

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50102711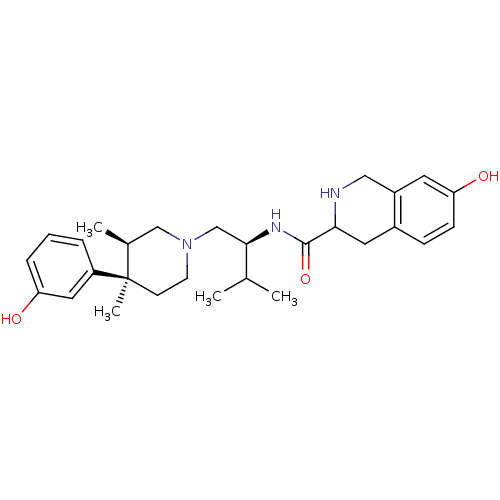 (7-Hydroxy-1,2,3,4-tetrahydro-isoquinoline-3-carbox...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.00600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Antagonist activity on agonist (U50,488) stimulated [35S]GTP-gamma-S, binding in cloned opioid receptor kappa 1 | J Med Chem 44: 2687-90 (2001) BindingDB Entry DOI: 10.7270/Q25T3M58 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM50064515 (3-[(3R,4R)-3,4-Dimethyl-1-((E)-3-o-tolyl-allyl)-pi...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description The compound was tested for antagonist activity by selective inhibition of [35S]-GTP-gammaS, binding to Opioid receptor mu 1 in Guinea pig caudate st... | J Med Chem 41: 1980-90 (1998) Article DOI: 10.1021/jm980063g BindingDB Entry DOI: 10.7270/Q2K35SSN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50102711 (7-Hydroxy-1,2,3,4-tetrahydro-isoquinoline-3-carbox...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Inhibition of stimulation of [35S]GTP-gamma-S, binding produced by the selective agonist (U69593, kappa-receptor), in guinea pig caudate membranes. | J Med Chem 44: 2687-90 (2001) BindingDB Entry DOI: 10.7270/Q25T3M58 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM50045775 ((3R,4R)3-[1-(3-Cyclohexyl-3-hydroxy-propyl)-3,4-di...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description The compound was tested for antagonist activity by selective inhibition of [35S]-GTP-gammaS, binding to Opioid receptor mu 1 in Guinea pig caudate st... | J Med Chem 41: 1980-90 (1998) Article DOI: 10.1021/jm980063g BindingDB Entry DOI: 10.7270/Q2K35SSN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM50064518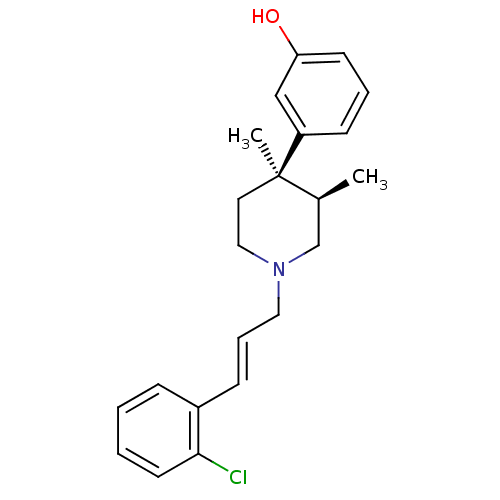 (3-{(3R,4R)-1-[(E)-3-(2-Chloro-phenyl)-allyl]-3,4-d...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description The compound was tested for antagonist activity by selective inhibition of [35S]-GTP-gammaS, binding to Opioid receptor mu 1 in Guinea pig caudate st... | J Med Chem 41: 1980-90 (1998) Article DOI: 10.1021/jm980063g BindingDB Entry DOI: 10.7270/Q2K35SSN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM82551 (C18130 | CAS_105618-26-6 | NOR-BNI (HCI)2 | NORBNI) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | 0.0380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Inhibition of [35S]GTP-gamma-S, binding from Opioid receptor kappa 1 in Guinea pig Caudate stimulated by U69,593 | J Med Chem 41: 5188-97 (1999) Article DOI: 10.1021/jm980511k BindingDB Entry DOI: 10.7270/Q2P26ZT4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM82551 (C18130 | CAS_105618-26-6 | NOR-BNI (HCI)2 | NORBNI) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem Similars | PubMed | 0.0380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Inhibition of stimulation of [35S]GTP-gamma-S, binding produced by the selective agonist (U69593, kappa-receptor), in guinea pig caudate membranes | J Med Chem 44: 2687-90 (2001) BindingDB Entry DOI: 10.7270/Q25T3M58 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM50291970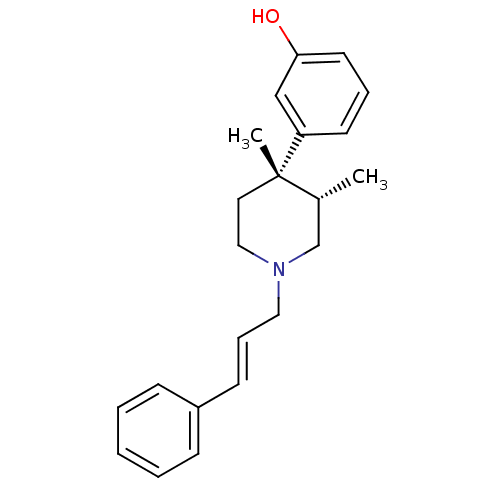 (3-[(3S,4S)-3,4-Dimethyl-1-((E)-3-phenyl-allyl)-pip...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Inhibition of [35S]GTP-gamma-S, binding from Opioid receptor mu 1 in Guinea pig Caudate stimulated by DAMGO | J Med Chem 41: 5188-97 (1999) Article DOI: 10.1021/jm980511k BindingDB Entry DOI: 10.7270/Q2P26ZT4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM50064521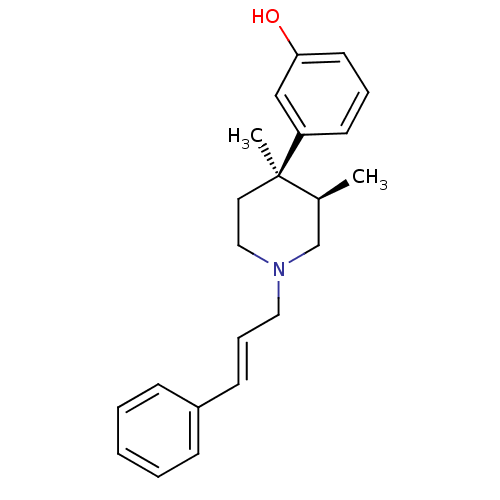 (3-[(3R,4R)-3,4-Dimethyl-1-((E)-3-phenyl-allyl)-pip...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description The compound was tested for antagonist activity by selective inhibition of [35S]-GTP-gammaS, binding to Opioid receptor mu 1 in Guinea pig caudate st... | J Med Chem 41: 1980-90 (1998) Article DOI: 10.1021/jm980063g BindingDB Entry DOI: 10.7270/Q2K35SSN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50075807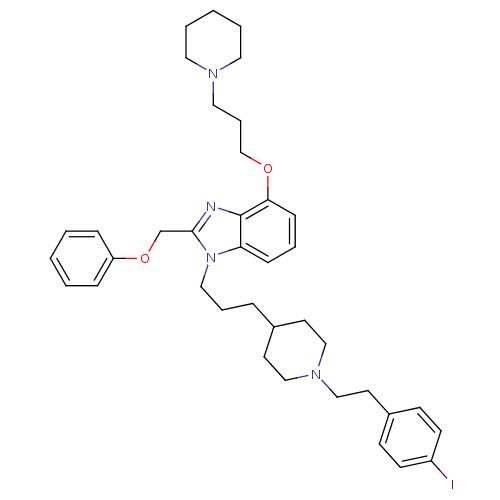 (1-(3-{1-[2-(4-Iodo-phenyl)-ethyl]-piperidin-4-yl}-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0520 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company Curated by ChEMBL | Assay Description Ability to displace [125I]-peptide YY binding to cloned human Neuropeptide Y receptor type 1 expressed in AV-12 cells | Bioorg Med Chem Lett 9: 647-52 (1999) BindingDB Entry DOI: 10.7270/Q2QN65ZK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50026614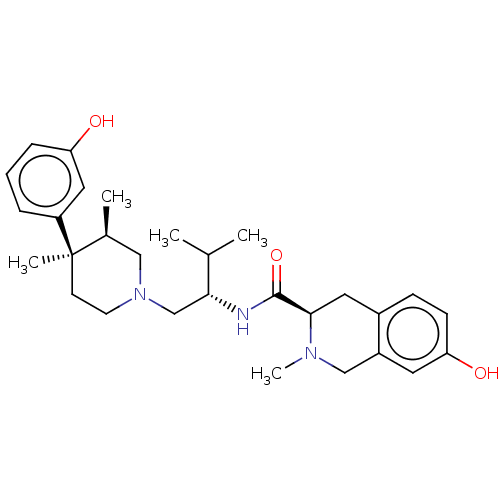 (CHEMBL575508) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0530 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Inhibition of [3H]U-69593 binding to Opioid receptor kappa 1 of guinea pig brain | J Med Chem 46: 3127-37 (2003) Article DOI: 10.1021/jm030094y BindingDB Entry DOI: 10.7270/Q2319WM4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM21864 ((21R)-22-(cyclopropylmethyl)-14-oxa-11,22-diazahep...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description [3H]-Cl-DPDPE competition binding assay using human cloned opioid receptor delta 1 | J Med Chem 47: 281-4 (2004) Article DOI: 10.1021/jm030419a BindingDB Entry DOI: 10.7270/Q20K2990 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50075796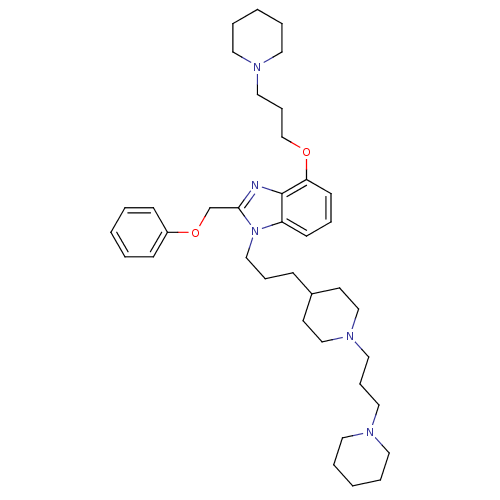 (2-Phenoxymethyl-4-(3-piperidin-1-yl-propoxy)-1-{3-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.106 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company Curated by ChEMBL | Assay Description Ability to displace [125I]-peptide YY binding to cloned human Neuropeptide Y receptor type 1 expressed in AV-12 cells | Bioorg Med Chem Lett 9: 647-52 (1999) BindingDB Entry DOI: 10.7270/Q2QN65ZK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50075811 (3-(4-{3-[2-Phenoxymethyl-4-(3-piperidin-1-yl-propo...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.112 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company Curated by ChEMBL | Assay Description Ability to displace [125I]-peptide YY binding to cloned human Neuropeptide Y receptor type 1 expressed in AV-12 cells | Bioorg Med Chem Lett 9: 647-52 (1999) BindingDB Entry DOI: 10.7270/Q2QN65ZK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50075803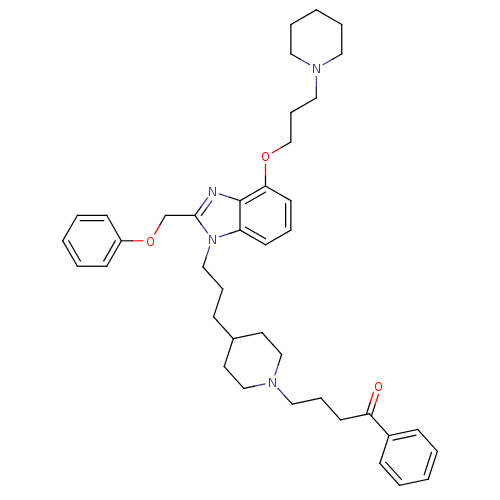 (4-(4-{3-[2-Phenoxymethyl-4-(3-piperidin-1-yl-propo...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.132 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company Curated by ChEMBL | Assay Description Ability to displace [125I]-peptide YY binding to cloned human Neuropeptide Y receptor type 1 expressed in AV-12 cells | Bioorg Med Chem Lett 9: 647-52 (1999) BindingDB Entry DOI: 10.7270/Q2QN65ZK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50064515 (3-[(3R,4R)-3,4-Dimethyl-1-((E)-3-o-tolyl-allyl)-pi...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description The compound was tested for antagonist activity by selective inhibition of [35S]-GTP-gammaS, binding in Guinea pig caudate stimulated by U69,593 to O... | J Med Chem 41: 1980-90 (1998) Article DOI: 10.1021/jm980063g BindingDB Entry DOI: 10.7270/Q2K35SSN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50045792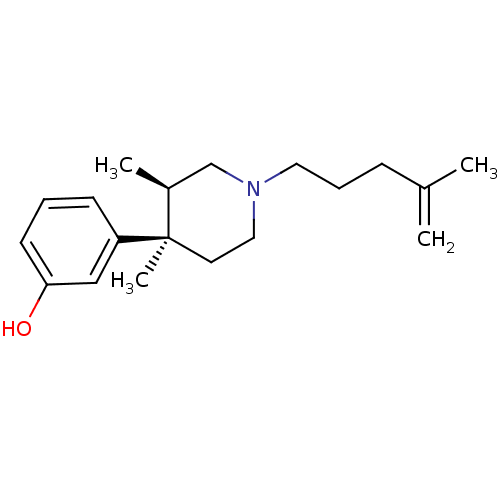 (3-[3,4-Dimethyl-1-(4-methyl-pent-4-enyl)-piperidin...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Binding affinity towards mu-opioid receptor by the displacement of [3H]-Nal in rat brain homogenates | J Med Chem 36: 2842-50 (1993) BindingDB Entry DOI: 10.7270/Q2R78D9W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50027039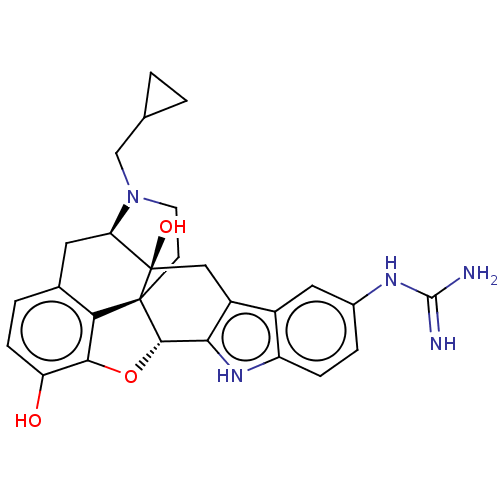 (5''-Guanidinonaltrindole | 5''-Guanidinylnaltrindo...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Inhibition of [3H]U-69593 binding to Opioid receptor kappa 1 of guinea pig brain | J Med Chem 46: 3127-37 (2003) Article DOI: 10.1021/jm030094y BindingDB Entry DOI: 10.7270/Q2319WM4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50075812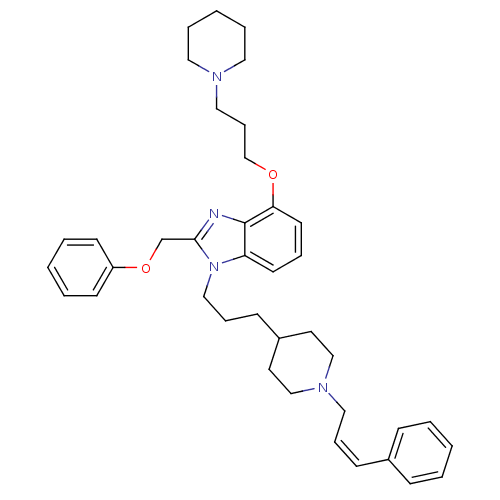 (2-Phenoxymethyl-1-{3-[1-((Z)-3-phenyl-allyl)-piper...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.183 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company Curated by ChEMBL | Assay Description Ability to displace [125I]-peptide YY binding to cloned human Neuropeptide Y receptor type 1 expressed in AV-12 cells | Bioorg Med Chem Lett 9: 647-52 (1999) BindingDB Entry DOI: 10.7270/Q2QN65ZK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50075809 (1-[3-(1-Phenethyl-piperidin-4-yl)-propyl]-2-phenox...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.191 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company Curated by ChEMBL | Assay Description Ability to displace [125I]-peptide YY binding to cloned human Neuropeptide Y receptor type 1 expressed in AV-12 cells | Bioorg Med Chem Lett 9: 647-52 (1999) BindingDB Entry DOI: 10.7270/Q2QN65ZK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50045733 (3-[3,4-Dimethyl-1-(3-thiophen-2-yl-propyl)-piperid...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Binding affinity towards mu-opioid receptor by displacement of [3H]-NAL at 10 nM from rat brain homogenates | J Med Chem 36: 2833-41 (1993) BindingDB Entry DOI: 10.7270/Q2W0951M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50045733 (3-[3,4-Dimethyl-1-(3-thiophen-2-yl-propyl)-piperid...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Binding affinity towards mu opioid receptor by displacement of [3H]-NAL from rat brain homogenates | J Med Chem 36: 2833-41 (1993) BindingDB Entry DOI: 10.7270/Q2W0951M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50018730 (3-(2-Phenethyl-octahydro-isoquinolin-4a-yl)-phenol...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company Curated by ChEMBL | Assay Description Binding affinity to Opioid receptor mu 1 in rat brain using [3H]-NAL as radioligand | J Med Chem 31: 555-60 (1988) BindingDB Entry DOI: 10.7270/Q2HQ40HR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50045742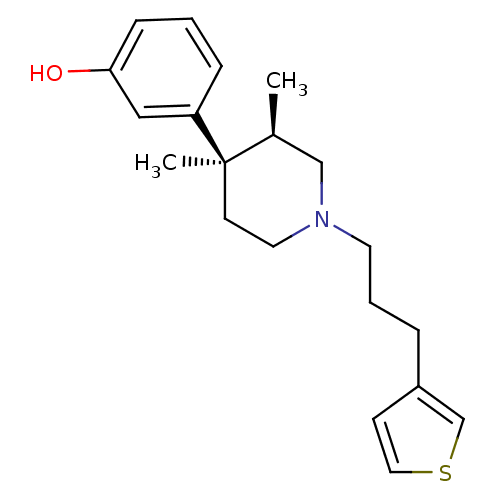 (3-[3,4-Dimethyl-1-(3-thiophen-3-yl-propyl)-piperid...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Binding affinity towards mu-opioid receptor by the displacement of [3H]-Nal in rat brain homogenates | J Med Chem 36: 2842-50 (1993) BindingDB Entry DOI: 10.7270/Q2R78D9W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50045742 (3-[3,4-Dimethyl-1-(3-thiophen-3-yl-propyl)-piperid...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Binding affinity towards mu-opioid receptor by displacement of [3H]-NAL at 10 nM from rat brain homogenates | J Med Chem 36: 2833-41 (1993) BindingDB Entry DOI: 10.7270/Q2W0951M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM82551 (C18130 | CAS_105618-26-6 | NOR-BNI (HCI)2 | NORBNI) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | 0.280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Binding affinity determined on Opioid receptor kappa 1 in Guinea pig brain membranes using radioligand [3H]U-69593 | J Med Chem 41: 5188-97 (1999) Article DOI: 10.1021/jm980511k BindingDB Entry DOI: 10.7270/Q2P26ZT4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50075797 (1-{3-[1-(2-Cyclohexyl-ethyl)-piperidin-4-yl]-propy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.285 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company Curated by ChEMBL | Assay Description Ability to displace [125I]-peptide YY binding to cloned human Neuropeptide Y receptor type 1 expressed in AV-12 cells | Bioorg Med Chem Lett 9: 647-52 (1999) BindingDB Entry DOI: 10.7270/Q2QN65ZK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50045738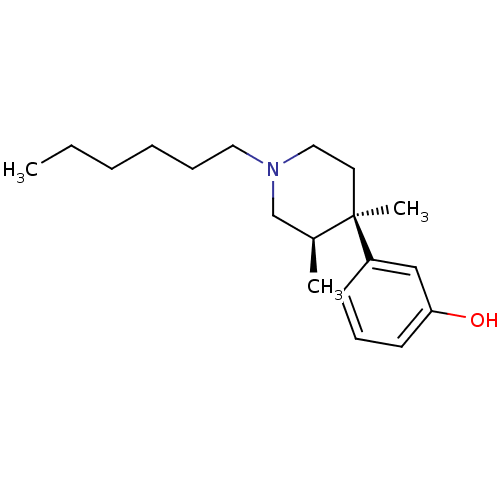 ((3R,4R)3-(1-Hexyl-3,4-dimethyl-piperidin-4-yl)-phe...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Binding affinity towards mu opioid receptor by displacement of [3H]-NAL from rat brain homogenates | J Med Chem 36: 2833-41 (1993) BindingDB Entry DOI: 10.7270/Q2W0951M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50045779 (3-[3,4-Dimethyl-1-(4-methyl-pentyl)-piperidin-4-yl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Binding affinity towards mu-opioid receptor by the displacement of [3H]-Nal in rat brain homogenates | J Med Chem 36: 2842-50 (1993) BindingDB Entry DOI: 10.7270/Q2R78D9W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50045776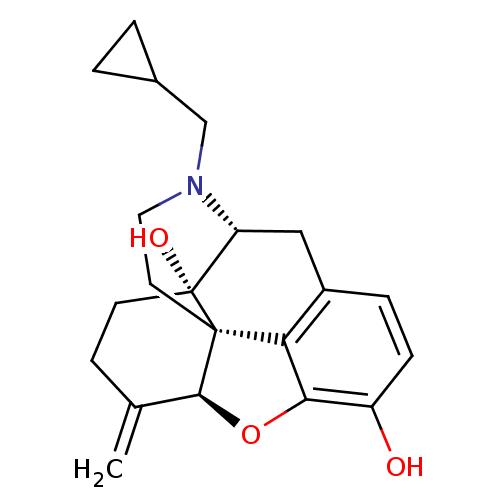 (CHEMBL982 | JF-1 | NALMEFENE | Nalmetrene | ORF-11...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | PubMed | 0.290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Binding affinity towards mu-opioid receptor by the displacement of [3H]-Nal in rat brain homogenates | J Med Chem 36: 2842-50 (1993) BindingDB Entry DOI: 10.7270/Q2R78D9W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50045780 (3-[1-(3-Cyclohexyl-3-hydroxy-propyl)-3,4-dimethyl-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 0.290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Binding affinity towards mu-opioid receptor by the displacement of [3H]-Nal in rat brain homogenates | J Med Chem 36: 2842-50 (1993) BindingDB Entry DOI: 10.7270/Q2R78D9W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50075806 (1-{3-[1-(3-Methyl-butyl)-piperidin-4-yl]-propyl}-2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company Curated by ChEMBL | Assay Description Ability to displace [125I]-peptide YY binding to cloned human Neuropeptide Y receptor type 1 expressed in AV-12 cells | Bioorg Med Chem Lett 9: 647-52 (1999) BindingDB Entry DOI: 10.7270/Q2QN65ZK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50045748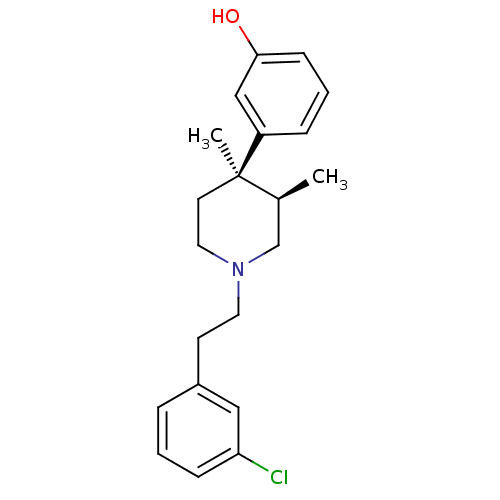 (3-{1-[2-(3-Chloro-phenyl)-ethyl]-3,4-dimethyl-pipe...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Binding affinity towards mu opioid receptor by displacement of [3H]-NAL from rat brain homogenates | J Med Chem 36: 2833-41 (1993) BindingDB Entry DOI: 10.7270/Q2W0951M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50045775 ((3R,4R)3-[1-(3-Cyclohexyl-3-hydroxy-propyl)-3,4-di...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 0.312 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description The compound was tested for antagonist activity by selective inhibition of [35S]-GTP-gammaS, binding in Guinea pig caudate stimulated by SMC-80 (Opio... | J Med Chem 41: 1980-90 (1998) Article DOI: 10.1021/jm980063g BindingDB Entry DOI: 10.7270/Q2K35SSN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50075802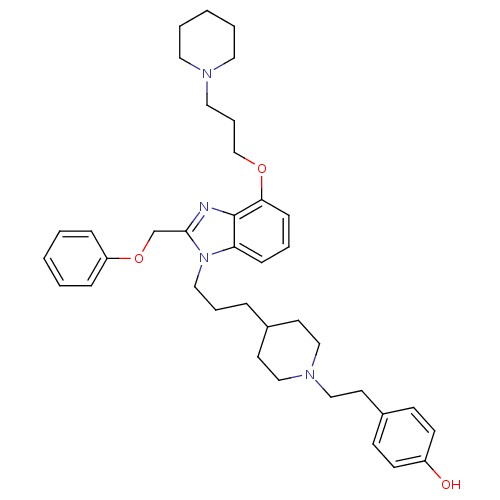 (4-[2-(4-{3-[2-Phenoxymethyl-4-(3-piperidin-1-yl-pr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.313 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company Curated by ChEMBL | Assay Description Ability to displace [125I]-peptide YY binding to cloned human Neuropeptide Y receptor type 1 expressed in AV-12 cells | Bioorg Med Chem Lett 9: 647-52 (1999) BindingDB Entry DOI: 10.7270/Q2QN65ZK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50045775 ((3R,4R)3-[1-(3-Cyclohexyl-3-hydroxy-propyl)-3,4-di...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 0.320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Inhibition of [3H]-DAMGO binding to Opioid receptor mu 1 of rat brain membranes | J Med Chem 41: 1980-90 (1998) Article DOI: 10.1021/jm980063g BindingDB Entry DOI: 10.7270/Q2K35SSN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50130563 ((3R)-7-Hydroxy-N-[(1S)-1-{[(3R,4R)-4-(3-hydroxyphe...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Inhibition of [3H]U-69593 binding to Opioid receptor kappa 1 of guinea pig brain | J Med Chem 46: 3127-37 (2003) Article DOI: 10.1021/jm030094y BindingDB Entry DOI: 10.7270/Q2319WM4 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50102711 (7-Hydroxy-1,2,3,4-tetrahydro-isoquinoline-3-carbox...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Binding affinity to opioid receptor kappa 1 of guinea pig brain, using [3H]U-69593 as radioligand | J Med Chem 44: 2687-90 (2001) BindingDB Entry DOI: 10.7270/Q25T3M58 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50130563 ((3R)-7-Hydroxy-N-[(1S)-1-{[(3R,4R)-4-(3-hydroxyphe...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Binding affinity of compound for Opioid receptor kappa 1 was determined using [3H]U-69593 as radioligand from guinea pig caudate | J Med Chem 47: 1070-3 (2004) Article DOI: 10.1021/jm030467v BindingDB Entry DOI: 10.7270/Q2BV7HC4 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50130563 ((3R)-7-Hydroxy-N-[(1S)-1-{[(3R,4R)-4-(3-hydroxyphe...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Binding affinity towards human cloned Opioid receptor kappa 1 using [3H]U-69593 | J Med Chem 46: 3127-37 (2003) Article DOI: 10.1021/jm030094y BindingDB Entry DOI: 10.7270/Q2319WM4 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50045775 ((3R,4R)3-[1-(3-Cyclohexyl-3-hydroxy-propyl)-3,4-di...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 0.330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description The compound was tested for antagonist activity by selective inhibition of [35S]-GTP-gammaS, binding in Guinea pig caudate stimulated by U69,593 to O... | J Med Chem 41: 1980-90 (1998) Article DOI: 10.1021/jm980063g BindingDB Entry DOI: 10.7270/Q2K35SSN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM50067447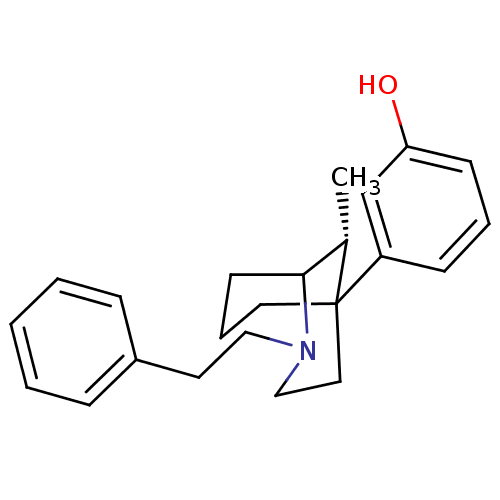 (3-((R)-9-Methyl-2-phenethyl-2-aza-bicyclo[3.3.1]no...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Inhibition of [35S]-GTP-gammaS, binding in guinea pig caudate stimulated by DAMGO (Opioid receptor mu 1) | J Med Chem 41: 4143-9 (1998) Article DOI: 10.1021/jm980290i BindingDB Entry DOI: 10.7270/Q2RB73Q6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50045781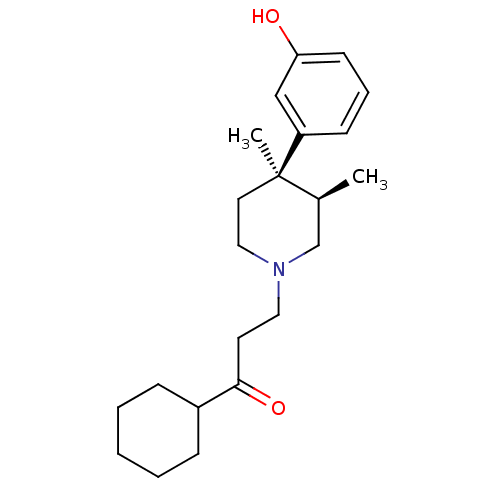 (1-Cyclohexyl-3-[4-(3-hydroxy-phenyl)-3,4-dimethyl-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Binding affinity towards mu-opioid receptor by the displacement of [3H]-Nal in rat brain homogenates | J Med Chem 36: 2842-50 (1993) BindingDB Entry DOI: 10.7270/Q2R78D9W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50064515 (3-[(3R,4R)-3,4-Dimethyl-1-((E)-3-o-tolyl-allyl)-pi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.355 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description The compound was tested for antagonist activity by selective inhibition of [35S]-GTP-gammaS, binding in Guinea pig caudate stimulated by SMC-80 (Opio... | J Med Chem 41: 1980-90 (1998) Article DOI: 10.1021/jm980063g BindingDB Entry DOI: 10.7270/Q2K35SSN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50075799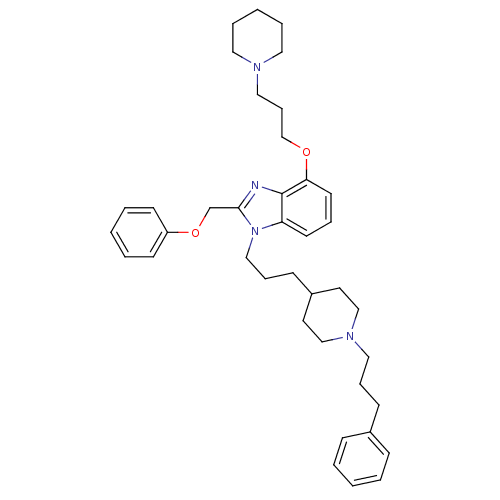 (2-Phenoxymethyl-1-{3-[1-(3-phenyl-propyl)-piperidi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.361 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company Curated by ChEMBL | Assay Description Ability to displace [125I]-peptide YY binding to cloned human Neuropeptide Y receptor type 1 expressed in AV-12 cells | Bioorg Med Chem Lett 9: 647-52 (1999) BindingDB Entry DOI: 10.7270/Q2QN65ZK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50045791 (3-[1-(3-Cyclopentyl-propyl)-3,4-dimethyl-piperidin...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Binding affinity towards mu-opioid receptor by the displacement of [3H]-Nal in rat brain homogenates | J Med Chem 36: 2842-50 (1993) BindingDB Entry DOI: 10.7270/Q2R78D9W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50075798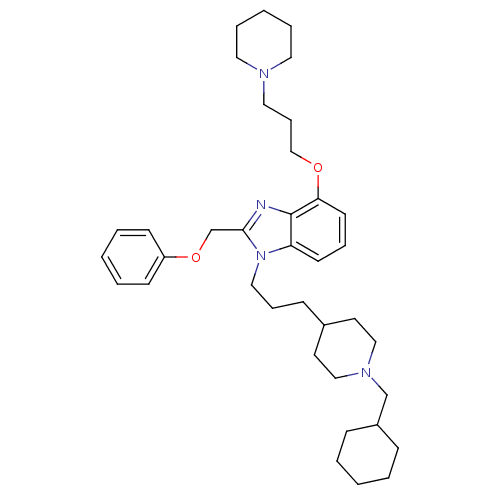 (1-[3-(1-Cyclohexylmethyl-piperidin-4-yl)-propyl]-2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.393 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company Curated by ChEMBL | Assay Description Ability to displace [125I]-peptide YY binding to cloned human Neuropeptide Y receptor type 1 expressed in AV-12 cells | Bioorg Med Chem Lett 9: 647-52 (1999) BindingDB Entry DOI: 10.7270/Q2QN65ZK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50045786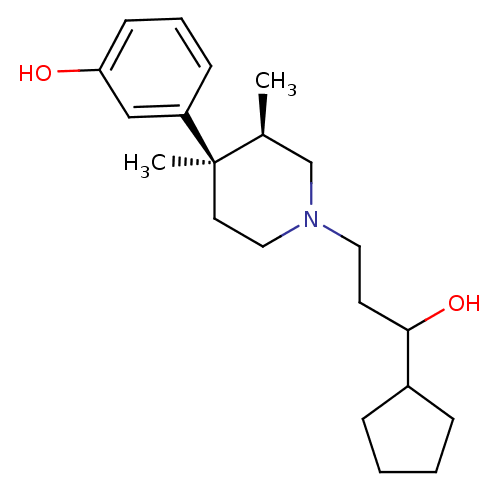 (3-[1-(3-Cyclopentyl-3-hydroxy-propyl)-3,4-dimethyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.410 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Binding affinity towards mu-opioid receptor by the displacement of [3H]-Nal in rat brain homogenates | J Med Chem 36: 2842-50 (1993) BindingDB Entry DOI: 10.7270/Q2R78D9W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50045775 ((3R,4R)3-[1-(3-Cyclohexyl-3-hydroxy-propyl)-3,4-di...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | PubMed | 0.410 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Binding affinity towards mu-opioid receptor by the displacement of [3H]-Nal in rat brain homogenates | J Med Chem 36: 2842-50 (1993) BindingDB Entry DOI: 10.7270/Q2R78D9W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50045785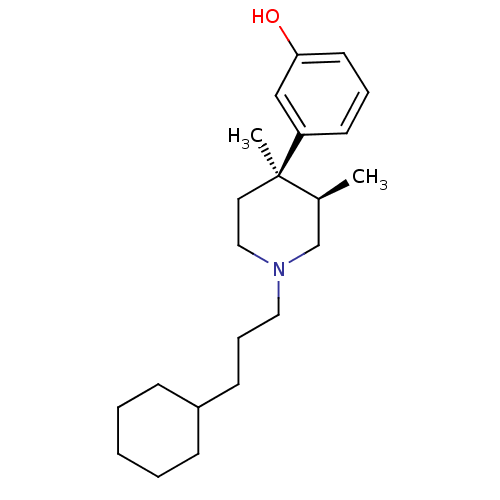 (3-[1-(3-Cyclohexyl-propyl)-3,4-dimethyl-piperidin-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.490 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Binding affinity towards mu-opioid receptor by the displacement of [3H]-Nal in rat brain homogenates | J Med Chem 36: 2842-50 (1993) BindingDB Entry DOI: 10.7270/Q2R78D9W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 657 total ) | Next | Last >> |