 Found 6563 hits with Last Name = 'chou' and Initial = 'c'
Found 6563 hits with Last Name = 'chou' and Initial = 'c' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Dihydrofolate reductase
(Mus musculus (Mouse)) | BDBM50016326
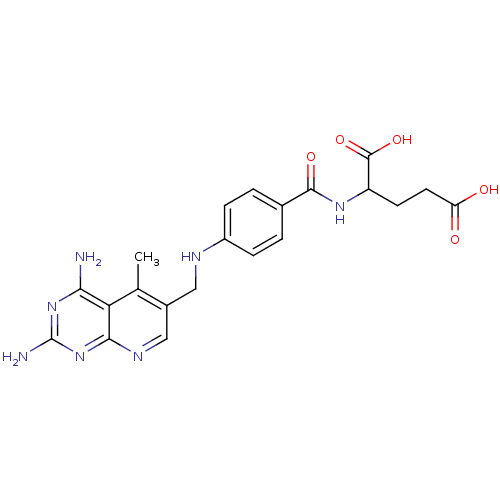
(2-{4-[(2,4-Diamino-5-methyl-pyrido[2,3-d]pyrimidin...)Show SMILES Cc1c(CNc2ccc(cc2)C(=O)NC(CCC(O)=O)C(O)=O)cnc2nc(N)nc(N)c12 Show InChI InChI=1S/C21H23N7O5/c1-10-12(9-25-18-16(10)17(22)27-21(23)28-18)8-24-13-4-2-11(3-5-13)19(31)26-14(20(32)33)6-7-15(29)30/h2-5,9,14,24H,6-8H2,1H3,(H,26,31)(H,29,30)(H,32,33)(H4,22,23,25,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cornell University
Curated by ChEMBL
| Assay Description
Inhibitory activity against dihydrofolate reductase (DHFR) of L-1210 cells |
J Med Chem 31: 1209-15 (1988)
BindingDB Entry DOI: 10.7270/Q2930S6N |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Mus musculus (Mouse)) | BDBM66082

((2S)-2-[[4-[(2,4-diaminopteridin-6-yl)methyl-methy...)Show SMILES CN(Cc1cnc2nc(N)nc(N)c2n1)c1ccc(cc1)C(=O)N[C@@H](CCC(O)=O)C(O)=O Show InChI InChI=1S/C20H22N8O5/c1-28(9-11-8-23-17-15(24-11)16(21)26-20(22)27-17)12-4-2-10(3-5-12)18(31)25-13(19(32)33)6-7-14(29)30/h2-5,8,13H,6-7,9H2,1H3,(H,25,31)(H,29,30)(H,32,33)(H4,21,22,23,26,27)/t13-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| PDB
PubMed
| 0 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cornell University
Curated by ChEMBL
| Assay Description
Inhibitory activity against dihydrofolate reductase (DHFR) of L-1210 cells |
J Med Chem 31: 1209-15 (1988)
BindingDB Entry DOI: 10.7270/Q2930S6N |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Dihydrofolate reductase
(Mus musculus (Mouse)) | BDBM50023681

(2-{4-[(2,4-Diamino-5,7-dimethyl-pyrido[2,3-d]pyrim...)Show SMILES Cc1nc2nc(N)nc(N)c2c(C)c1CNc1ccc(cc1)C(=O)NC(CCC(O)=O)C(O)=O Show InChI InChI=1S/C22H25N7O5/c1-10-14(11(2)26-19-17(10)18(23)28-22(24)29-19)9-25-13-5-3-12(4-6-13)20(32)27-15(21(33)34)7-8-16(30)31/h3-6,15,25H,7-9H2,1-2H3,(H,27,32)(H,30,31)(H,33,34)(H4,23,24,26,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.00200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cornell University
Curated by ChEMBL
| Assay Description
Inhibitory activity against dihydrofolate reductase (DHFR) of L-1210 cells |
J Med Chem 31: 1209-15 (1988)
BindingDB Entry DOI: 10.7270/Q2930S6N |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Mus musculus (Mouse)) | BDBM50023683
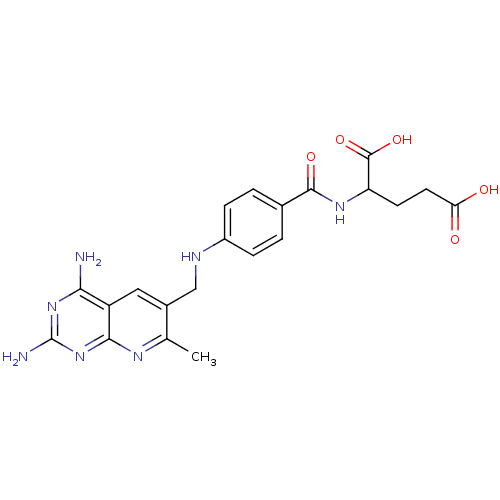
(2-{4-[(2,4-Diamino-7-methyl-pyrido[2,3-d]pyrimidin...)Show SMILES Cc1nc2nc(N)nc(N)c2cc1CNc1ccc(cc1)C(=O)NC(CCC(O)=O)C(O)=O Show InChI InChI=1S/C21H23N7O5/c1-10-12(8-14-17(22)27-21(23)28-18(14)25-10)9-24-13-4-2-11(3-5-13)19(31)26-15(20(32)33)6-7-16(29)30/h2-5,8,15,24H,6-7,9H2,1H3,(H,26,31)(H,29,30)(H,32,33)(H4,22,23,25,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| >0.00400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cornell University
Curated by ChEMBL
| Assay Description
Inhibitory activity against dihydrofolate reductase (DHFR) of L-1210 cells |
J Med Chem 31: 1209-15 (1988)
BindingDB Entry DOI: 10.7270/Q2930S6N |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Mus musculus (Mouse)) | BDBM50023682

(2-{4-[(2,4-Diamino-5-methyl-7-phenyl-pyrido[2,3-d]...)Show SMILES Cc1c(CNc2ccc(cc2)C(=O)NC(CCC(O)=O)C(O)=O)c(nc2nc(N)nc(N)c12)-c1ccccc1 Show InChI InChI=1S/C27H27N7O5/c1-14-18(22(15-5-3-2-4-6-15)32-24-21(14)23(28)33-27(29)34-24)13-30-17-9-7-16(8-10-17)25(37)31-19(26(38)39)11-12-20(35)36/h2-10,19,30H,11-13H2,1H3,(H,31,37)(H,35,36)(H,38,39)(H4,28,29,32,33,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| >0.00400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cornell University
Curated by ChEMBL
| Assay Description
Inhibitory activity against dihydrofolate reductase (DHFR) of L-1210 cells |
J Med Chem 31: 1209-15 (1988)
BindingDB Entry DOI: 10.7270/Q2930S6N |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Mus musculus (Mouse)) | BDBM50023684
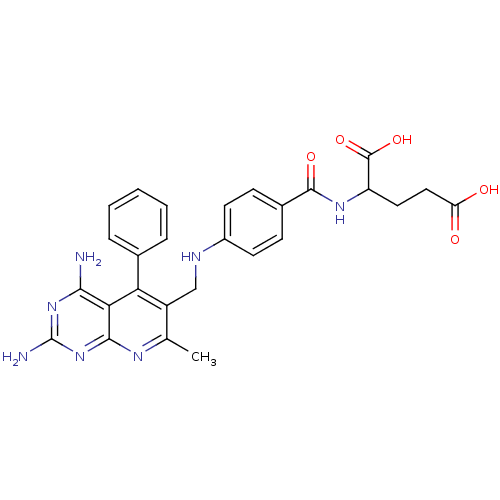
(2-{4-[(2,4-Diamino-7-methyl-5-phenyl-pyrido[2,3-d]...)Show SMILES Cc1nc2nc(N)nc(N)c2c(-c2ccccc2)c1CNc1ccc(cc1)C(=O)NC(CCC(O)=O)C(O)=O Show InChI InChI=1S/C27H27N7O5/c1-14-18(21(15-5-3-2-4-6-15)22-23(28)33-27(29)34-24(22)31-14)13-30-17-9-7-16(8-10-17)25(37)32-19(26(38)39)11-12-20(35)36/h2-10,19,30H,11-13H2,1H3,(H,32,37)(H,35,36)(H,38,39)(H4,28,29,31,33,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| >0.00400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cornell University
Curated by ChEMBL
| Assay Description
Inhibitory activity against dihydrofolate reductase (DHFR) of L-1210 cells |
J Med Chem 31: 1209-15 (1988)
BindingDB Entry DOI: 10.7270/Q2930S6N |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Mus musculus (Mouse)) | BDBM50023680

(2-{4-[(2,4-Diamino-7-phenyl-pyrido[2,3-d]pyrimidin...)Show SMILES Nc1nc(N)c2cc(CNc3ccc(cc3)C(=O)NC(CCC(O)=O)C(O)=O)c(nc2n1)-c1ccccc1 Show InChI InChI=1S/C26H25N7O5/c27-22-18-12-16(21(14-4-2-1-3-5-14)31-23(18)33-26(28)32-22)13-29-17-8-6-15(7-9-17)24(36)30-19(25(37)38)10-11-20(34)35/h1-9,12,19,29H,10-11,13H2,(H,30,36)(H,34,35)(H,37,38)(H4,27,28,31,32,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| >0.00400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cornell University
Curated by ChEMBL
| Assay Description
Inhibitory activity against dihydrofolate reductase (DHFR) of L-1210 cells |
J Med Chem 31: 1209-15 (1988)
BindingDB Entry DOI: 10.7270/Q2930S6N |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50123438

((3,5-Dichloro-pyridin-4-yl)-(4-methyl-4-{(S)-3-met...)Show SMILES C[C@H](N1CCN(C[C@@H]1C)C1(C)CCN(CC1)C(=O)c1c(Cl)cncc1Cl)c1ccc(cc1)C(F)(F)F Show InChI InChI=1S/C26H31Cl2F3N4O/c1-17-16-34(12-13-35(17)18(2)19-4-6-20(7-5-19)26(29,30)31)25(3)8-10-33(11-9-25)24(36)23-21(27)14-32-15-22(23)28/h4-7,14-15,17-18H,8-13,16H2,1-3H3/t17-,18-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Ability to inhibit [125I]-labeled RANTES binding to the CCR5 receptor expressed in membrane preparations from CHO cells |
Bioorg Med Chem Lett 13: 567-71 (2003)
BindingDB Entry DOI: 10.7270/Q28K78G6 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50439674
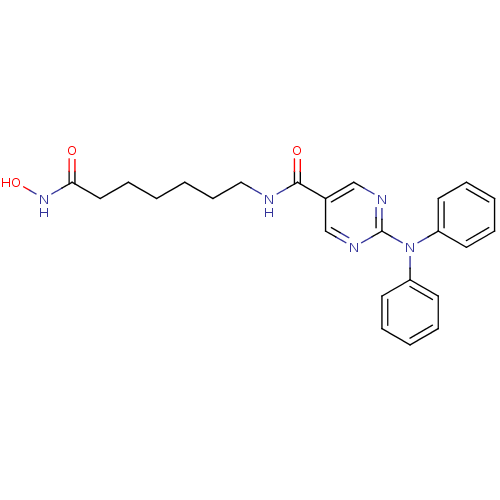
(RICOLINOSTAT | US10858323, Compound 2 | US11207431...)Show SMILES ONC(=O)CCCCCCNC(=O)c1cnc(nc1)N(c1ccccc1)c1ccccc1 Show InChI InChI=1S/C24H27N5O3/c30-22(28-32)15-9-1-2-10-16-25-23(31)19-17-26-24(27-18-19)29(20-11-5-3-6-12-20)21-13-7-4-8-14-21/h3-8,11-14,17-18,32H,1-2,9-10,15-16H2,(H,25,31)(H,28,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00853
BindingDB Entry DOI: 10.7270/Q2XD15N3 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50104946
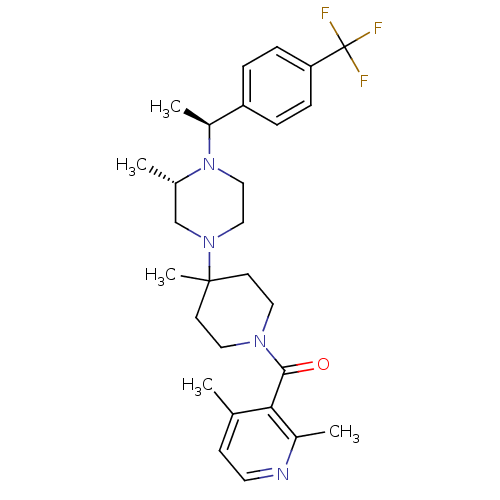
((2,4-Dimethyl-pyridin-3-yl)-(4-methyl-4-{(S)-3-met...)Show SMILES C[C@H](N1CCN(C[C@@H]1C)C1(C)CCN(CC1)C(=O)c1c(C)ccnc1C)c1ccc(cc1)C(F)(F)F Show InChI InChI=1S/C28H37F3N4O/c1-19-10-13-32-21(3)25(19)26(36)33-14-11-27(5,12-15-33)34-16-17-35(20(2)18-34)22(4)23-6-8-24(9-7-23)28(29,30)31/h6-10,13,20,22H,11-12,14-18H2,1-5H3/t20-,22-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Ability to inhibit [125I]-labeled RANTES binding to the CCR5 receptor expressed in membrane preparations from CHO cells |
Bioorg Med Chem Lett 13: 567-71 (2003)
BindingDB Entry DOI: 10.7270/Q28K78G6 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50123436
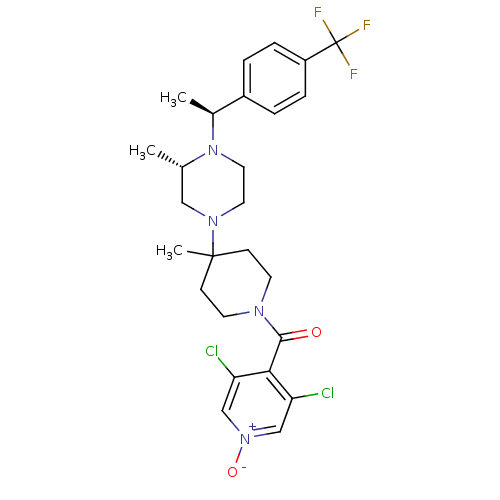
((3,5-Dichloro-1-oxy-pyridin-4-yl)-(4-methyl-4-{(S)...)Show SMILES C[C@H](N1CCN(C[C@@H]1C)C1(C)CCN(CC1)C(=O)c1c(Cl)c[n+]([O-])cc1Cl)c1ccc(cc1)C(F)(F)F Show InChI InChI=1S/C26H31Cl2F3N4O2/c1-17-14-33(12-13-35(17)18(2)19-4-6-20(7-5-19)26(29,30)31)25(3)8-10-32(11-9-25)24(36)23-21(27)15-34(37)16-22(23)28/h4-7,15-18H,8-14H2,1-3H3/t17-,18-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Ability to inhibit [125I]-labeled RANTES binding to the CCR5 receptor expressed in membrane preparations from CHO cells |
Bioorg Med Chem Lett 13: 567-71 (2003)
BindingDB Entry DOI: 10.7270/Q28K78G6 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50123435

((4,6-Dimethyl-pyrimidin-5-yl)-(4-methyl-4-{(S)-3-m...)Show SMILES C[C@H](N1CCN(C[C@@H]1C)C1(C)CCN(CC1)C(=O)c1c(C)ncnc1C)c1ccc(cc1)C(F)(F)F Show InChI InChI=1S/C27H36F3N5O/c1-18-16-34(14-15-35(18)21(4)22-6-8-23(9-7-22)27(28,29)30)26(5)10-12-33(13-11-26)25(36)24-19(2)31-17-32-20(24)3/h6-9,17-18,21H,10-16H2,1-5H3/t18-,21-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Ability of compound to inhibit [125I]-labeled RANTES binding to the CCR5 receptor expressed in membrane preparations from CHO cells |
Bioorg Med Chem Lett 13: 567-71 (2003)
BindingDB Entry DOI: 10.7270/Q28K78G6 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50593116
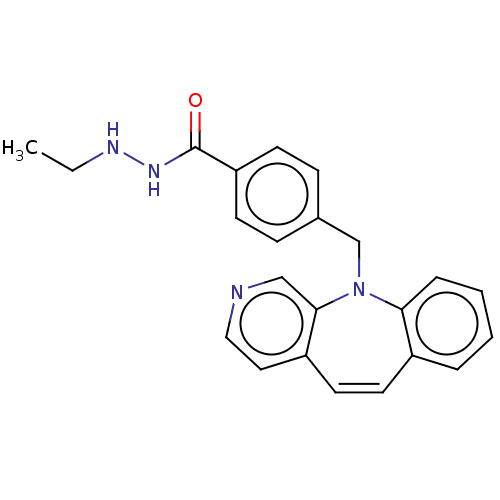
(CHEMBL5173493)Show SMILES CCNNC(=O)c1ccc(CN2c3ccccc3C=Cc3ccncc23)cc1 |c:19| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00853
BindingDB Entry DOI: 10.7270/Q2XD15N3 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50380399

(CHEMBL2018302 | Tubastatin A | US10227295, Compoun...)Show InChI InChI=1S/C20H21N3O2/c1-22-11-10-19-17(13-22)16-4-2-3-5-18(16)23(19)12-14-6-8-15(9-7-14)20(24)21-25/h2-9,25H,10-13H2,1H3,(H,21,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00853
BindingDB Entry DOI: 10.7270/Q2XD15N3 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50123445
((3,5-Dimethyl-pyridin-4-yl)-(4-methyl-4-{(S)-3-met...)Show SMILES C[C@H](N1CCN(C[C@@H]1C)C1(C)CCN(CC1)C(=O)c1c(C)cncc1C)c1ccc(cc1)C(F)(F)F Show InChI InChI=1S/C28H37F3N4O/c1-19-16-32-17-20(2)25(19)26(36)33-12-10-27(5,11-13-33)34-14-15-35(21(3)18-34)22(4)23-6-8-24(9-7-23)28(29,30)31/h6-9,16-17,21-22H,10-15,18H2,1-5H3/t21-,22-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 5.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Ability to inhibit [125I]-labeled RANTES binding to the CCR5 receptor expressed in membrane preparations from CHO cells |
Bioorg Med Chem Lett 13: 567-71 (2003)
BindingDB Entry DOI: 10.7270/Q28K78G6 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50104956
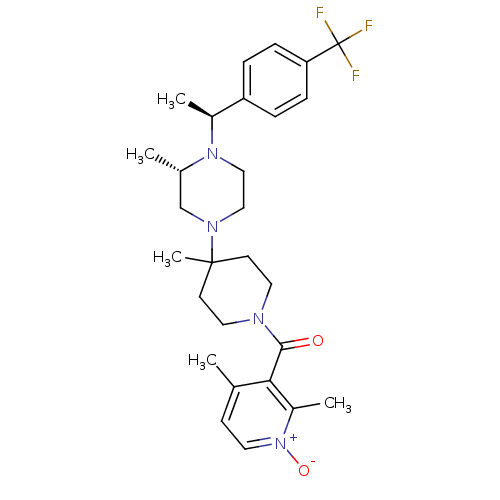
((2,4-Dimethyl-1-oxy-pyridin-3-yl)-(4-methyl-4-{(S)...)Show SMILES C[C@H](N1CCN(C[C@@H]1C)C1(C)CCN(CC1)C(=O)c1c(C)cc[n+]([O-])c1C)c1ccc(cc1)C(F)(F)F Show InChI InChI=1S/C28H37F3N4O2/c1-19-10-13-35(37)22(4)25(19)26(36)32-14-11-27(5,12-15-32)33-16-17-34(20(2)18-33)21(3)23-6-8-24(9-7-23)28(29,30)31/h6-10,13,20-21H,11-12,14-18H2,1-5H3/t20-,21-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Ability to inhibit [125I]-labeled RANTES binding to the CCR5 receptor expressed in membrane preparations from CHO cells |
Bioorg Med Chem Lett 13: 567-71 (2003)
BindingDB Entry DOI: 10.7270/Q28K78G6 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
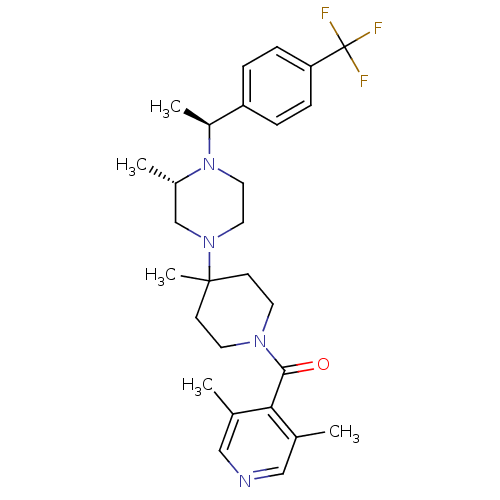
(Homo sapiens (Human)) | BDBM50123444

((4,6-Dimethyl-2-trifluoromethyl-pyrimidin-5-yl)-(4...)Show SMILES C[C@H](N1CCN(C[C@@H]1C)C1(C)CCN(CC1)C(=O)c1c(C)nc(nc1C)C(F)(F)F)c1ccc(cc1)C(F)(F)F Show InChI InChI=1S/C28H35F6N5O/c1-17-16-38(14-15-39(17)20(4)21-6-8-22(9-7-21)27(29,30)31)26(5)10-12-37(13-11-26)24(40)23-18(2)35-25(28(32,33)34)36-19(23)3/h6-9,17,20H,10-16H2,1-5H3/t17-,20-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Ability to inhibit [125I]-labeled RANTES binding to the CCR5 receptor expressed in membrane preparations from CHO cells |
Bioorg Med Chem Lett 13: 567-71 (2003)
BindingDB Entry DOI: 10.7270/Q28K78G6 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50123442
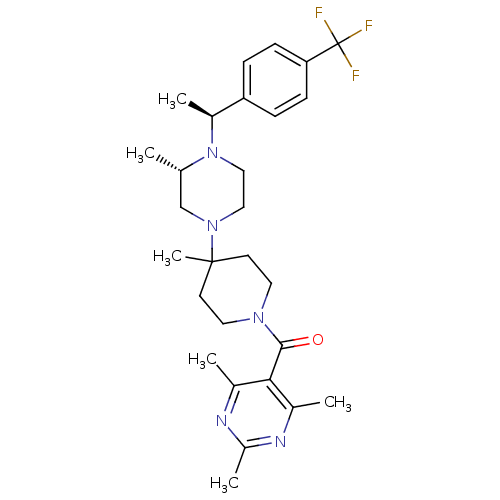
((4-Methyl-4-{(S)-3-methyl-4-[(S)-1-(4-trifluoromet...)Show SMILES C[C@H](N1CCN(C[C@@H]1C)C1(C)CCN(CC1)C(=O)c1c(C)nc(C)nc1C)c1ccc(cc1)C(F)(F)F Show InChI InChI=1S/C28H38F3N5O/c1-18-17-35(15-16-36(18)21(4)23-7-9-24(10-8-23)28(29,30)31)27(6)11-13-34(14-12-27)26(37)25-19(2)32-22(5)33-20(25)3/h7-10,18,21H,11-17H2,1-6H3/t18-,21-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Ability to inhibit [125I]-labeled RANTES binding to the CCR5 receptor expressed in membrane preparations from CHO cells |
Bioorg Med Chem Lett 13: 567-71 (2003)
BindingDB Entry DOI: 10.7270/Q28K78G6 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50123437
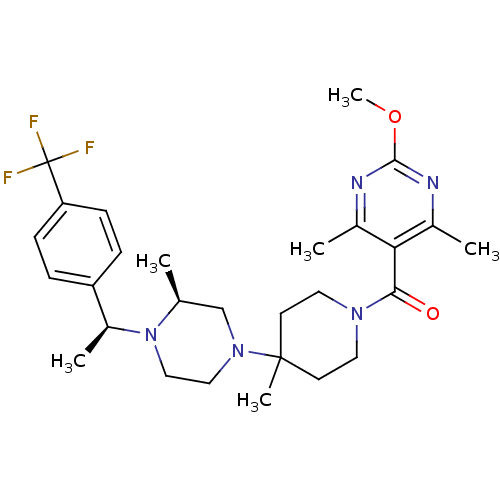
((2-Methoxy-4,6-dimethyl-pyrimidin-5-yl)-(4-methyl-...)Show SMILES COc1nc(C)c(C(=O)N2CCC(C)(CC2)N2CCN([C@@H](C)c3ccc(cc3)C(F)(F)F)[C@@H](C)C2)c(C)n1 Show InChI InChI=1S/C28H38F3N5O2/c1-18-17-35(15-16-36(18)21(4)22-7-9-23(10-8-22)28(29,30)31)27(5)11-13-34(14-12-27)25(37)24-19(2)32-26(38-6)33-20(24)3/h7-10,18,21H,11-17H2,1-6H3/t18-,21-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Ability to inhibit [125I]-labeled RANTES binding to the CCR5 receptor expressed in membrane preparations from CHO cells |
Bioorg Med Chem Lett 13: 567-71 (2003)
BindingDB Entry DOI: 10.7270/Q28K78G6 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50123446
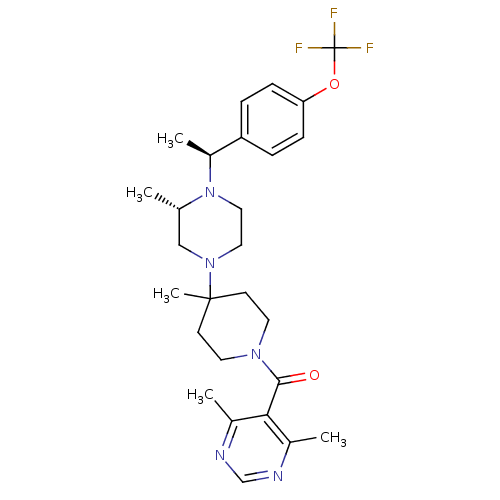
((4,6-Dimethyl-pyrimidin-5-yl)-(4-methyl-4-{(S)-3-m...)Show SMILES C[C@H](N1CCN(C[C@@H]1C)C1(C)CCN(CC1)C(=O)c1c(C)ncnc1C)c1ccc(OC(F)(F)F)cc1 Show InChI InChI=1S/C27H36F3N5O2/c1-18-16-34(14-15-35(18)21(4)22-6-8-23(9-7-22)37-27(28,29)30)26(5)10-12-33(13-11-26)25(36)24-19(2)31-17-32-20(24)3/h6-9,17-18,21H,10-16H2,1-5H3/t18-,21-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Ability to inhibit [125I]-labeled RANTES binding to the CCR5 receptor expressed in membrane preparations from CHO cells |
Bioorg Med Chem Lett 13: 567-71 (2003)
BindingDB Entry DOI: 10.7270/Q28K78G6 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50123443
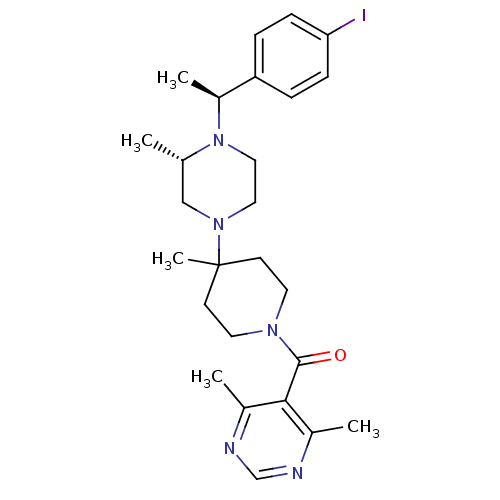
((4,6-Dimethyl-pyrimidin-5-yl)-(4-{(S)-4-[(S)-1-(4-...)Show SMILES C[C@H](N1CCN(C[C@@H]1C)C1(C)CCN(CC1)C(=O)c1c(C)ncnc1C)c1ccc(I)cc1 Show InChI InChI=1S/C26H36IN5O/c1-18-16-31(14-15-32(18)21(4)22-6-8-23(27)9-7-22)26(5)10-12-30(13-11-26)25(33)24-19(2)28-17-29-20(24)3/h6-9,17-18,21H,10-16H2,1-5H3/t18-,21-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Ability to inhibit [125I]-labeled RANTES binding to the CCR5 receptor expressed in membrane preparations from CHO cells |
Bioorg Med Chem Lett 13: 567-71 (2003)
BindingDB Entry DOI: 10.7270/Q28K78G6 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50123439
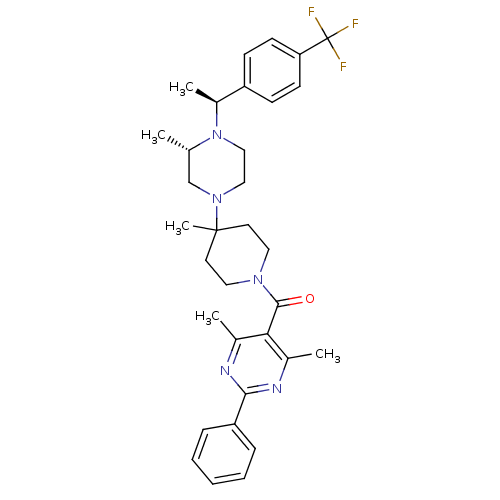
((4,6-Dimethyl-2-phenyl-pyrimidin-5-yl)-(4-methyl-4...)Show SMILES C[C@H](N1CCN(C[C@@H]1C)C1(C)CCN(CC1)C(=O)c1c(C)nc(nc1C)-c1ccccc1)c1ccc(cc1)C(F)(F)F Show InChI InChI=1S/C33H40F3N5O/c1-22-21-40(19-20-41(22)25(4)26-11-13-28(14-12-26)33(34,35)36)32(5)15-17-39(18-16-32)31(42)29-23(2)37-30(38-24(29)3)27-9-7-6-8-10-27/h6-14,22,25H,15-21H2,1-5H3/t22-,25-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Ability to inhibit [125I]-labeled RANTES binding to the CCR5 receptor expressed in membrane preparations from CHO cells |
Bioorg Med Chem Lett 13: 567-71 (2003)
BindingDB Entry DOI: 10.7270/Q28K78G6 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50123447
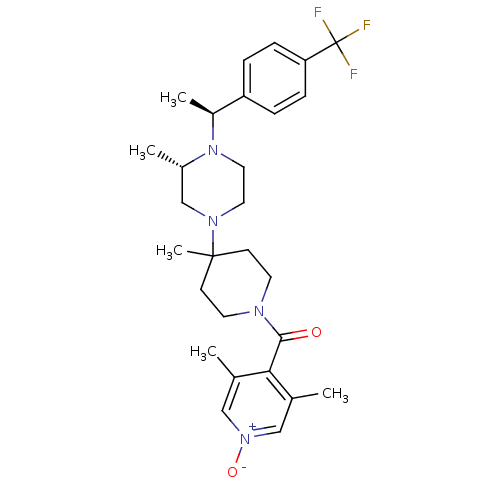
((3,5-Dimethyl-1-oxy-pyridin-4-yl)-(4-methyl-4-{(S)...)Show SMILES C[C@H](N1CCN(C[C@@H]1C)C1(C)CCN(CC1)C(=O)c1c(C)c[n+]([O-])cc1C)c1ccc(cc1)C(F)(F)F Show InChI InChI=1S/C28H37F3N4O2/c1-19-16-34(37)17-20(2)25(19)26(36)32-12-10-27(5,11-13-32)33-14-15-35(21(3)18-33)22(4)23-6-8-24(9-7-23)28(29,30)31/h6-9,16-17,21-22H,10-15,18H2,1-5H3/t21-,22-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Ability to inhibit [125I]-labeled RANTES binding to the CCR5 receptor expressed in membrane preparations from CHO cells |
Bioorg Med Chem Lett 13: 567-71 (2003)
BindingDB Entry DOI: 10.7270/Q28K78G6 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 1
(Homo sapiens (Human)) | BDBM50205477

((Z)-2-((1R,2S)-2-phenylcyclopropylamino)-5-(quinol...)Show SMILES O=C1N=C(N[C@@H]2C[C@H]2c2ccccc2)SC1=Cc1ccc2ncccc2c1 |w:16.19,t:2| Show InChI InChI=1S/C22H17N3OS/c26-21-20(12-14-8-9-18-16(11-14)7-4-10-23-18)27-22(25-21)24-19-13-17(19)15-5-2-1-3-6-15/h1-12,17,19H,13H2,(H,24,25,26)/t17-,19+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center
Curated by ChEMBL
| Assay Description
Inhibition of CDK1 |
Bioorg Med Chem Lett 17: 2134-8 (2007)
Article DOI: 10.1016/j.bmcl.2007.01.081
BindingDB Entry DOI: 10.7270/Q2BV7G9T |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50123440
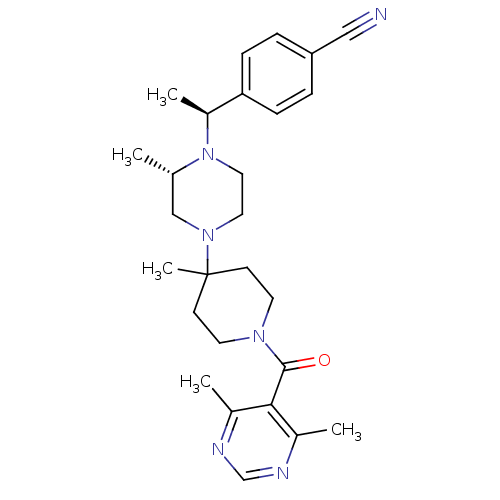
(4-((S)-1-{(S)-4-[1-(4,6-Dimethyl-pyrimidine-5-carb...)Show SMILES C[C@H](N1CCN(C[C@@H]1C)C1(C)CCN(CC1)C(=O)c1c(C)ncnc1C)c1ccc(cc1)C#N Show InChI InChI=1S/C27H36N6O/c1-19-17-32(14-15-33(19)22(4)24-8-6-23(16-28)7-9-24)27(5)10-12-31(13-11-27)26(34)25-20(2)29-18-30-21(25)3/h6-9,18-19,22H,10-15,17H2,1-5H3/t19-,22-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Ability to inhibit [125I]-labeled RANTES binding to the CCR5 receptor expressed in membrane preparations from CHO cells |
Bioorg Med Chem Lett 13: 567-71 (2003)
BindingDB Entry DOI: 10.7270/Q28K78G6 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 1
(Homo sapiens (Human)) | BDBM50205472

((5Z)-5-(quinolin-6-ylmethylidene)-2-[(thiophen-2-y...)Show SMILES O=C1N=C(NCc2cccs2)SC1=Cc1ccc2ncccc2c1 |w:13.15,t:2| Show InChI InChI=1S/C18H13N3OS2/c22-17-16(24-18(21-17)20-11-14-4-2-8-23-14)10-12-5-6-15-13(9-12)3-1-7-19-15/h1-10H,11H2,(H,20,21,22) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center
Curated by ChEMBL
| Assay Description
Inhibition of CDK1 |
Bioorg Med Chem Lett 17: 2134-8 (2007)
Article DOI: 10.1016/j.bmcl.2007.01.081
BindingDB Entry DOI: 10.7270/Q2BV7G9T |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50123441

((2-Amino-4,6-dimethyl-pyrimidin-5-yl)-(4-methyl-4-...)Show SMILES C[C@H](N1CCN(C[C@@H]1C)C1(C)CCN(CC1)C(=O)c1c(C)nc(N)nc1C)c1ccc(cc1)C(F)(F)F Show InChI InChI=1S/C27H37F3N6O/c1-17-16-35(14-15-36(17)20(4)21-6-8-22(9-7-21)27(28,29)30)26(5)10-12-34(13-11-26)24(37)23-18(2)32-25(31)33-19(23)3/h6-9,17,20H,10-16H2,1-5H3,(H2,31,32,33)/t17-,20-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 38 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Ability to inhibit [125I]-labeled RANTES binding to the CCR5 receptor expressed in membrane preparations from CHO cells |
Bioorg Med Chem Lett 13: 567-71 (2003)
BindingDB Entry DOI: 10.7270/Q28K78G6 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(Homo sapiens (Human)) | BDBM50123446

((4,6-Dimethyl-pyrimidin-5-yl)-(4-methyl-4-{(S)-3-m...)Show SMILES C[C@H](N1CCN(C[C@@H]1C)C1(C)CCN(CC1)C(=O)c1c(C)ncnc1C)c1ccc(OC(F)(F)F)cc1 Show InChI InChI=1S/C27H36F3N5O2/c1-18-16-34(14-15-35(18)21(4)22-6-8-23(9-7-22)37-27(28,29)30)26(5)10-12-33(13-11-26)25(36)24-19(2)31-17-32-20(24)3/h6-9,17-18,21H,10-16H2,1-5H3/t18-,21-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 68 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity towards muscarinic receptor M2 |
Bioorg Med Chem Lett 13: 567-71 (2003)
BindingDB Entry DOI: 10.7270/Q28K78G6 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50439674

(RICOLINOSTAT | US10858323, Compound 2 | US11207431...)Show SMILES ONC(=O)CCCCCCNC(=O)c1cnc(nc1)N(c1ccccc1)c1ccccc1 Show InChI InChI=1S/C24H27N5O3/c30-22(28-32)15-9-1-2-10-16-25-23(31)19-17-26-24(27-18-19)29(20-11-5-3-6-12-20)21-13-7-4-8-14-21/h3-8,11-14,17-18,32H,1-2,9-10,15-16H2,(H,25,31)(H,28,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 74 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00853
BindingDB Entry DOI: 10.7270/Q2XD15N3 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 3
(Homo sapiens (Human)) | BDBM50439674

(RICOLINOSTAT | US10858323, Compound 2 | US11207431...)Show SMILES ONC(=O)CCCCCCNC(=O)c1cnc(nc1)N(c1ccccc1)c1ccccc1 Show InChI InChI=1S/C24H27N5O3/c30-22(28-32)15-9-1-2-10-16-25-23(31)19-17-26-24(27-18-19)29(20-11-5-3-6-12-20)21-13-7-4-8-14-21/h3-8,11-14,17-18,32H,1-2,9-10,15-16H2,(H,25,31)(H,28,30) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 123 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00853
BindingDB Entry DOI: 10.7270/Q2XD15N3 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 1
(Homo sapiens (Human)) | BDBM50205468

((Z)-2-amino-5-(quinolin-6-ylmethylene)thiazol-4(5H...)Show SMILES NC1=NC(=O)C(S1)=Cc1ccc2ncccc2c1 |w:7.8,t:1| Show InChI InChI=1S/C13H9N3OS/c14-13-16-12(17)11(18-13)7-8-3-4-10-9(6-8)2-1-5-15-10/h1-7H,(H2,14,16,17) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center
Curated by ChEMBL
| Assay Description
Inhibition of CDK1 |
Bioorg Med Chem Lett 17: 2134-8 (2007)
Article DOI: 10.1016/j.bmcl.2007.01.081
BindingDB Entry DOI: 10.7270/Q2BV7G9T |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(Homo sapiens (Human)) | BDBM50123440

(4-((S)-1-{(S)-4-[1-(4,6-Dimethyl-pyrimidine-5-carb...)Show SMILES C[C@H](N1CCN(C[C@@H]1C)C1(C)CCN(CC1)C(=O)c1c(C)ncnc1C)c1ccc(cc1)C#N Show InChI InChI=1S/C27H36N6O/c1-19-17-32(14-15-33(19)22(4)24-8-6-23(16-28)7-9-24)27(5)10-12-31(13-11-27)26(34)25-20(2)29-18-30-21(25)3/h6-9,18-19,22H,10-15,17H2,1-5H3/t19-,22-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 164 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity towards muscarinic receptor M2 |
Bioorg Med Chem Lett 13: 567-71 (2003)
BindingDB Entry DOI: 10.7270/Q28K78G6 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(Homo sapiens (Human)) | BDBM50123443

((4,6-Dimethyl-pyrimidin-5-yl)-(4-{(S)-4-[(S)-1-(4-...)Show SMILES C[C@H](N1CCN(C[C@@H]1C)C1(C)CCN(CC1)C(=O)c1c(C)ncnc1C)c1ccc(I)cc1 Show InChI InChI=1S/C26H36IN5O/c1-18-16-31(14-15-32(18)21(4)22-6-8-23(27)9-7-22)26(5)10-12-30(13-11-26)25(33)24-19(2)28-17-29-20(24)3/h6-9,17-18,21H,10-16H2,1-5H3/t18-,21-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 169 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity towards muscarinic receptor M2 |
Bioorg Med Chem Lett 13: 567-71 (2003)
BindingDB Entry DOI: 10.7270/Q28K78G6 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(Homo sapiens (Human)) | BDBM50123437

((2-Methoxy-4,6-dimethyl-pyrimidin-5-yl)-(4-methyl-...)Show SMILES COc1nc(C)c(C(=O)N2CCC(C)(CC2)N2CCN([C@@H](C)c3ccc(cc3)C(F)(F)F)[C@@H](C)C2)c(C)n1 Show InChI InChI=1S/C28H38F3N5O2/c1-18-17-35(15-16-36(18)21(4)22-7-9-23(10-8-22)28(29,30)31)27(5)11-13-34(14-12-27)25(37)24-19(2)32-26(38-6)33-20(24)3/h7-10,18,21H,11-17H2,1-6H3/t18-,21-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 202 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity towards muscarinic receptor M2 |
Bioorg Med Chem Lett 13: 567-71 (2003)
BindingDB Entry DOI: 10.7270/Q28K78G6 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 2
(Homo sapiens (Human)) | BDBM50439674

(RICOLINOSTAT | US10858323, Compound 2 | US11207431...)Show SMILES ONC(=O)CCCCCCNC(=O)c1cnc(nc1)N(c1ccccc1)c1ccccc1 Show InChI InChI=1S/C24H27N5O3/c30-22(28-32)15-9-1-2-10-16-25-23(31)19-17-26-24(27-18-19)29(20-11-5-3-6-12-20)21-13-7-4-8-14-21/h3-8,11-14,17-18,32H,1-2,9-10,15-16H2,(H,25,31)(H,28,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 204 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00853
BindingDB Entry DOI: 10.7270/Q2XD15N3 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM50205477

((Z)-2-((1R,2S)-2-phenylcyclopropylamino)-5-(quinol...)Show SMILES O=C1N=C(N[C@@H]2C[C@H]2c2ccccc2)SC1=Cc1ccc2ncccc2c1 |w:16.19,t:2| Show InChI InChI=1S/C22H17N3OS/c26-21-20(12-14-8-9-18-16(11-14)7-4-10-23-18)27-22(25-21)24-19-13-17(19)15-5-2-1-3-6-15/h1-12,17,19H,13H2,(H,24,25,26)/t17-,19+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center
Curated by ChEMBL
| Assay Description
Inhibition of ERK |
Bioorg Med Chem Lett 17: 2134-8 (2007)
Article DOI: 10.1016/j.bmcl.2007.01.081
BindingDB Entry DOI: 10.7270/Q2BV7G9T |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(Homo sapiens (Human)) | BDBM50104956

((2,4-Dimethyl-1-oxy-pyridin-3-yl)-(4-methyl-4-{(S)...)Show SMILES C[C@H](N1CCN(C[C@@H]1C)C1(C)CCN(CC1)C(=O)c1c(C)cc[n+]([O-])c1C)c1ccc(cc1)C(F)(F)F Show InChI InChI=1S/C28H37F3N4O2/c1-19-10-13-35(37)22(4)25(19)26(36)32-14-11-27(5,12-15-32)33-16-17-34(20(2)18-33)21(3)23-6-8-24(9-7-23)28(29,30)31/h6-10,13,20-21H,11-12,14-18H2,1-5H3/t20-,21-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity towards muscarinic receptor M2 |
Bioorg Med Chem Lett 13: 567-71 (2003)
BindingDB Entry DOI: 10.7270/Q28K78G6 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(Homo sapiens (Human)) | BDBM50123447

((3,5-Dimethyl-1-oxy-pyridin-4-yl)-(4-methyl-4-{(S)...)Show SMILES C[C@H](N1CCN(C[C@@H]1C)C1(C)CCN(CC1)C(=O)c1c(C)c[n+]([O-])cc1C)c1ccc(cc1)C(F)(F)F Show InChI InChI=1S/C28H37F3N4O2/c1-19-16-34(37)17-20(2)25(19)26(36)32-12-10-27(5,11-13-32)33-14-15-35(21(3)18-33)22(4)23-6-8-24(9-7-23)28(29,30)31/h6-9,16-17,21-22H,10-15,18H2,1-5H3/t21-,22-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 255 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity towards muscarinic receptor M2 |
Bioorg Med Chem Lett 13: 567-71 (2003)
BindingDB Entry DOI: 10.7270/Q28K78G6 |
More data for this
Ligand-Target Pair | |
Protein kinase C delta type
(Homo sapiens (Human)) | BDBM50205472

((5Z)-5-(quinolin-6-ylmethylidene)-2-[(thiophen-2-y...)Show SMILES O=C1N=C(NCc2cccs2)SC1=Cc1ccc2ncccc2c1 |w:13.15,t:2| Show InChI InChI=1S/C18H13N3OS2/c22-17-16(24-18(21-17)20-11-14-4-2-8-23-14)10-12-5-6-15-13(9-12)3-1-7-19-15/h1-10H,11H2,(H,20,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center
Curated by ChEMBL
| Assay Description
Inhibition of PKCd |
Bioorg Med Chem Lett 17: 2134-8 (2007)
Article DOI: 10.1016/j.bmcl.2007.01.081
BindingDB Entry DOI: 10.7270/Q2BV7G9T |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM50205472

((5Z)-5-(quinolin-6-ylmethylidene)-2-[(thiophen-2-y...)Show SMILES O=C1N=C(NCc2cccs2)SC1=Cc1ccc2ncccc2c1 |w:13.15,t:2| Show InChI InChI=1S/C18H13N3OS2/c22-17-16(24-18(21-17)20-11-14-4-2-8-23-14)10-12-5-6-15-13(9-12)3-1-7-19-15/h1-10H,11H2,(H,20,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center
Curated by ChEMBL
| Assay Description
Inhibition of CDK2 |
Bioorg Med Chem Lett 17: 2134-8 (2007)
Article DOI: 10.1016/j.bmcl.2007.01.081
BindingDB Entry DOI: 10.7270/Q2BV7G9T |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(Homo sapiens (Human)) | BDBM50123436

((3,5-Dichloro-1-oxy-pyridin-4-yl)-(4-methyl-4-{(S)...)Show SMILES C[C@H](N1CCN(C[C@@H]1C)C1(C)CCN(CC1)C(=O)c1c(Cl)c[n+]([O-])cc1Cl)c1ccc(cc1)C(F)(F)F Show InChI InChI=1S/C26H31Cl2F3N4O2/c1-17-14-33(12-13-35(17)18(2)19-4-6-20(7-5-19)26(29,30)31)25(3)8-10-32(11-9-25)24(36)23-21(27)15-34(37)16-22(23)28/h4-7,15-18H,8-14H2,1-3H3/t17-,18-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 348 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity towards muscarinic receptor M2 |
Bioorg Med Chem Lett 13: 567-71 (2003)
BindingDB Entry DOI: 10.7270/Q28K78G6 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(Homo sapiens (Human)) | BDBM50104956

((2,4-Dimethyl-1-oxy-pyridin-3-yl)-(4-methyl-4-{(S)...)Show SMILES C[C@H](N1CCN(C[C@@H]1C)C1(C)CCN(CC1)C(=O)c1c(C)cc[n+]([O-])c1C)c1ccc(cc1)C(F)(F)F Show InChI InChI=1S/C28H37F3N4O2/c1-19-10-13-35(37)22(4)25(19)26(36)32-14-11-27(5,12-15-32)33-16-17-34(20(2)18-33)21(3)23-6-8-24(9-7-23)28(29,30)31/h6-10,13,20-21H,11-12,14-18H2,1-5H3/t20-,21-/m0/s1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity of the compound towards muscarinic receptor M1 |
Bioorg Med Chem Lett 13: 567-71 (2003)
BindingDB Entry DOI: 10.7270/Q28K78G6 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 3
(Homo sapiens (Human)) | BDBM50593116

(CHEMBL5173493)Show SMILES CCNNC(=O)c1ccc(CN2c3ccccc3C=Cc3ccncc23)cc1 |c:19| | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00853
BindingDB Entry DOI: 10.7270/Q2XD15N3 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(Homo sapiens (Human)) | BDBM50123435

((4,6-Dimethyl-pyrimidin-5-yl)-(4-methyl-4-{(S)-3-m...)Show SMILES C[C@H](N1CCN(C[C@@H]1C)C1(C)CCN(CC1)C(=O)c1c(C)ncnc1C)c1ccc(cc1)C(F)(F)F Show InChI InChI=1S/C27H36F3N5O/c1-18-16-34(14-15-35(18)21(4)22-6-8-23(9-7-22)27(28,29)30)26(5)10-12-33(13-11-26)25(36)24-19(2)31-17-32-20(24)3/h6-9,17-18,21H,10-16H2,1-5H3/t18-,21-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 456 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of binding affinity of the compound to muscarinic receptor M2 |
Bioorg Med Chem Lett 13: 567-71 (2003)
BindingDB Entry DOI: 10.7270/Q28K78G6 |
More data for this
Ligand-Target Pair | |
Protein kinase C delta type
(Homo sapiens (Human)) | BDBM50205477

((Z)-2-((1R,2S)-2-phenylcyclopropylamino)-5-(quinol...)Show SMILES O=C1N=C(N[C@@H]2C[C@H]2c2ccccc2)SC1=Cc1ccc2ncccc2c1 |w:16.19,t:2| Show InChI InChI=1S/C22H17N3OS/c26-21-20(12-14-8-9-18-16(11-14)7-4-10-23-18)27-22(25-21)24-19-13-17(19)15-5-2-1-3-6-15/h1-12,17,19H,13H2,(H,24,25,26)/t17-,19+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center
Curated by ChEMBL
| Assay Description
Inhibition of PKCd |
Bioorg Med Chem Lett 17: 2134-8 (2007)
Article DOI: 10.1016/j.bmcl.2007.01.081
BindingDB Entry DOI: 10.7270/Q2BV7G9T |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3
(Homo sapiens (Human)) | BDBM50205472

((5Z)-5-(quinolin-6-ylmethylidene)-2-[(thiophen-2-y...)Show SMILES O=C1N=C(NCc2cccs2)SC1=Cc1ccc2ncccc2c1 |w:13.15,t:2| Show InChI InChI=1S/C18H13N3OS2/c22-17-16(24-18(21-17)20-11-14-4-2-8-23-14)10-12-5-6-15-13(9-12)3-1-7-19-15/h1-10H,11H2,(H,20,21,22) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center
Curated by ChEMBL
| Assay Description
Inhibition of GSKp1 |
Bioorg Med Chem Lett 17: 2134-8 (2007)
Article DOI: 10.1016/j.bmcl.2007.01.081
BindingDB Entry DOI: 10.7270/Q2BV7G9T |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(Homo sapiens (Human)) | BDBM50123435

((4,6-Dimethyl-pyrimidin-5-yl)-(4-methyl-4-{(S)-3-m...)Show SMILES C[C@H](N1CCN(C[C@@H]1C)C1(C)CCN(CC1)C(=O)c1c(C)ncnc1C)c1ccc(cc1)C(F)(F)F Show InChI InChI=1S/C27H36F3N5O/c1-18-16-34(14-15-35(18)21(4)22-6-8-23(9-7-22)27(28,29)30)26(5)10-12-33(13-11-26)25(36)24-19(2)31-17-32-20(24)3/h6-9,17-18,21H,10-16H2,1-5H3/t18-,21-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 575 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity towards muscarinic receptor M2 |
Bioorg Med Chem Lett 13: 567-71 (2003)
BindingDB Entry DOI: 10.7270/Q28K78G6 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(Homo sapiens (Human)) | BDBM50123444

((4,6-Dimethyl-2-trifluoromethyl-pyrimidin-5-yl)-(4...)Show SMILES C[C@H](N1CCN(C[C@@H]1C)C1(C)CCN(CC1)C(=O)c1c(C)nc(nc1C)C(F)(F)F)c1ccc(cc1)C(F)(F)F Show InChI InChI=1S/C28H35F6N5O/c1-17-16-38(14-15-39(17)20(4)21-6-8-22(9-7-21)27(29,30)31)26(5)10-12-37(13-11-26)24(40)23-18(2)35-25(28(32,33)34)36-19(23)3/h6-9,17,20H,10-16H2,1-5H3/t17-,20-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 613 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity towards muscarinic receptor M2 |
Bioorg Med Chem Lett 13: 567-71 (2003)
BindingDB Entry DOI: 10.7270/Q28K78G6 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50593116

(CHEMBL5173493)Show SMILES CCNNC(=O)c1ccc(CN2c3ccccc3C=Cc3ccncc23)cc1 |c:19| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 875 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00853
BindingDB Entry DOI: 10.7270/Q2XD15N3 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(Homo sapiens (Human)) | BDBM50123442

((4-Methyl-4-{(S)-3-methyl-4-[(S)-1-(4-trifluoromet...)Show SMILES C[C@H](N1CCN(C[C@@H]1C)C1(C)CCN(CC1)C(=O)c1c(C)nc(C)nc1C)c1ccc(cc1)C(F)(F)F Show InChI InChI=1S/C28H38F3N5O/c1-18-17-35(15-16-36(18)21(4)23-7-9-24(10-8-23)28(29,30)31)27(6)11-13-34(14-12-27)26(37)25-19(2)32-22(5)33-20(25)3/h7-10,18,21H,11-17H2,1-6H3/t18-,21-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 985 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity towards muscarinic receptor M2 |
Bioorg Med Chem Lett 13: 567-71 (2003)
BindingDB Entry DOI: 10.7270/Q28K78G6 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data