

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Glutamate receptor ionotropic, NMDA 2C (Rattus norvegicus (Rat)) | BDBM83449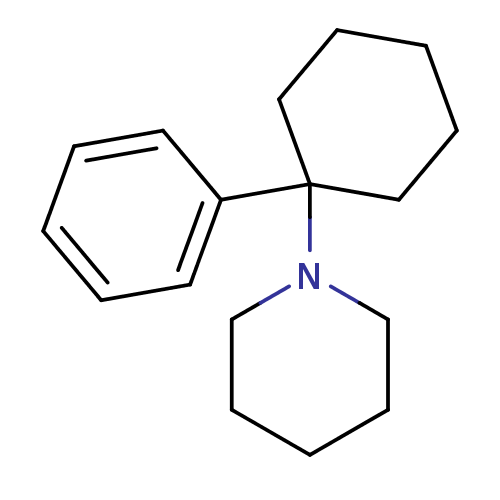 (1-(1-phenylcyclohexyl)piperidine;hydrochloride | M...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase DrugBank KEGG PC cid PC sid PDB UniChem Similars | PubMed | 91 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes, Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Binding affinity to sigma site of Phencyclidine receptor by displacement of [3H]-(+)-SKF- 10,047 | J Med Chem 31: 1571-5 (1988) BindingDB Entry DOI: 10.7270/Q2WD41S2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50071330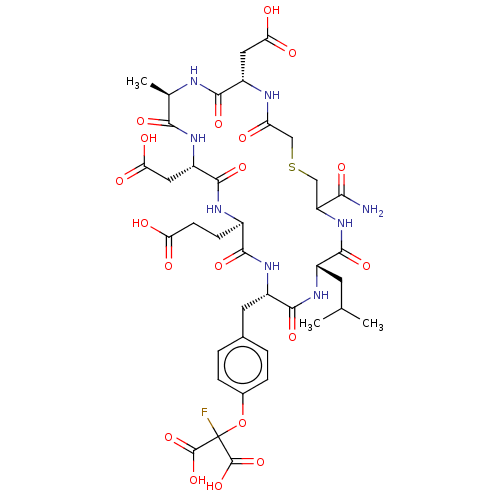 (CHEMBL2369450 | c(S-CH2-Ac-Asp-Ala-Asp-Glu-FOMT-Le...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute Curated by ChEMBL | Assay Description Compound was evaluated for the binding affinity against protein-tyrosine phosphatase 1B (PTP1B) | Bioorg Med Chem Lett 8: 2149-50 (1999) BindingDB Entry DOI: 10.7270/Q27M0737 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50103243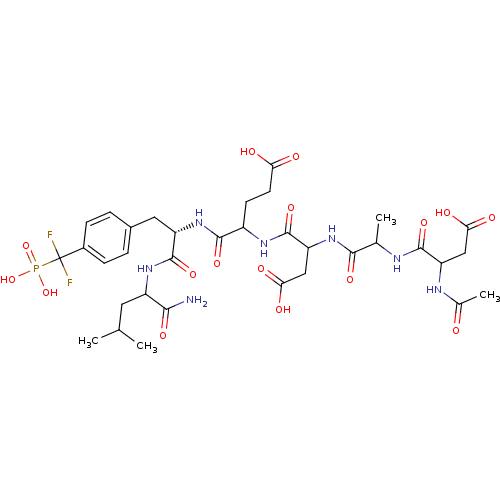 (4-{2-[2-(2-Acetylamino-3-carboxy-propionylamino)-p...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute Curated by ChEMBL | Assay Description Inhibitory activity against human Protein-tyrosinephosphatase 1B (PTP1B) | J Med Chem 44: 2869-78 (2001) BindingDB Entry DOI: 10.7270/Q237781C | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Glutamate receptor ionotropic, NMDA 2C (Rattus norvegicus (Rat)) | BDBM83449 (1-(1-phenylcyclohexyl)piperidine;hydrochloride | M...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase DrugBank KEGG PC cid PC sid PDB UniChem Similars | PubMed | 530 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes, Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Binding affinity to sigma site of Phencyclidine receptor by displacement of [3H]-(+)-SKF- 10,047 | J Med Chem 31: 1571-5 (1988) BindingDB Entry DOI: 10.7270/Q2WD41S2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2C (Rattus norvegicus (Rat)) | BDBM50405724 (CHEMBL1169565) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 630 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes, Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Binding affinity of compound against Phencyclidine receptor by displacement of [3H]TCP | J Med Chem 31: 1571-5 (1988) BindingDB Entry DOI: 10.7270/Q2WD41S2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2C (Rattus norvegicus (Rat)) | BDBM50405723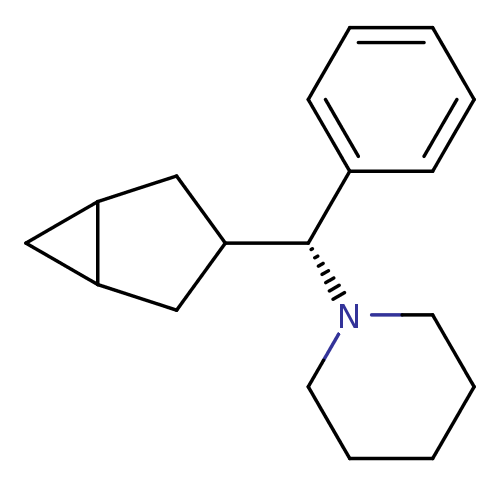 (CHEMBL1169564) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 680 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes, Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Binding affinity of compound against Phencyclidine receptor by displacement of [3H]TCP | J Med Chem 31: 1571-5 (1988) BindingDB Entry DOI: 10.7270/Q2WD41S2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2C (Rattus norvegicus (Rat)) | BDBM50405723 (CHEMBL1169564) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes, Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Binding affinity to sigma site of Phencyclidine receptor by displacement of [3H]-(+)-SKF- 10,047 | J Med Chem 31: 1571-5 (1988) BindingDB Entry DOI: 10.7270/Q2WD41S2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tumor susceptibility gene 101 protein (Homo sapiens (Human)) | BDBM50362892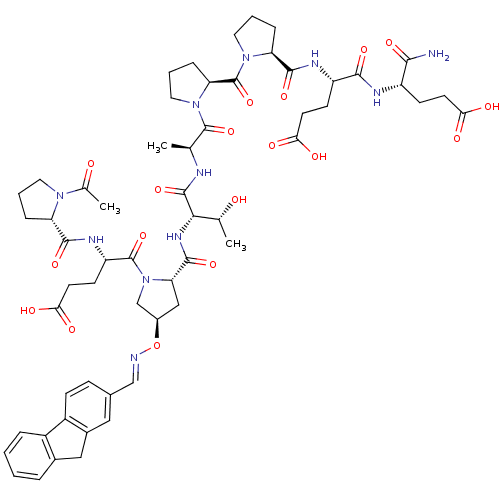 (CHEMBL1946564) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 2.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NCI-Frederick Curated by ChEMBL | Assay Description Displacement of FITC-conjugated (S)-4-((S)-1-acetylpyrrolidine-2-carboxamido)-5-((2S,4R)-2-((2S,3R)-1-((S)-1-((S)-2-((S)-2-((S)-1-((S)-1-amino-4-carb... | ACS Med Chem Lett 2: 337-341 (2011) Article DOI: 10.1021/ml1002579 BindingDB Entry DOI: 10.7270/Q22B8ZF4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tumor susceptibility gene 101 protein (Homo sapiens (Human)) | BDBM50362891 (CHEMBL1946260) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 2.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NCI-Frederick Curated by ChEMBL | Assay Description Displacement of FITC-conjugated (S)-4-((S)-1-acetylpyrrolidine-2-carboxamido)-5-((2S,4R)-2-((2S,3R)-1-((S)-1-((S)-2-((S)-2-((S)-1-((S)-1-amino-4-carb... | ACS Med Chem Lett 2: 337-341 (2011) Article DOI: 10.1021/ml1002579 BindingDB Entry DOI: 10.7270/Q22B8ZF4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2C (Rattus norvegicus (Rat)) | BDBM50405724 (CHEMBL1169565) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes, Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Binding affinity to sigma site of Phencyclidine receptor by displacement of [3H]-(+)-SKF- 10,047 | J Med Chem 31: 1571-5 (1988) BindingDB Entry DOI: 10.7270/Q2WD41S2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50103235 (5-[2-(2-{2-[2-(2-Acetylamino-3-carboxy-propionylam...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute Curated by ChEMBL | Assay Description Inhibitory activity against human Protein-tyrosinephosphatase 1B (PTP1B) | J Med Chem 44: 2869-78 (2001) BindingDB Entry DOI: 10.7270/Q237781C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tumor susceptibility gene 101 protein (Homo sapiens (Human)) | BDBM50362890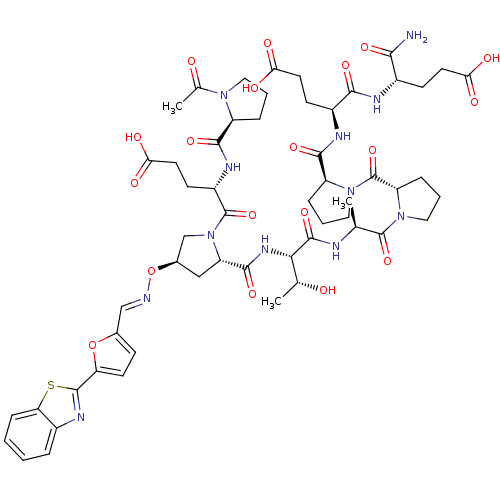 (CHEMBL1946259) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 5.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NCI-Frederick Curated by ChEMBL | Assay Description Displacement of FITC-conjugated (S)-4-((S)-1-acetylpyrrolidine-2-carboxamido)-5-((2S,4R)-2-((2S,3R)-1-((S)-1-((S)-2-((S)-2-((S)-1-((S)-1-amino-4-carb... | ACS Med Chem Lett 2: 337-341 (2011) Article DOI: 10.1021/ml1002579 BindingDB Entry DOI: 10.7270/Q22B8ZF4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tumor susceptibility gene 101 protein (Homo sapiens (Human)) | BDBM50362889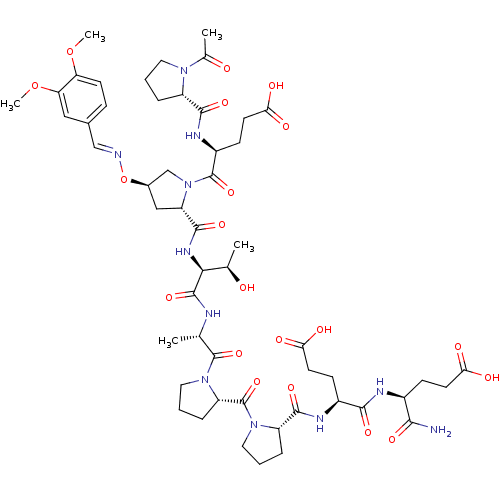 (CHEMBL1946129) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 8.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NCI-Frederick Curated by ChEMBL | Assay Description Displacement of FITC-conjugated (S)-4-((S)-1-acetylpyrrolidine-2-carboxamido)-5-((2S,4R)-2-((2S,3R)-1-((S)-1-((S)-2-((S)-2-((S)-1-((S)-1-amino-4-carb... | ACS Med Chem Lett 2: 337-341 (2011) Article DOI: 10.1021/ml1002579 BindingDB Entry DOI: 10.7270/Q22B8ZF4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50103240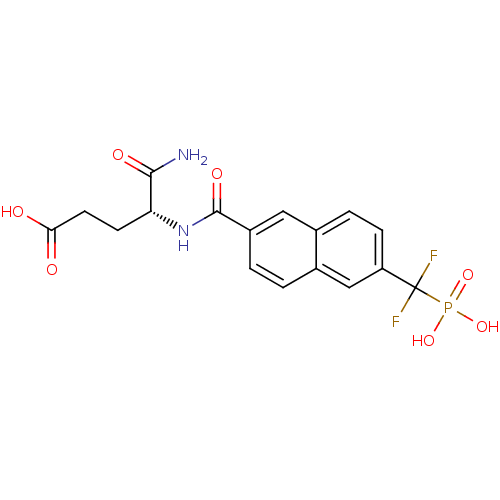 (4-Carbamoyl-4-{[6-(difluoro-phosphono-methyl)-naph...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute Curated by ChEMBL | Assay Description Inhibitory activity against human Protein-tyrosinephosphatase 1B (PTP1B) | J Med Chem 44: 2869-78 (2001) BindingDB Entry DOI: 10.7270/Q237781C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50050961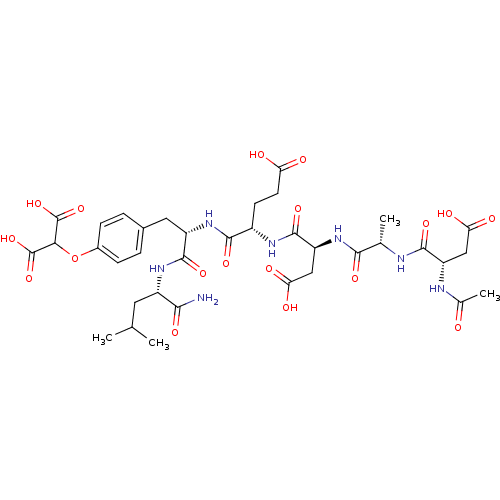 (2-{4-[(S)-2-((S)-2-{(S)-2-[(S)-2-((S)-2-Acetylamin...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute Curated by ChEMBL | Assay Description Compound was evaluated for the binding affinity against protein-tyrosine phosphatase 1B (PTP1B) | Bioorg Med Chem Lett 8: 2149-50 (1999) BindingDB Entry DOI: 10.7270/Q27M0737 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50132237 (CHEMBL320852 | [Difluoro-(6-methanesulfonylaminoca...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NIH Curated by ChEMBL | Assay Description Binding affinity of the compound towards recombinant human Protein-tyrosine phosphatase 1B was determined | Bioorg Med Chem Lett 13: 3005-7 (2003) BindingDB Entry DOI: 10.7270/Q22F7MV1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50103227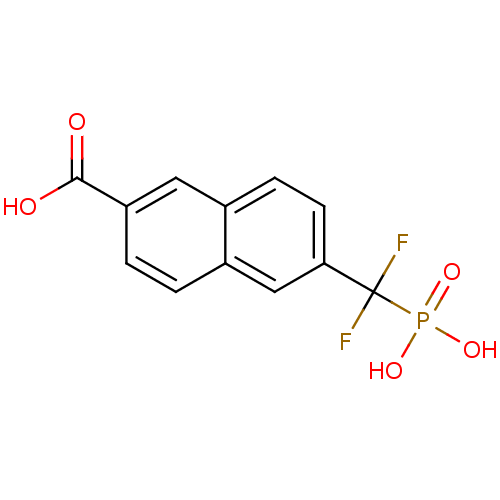 (6-(Difluoro-phosphono-methyl)-naphthalene-2-carbox...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB PubMed | 2.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute Curated by ChEMBL | Assay Description Inhibitory activity against human Protein-tyrosinephosphatase 1B (PTP1B) | J Med Chem 44: 2869-78 (2001) BindingDB Entry DOI: 10.7270/Q237781C | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50103227 (6-(Difluoro-phosphono-methyl)-naphthalene-2-carbox...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB PubMed | 2.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NIH Curated by ChEMBL | Assay Description Binding affinity of the compound towards recombinant human Protein-tyrosine phosphatase 1B was determined | Bioorg Med Chem Lett 13: 3005-7 (2003) BindingDB Entry DOI: 10.7270/Q22F7MV1 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50131098 (2-(4-{2-((S)-1-(S)-Carbamoyl-3-methyl-butylcarbamo...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute Curated by ChEMBL | Assay Description Inhibitory potency against human Protein-tyrosine phosphatase 1B expressed in E. coli BL21 (DE3) cells | Bioorg Med Chem Lett 13: 2577-81 (2003) BindingDB Entry DOI: 10.7270/Q2QC02VZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50075312 ((Difluoro-naphthalen-2-yl-methyl)-phosphonic acid ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.79E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NIH Curated by ChEMBL | Assay Description Binding affinity of the compound towards recombinant human Protein-tyrosine phosphatase 1B was determined | Bioorg Med Chem Lett 13: 3005-7 (2003) BindingDB Entry DOI: 10.7270/Q22F7MV1 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50103238 (3-Carboxymethoxy-naphthalene-2,7-dicarboxylic acid...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute Curated by ChEMBL | Assay Description Inhibitory activity against human Protein-tyrosinephosphatase 1B (PTP1B) | J Med Chem 44: 2869-78 (2001) BindingDB Entry DOI: 10.7270/Q237781C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50103234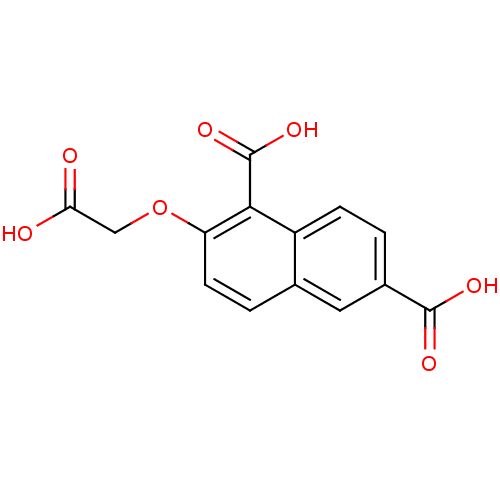 (2-Carboxymethoxy-naphthalene-1,6-dicarboxylic acid...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 9.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute Curated by ChEMBL | Assay Description Inhibitory activity against human Protein-tyrosinephosphatase 1B (PTP1B) | J Med Chem 44: 2869-78 (2001) BindingDB Entry DOI: 10.7270/Q237781C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50103231 (CHEMBL98615 | Naphthalene-2,7-dicarboxylic acid) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | >3.20E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute Curated by ChEMBL | Assay Description Inhibitory activity against human Protein-tyrosinephosphatase 1B (PTP1B) | J Med Chem 44: 2869-78 (2001) BindingDB Entry DOI: 10.7270/Q237781C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50103242 (CHEMBL316894 | Naphthalene trisulfonate (NTS) | na...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank PC cid PC sid PDB UniChem Similars | PubMed | >4.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute Curated by ChEMBL | Assay Description Inhibitory activity against human Protein-tyrosinephosphatase 1B (PTP1B) | J Med Chem 44: 2869-78 (2001) BindingDB Entry DOI: 10.7270/Q237781C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor/Kappa-type opioid receptor/Mu-type opioid receptor/Sigma non-opioid intracellular receptor 1 (Rattus norvegicus (rat)-RAT) | BDBM50021347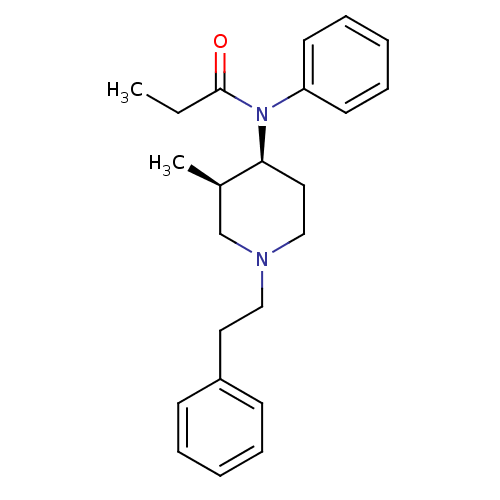 (CHEMBL12391 | N-(3-Methyl-1-phenethyl-piperidin-4-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vivo binding affinity to opioid receptor preparations from rat brain | J Med Chem 29: 1087-93 (1986) BindingDB Entry DOI: 10.7270/Q2JQ1364 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor/Kappa-type opioid receptor/Mu-type opioid receptor/Sigma non-opioid intracellular receptor 1 (Rattus norvegicus (rat)-RAT) | BDBM50021347 (CHEMBL12391 | N-(3-Methyl-1-phenethyl-piperidin-4-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vivo binding affinity to opioid receptor preparations from rat brain | J Med Chem 29: 1087-93 (1986) BindingDB Entry DOI: 10.7270/Q2JQ1364 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Growth factor receptor-bound protein 2 (Homo sapiens (Human)) | BDBM50139763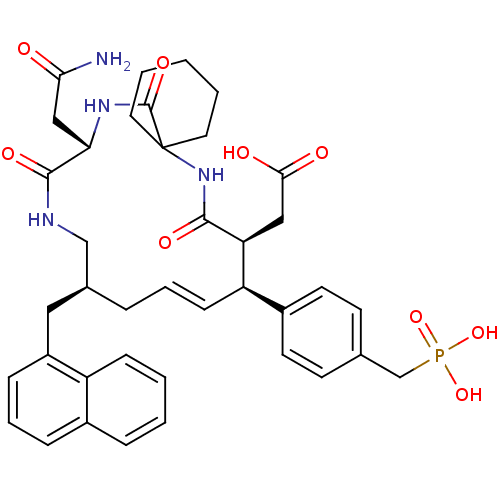 (CHEMBL306657 | [(E)-(9S,10S,14S,18S)-18-Carbamoylm...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute Curated by ChEMBL | Assay Description In vitro Growth factor receptor bound protein 2-binding affinity of the compound was determined in in surface plasmon resonance (SPR) method | J Med Chem 47: 2166-9 (2004) Article DOI: 10.1021/jm030510e BindingDB Entry DOI: 10.7270/Q2668CNN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Growth factor receptor-bound protein 2 (Homo sapiens (Human)) | BDBM50139763 (CHEMBL306657 | [(E)-(9S,10S,14S,18S)-18-Carbamoylm...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute Curated by ChEMBL | Assay Description In vitro Growth factor receptor bound protein 2-binding affinity of the compound was determined in in surface plasmon resonance (SPR) method | J Med Chem 47: 2166-9 (2004) Article DOI: 10.1021/jm030510e BindingDB Entry DOI: 10.7270/Q2668CNN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Growth factor receptor-bound protein 2 (Homo sapiens (Human)) | BDBM50139763 (CHEMBL306657 | [(E)-(9S,10S,14S,18S)-18-Carbamoylm...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute Curated by ChEMBL | Assay Description In vitro binding affinity in extracellular ELISA-based assay for Grb2 SH2 domain | J Med Chem 47: 788-91 (2004) Article DOI: 10.1021/jm030440b BindingDB Entry DOI: 10.7270/Q2XD114Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Growth factor receptor-bound protein 2 (Homo sapiens (Human)) | BDBM50085869 (CHEMBL367037 | N-[1-(1-{2-Carbamoyl-1-[3-(5-methyl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute Curated by ChEMBL | Assay Description Inhibition of Growth factor receptor bound protein 2 binding by ELISA assay method | J Med Chem 43: 911-20 (2000) BindingDB Entry DOI: 10.7270/Q2WD3ZRD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Growth factor receptor-bound protein 2 (Homo sapiens (Human)) | BDBM50122796 (CHEMBL84798 | [18-Carbamoylmethyl-14-(2,3-dihydro-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute-Frederick Curated by ChEMBL | Assay Description Inhibition against Growth factor receptor bound protein 2 binding using ELISA technique | J Med Chem 46: 244-54 (2003) Article DOI: 10.1021/jm0203635 BindingDB Entry DOI: 10.7270/Q2RR1XMN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Growth factor receptor-bound protein 2 (Homo sapiens (Human)) | BDBM50168312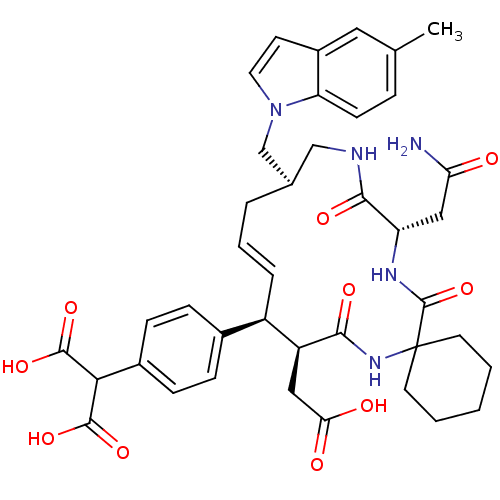 (2-{4-[(16R,20S)-18-((S)-Carbamoylmethyl)-9-carboxy...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute Curated by ChEMBL | Assay Description Inhibitory concentration against growth factor receptor bound protein 2 | J Med Chem 48: 3945-8 (2005) Article DOI: 10.1021/jm050059m BindingDB Entry DOI: 10.7270/Q2V69J48 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase PLK1 (Homo sapiens (Human)) | BDBM50403016 (CHEMBL2207845) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute-Frederick Curated by ChEMBL | Assay Description Inhibition of 5CF-GPMQSpTPLNG-NH2 binding to Plk1 C-terminal polo-box domain (unknown origin) preincubated for 30 mins followed by probe addition mea... | Bioorg Med Chem Lett 28: 3202-3205 (2018) Article DOI: 10.1016/j.bmcl.2018.08.018 BindingDB Entry DOI: 10.7270/Q25Q4ZST | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Growth factor receptor-bound protein 2 (Homo sapiens (Human)) | BDBM50073909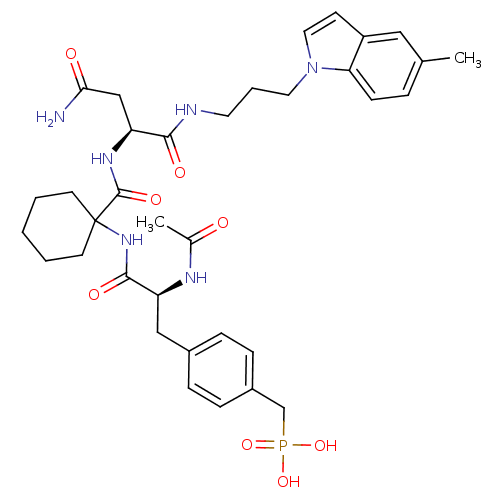 (CHEMBL72036 | {4-[(S)-2-Acetylamino-2-(1-{(S)-2-ca...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute Curated by ChEMBL | Assay Description Inhibition of Growth factor receptor bound protein 2 binding by ELISA assay method | J Med Chem 43: 911-20 (2000) BindingDB Entry DOI: 10.7270/Q2WD3ZRD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Growth factor receptor-bound protein 2 (Homo sapiens (Human)) | BDBM50143944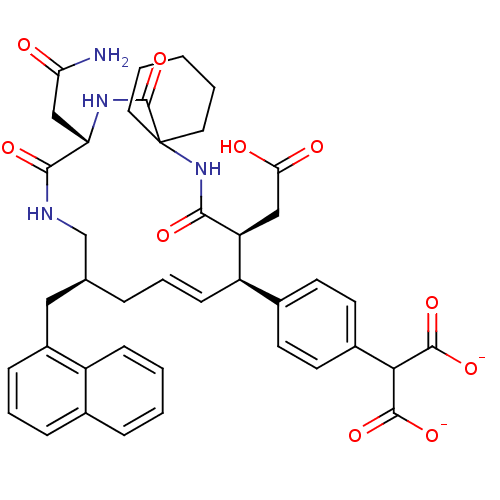 (2-[4-((E)-(9S,10S,14S,18S)-18-Carbamoylmethyl-9-ca...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute Curated by ChEMBL | Assay Description In vitro Growth factor receptor bound protein 2-binding affinity of the compound was determined in ELISA method | J Med Chem 47: 2166-9 (2004) Article DOI: 10.1021/jm030510e BindingDB Entry DOI: 10.7270/Q2668CNN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase PLK1 (Homo sapiens (Human)) | BDBM50403016 (CHEMBL2207845) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Chemical Biology Laboratory, Center for Cancer Research, National Cancer Institute, National Institutes of Health, Frederick, MD 21702, United States. Curated by ChEMBL | Assay Description Inhibition of 5CF-GPMQSpTPLNG-NH2 binding to myc-tagged human Plk1 PBD expressed in HEK-293T cells incubated for 1 hr by fluorescence polarization as... | Bioorg Med Chem 25: 5041-5049 (2017) Article DOI: 10.1016/j.bmc.2017.02.063 BindingDB Entry DOI: 10.7270/Q2QR50MP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| NAD(P)H dehydrogenase [quinone] 1 (Homo sapiens (Human)) | BDBM50055714 (3-(4,7-dihydroxy-2-oxo-2H-3-chromenylmethyl)-4,7-d...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester Curated by ChEMBL | Assay Description Inhibition of human recombinant NQO1 | J Med Chem 50: 6316-25 (2007) Article DOI: 10.1021/jm070472p BindingDB Entry DOI: 10.7270/Q2DV1JM1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| NAD(P)H dehydrogenase [quinone] 1 (Homo sapiens (Human)) | BDBM35525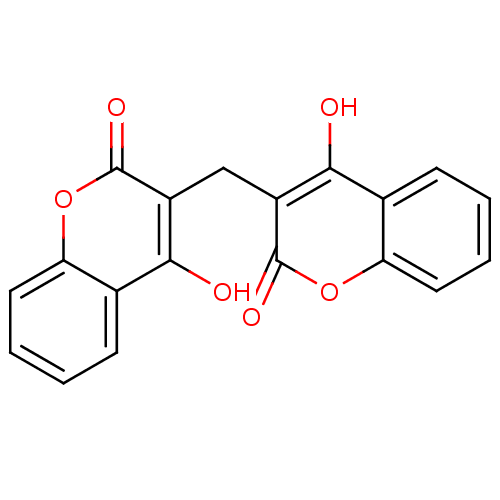 (3,3''''-methylenebis(4-hydroxy-coumarin | 3,3''''-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid UniChem Similars | DrugBank MMDB Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester Curated by ChEMBL | Assay Description Inhibition of human recombinant NQO1 | J Med Chem 50: 6316-25 (2007) Article DOI: 10.1021/jm070472p BindingDB Entry DOI: 10.7270/Q2DV1JM1 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Serine/threonine-protein kinase PLK1 (Homo sapiens (Human)) | BDBM50467110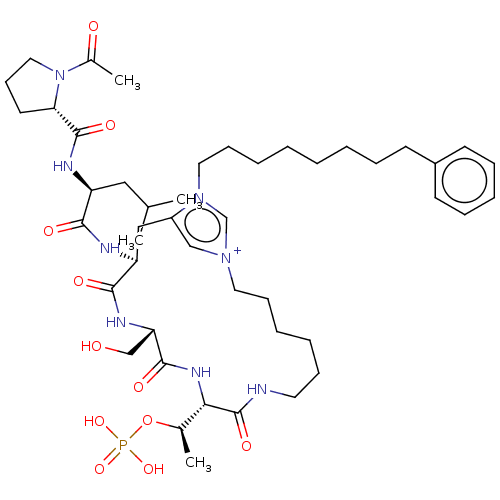 (CHEMBL4278200) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute-Frederick Curated by ChEMBL | Assay Description Inhibition of 5CF-GPMQSpTPLNG-NH2 binding to Plk1 C-terminal polo-box domain (unknown origin) preincubated for 30 mins followed by probe addition mea... | Bioorg Med Chem Lett 28: 3202-3205 (2018) Article DOI: 10.1016/j.bmcl.2018.08.018 BindingDB Entry DOI: 10.7270/Q25Q4ZST | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase PLK1 (Homo sapiens (Human)) | BDBM50260121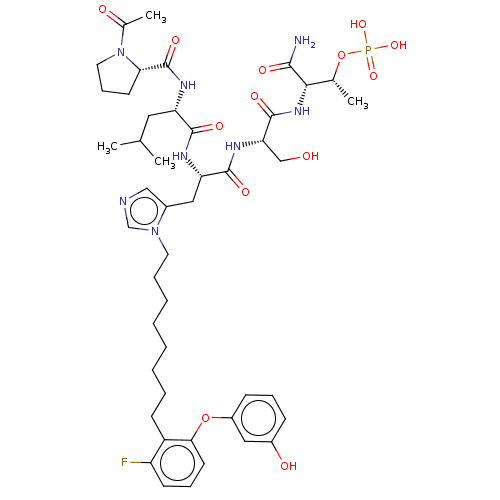 (CHEMBL4068949) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Chemical Biology Laboratory, Center for Cancer Research, National Cancer Institute, National Institutes of Health, Frederick, MD 21702, United States. Curated by ChEMBL | Assay Description Inhibition of 5CF-GPMQSpTPLNG-NH2 binding to myc-tagged human Plk1 PBD expressed in HEK-293T cells incubated for 1 hr by fluorescence polarization as... | Bioorg Med Chem 25: 5041-5049 (2017) Article DOI: 10.1016/j.bmc.2017.02.063 BindingDB Entry DOI: 10.7270/Q2QR50MP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase PLK1 (Homo sapiens (Human)) | BDBM50260122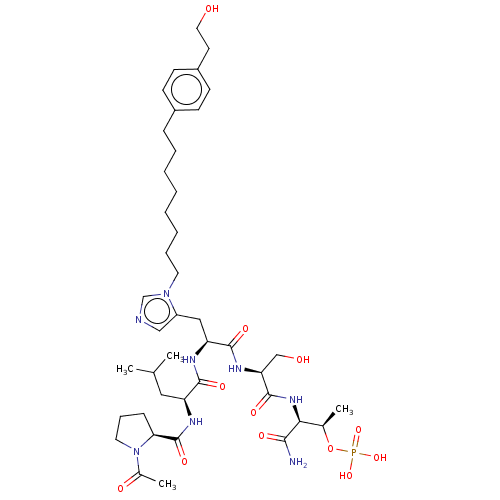 (CHEMBL4092848) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Chemical Biology Laboratory, Center for Cancer Research, National Cancer Institute, National Institutes of Health, Frederick, MD 21702, United States. Curated by ChEMBL | Assay Description Inhibition of 5CF-GPMQSpTPLNG-NH2 binding to myc-tagged human Plk1 PBD expressed in HEK-293T cells incubated for 1 hr by fluorescence polarization as... | Bioorg Med Chem 25: 5041-5049 (2017) Article DOI: 10.1016/j.bmc.2017.02.063 BindingDB Entry DOI: 10.7270/Q2QR50MP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase PLK1 (Homo sapiens (Human)) | BDBM50403016 (CHEMBL2207845) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Frederick National Laboratory for Cancer Research Curated by ChEMBL | Assay Description Competitive binding affinity to GFP-HA-fused PLK1 polo box domain expressed in HEK293A cells using biotinylated p-T38 peptide as substrate after 1 hr... | Bioorg Med Chem Lett 22: 7306-8 (2012) Article DOI: 10.1016/j.bmcl.2012.10.093 BindingDB Entry DOI: 10.7270/Q2Z89DK2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase PLK1 (Homo sapiens (Human)) | BDBM50467111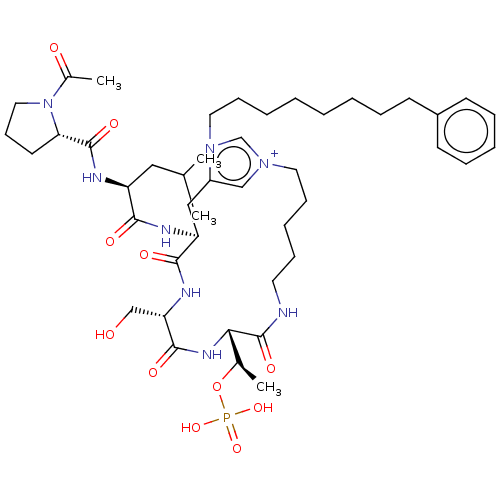 (CHEMBL4281659) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute-Frederick Curated by ChEMBL | Assay Description Inhibition of 5CF-GPMQSpTPLNG-NH2 binding to Plk1 C-terminal polo-box domain (unknown origin) preincubated for 30 mins followed by probe addition mea... | Bioorg Med Chem Lett 28: 3202-3205 (2018) Article DOI: 10.1016/j.bmcl.2018.08.018 BindingDB Entry DOI: 10.7270/Q25Q4ZST | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Growth factor receptor-bound protein 2 (Homo sapiens (Human)) | BDBM50168318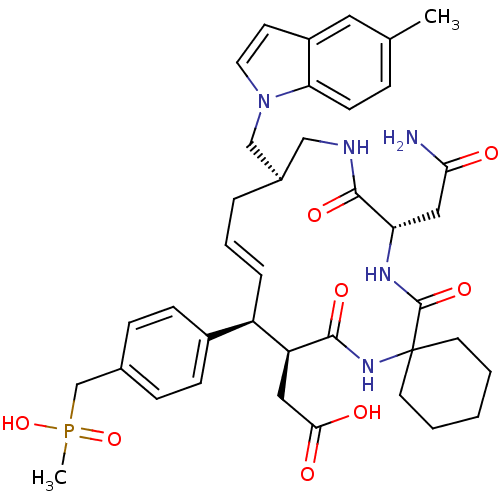 (CHEMBL424823 | [(15R,20S)-18-Carbamoylmethyl-10-[4...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute Curated by ChEMBL | Assay Description Inhibitory concentration against growth factor receptor bound protein 2 | J Med Chem 48: 3945-8 (2005) Article DOI: 10.1021/jm050059m BindingDB Entry DOI: 10.7270/Q2V69J48 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase PLK1 (Homo sapiens (Human)) | BDBM50194933 (CHEMBL3956625) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 6.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Chemical Biology Laboratory, Center for Cancer Research, National Cancer Institute, National Institutes of Health, Frederick, MD 21702, United States. Curated by ChEMBL | Assay Description Inhibition of 5CF-GPMQSpTPLNG-NH2 binding to myc-tagged human Plk1 PBD expressed in HEK-293T cells incubated for 1 hr by fluorescence polarization as... | Bioorg Med Chem 25: 5041-5049 (2017) Article DOI: 10.1016/j.bmc.2017.02.063 BindingDB Entry DOI: 10.7270/Q2QR50MP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Growth factor receptor-bound protein 2 (Homo sapiens (Human)) | BDBM50075017 (CHEMBL332862 | Phosphoric acid mono-[4-((R)-2-acet...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute Curated by ChEMBL | Assay Description Inhibitory concentration of the compound against Growth factor receptor bound protein 2 in MDA-MB-453 cells | Bioorg Med Chem Lett 9: 347-52 (1999) BindingDB Entry DOI: 10.7270/Q2VM4BF2 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Mu-type opioid receptor (MOUSE) | BDBM50027791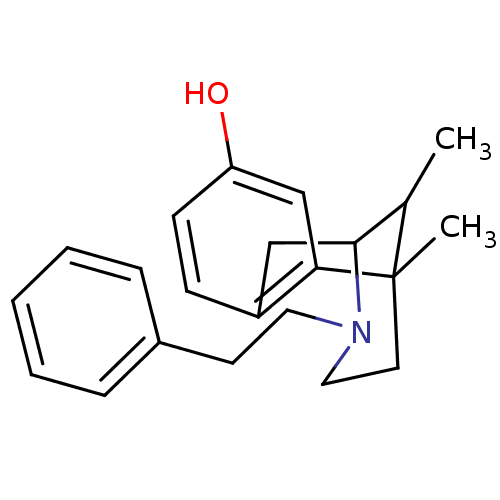 (6,11-Dimethyl-3-phenethyl-1,2,3,4,5,6-hexahydro-2,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Ability to induce 50% of maximal effect in mouse vas deferens expressing Opioid receptor delta 1 | J Med Chem 26: 1643-5 (1983) BindingDB Entry DOI: 10.7270/Q2N015J4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Growth factor receptor-bound protein 2 (Homo sapiens (Human)) | BDBM50122792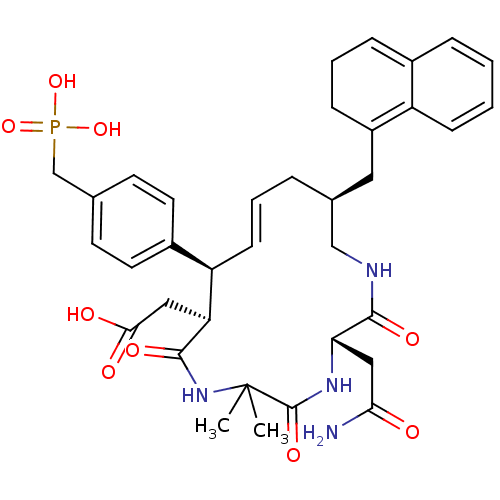 (CHEMBL312875 | [3-Carbamoylmethyl-14-(2,3-dihydro-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute-Frederick Curated by ChEMBL | Assay Description Inhibition against Growth factor receptor bound protein 2 binding using ELISA technique | J Med Chem 46: 244-54 (2003) Article DOI: 10.1021/jm0203635 BindingDB Entry DOI: 10.7270/Q2RR1XMN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Growth factor receptor-bound protein 2 (Homo sapiens (Human)) | BDBM50118687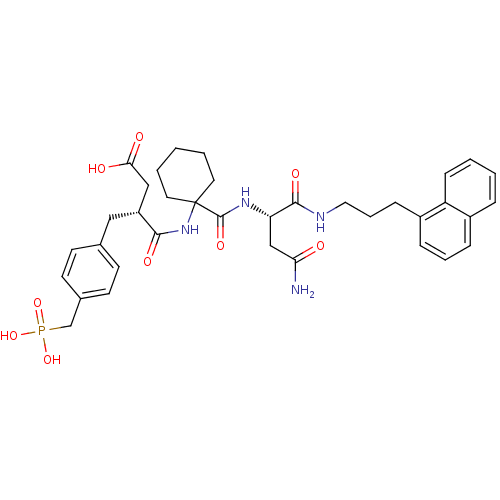 ((R)-N-{1-[(S)-2-Carbamoyl-1-(3-naphthalen-1-yl-pro...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health Curated by ChEMBL | Assay Description Binding affinity towards 5-hydroxytryptamine 3 receptor | Bioorg Med Chem Lett 12: 2781-4 (2002) BindingDB Entry DOI: 10.7270/Q23R0S6B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Growth factor receptor-bound protein 2 (Homo sapiens (Human)) | BDBM50085874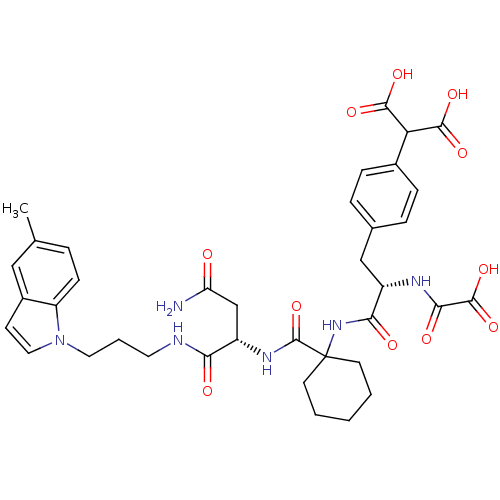 (2-{4-[(S)-2-(1-{(S)-2-Carbamoyl-1-[3-(5-methyl-ind...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute Curated by ChEMBL | Assay Description Inhibition of Growth factor receptor bound protein 2 binding by ELISA assay method | J Med Chem 43: 911-20 (2000) BindingDB Entry DOI: 10.7270/Q2WD3ZRD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1767 total ) | Next | Last >> |