 Found 1087 hits with Last Name = 'chin' and Initial = 'dn'
Found 1087 hits with Last Name = 'chin' and Initial = 'dn' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50261816
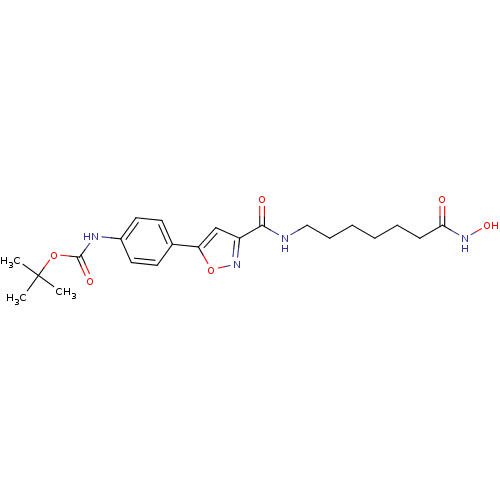
(CHEMBL511749 | tert-butyl 4-(3-((7-(hydroxyamino)-...)Show SMILES CC(C)(C)OC(=O)Nc1ccc(cc1)-c1cc(no1)C(=O)NCCCCCCC(=O)NO Show InChI InChI=1S/C22H30N4O6/c1-22(2,3)31-21(29)24-16-11-9-15(10-12-16)18-14-17(26-32-18)20(28)23-13-7-5-4-6-8-19(27)25-30/h9-12,14,30H,4-8,13H2,1-3H3,(H,23,28)(H,24,29)(H,25,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.00200 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Inhibition of HDAC6 (unknown origin) after 17 hrs |
J Med Chem 51: 4370-3 (2008)
Article DOI: 10.1021/jm8002894
BindingDB Entry DOI: 10.7270/Q27W6C11 |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50267760
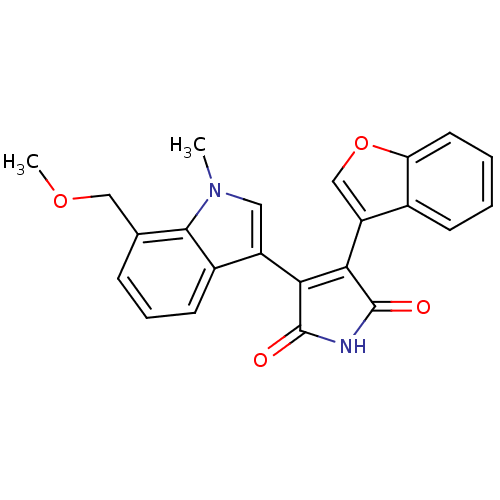
(3-Benzofuran-3-yl-4-(7-methoxymethyl-1-methyl-1H-i...)Show SMILES COCc1cccc2c(cn(C)c12)C1=C(C(=O)NC1=O)c1coc2ccccc12 |t:15| Show InChI InChI=1S/C23H18N2O4/c1-25-10-16(15-8-5-6-13(11-28-2)21(15)25)19-20(23(27)24-22(19)26)17-12-29-18-9-4-3-7-14(17)18/h3-10,12H,11H2,1-2H3,(H,24,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.230 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Inhibition of human GSK3-beta by scintillation counting |
J Med Chem 52: 1853-63 (2009)
Article DOI: 10.1021/jm801317h
BindingDB Entry DOI: 10.7270/Q24M94FZ |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50267461
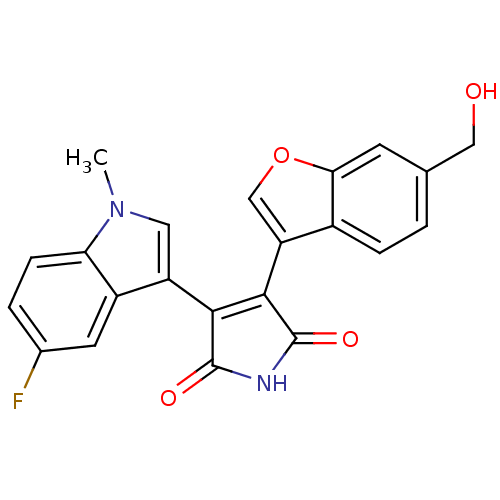
(3-(5-Fluoro-1-methyl-1H-indol-3-yl)-4-(6-hydroxyme...)Show SMILES Cn1cc(C2=C(C(=O)NC2=O)c2coc3cc(CO)ccc23)c2cc(F)ccc12 |t:4| Show InChI InChI=1S/C22H15FN2O4/c1-25-8-15(14-7-12(23)3-5-17(14)25)19-20(22(28)24-21(19)27)16-10-29-18-6-11(9-26)2-4-13(16)18/h2-8,10,26H,9H2,1H3,(H,24,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.350 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Inhibition of human GSK3-beta by scintillation counting |
J Med Chem 52: 1853-63 (2009)
Article DOI: 10.1021/jm801317h
BindingDB Entry DOI: 10.7270/Q24M94FZ |
More data for this
Ligand-Target Pair | |
Histone deacetylase 3
(Homo sapiens (Human)) | BDBM50261816

(CHEMBL511749 | tert-butyl 4-(3-((7-(hydroxyamino)-...)Show SMILES CC(C)(C)OC(=O)Nc1ccc(cc1)-c1cc(no1)C(=O)NCCCCCCC(=O)NO Show InChI InChI=1S/C22H30N4O6/c1-22(2,3)31-21(29)24-16-11-9-15(10-12-16)18-14-17(26-32-18)20(28)23-13-7-5-4-6-8-19(27)25-30/h9-12,14,30H,4-8,13H2,1-3H3,(H,23,28)(H,24,29)(H,25,27) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.420 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Inhibition of HDAC3 (unknown origin) after 17 hrs |
J Med Chem 51: 4370-3 (2008)
Article DOI: 10.1021/jm8002894
BindingDB Entry DOI: 10.7270/Q27W6C11 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase Chk1
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | <0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Inhibition of CHK1 |
J Med Chem 52: 1853-63 (2009)
Article DOI: 10.1021/jm801317h
BindingDB Entry DOI: 10.7270/Q24M94FZ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50267607

(3-(5-Bromo-1-methyl-1H-indol-3-yl)-4-(6-hydroxymet...)Show SMILES Cn1cc(C2=C(C(=O)NC2=O)c2coc3cc(CO)ccc23)c2cc(Br)ccc12 |t:4| Show InChI InChI=1S/C22H15BrN2O4/c1-25-8-15(14-7-12(23)3-5-17(14)25)19-20(22(28)24-21(19)27)16-10-29-18-6-11(9-26)2-4-13(16)18/h2-8,10,26H,9H2,1H3,(H,24,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.510 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Inhibition of human GSK3-beta by scintillation counting |
J Med Chem 52: 1853-63 (2009)
Article DOI: 10.1021/jm801317h
BindingDB Entry DOI: 10.7270/Q24M94FZ |
More data for this
Ligand-Target Pair | |
RAC-gamma serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.640 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Inhibition of AKT3 |
J Med Chem 52: 1853-63 (2009)
Article DOI: 10.1021/jm801317h
BindingDB Entry DOI: 10.7270/Q24M94FZ |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50267800
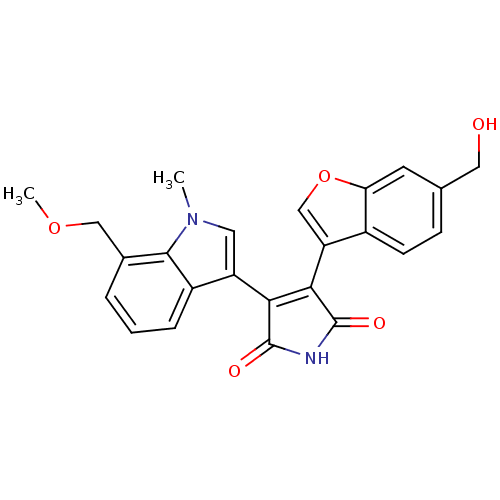
(3-(6-Hydroxymethylbenzofuran-3-yl)-4-(7-methoxymet...)Show SMILES COCc1cccc2c(cn(C)c12)C1=C(C(=O)NC1=O)c1coc2cc(CO)ccc12 |t:15| Show InChI InChI=1S/C24H20N2O5/c1-26-9-17(16-5-3-4-14(11-30-2)22(16)26)20-21(24(29)25-23(20)28)18-12-31-19-8-13(10-27)6-7-15(18)19/h3-9,12,27H,10-11H2,1-2H3,(H,25,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.730 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Inhibition of human GSK3-beta by scintillation counting |
J Med Chem 52: 1853-63 (2009)
Article DOI: 10.1021/jm801317h
BindingDB Entry DOI: 10.7270/Q24M94FZ |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50267523
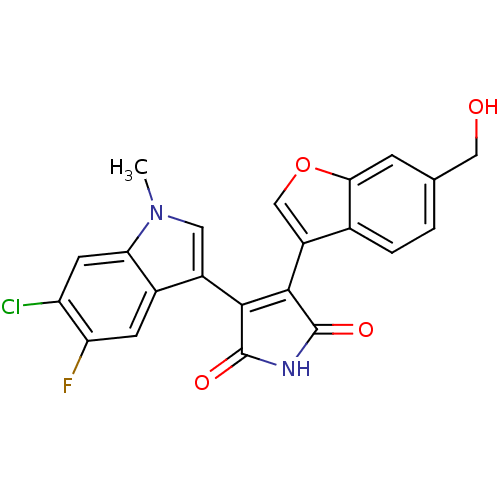
(3-(6-Chloro-5-fluoro-1-methyl-1H-indol-3-yl)-4-(6-...)Show SMILES Cn1cc(C2=C(C(=O)NC2=O)c2coc3cc(CO)ccc23)c2cc(F)c(Cl)cc12 |t:4| Show InChI InChI=1S/C22H14ClFN2O4/c1-26-7-13(12-5-16(24)15(23)6-17(12)26)19-20(22(29)25-21(19)28)14-9-30-18-4-10(8-27)2-3-11(14)18/h2-7,9,27H,8H2,1H3,(H,25,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.950 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Inhibition of human GSK3-beta by scintillation counting |
J Med Chem 52: 1853-63 (2009)
Article DOI: 10.1021/jm801317h
BindingDB Entry DOI: 10.7270/Q24M94FZ |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM19130

((2E,4E,6R)-7-[4-(dimethylamino)phenyl]-N-hydroxy-4...)Show SMILES CC(C=CC(=O)NO)C=C(C)C(=O)c1ccc(cc1)N(C)C |w:8.7,2.1| Show InChI InChI=1S/C17H22N2O3/c1-12(5-10-16(20)18-22)11-13(2)17(21)14-6-8-15(9-7-14)19(3)4/h5-12,22H,1-4H3,(H,18,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Inhibition of HDAC6 (unknown origin) after 17 hrs |
J Med Chem 51: 4370-3 (2008)
Article DOI: 10.1021/jm8002894
BindingDB Entry DOI: 10.7270/Q27W6C11 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Poly [ADP-ribose] polymerase tankyrase-2
(Homo sapiens (Human)) | BDBM191194
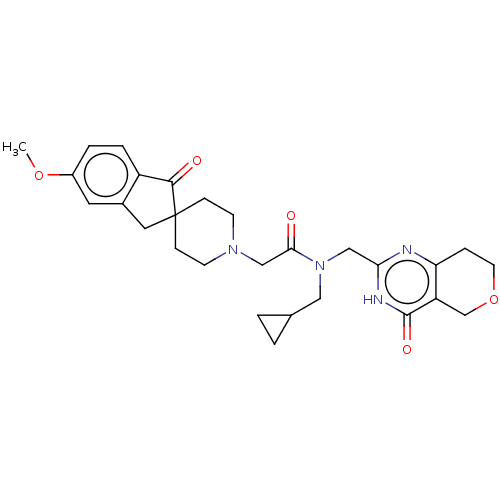
(US9181266, 63)Show SMILES COc1ccc2C(=O)C3(Cc2c1)CCN(CC(=O)N(CC1CC1)Cc1nc2CCOCc2c(=O)[nH]1)CC3 Show InChI InChI=1S/C28H34N4O5/c1-36-20-4-5-21-19(12-20)13-28(26(21)34)7-9-31(10-8-28)16-25(33)32(14-18-2-3-18)15-24-29-23-6-11-37-17-22(23)27(35)30-24/h4-5,12,18H,2-3,6-11,13-17H2,1H3,(H,29,30,35) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | 25 |
NOVARTIS AG
US Patent
| Assay Description
The autoparsylation activity of the TNKS 1/2 or PARP1/2 enzymes was measured by the liquid chromatography-mass spectrometry (LC/MS) detection of nico... |
US Patent US9181266 (2015)
BindingDB Entry DOI: 10.7270/Q20P0XTF |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50267802
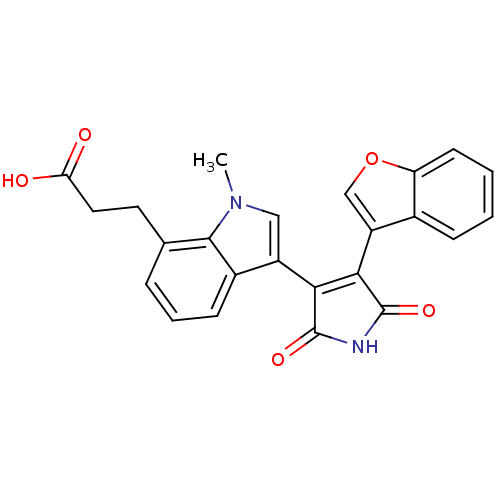
(3-[3-(4-Benzofuran-3-yl-2,5-dioxo-2,5-dihydro-1H-p...)Show SMILES Cn1cc(C2=C(C(=O)NC2=O)c2coc3ccccc23)c2cccc(CCC(O)=O)c12 |t:4| Show InChI InChI=1S/C24H18N2O5/c1-26-11-16(15-7-4-5-13(22(15)26)9-10-19(27)28)20-21(24(30)25-23(20)29)17-12-31-18-8-3-2-6-14(17)18/h2-8,11-12H,9-10H2,1H3,(H,27,28)(H,25,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Inhibition of human GSK3-beta by scintillation counting |
J Med Chem 52: 1853-63 (2009)
Article DOI: 10.1021/jm801317h
BindingDB Entry DOI: 10.7270/Q24M94FZ |
More data for this
Ligand-Target Pair | |
Histone deacetylase 8
(Homo sapiens (Human)) | BDBM19130

((2E,4E,6R)-7-[4-(dimethylamino)phenyl]-N-hydroxy-4...)Show SMILES CC(C=CC(=O)NO)C=C(C)C(=O)c1ccc(cc1)N(C)C |w:8.7,2.1| Show InChI InChI=1S/C17H22N2O3/c1-12(5-10-16(20)18-22)11-13(2)17(21)14-6-8-15(9-7-14)19(3)4/h5-12,22H,1-4H3,(H,18,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 1.38 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Inhibition of HDAC8 (unknown origin) after 17 hrs |
J Med Chem 51: 4370-3 (2008)
Article DOI: 10.1021/jm8002894
BindingDB Entry DOI: 10.7270/Q27W6C11 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Inhibition of CDK2/Cyclin A |
J Med Chem 52: 1853-63 (2009)
Article DOI: 10.1021/jm801317h
BindingDB Entry DOI: 10.7270/Q24M94FZ |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Histone deacetylase 3
(Homo sapiens (Human)) | BDBM19130

((2E,4E,6R)-7-[4-(dimethylamino)phenyl]-N-hydroxy-4...)Show SMILES CC(C=CC(=O)NO)C=C(C)C(=O)c1ccc(cc1)N(C)C |w:8.7,2.1| Show InChI InChI=1S/C17H22N2O3/c1-12(5-10-16(20)18-22)11-13(2)17(21)14-6-8-15(9-7-14)19(3)4/h5-12,22H,1-4H3,(H,18,20) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Inhibition of HDAC3 (unknown origin) after 17 hrs |
J Med Chem 51: 4370-3 (2008)
Article DOI: 10.1021/jm8002894
BindingDB Entry DOI: 10.7270/Q27W6C11 |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase tankyrase-2
(Homo sapiens (Human)) | BDBM191195
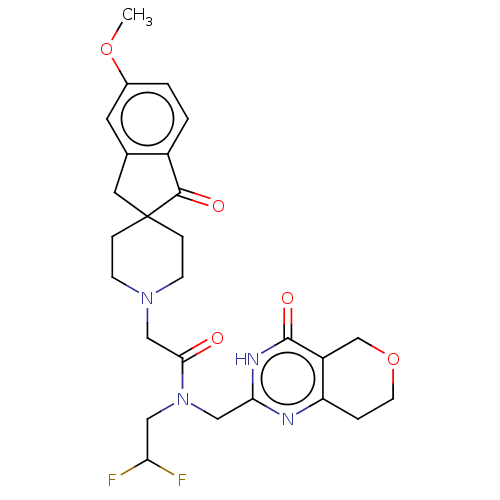
(US9181266, 64)Show SMILES COc1ccc2C(=O)C3(Cc2c1)CCN(CC(=O)N(CC(F)F)Cc1nc2CCOCc2c(=O)[nH]1)CC3 Show InChI InChI=1S/C26H30F2N4O5/c1-36-17-2-3-18-16(10-17)11-26(24(18)34)5-7-31(8-6-26)14-23(33)32(12-21(27)28)13-22-29-20-4-9-37-15-19(20)25(35)30-22/h2-3,10,21H,4-9,11-15H2,1H3,(H,29,30,35) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | 25 |
NOVARTIS AG
US Patent
| Assay Description
The autoparsylation activity of the TNKS 1/2 or PARP1/2 enzymes was measured by the liquid chromatography-mass spectrometry (LC/MS) detection of nico... |
US Patent US9181266 (2015)
BindingDB Entry DOI: 10.7270/Q20P0XTF |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50261753
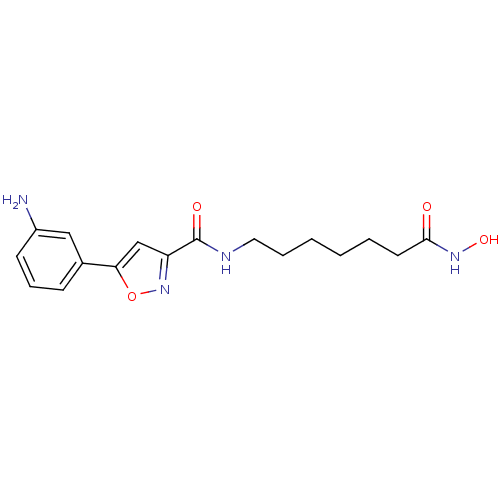
(5-(3-aminophenyl)-N-(7-(hydroxyamino)-7-oxoheptyl)...)Show InChI InChI=1S/C17H22N4O4/c18-13-7-5-6-12(10-13)15-11-14(21-25-15)17(23)19-9-4-2-1-3-8-16(22)20-24/h5-7,10-11,24H,1-4,8-9,18H2,(H,19,23)(H,20,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.54 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Inhibition of HDAC6 (unknown origin) after 17 hrs |
J Med Chem 51: 4370-3 (2008)
Article DOI: 10.1021/jm8002894
BindingDB Entry DOI: 10.7270/Q27W6C11 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50258646
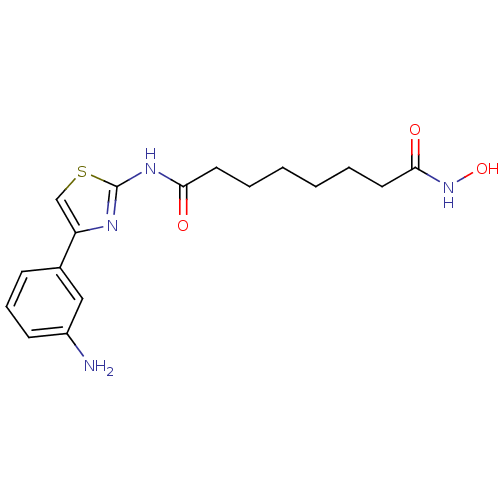
(CHEMBL468935 | N1-(4-(3-aminophenyl)thiazol-2-yl)-...)Show InChI InChI=1S/C17H22N4O3S/c18-13-7-5-6-12(10-13)14-11-25-17(19-14)20-15(22)8-3-1-2-4-9-16(23)21-24/h5-7,10-11,24H,1-4,8-9,18H2,(H,21,23)(H,19,20,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC6 |
Bioorg Med Chem Lett 19: 3023-6 (2009)
Article DOI: 10.1016/j.bmcl.2009.04.058
BindingDB Entry DOI: 10.7270/Q2833RXG |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50258645

(CHEMBL511212 | N1-hydroxy-N8-(4-phenylthiazol-2-yl...)Show InChI InChI=1S/C17H21N3O3S/c21-15(10-6-1-2-7-11-16(22)20-23)19-17-18-14(12-24-17)13-8-4-3-5-9-13/h3-5,8-9,12,23H,1-2,6-7,10-11H2,(H,20,22)(H,18,19,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC6 |
Bioorg Med Chem Lett 19: 3023-6 (2009)
Article DOI: 10.1016/j.bmcl.2009.04.058
BindingDB Entry DOI: 10.7270/Q2833RXG |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50258645

(CHEMBL511212 | N1-hydroxy-N8-(4-phenylthiazol-2-yl...)Show InChI InChI=1S/C17H21N3O3S/c21-15(10-6-1-2-7-11-16(22)20-23)19-17-18-14(12-24-17)13-8-4-3-5-9-13/h3-5,8-9,12,23H,1-2,6-7,10-11H2,(H,20,22)(H,18,19,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC1 |
Bioorg Med Chem Lett 19: 3023-6 (2009)
Article DOI: 10.1016/j.bmcl.2009.04.058
BindingDB Entry DOI: 10.7270/Q2833RXG |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase tankyrase-2
(Homo sapiens (Human)) | BDBM185805

(US9163003, 13 | USRE46942, Example 13)Show SMILES COc1ccc(cc1C)C(=O)C1CCN(CC1)[C@H]1CCN(Cc2nc3CCOCc3c(=O)[nH]2)C1=O |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | 25 |
NOVARTIS AG
US Patent
| Assay Description
The human tankyrase 1 PARP catalytic domain, TNKS1P, was cloned into a pDONR221 vector using the Invitrogen Gateway Technology. This entry clone was ... |
US Patent US9163003 (2015)
BindingDB Entry DOI: 10.7270/Q2QN65J6 |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase tankyrase-2
(Homo sapiens (Human)) | BDBM185805

(US9163003, 13 | USRE46942, Example 13)Show SMILES COc1ccc(cc1C)C(=O)C1CCN(CC1)[C@H]1CCN(Cc2nc3CCOCc3c(=O)[nH]2)C1=O |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG
US Patent
| Assay Description
The human tankyrase 1 PARP catalytic domain, TNKS1P, was cloned into a pDONR221 vector using the Invitrogen Gateway Technology. This entry clone was ... |
US Patent USRE46942 (2018)
BindingDB Entry DOI: 10.7270/Q2VX0JVH |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 1
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Inhibition of CDK1/Cyclin B |
J Med Chem 52: 1853-63 (2009)
Article DOI: 10.1021/jm801317h
BindingDB Entry DOI: 10.7270/Q24M94FZ |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 9
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Inhibition of CDK9/cyclin T1 |
J Med Chem 52: 1853-63 (2009)
Article DOI: 10.1021/jm801317h
BindingDB Entry DOI: 10.7270/Q24M94FZ |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50267520
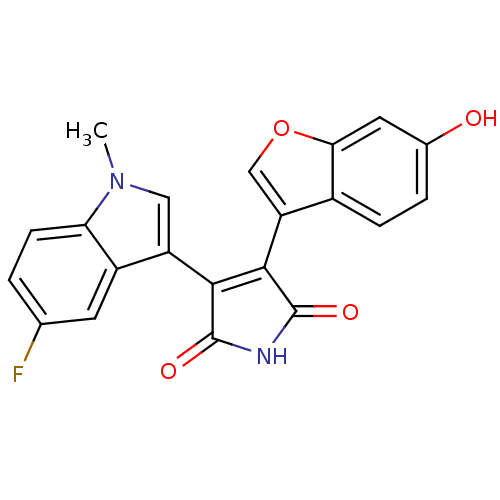
(3-(5-Fluoro-1-methyl-1H-indol-3-yl)-4-(6-hydroxybe...)Show SMILES Cn1cc(C2=C(C(=O)NC2=O)c2coc3cc(O)ccc23)c2cc(F)ccc12 |t:4| Show InChI InChI=1S/C21H13FN2O4/c1-24-8-14(13-6-10(22)2-5-16(13)24)18-19(21(27)23-20(18)26)15-9-28-17-7-11(25)3-4-12(15)17/h2-9,25H,1H3,(H,23,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Inhibition of human GSK3-beta by scintillation counting |
J Med Chem 52: 1853-63 (2009)
Article DOI: 10.1021/jm801317h
BindingDB Entry DOI: 10.7270/Q24M94FZ |
More data for this
Ligand-Target Pair | |
Protein Wnt-3a
(Homo sapiens (Human)) | BDBM50439791
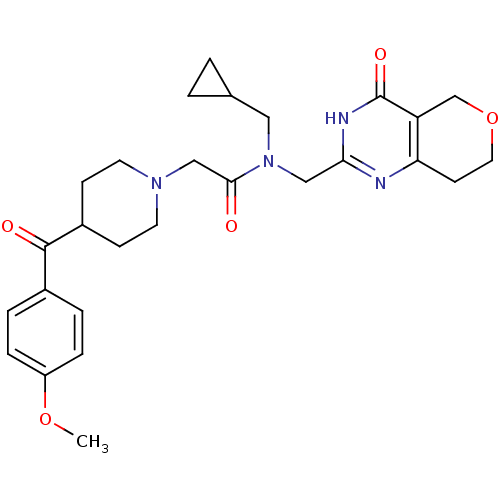
(CHEMBL2419706 | US9181266, 5)Show SMILES COc1ccc(cc1)C(=O)C1CCN(CC(=O)N(CC2CC2)Cc2nc3CCOCc3c(=O)[nH]2)CC1 Show InChI InChI=1S/C27H34N4O5/c1-35-21-6-4-19(5-7-21)26(33)20-8-11-30(12-9-20)16-25(32)31(14-18-2-3-18)15-24-28-23-10-13-36-17-22(23)27(34)29-24/h4-7,18,20H,2-3,8-17H2,1H3,(H,28,29,34) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of WNT3A signaling in HEK293 cells by luciferase reporter gene assay in presence of forskolin |
J Med Chem 56: 6495-511 (2013)
Article DOI: 10.1021/jm400807n
BindingDB Entry DOI: 10.7270/Q24Q7WFB |
More data for this
Ligand-Target Pair | |
Protein Wnt-3a
(Homo sapiens (Human)) | BDBM50439799
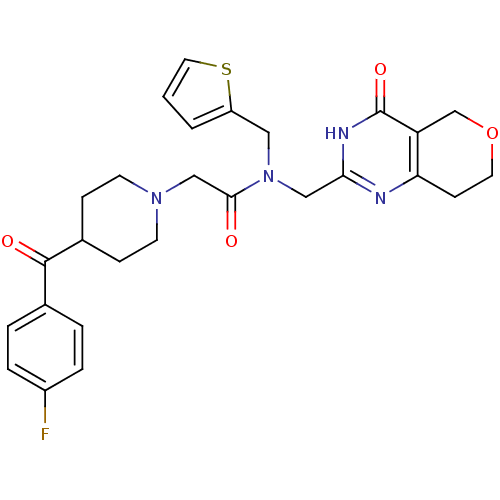
(CHEMBL2419698 | US9181266, 13)Show SMILES Fc1ccc(cc1)C(=O)C1CCN(CC(=O)N(Cc2cccs2)Cc2nc3CCOCc3c(=O)[nH]2)CC1 Show InChI InChI=1S/C27H29FN4O4S/c28-20-5-3-18(4-6-20)26(34)19-7-10-31(11-8-19)16-25(33)32(14-21-2-1-13-37-21)15-24-29-23-9-12-36-17-22(23)27(35)30-24/h1-6,13,19H,7-12,14-17H2,(H,29,30,35) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of WNT3A signaling in HEK293 cells by luciferase reporter gene assay in presence of forskolin |
J Med Chem 56: 6495-511 (2013)
Article DOI: 10.1021/jm400807n
BindingDB Entry DOI: 10.7270/Q24Q7WFB |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 5
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Inhibition of CDK5/P35 |
J Med Chem 52: 1853-63 (2009)
Article DOI: 10.1021/jm801317h
BindingDB Entry DOI: 10.7270/Q24M94FZ |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Inhibition of CDK2/Cyclin E (unknown origin) |
J Med Chem 52: 1853-63 (2009)
Article DOI: 10.1021/jm801317h
BindingDB Entry DOI: 10.7270/Q24M94FZ |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50261752
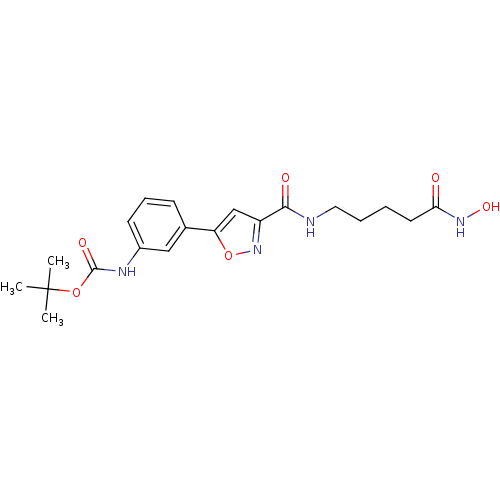
(CHEMBL469275 | tert-butyl 3-(3-((5-(hydroxyamino)-...)Show SMILES CC(C)(C)OC(=O)Nc1cccc(c1)-c1cc(no1)C(=O)NCCCCC(=O)NO Show InChI InChI=1S/C20H26N4O6/c1-20(2,3)29-19(27)22-14-8-6-7-13(11-14)16-12-15(24-30-16)18(26)21-10-5-4-9-17(25)23-28/h6-8,11-12,28H,4-5,9-10H2,1-3H3,(H,21,26)(H,22,27)(H,23,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.98 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Inhibition of HDAC6 (unknown origin) after 17 hrs |
J Med Chem 51: 4370-3 (2008)
Article DOI: 10.1021/jm8002894
BindingDB Entry DOI: 10.7270/Q27W6C11 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM19130

((2E,4E,6R)-7-[4-(dimethylamino)phenyl]-N-hydroxy-4...)Show SMILES CC(C=CC(=O)NO)C=C(C)C(=O)c1ccc(cc1)N(C)C |w:8.7,2.1| Show InChI InChI=1S/C17H22N2O3/c1-12(5-10-16(20)18-22)11-13(2)17(21)14-6-8-15(9-7-14)19(3)4/h5-12,22H,1-4H3,(H,18,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 (unknown origin) after 17 hrs |
J Med Chem 51: 4370-3 (2008)
Article DOI: 10.1021/jm8002894
BindingDB Entry DOI: 10.7270/Q27W6C11 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50258646

(CHEMBL468935 | N1-(4-(3-aminophenyl)thiazol-2-yl)-...)Show InChI InChI=1S/C17H22N4O3S/c18-13-7-5-6-12(10-13)14-11-25-17(19-14)20-15(22)8-3-1-2-4-9-16(23)21-24/h5-7,10-11,24H,1-4,8-9,18H2,(H,21,23)(H,19,20,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC1 |
Bioorg Med Chem Lett 19: 3023-6 (2009)
Article DOI: 10.1016/j.bmcl.2009.04.058
BindingDB Entry DOI: 10.7270/Q2833RXG |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 5
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Inhibition of CDK5/P25 (unknown origin) |
J Med Chem 52: 1853-63 (2009)
Article DOI: 10.1021/jm801317h
BindingDB Entry DOI: 10.7270/Q24M94FZ |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase tankyrase-2
(Homo sapiens (Human)) | BDBM191193
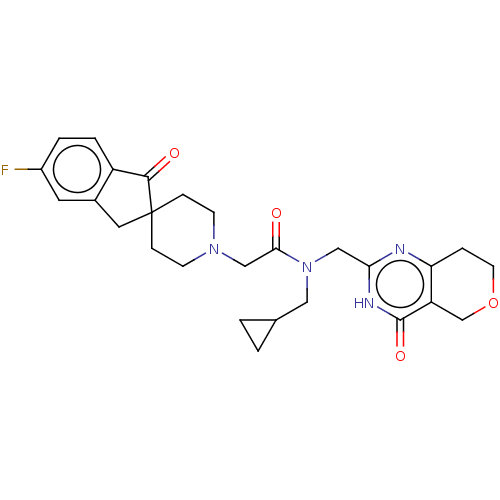
(US9181266, 62)Show SMILES Fc1ccc2C(=O)C3(Cc2c1)CCN(CC(=O)N(CC1CC1)Cc1nc2CCOCc2c(=O)[nH]1)CC3 Show InChI InChI=1S/C27H31FN4O4/c28-19-3-4-20-18(11-19)12-27(25(20)34)6-8-31(9-7-27)15-24(33)32(13-17-1-2-17)14-23-29-22-5-10-36-16-21(22)26(35)30-23/h3-4,11,17H,1-2,5-10,12-16H2,(H,29,30,35) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | 25 |
NOVARTIS AG
US Patent
| Assay Description
The autoparsylation activity of the TNKS 1/2 or PARP1/2 enzymes was measured by the liquid chromatography-mass spectrometry (LC/MS) detection of nico... |
US Patent US9181266 (2015)
BindingDB Entry DOI: 10.7270/Q20P0XTF |
More data for this
Ligand-Target Pair | |
Histone deacetylase 3
(Homo sapiens (Human)) | BDBM50261753

(5-(3-aminophenyl)-N-(7-(hydroxyamino)-7-oxoheptyl)...)Show InChI InChI=1S/C17H22N4O4/c18-13-7-5-6-12(10-13)15-11-14(21-25-15)17(23)19-9-4-2-1-3-8-16(22)20-24/h5-7,10-11,24H,1-4,8-9,18H2,(H,19,23)(H,20,22) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.04 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Inhibition of HDAC3 (unknown origin) after 17 hrs |
J Med Chem 51: 4370-3 (2008)
Article DOI: 10.1021/jm8002894
BindingDB Entry DOI: 10.7270/Q27W6C11 |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase tankyrase-2
(Homo sapiens (Human)) | BDBM404370
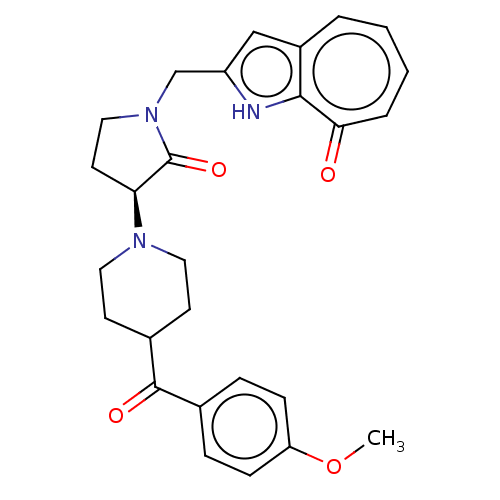
(2-{(S)-3-[4-(4-Methoxy-benzoyl)-piperidin-1-yl]-2-...)Show SMILES COc1ccc(cc1)C(=O)C1CCN(CC1)[C@H]1CCN(Cc2cc3ccccc(=O)c3[nH]2)C1=O |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG
US Patent
| Assay Description
The human tankyrase 1 PARP catalytic domain, TNKS1P, was cloned into a pDONR221 vector using the Invitrogen Gateway Technology. This entry clone was ... |
US Patent USRE46942 (2018)
BindingDB Entry DOI: 10.7270/Q2VX0JVH |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase tankyrase-2
(Homo sapiens (Human)) | BDBM50318567
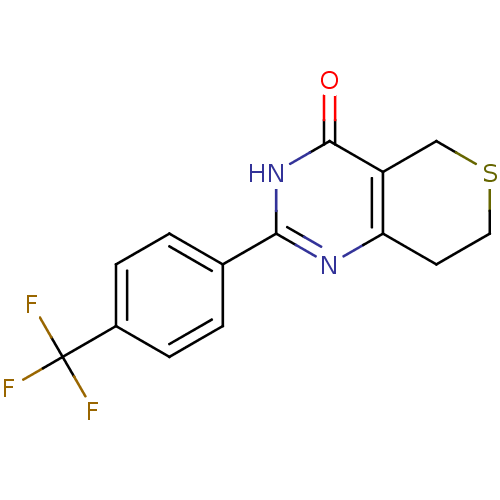
(2-(4-(Trifluoromethyl)phenyl)-7,8-dihydro-5H-thiop...)Show InChI InChI=1S/C14H11F3N2OS/c15-14(16,17)9-3-1-8(2-4-9)12-18-11-5-6-21-7-10(11)13(20)19-12/h1-4H,5-7H2,(H,18,19,20) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of GST-tagged TNKS2P catalytic domain autoPARsylation measuring nicotinamide concentration after 2 hrs by LC-MS analysis |
J Med Chem 55: 1127-36 (2012)
Article DOI: 10.1021/jm2011222
BindingDB Entry DOI: 10.7270/Q2BK1DCV |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Poly [ADP-ribose] polymerase tankyrase-2
(Homo sapiens (Human)) | BDBM185818

(US9163003, 30)Show SMILES COc1ccc(cc1)C(=O)C1CCN(CC1)[C@H]1CCN(Cc2nc3ccccc(=O)c3[nH]2)C1=O |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | 25 |
NOVARTIS AG
US Patent
| Assay Description
The human tankyrase 1 PARP catalytic domain, TNKS1P, was cloned into a pDONR221 vector using the Invitrogen Gateway Technology. This entry clone was ... |
US Patent US9163003 (2015)
BindingDB Entry DOI: 10.7270/Q2QN65J6 |
More data for this
Ligand-Target Pair | |
Polyamine deacetylase HDAC10
(Homo sapiens (Human)) | BDBM19130

((2E,4E,6R)-7-[4-(dimethylamino)phenyl]-N-hydroxy-4...)Show SMILES CC(C=CC(=O)NO)C=C(C)C(=O)c1ccc(cc1)N(C)C |w:8.7,2.1| Show InChI InChI=1S/C17H22N2O3/c1-12(5-10-16(20)18-22)11-13(2)17(21)14-6-8-15(9-7-14)19(3)4/h5-12,22H,1-4H3,(H,18,20) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Inhibition of HDAC10 (unknown origin) after 17 hrs |
J Med Chem 51: 4370-3 (2008)
Article DOI: 10.1021/jm8002894
BindingDB Entry DOI: 10.7270/Q27W6C11 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50258647
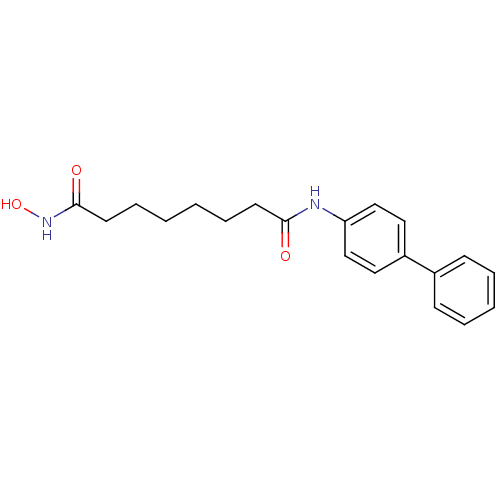
(CHEMBL512644 | N1-(biphenyl-4-yl)-N8-hydroxyoctane...)Show InChI InChI=1S/C20H24N2O3/c23-19(10-6-1-2-7-11-20(24)22-25)21-18-14-12-17(13-15-18)16-8-4-3-5-9-16/h3-5,8-9,12-15,25H,1-2,6-7,10-11H2,(H,21,23)(H,22,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC6 |
Bioorg Med Chem Lett 19: 3023-6 (2009)
Article DOI: 10.1016/j.bmcl.2009.04.058
BindingDB Entry DOI: 10.7270/Q2833RXG |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase tankyrase-2
(Homo sapiens (Human)) | BDBM191199
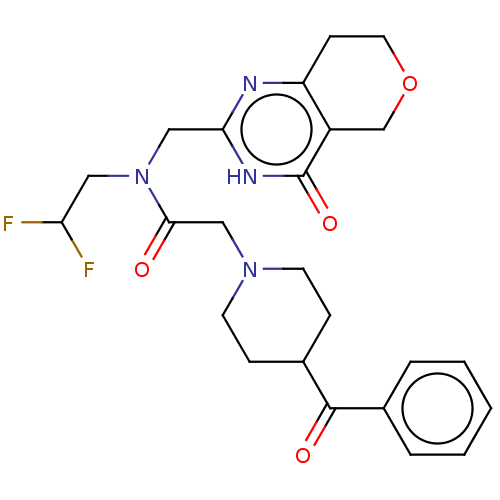
(US9181266, 68)Show SMILES FC(F)CN(Cc1nc2CCOCc2c(=O)[nH]1)C(=O)CN1CCC(CC1)C(=O)c1ccccc1 Show InChI InChI=1S/C24H28F2N4O4/c25-20(26)12-30(13-21-27-19-8-11-34-15-18(19)24(33)28-21)22(31)14-29-9-6-17(7-10-29)23(32)16-4-2-1-3-5-16/h1-5,17,20H,6-15H2,(H,27,28,33) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | 25 |
NOVARTIS AG
US Patent
| Assay Description
The autoparsylation activity of the TNKS 1/2 or PARP1/2 enzymes was measured by the liquid chromatography-mass spectrometry (LC/MS) detection of nico... |
US Patent US9181266 (2015)
BindingDB Entry DOI: 10.7270/Q20P0XTF |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50267759
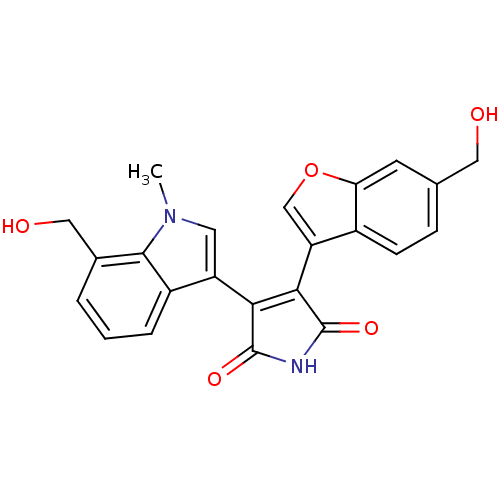
(3-(6-Hydroxymethylbenzofuran-3-yl)-4-(7-hydroxymet...)Show SMILES Cn1cc(C2=C(C(=O)NC2=O)c2coc3cc(CO)ccc23)c2cccc(CO)c12 |t:4| Show InChI InChI=1S/C23H18N2O5/c1-25-8-16(15-4-2-3-13(10-27)21(15)25)19-20(23(29)24-22(19)28)17-11-30-18-7-12(9-26)5-6-14(17)18/h2-8,11,26-27H,9-10H2,1H3,(H,24,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.10 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Inhibition of human GSK3-beta by scintillation counting |
J Med Chem 52: 1853-63 (2009)
Article DOI: 10.1021/jm801317h
BindingDB Entry DOI: 10.7270/Q24M94FZ |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50267758

(3-Benzofuran-3-yl-4-(7-hydroxymethyl-1-methyl-1H-i...)Show SMILES Cn1cc(C2=C(C(=O)NC2=O)c2coc3ccccc23)c2cccc(CO)c12 |t:4| Show InChI InChI=1S/C22H16N2O4/c1-24-9-15(14-7-4-5-12(10-25)20(14)24)18-19(22(27)23-21(18)26)16-11-28-17-8-3-2-6-13(16)17/h2-9,11,25H,10H2,1H3,(H,23,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.40 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Inhibition of human GSK3-beta by scintillation counting |
J Med Chem 52: 1853-63 (2009)
Article DOI: 10.1021/jm801317h
BindingDB Entry DOI: 10.7270/Q24M94FZ |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50261818
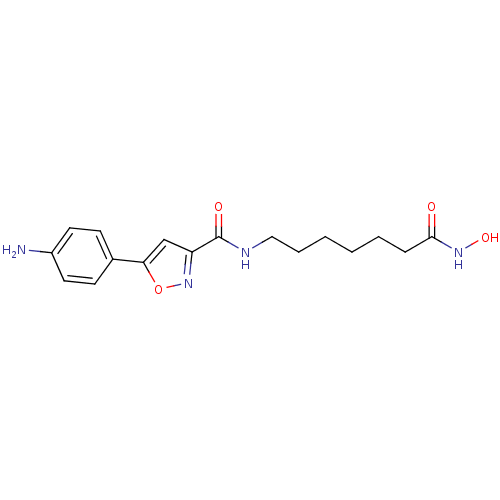
(5-(4-aminophenyl)-N-(7-(hydroxyamino)-7-oxoheptyl)...)Show InChI InChI=1S/C17H22N4O4/c18-13-8-6-12(7-9-13)15-11-14(21-25-15)17(23)19-10-4-2-1-3-5-16(22)20-24/h6-9,11,24H,1-5,10,18H2,(H,19,23)(H,20,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.86 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Inhibition of HDAC6 (unknown origin) after 17 hrs |
J Med Chem 51: 4370-3 (2008)
Article DOI: 10.1021/jm8002894
BindingDB Entry DOI: 10.7270/Q27W6C11 |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase tankyrase-2
(Homo sapiens (Human)) | BDBM185802

(US9163003, 10 | USRE46942, Example 10)Show SMILES Fc1ccc(cc1)C(=O)C1CCN(CC1)C1CCN(Cc2nc3CCOCc3c(=O)[nH]2)C1=O | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG
US Patent
| Assay Description
The human tankyrase 1 PARP catalytic domain, TNKS1P, was cloned into a pDONR221 vector using the Invitrogen Gateway Technology. This entry clone was ... |
US Patent USRE46942 (2018)
BindingDB Entry DOI: 10.7270/Q2VX0JVH |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase tankyrase-1
(Homo sapiens (Human)) | BDBM185805

(US9163003, 13 | USRE46942, Example 13)Show SMILES COc1ccc(cc1C)C(=O)C1CCN(CC1)[C@H]1CCN(Cc2nc3CCOCc3c(=O)[nH]2)C1=O |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG
US Patent
| Assay Description
The human tankyrase 1 PARP catalytic domain, TNKS1P, was cloned into a pDONR221 vector using the Invitrogen Gateway Technology. This entry clone was ... |
US Patent USRE46942 (2018)
BindingDB Entry DOI: 10.7270/Q2VX0JVH |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase tankyrase-2
(Homo sapiens (Human)) | BDBM185802

(US9163003, 10 | USRE46942, Example 10)Show SMILES Fc1ccc(cc1)C(=O)C1CCN(CC1)C1CCN(Cc2nc3CCOCc3c(=O)[nH]2)C1=O | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | 25 |
NOVARTIS AG
US Patent
| Assay Description
The human tankyrase 1 PARP catalytic domain, TNKS1P, was cloned into a pDONR221 vector using the Invitrogen Gateway Technology. This entry clone was ... |
US Patent US9163003 (2015)
BindingDB Entry DOI: 10.7270/Q2QN65J6 |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase tankyrase-1
(Homo sapiens (Human)) | BDBM185805

(US9163003, 13 | USRE46942, Example 13)Show SMILES COc1ccc(cc1C)C(=O)C1CCN(CC1)[C@H]1CCN(Cc2nc3CCOCc3c(=O)[nH]2)C1=O |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | 25 |
NOVARTIS AG
US Patent
| Assay Description
The human tankyrase 1 PARP catalytic domain, TNKS1P, was cloned into a pDONR221 vector using the Invitrogen Gateway Technology. This entry clone was ... |
US Patent US9163003 (2015)
BindingDB Entry DOI: 10.7270/Q2QN65J6 |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase tankyrase-2
(Homo sapiens (Human)) | BDBM191219
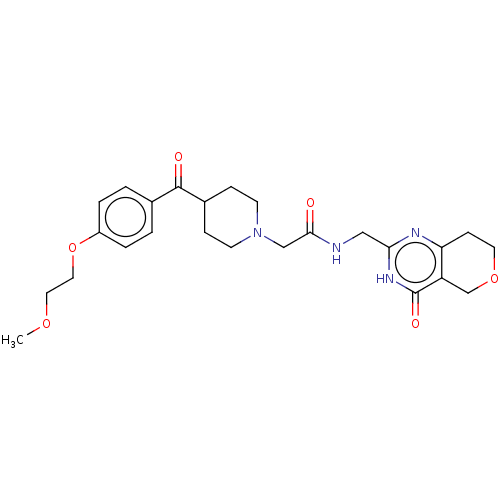
(US9181266, 88)Show SMILES COCCOc1ccc(cc1)C(=O)C1CCN(CC(=O)NCc2nc3CCOCc3c(=O)[nH]2)CC1 Show InChI InChI=1S/C25H32N4O6/c1-33-12-13-35-19-4-2-17(3-5-19)24(31)18-6-9-29(10-7-18)15-23(30)26-14-22-27-21-8-11-34-16-20(21)25(32)28-22/h2-5,18H,6-16H2,1H3,(H,26,30)(H,27,28,32) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | 25 |
NOVARTIS AG
US Patent
| Assay Description
The autoparsylation activity of the TNKS 1/2 or PARP1/2 enzymes was measured by the liquid chromatography-mass spectrometry (LC/MS) detection of nico... |
US Patent US9181266 (2015)
BindingDB Entry DOI: 10.7270/Q20P0XTF |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase tankyrase-2
(Homo sapiens (Human)) | BDBM50439791

(CHEMBL2419706 | US9181266, 5)Show SMILES COc1ccc(cc1)C(=O)C1CCN(CC(=O)N(CC2CC2)Cc2nc3CCOCc3c(=O)[nH]2)CC1 Show InChI InChI=1S/C27H34N4O5/c1-35-21-6-4-19(5-7-21)26(33)20-8-11-30(12-9-20)16-25(32)31(14-18-2-3-18)15-24-28-23-10-13-36-17-22(23)27(34)29-24/h4-7,18,20H,2-3,8-17H2,1H3,(H,28,29,34) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | 25 |
NOVARTIS AG
US Patent
| Assay Description
The autoparsylation activity of the TNKS 1/2 or PARP1/2 enzymes was measured by the liquid chromatography-mass spectrometry (LC/MS) detection of nico... |
US Patent US9181266 (2015)
BindingDB Entry DOI: 10.7270/Q20P0XTF |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data