(H,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]-L-3,5,3'-triiodothyronine from human TRbeta expressed in human Hela cell lysate measured after overnight incubation by competi... |
Bioorg Med Chem Lett 25: 1377-80 (2015)
Article DOI: 10.1016/j.bmcl.2015.02.062
BindingDB Entry DOI: 10.7270/Q2PV6N2G |
More data for this
Ligand-Target Pair | |
Tubulin beta chain
(Sus scrofa) | BDBM50485946
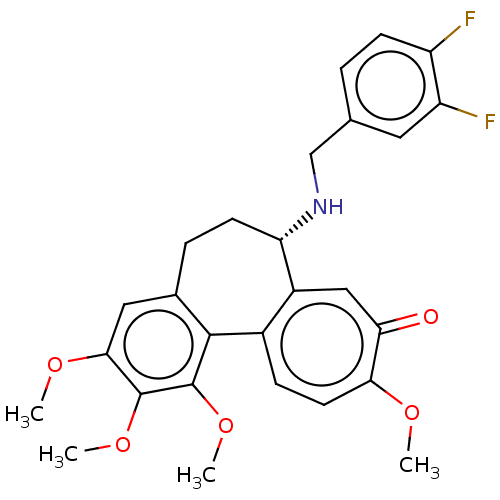
(CHEMBL2181000)Show SMILES COc1cc2CC[C@H](NCc3ccc(F)c(F)c3)c3cc(=O)c(OC)ccc3-c2c(OC)c1OC |r| Show InChI InChI=1S/C27H27F2NO5/c1-32-23-10-7-17-18(13-22(23)31)21(30-14-15-5-8-19(28)20(29)11-15)9-6-16-12-24(33-2)26(34-3)27(35-4)25(16)17/h5,7-8,10-13,21,30H,6,9,14H2,1-4H3/t21-/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.143 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bradford
Curated by ChEMBL
| Assay Description
Binding affinity to pig brain tubulin |
J Med Chem 55: 11062-6 (2012)
Article DOI: 10.1021/jm301151t
BindingDB Entry DOI: 10.7270/Q22V2K0N |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4
(Rattus norvegicus (Rat)) | BDBM86809

(3'-(4-fluorophenyl)deschloroepibatidine | CAS_...)Show SMILES Fc1ccc(cc1)-c1cncc(c1)C1CC2CCC1N2 |TLB:11:13:16.17:19| Show InChI InChI=1S/C17H17FN2/c18-14-3-1-11(2-4-14)12-7-13(10-19-9-12)16-8-15-5-6-17(16)20-15/h1-4,7,9-10,15-17,20H,5-6,8H2 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute
Curated by PDSP Ki Database
| |
Bioorg Med Chem 16: 746-54 (2008)
Article DOI: 10.1016/j.bmc.2007.10.027
BindingDB Entry DOI: 10.7270/Q2H130KC |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50085662

((2S,3S,4R,5R)-5-[6-(2,2-Diphenyl-ethylamino)-2-((1...)Show SMILES CCNC(=O)[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(NCC(c3ccccc3)c3ccccc3)nc(N[C@@H]3CCC[C@H]3O)nc12 Show InChI InChI=1S/C31H37N7O5/c1-2-32-29(42)26-24(40)25(41)30(43-26)38-17-34-23-27(36-31(37-28(23)38)35-21-14-9-15-22(21)39)33-16-20(18-10-5-3-6-11-18)19-12-7-4-8-13-19/h3-8,10-13,17,20-22,24-26,30,39-41H,2,9,14-16H2,1H3,(H,32,42)(H2,33,35,36,37)/t21-,22-,24+,25-,26+,30-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre
Curated by ChEMBL
| Assay Description
In vitro inhibition of human neutrophil activation via Adenosine A2A receptor. |
Bioorg Med Chem Lett 10: 403-6 (2000)
BindingDB Entry DOI: 10.7270/Q2XK8G2K |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4
(Rattus norvegicus (Rat)) | BDBM86822

(CAS_45263767 | NSC_45263767 | rac-3'-(3-nitrop...)Show SMILES [O-][N+](=O)c1cccc(c1)-c1cncc(c1)C1CC2CCC1N2 |TLB:13:15:18.19:21| Show InChI InChI=1S/C17H17N3O2/c21-20(22)15-3-1-2-11(7-15)12-6-13(10-18-9-12)16-8-14-4-5-17(16)19-14/h1-3,6-7,9-10,14,16-17,19H,4-5,8H2 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute
Curated by PDSP Ki Database
| |
Bioorg Med Chem 16: 746-54 (2008)
Article DOI: 10.1016/j.bmc.2007.10.027
BindingDB Entry DOI: 10.7270/Q2H130KC |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4
(Rattus norvegicus (Rat)) | BDBM86820

(CAS_45263774 | NSC_45263774 | rac-3'-(3-chloro...)Show SMILES Clc1cccc(c1)-c1cncc(c1)C1CC2CCC1N2 |TLB:11:13:16.17:19| Show InChI InChI=1S/C17H17ClN2/c18-14-3-1-2-11(7-14)12-6-13(10-19-9-12)16-8-15-4-5-17(16)20-15/h1-3,6-7,9-10,15-17,20H,4-5,8H2 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute
Curated by PDSP Ki Database
| |
Bioorg Med Chem 16: 746-54 (2008)
Article DOI: 10.1016/j.bmc.2007.10.027
BindingDB Entry DOI: 10.7270/Q2H130KC |
More data for this
Ligand-Target Pair | |
Thyroid hormone receptor beta
(Homo sapiens (Human)) | BDBM50063686
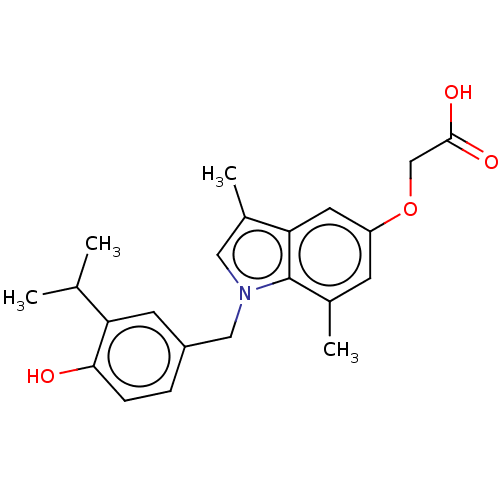
(CHEMBL3397340)Show SMILES CC(C)c1cc(Cn2cc(C)c3cc(OCC(O)=O)cc(C)c23)ccc1O Show InChI InChI=1S/C22H25NO4/c1-13(2)18-8-16(5-6-20(18)24)11-23-10-15(4)19-9-17(27-12-21(25)26)7-14(3)22(19)23/h5-10,13,24H,11-12H2,1-4H3,(H,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]-L-3,5,3'-triiodothyronine from human TRbeta expressed in human Hela cell lysate measured after overnight incubation by competi... |
Bioorg Med Chem Lett 25: 1377-80 (2015)
Article DOI: 10.1016/j.bmcl.2015.02.062
BindingDB Entry DOI: 10.7270/Q2PV6N2G |
More data for this
Ligand-Target Pair | |
Thyroid hormone receptor beta
(Homo sapiens (Human)) | BDBM50063686

(CHEMBL3397340)Show SMILES CC(C)c1cc(Cn2cc(C)c3cc(OCC(O)=O)cc(C)c23)ccc1O Show InChI InChI=1S/C22H25NO4/c1-13(2)18-8-16(5-6-20(18)24)11-23-10-15(4)19-9-17(27-12-21(25)26)7-14(3)22(19)23/h5-10,13,24H,11-12H2,1-4H3,(H,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]-L-3,5,3'-triiodothyronine from human TRbeta expressed in human Hela cell lysate measured after overnight incubation by competi... |
Bioorg Med Chem Lett 25: 1377-80 (2015)
Article DOI: 10.1016/j.bmcl.2015.02.062
BindingDB Entry DOI: 10.7270/Q2PV6N2G |
More data for this
Ligand-Target Pair | |
Tubulin beta chain
(Sus scrofa) | BDBM50485947
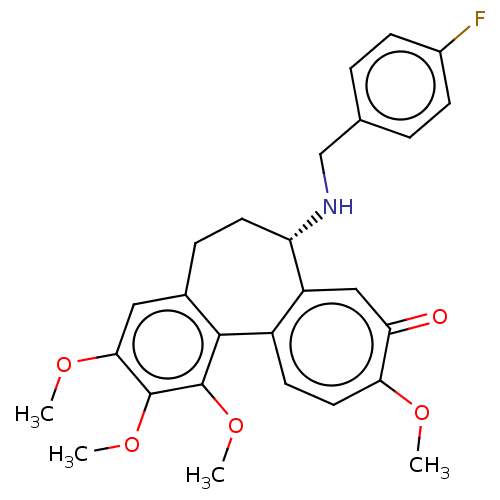
(CHEMBL2181008)Show SMILES COc1cc2CC[C@H](NCc3ccc(F)cc3)c3cc(=O)c(OC)ccc3-c2c(OC)c1OC |r| Show InChI InChI=1S/C27H28FNO5/c1-31-23-12-10-19-20(14-22(23)30)21(29-15-16-5-8-18(28)9-6-16)11-7-17-13-24(32-2)26(33-3)27(34-4)25(17)19/h5-6,8-10,12-14,21,29H,7,11,15H2,1-4H3/t21-/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.183 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bradford
Curated by ChEMBL
| Assay Description
Binding affinity to pig brain tubulin |
J Med Chem 55: 11062-6 (2012)
Article DOI: 10.1021/jm301151t
BindingDB Entry DOI: 10.7270/Q22V2K0N |
More data for this
Ligand-Target Pair | |
Tubulin beta chain
(Sus scrofa) | BDBM50485949

(CHEMBL2180999)Show SMILES COc1cc2CC[C@H](NCc3cccc(F)c3F)c3cc(=O)c(OC)ccc3-c2c(OC)c1OC |r| Show InChI InChI=1S/C27H27F2NO5/c1-32-22-11-9-17-18(13-21(22)31)20(30-14-16-6-5-7-19(28)25(16)29)10-8-15-12-23(33-2)26(34-3)27(35-4)24(15)17/h5-7,9,11-13,20,30H,8,10,14H2,1-4H3/t20-/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.198 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bradford
Curated by ChEMBL
| Assay Description
Binding affinity to pig brain tubulin |
J Med Chem 55: 11062-6 (2012)
Article DOI: 10.1021/jm301151t
BindingDB Entry DOI: 10.7270/Q22V2K0N |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50001450

((SP)Arg-Pro-Lys-Pro-Gln-Gln-Phe-Phe-Gly-Leu-Met-NH...)Show SMILES [#6]-[#16]-[#6]-[#6]-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#6])-[#6])-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccccc1)-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccccc1)-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6](-[#7])=O)-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6](-[#7])=O)-[#7]-[#6](=O)-[#6@@H]-1-[#6]-[#6]-[#6]-[#7]-1-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#7]-[#6](=O)-[#6@@H]-1-[#6]-[#6]-[#6]-[#7]-1-[#6](=O)-[#6@@H](-[#7])-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](-[#7])=O |r| Show InChI InChI=1S/C63H98N18O13S/c1-37(2)33-45(57(89)74-41(53(68)85)27-32-95-3)73-52(84)36-72-54(86)46(34-38-15-6-4-7-16-38)78-58(90)47(35-39-17-8-5-9-18-39)79-56(88)42(23-25-50(66)82)75-55(87)43(24-26-51(67)83)76-59(91)49-22-14-31-81(49)62(94)44(20-10-11-28-64)77-60(92)48-21-13-30-80(48)61(93)40(65)19-12-29-71-63(69)70/h4-9,15-18,37,40-49H,10-14,19-36,64-65H2,1-3H3,(H2,66,82)(H2,67,83)(H2,68,85)(H,72,86)(H,73,84)(H,74,89)(H,75,87)(H,76,91)(H,77,92)(H,78,90)(H,79,88)(H4,69,70,71)/t40-,41-,42-,43-,44-,45-,46-,47-,48-,49-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Marion Roussel
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 277: 840-51 (1996)
BindingDB Entry DOI: 10.7270/Q2KD1WF0 |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4
(Rattus norvegicus (Rat)) | BDBM86821
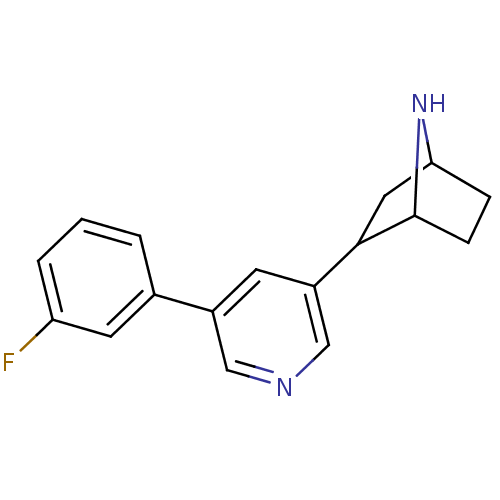
(CAS_45263766 | NSC_45263766 | rac-3'-(3-fluoro...)Show SMILES Fc1cccc(c1)-c1cncc(c1)C1CC2CCC1N2 |TLB:11:13:16.17:19| Show InChI InChI=1S/C17H17FN2/c18-14-3-1-2-11(7-14)12-6-13(10-19-9-12)16-8-15-4-5-17(16)20-15/h1-3,6-7,9-10,15-17,20H,4-5,8H2 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute
Curated by PDSP Ki Database
| |
Bioorg Med Chem 16: 746-54 (2008)
Article DOI: 10.1016/j.bmc.2007.10.027
BindingDB Entry DOI: 10.7270/Q2H130KC |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4
(Rattus norvegicus (Rat)) | BDBM86811

(3-(4-chlorophenyl)deschloroepibatidine | CAS_44452...)Show SMILES Clc1ccc(cc1)-c1cncc(c1)C1CC2CCC1N2 |TLB:11:13:16.17:19| Show InChI InChI=1S/C17H17ClN2/c18-14-3-1-11(2-4-14)12-7-13(10-19-9-12)16-8-15-5-6-17(16)20-15/h1-4,7,9-10,15-17,20H,5-6,8H2 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute
Curated by PDSP Ki Database
| |
Bioorg Med Chem 16: 746-54 (2008)
Article DOI: 10.1016/j.bmc.2007.10.027
BindingDB Entry DOI: 10.7270/Q2H130KC |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(GUINEA PIG) | BDBM50001450

((SP)Arg-Pro-Lys-Pro-Gln-Gln-Phe-Phe-Gly-Leu-Met-NH...)Show SMILES [#6]-[#16]-[#6]-[#6]-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#6])-[#6])-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccccc1)-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccccc1)-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6](-[#7])=O)-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6](-[#7])=O)-[#7]-[#6](=O)-[#6@@H]-1-[#6]-[#6]-[#6]-[#7]-1-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#7]-[#6](=O)-[#6@@H]-1-[#6]-[#6]-[#6]-[#7]-1-[#6](=O)-[#6@@H](-[#7])-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](-[#7])=O |r| Show InChI InChI=1S/C63H98N18O13S/c1-37(2)33-45(57(89)74-41(53(68)85)27-32-95-3)73-52(84)36-72-54(86)46(34-38-15-6-4-7-16-38)78-58(90)47(35-39-17-8-5-9-18-39)79-56(88)42(23-25-50(66)82)75-55(87)43(24-26-51(67)83)76-59(91)49-22-14-31-81(49)62(94)44(20-10-11-28-64)77-60(92)48-21-13-30-80(48)61(93)40(65)19-12-29-71-63(69)70/h4-9,15-18,37,40-49H,10-14,19-36,64-65H2,1-3H3,(H2,66,82)(H2,67,83)(H2,68,85)(H,72,86)(H,73,84)(H,74,89)(H,75,87)(H,76,91)(H,77,92)(H,78,90)(H,79,88)(H4,69,70,71)/t40-,41-,42-,43-,44-,45-,46-,47-,48-,49-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Marion Roussel
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 277: 840-51 (1996)
BindingDB Entry DOI: 10.7270/Q2KD1WF0 |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4
(Rattus norvegicus (Rat)) | BDBM86806
(CAS_45263762 | NSC_45263762 | US9150581, RTI-7527-...)Show SMILES Fc1ncc(cc1-c1ccccc1)C1CC2CCC1N2 |TLB:4:13:16.17:19| Show InChI InChI=1S/C17H17FN2/c18-17-15(11-4-2-1-3-5-11)8-12(10-19-17)14-9-13-6-7-16(14)20-13/h1-5,8,10,13-14,16,20H,6-7,9H2 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute
Curated by PDSP Ki Database
| |
Bioorg Med Chem 16: 746-54 (2008)
Article DOI: 10.1016/j.bmc.2007.10.027
BindingDB Entry DOI: 10.7270/Q2H130KC |
More data for this
Ligand-Target Pair | |
Thyroid hormone receptor beta
(Homo sapiens (Human)) | BDBM50063690
(CHEMBL3397336)Show SMILES CC(C)c1cc(Cn2ccc3cc(OCC(O)=O)cc(C)c23)ccc1O Show InChI InChI=1S/C21H23NO4/c1-13(2)18-9-15(4-5-19(18)23)11-22-7-6-16-10-17(26-12-20(24)25)8-14(3)21(16)22/h4-10,13,23H,11-12H2,1-3H3,(H,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]-L-3,5,3'-triiodothyronine from human TRbeta expressed in human Hela cell lysate measured after overnight incubation by competi... |
Bioorg Med Chem Lett 25: 1377-80 (2015)
Article DOI: 10.1016/j.bmcl.2015.02.062
BindingDB Entry DOI: 10.7270/Q2PV6N2G |
More data for this
Ligand-Target Pair | |
Thyroid hormone receptor beta
(Homo sapiens (Human)) | BDBM50063690

(CHEMBL3397336)Show SMILES CC(C)c1cc(Cn2ccc3cc(OCC(O)=O)cc(C)c23)ccc1O Show InChI InChI=1S/C21H23NO4/c1-13(2)18-9-15(4-5-19(18)23)11-22-7-6-16-10-17(26-12-20(24)25)8-14(3)21(16)22/h4-10,13,23H,11-12H2,1-3H3,(H,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]-L-3,5,3'-triiodothyronine from human TRbeta expressed in human Hela cell lysate measured after overnight incubation by competi... |
Bioorg Med Chem Lett 25: 1377-80 (2015)
Article DOI: 10.1016/j.bmcl.2015.02.062
BindingDB Entry DOI: 10.7270/Q2PV6N2G |
More data for this
Ligand-Target Pair | |
Tubulin beta chain
(Sus scrofa) | BDBM50485948
(CHEMBL2181007)Show SMILES COc1cc2CC[C@H](NCc3ccc(I)cc3)c3cc(=O)c(OC)ccc3-c2c(OC)c1OC |r| Show InChI InChI=1S/C27H28INO5/c1-31-23-12-10-19-20(14-22(23)30)21(29-15-16-5-8-18(28)9-6-16)11-7-17-13-24(32-2)26(33-3)27(34-4)25(17)19/h5-6,8-10,12-14,21,29H,7,11,15H2,1-4H3/t21-/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bradford
Curated by ChEMBL
| Assay Description
Binding affinity to pig brain tubulin |
J Med Chem 55: 11062-6 (2012)
Article DOI: 10.1021/jm301151t
BindingDB Entry DOI: 10.7270/Q22V2K0N |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50181362
((R)-(+)-7,9-difluoro-5-[4-(2-piperidin-1-ylethoxy)...)Show SMILES Oc1ccc2c3[C@H](Oc4c(F)cc(F)cc4-c3ccc2c1)c1ccc(OCCN2CCCCC2)cc1 Show InChI InChI=1S/C30H27F2NO3/c31-21-17-26-25-10-6-20-16-22(34)7-11-24(20)28(25)29(36-30(26)27(32)18-21)19-4-8-23(9-5-19)35-15-14-33-12-2-1-3-13-33/h4-11,16-18,29,34H,1-3,12-15H2/t29-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]17beta-estradiol from ERalpha |
J Med Chem 49: 843-6 (2006)
Article DOI: 10.1021/jm0509795
BindingDB Entry DOI: 10.7270/Q20R9Q60 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data