 Found 104 hits with Last Name = 'carrasco' and Initial = 'm'
Found 104 hits with Last Name = 'carrasco' and Initial = 'm' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50303364
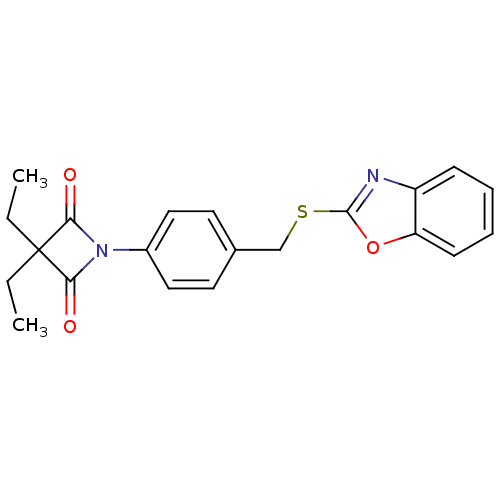
(1-(4-((Benzo[d]oxazol-2-ylthio)methyl)phenyl)-3,3-...)Show SMILES CCC1(CC)C(=O)N(C1=O)c1ccc(CSc2nc3ccccc3o2)cc1 Show InChI InChI=1S/C21H20N2O3S/c1-3-21(4-2)18(24)23(19(21)25)15-11-9-14(10-12-15)13-27-20-22-16-7-5-6-8-17(16)26-20/h5-12H,3-4,13H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Lisbon
Curated by ChEMBL
| Assay Description
Inhibition of human leukocyte elastase after 20 mins |
J Med Chem 53: 241-53 (2010)
Article DOI: 10.1021/jm901082k
BindingDB Entry DOI: 10.7270/Q23B607D |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50303371

(1-(6-((Benzo[d]thiazol-2-ylthio)methyl)pyridin-3-y...)Show SMILES CCC1(CC)C(=O)N(C1=O)c1ccc(CSc2nc3ccccc3s2)nc1 Show InChI InChI=1S/C20H19N3O2S2/c1-3-20(4-2)17(24)23(18(20)25)14-10-9-13(21-11-14)12-26-19-22-15-7-5-6-8-16(15)27-19/h5-11H,3-4,12H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Lisbon
Curated by ChEMBL
| Assay Description
Inhibition of human leukocyte elastase after 20 mins |
J Med Chem 53: 241-53 (2010)
Article DOI: 10.1021/jm901082k
BindingDB Entry DOI: 10.7270/Q23B607D |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50235615
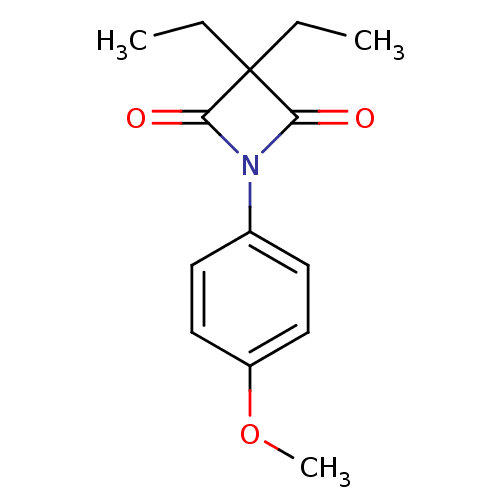
(3,3-diethyl-1-(4-methoxyphenyl)azetidine-2,4-dione...)Show InChI InChI=1S/C14H17NO3/c1-4-14(5-2)12(16)15(13(14)17)10-6-8-11(18-3)9-7-10/h6-9H,4-5H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.710 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Lisbon
Curated by ChEMBL
| Assay Description
Inhibition of human leukocyte elastase after 20 mins |
J Med Chem 53: 241-53 (2010)
Article DOI: 10.1021/jm901082k
BindingDB Entry DOI: 10.7270/Q23B607D |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50303369

(1-(4-((Benzo[d]thiazol-2-ylthio)methyl)phenyl)-3,3...)Show SMILES CCC1(CC)C(=O)N(C1=O)c1ccc(CSc2nc3ccccc3s2)cc1 Show InChI InChI=1S/C21H20N2O2S2/c1-3-21(4-2)18(24)23(19(21)25)15-11-9-14(10-12-15)13-26-20-22-16-7-5-6-8-17(16)27-20/h5-12H,3-4,13H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.820 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Lisbon
Curated by ChEMBL
| Assay Description
Inhibition of human leukocyte elastase after 20 mins |
J Med Chem 53: 241-53 (2010)
Article DOI: 10.1021/jm901082k
BindingDB Entry DOI: 10.7270/Q23B607D |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50303373
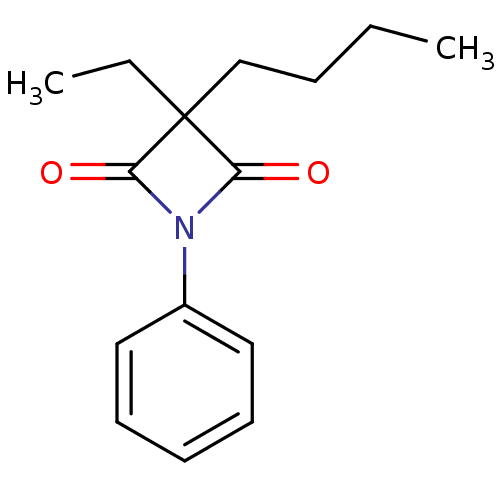
(3-butyl-3-ethyl-1-phenylazetidine-2,4-dione | CHEM...)Show InChI InChI=1S/C15H19NO2/c1-3-5-11-15(4-2)13(17)16(14(15)18)12-9-7-6-8-10-12/h6-10H,3-5,11H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.850 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Lisbon
Curated by ChEMBL
| Assay Description
Inhibition of human leukocyte elastase after 20 mins |
J Med Chem 53: 241-53 (2010)
Article DOI: 10.1021/jm901082k
BindingDB Entry DOI: 10.7270/Q23B607D |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50303363
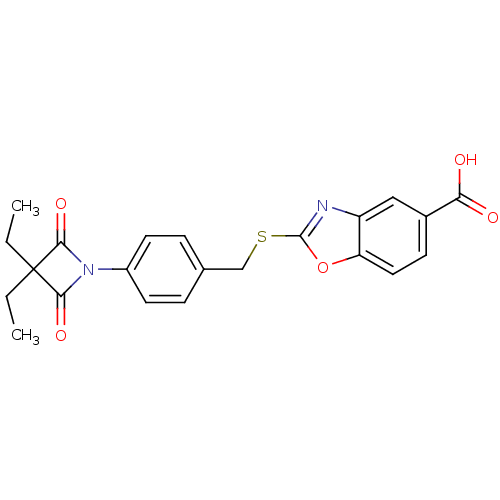
(2-(4-(3,3-Diethyl-2,4-dioxoazetidin-1-yl)benzylthi...)Show SMILES CCC1(CC)C(=O)N(C1=O)c1ccc(CSc2nc3cc(ccc3o2)C(O)=O)cc1 Show InChI InChI=1S/C22H20N2O5S/c1-3-22(4-2)19(27)24(20(22)28)15-8-5-13(6-9-15)12-30-21-23-16-11-14(18(25)26)7-10-17(16)29-21/h5-11H,3-4,12H2,1-2H3,(H,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Lisbon
Curated by ChEMBL
| Assay Description
Inhibition of human leukocyte elastase after 20 mins |
J Med Chem 53: 241-53 (2010)
Article DOI: 10.1021/jm901082k
BindingDB Entry DOI: 10.7270/Q23B607D |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50303370
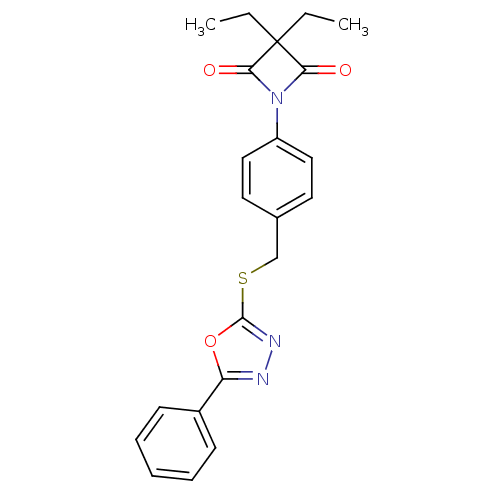
(1-(4-((5-Phenyl-1,3,4-oxadiazol-2-ylthio)methyl)ph...)Show SMILES CCC1(CC)C(=O)N(C1=O)c1ccc(CSc2nnc(o2)-c2ccccc2)cc1 Show InChI InChI=1S/C22H21N3O3S/c1-3-22(4-2)19(26)25(20(22)27)17-12-10-15(11-13-17)14-29-21-24-23-18(28-21)16-8-6-5-7-9-16/h5-13H,3-4,14H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Lisbon
Curated by ChEMBL
| Assay Description
Inhibition of human leukocyte elastase after 20 mins |
J Med Chem 53: 241-53 (2010)
Article DOI: 10.1021/jm901082k
BindingDB Entry DOI: 10.7270/Q23B607D |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50235613

(3,3-diethyl-1-phenylazetidine-2,4-dione | CHEMBL27...)Show InChI InChI=1S/C13H15NO2/c1-3-13(4-2)11(15)14(12(13)16)10-8-6-5-7-9-10/h5-9H,3-4H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Lisbon
Curated by ChEMBL
| Assay Description
Inhibition of human leukocyte elastase after 20 mins |
J Med Chem 53: 241-53 (2010)
Article DOI: 10.1021/jm901082k
BindingDB Entry DOI: 10.7270/Q23B607D |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50303357
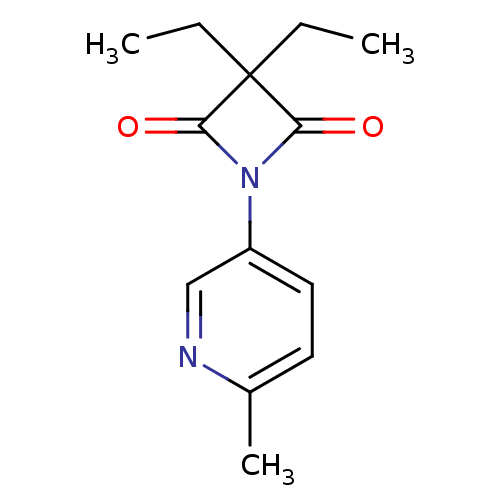
(3,3-Diethyl-1-(6-methylpyridin-3-yl)azetidine-2,4-...)Show InChI InChI=1S/C13H16N2O2/c1-4-13(5-2)11(16)15(12(13)17)10-7-6-9(3)14-8-10/h6-8H,4-5H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Lisbon
Curated by ChEMBL
| Assay Description
Inhibition of human leukocyte elastase after 20 mins |
J Med Chem 53: 241-53 (2010)
Article DOI: 10.1021/jm901082k
BindingDB Entry DOI: 10.7270/Q23B607D |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50303372
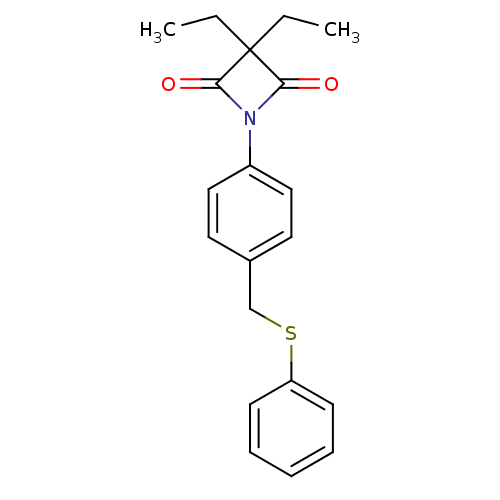
(3,3-Diethyl-1-(4-((phenylthio)methyl)phenyl)azetid...)Show InChI InChI=1S/C20H21NO2S/c1-3-20(4-2)18(22)21(19(20)23)16-12-10-15(11-13-16)14-24-17-8-6-5-7-9-17/h5-13H,3-4,14H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Lisbon
Curated by ChEMBL
| Assay Description
Inhibition of human leukocyte elastase after 20 mins |
J Med Chem 53: 241-53 (2010)
Article DOI: 10.1021/jm901082k
BindingDB Entry DOI: 10.7270/Q23B607D |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50303356
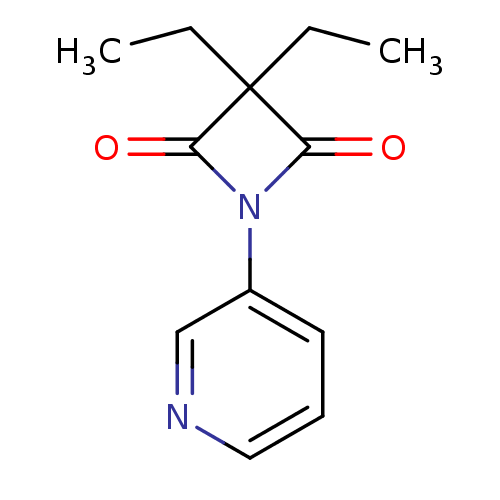
(3,3-Diethyl-1-(pyridin-3-yl)azetidine-2,4-dione | ...)Show InChI InChI=1S/C12H14N2O2/c1-3-12(4-2)10(15)14(11(12)16)9-6-5-7-13-8-9/h5-8H,3-4H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Lisbon
Curated by ChEMBL
| Assay Description
Inhibition of human leukocyte elastase after 20 mins |
J Med Chem 53: 241-53 (2010)
Article DOI: 10.1021/jm901082k
BindingDB Entry DOI: 10.7270/Q23B607D |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50303361
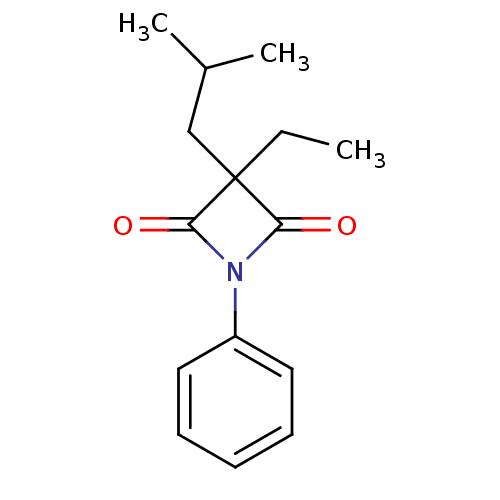
(3-Ethyl-3-isobutyl-1-phenylazetidine-2,4-dione | C...)Show InChI InChI=1S/C15H19NO2/c1-4-15(10-11(2)3)13(17)16(14(15)18)12-8-6-5-7-9-12/h5-9,11H,4,10H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Lisbon
Curated by ChEMBL
| Assay Description
Inhibition of human leukocyte elastase after 20 mins |
J Med Chem 53: 241-53 (2010)
Article DOI: 10.1021/jm901082k
BindingDB Entry DOI: 10.7270/Q23B607D |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50303365
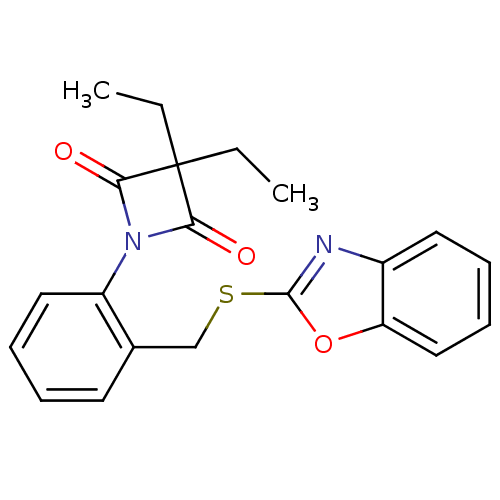
(1-(2-((Benzo[d]oxazol-2-ylthio)methyl)phenyl)-3,3-...)Show SMILES CCC1(CC)C(=O)N(C1=O)c1ccccc1CSc1nc2ccccc2o1 Show InChI InChI=1S/C21H20N2O3S/c1-3-21(4-2)18(24)23(19(21)25)16-11-7-5-9-14(16)13-27-20-22-15-10-6-8-12-17(15)26-20/h5-12H,3-4,13H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Lisbon
Curated by ChEMBL
| Assay Description
Inhibition of human leukocyte elastase after 20 mins |
J Med Chem 53: 241-53 (2010)
Article DOI: 10.1021/jm901082k
BindingDB Entry DOI: 10.7270/Q23B607D |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50235616
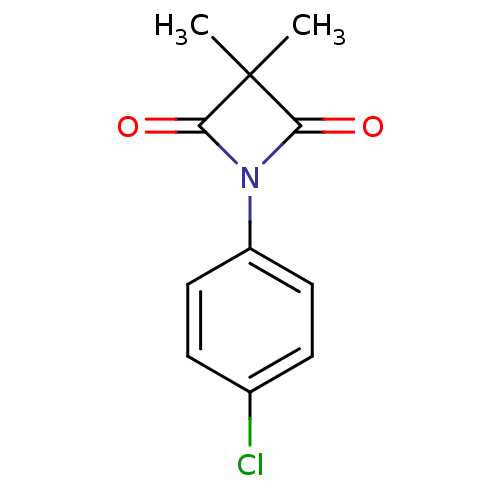
(1-(4-chlorophenyl)-3,3-dimethylazetidine-2,4-dione...)Show InChI InChI=1S/C11H10ClNO2/c1-11(2)9(14)13(10(11)15)8-5-3-7(12)4-6-8/h3-6H,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 23.8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Lisbon
Curated by ChEMBL
| Assay Description
Inhibition of human leukocyte elastase after 20 mins |
J Med Chem 53: 241-53 (2010)
Article DOI: 10.1021/jm901082k
BindingDB Entry DOI: 10.7270/Q23B607D |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50303358

(3,3-Diethyl-1-(naphthalen-1-yl)azetidine-2,4-dione...)Show InChI InChI=1S/C17H17NO2/c1-3-17(4-2)15(19)18(16(17)20)14-11-7-9-12-8-5-6-10-13(12)14/h5-11H,3-4H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 33.9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Lisbon
Curated by ChEMBL
| Assay Description
Inhibition of human leukocyte elastase after 20 mins |
J Med Chem 53: 241-53 (2010)
Article DOI: 10.1021/jm901082k
BindingDB Entry DOI: 10.7270/Q23B607D |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50235610
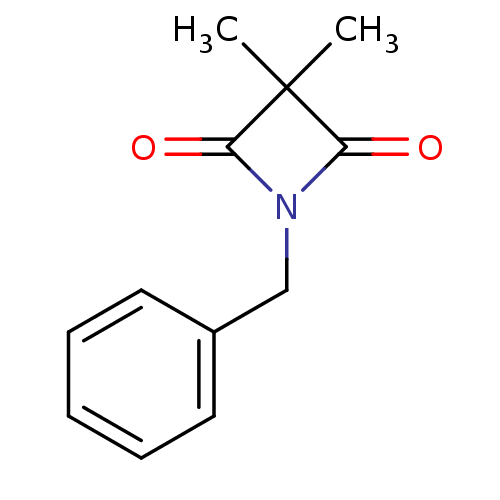
(1-benzyl-3,3-dimethylazetidine-2,4-dione | CHEMBL5...)Show InChI InChI=1S/C12H13NO2/c1-12(2)10(14)13(11(12)15)8-9-6-4-3-5-7-9/h3-7H,8H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 34.4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Lisbon
Curated by ChEMBL
| Assay Description
Inhibition of human leukocyte elastase after 20 mins |
J Med Chem 53: 241-53 (2010)
Article DOI: 10.1021/jm901082k
BindingDB Entry DOI: 10.7270/Q23B607D |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50235611
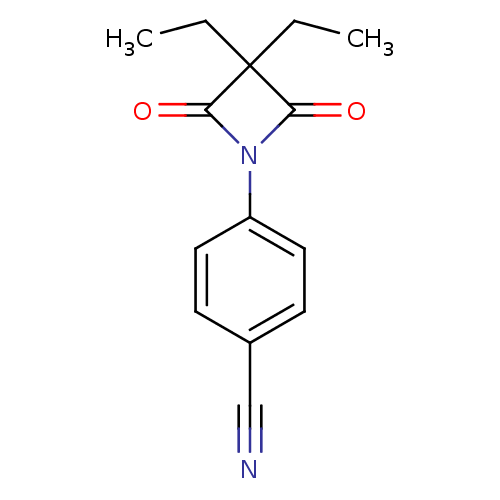
(4-(3,3-diethyl-2,4-dioxoazetidin-1-yl)benzonitrile...)Show InChI InChI=1S/C14H14N2O2/c1-3-14(4-2)12(17)16(13(14)18)11-7-5-10(9-15)6-8-11/h5-8H,3-4H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 63.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Lisbon
Curated by ChEMBL
| Assay Description
Inhibition of human leukocyte elastase after 20 mins |
J Med Chem 53: 241-53 (2010)
Article DOI: 10.1021/jm901082k
BindingDB Entry DOI: 10.7270/Q23B607D |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50303362
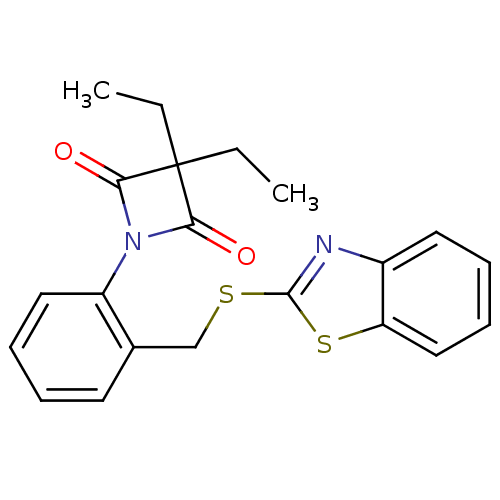
(1-(2-((Benzo[d]thiazol-2-ylthio)methyl)phenyl)-3,3...)Show SMILES CCC1(CC)C(=O)N(C1=O)c1ccccc1CSc1nc2ccccc2s1 Show InChI InChI=1S/C21H20N2O2S2/c1-3-21(4-2)18(24)23(19(21)25)16-11-7-5-9-14(16)13-26-20-22-15-10-6-8-12-17(15)27-20/h5-12H,3-4,13H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 99.3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Lisbon
Curated by ChEMBL
| Assay Description
Inhibition of human leukocyte elastase after 20 mins |
J Med Chem 53: 241-53 (2010)
Article DOI: 10.1021/jm901082k
BindingDB Entry DOI: 10.7270/Q23B607D |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50303359

(1-benzyl-3,3-diethylazetidine-2,4-dione | CHEMBL27...)Show InChI InChI=1S/C14H17NO2/c1-3-14(4-2)12(16)15(13(14)17)10-11-8-6-5-7-9-11/h5-9H,3-4,10H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 114 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Lisbon
Curated by ChEMBL
| Assay Description
Inhibition of human leukocyte elastase after 20 mins |
J Med Chem 53: 241-53 (2010)
Article DOI: 10.1021/jm901082k
BindingDB Entry DOI: 10.7270/Q23B607D |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50303374
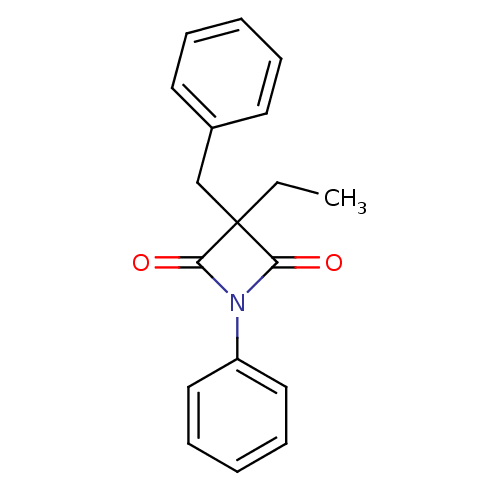
(3-Benzyl-3-ethyl-1-phenylazetidine-2,4-dione | CHE...)Show InChI InChI=1S/C18H17NO2/c1-2-18(13-14-9-5-3-6-10-14)16(20)19(17(18)21)15-11-7-4-8-12-15/h3-12H,2,13H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 127 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Lisbon
Curated by ChEMBL
| Assay Description
Inhibition of human leukocyte elastase after 20 mins |
J Med Chem 53: 241-53 (2010)
Article DOI: 10.1021/jm901082k
BindingDB Entry DOI: 10.7270/Q23B607D |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50303368
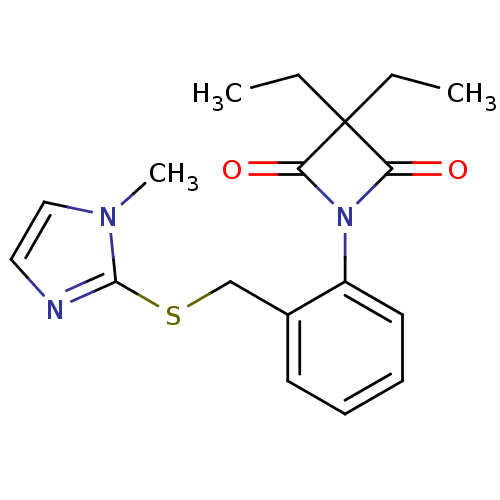
(1-(2-((1-Methyl-1H-imidazol-2-ylthio)methyl)phenyl...)Show InChI InChI=1S/C18H21N3O2S/c1-4-18(5-2)15(22)21(16(18)23)14-9-7-6-8-13(14)12-24-17-19-10-11-20(17)3/h6-11H,4-5,12H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 203 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Lisbon
Curated by ChEMBL
| Assay Description
Inhibition of human leukocyte elastase after 20 mins |
J Med Chem 53: 241-53 (2010)
Article DOI: 10.1021/jm901082k
BindingDB Entry DOI: 10.7270/Q23B607D |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50303360
(CHEMBL271820 | ethyl 2-(3,3-Diethyl-2,4-dioxoazeti...)Show InChI InChI=1S/C11H17NO4/c1-4-11(5-2)9(14)12(10(11)15)7-8(13)16-6-3/h4-7H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 219 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Lisbon
Curated by ChEMBL
| Assay Description
Inhibition of human leukocyte elastase after 20 mins |
J Med Chem 53: 241-53 (2010)
Article DOI: 10.1021/jm901082k
BindingDB Entry DOI: 10.7270/Q23B607D |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50303355
(3,3-Diethyl-1-o-tolylazetidine-2,4-dione | CHEMBL5...)Show InChI InChI=1S/C14H17NO2/c1-4-14(5-2)12(16)15(13(14)17)11-9-7-6-8-10(11)3/h6-9H,4-5H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 233 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Lisbon
Curated by ChEMBL
| Assay Description
Inhibition of human leukocyte elastase after 20 mins |
J Med Chem 53: 241-53 (2010)
Article DOI: 10.1021/jm901082k
BindingDB Entry DOI: 10.7270/Q23B607D |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50303366
(3-Benzyl-3-methyl-1-phenylazetidine-2,4-dione | CH...)Show InChI InChI=1S/C17H15NO2/c1-17(12-13-8-4-2-5-9-13)15(19)18(16(17)20)14-10-6-3-7-11-14/h2-11H,12H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.45E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Lisbon
Curated by ChEMBL
| Assay Description
Inhibition of human leukocyte elastase after 20 mins |
J Med Chem 53: 241-53 (2010)
Article DOI: 10.1021/jm901082k
BindingDB Entry DOI: 10.7270/Q23B607D |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase receptor Ret
(Homo sapiens (Human)) | BDBM50604441
(CHEMBL5178384)Show SMILES CC(C)n1nc(-c2ccc(NC(=O)Nc3cc(ccc3F)C(F)(F)F)cc2)c2c(N)ncnc12 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114226
BindingDB Entry DOI: 10.7270/Q2RJ4PJX |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase receptor Ret
(Homo sapiens (Human)) | BDBM50363397

(CHEMBL1946170 | REGORAFENIB | US10183928, Regorafe...)Show SMILES CNC(=O)c1cc(Oc2ccc(NC(=O)Nc3ccc(Cl)c(c3)C(F)(F)F)c(F)c2)ccn1 Show InChI InChI=1S/C21H15ClF4N4O3/c1-27-19(31)18-10-13(6-7-28-18)33-12-3-5-17(16(23)9-12)30-20(32)29-11-2-4-15(22)14(8-11)21(24,25)26/h2-10H,1H3,(H,27,31)(H2,29,30,32) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114226
BindingDB Entry DOI: 10.7270/Q2RJ4PJX |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase receptor Ret
(Homo sapiens (Human)) | BDBM50382959

(CEP-32496 | CHEMBL2029988 | US9730937, Example 261)Show SMILES COc1cc2ncnc(Oc3cccc(NC(=O)Nc4cc(on4)C(C)(C)C(F)(F)F)c3)c2cc1OC Show InChI InChI=1S/C24H22F3N5O5/c1-23(2,24(25,26)27)19-11-20(32-37-19)31-22(33)30-13-6-5-7-14(8-13)36-21-15-9-17(34-3)18(35-4)10-16(15)28-12-29-21/h5-12H,1-4H3,(H2,30,31,32,33) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114226
BindingDB Entry DOI: 10.7270/Q2RJ4PJX |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase receptor Ret
(Homo sapiens (Human)) | BDBM50604450
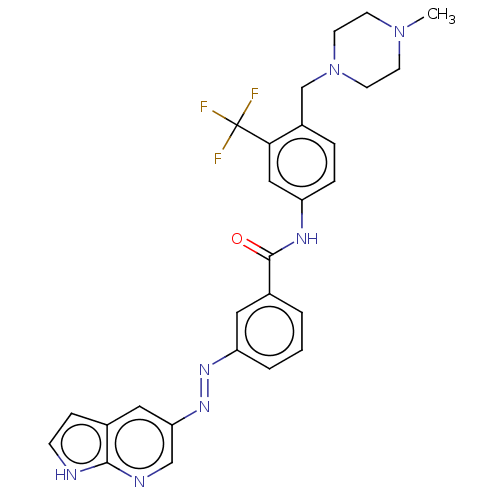
(CHEMBL5180040)Show SMILES CN1CCN(Cc2ccc(NC(=O)c3cccc(c3)\N=N\c3cnc4[nH]ccc4c3)cc2C(F)(F)F)CC1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114226
BindingDB Entry DOI: 10.7270/Q2RJ4PJX |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase receptor Ret
(Homo sapiens (Human)) | BDBM50021574
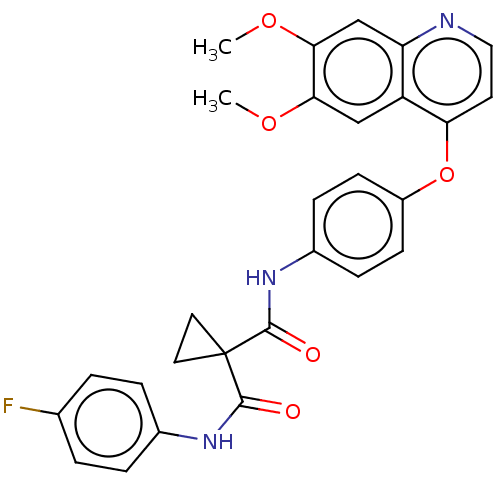
(BMS-907351 | CABOZANTINIB | CHEBI:72317 | Cabomety...)Show SMILES COc1cc2nccc(Oc3ccc(NC(=O)C4(CC4)C(=O)Nc4ccc(F)cc4)cc3)c2cc1OC Show InChI InChI=1S/C28H24FN3O5/c1-35-24-15-21-22(16-25(24)36-2)30-14-11-23(21)37-20-9-7-19(8-10-20)32-27(34)28(12-13-28)26(33)31-18-5-3-17(29)4-6-18/h3-11,14-16H,12-13H2,1-2H3,(H,31,33)(H,32,34) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114226
BindingDB Entry DOI: 10.7270/Q2RJ4PJX |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase receptor Ret
(Homo sapiens (Human)) | BDBM16673

(4-[4-({[4-chloro-3-(trifluoromethyl)phenyl]carbamo...)Show SMILES CNC(=O)c1cc(Oc2ccc(NC(=O)Nc3ccc(Cl)c(c3)C(F)(F)F)cc2)ccn1 Show InChI InChI=1S/C21H16ClF3N4O3/c1-26-19(30)18-11-15(8-9-27-18)32-14-5-2-12(3-6-14)28-20(31)29-13-4-7-17(22)16(10-13)21(23,24)25/h2-11H,1H3,(H,26,30)(H2,28,29,31) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 5.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114226
BindingDB Entry DOI: 10.7270/Q2RJ4PJX |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase receptor Ret
(Homo sapiens (Human)) | BDBM50322535

(3-(imidazo[1,2-b]pyridazin-3-ylethynyl)-4-methyl-N...)Show SMILES CN1CCN(Cc2ccc(NC(=O)c3ccc(C)c(c3)C#Cc3cnc4cccnn34)cc2C(F)(F)F)CC1 Show InChI InChI=1S/C29H27F3N6O/c1-20-5-6-22(16-21(20)8-10-25-18-33-27-4-3-11-34-38(25)27)28(39)35-24-9-7-23(26(17-24)29(30,31)32)19-37-14-12-36(2)13-15-37/h3-7,9,11,16-18H,12-15,19H2,1-2H3,(H,35,39) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114226
BindingDB Entry DOI: 10.7270/Q2RJ4PJX |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase receptor Ret
(Homo sapiens (Human)) | BDBM50604446
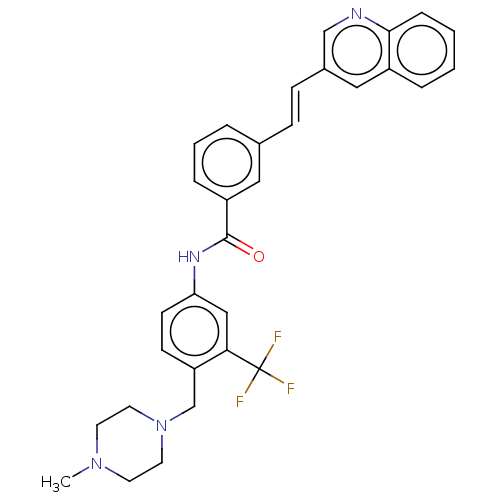
(CHEMBL5200675)Show SMILES CN1CCN(Cc2ccc(NC(=O)c3cccc(\C=C\c4cnc5ccccc5c4)c3)cc2C(F)(F)F)CC1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114226
BindingDB Entry DOI: 10.7270/Q2RJ4PJX |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase receptor Ret
(Homo sapiens (Human)) | BDBM50604444
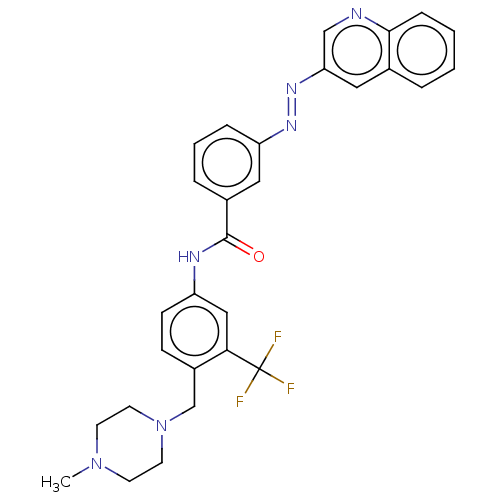
(CHEMBL5189744)Show SMILES CN1CCN(Cc2ccc(NC(=O)c3cccc(c3)\N=N\c3cnc4ccccc4c3)cc2C(F)(F)F)CC1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 9.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114226
BindingDB Entry DOI: 10.7270/Q2RJ4PJX |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase receptor Ret
(Homo sapiens (Human)) | BDBM50604443
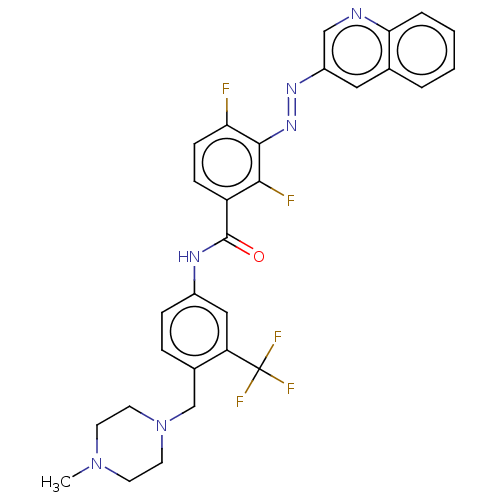
(CHEMBL5184072)Show SMILES CN1CCN(Cc2ccc(NC(=O)c3ccc(F)c(\N=N\c4cnc5ccccc5c4)c3F)cc2C(F)(F)F)CC1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114226
BindingDB Entry DOI: 10.7270/Q2RJ4PJX |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase receptor Ret
(Homo sapiens (Human)) | BDBM50604433
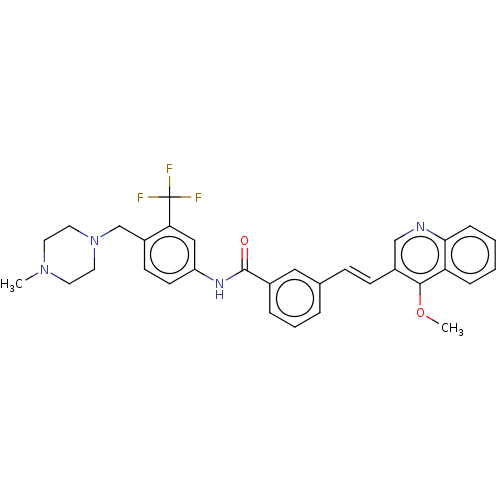
(CHEMBL5179230)Show SMILES COc1c(\C=C\c2cccc(c2)C(=O)Nc2ccc(CN3CCN(C)CC3)c(c2)C(F)(F)F)cnc2ccccc12 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114226
BindingDB Entry DOI: 10.7270/Q2RJ4PJX |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase receptor Ret
(Homo sapiens (Human)) | BDBM50322535

(3-(imidazo[1,2-b]pyridazin-3-ylethynyl)-4-methyl-N...)Show SMILES CN1CCN(Cc2ccc(NC(=O)c3ccc(C)c(c3)C#Cc3cnc4cccnn34)cc2C(F)(F)F)CC1 Show InChI InChI=1S/C29H27F3N6O/c1-20-5-6-22(16-21(20)8-10-25-18-33-27-4-3-11-34-38(25)27)28(39)35-24-9-7-23(26(17-24)29(30,31)32)19-37-14-12-36(2)13-15-37/h3-7,9,11,16-18H,12-15,19H2,1-2H3,(H,35,39) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114226
BindingDB Entry DOI: 10.7270/Q2RJ4PJX |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase receptor Ret
(Homo sapiens (Human)) | BDBM50604445
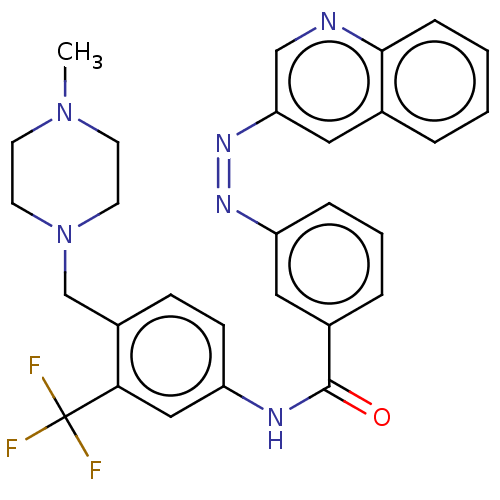
(CHEMBL5200720)Show SMILES CN1CCN(Cc2ccc(NC(=O)c3cccc(c3)\N=N/c3cnc4ccccc4c3)cc2C(F)(F)F)CC1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114226
BindingDB Entry DOI: 10.7270/Q2RJ4PJX |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase receptor Ret
(Homo sapiens (Human)) | BDBM50604449
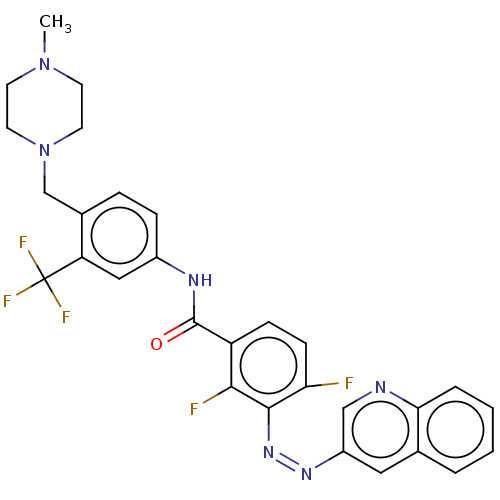
(CHEMBL5185302)Show SMILES CN1CCN(Cc2ccc(NC(=O)c3ccc(F)c(\N=N/c4cnc5ccccc5c4)c3F)cc2C(F)(F)F)CC1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114226
BindingDB Entry DOI: 10.7270/Q2RJ4PJX |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase receptor Ret
(Homo sapiens (Human)) | BDBM50604442
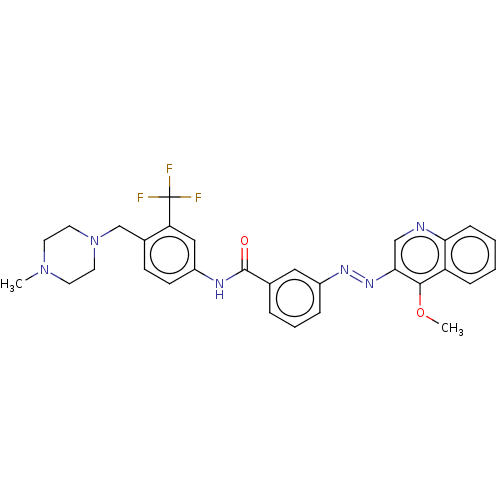
(CHEMBL5191715)Show SMILES COc1c(cnc2ccccc12)\N=N\c1cccc(c1)C(=O)Nc1ccc(CN2CCN(C)CC2)c(c1)C(F)(F)F | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114226
BindingDB Entry DOI: 10.7270/Q2RJ4PJX |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase receptor Ret
(Homo sapiens (Human)) | BDBM50604437
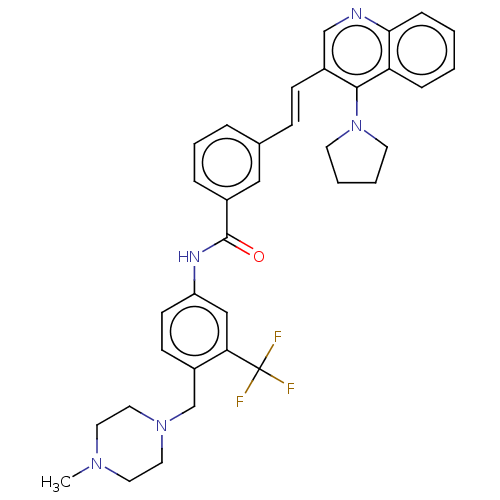
(CHEMBL5182347)Show SMILES CN1CCN(Cc2ccc(NC(=O)c3cccc(\C=C\c4cnc5ccccc5c4N4CCCC4)c3)cc2C(F)(F)F)CC1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 66 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114226
BindingDB Entry DOI: 10.7270/Q2RJ4PJX |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase receptor Ret
(Homo sapiens (Human)) | BDBM50604435
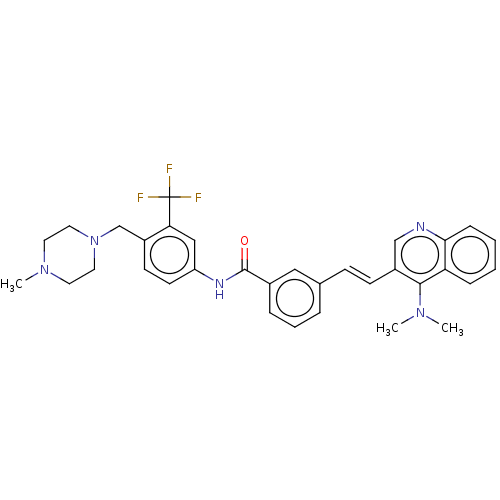
(CHEMBL5200195)Show SMILES CN(C)c1c(\C=C\c2cccc(c2)C(=O)Nc2ccc(CN3CCN(C)CC3)c(c2)C(F)(F)F)cnc2ccccc12 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 98 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114226
BindingDB Entry DOI: 10.7270/Q2RJ4PJX |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase receptor Ret
(Homo sapiens (Human)) | BDBM50604431
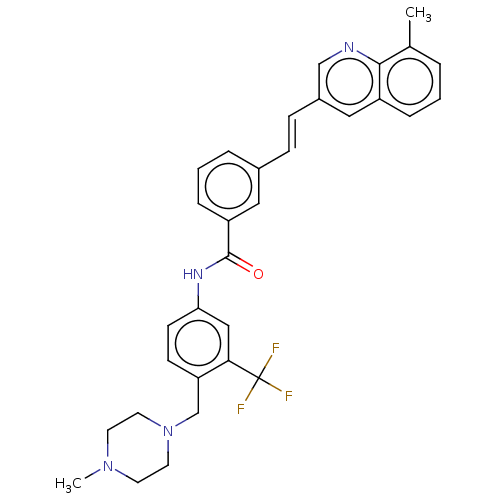
(CHEMBL5182833)Show SMILES CN1CCN(Cc2ccc(NC(=O)c3cccc(\C=C\c4cnc5c(C)cccc5c4)c3)cc2C(F)(F)F)CC1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 257 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114226
BindingDB Entry DOI: 10.7270/Q2RJ4PJX |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase receptor Ret
(Homo sapiens (Human)) | BDBM50604448
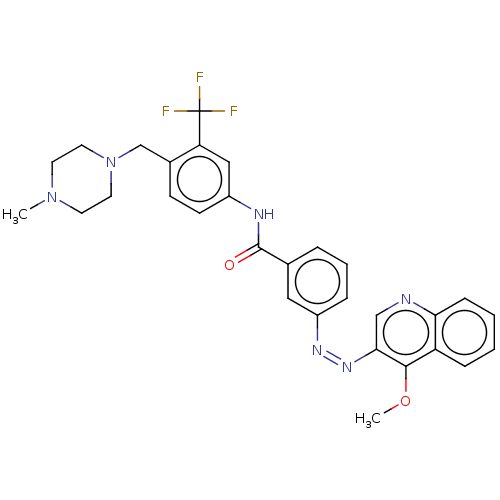
(CHEMBL5193068)Show SMILES COc1c(cnc2ccccc12)\N=N/c1cccc(c1)C(=O)Nc1ccc(CN2CCN(C)CC2)c(c1)C(F)(F)F | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 363 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114226
BindingDB Entry DOI: 10.7270/Q2RJ4PJX |
More data for this
Ligand-Target Pair | |
Choline kinase alpha
(Homo sapiens (Human)) | BDBM50342874
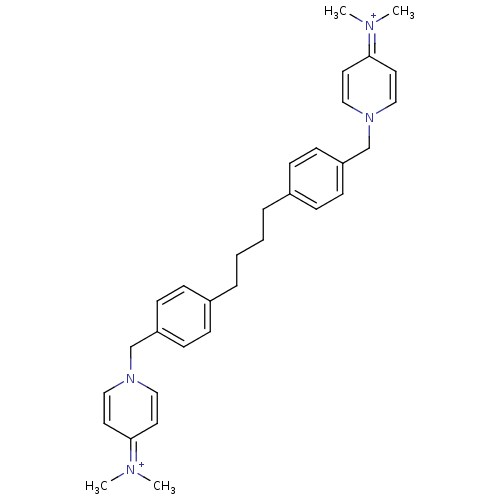
(1,1'-[4,4'-(Butane-1,4-diyl)bis(1,4-phenylene)]bis...)Show SMILES C[N+](C)=c1ccn(Cc2ccc(CCCCc3ccc(Cn4ccc(cc4)=[N+](C)C)cc3)cc2)cc1 |(16.16,-7.42,;16.14,-5.88,;17.47,-5.1,;14.79,-5.13,;13.47,-5.91,;12.13,-5.16,;12.12,-3.62,;10.78,-2.86,;9.45,-3.65,;9.46,-5.18,;8.14,-5.95,;6.81,-5.2,;5.48,-5.98,;4.15,-5.21,;2.82,-5.99,;1.48,-5.22,;.15,-6,;.15,-7.55,;-1.19,-8.32,;-2.52,-7.55,;-3.86,-8.32,;-5.19,-7.55,;-5.18,-6,;-6.52,-5.24,;-7.85,-6,;-7.84,-7.55,;-6.52,-8.32,;-9.18,-5.24,;-9.18,-3.7,;-10.52,-6,;-2.52,-6,;-1.19,-5.23,;6.79,-3.67,;8.1,-2.89,;13.43,-2.83,;14.77,-3.58,)| Show InChI InChI=1S/C32H40N4/c1-33(2)31-17-21-35(22-18-31)25-29-13-9-27(10-14-29)7-5-6-8-28-11-15-30(16-12-28)26-36-23-19-32(20-24-36)34(3)4/h9-24H,5-8,25-26H2,1-4H3/q+2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 780 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Granada
Curated by ChEMBL
| Assay Description
Inhibition of human choline kinase alpha1 using [methyl-14C]choline as substrate assessed as reduction in rate of incorporation of 14C from [methyl-1... |
Bioorg Med Chem Lett 28: 2485-2489 (2018)
Article DOI: 10.1016/j.bmcl.2018.05.060
BindingDB Entry DOI: 10.7270/Q20Z75RV |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase receptor Ret
(Homo sapiens (Human)) | BDBM50604447
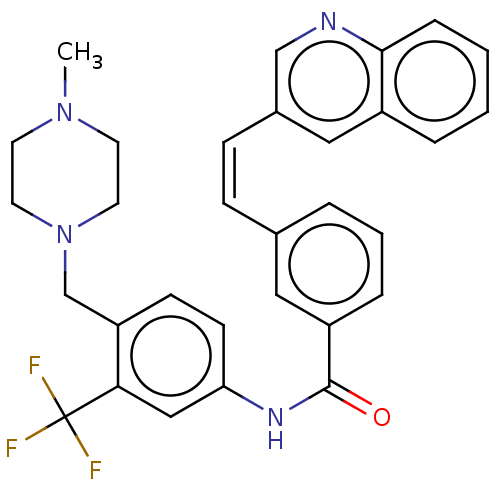
(CHEMBL5203860)Show SMILES CN1CCN(Cc2ccc(NC(=O)c3cccc(\C=C/c4cnc5ccccc5c4)c3)cc2C(F)(F)F)CC1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 900 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114226
BindingDB Entry DOI: 10.7270/Q2RJ4PJX |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase receptor Ret
(Homo sapiens (Human)) | BDBM50604442

(CHEMBL5191715)Show SMILES COc1c(cnc2ccccc12)\N=N\c1cccc(c1)C(=O)Nc1ccc(CN2CCN(C)CC2)c(c1)C(F)(F)F | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 920 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114226
BindingDB Entry DOI: 10.7270/Q2RJ4PJX |
More data for this
Ligand-Target Pair | |
Choline kinase alpha
(Homo sapiens (Human)) | BDBM50275672
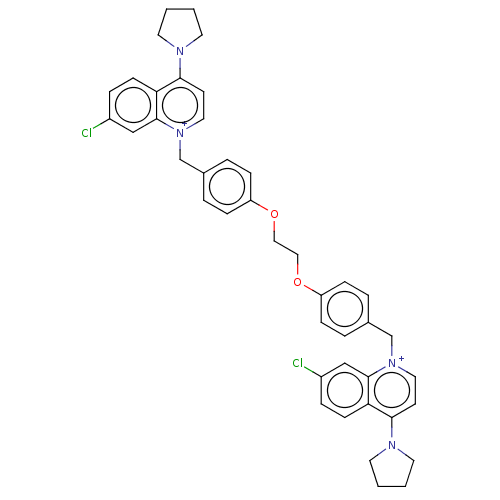
(CHEMBL4128522)Show SMILES [Br-].[Br-].Clc1ccc2c(cc[n+](Cc3ccc(OCCOc4ccc(C[n+]5ccc(N6CCCC6)c6ccc(Cl)cc56)cc4)cc3)c2c1)N1CCCC1 Show InChI InChI=1S/C42H42Cl2N4O2/c43-33-9-15-37-39(45-19-1-2-20-45)17-23-47(41(37)27-33)29-31-5-11-35(12-6-31)49-25-26-50-36-13-7-32(8-14-36)30-48-24-18-40(46-21-3-4-22-46)38-16-10-34(44)28-42(38)48/h5-18,23-24,27-28H,1-4,19-22,25-26,29-30H2/q+2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 920 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Granada
Curated by ChEMBL
| Assay Description
Inhibition of human choline kinase alpha1 using [methyl-14C]choline as substrate assessed as reduction in rate of incorporation of 14C from [methyl-1... |
Bioorg Med Chem Lett 28: 2485-2489 (2018)
Article DOI: 10.1016/j.bmcl.2018.05.060
BindingDB Entry DOI: 10.7270/Q20Z75RV |
More data for this
Ligand-Target Pair | |
Choline kinase alpha
(Homo sapiens (Human)) | BDBM50275707
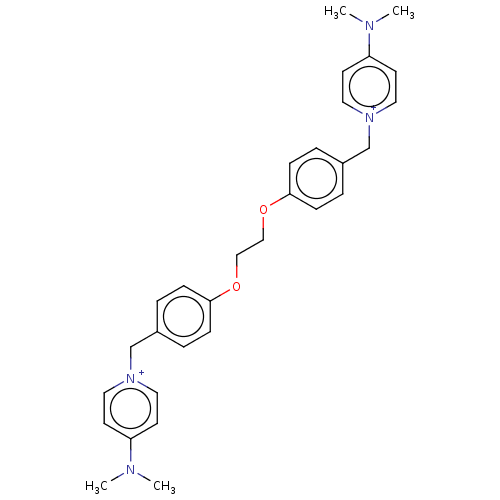
(CHEMBL4128899)Show SMILES [Br-].[Br-].CN(C)c1cc[n+](Cc2ccc(OCCOc3ccc(C[n+]4ccc(cc4)N(C)C)cc3)cc2)cc1 Show InChI InChI=1S/C30H36N4O2/c1-31(2)27-13-17-33(18-14-27)23-25-5-9-29(10-6-25)35-21-22-36-30-11-7-26(8-12-30)24-34-19-15-28(16-20-34)32(3)4/h5-20H,21-24H2,1-4H3/q+2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Granada
Curated by ChEMBL
| Assay Description
Inhibition of human choline kinase alpha1 using [methyl-14C]choline as substrate assessed as reduction in rate of incorporation of 14C from [methyl-1... |
Bioorg Med Chem Lett 28: 2485-2489 (2018)
Article DOI: 10.1016/j.bmcl.2018.05.060
BindingDB Entry DOI: 10.7270/Q20Z75RV |
More data for this
Ligand-Target Pair | |
Choline kinase alpha
(Homo sapiens (Human)) | BDBM50275705
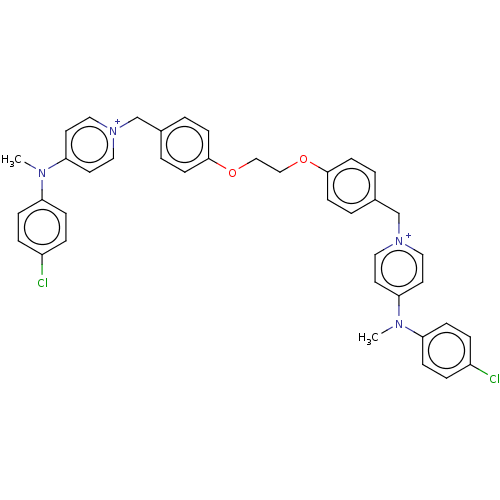
(CHEMBL4129845)Show SMILES [Br-].[Br-].CN(c1ccc(Cl)cc1)c1cc[n+](Cc2ccc(OCCOc3ccc(C[n+]4ccc(cc4)N(C)c4ccc(Cl)cc4)cc3)cc2)cc1 Show InChI InChI=1S/C40H38Cl2N4O2/c1-43(35-11-7-33(41)8-12-35)37-19-23-45(24-20-37)29-31-3-15-39(16-4-31)47-27-28-48-40-17-5-32(6-18-40)30-46-25-21-38(22-26-46)44(2)36-13-9-34(42)10-14-36/h3-26H,27-30H2,1-2H3/q+2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.63E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Granada
Curated by ChEMBL
| Assay Description
Inhibition of human choline kinase alpha1 using [methyl-14C]choline as substrate assessed as reduction in rate of incorporation of 14C from [methyl-1... |
Bioorg Med Chem Lett 28: 2485-2489 (2018)
Article DOI: 10.1016/j.bmcl.2018.05.060
BindingDB Entry DOI: 10.7270/Q20Z75RV |
More data for this
Ligand-Target Pair | |
Choline kinase alpha
(Homo sapiens (Human)) | BDBM50275678
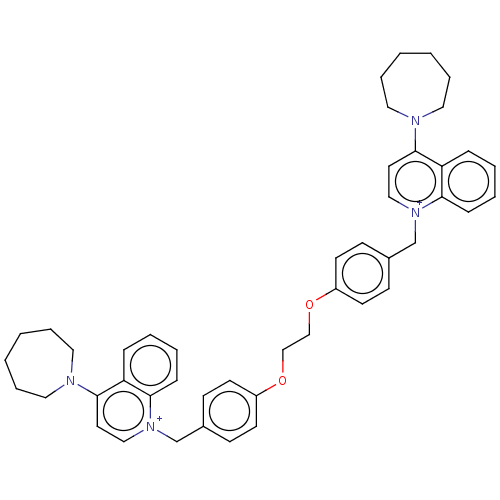
(CHEMBL4125997)Show SMILES [Br-].[Br-].C(COc1ccc(C[n+]2ccc(N3CCCCCC3)c3ccccc23)cc1)Oc1ccc(C[n+]2ccc(N3CCCCCC3)c3ccccc23)cc1 Show InChI InChI=1S/C46H52N4O2/c1-2-10-28-47(27-9-1)45-25-31-49(43-15-7-5-13-41(43)45)35-37-17-21-39(22-18-37)51-33-34-52-40-23-19-38(20-24-40)36-50-32-26-46(42-14-6-8-16-44(42)50)48-29-11-3-4-12-30-48/h5-8,13-26,31-32H,1-4,9-12,27-30,33-36H2/q+2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.66E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Granada
Curated by ChEMBL
| Assay Description
Inhibition of human choline kinase alpha1 using [methyl-14C]choline as substrate assessed as reduction in rate of incorporation of 14C from [methyl-1... |
Bioorg Med Chem Lett 28: 2485-2489 (2018)
Article DOI: 10.1016/j.bmcl.2018.05.060
BindingDB Entry DOI: 10.7270/Q20Z75RV |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data