 Found 391 hits with Last Name = 'cervi' and Initial = 'g'
Found 391 hits with Last Name = 'cervi' and Initial = 'g' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Cyclin-A2/Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM31541

(pyrazolo[4,3-h]quinazoline-3-carboxamide, 24)Show SMILES CN1CCN(CC1)c1cccc(Nc2ncc3CCc4c(nn(C)c4-c3n2)C(N)=O)c1 Show InChI InChI=1S/C22H26N8O/c1-28-8-10-30(11-9-28)16-5-3-4-15(12-16)25-22-24-13-14-6-7-17-19(21(23)31)27-29(2)20(17)18(14)26-22/h3-5,12-13H,6-11H2,1-2H3,(H2,23,31)(H,24,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.4 | 23 |
Nerviano Medical Sciences Srl
| Assay Description
The biochemical activity of compounds was determined by incubation with specific enzymes and substrates, followed by quantitation of the phosphorylat... |
J Med Chem 52: 5152-63 (2009)
Article DOI: 10.1021/jm9006559
BindingDB Entry DOI: 10.7270/Q2JH3JJP |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Serine/threonine-protein kinase pim-1
(Homo sapiens (Human)) | BDBM50021624
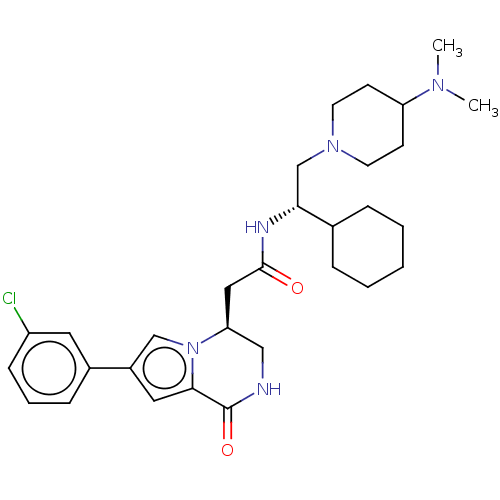
(CHEMBL3297766 | US9145418, 23)Show SMILES CN(C)C1CCN(C[C@@H](NC(=O)C[C@H]2CNC(=O)c3cc(cn23)-c2cccc(Cl)c2)C2CCCCC2)CC1 |r| Show InChI InChI=1S/C30H42ClN5O2/c1-34(2)25-11-13-35(14-12-25)20-27(21-7-4-3-5-8-21)33-29(37)17-26-18-32-30(38)28-16-23(19-36(26)28)22-9-6-10-24(31)15-22/h6,9-10,15-16,19,21,25-27H,3-5,7-8,11-14,17-18,20H2,1-2H3,(H,32,38)(H,33,37)/t26-,27+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences
Curated by ChEMBL
| Assay Description
Inhibition of human PIM1 using ARK-RERTYSFGHHA as substrate incubated for 60 mins prior to substrate addition by topcount scintillation counting anal... |
Bioorg Med Chem 21: 7364-80 (2013)
Article DOI: 10.1016/j.bmc.2013.09.054
BindingDB Entry DOI: 10.7270/Q23F4R7S |
More data for this
Ligand-Target Pair | |
Cyclin-A2/Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM31532
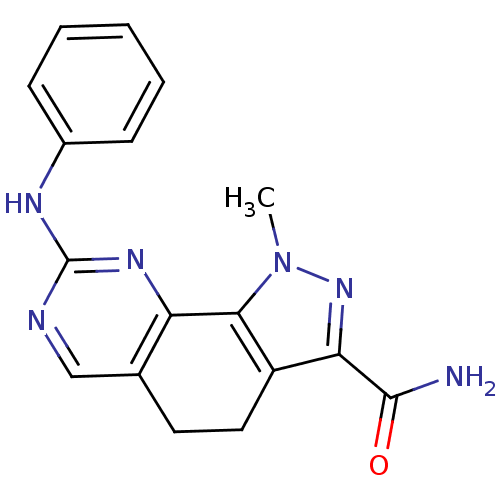
(pyrazolo[4,3-h]quinazoline-3-carboxamide, 1)Show InChI InChI=1S/C17H16N6O/c1-23-15-12(14(22-23)16(18)24)8-7-10-9-19-17(21-13(10)15)20-11-5-3-2-4-6-11/h2-6,9H,7-8H2,1H3,(H2,18,24)(H,19,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | 7.4 | 23 |
Nerviano Medical Sciences Srl
| Assay Description
The biochemical activity of compounds was determined by incubation with specific enzymes and substrates, followed by quantitation of the phosphorylat... |
J Med Chem 52: 5152-63 (2009)
Article DOI: 10.1021/jm9006559
BindingDB Entry DOI: 10.7270/Q2JH3JJP |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-1
(Homo sapiens (Human)) | BDBM50021620
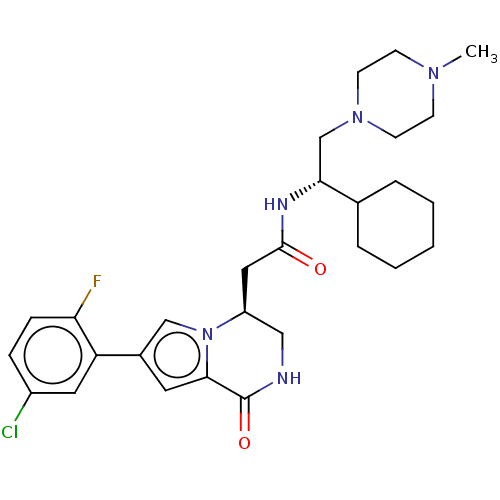
(CHEMBL3297764 | US9145418, 26)Show SMILES CN1CCN(C[C@@H](NC(=O)C[C@H]2CNC(=O)c3cc(cn23)-c2cc(Cl)ccc2F)C2CCCCC2)CC1 |r| Show InChI InChI=1S/C28H37ClFN5O2/c1-33-9-11-34(12-10-33)18-25(19-5-3-2-4-6-19)32-27(36)15-22-16-31-28(37)26-13-20(17-35(22)26)23-14-21(29)7-8-24(23)30/h7-8,13-14,17,19,22,25H,2-6,9-12,15-16,18H2,1H3,(H,31,37)(H,32,36)/t22-,25+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences
Curated by ChEMBL
| Assay Description
Inhibition of human PIM1 using ARK-RERTYSFGHHA as substrate incubated for 60 mins prior to substrate addition by topcount scintillation counting anal... |
Bioorg Med Chem 21: 7364-80 (2013)
Article DOI: 10.1016/j.bmc.2013.09.054
BindingDB Entry DOI: 10.7270/Q23F4R7S |
More data for this
Ligand-Target Pair | |
Cyclin-A2/Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM31539
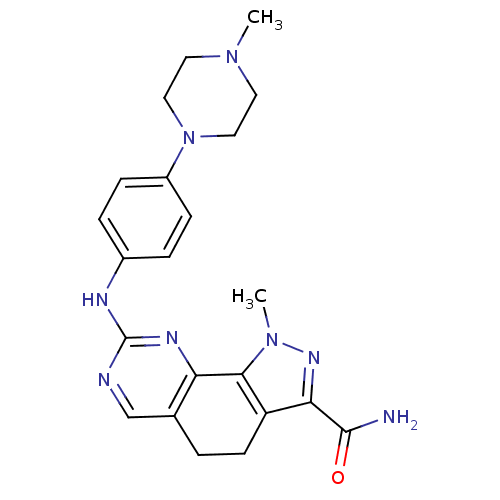
(pyrazolo[4,3-h]quinazoline-3-carboxamide, 22)Show SMILES CN1CCN(CC1)c1ccc(Nc2ncc3CCc4c(nn(C)c4-c3n2)C(N)=O)cc1 Show InChI InChI=1S/C22H26N8O/c1-28-9-11-30(12-10-28)16-6-4-15(5-7-16)25-22-24-13-14-3-8-17-19(21(23)31)27-29(2)20(17)18(14)26-22/h4-7,13H,3,8-12H2,1-2H3,(H2,23,31)(H,24,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | 7.4 | 23 |
Nerviano Medical Sciences Srl
| Assay Description
The biochemical activity of compounds was determined by incubation with specific enzymes and substrates, followed by quantitation of the phosphorylat... |
J Med Chem 52: 5152-63 (2009)
Article DOI: 10.1021/jm9006559
BindingDB Entry DOI: 10.7270/Q2JH3JJP |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Serine/threonine-protein kinase pim-1
(Homo sapiens (Human)) | BDBM50021622
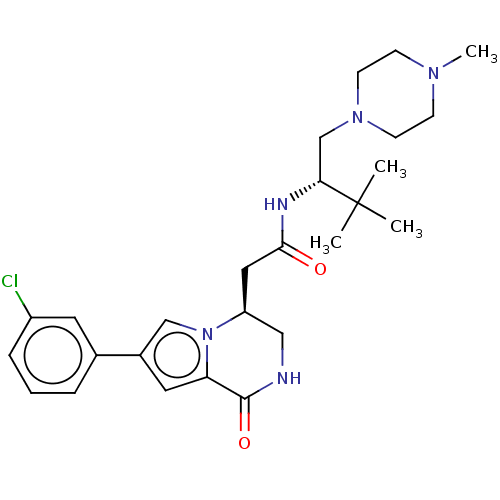
(CHEMBL3297767)Show SMILES CN1CCN(C[C@@H](NC(=O)C[C@H]2CNC(=O)c3cc(cn23)-c2cccc(Cl)c2)C(C)(C)C)CC1 |r| Show InChI InChI=1S/C26H36ClN5O2/c1-26(2,3)23(17-31-10-8-30(4)9-11-31)29-24(33)14-21-15-28-25(34)22-13-19(16-32(21)22)18-6-5-7-20(27)12-18/h5-7,12-13,16,21,23H,8-11,14-15,17H2,1-4H3,(H,28,34)(H,29,33)/t21-,23+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences
Curated by ChEMBL
| Assay Description
Inhibition of human PIM1 using ARK-RERTYSFGHHA as substrate incubated for 60 mins prior to substrate addition by topcount scintillation counting anal... |
Bioorg Med Chem 21: 7364-80 (2013)
Article DOI: 10.1016/j.bmc.2013.09.054
BindingDB Entry DOI: 10.7270/Q23F4R7S |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM50327930

(3-(4-Morpholin-4-yl-benzoylamino)-1H-thieno[3,2-c]...)Show SMILES C[C@@H](NC(=O)c1cc2n[nH]c(NC(=O)c3ccc(cc3)N3CCOCC3)c2s1)c1ccccc1 |r| Show InChI InChI=1S/C25H25N5O3S/c1-16(17-5-3-2-4-6-17)26-25(32)21-15-20-22(34-21)23(29-28-20)27-24(31)18-7-9-19(10-8-18)30-11-13-33-14-12-30/h2-10,15-16H,11-14H2,1H3,(H,26,32)(H2,27,28,29,31)/t16-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences-Oncology
Curated by ChEMBL
| Assay Description
Inhibition of AurA |
Bioorg Med Chem 18: 7113-20 (2010)
Article DOI: 10.1016/j.bmc.2010.07.048
BindingDB Entry DOI: 10.7270/Q2Q240GM |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM50327929
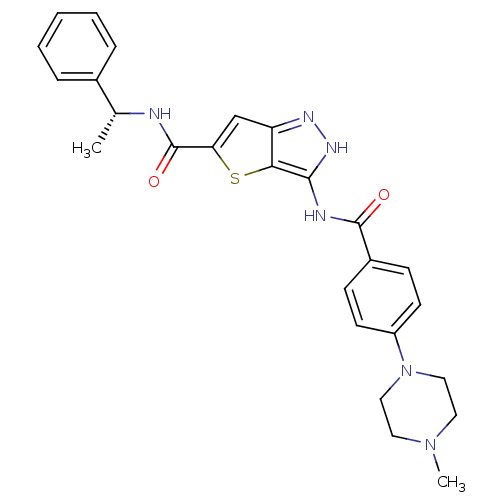
(3-[4-(4-Methyl-piperazin-1-yl)-benzoylamino]-1H-th...)Show SMILES C[C@@H](NC(=O)c1cc2n[nH]c(NC(=O)c3ccc(cc3)N3CCN(C)CC3)c2s1)c1ccccc1 |r| Show InChI InChI=1S/C26H28N6O2S/c1-17(18-6-4-3-5-7-18)27-26(34)22-16-21-23(35-22)24(30-29-21)28-25(33)19-8-10-20(11-9-19)32-14-12-31(2)13-15-32/h3-11,16-17H,12-15H2,1-2H3,(H,27,34)(H2,28,29,30,33)/t17-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences-Oncology
Curated by ChEMBL
| Assay Description
Inhibition of AurA |
Bioorg Med Chem 18: 7113-20 (2010)
Article DOI: 10.1016/j.bmc.2010.07.048
BindingDB Entry DOI: 10.7270/Q2Q240GM |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-1
(Homo sapiens (Human)) | BDBM50021617
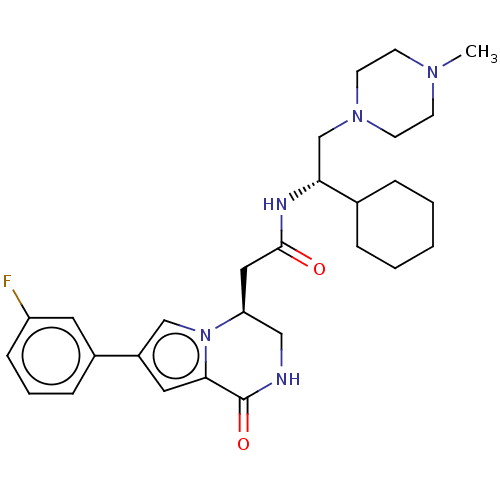
(CHEMBL3297761)Show SMILES CN1CCN(C[C@@H](NC(=O)C[C@H]2CNC(=O)c3cc(cn23)-c2cccc(F)c2)C2CCCCC2)CC1 |r| Show InChI InChI=1S/C28H38FN5O2/c1-32-10-12-33(13-11-32)19-25(20-6-3-2-4-7-20)31-27(35)16-24-17-30-28(36)26-15-22(18-34(24)26)21-8-5-9-23(29)14-21/h5,8-9,14-15,18,20,24-25H,2-4,6-7,10-13,16-17,19H2,1H3,(H,30,36)(H,31,35)/t24-,25+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences
Curated by ChEMBL
| Assay Description
Inhibition of human PIM1 using ARK-RERTYSFGHHA as substrate incubated for 60 mins prior to substrate addition by topcount scintillation counting anal... |
Bioorg Med Chem 21: 7364-80 (2013)
Article DOI: 10.1016/j.bmc.2013.09.054
BindingDB Entry DOI: 10.7270/Q23F4R7S |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM50327928
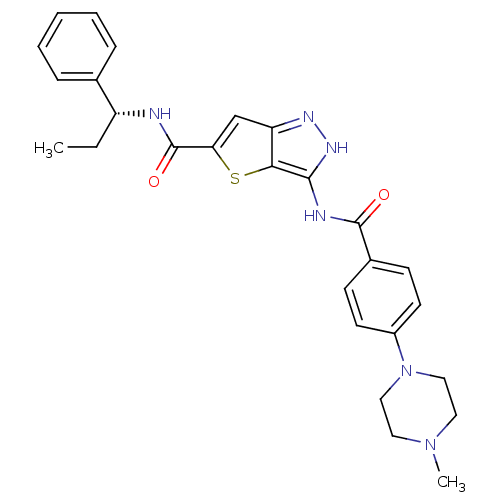
(3-({[4-(4-METHYLPIPERAZIN-1-YL)PHENYL]CARBONYL}AMI...)Show SMILES CC[C@@H](NC(=O)c1cc2n[nH]c(NC(=O)c3ccc(cc3)N3CCN(C)CC3)c2s1)c1ccccc1 |r| Show InChI InChI=1S/C27H30N6O2S/c1-3-21(18-7-5-4-6-8-18)28-27(35)23-17-22-24(36-23)25(31-30-22)29-26(34)19-9-11-20(12-10-19)33-15-13-32(2)14-16-33/h4-12,17,21H,3,13-16H2,1-2H3,(H,28,35)(H2,29,30,31,34)/t21-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences-Oncology
Curated by ChEMBL
| Assay Description
Inhibition of AurA |
Bioorg Med Chem 18: 7113-20 (2010)
Article DOI: 10.1016/j.bmc.2010.07.048
BindingDB Entry DOI: 10.7270/Q2Q240GM |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-1
(Homo sapiens (Human)) | BDBM50021649
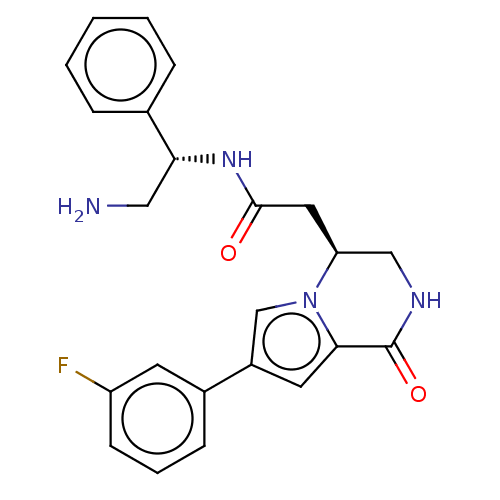
(CHEMBL3298891)Show SMILES NC[C@@H](NC(=O)C[C@H]1CNC(=O)c2cc(cn12)-c1cccc(F)c1)c1ccccc1 |r| Show InChI InChI=1S/C23H23FN4O2/c24-18-8-4-7-16(9-18)17-10-21-23(30)26-13-19(28(21)14-17)11-22(29)27-20(12-25)15-5-2-1-3-6-15/h1-10,14,19-20H,11-13,25H2,(H,26,30)(H,27,29)/t19-,20+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences
Curated by ChEMBL
| Assay Description
Inhibition of human PIM1 using ARK-RERTYSFGHHA as substrate incubated for 60 mins prior to substrate addition by topcount scintillation counting anal... |
Bioorg Med Chem 21: 7364-80 (2013)
Article DOI: 10.1016/j.bmc.2013.09.054
BindingDB Entry DOI: 10.7270/Q23F4R7S |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM50327923
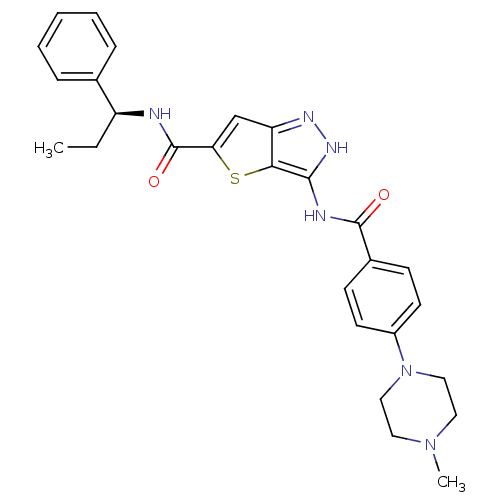
(3-[4-(4-Methyl-piperazin-1-yl)-benzoylamino]-1H-th...)Show SMILES CC[C@H](NC(=O)c1cc2n[nH]c(NC(=O)c3ccc(cc3)N3CCN(C)CC3)c2s1)c1ccccc1 |r| Show InChI InChI=1S/C27H30N6O2S/c1-3-21(18-7-5-4-6-8-18)28-27(35)23-17-22-24(36-23)25(31-30-22)29-26(34)19-9-11-20(12-10-19)33-15-13-32(2)14-16-33/h4-12,17,21H,3,13-16H2,1-2H3,(H,28,35)(H2,29,30,31,34)/t21-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences-Oncology
Curated by ChEMBL
| Assay Description
Inhibition of AurA |
Bioorg Med Chem 18: 7113-20 (2010)
Article DOI: 10.1016/j.bmc.2010.07.048
BindingDB Entry DOI: 10.7270/Q2Q240GM |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM50327927
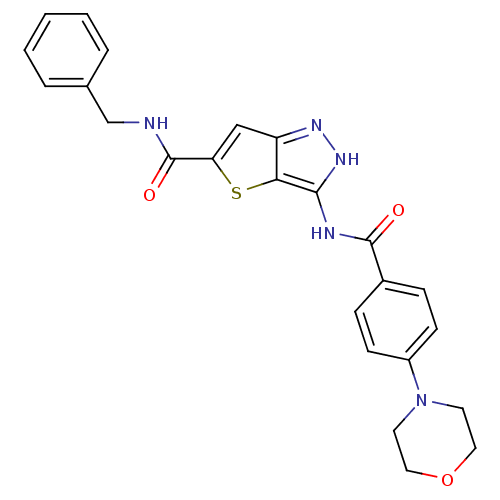
(3-(4-Morpholin-4-yl-benzoylamino)-1H-thieno[3,2-c]...)Show SMILES O=C(NCc1ccccc1)c1cc2n[nH]c(NC(=O)c3ccc(cc3)N3CCOCC3)c2s1 Show InChI InChI=1S/C24H23N5O3S/c30-23(17-6-8-18(9-7-17)29-10-12-32-13-11-29)26-22-21-19(27-28-22)14-20(33-21)24(31)25-15-16-4-2-1-3-5-16/h1-9,14H,10-13,15H2,(H,25,31)(H2,26,27,28,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences-Oncology
Curated by ChEMBL
| Assay Description
Inhibition of AurA |
Bioorg Med Chem 18: 7113-20 (2010)
Article DOI: 10.1016/j.bmc.2010.07.048
BindingDB Entry DOI: 10.7270/Q2Q240GM |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-1
(Homo sapiens (Human)) | BDBM50021653
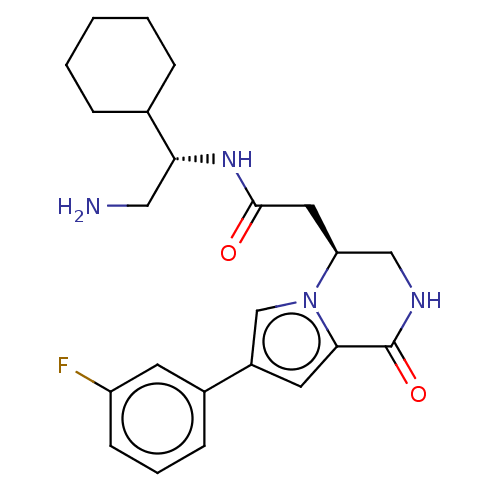
(CHEMBL3297759)Show SMILES NC[C@@H](NC(=O)C[C@H]1CNC(=O)c2cc(cn12)-c1cccc(F)c1)C1CCCCC1 |r| Show InChI InChI=1S/C23H29FN4O2/c24-18-8-4-7-16(9-18)17-10-21-23(30)26-13-19(28(21)14-17)11-22(29)27-20(12-25)15-5-2-1-3-6-15/h4,7-10,14-15,19-20H,1-3,5-6,11-13,25H2,(H,26,30)(H,27,29)/t19-,20+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences
Curated by ChEMBL
| Assay Description
Inhibition of human PIM1 using ARK-RERTYSFGHHA as substrate incubated for 60 mins prior to substrate addition by topcount scintillation counting anal... |
Bioorg Med Chem 21: 7364-80 (2013)
Article DOI: 10.1016/j.bmc.2013.09.054
BindingDB Entry DOI: 10.7270/Q23F4R7S |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-3
(Homo sapiens (Human)) | BDBM50021618
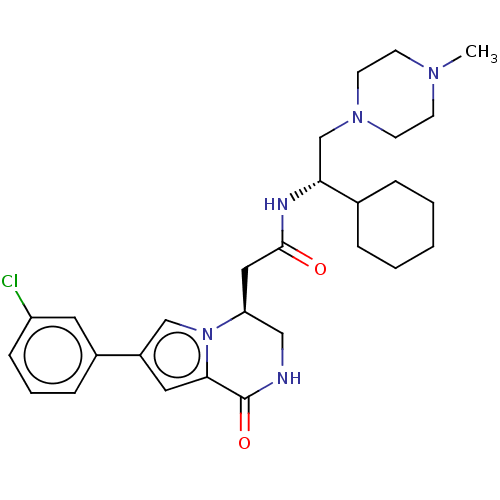
(CHEMBL3297762 | US9145418, 2)Show SMILES CN1CCN(C[C@@H](NC(=O)C[C@H]2CNC(=O)c3cc(cn23)-c2cccc(Cl)c2)C2CCCCC2)CC1 |r| Show InChI InChI=1S/C28H38ClN5O2/c1-32-10-12-33(13-11-32)19-25(20-6-3-2-4-7-20)31-27(35)16-24-17-30-28(36)26-15-22(18-34(24)26)21-8-5-9-23(29)14-21/h5,8-9,14-15,18,20,24-25H,2-4,6-7,10-13,16-17,19H2,1H3,(H,30,36)(H,31,35)/t24-,25+/m0/s1 | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences
Curated by ChEMBL
| Assay Description
Inhibition of human PIM3 using ARK-RERTYSFGHHA as substrate incubated for 60 mins prior to substrate addition by topcount scintillation counting anal... |
Bioorg Med Chem 21: 7364-80 (2013)
Article DOI: 10.1016/j.bmc.2013.09.054
BindingDB Entry DOI: 10.7270/Q23F4R7S |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM50327915
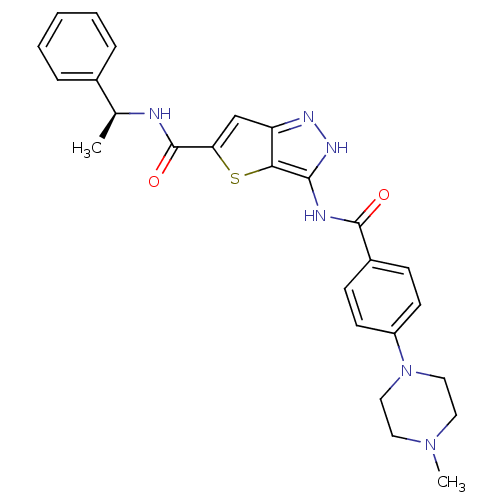
(3-[4-(4-Methyl-piperazin-1-yl)-benzoylamino]-1H-th...)Show SMILES C[C@H](NC(=O)c1cc2n[nH]c(NC(=O)c3ccc(cc3)N3CCN(C)CC3)c2s1)c1ccccc1 |r| Show InChI InChI=1S/C26H28N6O2S/c1-17(18-6-4-3-5-7-18)27-26(34)22-16-21-23(35-22)24(30-29-21)28-25(33)19-8-10-20(11-9-19)32-14-12-31(2)13-15-32/h3-11,16-17H,12-15H2,1-2H3,(H,27,34)(H2,28,29,30,33)/t17-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences-Oncology
Curated by ChEMBL
| Assay Description
Inhibition of AurA |
Bioorg Med Chem 18: 7113-20 (2010)
Article DOI: 10.1016/j.bmc.2010.07.048
BindingDB Entry DOI: 10.7270/Q2Q240GM |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-1
(Homo sapiens (Human)) | BDBM50021647
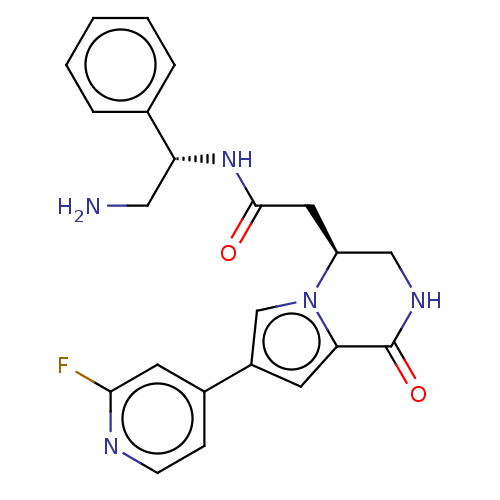
(CHEMBL3298889)Show SMILES NC[C@@H](NC(=O)C[C@H]1CNC(=O)c2cc(cn12)-c1ccnc(F)c1)c1ccccc1 |r| Show InChI InChI=1S/C22H22FN5O2/c23-20-9-15(6-7-25-20)16-8-19-22(30)26-12-17(28(19)13-16)10-21(29)27-18(11-24)14-4-2-1-3-5-14/h1-9,13,17-18H,10-12,24H2,(H,26,30)(H,27,29)/t17-,18+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences
Curated by ChEMBL
| Assay Description
Inhibition of human PIM1 using ARK-RERTYSFGHHA as substrate incubated for 60 mins prior to substrate addition by topcount scintillation counting anal... |
Bioorg Med Chem 21: 7364-80 (2013)
Article DOI: 10.1016/j.bmc.2013.09.054
BindingDB Entry DOI: 10.7270/Q23F4R7S |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-1
(Homo sapiens (Human)) | BDBM50021621
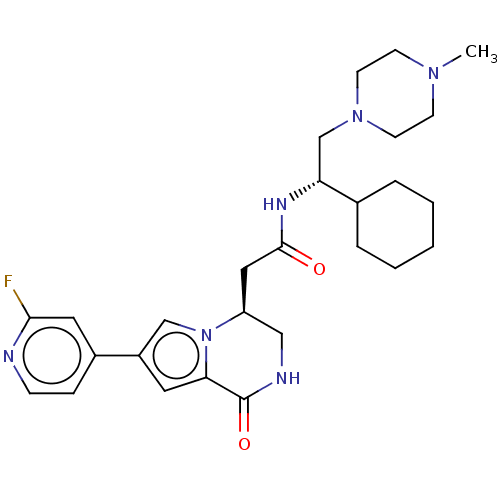
(CHEMBL3297765)Show SMILES CN1CCN(C[C@@H](NC(=O)C[C@H]2CNC(=O)c3cc(cn23)-c2ccnc(F)c2)C2CCCCC2)CC1 |r| Show InChI InChI=1S/C27H37FN6O2/c1-32-9-11-33(12-10-32)18-23(19-5-3-2-4-6-19)31-26(35)15-22-16-30-27(36)24-13-21(17-34(22)24)20-7-8-29-25(28)14-20/h7-8,13-14,17,19,22-23H,2-6,9-12,15-16,18H2,1H3,(H,30,36)(H,31,35)/t22-,23+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences
Curated by ChEMBL
| Assay Description
Inhibition of human PIM1 using ARK-RERTYSFGHHA as substrate incubated for 60 mins prior to substrate addition by topcount scintillation counting anal... |
Bioorg Med Chem 21: 7364-80 (2013)
Article DOI: 10.1016/j.bmc.2013.09.054
BindingDB Entry DOI: 10.7270/Q23F4R7S |
More data for this
Ligand-Target Pair | |
Cyclin-A2/Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM31542

(pyrazolo[4,3-h]quinazoline-3-carboxamide, 25)Show SMILES Cn1nc(C(N)=O)c2CCc3cnc(Nc4ccc(cc4)N4CCOCC4)nc3-c12 Show InChI InChI=1S/C21H23N7O2/c1-27-19-16(18(26-27)20(22)29)7-2-13-12-23-21(25-17(13)19)24-14-3-5-15(6-4-14)28-8-10-30-11-9-28/h3-6,12H,2,7-11H2,1H3,(H2,22,29)(H,23,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | 7.4 | 23 |
Nerviano Medical Sciences Srl
| Assay Description
The biochemical activity of compounds was determined by incubation with specific enzymes and substrates, followed by quantitation of the phosphorylat... |
J Med Chem 52: 5152-63 (2009)
Article DOI: 10.1021/jm9006559
BindingDB Entry DOI: 10.7270/Q2JH3JJP |
More data for this
Ligand-Target Pair | |
Fibroblast growth factor receptor 1
(Homo sapiens (Human)) | BDBM50327912
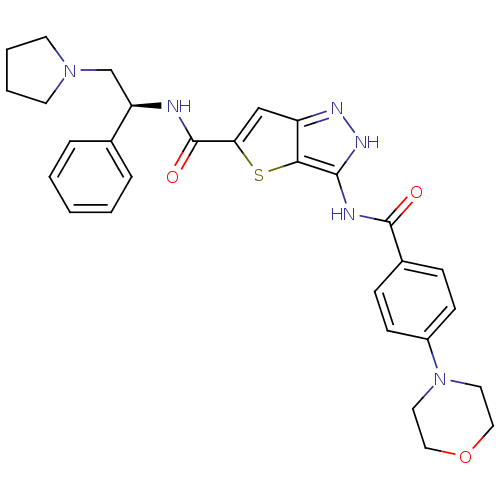
(3-(4-Morpholin-4-yl-benzoylamino)-1H-thieno[3,2-c]...)Show SMILES O=C(N[C@H](CN1CCCC1)c1ccccc1)c1cc2n[nH]c(NC(=O)c3ccc(cc3)N3CCOCC3)c2s1 |r| Show InChI InChI=1S/C29H32N6O3S/c36-28(21-8-10-22(11-9-21)35-14-16-38-17-15-35)31-27-26-23(32-33-27)18-25(39-26)29(37)30-24(19-34-12-4-5-13-34)20-6-2-1-3-7-20/h1-3,6-11,18,24H,4-5,12-17,19H2,(H,30,37)(H2,31,32,33,36)/t24-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences-Oncology
Curated by ChEMBL
| Assay Description
Inhibition of FGFR1 |
Bioorg Med Chem 18: 7113-20 (2010)
Article DOI: 10.1016/j.bmc.2010.07.048
BindingDB Entry DOI: 10.7270/Q2Q240GM |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-1
(Homo sapiens (Human)) | BDBM50021650
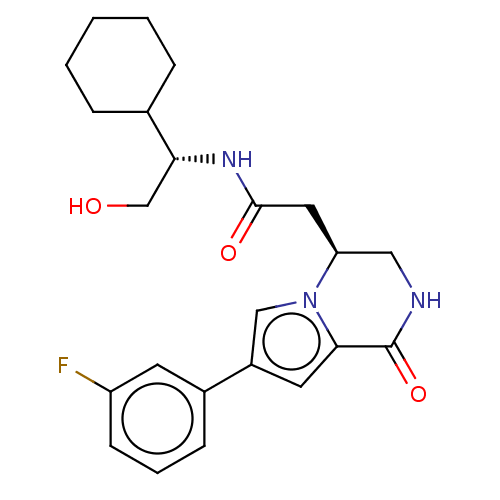
(CHEMBL3298892)Show SMILES OC[C@@H](NC(=O)C[C@H]1CNC(=O)c2cc(cn12)-c1cccc(F)c1)C1CCCCC1 |r| Show InChI InChI=1S/C23H28FN3O3/c24-18-8-4-7-16(9-18)17-10-21-23(30)25-12-19(27(21)13-17)11-22(29)26-20(14-28)15-5-2-1-3-6-15/h4,7-10,13,15,19-20,28H,1-3,5-6,11-12,14H2,(H,25,30)(H,26,29)/t19-,20+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences
Curated by ChEMBL
| Assay Description
Inhibition of human PIM1 using ARK-RERTYSFGHHA as substrate incubated for 60 mins prior to substrate addition by topcount scintillation counting anal... |
Bioorg Med Chem 21: 7364-80 (2013)
Article DOI: 10.1016/j.bmc.2013.09.054
BindingDB Entry DOI: 10.7270/Q23F4R7S |
More data for this
Ligand-Target Pair | |
Cyclin-A2/Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM31544

(pyrazolo[4,3-h]quinazoline-3-carboxamide, 27)Show SMILES CN1CCN(CC1)c1ccc(Nc2ncc3CC(C)(C)c4c(nn(C)c4-c3n2)C(N)=O)cc1 Show InChI InChI=1S/C24H30N8O/c1-24(2)13-15-14-26-23(28-19(15)21-18(24)20(22(25)33)29-31(21)4)27-16-5-7-17(8-6-16)32-11-9-30(3)10-12-32/h5-8,14H,9-13H2,1-4H3,(H2,25,33)(H,26,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | 7.4 | 23 |
Nerviano Medical Sciences Srl
| Assay Description
The biochemical activity of compounds was determined by incubation with specific enzymes and substrates, followed by quantitation of the phosphorylat... |
J Med Chem 52: 5152-63 (2009)
Article DOI: 10.1021/jm9006559
BindingDB Entry DOI: 10.7270/Q2JH3JJP |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Aurora kinase A
(Homo sapiens (Human)) | BDBM50327926
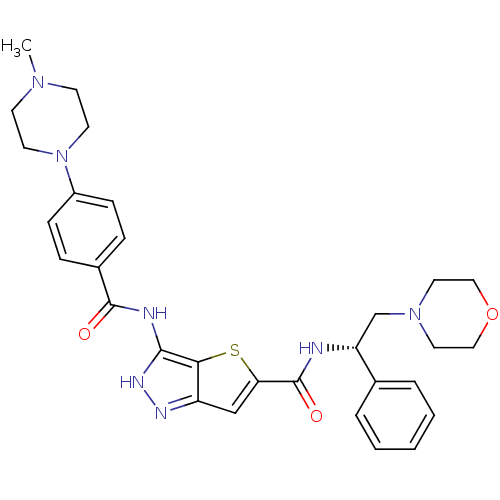
(3-[4-(4-Methyl-piperazin-1-yl)-benzoylamino]-1H-th...)Show SMILES CN1CCN(CC1)c1ccc(cc1)C(=O)Nc1[nH]nc2cc(sc12)C(=O)N[C@H](CN1CCOCC1)c1ccccc1 |r| Show InChI InChI=1S/C30H35N7O3S/c1-35-11-13-37(14-12-35)23-9-7-22(8-10-23)29(38)32-28-27-24(33-34-28)19-26(41-27)30(39)31-25(21-5-3-2-4-6-21)20-36-15-17-40-18-16-36/h2-10,19,25H,11-18,20H2,1H3,(H,31,39)(H2,32,33,34,38)/t25-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences-Oncology
Curated by ChEMBL
| Assay Description
Inhibition of AurA |
Bioorg Med Chem 18: 7113-20 (2010)
Article DOI: 10.1016/j.bmc.2010.07.048
BindingDB Entry DOI: 10.7270/Q2Q240GM |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM50327912

(3-(4-Morpholin-4-yl-benzoylamino)-1H-thieno[3,2-c]...)Show SMILES O=C(N[C@H](CN1CCCC1)c1ccccc1)c1cc2n[nH]c(NC(=O)c3ccc(cc3)N3CCOCC3)c2s1 |r| Show InChI InChI=1S/C29H32N6O3S/c36-28(21-8-10-22(11-9-21)35-14-16-38-17-15-35)31-27-26-23(32-33-27)18-25(39-26)29(37)30-24(19-34-12-4-5-13-34)20-6-2-1-3-7-20/h1-3,6-11,18,24H,4-5,12-17,19H2,(H,30,37)(H2,31,32,33,36)/t24-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences-Oncology
Curated by ChEMBL
| Assay Description
Inhibition of AurA |
Bioorg Med Chem 18: 7113-20 (2010)
Article DOI: 10.1016/j.bmc.2010.07.048
BindingDB Entry DOI: 10.7270/Q2Q240GM |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 3
(Homo sapiens (Human)) | BDBM50327912

(3-(4-Morpholin-4-yl-benzoylamino)-1H-thieno[3,2-c]...)Show SMILES O=C(N[C@H](CN1CCCC1)c1ccccc1)c1cc2n[nH]c(NC(=O)c3ccc(cc3)N3CCOCC3)c2s1 |r| Show InChI InChI=1S/C29H32N6O3S/c36-28(21-8-10-22(11-9-21)35-14-16-38-17-15-35)31-27-26-23(32-33-27)18-25(39-26)29(37)30-24(19-34-12-4-5-13-34)20-6-2-1-3-7-20/h1-3,6-11,18,24H,4-5,12-17,19H2,(H,30,37)(H2,31,32,33,36)/t24-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences-Oncology
Curated by ChEMBL
| Assay Description
Inhibition of VEGFR3 |
Bioorg Med Chem 18: 7113-20 (2010)
Article DOI: 10.1016/j.bmc.2010.07.048
BindingDB Entry DOI: 10.7270/Q2Q240GM |
More data for this
Ligand-Target Pair | |
Cyclin-A2/Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM31533

(pyrazolo[4,3-h]quinazoline-3-carboxamide, 16)Show InChI InChI=1S/C18H18N6O/c1-19-17(25)15-13-9-8-11-10-20-18(21-12-6-4-3-5-7-12)22-14(11)16(13)24(2)23-15/h3-7,10H,8-9H2,1-2H3,(H,19,25)(H,20,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | 7.4 | 23 |
Nerviano Medical Sciences Srl
| Assay Description
The biochemical activity of compounds was determined by incubation with specific enzymes and substrates, followed by quantitation of the phosphorylat... |
J Med Chem 52: 5152-63 (2009)
Article DOI: 10.1021/jm9006559
BindingDB Entry DOI: 10.7270/Q2JH3JJP |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Cyclin-A2/Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM31543

(pyrazolo[4,3-h]quinazoline-3-carboxamide, 26)Show SMILES Cn1nc(C(N)=O)c2c1-c1nc(Nc3ccccc3)ncc1CC2(C)C Show InChI InChI=1S/C19H20N6O/c1-19(2)9-11-10-21-18(22-12-7-5-4-6-8-12)23-14(11)16-13(19)15(17(20)26)24-25(16)3/h4-8,10H,9H2,1-3H3,(H2,20,26)(H,21,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 24 | n/a | n/a | n/a | n/a | 7.4 | 23 |
Nerviano Medical Sciences Srl
| Assay Description
The biochemical activity of compounds was determined by incubation with specific enzymes and substrates, followed by quantitation of the phosphorylat... |
J Med Chem 52: 5152-63 (2009)
Article DOI: 10.1021/jm9006559
BindingDB Entry DOI: 10.7270/Q2JH3JJP |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Aurora kinase A
(Homo sapiens (Human)) | BDBM50327925
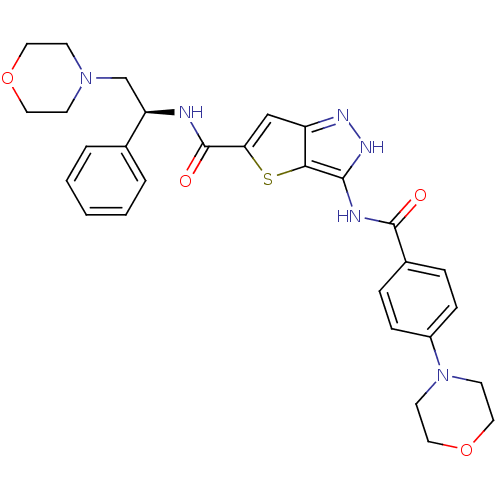
(3-(4-Morpholin-4-yl-benzoylamino)-1H-thieno[3,2-c]...)Show SMILES O=C(N[C@H](CN1CCOCC1)c1ccccc1)c1cc2n[nH]c(NC(=O)c3ccc(cc3)N3CCOCC3)c2s1 |r| Show InChI InChI=1S/C29H32N6O4S/c36-28(21-6-8-22(9-7-21)35-12-16-39-17-13-35)31-27-26-23(32-33-27)18-25(40-26)29(37)30-24(20-4-2-1-3-5-20)19-34-10-14-38-15-11-34/h1-9,18,24H,10-17,19H2,(H,30,37)(H2,31,32,33,36)/t24-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences-Oncology
Curated by ChEMBL
| Assay Description
Inhibition of AurA |
Bioorg Med Chem 18: 7113-20 (2010)
Article DOI: 10.1016/j.bmc.2010.07.048
BindingDB Entry DOI: 10.7270/Q2Q240GM |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-1
(Homo sapiens (Human)) | BDBM50021619
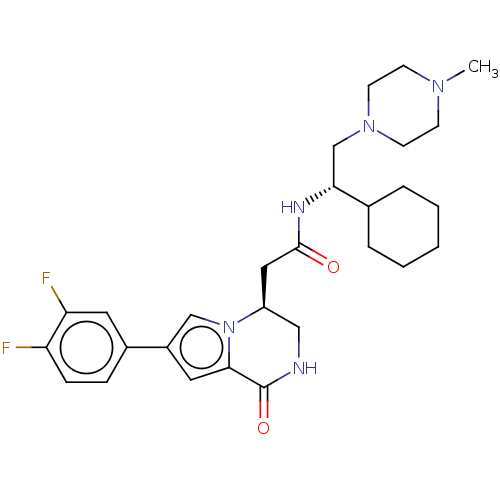
(CHEMBL3297763)Show SMILES CN1CCN(C[C@@H](NC(=O)C[C@H]2CNC(=O)c3cc(cn23)-c2ccc(F)c(F)c2)C2CCCCC2)CC1 |r| Show InChI InChI=1S/C28H37F2N5O2/c1-33-9-11-34(12-10-33)18-25(19-5-3-2-4-6-19)32-27(36)15-22-16-31-28(37)26-14-21(17-35(22)26)20-7-8-23(29)24(30)13-20/h7-8,13-14,17,19,22,25H,2-6,9-12,15-16,18H2,1H3,(H,31,37)(H,32,36)/t22-,25+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences
Curated by ChEMBL
| Assay Description
Inhibition of human PIM1 using ARK-RERTYSFGHHA as substrate incubated for 60 mins prior to substrate addition by topcount scintillation counting anal... |
Bioorg Med Chem 21: 7364-80 (2013)
Article DOI: 10.1016/j.bmc.2013.09.054
BindingDB Entry DOI: 10.7270/Q23F4R7S |
More data for this
Ligand-Target Pair | |
Transitional endoplasmic reticulum ATPase
(Homo sapiens (Human)) | BDBM50040551
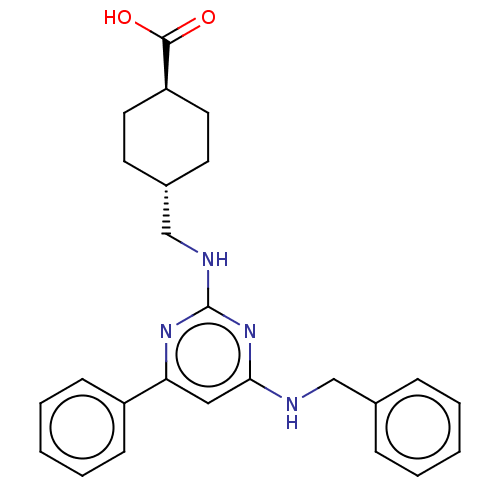
(CHEMBL3361184)Show SMILES OC(=O)[C@H]1CC[C@H](CNc2nc(NCc3ccccc3)cc(n2)-c2ccccc2)CC1 |r,wU:3.2,wD:6.6,(37.31,-57.24,;35.98,-58.01,;35.98,-59.55,;34.65,-57.24,;33.31,-58.01,;31.98,-57.24,;31.98,-55.7,;30.64,-54.93,;30.64,-53.39,;29.31,-52.62,;27.98,-53.39,;26.64,-52.62,;25.31,-53.39,;25.31,-54.93,;23.97,-55.7,;23.97,-57.24,;22.64,-58.01,;21.31,-57.24,;21.31,-55.7,;22.64,-54.93,;26.64,-51.08,;27.98,-50.31,;29.31,-51.08,;27.98,-48.77,;26.64,-48,;26.64,-46.46,;27.98,-45.69,;29.31,-46.46,;29.31,-48,;33.31,-54.93,;34.65,-55.7,)| Show InChI InChI=1S/C25H28N4O2/c30-24(31)21-13-11-19(12-14-21)17-27-25-28-22(20-9-5-2-6-10-20)15-23(29-25)26-16-18-7-3-1-4-8-18/h1-10,15,19,21H,11-14,16-17H2,(H,30,31)(H2,26,27,28,29)/t19-,21- | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences S.r.l.
Curated by ChEMBL
| Assay Description
Inhibition of human full length VCP (2 to 806 residues) expressed in High5 insect cells assessed as reduction in ATPase activity by measuring ADP for... |
J Med Chem 57: 10443-54 (2014)
Article DOI: 10.1021/jm501313x
BindingDB Entry DOI: 10.7270/Q2T1558Z |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM50327924
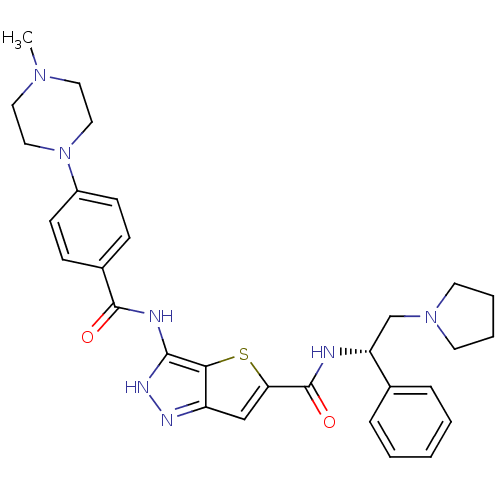
(3-[4-(4-Methyl-piperazin-1-yl)-benzoylamino]-1H-th...)Show SMILES CN1CCN(CC1)c1ccc(cc1)C(=O)Nc1[nH]nc2cc(sc12)C(=O)N[C@H](CN1CCCC1)c1ccccc1 |r| Show InChI InChI=1S/C30H35N7O2S/c1-35-15-17-37(18-16-35)23-11-9-22(10-12-23)29(38)32-28-27-24(33-34-28)19-26(40-27)30(39)31-25(20-36-13-5-6-14-36)21-7-3-2-4-8-21/h2-4,7-12,19,25H,5-6,13-18,20H2,1H3,(H,31,39)(H2,32,33,34,38)/t25-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences-Oncology
Curated by ChEMBL
| Assay Description
Inhibition of AurA |
Bioorg Med Chem 18: 7113-20 (2010)
Article DOI: 10.1016/j.bmc.2010.07.048
BindingDB Entry DOI: 10.7270/Q2Q240GM |
More data for this
Ligand-Target Pair | |
Cyclin-A2/Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM31549

(pyrazolo[4,3-h]quinazoline-3-carboxamide, 32)Show SMILES CNC(=O)c1nn(C)c-2c1C(C)(C)Cc1cnc(Nc3ccc(OC4CCN(C)CC4)cc3)nc-21 Show InChI InChI=1S/C26H33N7O2/c1-26(2)14-16-15-28-25(30-21(16)23-20(26)22(24(34)27-3)31-33(23)5)29-17-6-8-18(9-7-17)35-19-10-12-32(4)13-11-19/h6-9,15,19H,10-14H2,1-5H3,(H,27,34)(H,28,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 29 | n/a | n/a | n/a | n/a | 7.4 | 23 |
Nerviano Medical Sciences Srl
| Assay Description
The biochemical activity of compounds was determined by incubation with specific enzymes and substrates, followed by quantitation of the phosphorylat... |
J Med Chem 52: 5152-63 (2009)
Article DOI: 10.1021/jm9006559
BindingDB Entry DOI: 10.7270/Q2JH3JJP |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-1
(Homo sapiens (Human)) | BDBM50021618

(CHEMBL3297762 | US9145418, 2)Show SMILES CN1CCN(C[C@@H](NC(=O)C[C@H]2CNC(=O)c3cc(cn23)-c2cccc(Cl)c2)C2CCCCC2)CC1 |r| Show InChI InChI=1S/C28H38ClN5O2/c1-32-10-12-33(13-11-32)19-25(20-6-3-2-4-7-20)31-27(35)16-24-17-30-28(36)26-15-22(18-34(24)26)21-8-5-9-23(29)14-21/h5,8-9,14-15,18,20,24-25H,2-4,6-7,10-13,16-17,19H2,1H3,(H,30,36)(H,31,35)/t24-,25+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences
Curated by ChEMBL
| Assay Description
Inhibition of human PIM1 using ARK-RERTYSFGHHA as substrate incubated for 60 mins prior to substrate addition by topcount scintillation counting anal... |
Bioorg Med Chem 21: 7364-80 (2013)
Article DOI: 10.1016/j.bmc.2013.09.054
BindingDB Entry DOI: 10.7270/Q23F4R7S |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM31541

(pyrazolo[4,3-h]quinazoline-3-carboxamide, 24)Show SMILES CN1CCN(CC1)c1cccc(Nc2ncc3CCc4c(nn(C)c4-c3n2)C(N)=O)c1 Show InChI InChI=1S/C22H26N8O/c1-28-8-10-30(11-9-28)16-5-3-4-15(12-16)25-22-24-13-14-6-7-17-19(21(23)31)27-29(2)20(17)18(14)26-22/h3-5,12-13H,6-11H2,1-2H3,(H2,23,31)(H,24,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | 25 |
Nerviano Medical Sciences Srl
| Assay Description
The biochemical activity of compounds was determined by incubation with specific enzymes and substrates, followed by quantitation of the phosphorylat... |
J Med Chem 52: 5152-63 (2009)
Article DOI: 10.1021/jm9006559
BindingDB Entry DOI: 10.7270/Q2JH3JJP |
More data for this
Ligand-Target Pair | |
Cyclin-A2/Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM31546

(pyrazolo[4,3-h]quinazoline-3-carboxamide, 29)Show SMILES CNC(=O)c1nn(C)c-2c1C(C)(C)Cc1cnc(Nc3cccc(c3)N3CCN(C)CC3)nc-21 Show InChI InChI=1S/C25H32N8O/c1-25(2)14-16-15-27-24(29-20(16)22-19(25)21(23(34)26-3)30-32(22)5)28-17-7-6-8-18(13-17)33-11-9-31(4)10-12-33/h6-8,13,15H,9-12,14H2,1-5H3,(H,26,34)(H,27,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 31 | n/a | n/a | n/a | n/a | 7.4 | 23 |
Nerviano Medical Sciences Srl
| Assay Description
The biochemical activity of compounds was determined by incubation with specific enzymes and substrates, followed by quantitation of the phosphorylat... |
J Med Chem 52: 5152-63 (2009)
Article DOI: 10.1021/jm9006559
BindingDB Entry DOI: 10.7270/Q2JH3JJP |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Aurora kinase A
(Homo sapiens (Human)) | BDBM50327923

(3-[4-(4-Methyl-piperazin-1-yl)-benzoylamino]-1H-th...)Show SMILES CC[C@H](NC(=O)c1cc2n[nH]c(NC(=O)c3ccc(cc3)N3CCN(C)CC3)c2s1)c1ccccc1 |r| Show InChI InChI=1S/C27H30N6O2S/c1-3-21(18-7-5-4-6-8-18)28-27(35)23-17-22-24(36-23)25(31-30-22)29-26(34)19-9-11-20(12-10-19)33-15-13-32(2)14-16-33/h4-12,17,21H,3,13-16H2,1-2H3,(H,28,35)(H2,29,30,31,34)/t21-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences-Oncology
Curated by ChEMBL
| Assay Description
Inhibition of AurA |
Bioorg Med Chem 18: 7113-20 (2010)
Article DOI: 10.1016/j.bmc.2010.07.048
BindingDB Entry DOI: 10.7270/Q2Q240GM |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM50327922
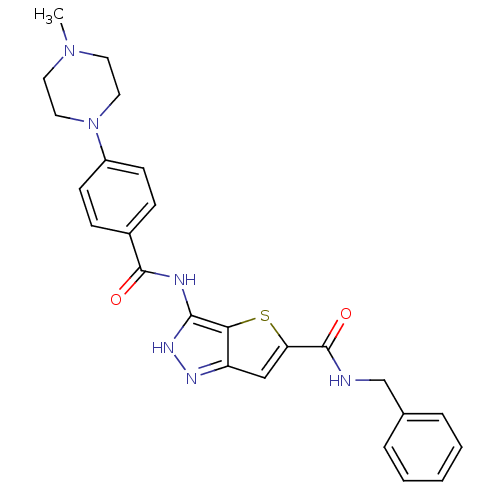
(3-[4-(4-Methyl-piperazin-1-yl)-benzoylamino]-1H-th...)Show SMILES CN1CCN(CC1)c1ccc(cc1)C(=O)Nc1[nH]nc2cc(sc12)C(=O)NCc1ccccc1 Show InChI InChI=1S/C25H26N6O2S/c1-30-11-13-31(14-12-30)19-9-7-18(8-10-19)24(32)27-23-22-20(28-29-23)15-21(34-22)25(33)26-16-17-5-3-2-4-6-17/h2-10,15H,11-14,16H2,1H3,(H,26,33)(H2,27,28,29,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences-Oncology
Curated by ChEMBL
| Assay Description
Inhibition of AurA |
Bioorg Med Chem 18: 7113-20 (2010)
Article DOI: 10.1016/j.bmc.2010.07.048
BindingDB Entry DOI: 10.7270/Q2Q240GM |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-1
(Homo sapiens (Human)) | BDBM50021641
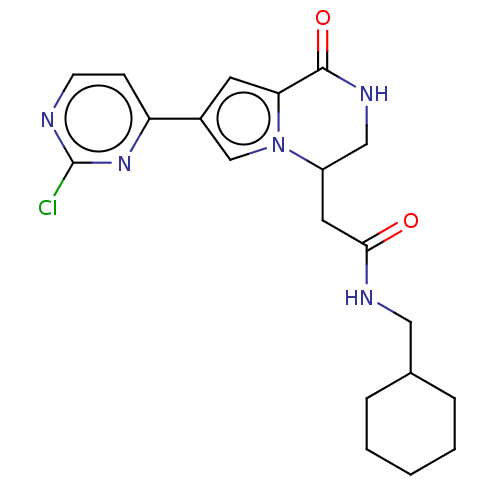
(CHEMBL3298822)Show SMILES Clc1nccc(n1)-c1cc2C(=O)NCC(CC(=O)NCC3CCCCC3)n2c1 Show InChI InChI=1S/C20H24ClN5O2/c21-20-22-7-6-16(25-20)14-8-17-19(28)24-11-15(26(17)12-14)9-18(27)23-10-13-4-2-1-3-5-13/h6-8,12-13,15H,1-5,9-11H2,(H,23,27)(H,24,28) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences
Curated by ChEMBL
| Assay Description
Inhibition of human PIM1 using ARK-RERTYSFGHHA as substrate incubated for 60 mins prior to substrate addition by topcount scintillation counting anal... |
Bioorg Med Chem 21: 7364-80 (2013)
Article DOI: 10.1016/j.bmc.2013.09.054
BindingDB Entry DOI: 10.7270/Q23F4R7S |
More data for this
Ligand-Target Pair | |
Mast/stem cell growth factor receptor Kit
(Homo sapiens (Human)) | BDBM50327912

(3-(4-Morpholin-4-yl-benzoylamino)-1H-thieno[3,2-c]...)Show SMILES O=C(N[C@H](CN1CCCC1)c1ccccc1)c1cc2n[nH]c(NC(=O)c3ccc(cc3)N3CCOCC3)c2s1 |r| Show InChI InChI=1S/C29H32N6O3S/c36-28(21-8-10-22(11-9-21)35-14-16-38-17-15-35)31-27-26-23(32-33-27)18-25(39-26)29(37)30-24(19-34-12-4-5-13-34)20-6-2-1-3-7-20/h1-3,6-11,18,24H,4-5,12-17,19H2,(H,30,37)(H2,31,32,33,36)/t24-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences-Oncology
Curated by ChEMBL
| Assay Description
Inhibition of KIT |
Bioorg Med Chem 18: 7113-20 (2010)
Article DOI: 10.1016/j.bmc.2010.07.048
BindingDB Entry DOI: 10.7270/Q2Q240GM |
More data for this
Ligand-Target Pair | |
Cyclin-A2/Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM31557

(pyrazolo[4,3-h]quinazoline-3-carboxamide, 40)Show SMILES CNC(=O)c1nn(C)c-2c1C(C)(C)Cc1cnc(Nc3ccc(CN(C)C)cc3)nc-21 Show InChI InChI=1S/C23H29N7O/c1-23(2)11-15-12-25-22(26-16-9-7-14(8-10-16)13-29(4)5)27-18(15)20-17(23)19(21(31)24-3)28-30(20)6/h7-10,12H,11,13H2,1-6H3,(H,24,31)(H,25,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 35 | n/a | n/a | n/a | n/a | 7.4 | 23 |
Nerviano Medical Sciences Srl
| Assay Description
The biochemical activity of compounds was determined by incubation with specific enzymes and substrates, followed by quantitation of the phosphorylat... |
J Med Chem 52: 5152-63 (2009)
Article DOI: 10.1021/jm9006559
BindingDB Entry DOI: 10.7270/Q2JH3JJP |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Serine/threonine-protein kinase pim-2
(Homo sapiens (Human)) | BDBM50021624

(CHEMBL3297766 | US9145418, 23)Show SMILES CN(C)C1CCN(C[C@@H](NC(=O)C[C@H]2CNC(=O)c3cc(cn23)-c2cccc(Cl)c2)C2CCCCC2)CC1 |r| Show InChI InChI=1S/C30H42ClN5O2/c1-34(2)25-11-13-35(14-12-25)20-27(21-7-4-3-5-8-21)33-29(37)17-26-18-32-30(38)28-16-23(19-36(26)28)22-9-6-10-24(31)15-22/h6,9-10,15-16,19,21,25-27H,3-5,7-8,11-14,17-18,20H2,1-2H3,(H,32,38)(H,33,37)/t26-,27+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences
Curated by ChEMBL
| Assay Description
Inhibition of human PIM2 using ARK-RERTYSFGHHA as substrate incubated for 60 mins prior to substrate addition by topcount scintillation counting anal... |
Bioorg Med Chem 21: 7364-80 (2013)
Article DOI: 10.1016/j.bmc.2013.09.054
BindingDB Entry DOI: 10.7270/Q23F4R7S |
More data for this
Ligand-Target Pair | |
Transitional endoplasmic reticulum ATPase
(Homo sapiens (Human)) | BDBM50040550
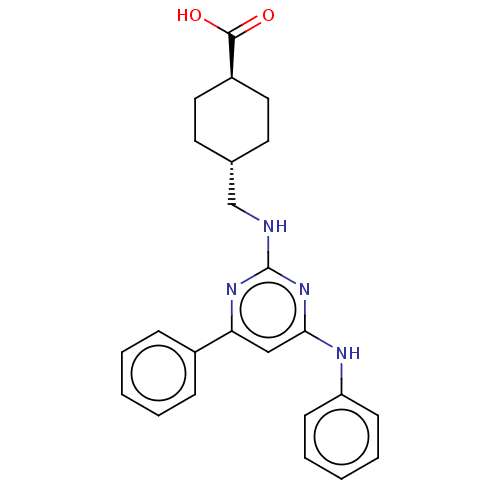
(CHEMBL3361185)Show SMILES OC(=O)[C@H]1CC[C@H](CNc2nc(Nc3ccccc3)cc(n2)-c2ccccc2)CC1 |r,wU:3.2,wD:6.6,(59.29,-56.65,;57.96,-57.42,;57.96,-58.96,;56.63,-56.65,;55.29,-57.42,;53.96,-56.65,;53.96,-55.11,;52.62,-54.34,;51.29,-55.11,;49.96,-54.34,;49.96,-52.8,;48.62,-52.03,;48.62,-50.49,;49.96,-49.72,;49.96,-48.18,;51.29,-47.41,;52.62,-48.18,;52.62,-49.72,;51.29,-50.49,;47.29,-52.8,;47.29,-54.34,;48.62,-55.11,;45.95,-55.11,;45.95,-56.65,;44.62,-57.42,;43.29,-56.65,;43.29,-55.11,;44.62,-54.34,;55.29,-54.34,;56.63,-55.11,)| Show InChI InChI=1S/C24H26N4O2/c29-23(30)19-13-11-17(12-14-19)16-25-24-27-21(18-7-3-1-4-8-18)15-22(28-24)26-20-9-5-2-6-10-20/h1-10,15,17,19H,11-14,16H2,(H,29,30)(H2,25,26,27,28)/t17-,19- | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences S.r.l.
Curated by ChEMBL
| Assay Description
Inhibition of human full length VCP (2 to 806 residues) expressed in High5 insect cells assessed as reduction in ATPase activity by measuring ADP for... |
J Med Chem 57: 10443-54 (2014)
Article DOI: 10.1021/jm501313x
BindingDB Entry DOI: 10.7270/Q2T1558Z |
More data for this
Ligand-Target Pair | |
Cyclin-A2/Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM31553
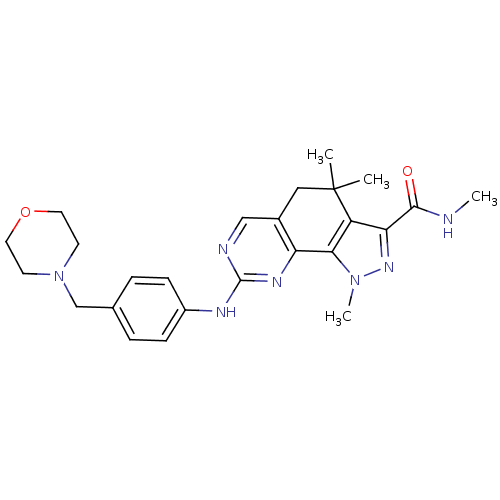
(pyrazolo[4,3-h]quinazoline-3-carboxamide, 36)Show SMILES CNC(=O)c1nn(C)c-2c1C(C)(C)Cc1cnc(Nc3ccc(CN4CCOCC4)cc3)nc-21 Show InChI InChI=1S/C25H31N7O2/c1-25(2)13-17-14-27-24(29-20(17)22-19(25)21(23(33)26-3)30-31(22)4)28-18-7-5-16(6-8-18)15-32-9-11-34-12-10-32/h5-8,14H,9-13,15H2,1-4H3,(H,26,33)(H,27,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 36 | n/a | n/a | n/a | n/a | 7.4 | 23 |
Nerviano Medical Sciences Srl
| Assay Description
The biochemical activity of compounds was determined by incubation with specific enzymes and substrates, followed by quantitation of the phosphorylat... |
J Med Chem 52: 5152-63 (2009)
Article DOI: 10.1021/jm9006559
BindingDB Entry DOI: 10.7270/Q2JH3JJP |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-1
(Homo sapiens (Human)) | BDBM50021643
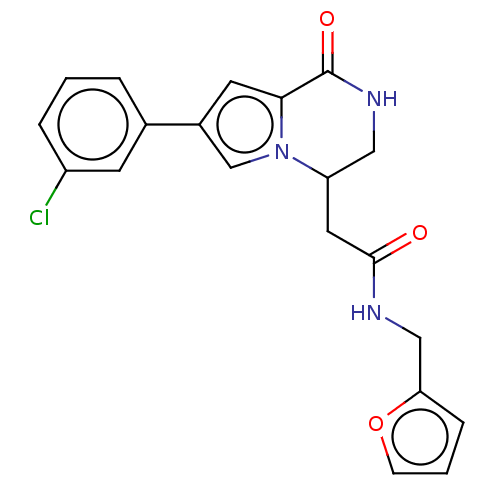
(CHEMBL3298886)Show SMILES Clc1cccc(c1)-c1cc2C(=O)NCC(CC(=O)NCc3ccco3)n2c1 Show InChI InChI=1S/C20H18ClN3O3/c21-15-4-1-3-13(7-15)14-8-18-20(26)23-10-16(24(18)12-14)9-19(25)22-11-17-5-2-6-27-17/h1-8,12,16H,9-11H2,(H,22,25)(H,23,26) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences
Curated by ChEMBL
| Assay Description
Inhibition of human PIM1 using ARK-RERTYSFGHHA as substrate incubated for 60 mins prior to substrate addition by topcount scintillation counting anal... |
Bioorg Med Chem 21: 7364-80 (2013)
Article DOI: 10.1016/j.bmc.2013.09.054
BindingDB Entry DOI: 10.7270/Q23F4R7S |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM50327921
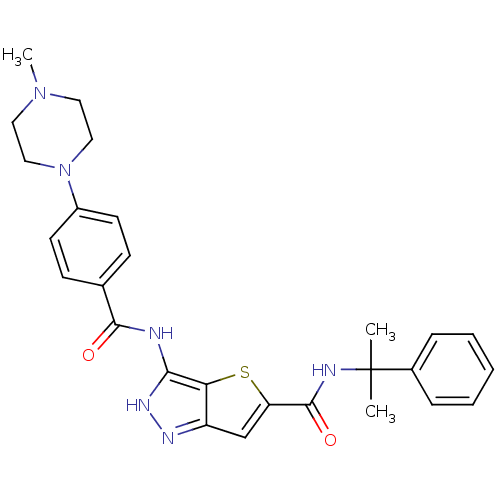
(3-[4-(4-Methyl-piperazin-1-yl)-benzoylamino]-1H-th...)Show SMILES CN1CCN(CC1)c1ccc(cc1)C(=O)Nc1[nH]nc2cc(sc12)C(=O)NC(C)(C)c1ccccc1 Show InChI InChI=1S/C27H30N6O2S/c1-27(2,19-7-5-4-6-8-19)29-26(35)22-17-21-23(36-22)24(31-30-21)28-25(34)18-9-11-20(12-10-18)33-15-13-32(3)14-16-33/h4-12,17H,13-16H2,1-3H3,(H,29,35)(H2,28,30,31,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 44 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences-Oncology
Curated by ChEMBL
| Assay Description
Inhibition of AurA |
Bioorg Med Chem 18: 7113-20 (2010)
Article DOI: 10.1016/j.bmc.2010.07.048
BindingDB Entry DOI: 10.7270/Q2Q240GM |
More data for this
Ligand-Target Pair | |
Cyclin-A2/Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM31540
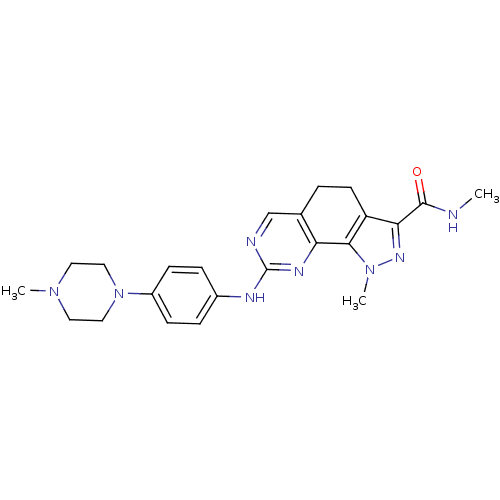
(pyrazolo[4,3-h]quinazoline-3-carboxamide, 23)Show SMILES CNC(=O)c1nn(C)c-2c1CCc1cnc(Nc3ccc(cc3)N3CCN(C)CC3)nc-21 Show InChI InChI=1S/C23H28N8O/c1-24-22(32)20-18-9-4-15-14-25-23(27-19(15)21(18)30(3)28-20)26-16-5-7-17(8-6-16)31-12-10-29(2)11-13-31/h5-8,14H,4,9-13H2,1-3H3,(H,24,32)(H,25,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 45 | n/a | n/a | n/a | n/a | 7.4 | 23 |
Nerviano Medical Sciences Srl
| Assay Description
The biochemical activity of compounds was determined by incubation with specific enzymes and substrates, followed by quantitation of the phosphorylat... |
J Med Chem 52: 5152-63 (2009)
Article DOI: 10.1021/jm9006559
BindingDB Entry DOI: 10.7270/Q2JH3JJP |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Aurora kinase A
(Homo sapiens (Human)) | BDBM50327920
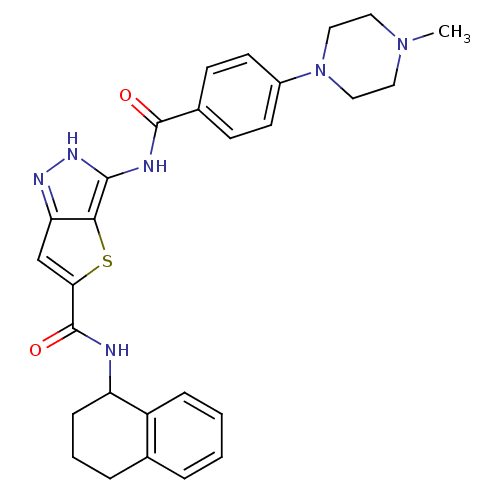
(3-[4-(4-Methyl-piperazin-1-yl)-benzoylamino]-1H-th...)Show SMILES CN1CCN(CC1)c1ccc(cc1)C(=O)Nc1[nH]nc2cc(sc12)C(=O)NC1CCCc2ccccc12 Show InChI InChI=1S/C28H30N6O2S/c1-33-13-15-34(16-14-33)20-11-9-19(10-12-20)27(35)30-26-25-23(31-32-26)17-24(37-25)28(36)29-22-8-4-6-18-5-2-3-7-21(18)22/h2-3,5,7,9-12,17,22H,4,6,8,13-16H2,1H3,(H,29,36)(H2,30,31,32,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences-Oncology
Curated by ChEMBL
| Assay Description
Inhibition of AurA |
Bioorg Med Chem 18: 7113-20 (2010)
Article DOI: 10.1016/j.bmc.2010.07.048
BindingDB Entry DOI: 10.7270/Q2Q240GM |
More data for this
Ligand-Target Pair | |
Cyclin-A2/Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM31545
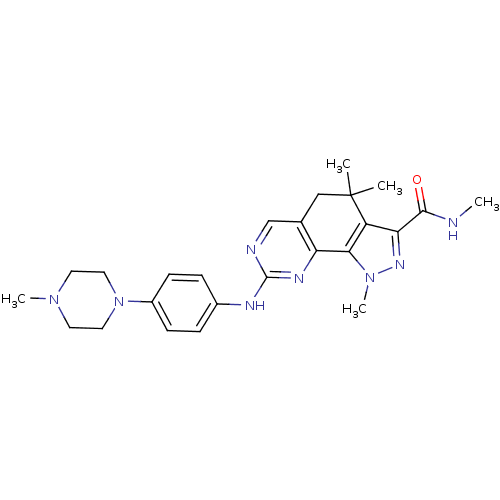
(Milciclib | pyrazolo[4,3-h]quinazoline-3-carboxami...)Show SMILES CNC(=O)c1nn(C)c-2c1C(C)(C)Cc1cnc(Nc3ccc(cc3)N3CCN(C)CC3)nc-21 Show InChI InChI=1S/C25H32N8O/c1-25(2)14-16-15-27-24(29-20(16)22-19(25)21(23(34)26-3)30-32(22)5)28-17-6-8-18(9-7-17)33-12-10-31(4)11-13-33/h6-9,15H,10-14H2,1-5H3,(H,26,34)(H,27,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 45 | n/a | n/a | n/a | n/a | 7.4 | 23 |
Nerviano Medical Sciences Srl
| Assay Description
The biochemical activity of compounds was determined by incubation with specific enzymes and substrates, followed by quantitation of the phosphorylat... |
J Med Chem 52: 5152-63 (2009)
Article DOI: 10.1021/jm9006559
BindingDB Entry DOI: 10.7270/Q2JH3JJP |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Aurora kinase B
(Homo sapiens (Human)) | BDBM50327912

(3-(4-Morpholin-4-yl-benzoylamino)-1H-thieno[3,2-c]...)Show SMILES O=C(N[C@H](CN1CCCC1)c1ccccc1)c1cc2n[nH]c(NC(=O)c3ccc(cc3)N3CCOCC3)c2s1 |r| Show InChI InChI=1S/C29H32N6O3S/c36-28(21-8-10-22(11-9-21)35-14-16-38-17-15-35)31-27-26-23(32-33-27)18-25(39-26)29(37)30-24(19-34-12-4-5-13-34)20-6-2-1-3-7-20/h1-3,6-11,18,24H,4-5,12-17,19H2,(H,30,37)(H2,31,32,33,36)/t24-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences-Oncology
Curated by ChEMBL
| Assay Description
Inhibition of aurora B |
Bioorg Med Chem 18: 7113-20 (2010)
Article DOI: 10.1016/j.bmc.2010.07.048
BindingDB Entry DOI: 10.7270/Q2Q240GM |
More data for this
Ligand-Target Pair | |
Transitional endoplasmic reticulum ATPase
(Homo sapiens (Human)) | BDBM50040513

(CHEMBL3361210)Show SMILES NC(=O)[C@H]1CC[C@H](CNc2nc(NCc3ccccc3)cc(n2)-c2ccccc2F)CC1 |r,wU:3.2,wD:6.6,(46.52,-53.26,;47.86,-54.03,;47.86,-55.57,;49.2,-53.26,;49.2,-51.72,;50.53,-50.95,;51.86,-51.72,;53.2,-50.95,;54.53,-51.72,;55.86,-50.95,;55.86,-49.41,;57.2,-48.64,;57.2,-47.1,;55.86,-46.33,;55.86,-44.79,;54.53,-44.02,;54.53,-42.48,;55.86,-41.71,;57.2,-42.48,;57.2,-44.02,;58.53,-49.41,;58.53,-50.95,;57.2,-51.72,;59.86,-51.72,;59.86,-53.26,;61.2,-54.03,;62.53,-53.26,;62.53,-51.72,;61.2,-50.95,;61.2,-49.41,;51.86,-53.26,;50.53,-54.03,)| Show InChI InChI=1S/C25H28FN5O/c26-21-9-5-4-8-20(21)22-14-23(28-15-17-6-2-1-3-7-17)31-25(30-22)29-16-18-10-12-19(13-11-18)24(27)32/h1-9,14,18-19H,10-13,15-16H2,(H2,27,32)(H2,28,29,30,31)/t18-,19- | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 46 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences S.r.l.
Curated by ChEMBL
| Assay Description
Inhibition of human full length VCP (2 to 806 residues) expressed in High5 insect cells assessed as reduction in ATPase activity by measuring ADP for... |
J Med Chem 57: 10443-54 (2014)
Article DOI: 10.1021/jm501313x
BindingDB Entry DOI: 10.7270/Q2T1558Z |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data