

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50246381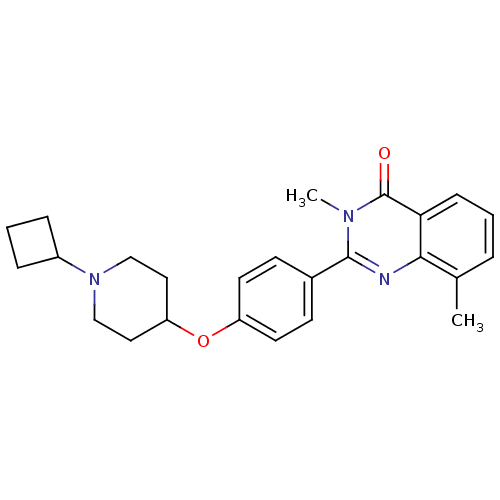 (2-(4-(1-cyclobutylpiperidin-4-yloxy)phenyl)-3,8-di...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0313 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bioprojet-Biotech Curated by ChEMBL | Assay Description Displacement of [125I]Iodoproxyfan from human recombinant histamine H3 receptor by Competitive binding assay | Bioorg Med Chem Lett 21: 5378-83 (2011) Article DOI: 10.1016/j.bmcl.2011.07.006 BindingDB Entry DOI: 10.7270/Q2VX0GXK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50492784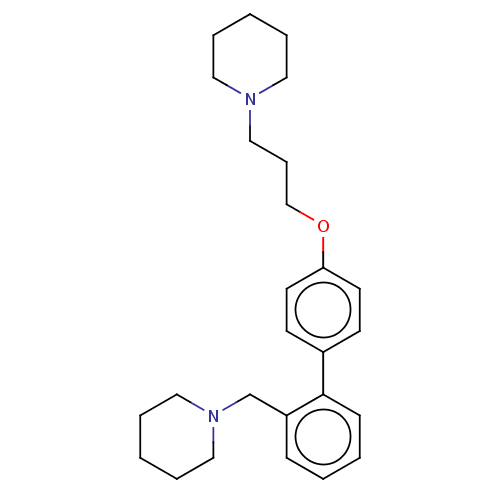 (CHEMBL2413837) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BIOPROJET-BIOTECH Curated by ChEMBL | Assay Description Displacement of [125I]iodoproxyfan from histamine H3 receptor (unknown origin) expressed in CHO cells | Bioorg Med Chem 21: 4526-9 (2013) Article DOI: 10.1016/j.bmc.2013.05.035 BindingDB Entry DOI: 10.7270/Q2J9699Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50319536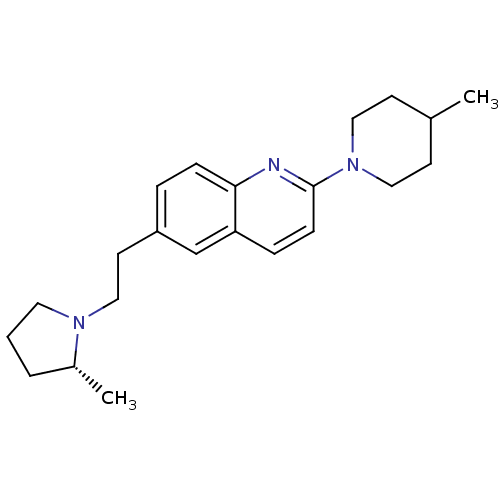 ((R)-2-(4-methylpiperidin-1-yl)-6-(2-(2-methylpyrro...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bioprojet-Biotech Curated by ChEMBL | Assay Description Displacement of [125I]Iodoproxyfan from human recombinant histamine H3 receptor by Competitive binding assay | Bioorg Med Chem Lett 21: 5378-83 (2011) Article DOI: 10.1016/j.bmcl.2011.07.006 BindingDB Entry DOI: 10.7270/Q2VX0GXK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1D (Homo sapiens (Human)) | BDBM50413549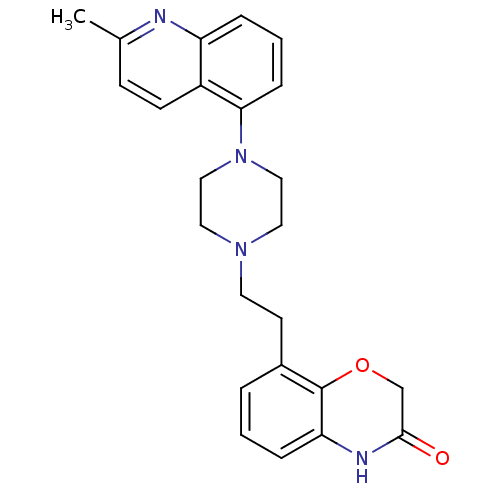 (CHEMBL513715) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity at human 5-HT1D receptor expressed in CHO cells assessed as inhibition of GTPgammaS binding by scintillation proximity assay | Bioorg Med Chem Lett 20: 7092-6 (2010) Article DOI: 10.1016/j.bmcl.2010.09.085 BindingDB Entry DOI: 10.7270/Q2MC919V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50492781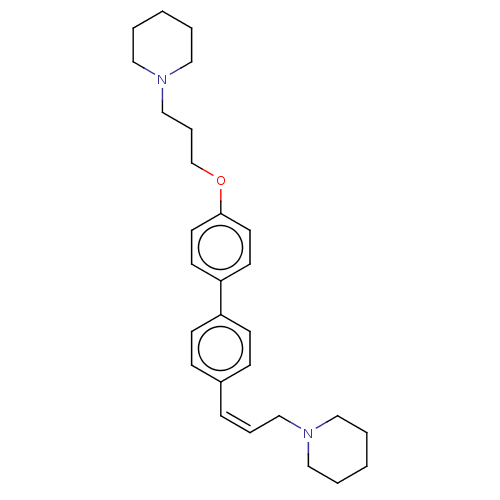 (CHEMBL2413835) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0570 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BIOPROJET-BIOTECH Curated by ChEMBL | Assay Description Displacement of [125I]iodoproxyfan from histamine H3 receptor (unknown origin) expressed in CHO cells | Bioorg Med Chem 21: 4526-9 (2013) Article DOI: 10.1016/j.bmc.2013.05.035 BindingDB Entry DOI: 10.7270/Q2J9699Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50246382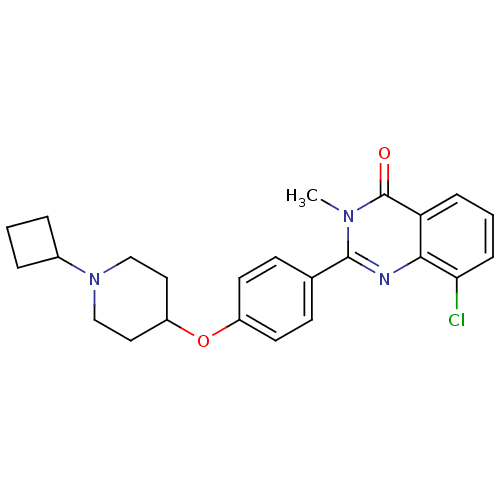 (8-chloro-2-(4-(1-cyclobutylpiperidin-4-yloxy)pheny...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0625 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bioprojet-Biotech Curated by ChEMBL | Assay Description Displacement of [125I]Iodoproxyfan from human recombinant histamine H3 receptor by Competitive binding assay | Bioorg Med Chem Lett 21: 5378-83 (2011) Article DOI: 10.1016/j.bmcl.2011.07.006 BindingDB Entry DOI: 10.7270/Q2VX0GXK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50278495 (4-chloro-1-(4-(3-(piperidin-1-yl)propoxy)benzyl)pi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0680 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johann Wolfgang Goethe-Universität Curated by ChEMBL | Assay Description Displacement of [125I]iodoproxyfan from human histamine H3 receptor expressed in HEL293 cells | Bioorg Med Chem Lett 19: 2172-5 (2009) Article DOI: 10.1016/j.bmcl.2009.02.110 BindingDB Entry DOI: 10.7270/Q2H41RBM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50246290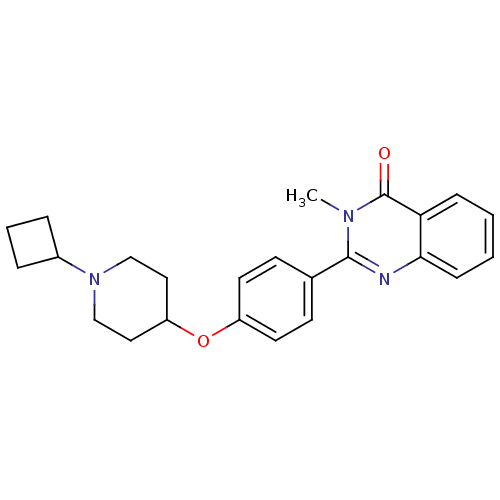 (2-(4-(1-cyclobutylpiperidin-4-yloxy)phenyl)-3-meth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0688 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bioprojet-Biotech Curated by ChEMBL | Assay Description Displacement of [125I]Iodoproxyfan from human recombinant histamine H3 receptor by Competitive binding assay | Bioorg Med Chem Lett 21: 5378-83 (2011) Article DOI: 10.1016/j.bmcl.2011.07.006 BindingDB Entry DOI: 10.7270/Q2VX0GXK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50246434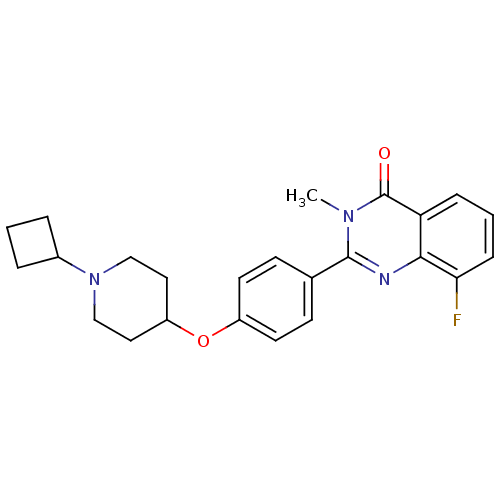 (2-(4-(1-cyclobutylpiperidin-4-yloxy)phenyl)-8-fluo...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0781 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bioprojet-Biotech Curated by ChEMBL | Assay Description Displacement of [125I]Iodoproxyfan from human recombinant histamine H3 receptor by Competitive binding assay | Bioorg Med Chem Lett 21: 5378-83 (2011) Article DOI: 10.1016/j.bmcl.2011.07.006 BindingDB Entry DOI: 10.7270/Q2VX0GXK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1D (Homo sapiens (Human)) | BDBM50417409 (CHEMBL1290487) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity at human 5-HT1D receptor expressed in CHO cells assessed as inhibition of GTPgammaS binding by scintillation proximity assay | Bioorg Med Chem Lett 20: 7092-6 (2010) Article DOI: 10.1016/j.bmcl.2010.09.085 BindingDB Entry DOI: 10.7270/Q2MC919V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50246334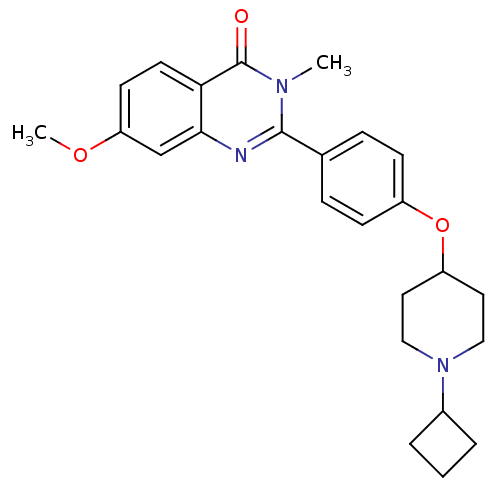 (2-(4-(1-cyclobutylpiperidin-4-yloxy)phenyl)-7-meth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0938 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bioprojet-Biotech Curated by ChEMBL | Assay Description Displacement of [125I]Iodoproxyfan from human recombinant histamine H3 receptor by Competitive binding assay | Bioorg Med Chem Lett 21: 5378-83 (2011) Article DOI: 10.1016/j.bmcl.2011.07.006 BindingDB Entry DOI: 10.7270/Q2VX0GXK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50246333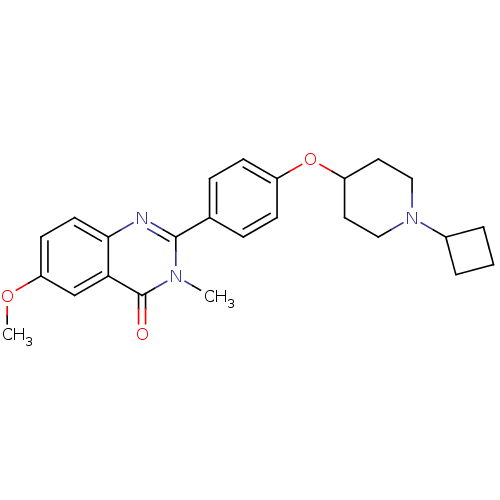 (2-(4-(1-cyclobutylpiperidin-4-yloxy)phenyl)-6-meth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0938 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bioprojet-Biotech Curated by ChEMBL | Assay Description Displacement of [125I]Iodoproxyfan from human recombinant histamine H3 receptor by Competitive binding assay | Bioorg Med Chem Lett 21: 5378-83 (2011) Article DOI: 10.1016/j.bmcl.2011.07.006 BindingDB Entry DOI: 10.7270/Q2VX0GXK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50246435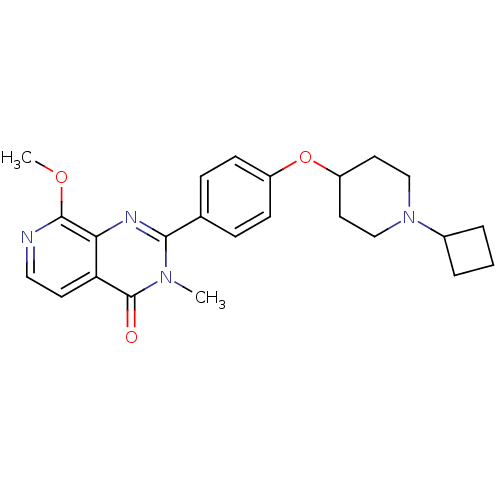 (2-(4-(1-cyclobutylpiperidin-4-yloxy)phenyl)-8-meth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.0938 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bioprojet-Biotech Curated by ChEMBL | Assay Description Displacement of [125I]Iodoproxyfan from human recombinant histamine H3 receptor by Competitive binding assay | Bioorg Med Chem Lett 21: 5378-83 (2011) Article DOI: 10.1016/j.bmcl.2011.07.006 BindingDB Entry DOI: 10.7270/Q2VX0GXK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50278350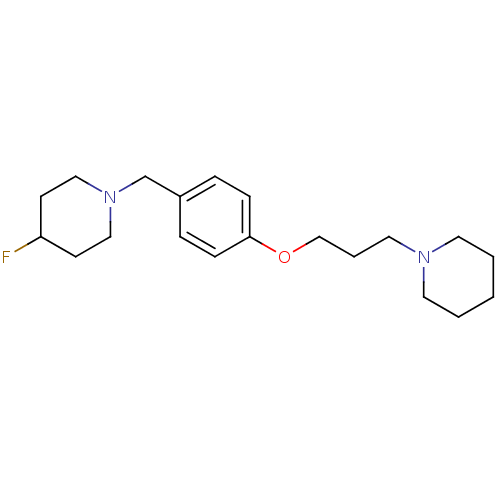 (4-fluoro-1-(4-(3-(piperidin-1-yl)propoxy)benzyl)pi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0940 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johann Wolfgang Goethe-Universität Curated by ChEMBL | Assay Description Displacement of [125I]iodoproxyfan from human histamine H3 receptor expressed in HEL293 cells | Bioorg Med Chem Lett 19: 2172-5 (2009) Article DOI: 10.1016/j.bmcl.2009.02.110 BindingDB Entry DOI: 10.7270/Q2H41RBM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50246287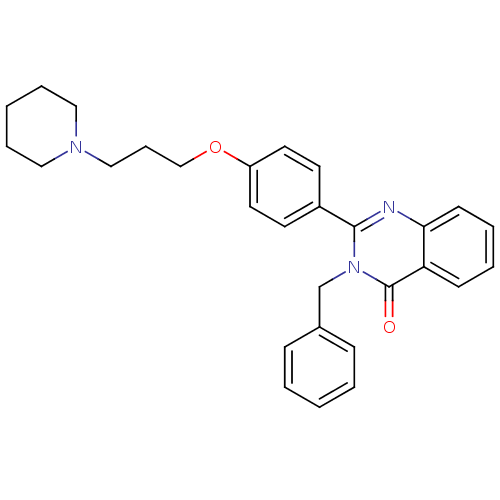 (3-benzyl-2-(4-(3-(piperidin-1-yl)propoxy)phenyl)qu...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0969 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bioprojet-Biotech Curated by ChEMBL | Assay Description Displacement of [125I]Iodoproxyfan from human recombinant histamine H3 receptor by Competitive binding assay | Bioorg Med Chem Lett 21: 5378-83 (2011) Article DOI: 10.1016/j.bmcl.2011.07.006 BindingDB Entry DOI: 10.7270/Q2VX0GXK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50319554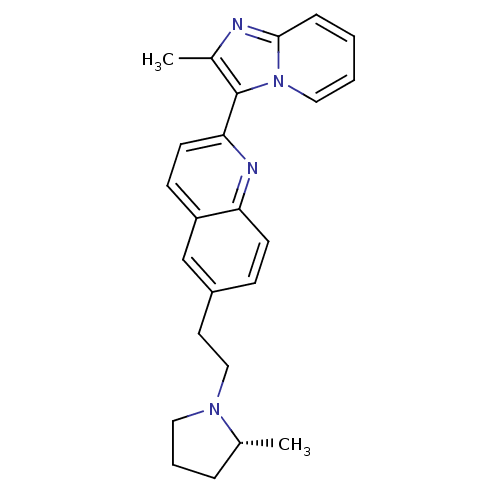 ((R)-2-(2-methylimidazo[1,2-a]pyridin-3-yl)-6-(2-(2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bioprojet-Biotech Curated by ChEMBL | Assay Description Displacement of [125I]Iodoproxyfan from human recombinant histamine H3 receptor by Competitive binding assay | Bioorg Med Chem Lett 21: 5378-83 (2011) Article DOI: 10.1016/j.bmcl.2011.07.006 BindingDB Entry DOI: 10.7270/Q2VX0GXK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50319552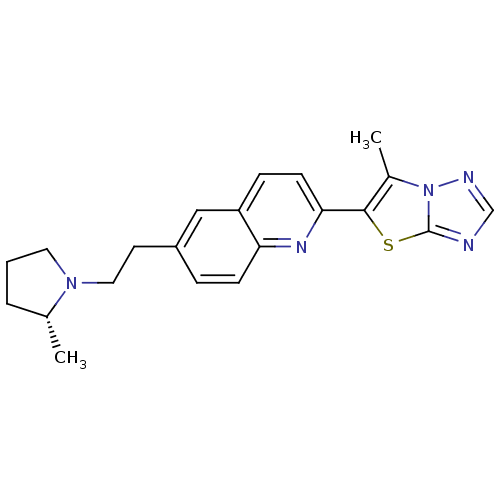 ((R)-6-methyl-5-(6-(2-(2-methylpyrrolidin-1-yl)ethy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bioprojet-Biotech Curated by ChEMBL | Assay Description Displacement of [125I]Iodoproxyfan from human recombinant histamine H3 receptor by Competitive binding assay | Bioorg Med Chem Lett 21: 5378-83 (2011) Article DOI: 10.1016/j.bmcl.2011.07.006 BindingDB Entry DOI: 10.7270/Q2VX0GXK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50319549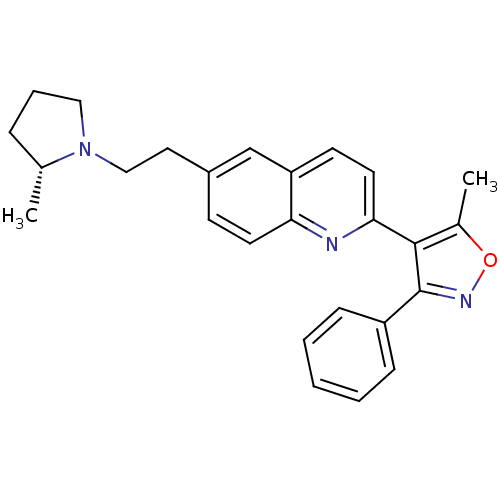 ((R)-5-methyl-4-(6-(2-(2-methylpyrrolidin-1-yl)ethy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bioprojet-Biotech Curated by ChEMBL | Assay Description Displacement of [125I]Iodoproxyfan from human recombinant histamine H3 receptor by Competitive binding assay | Bioorg Med Chem Lett 21: 5378-83 (2011) Article DOI: 10.1016/j.bmcl.2011.07.006 BindingDB Entry DOI: 10.7270/Q2VX0GXK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50492783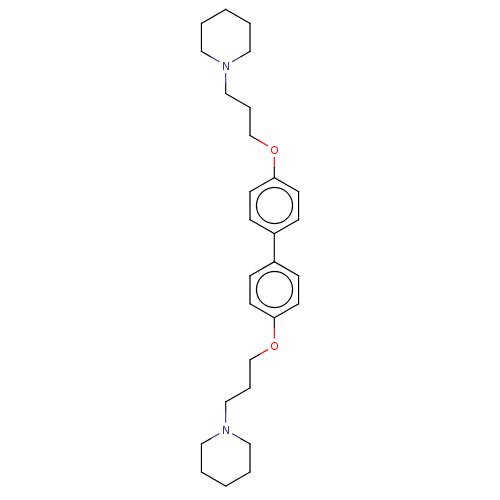 (CHEMBL2413824) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BIOPROJET-BIOTECH Curated by ChEMBL | Assay Description Displacement of [125I]iodoproxyfan from histamine H3 receptor (unknown origin) expressed in CHO cells | Bioorg Med Chem 21: 4526-9 (2013) Article DOI: 10.1016/j.bmc.2013.05.035 BindingDB Entry DOI: 10.7270/Q2J9699Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50319509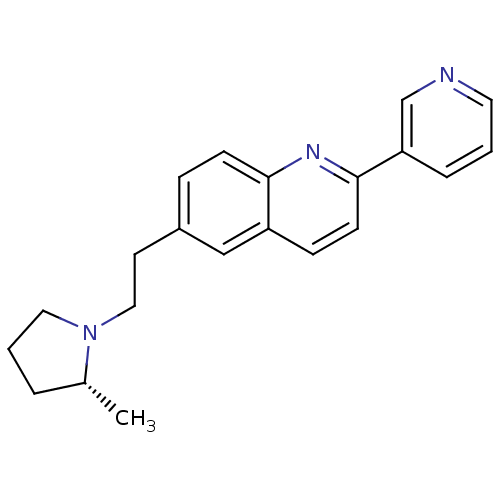 ((R)-6-(2-(2-methylpyrrolidin-1-yl)ethyl)-2-(pyridi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bioprojet-Biotech Curated by ChEMBL | Assay Description Displacement of [125I]Iodoproxyfan from human recombinant histamine H3 receptor by Competitive binding assay | Bioorg Med Chem Lett 21: 5378-83 (2011) Article DOI: 10.1016/j.bmcl.2011.07.006 BindingDB Entry DOI: 10.7270/Q2VX0GXK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1D (Homo sapiens (Human)) | BDBM50413550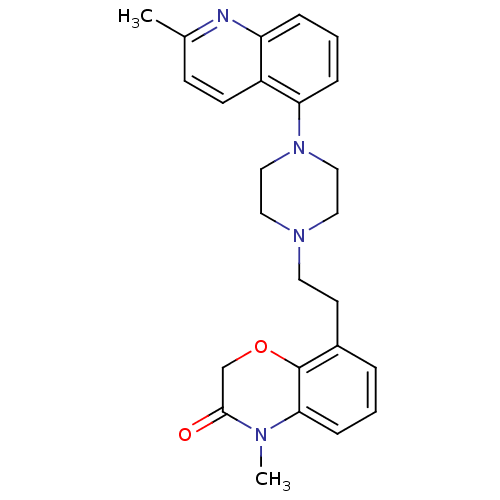 (CHEMBL469345) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity at human 5-HT1D receptor expressed in CHO cells assessed as inhibition of GTPgammaS binding by scintillation proximity assay | Bioorg Med Chem Lett 20: 7092-6 (2010) Article DOI: 10.1016/j.bmcl.2010.09.085 BindingDB Entry DOI: 10.7270/Q2MC919V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1D (Homo sapiens (Human)) | BDBM50417420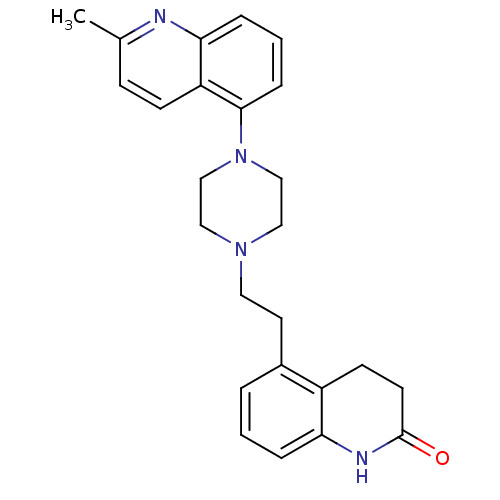 (CHEMBL1290486) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity at human 5-HT1D receptor expressed in CHO cells assessed as inhibition of GTPgammaS binding by scintillation proximity assay | Bioorg Med Chem Lett 20: 7092-6 (2010) Article DOI: 10.1016/j.bmcl.2010.09.085 BindingDB Entry DOI: 10.7270/Q2MC919V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50319538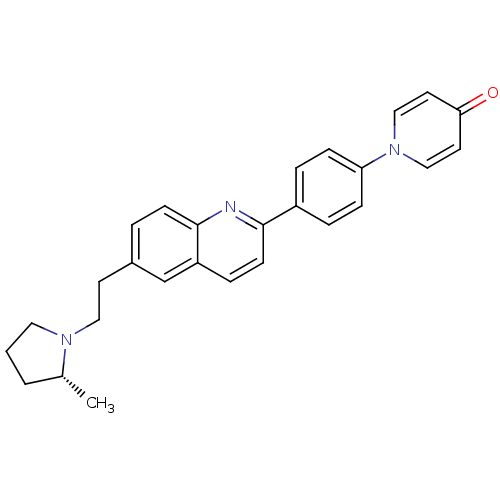 ((R)-1-(4-(6-(2-(2-methylpyrrolidin-1-yl)ethyl)quin...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bioprojet-Biotech Curated by ChEMBL | Assay Description Displacement of [125I]Iodoproxyfan from human recombinant histamine H3 receptor by Competitive binding assay | Bioorg Med Chem Lett 21: 5378-83 (2011) Article DOI: 10.1016/j.bmcl.2011.07.006 BindingDB Entry DOI: 10.7270/Q2VX0GXK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50319517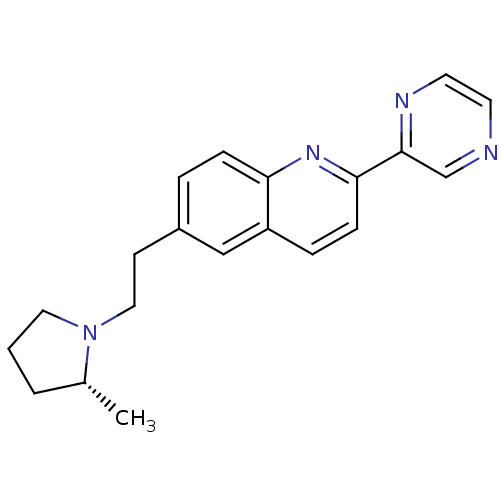 ((R)-6-(2-(2-methylpyrrolidin-1-yl)ethyl)-2-(pyrazi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bioprojet-Biotech Curated by ChEMBL | Assay Description Displacement of [125I]Iodoproxyfan from human recombinant histamine H3 receptor by Competitive binding assay | Bioorg Med Chem Lett 21: 5378-83 (2011) Article DOI: 10.1016/j.bmcl.2011.07.006 BindingDB Entry DOI: 10.7270/Q2VX0GXK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1D (Homo sapiens (Human)) | BDBM50417424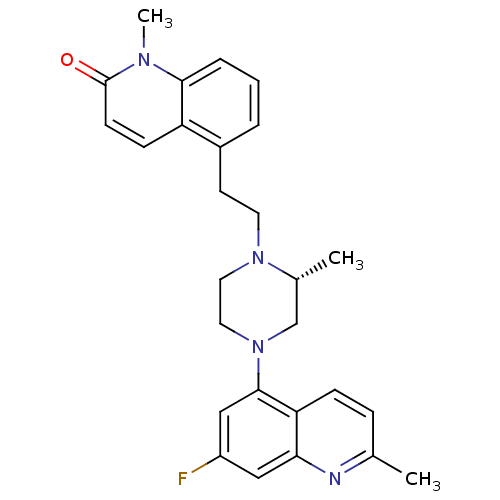 (CHEMBL1289394) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.126 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity at human 5-HT1D receptor expressed in CHO cells assessed as inhibition of GTPgammaS binding by scintillation proximity assay | Bioorg Med Chem Lett 20: 7092-6 (2010) Article DOI: 10.1016/j.bmcl.2010.09.085 BindingDB Entry DOI: 10.7270/Q2MC919V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1D (Homo sapiens (Human)) | BDBM50417411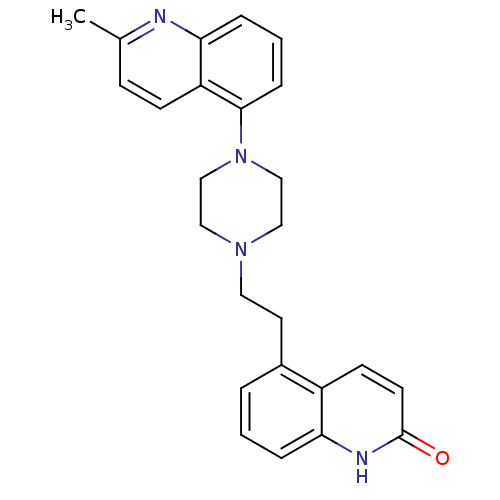 (CHEMBL1290715) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.126 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity at human 5-HT1D receptor expressed in CHO cells assessed as inhibition of GTPgammaS binding by scintillation proximity assay | Bioorg Med Chem Lett 20: 7092-6 (2010) Article DOI: 10.1016/j.bmcl.2010.09.085 BindingDB Entry DOI: 10.7270/Q2MC919V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50492772 (CHEMBL2413828) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BIOPROJET-BIOTECH Curated by ChEMBL | Assay Description Displacement of [125I]iodoproxyfan from histamine H3 receptor (unknown origin) expressed in CHO cells | Bioorg Med Chem 21: 4526-9 (2013) Article DOI: 10.1016/j.bmc.2013.05.035 BindingDB Entry DOI: 10.7270/Q2J9699Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (RAT) | BDBM50005463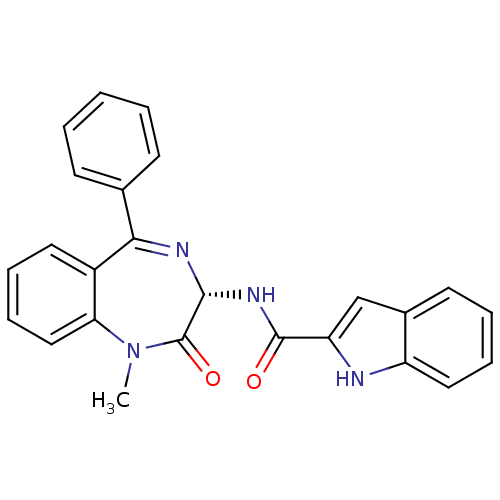 ((R)-1H-Indole-2-carboxylic acid (1-methyl-2-oxo-5-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.148 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre Curated by ChEMBL | Assay Description Inhibition by displacing [3H]CCK-8S against Cholecystokinin type A receptor of rat pancreatic membranes | J Med Chem 43: 3596-613 (2000) Article DOI: 10.1021/jm990967h BindingDB Entry DOI: 10.7270/Q26H4M5V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (RAT) | BDBM50449787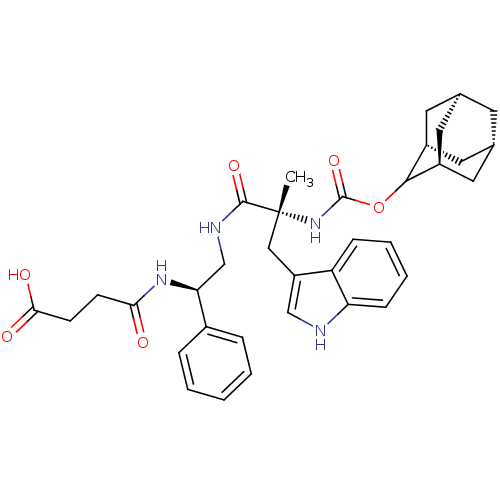 (CHEMBL2062154 | PD-134308) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.148 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre Curated by ChEMBL | Assay Description Inhibition by displacing [3H]CCK-8S against Cholecystokinin type A receptor of rat pancreatic membranes | J Med Chem 43: 3596-613 (2000) Article DOI: 10.1021/jm990967h BindingDB Entry DOI: 10.7270/Q26H4M5V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (RAT) | BDBM81962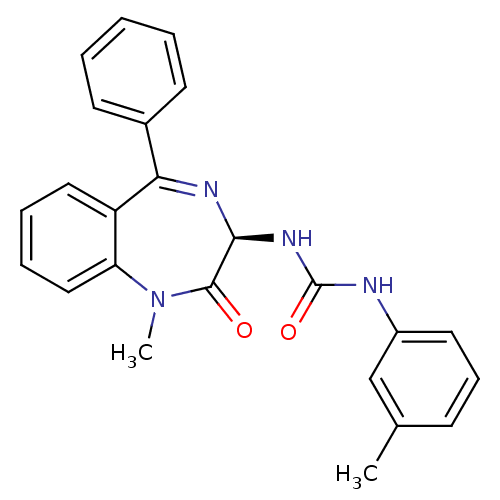 (S-L-365,260) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 0.148 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre Curated by ChEMBL | Assay Description Inhibition by displacing [3H]CCK-8S against Cholecystokinin type A receptor of rat pancreatic membranes | J Med Chem 43: 3596-613 (2000) Article DOI: 10.1021/jm990967h BindingDB Entry DOI: 10.7270/Q26H4M5V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50319512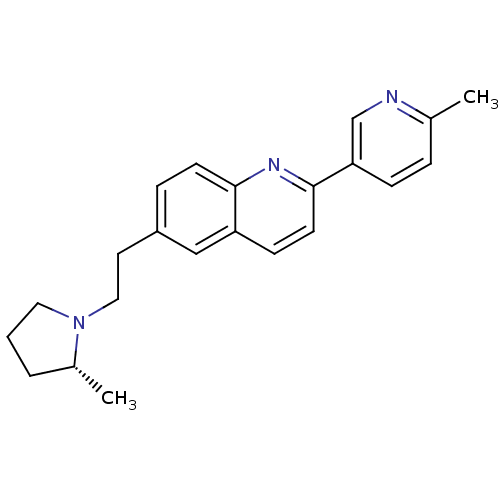 ((R)-2-(6-methylpyridin-3-yl)-6-(2-(2-methylpyrroli...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bioprojet-Biotech Curated by ChEMBL | Assay Description Displacement of [125I]Iodoproxyfan from human recombinant histamine H3 receptor by Competitive binding assay | Bioorg Med Chem Lett 21: 5378-83 (2011) Article DOI: 10.1016/j.bmcl.2011.07.006 BindingDB Entry DOI: 10.7270/Q2VX0GXK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50246380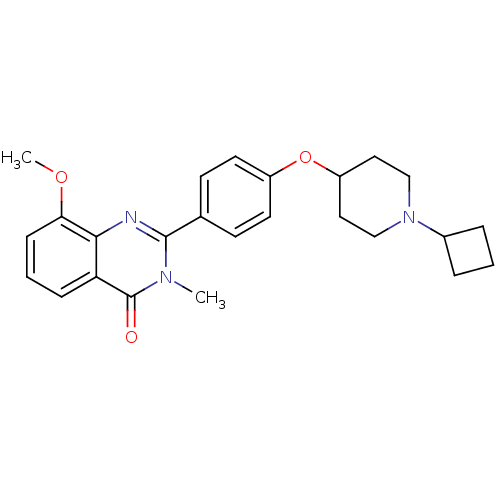 (2-(4-(1-cyclobutylpiperidin-4-yloxy)phenyl)-8-meth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.156 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bioprojet-Biotech Curated by ChEMBL | Assay Description Displacement of [125I]Iodoproxyfan from human recombinant histamine H3 receptor by Competitive binding assay | Bioorg Med Chem Lett 21: 5378-83 (2011) Article DOI: 10.1016/j.bmcl.2011.07.006 BindingDB Entry DOI: 10.7270/Q2VX0GXK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50246332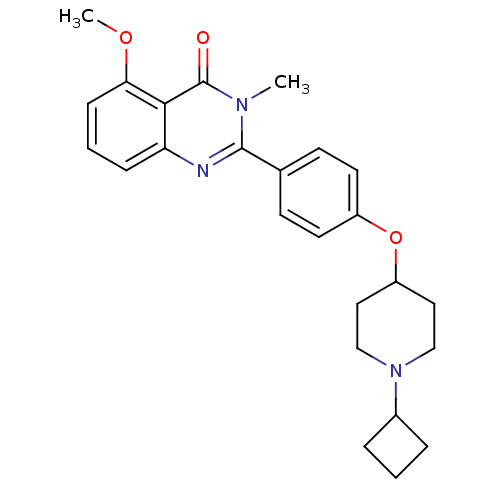 (2-(4-(1-cyclobutylpiperidin-4-yloxy)phenyl)-5-meth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.156 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bioprojet-Biotech Curated by ChEMBL | Assay Description Displacement of [125I]Iodoproxyfan from human recombinant histamine H3 receptor by Competitive binding assay | Bioorg Med Chem Lett 21: 5378-83 (2011) Article DOI: 10.1016/j.bmcl.2011.07.006 BindingDB Entry DOI: 10.7270/Q2VX0GXK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50246243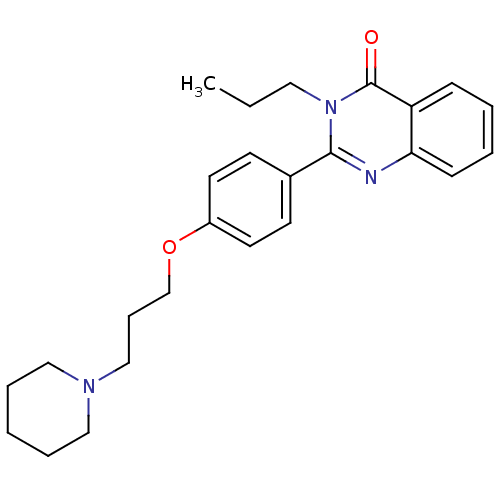 (2-(4-(3-(piperidin-1-yl)propoxy)phenyl)-3-propylqu...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.156 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bioprojet-Biotech Curated by ChEMBL | Assay Description Displacement of [125I]Iodoproxyfan from human recombinant histamine H3 receptor by Competitive binding assay | Bioorg Med Chem Lett 21: 5378-83 (2011) Article DOI: 10.1016/j.bmcl.2011.07.006 BindingDB Entry DOI: 10.7270/Q2VX0GXK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50413550 (CHEMBL469345) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.158 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity at human 5-HT1A receptor expressed in HEK293 cells assessed as inhibition of GTPgammaS binding by scintillation proximity assay | Bioorg Med Chem Lett 20: 7092-6 (2010) Article DOI: 10.1016/j.bmcl.2010.09.085 BindingDB Entry DOI: 10.7270/Q2MC919V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50352357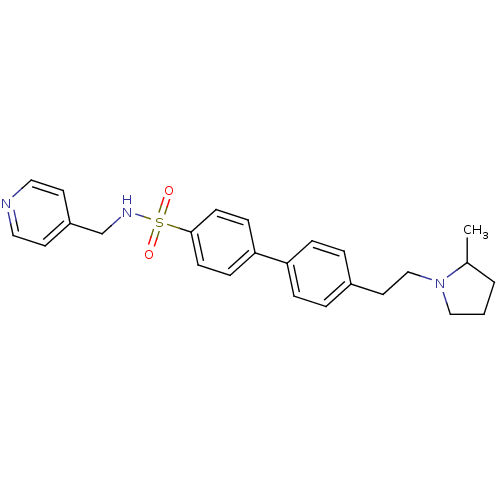 (CHEMBL558655) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.158 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bioprojet-Biotech Curated by ChEMBL | Assay Description Displacement of [125I]Iodoproxyfan from human recombinant histamine H3 receptor by Competitive binding assay | Bioorg Med Chem Lett 21: 5378-83 (2011) Article DOI: 10.1016/j.bmcl.2011.07.006 BindingDB Entry DOI: 10.7270/Q2VX0GXK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50319528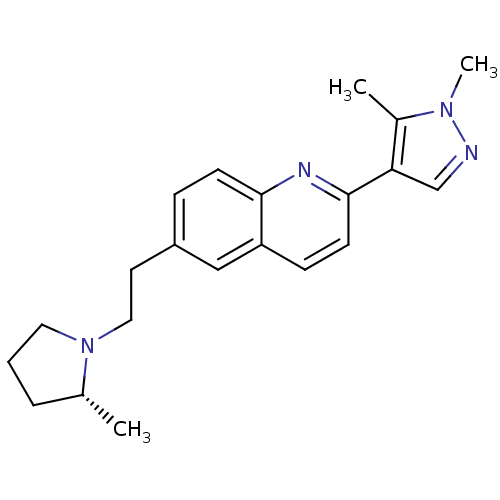 ((R)-2-(1,5-dimethyl-1H-pyrazol-4-yl)-6-(2-(2-methy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bioprojet-Biotech Curated by ChEMBL | Assay Description Displacement of [125I]Iodoproxyfan from human recombinant histamine H3 receptor by Competitive binding assay | Bioorg Med Chem Lett 21: 5378-83 (2011) Article DOI: 10.1016/j.bmcl.2011.07.006 BindingDB Entry DOI: 10.7270/Q2VX0GXK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50296178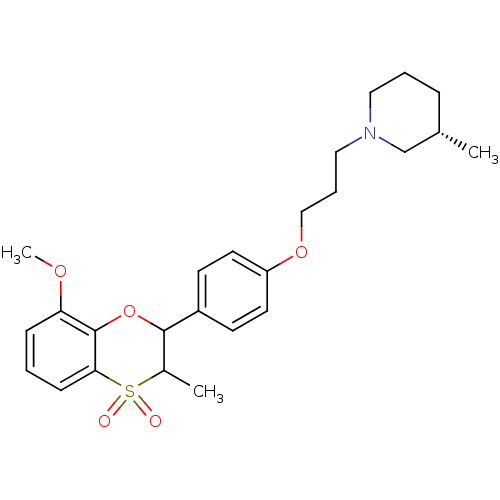 ((+/-)-(S)-1-{3-[4-(8-Methoxy-3-methyl-4,4-dioxo-3,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bioprojet-Biotech Curated by ChEMBL | Assay Description Displacement of [125I]Iodoproxyfan from human recombinant histamine H3 receptor by Competitive binding assay | Bioorg Med Chem Lett 21: 5378-83 (2011) Article DOI: 10.1016/j.bmcl.2011.07.006 BindingDB Entry DOI: 10.7270/Q2VX0GXK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50319547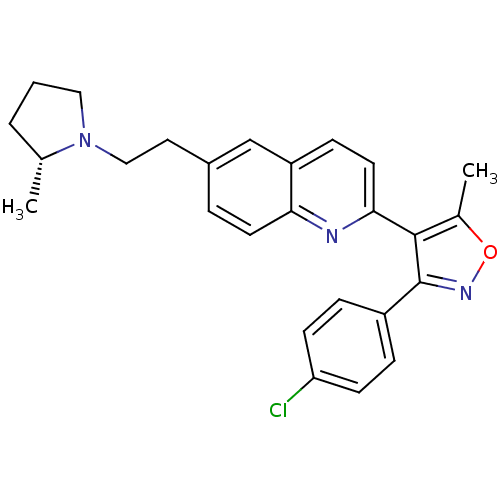 ((R)-3-(4-chlorophenyl)-5-methyl-4-(6-(2-(2-methylp...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bioprojet-Biotech Curated by ChEMBL | Assay Description Displacement of [125I]Iodoproxyfan from human recombinant histamine H3 receptor by Competitive binding assay | Bioorg Med Chem Lett 21: 5378-83 (2011) Article DOI: 10.1016/j.bmcl.2011.07.006 BindingDB Entry DOI: 10.7270/Q2VX0GXK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50296179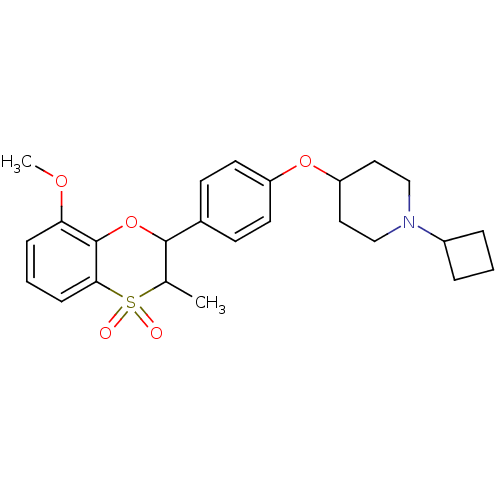 ((+/-)-1-Cyclobutyl-4-[4-(8-methoxy-3-methyl-4,4-di...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.161 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bioprojet-Biotech Curated by ChEMBL | Assay Description Displacement of [125I]Iodoproxyfan from human recombinant histamine H3 receptor by Competitive binding assay | Bioorg Med Chem Lett 21: 5378-83 (2011) Article DOI: 10.1016/j.bmcl.2011.07.006 BindingDB Entry DOI: 10.7270/Q2VX0GXK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50352086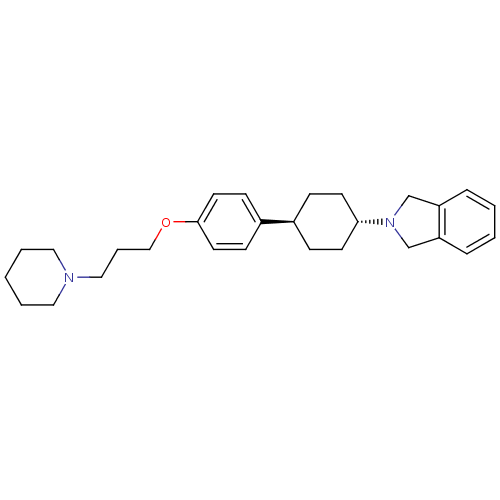 (CHEMBL1824245) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bioprojet-Biotech Curated by ChEMBL | Assay Description Displacement of [125I]Iodoproxyfan from recombinant human histamine H3 receptor expressed in HEK293 cells after 60 mins | Bioorg Med Chem Lett 21: 5384-8 (2011) Article DOI: 10.1016/j.bmcl.2011.06.102 BindingDB Entry DOI: 10.7270/Q2DV1K7H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50492769 (CHEMBL2413836) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BIOPROJET-BIOTECH Curated by ChEMBL | Assay Description Displacement of [125I]iodoproxyfan from histamine H3 receptor (unknown origin) expressed in CHO cells | Bioorg Med Chem 21: 4526-9 (2013) Article DOI: 10.1016/j.bmc.2013.05.035 BindingDB Entry DOI: 10.7270/Q2J9699Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (Homo sapiens (Human)) | BDBM50472889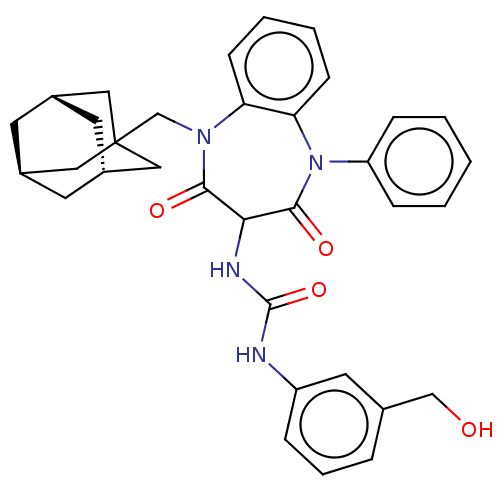 (CHEMBL415240) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.178 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre Curated by ChEMBL | Assay Description In vitro inhibition of binding of [3H]pCCK-8 against Cholecystokinin type B receptor of guinea pig cerebral cortex membranes | J Med Chem 43: 3596-613 (2000) Article DOI: 10.1021/jm990967h BindingDB Entry DOI: 10.7270/Q26H4M5V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50278449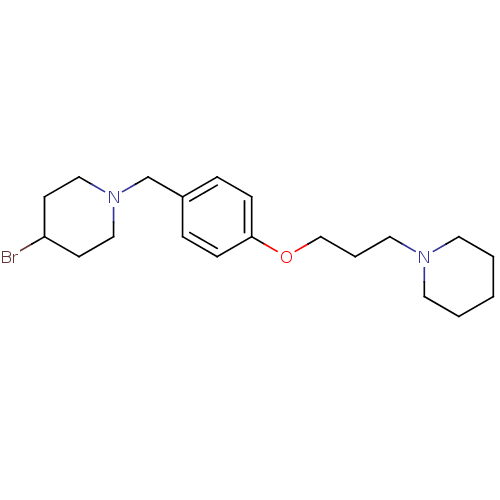 (4-bromo-1-(4-(3-(piperidin-1-yl)propoxy)benzyl)pip...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johann Wolfgang Goethe-Universität Curated by ChEMBL | Assay Description Displacement of [125I]iodoproxyfan from human histamine H3 receptor expressed in HEL293 cells | Bioorg Med Chem Lett 19: 2172-5 (2009) Article DOI: 10.1016/j.bmcl.2009.02.110 BindingDB Entry DOI: 10.7270/Q2H41RBM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM11626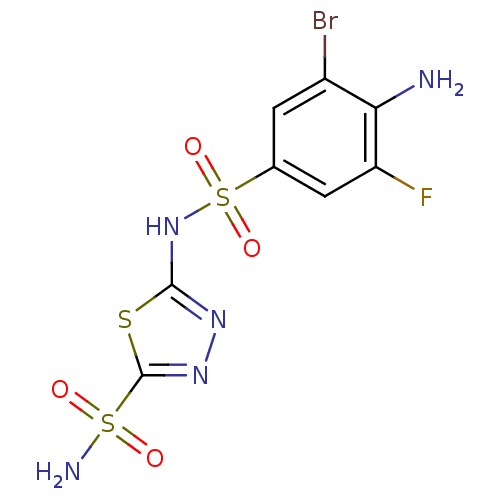 (β-CA inhibitor, 2 | 2-N-(4-amino-3-bromo-5-fl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Agricultural Sciences and Veterinary Medicine | Assay Description An Applied Photophysics stopped-flow instrument has been used for assaying the CA-catalyzed CO2 hydration activity. Phenol red has been used as indic... | J Med Chem 46: 2187-96 (2003) Article DOI: 10.1021/jm021123s BindingDB Entry DOI: 10.7270/Q2XK8CR9 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50319527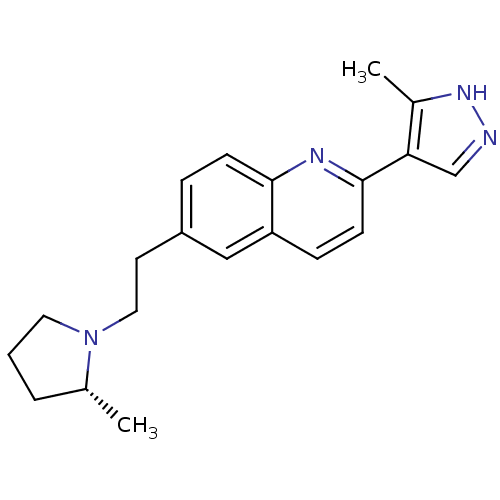 ((R)-2-(3-methyl-1H-pyrazol-4-yl)-6-(2-(2-methylpyr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bioprojet-Biotech Curated by ChEMBL | Assay Description Displacement of [125I]Iodoproxyfan from human recombinant histamine H3 receptor by Competitive binding assay | Bioorg Med Chem Lett 21: 5378-83 (2011) Article DOI: 10.1016/j.bmcl.2011.07.006 BindingDB Entry DOI: 10.7270/Q2VX0GXK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50319566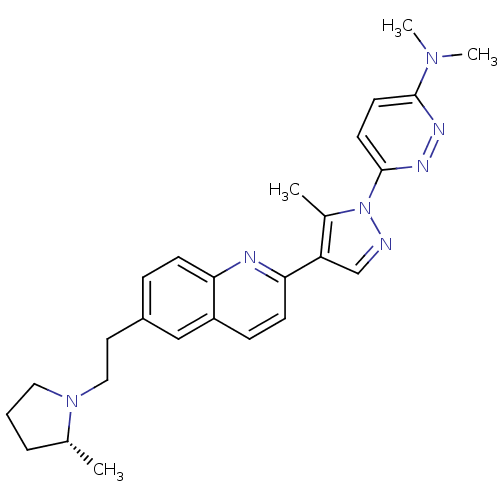 ((R)-N,N-dimethyl-6-(5-methyl-4-(6-(2-(2-methylpyrr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bioprojet-Biotech Curated by ChEMBL | Assay Description Displacement of [125I]Iodoproxyfan from human recombinant histamine H3 receptor by Competitive binding assay | Bioorg Med Chem Lett 21: 5378-83 (2011) Article DOI: 10.1016/j.bmcl.2011.07.006 BindingDB Entry DOI: 10.7270/Q2VX0GXK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50224191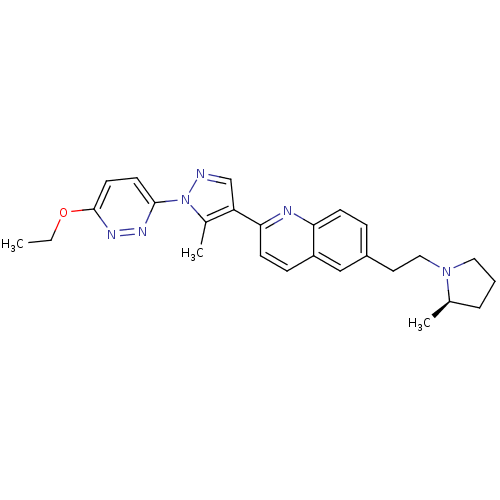 ((R)-2-(1-(6-ethoxypyridazin-3-yl)-5-methyl-1H-pyra...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bioprojet-Biotech Curated by ChEMBL | Assay Description Displacement of [125I]Iodoproxyfan from human recombinant histamine H3 receptor by Competitive binding assay | Bioorg Med Chem Lett 21: 5378-83 (2011) Article DOI: 10.1016/j.bmcl.2011.07.006 BindingDB Entry DOI: 10.7270/Q2VX0GXK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50319534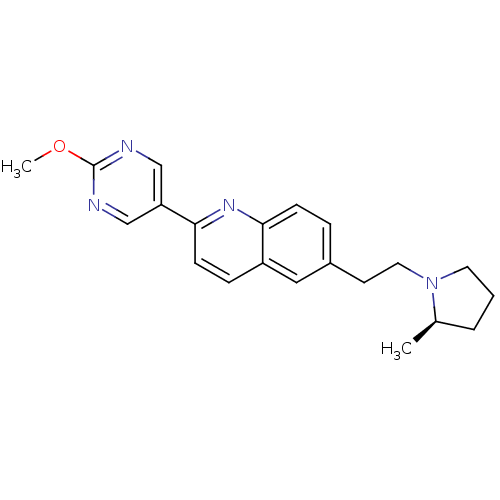 ((R)-2-(2-methoxypyrimidin-5-yl)-6-(2-(2-methylpyrr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bioprojet-Biotech Curated by ChEMBL | Assay Description Displacement of [125I]Iodoproxyfan from human recombinant histamine H3 receptor by Competitive binding assay | Bioorg Med Chem Lett 21: 5378-83 (2011) Article DOI: 10.1016/j.bmcl.2011.07.006 BindingDB Entry DOI: 10.7270/Q2VX0GXK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50319533 ((R)-6-(2-(2-methylpyrrolidin-1-yl)ethyl)-2-(pyrimi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bioprojet-Biotech Curated by ChEMBL | Assay Description Displacement of [125I]Iodoproxyfan from human recombinant histamine H3 receptor by Competitive binding assay | Bioorg Med Chem Lett 21: 5378-83 (2011) Article DOI: 10.1016/j.bmcl.2011.07.006 BindingDB Entry DOI: 10.7270/Q2VX0GXK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 4683 total ) | Next | Last >> |