 Found 775 hits with Last Name = 'dalgarno' and Initial = 'dc'
Found 775 hits with Last Name = 'dalgarno' and Initial = 'dc' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
L-lactate dehydrogenase A chain
(Homo sapiens (Human)) | BDBM23222
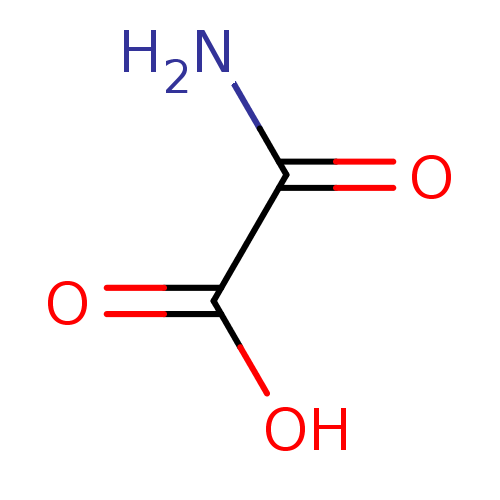
(Oxalamic acid | Oxamate | Oxamate, 3 | Oxamidic Ac...)Show InChI InChI=1S/C2H3NO3/c3-1(4)2(5)6/h(H2,3,4)(H,5,6) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
| PDB
Article
PubMed
| 2.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human LDH-A |
J Med Chem 56: 1023-40 (2013)
Article DOI: 10.1021/jm3014844
BindingDB Entry DOI: 10.7270/Q2QC04TB |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase Lyn
(Homo sapiens (Human)) | BDBM50345579

(5-((5-(4-((4-(2-hydroxyethyl)piperazin-1-yl)methyl...)Show SMILES CNC(=O)c1ncc(C#Cc2cc(ccc2C)C(=O)Nc2ccc(CN3CCN(CCO)CC3)c(c2)C(F)(F)F)n1C Show InChI InChI=1S/C30H33F3N6O3/c1-20-4-5-22(16-21(20)7-9-25-18-35-27(37(25)3)29(42)34-2)28(41)36-24-8-6-23(26(17-24)30(31,32)33)19-39-12-10-38(11-13-39)14-15-40/h4-6,8,16-18,40H,10-15,19H2,1-3H3,(H,34,42)(H,36,41) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <0.150 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human LYN using poly[Glu:Tyr] by Hotspot assay |
Bioorg Med Chem Lett 21: 3743-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.060
BindingDB Entry DOI: 10.7270/Q21G0MM8 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM50345579

(5-((5-(4-((4-(2-hydroxyethyl)piperazin-1-yl)methyl...)Show SMILES CNC(=O)c1ncc(C#Cc2cc(ccc2C)C(=O)Nc2ccc(CN3CCN(CCO)CC3)c(c2)C(F)(F)F)n1C Show InChI InChI=1S/C30H33F3N6O3/c1-20-4-5-22(16-21(20)7-9-25-18-35-27(37(25)3)29(42)34-2)28(41)36-24-8-6-23(26(17-24)30(31,32)33)19-39-12-10-38(11-13-39)14-15-40/h4-6,8,16-18,40H,10-15,19H2,1-3H3,(H,34,42)(H,36,41) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of wild type human ABL using [EAIYAAPFAKKK] peptide substrate by Hotspot assay |
Bioorg Med Chem Lett 21: 3743-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.060
BindingDB Entry DOI: 10.7270/Q21G0MM8 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM50345579

(5-((5-(4-((4-(2-hydroxyethyl)piperazin-1-yl)methyl...)Show SMILES CNC(=O)c1ncc(C#Cc2cc(ccc2C)C(=O)Nc2ccc(CN3CCN(CCO)CC3)c(c2)C(F)(F)F)n1C Show InChI InChI=1S/C30H33F3N6O3/c1-20-4-5-22(16-21(20)7-9-25-18-35-27(37(25)3)29(42)34-2)28(41)36-24-8-6-23(26(17-24)30(31,32)33)19-39-12-10-38(11-13-39)14-15-40/h4-6,8,16-18,40H,10-15,19H2,1-3H3,(H,34,42)(H,36,41) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of wild type human ABL using [EAIYAAPFAKKK] peptide substrate by Hotspot assay |
Bioorg Med Chem Lett 21: 3743-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.060
BindingDB Entry DOI: 10.7270/Q21G0MM8 |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase receptor Ret
(Homo sapiens (Human)) | BDBM50345579

(5-((5-(4-((4-(2-hydroxyethyl)piperazin-1-yl)methyl...)Show SMILES CNC(=O)c1ncc(C#Cc2cc(ccc2C)C(=O)Nc2ccc(CN3CCN(CCO)CC3)c(c2)C(F)(F)F)n1C Show InChI InChI=1S/C30H33F3N6O3/c1-20-4-5-22(16-21(20)7-9-25-18-35-27(37(25)3)29(42)34-2)28(41)36-24-8-6-23(26(17-24)30(31,32)33)19-39-12-10-38(11-13-39)14-15-40/h4-6,8,16-18,40H,10-15,19H2,1-3H3,(H,34,42)(H,36,41) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human RET using KKKSPGEYVNIEFG by Hotspot assay |
Bioorg Med Chem Lett 21: 3743-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.060
BindingDB Entry DOI: 10.7270/Q21G0MM8 |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50132351
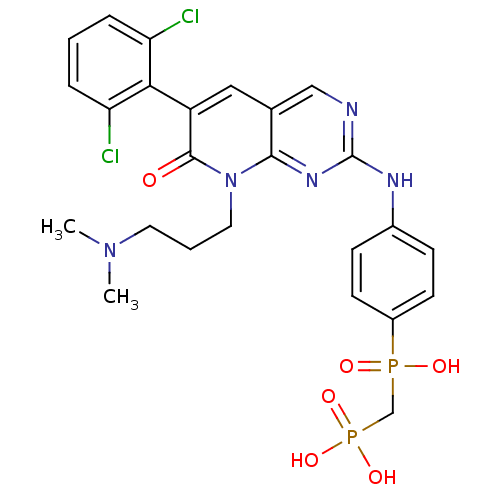
(({4-[6-(2,6-Dichloro-phenyl)-8-(3-dimethylamino-pr...)Show SMILES CN(C)CCCn1c2nc(Nc3ccc(cc3)P(O)(=O)CP(O)(O)=O)ncc2cc(-c2c(Cl)cccc2Cl)c1=O |(3.9,-7.22,;3.9,-5.68,;2.57,-4.91,;5.23,-4.91,;5.23,-3.37,;6.56,-2.59,;6.56,-1.05,;5.21,-.28,;3.88,-1.05,;2.55,-.29,;1.22,-1.06,;-.11,-.29,;-.11,1.25,;-1.44,2.01,;-2.78,1.23,;-2.77,-.31,;-1.44,-1.07,;-4.12,2,;-4.89,.67,;-3.35,3.34,;-5.45,2.77,;-5.45,4.31,;-6.99,4.32,;-5.86,5.8,;-4.12,5.09,;2.55,1.26,;3.88,2.03,;5.21,1.26,;6.54,2.03,;7.89,1.25,;9.22,2.02,;9.22,3.57,;7.89,4.33,;10.55,4.36,;11.88,3.57,;11.88,2.02,;10.55,1.25,;10.55,-.29,;7.89,-.28,;9.22,-1.05,)| Show InChI InChI=1S/C25H27Cl2N5O6P2/c1-31(2)11-4-12-32-23-16(13-19(24(32)33)22-20(26)5-3-6-21(22)27)14-28-25(30-23)29-17-7-9-18(10-8-17)39(34,35)15-40(36,37)38/h3,5-10,13-14H,4,11-12,15H2,1-2H3,(H,34,35)(H,28,29,30)(H2,36,37,38) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Src protein tryrosine kinase |
Bioorg Med Chem Lett 13: 3071-4 (2003)
BindingDB Entry DOI: 10.7270/Q21J995T |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM50322535

(3-(imidazo[1,2-b]pyridazin-3-ylethynyl)-4-methyl-N...)Show SMILES CN1CCN(Cc2ccc(NC(=O)c3ccc(C)c(c3)C#Cc3cnc4cccnn34)cc2C(F)(F)F)CC1 Show InChI InChI=1S/C29H27F3N6O/c1-20-5-6-22(16-21(20)8-10-25-18-33-27-4-3-11-34-38(25)27)28(39)35-24-9-7-23(26(17-24)29(30,31)32)19-37-14-12-36(2)13-15-37/h3-7,9,11,16-18H,12-15,19H2,1-2H3,(H,35,39) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| n/a | n/a | 0.370 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of ABL autophosphorylation |
J Med Chem 53: 4701-19 (2010)
Article DOI: 10.1021/jm100395q
BindingDB Entry DOI: 10.7270/Q27P8ZKX |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM82130
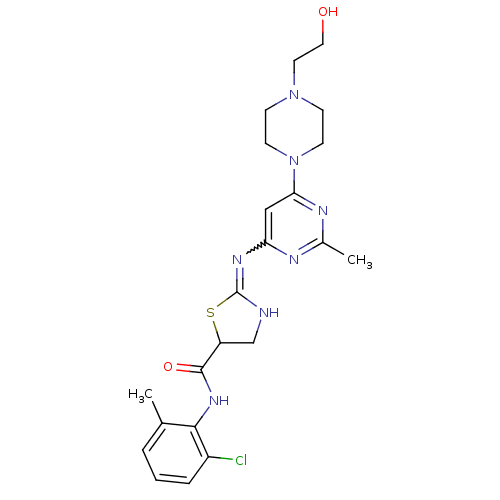
(Dasatinib)Show SMILES Cc1nc(cc(n1)N1CCN(CCO)CC1)N=C1NCC(S1)C(=O)Nc1c(C)cccc1Cl |w:16.17| Show InChI InChI=1S/C22H28ClN7O2S/c1-14-4-3-5-16(23)20(14)28-21(32)17-13-24-22(33-17)27-18-12-19(26-15(2)25-18)30-8-6-29(7-9-30)10-11-31/h3-5,12,17,31H,6-11,13H2,1-2H3,(H,28,32)(H,24,25,26,27) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals Inc
| Assay Description
In vitro kinase assay using Abl, Abl T315I or Src kinase. |
Chem Biol Drug Des 75: 18-28 (2010)
Article DOI: 10.1111/j.1747-0285.2009.00905.x
BindingDB Entry DOI: 10.7270/Q2MC8XHX |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM82130

(Dasatinib)Show SMILES Cc1nc(cc(n1)N1CCN(CCO)CC1)N=C1NCC(S1)C(=O)Nc1c(C)cccc1Cl |w:16.17| Show InChI InChI=1S/C22H28ClN7O2S/c1-14-4-3-5-16(23)20(14)28-21(32)17-13-24-22(33-17)27-18-12-19(26-15(2)25-18)30-8-6-29(7-9-30)10-11-31/h3-5,12,17,31H,6-11,13H2,1-2H3,(H,28,32)(H,24,25,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals Inc
| Assay Description
In vitro kinase assay using Abl, Abl T315I or Src kinase. |
Chem Biol Drug Des 75: 18-28 (2010)
Article DOI: 10.1111/j.1747-0285.2009.00905.x
BindingDB Entry DOI: 10.7270/Q2MC8XHX |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM50244569
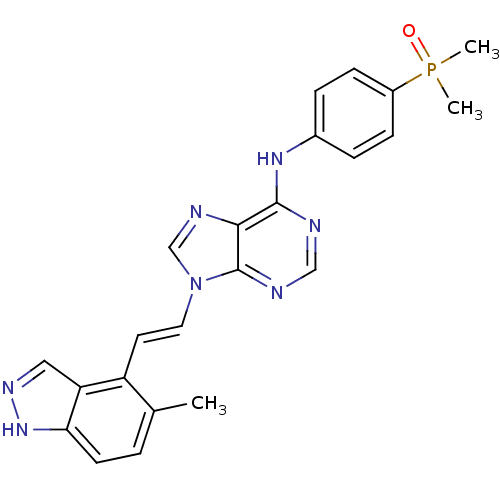
(AP24283 | CHEMBL510893 | N-(4-(dimethylphosphoryl)...)Show SMILES Cc1ccc2[nH]ncc2c1\C=C\n1cnc2c(Nc3ccc(cc3)P(C)(C)=O)ncnc12 Show InChI InChI=1S/C23H22N7OP/c1-15-4-9-20-19(12-27-29-20)18(15)10-11-30-14-26-21-22(24-13-25-23(21)30)28-16-5-7-17(8-6-16)32(2,3)31/h4-14H,1-3H3,(H,27,29)(H,24,25,28)/b11-10+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | <0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals Inc
| Assay Description
In vitro kinase assay using Abl, Abl T315I or Src kinase. |
Chem Biol Drug Des 75: 18-28 (2010)
Article DOI: 10.1111/j.1747-0285.2009.00905.x
BindingDB Entry DOI: 10.7270/Q2MC8XHX |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Isoform 1 of Fibronectin (1)
(Homo sapiens (Human)) | BDBM50244569

(AP24283 | CHEMBL510893 | N-(4-(dimethylphosphoryl)...)Show SMILES Cc1ccc2[nH]ncc2c1\C=C\n1cnc2c(Nc3ccc(cc3)P(C)(C)=O)ncnc12 Show InChI InChI=1S/C23H22N7OP/c1-15-4-9-20-19(12-27-29-20)18(15)10-11-30-14-26-21-22(24-13-25-23(21)30)28-16-5-7-17(8-6-16)32(2,3)31/h4-14H,1-3H3,(H,27,29)(H,24,25,28)/b11-10+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | <0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals Inc
| Assay Description
In vitro kinase assay using Abl, Abl T315I or Src kinase. |
Chem Biol Drug Des 75: 18-28 (2010)
Article DOI: 10.1111/j.1747-0285.2009.00905.x
BindingDB Entry DOI: 10.7270/Q2MC8XHX |
More data for this
Ligand-Target Pair | |
Ephrin type-A receptor 2
(Homo sapiens (Human)) | BDBM50345579

(5-((5-(4-((4-(2-hydroxyethyl)piperazin-1-yl)methyl...)Show SMILES CNC(=O)c1ncc(C#Cc2cc(ccc2C)C(=O)Nc2ccc(CN3CCN(CCO)CC3)c(c2)C(F)(F)F)n1C Show InChI InChI=1S/C30H33F3N6O3/c1-20-4-5-22(16-21(20)7-9-25-18-35-27(37(25)3)29(42)34-2)28(41)36-24-8-6-23(26(17-24)30(31,32)33)19-39-12-10-38(11-13-39)14-15-40/h4-6,8,16-18,40H,10-15,19H2,1-3H3,(H,34,42)(H,36,41) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human EPHA2 using poly[Glu:Tyr] peptide substrate by Hotspot assay |
Bioorg Med Chem Lett 21: 3743-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.060
BindingDB Entry DOI: 10.7270/Q21G0MM8 |
More data for this
Ligand-Target Pair | |
Ephrin type-A receptor 3
(Homo sapiens (Human)) | BDBM50345579

(5-((5-(4-((4-(2-hydroxyethyl)piperazin-1-yl)methyl...)Show SMILES CNC(=O)c1ncc(C#Cc2cc(ccc2C)C(=O)Nc2ccc(CN3CCN(CCO)CC3)c(c2)C(F)(F)F)n1C Show InChI InChI=1S/C30H33F3N6O3/c1-20-4-5-22(16-21(20)7-9-25-18-35-27(37(25)3)29(42)34-2)28(41)36-24-8-6-23(26(17-24)30(31,32)33)19-39-12-10-38(11-13-39)14-15-40/h4-6,8,16-18,40H,10-15,19H2,1-3H3,(H,34,42)(H,36,41) | PDB
MMDB
Reactome pathway
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human EPHA3 using poly[Glu:Tyr] peptide substrate by Hotspot assay |
Bioorg Med Chem Lett 21: 3743-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.060
BindingDB Entry DOI: 10.7270/Q21G0MM8 |
More data for this
Ligand-Target Pair | |
Platelet-derived growth factor receptor alpha
(Homo sapiens (Human)) | BDBM50345579

(5-((5-(4-((4-(2-hydroxyethyl)piperazin-1-yl)methyl...)Show SMILES CNC(=O)c1ncc(C#Cc2cc(ccc2C)C(=O)Nc2ccc(CN3CCN(CCO)CC3)c(c2)C(F)(F)F)n1C Show InChI InChI=1S/C30H33F3N6O3/c1-20-4-5-22(16-21(20)7-9-25-18-35-27(37(25)3)29(42)34-2)28(41)36-24-8-6-23(26(17-24)30(31,32)33)19-39-12-10-38(11-13-39)14-15-40/h4-6,8,16-18,40H,10-15,19H2,1-3H3,(H,34,42)(H,36,41) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human PDGFRalpha using poly[Glu:Tyr] by Hotspot assay |
Bioorg Med Chem Lett 21: 3743-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.060
BindingDB Entry DOI: 10.7270/Q21G0MM8 |
More data for this
Ligand-Target Pair | |
Fibroblast growth factor receptor 2
(Homo sapiens (Human)) | BDBM50345579

(5-((5-(4-((4-(2-hydroxyethyl)piperazin-1-yl)methyl...)Show SMILES CNC(=O)c1ncc(C#Cc2cc(ccc2C)C(=O)Nc2ccc(CN3CCN(CCO)CC3)c(c2)C(F)(F)F)n1C Show InChI InChI=1S/C30H33F3N6O3/c1-20-4-5-22(16-21(20)7-9-25-18-35-27(37(25)3)29(42)34-2)28(41)36-24-8-6-23(26(17-24)30(31,32)33)19-39-12-10-38(11-13-39)14-15-40/h4-6,8,16-18,40H,10-15,19H2,1-3H3,(H,34,42)(H,36,41) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human FGFR2 using poly[Glu:Tyr] peptide substrate by Hotspot assay |
Bioorg Med Chem Lett 21: 3743-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.060
BindingDB Entry DOI: 10.7270/Q21G0MM8 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM50322533
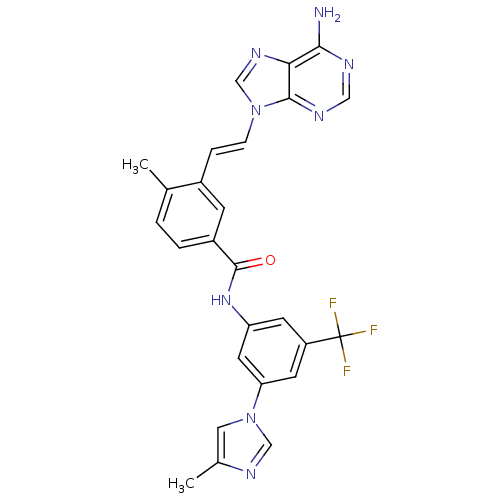
(3-(2-(6-amino-9H-purin-9-yl)vinyl)-4-methyl-N-(3-(...)Show SMILES Cc1cn(cn1)-c1cc(NC(=O)c2ccc(C)c(\C=C\n3cnc4c(N)ncnc34)c2)cc(c1)C(F)(F)F Show InChI InChI=1S/C26H21F3N8O/c1-15-3-4-18(7-17(15)5-6-36-14-34-22-23(30)31-12-32-24(22)36)25(38)35-20-8-19(26(27,28)29)9-21(10-20)37-11-16(2)33-13-37/h3-14H,1-2H3,(H,35,38)(H2,30,31,32)/b6-5+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of wild type human ABL after 1 hr by TR-FRET assay |
J Med Chem 53: 4701-19 (2010)
Article DOI: 10.1021/jm100395q
BindingDB Entry DOI: 10.7270/Q27P8ZKX |
More data for this
Ligand-Target Pair | |
Fibroblast growth factor receptor 1
(Homo sapiens (Human)) | BDBM50345579

(5-((5-(4-((4-(2-hydroxyethyl)piperazin-1-yl)methyl...)Show SMILES CNC(=O)c1ncc(C#Cc2cc(ccc2C)C(=O)Nc2ccc(CN3CCN(CCO)CC3)c(c2)C(F)(F)F)n1C Show InChI InChI=1S/C30H33F3N6O3/c1-20-4-5-22(16-21(20)7-9-25-18-35-27(37(25)3)29(42)34-2)28(41)36-24-8-6-23(26(17-24)30(31,32)33)19-39-12-10-38(11-13-39)14-15-40/h4-6,8,16-18,40H,10-15,19H2,1-3H3,(H,34,42)(H,36,41) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human FGFR1 using KKKSPGEYVNIEFG peptide substrate by Hotspot assay |
Bioorg Med Chem Lett 21: 3743-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.060
BindingDB Entry DOI: 10.7270/Q21G0MM8 |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50132348
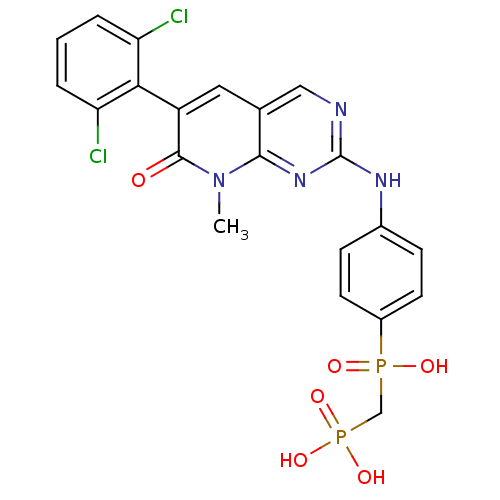
(({4-[6-(2,6-Dichloro-phenyl)-8-methyl-7-oxo-7,8-di...)Show SMILES Cn1c2nc(Nc3ccc(cc3)P(O)(=O)CP(O)(O)=O)ncc2cc(-c2c(Cl)cccc2Cl)c1=O |(3.94,-2.74,;3.93,-1.2,;2.6,-.43,;1.27,-1.2,;-.06,-.43,;-1.39,-1.21,;-2.73,-.45,;-2.73,1.1,;-4.06,1.86,;-5.41,1.09,;-5.38,-.47,;-4.05,-1.22,;-6.74,1.86,;-7.53,.53,;-5.97,3.19,;-8.09,2.61,;-9.4,1.82,;-10.19,3.17,;-10.91,1.42,;-9.02,.34,;-.07,1.11,;1.27,1.88,;2.6,1.11,;3.93,1.88,;5.26,1.11,;6.59,1.88,;6.59,3.42,;5.26,4.19,;7.92,4.19,;9.26,3.42,;9.26,1.88,;7.92,1.11,;7.92,-.43,;5.26,-.43,;6.6,-1.2,)| Show InChI InChI=1S/C21H18Cl2N4O6P2/c1-27-19-12(9-15(20(27)28)18-16(22)3-2-4-17(18)23)10-24-21(26-19)25-13-5-7-14(8-6-13)34(29,30)11-35(31,32)33/h2-10H,11H2,1H3,(H,29,30)(H,24,25,26)(H2,31,32,33) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Src protein tryrosine kinase |
Bioorg Med Chem Lett 13: 3071-4 (2003)
BindingDB Entry DOI: 10.7270/Q21J995T |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50345579

(5-((5-(4-((4-(2-hydroxyethyl)piperazin-1-yl)methyl...)Show SMILES CNC(=O)c1ncc(C#Cc2cc(ccc2C)C(=O)Nc2ccc(CN3CCN(CCO)CC3)c(c2)C(F)(F)F)n1C Show InChI InChI=1S/C30H33F3N6O3/c1-20-4-5-22(16-21(20)7-9-25-18-35-27(37(25)3)29(42)34-2)28(41)36-24-8-6-23(26(17-24)30(31,32)33)19-39-12-10-38(11-13-39)14-15-40/h4-6,8,16-18,40H,10-15,19H2,1-3H3,(H,34,42)(H,36,41) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human FLT3 using EAIYAAPFAKKK peptide substrate by Hotspot assay |
Bioorg Med Chem Lett 21: 3743-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.060
BindingDB Entry DOI: 10.7270/Q21G0MM8 |
More data for this
Ligand-Target Pair | |
Angiopoietin-1 receptor
(Homo sapiens (Human)) | BDBM50345579

(5-((5-(4-((4-(2-hydroxyethyl)piperazin-1-yl)methyl...)Show SMILES CNC(=O)c1ncc(C#Cc2cc(ccc2C)C(=O)Nc2ccc(CN3CCN(CCO)CC3)c(c2)C(F)(F)F)n1C Show InChI InChI=1S/C30H33F3N6O3/c1-20-4-5-22(16-21(20)7-9-25-18-35-27(37(25)3)29(42)34-2)28(41)36-24-8-6-23(26(17-24)30(31,32)33)19-39-12-10-38(11-13-39)14-15-40/h4-6,8,16-18,40H,10-15,19H2,1-3H3,(H,34,42)(H,36,41) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human Tie2/TEK using poly[Glu:Tyr] by Hotspot assay |
Bioorg Med Chem Lett 21: 3743-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.060
BindingDB Entry DOI: 10.7270/Q21G0MM8 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM50322564
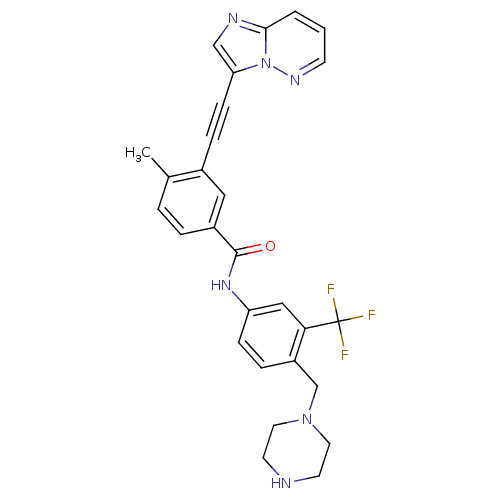
(3-[2-(Imidazo[1,2-b]pyridazin-3-yl)ethynyl]-4-meth...)Show SMILES Cc1ccc(cc1C#Cc1cnc2cccnn12)C(=O)Nc1ccc(CN2CCNCC2)c(c1)C(F)(F)F Show InChI InChI=1S/C28H25F3N6O/c1-19-4-5-21(15-20(19)7-9-24-17-33-26-3-2-10-34-37(24)26)27(38)35-23-8-6-22(25(16-23)28(29,30)31)18-36-13-11-32-12-14-36/h2-6,8,10,15-17,32H,11-14,18H2,1H3,(H,35,38) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of wild type human ABL after 1 hr by TR-FRET assay |
J Med Chem 53: 4701-19 (2010)
Article DOI: 10.1021/jm100395q
BindingDB Entry DOI: 10.7270/Q27P8ZKX |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM50322561

(3-[2-(Imidazo[1,2-a]pyrazin-3-yl)ethynyl]-4-methyl...)Show SMILES CN1CCN(Cc2ccc(NC(=O)c3ccc(C)c(c3)C#Cc3cnc4cnccn34)cc2C(F)(F)F)CC1 Show InChI InChI=1S/C29H27F3N6O/c1-20-3-4-22(15-21(20)6-8-25-17-34-27-18-33-9-10-38(25)27)28(39)35-24-7-5-23(26(16-24)29(30,31)32)19-37-13-11-36(2)12-14-37/h3-5,7,9-10,15-18H,11-14,19H2,1-2H3,(H,35,39) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of wild type human ABL after 1 hr by TR-FRET assay |
J Med Chem 53: 4701-19 (2010)
Article DOI: 10.1021/jm100395q
BindingDB Entry DOI: 10.7270/Q27P8ZKX |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM50322538
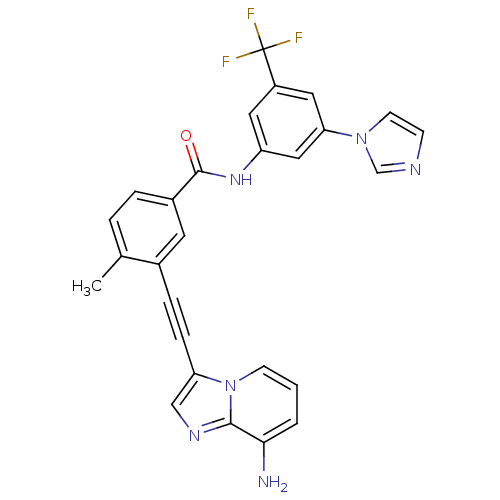
(3-[2-(8-Aminoimidazo[1,2-a]pyridin-3-yl)ethynyl]-N...)Show SMILES Cc1ccc(cc1C#Cc1cnc2c(N)cccn12)C(=O)Nc1cc(cc(c1)C(F)(F)F)-n1ccnc1 Show InChI InChI=1S/C27H19F3N6O/c1-17-4-5-19(11-18(17)6-7-22-15-33-25-24(31)3-2-9-36(22)25)26(37)34-21-12-20(27(28,29)30)13-23(14-21)35-10-8-32-16-35/h2-5,8-16H,31H2,1H3,(H,34,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of wild type human ABL after 1 hr by TR-FRET assay |
J Med Chem 53: 4701-19 (2010)
Article DOI: 10.1021/jm100395q
BindingDB Entry DOI: 10.7270/Q27P8ZKX |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50345579

(5-((5-(4-((4-(2-hydroxyethyl)piperazin-1-yl)methyl...)Show SMILES CNC(=O)c1ncc(C#Cc2cc(ccc2C)C(=O)Nc2ccc(CN3CCN(CCO)CC3)c(c2)C(F)(F)F)n1C Show InChI InChI=1S/C30H33F3N6O3/c1-20-4-5-22(16-21(20)7-9-25-18-35-27(37(25)3)29(42)34-2)28(41)36-24-8-6-23(26(17-24)30(31,32)33)19-39-12-10-38(11-13-39)14-15-40/h4-6,8,16-18,40H,10-15,19H2,1-3H3,(H,34,42)(H,36,41) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human VEGFR2 using poly[Glu:Tyr] by Hotspot assay |
Bioorg Med Chem Lett 21: 3743-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.060
BindingDB Entry DOI: 10.7270/Q21G0MM8 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM50244527
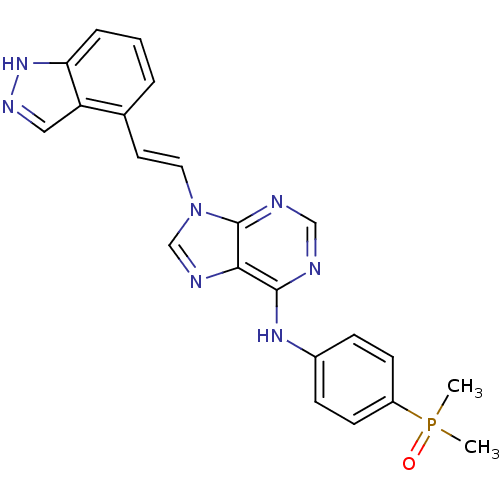
(9-(2-(1H-indazol-4-yl)vinyl)-N-(4-(dimethylphospho...)Show SMILES CP(C)(=O)c1ccc(Nc2ncnc3n(\C=C\c4cccc5[nH]ncc45)cnc23)cc1 Show InChI InChI=1S/C22H20N7OP/c1-31(2,30)17-8-6-16(7-9-17)27-21-20-22(24-13-23-21)29(14-25-20)11-10-15-4-3-5-19-18(15)12-26-28-19/h3-14H,1-2H3,(H,26,28)(H,23,24,27)/b11-10+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals Inc
| Assay Description
In vitro kinase assay using Abl, Abl T315I or Src kinase. |
Chem Biol Drug Des 75: 18-28 (2010)
Article DOI: 10.1111/j.1747-0285.2009.00905.x
BindingDB Entry DOI: 10.7270/Q2MC8XHX |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM50322568
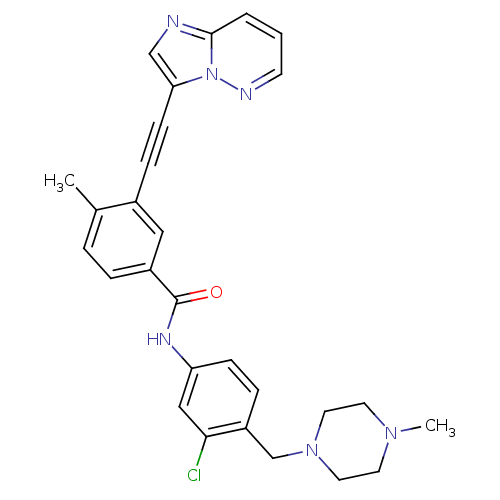
(CHEMBL1172718 | N-{3-Chloro-4-[(4-methylpiperazin-...)Show SMILES CN1CCN(Cc2ccc(NC(=O)c3ccc(C)c(c3)C#Cc3cnc4cccnn34)cc2Cl)CC1 Show InChI InChI=1S/C28H27ClN6O/c1-20-5-6-22(16-21(20)8-10-25-18-30-27-4-3-11-31-35(25)27)28(36)32-24-9-7-23(26(29)17-24)19-34-14-12-33(2)13-15-34/h3-7,9,11,16-18H,12-15,19H2,1-2H3,(H,32,36) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of wild type human ABL after 1 hr by TR-FRET assay |
J Med Chem 53: 4701-19 (2010)
Article DOI: 10.1021/jm100395q
BindingDB Entry DOI: 10.7270/Q27P8ZKX |
More data for this
Ligand-Target Pair | |
Platelet-derived growth factor receptor beta
(Homo sapiens (Human)) | BDBM50345579

(5-((5-(4-((4-(2-hydroxyethyl)piperazin-1-yl)methyl...)Show SMILES CNC(=O)c1ncc(C#Cc2cc(ccc2C)C(=O)Nc2ccc(CN3CCN(CCO)CC3)c(c2)C(F)(F)F)n1C Show InChI InChI=1S/C30H33F3N6O3/c1-20-4-5-22(16-21(20)7-9-25-18-35-27(37(25)3)29(42)34-2)28(41)36-24-8-6-23(26(17-24)30(31,32)33)19-39-12-10-38(11-13-39)14-15-40/h4-6,8,16-18,40H,10-15,19H2,1-3H3,(H,34,42)(H,36,41) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human PDGFRbeta using poly[Glu:Tyr] by Hotspot assay |
Bioorg Med Chem Lett 21: 3743-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.060
BindingDB Entry DOI: 10.7270/Q21G0MM8 |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 3
(Homo sapiens (Human)) | BDBM50345579

(5-((5-(4-((4-(2-hydroxyethyl)piperazin-1-yl)methyl...)Show SMILES CNC(=O)c1ncc(C#Cc2cc(ccc2C)C(=O)Nc2ccc(CN3CCN(CCO)CC3)c(c2)C(F)(F)F)n1C Show InChI InChI=1S/C30H33F3N6O3/c1-20-4-5-22(16-21(20)7-9-25-18-35-27(37(25)3)29(42)34-2)28(41)36-24-8-6-23(26(17-24)30(31,32)33)19-39-12-10-38(11-13-39)14-15-40/h4-6,8,16-18,40H,10-15,19H2,1-3H3,(H,34,42)(H,36,41) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human VEGFR3 using poly[Glu:Tyr] by Hotspot assay |
Bioorg Med Chem Lett 21: 3743-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.060
BindingDB Entry DOI: 10.7270/Q21G0MM8 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50322823

((S)-N-(4-(3-chloro-4-fluorophenylamino)-7-(tetrahy...)Show SMILES CN(C)C\C=C\C(=O)Nc1cc2c(Nc3ccc(F)c(Cl)c3)ncnc2cc1O[C@H]1CCOC1 |r| Show InChI InChI=1S/C24H25ClFN5O3/c1-31(2)8-3-4-23(32)30-21-11-17-20(12-22(21)34-16-7-9-33-13-16)27-14-28-24(17)29-15-5-6-19(26)18(25)10-15/h3-6,10-12,14,16H,7-9,13H2,1-2H3,(H,30,32)(H,27,28,29)/b4-3+/t16-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
| n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM50345584

(5-((5-(4-((4-(2-hydroxyethyl)piperazin-1-yl)methyl...)Show SMILES Cc1ccc(cc1C#Cc1cnc(C(N)=O)n1C)C(=O)Nc1ccc(CN2CCN(CCO)CC2)c(c1)C(F)(F)F Show InChI InChI=1S/C29H31F3N6O3/c1-19-3-4-21(15-20(19)6-8-24-17-34-27(26(33)40)36(24)2)28(41)35-23-7-5-22(25(16-23)29(30,31)32)18-38-11-9-37(10-12-38)13-14-39/h3-5,7,15-17,39H,9-14,18H2,1-2H3,(H2,33,40)(H,35,41) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of wild type human ABL using [EAIYAAPFAKKK] peptide substrate by Hotspot assay |
Bioorg Med Chem Lett 21: 3743-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.060
BindingDB Entry DOI: 10.7270/Q21G0MM8 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM50244430
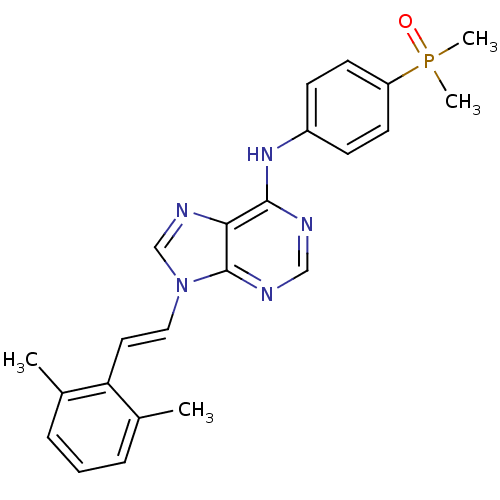
(9-(2,6-dimethylstyryl)-N-(4-(dimethylphosphoryl)ph...)Show SMILES Cc1cccc(C)c1\C=C\n1cnc2c(Nc3ccc(cc3)P(C)(C)=O)ncnc12 Show InChI InChI=1S/C23H24N5OP/c1-16-6-5-7-17(2)20(16)12-13-28-15-26-21-22(24-14-25-23(21)28)27-18-8-10-19(11-9-18)30(3,4)29/h5-15H,1-4H3,(H,24,25,27)/b13-12+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals Inc
| Assay Description
In vitro kinase assay using Abl, Abl T315I or Src kinase. |
Chem Biol Drug Des 75: 18-28 (2010)
Article DOI: 10.1111/j.1747-0285.2009.00905.x
BindingDB Entry DOI: 10.7270/Q2MC8XHX |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM50345587
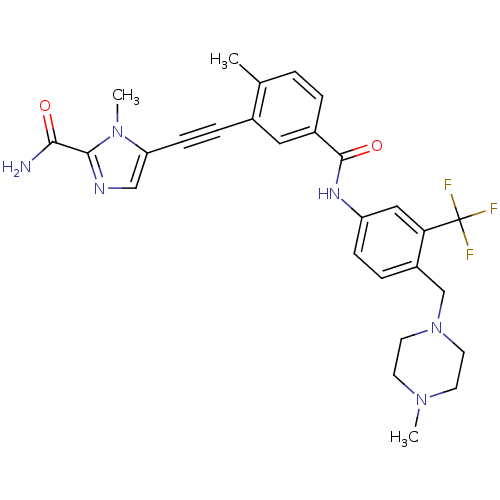
(1-methyl-5-((2-methyl-5-(4-((4-methylpiperazin-1-y...)Show SMILES CN1CCN(Cc2ccc(NC(=O)c3ccc(C)c(c3)C#Cc3cnc(C(N)=O)n3C)cc2C(F)(F)F)CC1 Show InChI InChI=1S/C28H29F3N6O2/c1-18-4-5-20(14-19(18)7-9-23-16-33-26(25(32)38)36(23)3)27(39)34-22-8-6-21(24(15-22)28(29,30)31)17-37-12-10-35(2)11-13-37/h4-6,8,14-16H,10-13,17H2,1-3H3,(H2,32,38)(H,34,39) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of wild type human ABL using [EAIYAAPFAKKK] peptide substrate by Hotspot assay |
Bioorg Med Chem Lett 21: 3743-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.060
BindingDB Entry DOI: 10.7270/Q21G0MM8 |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50345579

(5-((5-(4-((4-(2-hydroxyethyl)piperazin-1-yl)methyl...)Show SMILES CNC(=O)c1ncc(C#Cc2cc(ccc2C)C(=O)Nc2ccc(CN3CCN(CCO)CC3)c(c2)C(F)(F)F)n1C Show InChI InChI=1S/C30H33F3N6O3/c1-20-4-5-22(16-21(20)7-9-25-18-35-27(37(25)3)29(42)34-2)28(41)36-24-8-6-23(26(17-24)30(31,32)33)19-39-12-10-38(11-13-39)14-15-40/h4-6,8,16-18,40H,10-15,19H2,1-3H3,(H,34,42)(H,36,41) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human Src using poly[Glu:Tyr] by Hotspot assay |
Bioorg Med Chem Lett 21: 3743-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.060
BindingDB Entry DOI: 10.7270/Q21G0MM8 |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50132322

(({2-[(2-{4-[4-Amino-5-(3-hydroxy-phenyl)-pyrrolo[2...)Show SMILES CN(CCOP(O)(=O)CP(O)(O)=O)CCc1ccc(cc1)-n1cc(-c2cccc(O)c2)c2c(N)ncnc12 Show InChI InChI=1S/C24H29N5O7P2/c1-28(11-12-36-38(34,35)16-37(31,32)33)10-9-17-5-7-19(8-6-17)29-14-21(18-3-2-4-20(30)13-18)22-23(25)26-15-27-24(22)29/h2-8,13-15,30H,9-12,16H2,1H3,(H,34,35)(H2,25,26,27)(H2,31,32,33) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibition of Src protein tryrosine kinase using the Scintillation proximity kinase assay. |
Bioorg Med Chem Lett 13: 3063-6 (2003)
BindingDB Entry DOI: 10.7270/Q290235N |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM27216

((2R)-2-({6-[(3-chlorophenyl)amino]-9-(propan-2-yl)...)Show SMILES CC(C)[C@H](CO)Nc1nc(Nc2cccc(Cl)c2)c2ncn(C(C)C)c2n1 Show InChI InChI=1S/C19H25ClN6O/c1-11(2)15(9-27)23-19-24-17(22-14-7-5-6-13(20)8-14)16-18(25-19)26(10-21-16)12(3)4/h5-8,10-12,15,27H,9H2,1-4H3,(H2,22,23,24,25)/t15-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Cyclin-dependent kinase 2 (CDK2) |
Bioorg Med Chem Lett 13: 3067-70 (2003)
BindingDB Entry DOI: 10.7270/Q25B01V7 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Ephrin type-A receptor 7
(Homo sapiens (Human)) | BDBM50345579

(5-((5-(4-((4-(2-hydroxyethyl)piperazin-1-yl)methyl...)Show SMILES CNC(=O)c1ncc(C#Cc2cc(ccc2C)C(=O)Nc2ccc(CN3CCN(CCO)CC3)c(c2)C(F)(F)F)n1C Show InChI InChI=1S/C30H33F3N6O3/c1-20-4-5-22(16-21(20)7-9-25-18-35-27(37(25)3)29(42)34-2)28(41)36-24-8-6-23(26(17-24)30(31,32)33)19-39-12-10-38(11-13-39)14-15-40/h4-6,8,16-18,40H,10-15,19H2,1-3H3,(H,34,42)(H,36,41) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human EPHA7 using poly[Glu:Tyr] peptide substrate by Hotspot assay |
Bioorg Med Chem Lett 21: 3743-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.060
BindingDB Entry DOI: 10.7270/Q21G0MM8 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM50345582
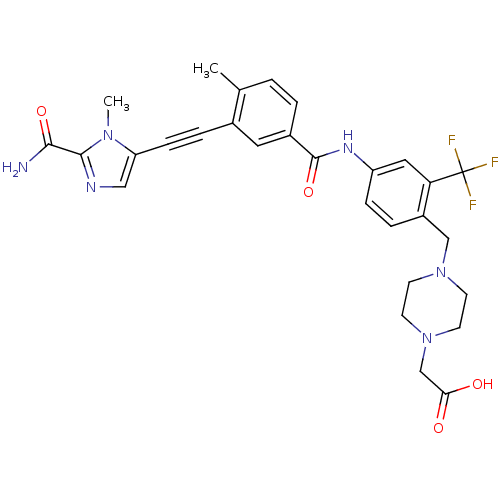
(2-(4-(4-(3-((2-carbamoyl-1-methyl-1H-imidazol-5-yl...)Show SMILES Cc1ccc(cc1C#Cc1cnc(C(N)=O)n1C)C(=O)Nc1ccc(CN2CCN(CC(O)=O)CC2)c(c1)C(F)(F)F Show InChI InChI=1S/C29H29F3N6O4/c1-18-3-4-20(13-19(18)6-8-23-15-34-27(26(33)41)36(23)2)28(42)35-22-7-5-21(24(14-22)29(30,31)32)16-37-9-11-38(12-10-37)17-25(39)40/h3-5,7,13-15H,9-12,16-17H2,1-2H3,(H2,33,41)(H,35,42)(H,39,40) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of wild type human ABL using [EAIYAAPFAKKK] peptide substrate by Hotspot assay |
Bioorg Med Chem Lett 21: 3743-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.060
BindingDB Entry DOI: 10.7270/Q21G0MM8 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM50345583
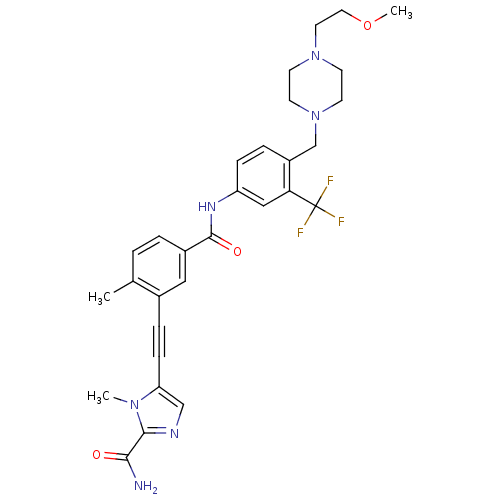
(5-((5-(4-((4-(2-methoxyethyl)piperazin-1-yl)methyl...)Show SMILES COCCN1CCN(Cc2ccc(NC(=O)c3ccc(C)c(c3)C#Cc3cnc(C(N)=O)n3C)cc2C(F)(F)F)CC1 Show InChI InChI=1S/C30H33F3N6O3/c1-20-4-5-22(16-21(20)7-9-25-18-35-28(27(34)40)37(25)2)29(41)36-24-8-6-23(26(17-24)30(31,32)33)19-39-12-10-38(11-13-39)14-15-42-3/h4-6,8,16-18H,10-15,19H2,1-3H3,(H2,34,40)(H,36,41) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of wild type human ABL using [EAIYAAPFAKKK] peptide substrate by Hotspot assay |
Bioorg Med Chem Lett 21: 3743-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.060
BindingDB Entry DOI: 10.7270/Q21G0MM8 |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 1
(Homo sapiens (Human)) | BDBM50345579

(5-((5-(4-((4-(2-hydroxyethyl)piperazin-1-yl)methyl...)Show SMILES CNC(=O)c1ncc(C#Cc2cc(ccc2C)C(=O)Nc2ccc(CN3CCN(CCO)CC3)c(c2)C(F)(F)F)n1C Show InChI InChI=1S/C30H33F3N6O3/c1-20-4-5-22(16-21(20)7-9-25-18-35-27(37(25)3)29(42)34-2)28(41)36-24-8-6-23(26(17-24)30(31,32)33)19-39-12-10-38(11-13-39)14-15-40/h4-6,8,16-18,40H,10-15,19H2,1-3H3,(H,34,42)(H,36,41) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human VEGFR1 using poly[Glu:Tyr] by Hotspot assay |
Bioorg Med Chem Lett 21: 3743-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.060
BindingDB Entry DOI: 10.7270/Q21G0MM8 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM50322569
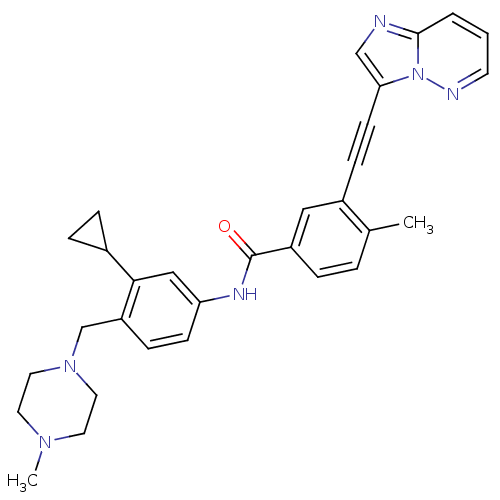
(CHEMBL1172719 | N-{3-Cyclopropyl-4-[(4-methylpiper...)Show SMILES CN1CCN(Cc2ccc(NC(=O)c3ccc(C)c(c3)C#Cc3cnc4cccnn34)cc2C2CC2)CC1 Show InChI InChI=1S/C31H32N6O/c1-22-5-6-25(18-24(22)10-12-28-20-32-30-4-3-13-33-37(28)30)31(38)34-27-11-9-26(29(19-27)23-7-8-23)21-36-16-14-35(2)15-17-36/h3-6,9,11,13,18-20,23H,7-8,14-17,21H2,1-2H3,(H,34,38) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5.30 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of wild type human ABL after 1 hr by TR-FRET assay |
J Med Chem 53: 4701-19 (2010)
Article DOI: 10.1021/jm100395q
BindingDB Entry DOI: 10.7270/Q27P8ZKX |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 2/G1/S-specific cyclin-E1
(Homo sapiens (Human)) | BDBM50345579

(5-((5-(4-((4-(2-hydroxyethyl)piperazin-1-yl)methyl...)Show SMILES CNC(=O)c1ncc(C#Cc2cc(ccc2C)C(=O)Nc2ccc(CN3CCN(CCO)CC3)c(c2)C(F)(F)F)n1C Show InChI InChI=1S/C30H33F3N6O3/c1-20-4-5-22(16-21(20)7-9-25-18-35-27(37(25)3)29(42)34-2)28(41)36-24-8-6-23(26(17-24)30(31,32)33)19-39-12-10-38(11-13-39)14-15-40/h4-6,8,16-18,40H,10-15,19H2,1-3H3,(H,34,42)(H,36,41) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.40 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human CDK2/Cyclin E using Histone H1 substrate by Hotspot assay |
Bioorg Med Chem Lett 21: 3743-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.060
BindingDB Entry DOI: 10.7270/Q21G0MM8 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM50322539
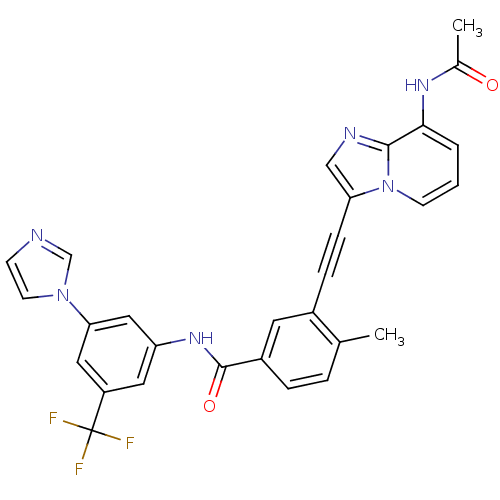
(3-{2-[8-(Acetylamino)imidazo-[1,2-a]pyridin-3-yl]e...)Show SMILES CC(=O)Nc1cccn2c(cnc12)C#Cc1cc(ccc1C)C(=O)Nc1cc(cc(c1)C(F)(F)F)-n1ccnc1 Show InChI InChI=1S/C29H21F3N6O2/c1-18-5-6-21(12-20(18)7-8-24-16-34-27-26(35-19(2)39)4-3-10-38(24)27)28(40)36-23-13-22(29(30,31)32)14-25(15-23)37-11-9-33-17-37/h3-6,9-17H,1-2H3,(H,35,39)(H,36,40) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6.40 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of wild type human ABL after 1 hr by TR-FRET assay |
J Med Chem 53: 4701-19 (2010)
Article DOI: 10.1021/jm100395q
BindingDB Entry DOI: 10.7270/Q27P8ZKX |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1
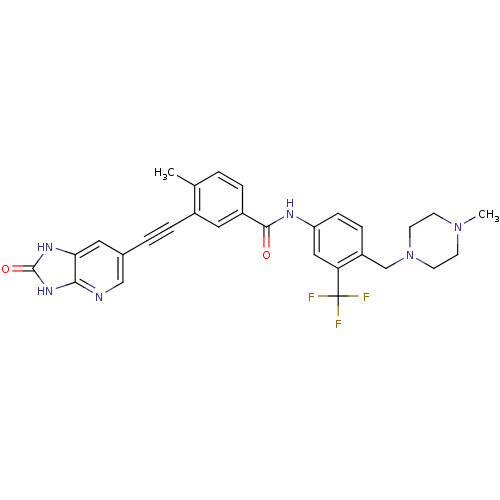
(Homo sapiens (Human)) | BDBM50322558
(4-Methyl-N-{4-[(4-methylpiperazin-1-yl)methyl]-3-(...)Show SMILES CN1CCN(Cc2ccc(NC(=O)c3ccc(C)c(c3)C#Cc3cnc4[nH]c(=O)[nH]c4c3)cc2C(F)(F)F)CC1 Show InChI InChI=1S/C29H27F3N6O2/c1-18-3-5-21(14-20(18)6-4-19-13-25-26(33-16-19)36-28(40)35-25)27(39)34-23-8-7-22(24(15-23)29(30,31)32)17-38-11-9-37(2)10-12-38/h3,5,7-8,13-16H,9-12,17H2,1-2H3,(H,34,39)(H2,33,35,36,40) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.90 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of wild type human ABL after 1 hr by TR-FRET assay |
J Med Chem 53: 4701-19 (2010)
Article DOI: 10.1021/jm100395q
BindingDB Entry DOI: 10.7270/Q27P8ZKX |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50345579

(5-((5-(4-((4-(2-hydroxyethyl)piperazin-1-yl)methyl...)Show SMILES CNC(=O)c1ncc(C#Cc2cc(ccc2C)C(=O)Nc2ccc(CN3CCN(CCO)CC3)c(c2)C(F)(F)F)n1C Show InChI InChI=1S/C30H33F3N6O3/c1-20-4-5-22(16-21(20)7-9-25-18-35-27(37(25)3)29(42)34-2)28(41)36-24-8-6-23(26(17-24)30(31,32)33)19-39-12-10-38(11-13-39)14-15-40/h4-6,8,16-18,40H,10-15,19H2,1-3H3,(H,34,42)(H,36,41) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.20 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human FMS using poly[Glu:Tyr] by Hotspot assay |
Bioorg Med Chem Lett 21: 3743-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.060
BindingDB Entry DOI: 10.7270/Q21G0MM8 |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50294011

((E)-3-(2-(6-(Cyclopropylamino)-9H-purin-9-yl)vinyl...)Show SMILES Cc1cn(cn1)-c1cc(NC(=O)c2ccc(C)c(\C=C\n3cnc4c(NC5CC5)ncnc34)c2)cc(c1)C(F)(F)F Show InChI InChI=1S/C29H25F3N8O/c1-17-3-4-20(9-19(17)7-8-39-16-36-25-26(37-22-5-6-22)33-14-34-27(25)39)28(41)38-23-10-21(29(30,31)32)11-24(12-23)40-13-18(2)35-15-40/h3-4,7-16,22H,5-6H2,1-2H3,(H,38,41)(H,33,34,37)/b8-7+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.60 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals Inc
| Assay Description
In vitro kinase assay using Abl, Abl T315I or Src kinase. |
Chem Biol Drug Des 75: 18-28 (2010)
Article DOI: 10.1111/j.1747-0285.2009.00905.x
BindingDB Entry DOI: 10.7270/Q2MC8XHX |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM50322535

(3-(imidazo[1,2-b]pyridazin-3-ylethynyl)-4-methyl-N...)Show SMILES CN1CCN(Cc2ccc(NC(=O)c3ccc(C)c(c3)C#Cc3cnc4cccnn34)cc2C(F)(F)F)CC1 Show InChI InChI=1S/C29H27F3N6O/c1-20-5-6-22(16-21(20)8-10-25-18-33-27-4-3-11-34-38(25)27)28(39)35-24-9-7-23(26(17-24)29(30,31)32)19-37-14-12-36(2)13-15-37/h3-7,9,11,16-18H,12-15,19H2,1-2H3,(H,35,39) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| n/a | n/a | 8.60 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of wild type human ABL using [EAIYAAPFAKKK] peptide substrate by Hotspot assay |
Bioorg Med Chem Lett 21: 3743-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.060
BindingDB Entry DOI: 10.7270/Q21G0MM8 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM50322535

(3-(imidazo[1,2-b]pyridazin-3-ylethynyl)-4-methyl-N...)Show SMILES CN1CCN(Cc2ccc(NC(=O)c3ccc(C)c(c3)C#Cc3cnc4cccnn34)cc2C(F)(F)F)CC1 Show InChI InChI=1S/C29H27F3N6O/c1-20-5-6-22(16-21(20)8-10-25-18-33-27-4-3-11-34-38(25)27)28(39)35-24-9-7-23(26(17-24)29(30,31)32)19-37-14-12-36(2)13-15-37/h3-7,9,11,16-18H,12-15,19H2,1-2H3,(H,35,39) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| n/a | n/a | 8.60 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of wild type human ABL after 1 hr by TR-FRET assay |
J Med Chem 53: 4701-19 (2010)
Article DOI: 10.1021/jm100395q
BindingDB Entry DOI: 10.7270/Q27P8ZKX |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase ABL1
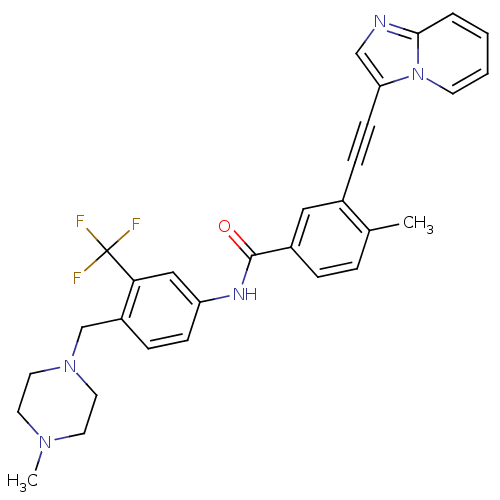
(Homo sapiens (Human)) | BDBM50322543
(3-[2-(Imidazo[1,2-a]pyridin-3-yl)ethynyl]-4-methyl...)Show SMILES CN1CCN(Cc2ccc(NC(=O)c3ccc(C)c(c3)C#Cc3cnc4ccccn34)cc2C(F)(F)F)CC1 Show InChI InChI=1S/C30H28F3N5O/c1-21-6-7-23(17-22(21)9-11-26-19-34-28-5-3-4-12-38(26)28)29(39)35-25-10-8-24(27(18-25)30(31,32)33)20-37-15-13-36(2)14-16-37/h3-8,10,12,17-19H,13-16,20H2,1-2H3,(H,35,39) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of wild type human ABL after 1 hr by TR-FRET assay |
J Med Chem 53: 4701-19 (2010)
Article DOI: 10.1021/jm100395q
BindingDB Entry DOI: 10.7270/Q27P8ZKX |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM50322544

(CHEMBL1171281 | N-{3-[2-(Imidazo[1,2-a]pyridin-3-y...)Show SMILES CN1CCN(Cc2ccc(cc2C(F)(F)F)C(=O)Nc2ccc(C)c(c2)C#Cc2cnc3ccccn23)CC1 Show InChI InChI=1S/C30H28F3N5O/c1-21-6-10-25(17-22(21)9-11-26-19-34-28-5-3-4-12-38(26)28)35-29(39)23-7-8-24(27(18-23)30(31,32)33)20-37-15-13-36(2)14-16-37/h3-8,10,12,17-19H,13-16,20H2,1-2H3,(H,35,39) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of wild type human ABL after 1 hr by TR-FRET assay |
J Med Chem 53: 4701-19 (2010)
Article DOI: 10.1021/jm100395q
BindingDB Entry DOI: 10.7270/Q27P8ZKX |
More data for this
Ligand-Target Pair | |
Isoform 1 of Fibronectin (1)
(Homo sapiens (Human)) | BDBM50244430

(9-(2,6-dimethylstyryl)-N-(4-(dimethylphosphoryl)ph...)Show SMILES Cc1cccc(C)c1\C=C\n1cnc2c(Nc3ccc(cc3)P(C)(C)=O)ncnc12 Show InChI InChI=1S/C23H24N5OP/c1-16-6-5-7-17(2)20(16)12-13-28-15-26-21-22(24-14-25-23(21)28)27-18-8-10-19(11-9-18)30(3,4)29/h5-15H,1-4H3,(H,24,25,27)/b13-12+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9.10 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals Inc
| Assay Description
In vitro kinase assay using Abl, Abl T315I or Src kinase. |
Chem Biol Drug Des 75: 18-28 (2010)
Article DOI: 10.1111/j.1747-0285.2009.00905.x
BindingDB Entry DOI: 10.7270/Q2MC8XHX |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data