

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50568146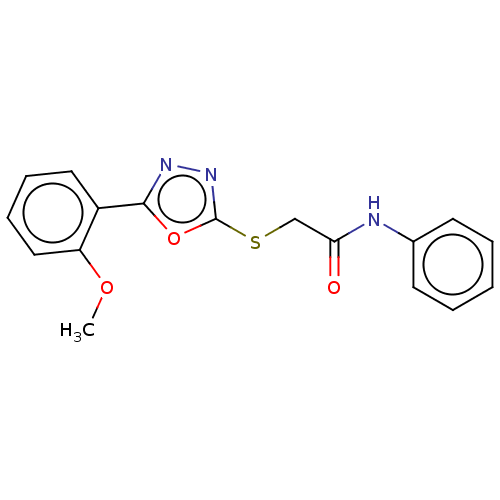 (CHEMBL1481541) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Noncompetitive inhibition of mushroom tyrosinase assessed as dissociation constant using L-DOPA as substrate by Lineweaver-Burk plot analysis | Citation and Details Article DOI: 10.1016/j.bmc.2021.116222 BindingDB Entry DOI: 10.7270/Q24171T1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM250432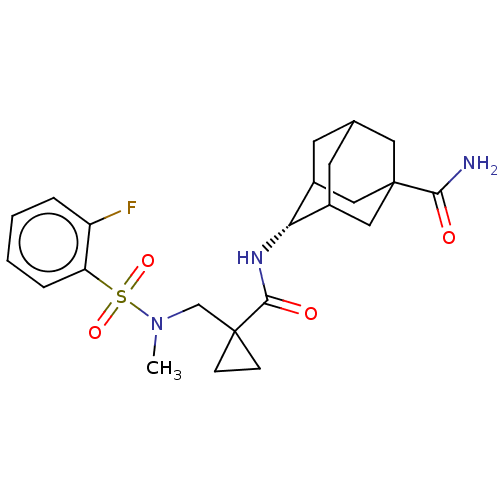 (US9464044, 22) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.0200 | n/a | n/a | n/a | n/a | 6.0 | 25 |
AHN-GOOK PHARMACEUTICAL CO., LTD.; BAMICHEM CO., LTD; INCHEON UNIVERSITY INDUSTRY ACADEMIC COOPERATION FOUNDATION US Patent | Assay Description The inhibiting activity of 11β-HSD1 derived from microsomal fractions was measured using the HTRF assay (62CO2PEB, Cisbio). Different concentrat... | US Patent US9464044 (2016) BindingDB Entry DOI: 10.7270/Q2SJ1JH0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM250460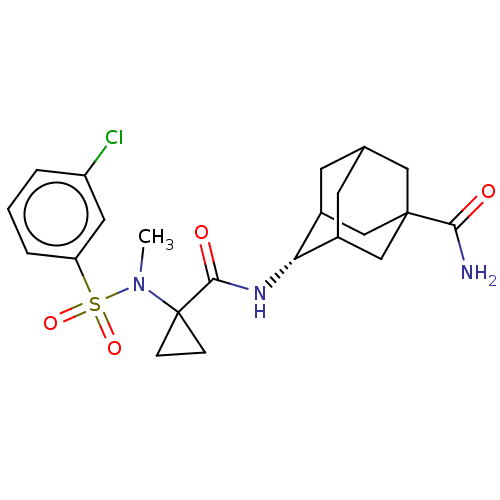 (US9464044, 62) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.120 | n/a | n/a | n/a | n/a | 6.0 | 25 |
AHN-GOOK PHARMACEUTICAL CO., LTD.; BAMICHEM CO., LTD; INCHEON UNIVERSITY INDUSTRY ACADEMIC COOPERATION FOUNDATION US Patent | Assay Description The inhibiting activity of 11β-HSD1 derived from microsomal fractions was measured using the HTRF assay (62CO2PEB, Cisbio). Different concentrat... | US Patent US9464044 (2016) BindingDB Entry DOI: 10.7270/Q2SJ1JH0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM250418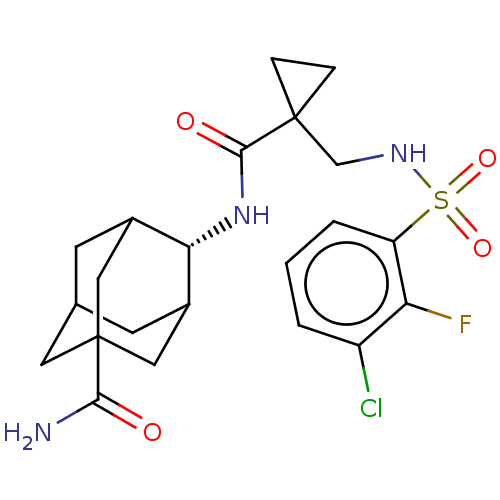 (US9464044, 8) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | 6.0 | 25 |
AHN-GOOK PHARMACEUTICAL CO., LTD.; BAMICHEM CO., LTD; INCHEON UNIVERSITY INDUSTRY ACADEMIC COOPERATION FOUNDATION US Patent | Assay Description The inhibiting activity of 11β-HSD1 derived from microsomal fractions was measured using the HTRF assay (62CO2PEB, Cisbio). Different concentrat... | US Patent US9464044 (2016) BindingDB Entry DOI: 10.7270/Q2SJ1JH0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Focal adhesion kinase 1 (Homo sapiens (Human)) | BDBM155552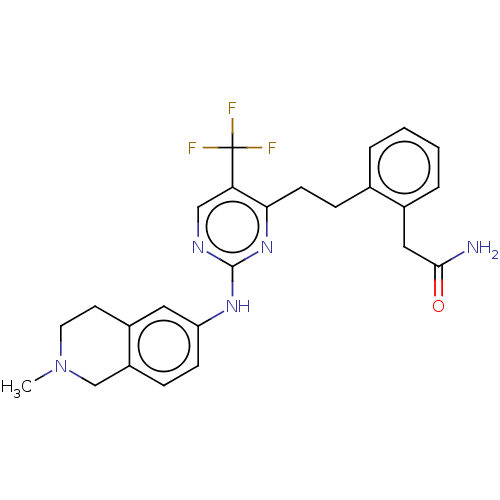 (US9012461, 28) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Cancer Therapeutics CRC Pty Ltd US Patent | Assay Description A biotin labeled peptide is used as substrate (amino acid sequence: Biotin-Glu-Gly-Pro-Trp-Leu-Glu-Glu-Glu-Glu-Glu-Ala-Tyr-Gly-Trp-Met-Asp-Phe-NH2)1S... | US Patent US9012461 (2015) BindingDB Entry DOI: 10.7270/Q2RN36K7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM50112152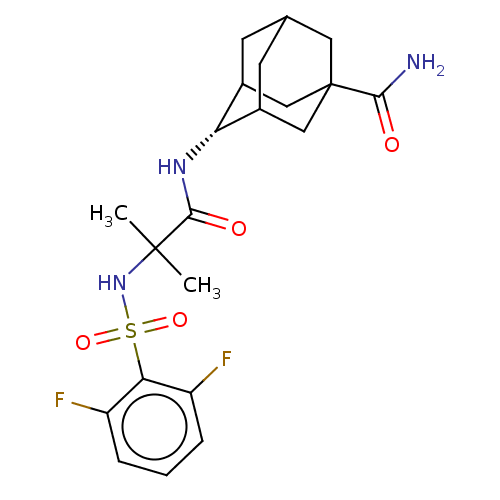 (CHEMBL3608401 | US9464044, 84) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.360 | n/a | n/a | n/a | n/a | 6.0 | 25 |
AHN-GOOK PHARMACEUTICAL CO., LTD.; BAMICHEM CO., LTD; INCHEON UNIVERSITY INDUSTRY ACADEMIC COOPERATION FOUNDATION US Patent | Assay Description The inhibiting activity of 11β-HSD1 derived from microsomal fractions was measured using the HTRF assay (62CO2PEB, Cisbio). Different concentrat... | US Patent US9464044 (2016) BindingDB Entry DOI: 10.7270/Q2SJ1JH0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Focal adhesion kinase 1 (Homo sapiens (Human)) | BDBM155547 (US9012461, 23) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.390 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Cancer Therapeutics CRC Pty Ltd US Patent | Assay Description A biotin labeled peptide is used as substrate (amino acid sequence: Biotin-Glu-Gly-Pro-Trp-Leu-Glu-Glu-Glu-Glu-Glu-Ala-Tyr-Gly-Trp-Met-Asp-Phe-NH2)1S... | US Patent US9012461 (2015) BindingDB Entry DOI: 10.7270/Q2RN36K7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM250453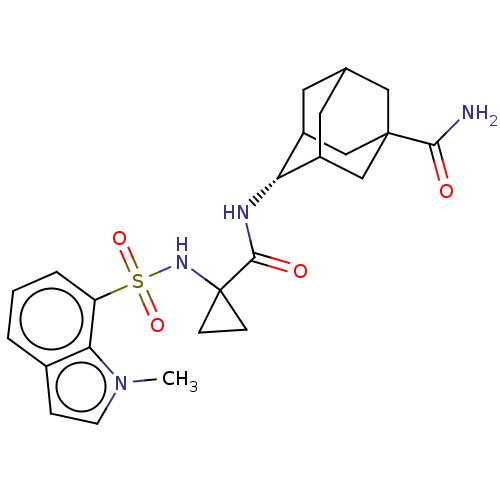 (US9464044, 50) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | 6.0 | 25 |
AHN-GOOK PHARMACEUTICAL CO., LTD.; BAMICHEM CO., LTD; INCHEON UNIVERSITY INDUSTRY ACADEMIC COOPERATION FOUNDATION US Patent | Assay Description The inhibiting activity of 11β-HSD1 derived from microsomal fractions was measured using the HTRF assay (62CO2PEB, Cisbio). Different concentrat... | US Patent US9464044 (2016) BindingDB Entry DOI: 10.7270/Q2SJ1JH0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM50112133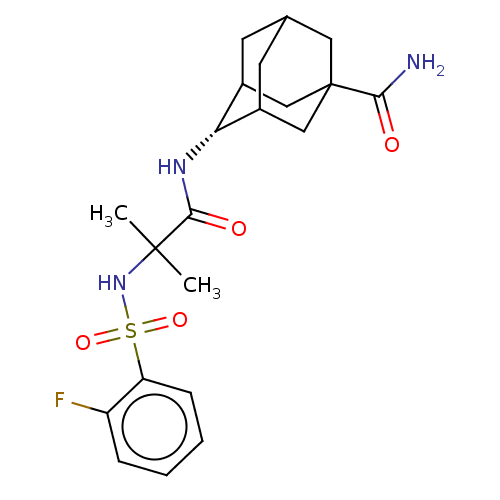 (CHEMBL3609861 | US9464044, 71) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | 6.0 | 25 |
AHN-GOOK PHARMACEUTICAL CO., LTD.; BAMICHEM CO., LTD; INCHEON UNIVERSITY INDUSTRY ACADEMIC COOPERATION FOUNDATION US Patent | Assay Description The inhibiting activity of 11β-HSD1 derived from microsomal fractions was measured using the HTRF assay (62CO2PEB, Cisbio). Different concentrat... | US Patent US9464044 (2016) BindingDB Entry DOI: 10.7270/Q2SJ1JH0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Focal adhesion kinase 1 (Homo sapiens (Human)) | BDBM155537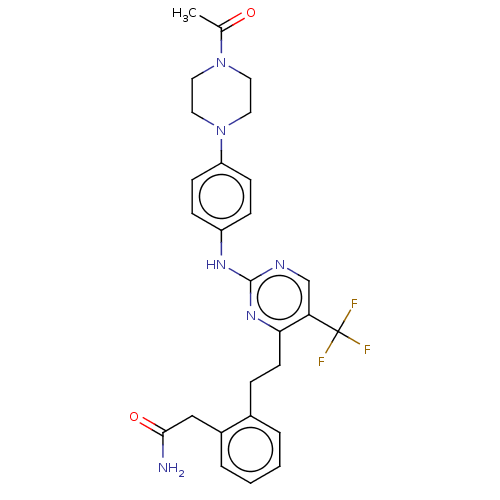 (US9012461, 13) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Cancer Therapeutics CRC Pty Ltd US Patent | Assay Description A biotin labeled peptide is used as substrate (amino acid sequence: Biotin-Glu-Gly-Pro-Trp-Leu-Glu-Glu-Glu-Glu-Glu-Ala-Tyr-Gly-Trp-Met-Asp-Phe-NH2)1S... | US Patent US9012461 (2015) BindingDB Entry DOI: 10.7270/Q2RN36K7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Bos taurus) | BDBM50304788 (CHEMBL594160 | methyl 4-(3-chloro-4-methoxybenzyla...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Chong Kun Dang Research Institute Curated by ChEMBL | Assay Description Inhibition of bovine platelet PDE5 | Bioorg Med Chem Lett 20: 383-6 (2010) Article DOI: 10.1016/j.bmcl.2009.10.071 BindingDB Entry DOI: 10.7270/Q28P60KW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Focal adhesion kinase 1 (Homo sapiens (Human)) | BDBM155548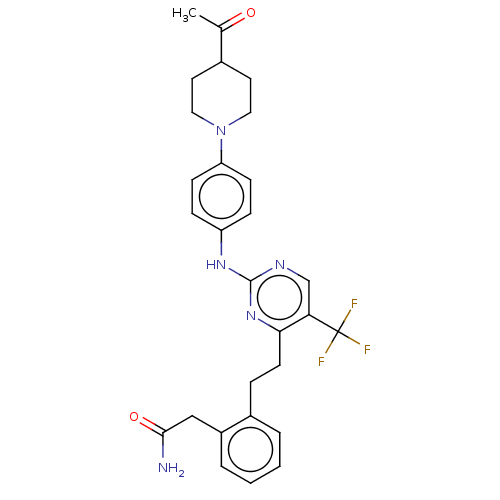 (US9012461, 24) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.770 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Cancer Therapeutics CRC Pty Ltd US Patent | Assay Description A biotin labeled peptide is used as substrate (amino acid sequence: Biotin-Glu-Gly-Pro-Trp-Leu-Glu-Glu-Glu-Glu-Glu-Ala-Tyr-Gly-Trp-Met-Asp-Phe-NH2)1S... | US Patent US9012461 (2015) BindingDB Entry DOI: 10.7270/Q2RN36K7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Focal adhesion kinase 1 (Homo sapiens (Human)) | BDBM155544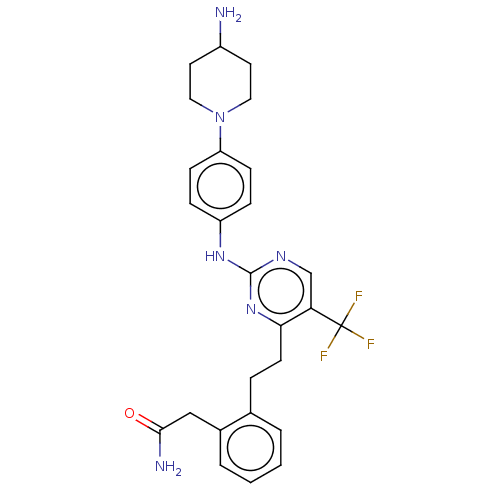 (US9012461, 20) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.810 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Cancer Therapeutics CRC Pty Ltd US Patent | Assay Description A biotin labeled peptide is used as substrate (amino acid sequence: Biotin-Glu-Gly-Pro-Trp-Leu-Glu-Glu-Glu-Glu-Glu-Ala-Tyr-Gly-Trp-Met-Asp-Phe-NH2)1S... | US Patent US9012461 (2015) BindingDB Entry DOI: 10.7270/Q2RN36K7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM50112143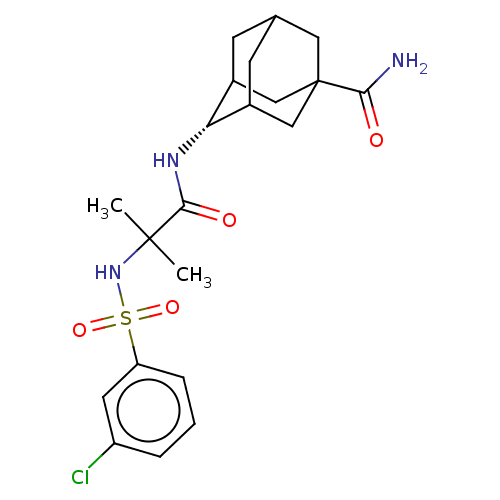 (CHEMBL3609871 | US9464044, 73) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.830 | n/a | n/a | n/a | n/a | 6.0 | 25 |
AHN-GOOK PHARMACEUTICAL CO., LTD.; BAMICHEM CO., LTD; INCHEON UNIVERSITY INDUSTRY ACADEMIC COOPERATION FOUNDATION US Patent | Assay Description The inhibiting activity of 11β-HSD1 derived from microsomal fractions was measured using the HTRF assay (62CO2PEB, Cisbio). Different concentrat... | US Patent US9464044 (2016) BindingDB Entry DOI: 10.7270/Q2SJ1JH0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM250486 (US9464044, 119) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | 6.0 | 25 |
AHN-GOOK PHARMACEUTICAL CO., LTD.; BAMICHEM CO., LTD; INCHEON UNIVERSITY INDUSTRY ACADEMIC COOPERATION FOUNDATION US Patent | Assay Description The inhibiting activity of 11β-HSD1 derived from microsomal fractions was measured using the HTRF assay (62CO2PEB, Cisbio). Different concentrat... | US Patent US9464044 (2016) BindingDB Entry DOI: 10.7270/Q2SJ1JH0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Bos taurus) | BDBM50304792 (CHEMBL595104 | N-(4-(3-chloro-4-methoxybenzylamino...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Chong Kun Dang Research Institute Curated by ChEMBL | Assay Description Inhibition of bovine platelet PDE5 | Bioorg Med Chem Lett 20: 383-6 (2010) Article DOI: 10.1016/j.bmcl.2009.10.071 BindingDB Entry DOI: 10.7270/Q28P60KW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Bos taurus) | BDBM50246252 (CHEMBL521075 | N-(4-(3-chloro-4-methoxybenzylamino...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Chong Kun Dang Research Institute Curated by ChEMBL | Assay Description Inhibition of bovine platelet PDE5 | Bioorg Med Chem Lett 20: 383-6 (2010) Article DOI: 10.1016/j.bmcl.2009.10.071 BindingDB Entry DOI: 10.7270/Q28P60KW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Bos taurus) | BDBM50246252 (CHEMBL521075 | N-(4-(3-chloro-4-methoxybenzylamino...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Chong Kun Dang Research Institute Curated by ChEMBL | Assay Description Inhibition of bovine PDE5 | Bioorg Med Chem Lett 18: 6279-82 (2008) Article DOI: 10.1016/j.bmcl.2008.09.108 BindingDB Entry DOI: 10.7270/Q2SB45MV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Bos taurus) | BDBM50304790 (CHEMBL595330 | N-(4-(3-chloro-4-methoxybenzylamino...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Chong Kun Dang Research Institute Curated by ChEMBL | Assay Description Inhibition of bovine platelet PDE5 | Bioorg Med Chem Lett 20: 383-6 (2010) Article DOI: 10.1016/j.bmcl.2009.10.071 BindingDB Entry DOI: 10.7270/Q28P60KW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Bos taurus) | BDBM50304789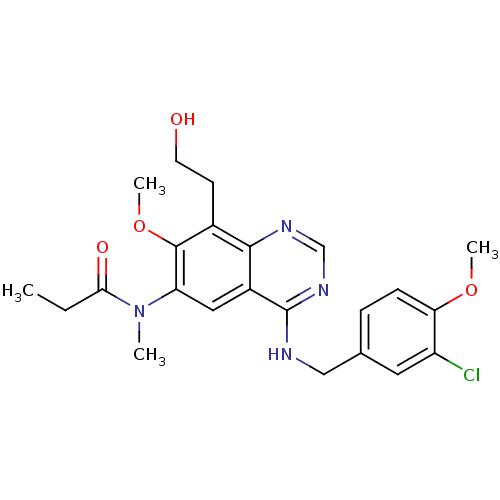 (CHEMBL604271 | N-(4-(3-chloro-4-methoxybenzylamino...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Chong Kun Dang Research Institute Curated by ChEMBL | Assay Description Inhibition of bovine platelet PDE5 | Bioorg Med Chem Lett 20: 383-6 (2010) Article DOI: 10.1016/j.bmcl.2009.10.071 BindingDB Entry DOI: 10.7270/Q28P60KW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Focal adhesion kinase 1 (Homo sapiens (Human)) | BDBM155527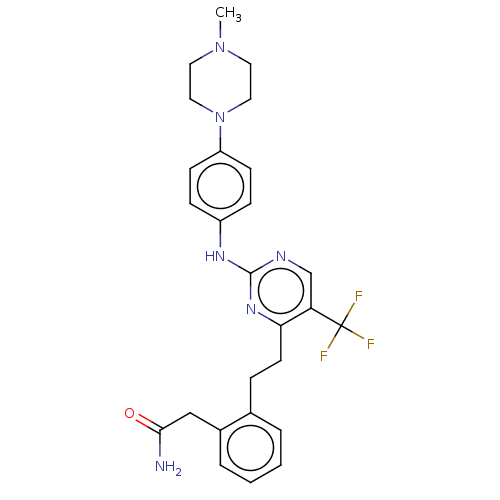 (US9012461, 3) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Cancer Therapeutics CRC Pty Ltd US Patent | Assay Description Compounds of the invention may be tested for in vitro activity in the following assay: A biotin labeled peptide is used as substrate (amino acid sequ... | US Patent US9012461 (2015) BindingDB Entry DOI: 10.7270/Q2RN36K7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM250474 (US9464044, 102) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | 6.0 | 25 |
AHN-GOOK PHARMACEUTICAL CO., LTD.; BAMICHEM CO., LTD; INCHEON UNIVERSITY INDUSTRY ACADEMIC COOPERATION FOUNDATION US Patent | Assay Description The inhibiting activity of 11β-HSD1 derived from microsomal fractions was measured using the HTRF assay (62CO2PEB, Cisbio). Different concentrat... | US Patent US9464044 (2016) BindingDB Entry DOI: 10.7270/Q2SJ1JH0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM250435 (US9464044, 26) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | 6.0 | 25 |
AHN-GOOK PHARMACEUTICAL CO., LTD.; BAMICHEM CO., LTD; INCHEON UNIVERSITY INDUSTRY ACADEMIC COOPERATION FOUNDATION US Patent | Assay Description The inhibiting activity of 11β-HSD1 derived from microsomal fractions was measured using the HTRF assay (62CO2PEB, Cisbio). Different concentrat... | US Patent US9464044 (2016) BindingDB Entry DOI: 10.7270/Q2SJ1JH0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM250466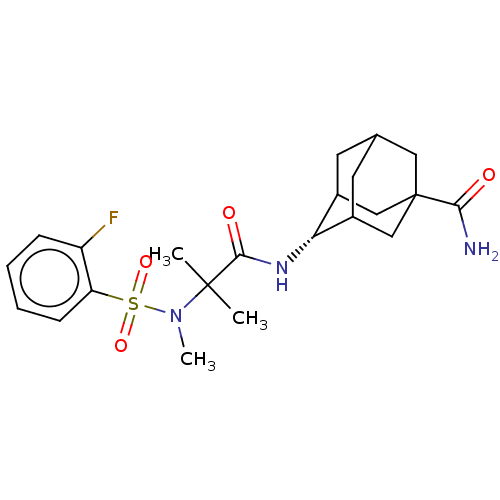 (US9464044, 72) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | 6.0 | 25 |
AHN-GOOK PHARMACEUTICAL CO., LTD.; BAMICHEM CO., LTD; INCHEON UNIVERSITY INDUSTRY ACADEMIC COOPERATION FOUNDATION US Patent | Assay Description The inhibiting activity of 11β-HSD1 derived from microsomal fractions was measured using the HTRF assay (62CO2PEB, Cisbio). Different concentrat... | US Patent US9464044 (2016) BindingDB Entry DOI: 10.7270/Q2SJ1JH0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM250436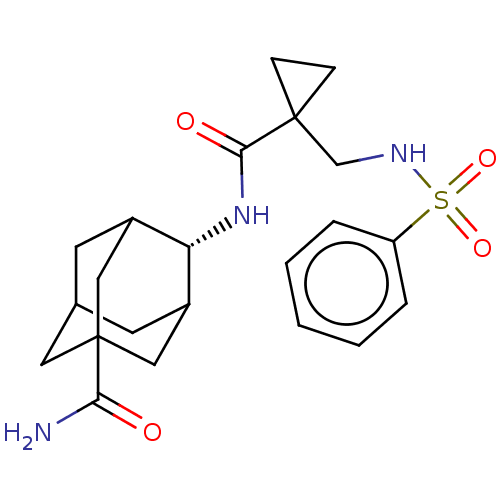 (US9464044, 28) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | 6.0 | 25 |
AHN-GOOK PHARMACEUTICAL CO., LTD.; BAMICHEM CO., LTD; INCHEON UNIVERSITY INDUSTRY ACADEMIC COOPERATION FOUNDATION US Patent | Assay Description The inhibiting activity of 11β-HSD1 derived from microsomal fractions was measured using the HTRF assay (62CO2PEB, Cisbio). Different concentrat... | US Patent US9464044 (2016) BindingDB Entry DOI: 10.7270/Q2SJ1JH0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM250437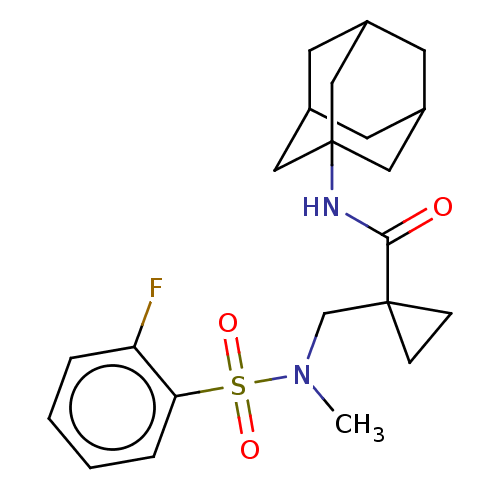 (US9464044, 31) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | 6.0 | 25 |
AHN-GOOK PHARMACEUTICAL CO., LTD.; BAMICHEM CO., LTD; INCHEON UNIVERSITY INDUSTRY ACADEMIC COOPERATION FOUNDATION US Patent | Assay Description The inhibiting activity of 11β-HSD1 derived from microsomal fractions was measured using the HTRF assay (62CO2PEB, Cisbio). Different concentrat... | US Patent US9464044 (2016) BindingDB Entry DOI: 10.7270/Q2SJ1JH0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM250438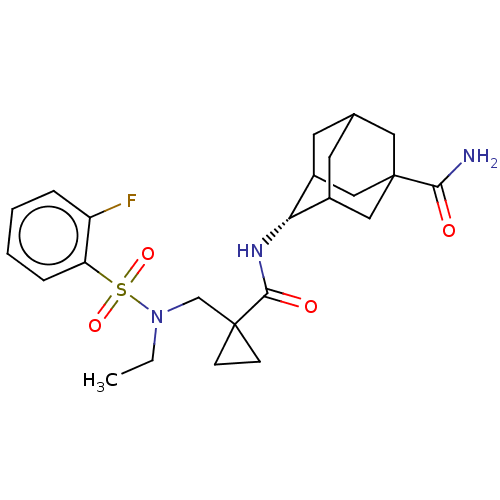 (US9464044, 32) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | 6.0 | 25 |
AHN-GOOK PHARMACEUTICAL CO., LTD.; BAMICHEM CO., LTD; INCHEON UNIVERSITY INDUSTRY ACADEMIC COOPERATION FOUNDATION US Patent | Assay Description The inhibiting activity of 11β-HSD1 derived from microsomal fractions was measured using the HTRF assay (62CO2PEB, Cisbio). Different concentrat... | US Patent US9464044 (2016) BindingDB Entry DOI: 10.7270/Q2SJ1JH0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM250439 (US9464044, 33) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | 6.0 | 25 |
AHN-GOOK PHARMACEUTICAL CO., LTD.; BAMICHEM CO., LTD; INCHEON UNIVERSITY INDUSTRY ACADEMIC COOPERATION FOUNDATION US Patent | Assay Description The inhibiting activity of 11β-HSD1 derived from microsomal fractions was measured using the HTRF assay (62CO2PEB, Cisbio). Different concentrat... | US Patent US9464044 (2016) BindingDB Entry DOI: 10.7270/Q2SJ1JH0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Focal adhesion kinase 1 (Homo sapiens (Human)) | BDBM155528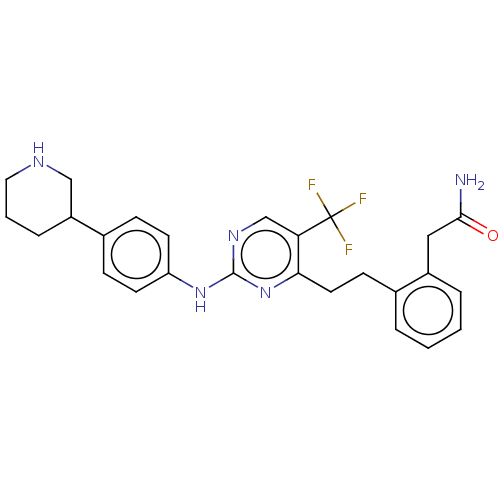 (US9012461, 4) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Cancer Therapeutics CRC Pty Ltd US Patent | Assay Description A biotin labeled peptide is used as substrate (amino acid sequence: Biotin-Glu-Gly-Pro-Trp-Leu-Glu-Glu-Glu-Glu-Glu-Ala-Tyr-Gly-Trp-Met-Asp-Phe-NH2)1S... | US Patent US9012461 (2015) BindingDB Entry DOI: 10.7270/Q2RN36K7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM250444 (US9464044, 40) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | 6.0 | 25 |
AHN-GOOK PHARMACEUTICAL CO., LTD.; BAMICHEM CO., LTD; INCHEON UNIVERSITY INDUSTRY ACADEMIC COOPERATION FOUNDATION US Patent | Assay Description The inhibiting activity of 11β-HSD1 derived from microsomal fractions was measured using the HTRF assay (62CO2PEB, Cisbio). Different concentrat... | US Patent US9464044 (2016) BindingDB Entry DOI: 10.7270/Q2SJ1JH0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Focal adhesion kinase 1 (Homo sapiens (Human)) | BDBM155527 (US9012461, 3) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Cancer Therapeutics CRC Pty Ltd US Patent | Assay Description A biotin labeled peptide is used as substrate (amino acid sequence: Biotin-Glu-Gly-Pro-Trp-Leu-Glu-Glu-Glu-Glu-Glu-Ala-Tyr-Gly-Trp-Met-Asp-Phe-NH2)1S... | US Patent US9012461 (2015) BindingDB Entry DOI: 10.7270/Q2RN36K7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM250472 (US9464044, 99) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | 6.0 | 25 |
AHN-GOOK PHARMACEUTICAL CO., LTD.; BAMICHEM CO., LTD; INCHEON UNIVERSITY INDUSTRY ACADEMIC COOPERATION FOUNDATION US Patent | Assay Description The inhibiting activity of 11β-HSD1 derived from microsomal fractions was measured using the HTRF assay (62CO2PEB, Cisbio). Different concentrat... | US Patent US9464044 (2016) BindingDB Entry DOI: 10.7270/Q2SJ1JH0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Focal adhesion kinase 1 (Homo sapiens (Human)) | BDBM155540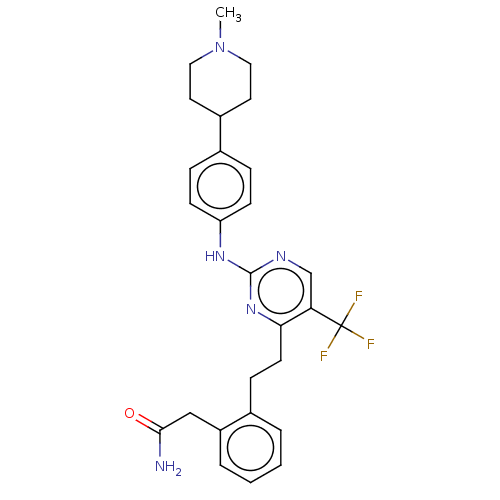 (US9012461, 16) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Cancer Therapeutics CRC Pty Ltd US Patent | Assay Description A biotin labeled peptide is used as substrate (amino acid sequence: Biotin-Glu-Gly-Pro-Trp-Leu-Glu-Glu-Glu-Glu-Glu-Ala-Tyr-Gly-Trp-Met-Asp-Phe-NH2)1S... | US Patent US9012461 (2015) BindingDB Entry DOI: 10.7270/Q2RN36K7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM50112142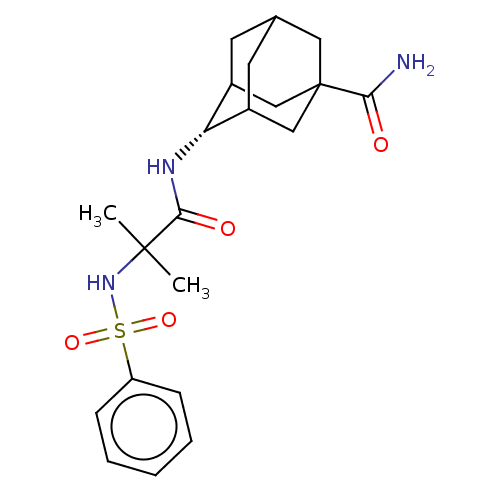 (CHEMBL3609870 | US9464044, 81) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | 6.0 | 25 |
AHN-GOOK PHARMACEUTICAL CO., LTD.; BAMICHEM CO., LTD; INCHEON UNIVERSITY INDUSTRY ACADEMIC COOPERATION FOUNDATION US Patent | Assay Description The inhibiting activity of 11β-HSD1 derived from microsomal fractions was measured using the HTRF assay (62CO2PEB, Cisbio). Different concentrat... | US Patent US9464044 (2016) BindingDB Entry DOI: 10.7270/Q2SJ1JH0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Focal adhesion kinase 1 (Homo sapiens (Human)) | BDBM155525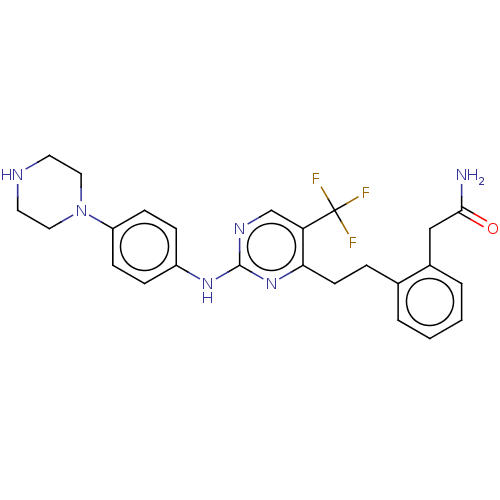 (US9012461, 1) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Cancer Therapeutics CRC Pty Ltd US Patent | Assay Description A biotin labeled peptide is used as substrate (amino acid sequence: Biotin-Glu-Gly-Pro-Trp-Leu-Glu-Glu-Glu-Glu-Glu-Ala-Tyr-Gly-Trp-Met-Asp-Phe-NH2)1S... | US Patent US9012461 (2015) BindingDB Entry DOI: 10.7270/Q2RN36K7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Focal adhesion kinase 1 (Homo sapiens (Human)) | BDBM155560 (US9012461, 36) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Cancer Therapeutics CRC Pty Ltd US Patent | Assay Description A biotin labeled peptide is used as substrate (amino acid sequence: Biotin-Glu-Gly-Pro-Trp-Leu-Glu-Glu-Glu-Glu-Glu-Ala-Tyr-Gly-Trp-Met-Asp-Phe-NH2)1S... | US Patent US9012461 (2015) BindingDB Entry DOI: 10.7270/Q2RN36K7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM50112127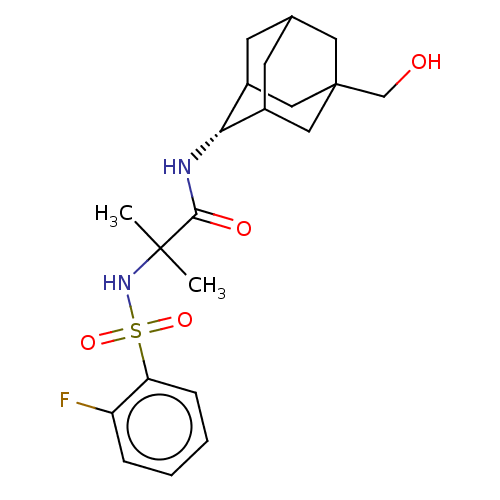 (CHEMBL3608407 | US9464044, 76) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | 6.0 | 25 |
AHN-GOOK PHARMACEUTICAL CO., LTD.; BAMICHEM CO., LTD; INCHEON UNIVERSITY INDUSTRY ACADEMIC COOPERATION FOUNDATION US Patent | Assay Description The inhibiting activity of 11β-HSD1 derived from microsomal fractions was measured using the HTRF assay (62CO2PEB, Cisbio). Different concentrat... | US Patent US9464044 (2016) BindingDB Entry DOI: 10.7270/Q2SJ1JH0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Focal adhesion kinase 1 (Homo sapiens (Human)) | BDBM155529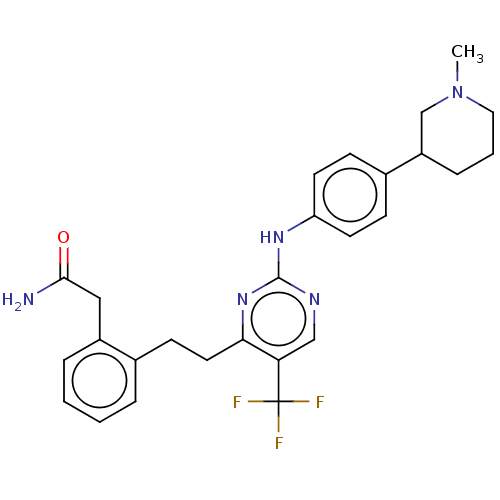 (US9012461, 5 | US9012461, 7) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Cancer Therapeutics CRC Pty Ltd US Patent | Assay Description A biotin labeled peptide is used as substrate (amino acid sequence: Biotin-Glu-Gly-Pro-Trp-Leu-Glu-Glu-Glu-Glu-Glu-Ala-Tyr-Gly-Trp-Met-Asp-Phe-NH2)1S... | US Patent US9012461 (2015) BindingDB Entry DOI: 10.7270/Q2RN36K7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Focal adhesion kinase 1 (Homo sapiens (Human)) | BDBM155545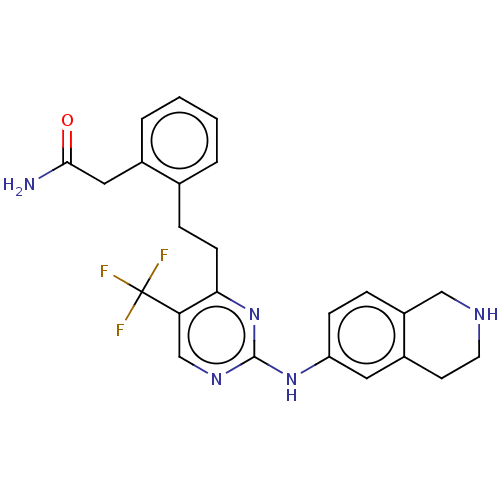 (US9012461, 21) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Cancer Therapeutics CRC Pty Ltd US Patent | Assay Description A biotin labeled peptide is used as substrate (amino acid sequence: Biotin-Glu-Gly-Pro-Trp-Leu-Glu-Glu-Glu-Glu-Glu-Ala-Tyr-Gly-Trp-Met-Asp-Phe-NH2)1S... | US Patent US9012461 (2015) BindingDB Entry DOI: 10.7270/Q2RN36K7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Focal adhesion kinase 1 (Homo sapiens (Human)) | BDBM155526 (US9012461, 2) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Cancer Therapeutics CRC Pty Ltd US Patent | Assay Description A biotin labeled peptide is used as substrate (amino acid sequence: Biotin-Glu-Gly-Pro-Trp-Leu-Glu-Glu-Glu-Glu-Glu-Ala-Tyr-Gly-Trp-Met-Asp-Phe-NH2)1S... | US Patent US9012461 (2015) BindingDB Entry DOI: 10.7270/Q2RN36K7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Focal adhesion kinase 1 (Homo sapiens (Human)) | BDBM155535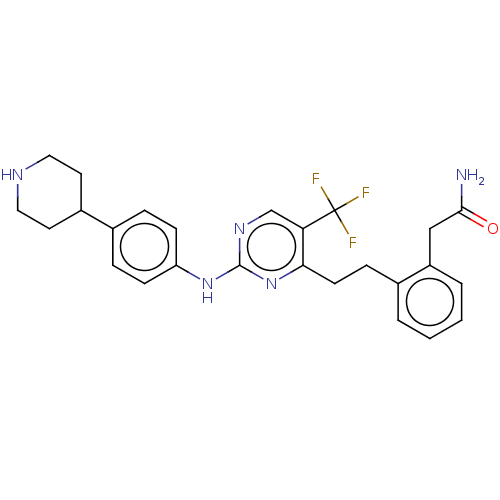 (US9012461, 11) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Cancer Therapeutics CRC Pty Ltd US Patent | Assay Description Compounds of the invention may be tested for in vitro activity in the following assay: A biotin labeled peptide is used as substrate (amino acid sequ... | US Patent US9012461 (2015) BindingDB Entry DOI: 10.7270/Q2RN36K7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Focal adhesion kinase 1 (Homo sapiens (Human)) | BDBM155533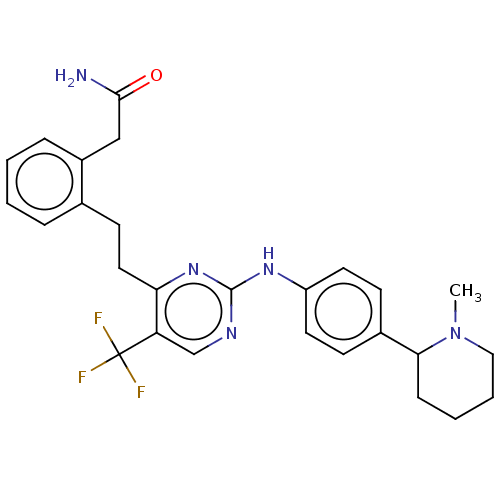 (US9012461, 9) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Cancer Therapeutics CRC Pty Ltd US Patent | Assay Description Compounds of the invention may be tested for in vitro activity in the following assay: A biotin labeled peptide is used as substrate (amino acid sequ... | US Patent US9012461 (2015) BindingDB Entry DOI: 10.7270/Q2RN36K7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM250420 (US9464044, 10) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | 6.0 | 25 |
AHN-GOOK PHARMACEUTICAL CO., LTD.; BAMICHEM CO., LTD; INCHEON UNIVERSITY INDUSTRY ACADEMIC COOPERATION FOUNDATION US Patent | Assay Description The inhibiting activity of 11β-HSD1 derived from microsomal fractions was measured using the HTRF assay (62CO2PEB, Cisbio). Different concentrat... | US Patent US9464044 (2016) BindingDB Entry DOI: 10.7270/Q2SJ1JH0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM50112126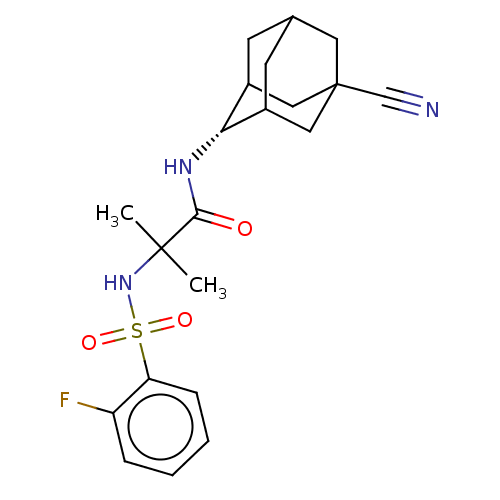 (CHEMBL3608406 | US9464044, 74) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | 6.0 | 25 |
AHN-GOOK PHARMACEUTICAL CO., LTD.; BAMICHEM CO., LTD; INCHEON UNIVERSITY INDUSTRY ACADEMIC COOPERATION FOUNDATION US Patent | Assay Description The inhibiting activity of 11β-HSD1 derived from microsomal fractions was measured using the HTRF assay (62CO2PEB, Cisbio). Different concentrat... | US Patent US9464044 (2016) BindingDB Entry DOI: 10.7270/Q2SJ1JH0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM250417 (US9464044, 7) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | 6.0 | 25 |
AHN-GOOK PHARMACEUTICAL CO., LTD.; BAMICHEM CO., LTD; INCHEON UNIVERSITY INDUSTRY ACADEMIC COOPERATION FOUNDATION US Patent | Assay Description The inhibiting activity of 11β-HSD1 derived from microsomal fractions was measured using the HTRF assay (62CO2PEB, Cisbio). Different concentrat... | US Patent US9464044 (2016) BindingDB Entry DOI: 10.7270/Q2SJ1JH0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM250480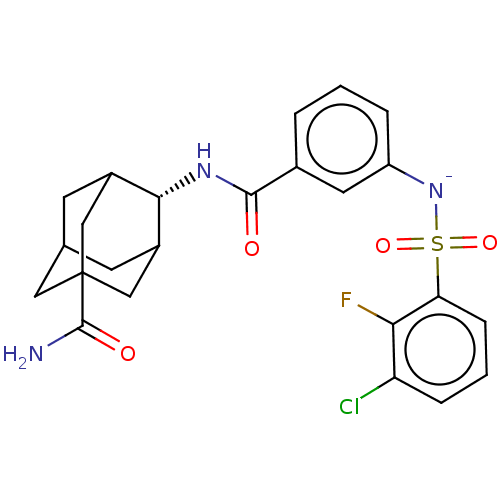 (US9464044, 113) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | 6.0 | 25 |
AHN-GOOK PHARMACEUTICAL CO., LTD.; BAMICHEM CO., LTD; INCHEON UNIVERSITY INDUSTRY ACADEMIC COOPERATION FOUNDATION US Patent | Assay Description The inhibiting activity of 11β-HSD1 derived from microsomal fractions was measured using the HTRF assay (62CO2PEB, Cisbio). Different concentrat... | US Patent US9464044 (2016) BindingDB Entry DOI: 10.7270/Q2SJ1JH0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM250416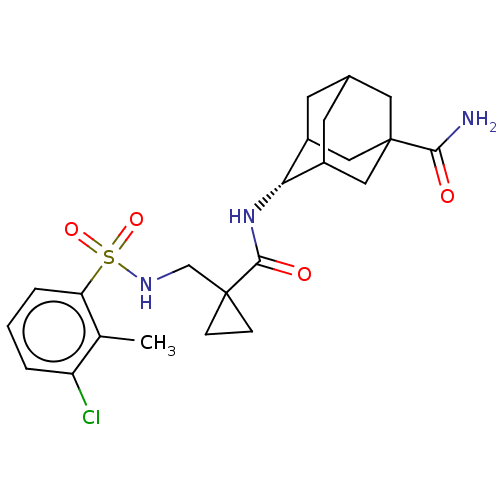 (US9464044, 5) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | 6.0 | 25 |
AHN-GOOK PHARMACEUTICAL CO., LTD.; BAMICHEM CO., LTD; INCHEON UNIVERSITY INDUSTRY ACADEMIC COOPERATION FOUNDATION US Patent | Assay Description The inhibiting activity of 11β-HSD1 derived from microsomal fractions was measured using the HTRF assay (62CO2PEB, Cisbio). Different concentrat... | US Patent US9464044 (2016) BindingDB Entry DOI: 10.7270/Q2SJ1JH0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM250459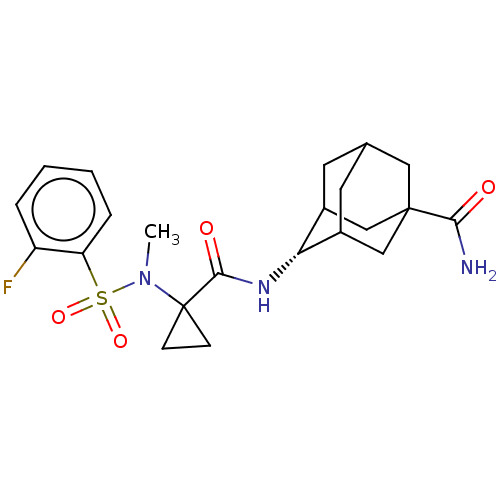 (US9464044, 61) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | 6.0 | 25 |
AHN-GOOK PHARMACEUTICAL CO., LTD.; BAMICHEM CO., LTD; INCHEON UNIVERSITY INDUSTRY ACADEMIC COOPERATION FOUNDATION US Patent | Assay Description The inhibiting activity of 11β-HSD1 derived from microsomal fractions was measured using the HTRF assay (62CO2PEB, Cisbio). Different concentrat... | US Patent US9464044 (2016) BindingDB Entry DOI: 10.7270/Q2SJ1JH0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM250423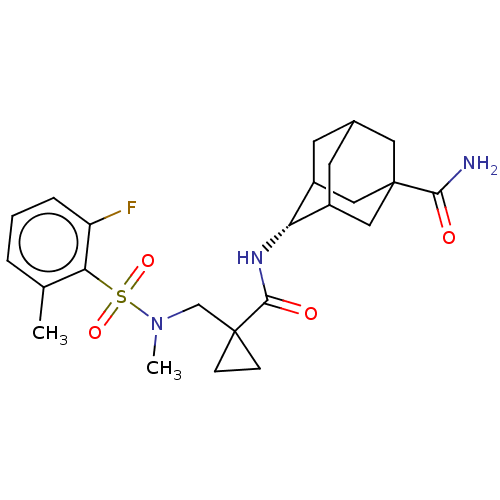 (US9464044, 13) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | 6.0 | 25 |
AHN-GOOK PHARMACEUTICAL CO., LTD.; BAMICHEM CO., LTD; INCHEON UNIVERSITY INDUSTRY ACADEMIC COOPERATION FOUNDATION US Patent | Assay Description The inhibiting activity of 11β-HSD1 derived from microsomal fractions was measured using the HTRF assay (62CO2PEB, Cisbio). Different concentrat... | US Patent US9464044 (2016) BindingDB Entry DOI: 10.7270/Q2SJ1JH0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Focal adhesion kinase 1 (Homo sapiens (Human)) | BDBM155546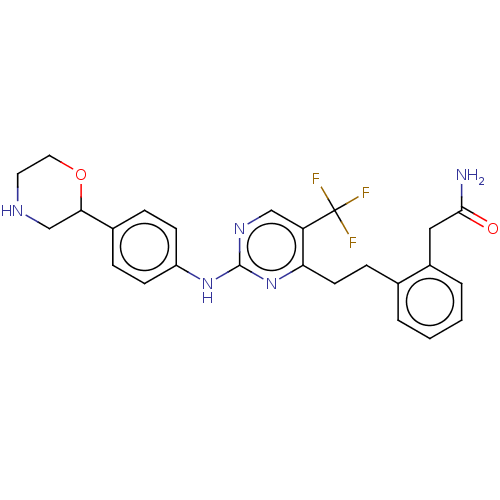 (US9012461, 22) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Cancer Therapeutics CRC Pty Ltd US Patent | Assay Description A biotin labeled peptide is used as substrate (amino acid sequence: Biotin-Glu-Gly-Pro-Trp-Leu-Glu-Glu-Glu-Glu-Glu-Ala-Tyr-Gly-Trp-Met-Asp-Phe-NH2)1S... | US Patent US9012461 (2015) BindingDB Entry DOI: 10.7270/Q2RN36K7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 364 total ) | Next | Last >> |