 Found 6881 hits with Last Name = 'davis' and Initial = 'r'
Found 6881 hits with Last Name = 'davis' and Initial = 'r' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Dimer of Gag-Pol polyprotein [501-599,Q508K,L534I,L564I,C568A,C596A]
(Human immunodeficiency virus type 1) | BDBM9192
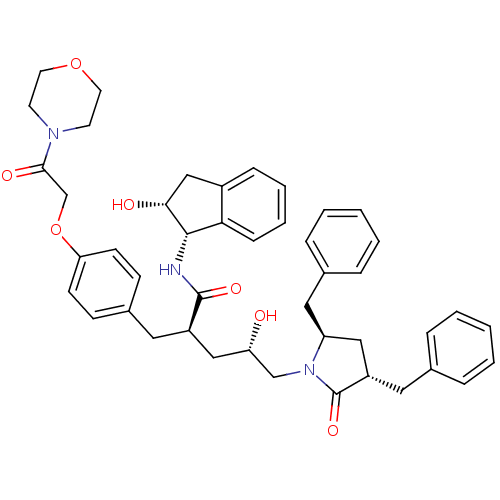
((2R,4S)-5-[(3S,5R)-3,5-dibenzyl-2-oxopyrrolidin-1-...)Show SMILES O[C@@H](C[C@@H](Cc1ccc(OCC(=O)N2CCOCC2)cc1)C(=O)N[C@@H]1[C@H](O)Cc2ccccc12)CN1[C@@H](Cc2ccccc2)C[C@H](Cc2ccccc2)C1=O |r| Show InChI InChI=1S/C45H51N3O7/c49-38(29-48-37(25-32-11-5-2-6-12-32)26-36(45(48)53)24-31-9-3-1-4-10-31)27-35(44(52)46-43-40-14-8-7-13-34(40)28-41(43)50)23-33-15-17-39(18-16-33)55-30-42(51)47-19-21-54-22-20-47/h1-18,35-38,41,43,49-50H,19-30H2,(H,46,52)/t35-,36+,37+,38+,41-,43+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0200 | -61.1 | 63 | n/a | n/a | n/a | n/a | 6.4 | 25 |
GlaxoSmithKline
| Assay Description
The Ki values were determined using fluorogenic substrate, 2-(aminobenzoyl)-Thr-Ile-Nle-Phe(p-NO2)-Gln-ArgNH2. A standard curve relating changes in f... |
Bioorg Med Chem Lett 15: 81-4 (2005)
Article DOI: 10.1016/j.bmcl.2004.10.029
BindingDB Entry DOI: 10.7270/Q29G5K1M |
More data for this
Ligand-Target Pair | |
Dimer of Gag-Pol polyprotein [501-599,Q508K,L534I,L564I,C568A,C596A]
(Human immunodeficiency virus type 1) | BDBM9195

((2R,4S)-5-[(3S,5R)-3,5-dibenzyl-2-oxopyrrolidin-1-...)Show SMILES COCCOCCOc1ccc(C[C@H](C[C@H](O)CN2[C@@H](Cc3ccccc3)C[C@H](Cc3ccccc3)C2=O)C(=O)N[C@@H]2[C@H](O)Cc3ccccc23)cc1 |r| Show InChI InChI=1S/C44H52N2O7/c1-51-20-21-52-22-23-53-39-18-16-33(17-19-39)24-35(43(49)45-42-40-15-9-8-14-34(40)29-41(42)48)28-38(47)30-46-37(26-32-12-6-3-7-13-32)27-36(44(46)50)25-31-10-4-2-5-11-31/h2-19,35-38,41-42,47-48H,20-30H2,1H3,(H,45,49)/t35-,36+,37+,38+,41-,42+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0200 | -61.1 | 93 | n/a | n/a | n/a | n/a | 6.4 | 25 |
GlaxoSmithKline
| Assay Description
The Ki values were determined using fluorogenic substrate, 2-(aminobenzoyl)-Thr-Ile-Nle-Phe(p-NO2)-Gln-ArgNH2. A standard curve relating changes in f... |
Bioorg Med Chem Lett 15: 81-4 (2005)
Article DOI: 10.1016/j.bmcl.2004.10.029
BindingDB Entry DOI: 10.7270/Q29G5K1M |
More data for this
Ligand-Target Pair | |
Dimer of Gag-Pol polyprotein [501-599,Q508K,L534I,L564I,C568A,C596A]
(Human immunodeficiency virus type 1) | BDBM9190
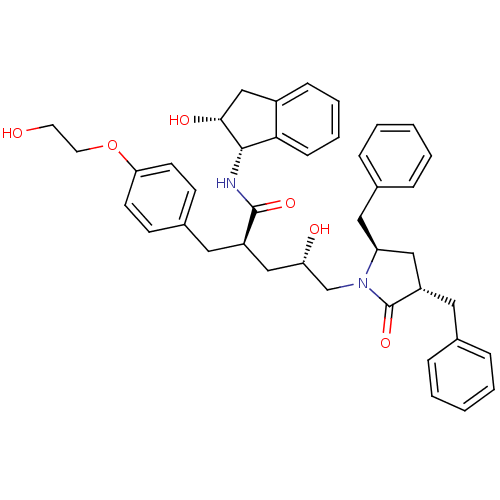
((2R,4S)-5-[(3S,5R)-3,5-dibenzyl-2-oxopyrrolidin-1-...)Show SMILES OCCOc1ccc(C[C@H](C[C@H](O)CN2[C@@H](Cc3ccccc3)C[C@H](Cc3ccccc3)C2=O)C(=O)N[C@@H]2[C@H](O)Cc3ccccc23)cc1 |r| Show InChI InChI=1S/C41H46N2O6/c44-19-20-49-36-17-15-30(16-18-36)21-32(40(47)42-39-37-14-8-7-13-31(37)26-38(39)46)25-35(45)27-43-34(23-29-11-5-2-6-12-29)24-33(41(43)48)22-28-9-3-1-4-10-28/h1-18,32-35,38-39,44-46H,19-27H2,(H,42,47)/t32-,33+,34+,35+,38-,39+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0200 | -61.1 | 94 | n/a | n/a | n/a | n/a | 6.4 | 25 |
GlaxoSmithKline
| Assay Description
The Ki values were determined using fluorogenic substrate, 2-(aminobenzoyl)-Thr-Ile-Nle-Phe(p-NO2)-Gln-ArgNH2. A standard curve relating changes in f... |
Bioorg Med Chem Lett 15: 81-4 (2005)
Article DOI: 10.1016/j.bmcl.2004.10.029
BindingDB Entry DOI: 10.7270/Q29G5K1M |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM21397

(8-[4-(4-fluorophenyl)-4-keto-butyl]-1-phenyl-1,3,8...)Show SMILES Fc1ccc(cc1)C(=O)CCCN1CCC2(CC1)N(CNC2=O)c1ccccc1 Show InChI InChI=1S/C23H26FN3O2/c24-19-10-8-18(9-11-19)21(28)7-4-14-26-15-12-23(13-16-26)22(29)25-17-27(23)20-5-2-1-3-6-20/h1-3,5-6,8-11H,4,7,12-17H2,(H,25,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ACADIA Pharmaceuticals
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 315: 1278-87 (2005)
Article DOI: 10.1124/jpet.105.092155
BindingDB Entry DOI: 10.7270/Q2Z60MNB |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50232153
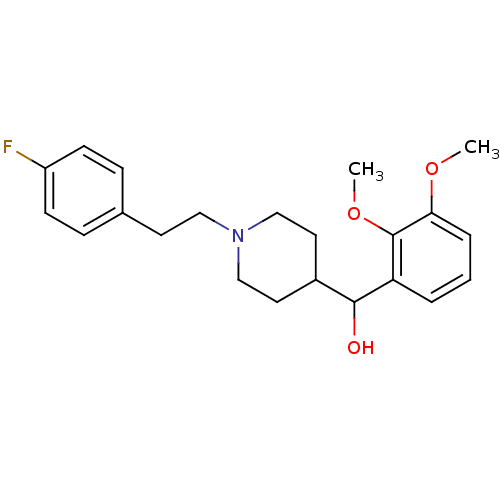
((1-(4-fluorophenethyl)piperidin-4-yl)(2,3-dimethox...)Show InChI InChI=1S/C22H28FNO3/c1-26-20-5-3-4-19(22(20)27-2)21(25)17-11-14-24(15-12-17)13-10-16-6-8-18(23)9-7-16/h3-9,17,21,25H,10-15H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0300 | -55.8 | n/a | n/a | n/a | n/a | n/a | n/a | 4 |
ACADIA Pharmaceuticals Inc.
| Assay Description
For the membrane binding, NIH-3T3 cells were grown to 70% confluence in 15-cm2 dishes and transfected with 10 ug of receptor plasmid DNA using Polyfe... |
J Pharmacol Exp Ther 317: 910-8 (2006)
Article DOI: 10.1124/jpet.105.097006
BindingDB Entry DOI: 10.7270/Q269728N |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM86187
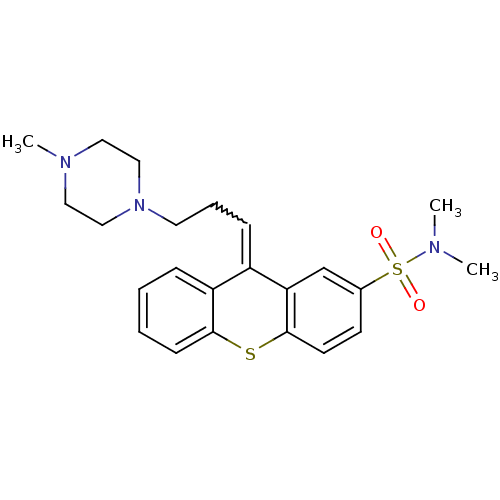
(CAS_22189-31-7 | NSC_5454 | THIOTHIXENE)Show SMILES CN(C)S(=O)(=O)c1ccc2Sc3ccccc3C(=CCCN3CCN(C)CC3)c2c1 |w:18.19| Show InChI InChI=1S/C23H29N3O2S2/c1-24(2)30(27,28)18-10-11-23-21(17-18)19(20-7-4-5-9-22(20)29-23)8-6-12-26-15-13-25(3)14-16-26/h4-5,7-11,17H,6,12-16H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ACADIA Pharmaceuticals
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 315: 1278-87 (2005)
Article DOI: 10.1124/jpet.105.092155
BindingDB Entry DOI: 10.7270/Q2Z60MNB |
More data for this
Ligand-Target Pair | |
Dimer of Gag-Pol polyprotein [501-599,Q508K,L534I,L564I,C568A,C596A]
(Human immunodeficiency virus type 1) | BDBM577

((3S)-oxolan-3-yl N-[(2S,3R)-4-[(4-aminobenzene)(2-...)Show SMILES CC(C)CN(C[C@@H](O)[C@H](Cc1ccccc1)NC(=O)O[C@H]1CCOC1)S(=O)(=O)c1ccc(N)cc1 |r| Show InChI InChI=1S/C25H35N3O6S/c1-18(2)15-28(35(31,32)22-10-8-20(26)9-11-22)16-24(29)23(14-19-6-4-3-5-7-19)27-25(30)34-21-12-13-33-17-21/h3-11,18,21,23-24,29H,12-17,26H2,1-2H3,(H,27,30)/t21-,23-,24+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 0.0400 | -59.3 | 150 | n/a | n/a | n/a | n/a | 6.4 | 25 |
GlaxoSmithKline
| Assay Description
The Ki values were determined using fluorogenic substrate, 2-(aminobenzoyl)-Thr-Ile-Nle-Phe(p-NO2)-Gln-ArgNH2. A standard curve relating changes in f... |
Bioorg Med Chem Lett 15: 81-4 (2005)
Article DOI: 10.1016/j.bmcl.2004.10.029
BindingDB Entry DOI: 10.7270/Q29G5K1M |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM139371
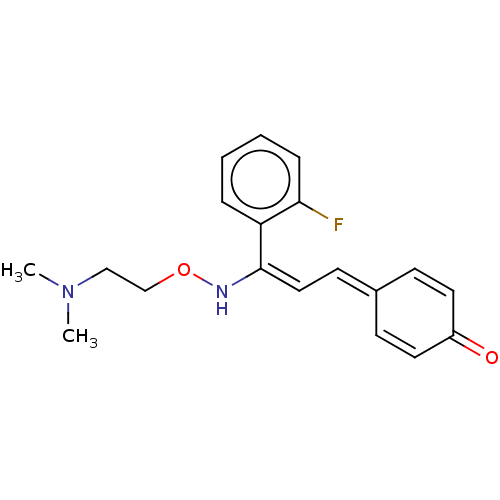
(eplivanserin)Show SMILES [#6]-[#7](-[#6])-[#6]-[#6]-[#8]-[#7]\[#6](=[#6]\[#6]=[#6]-1\[#6]=[#6]-[#6](=O)-[#6]=[#6]-1)-c1ccccc1F |c:11,15| Show InChI InChI=1S/C19H21FN2O2/c1-22(2)13-14-24-21-19(17-5-3-4-6-18(17)20)12-9-15-7-10-16(23)11-8-15/h3-12,21H,13-14H2,1-2H3/b19-12+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| 0.0460 | -54.8 | n/a | n/a | n/a | n/a | n/a | n/a | 4 |
ACADIA Pharmaceuticals Inc.
| Assay Description
For the membrane binding, NIH-3T3 cells were grown to 70% confluence in 15-cm2 dishes and transfected with 10 ug of receptor plasmid DNA using Polyfe... |
J Pharmacol Exp Ther 317: 910-8 (2006)
Article DOI: 10.1124/jpet.105.097006
BindingDB Entry DOI: 10.7270/Q269728N |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50080514
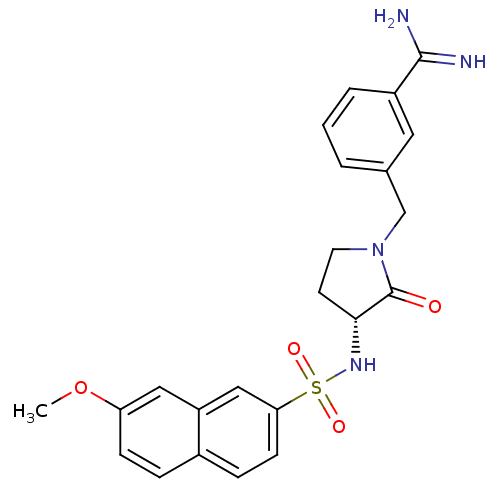
(3-[(R)-3-(7-Methoxy-naphthalene-2-sulfonylamino)-2...)Show SMILES COc1ccc2ccc(cc2c1)S(=O)(=O)N[C@@H]1CCN(Cc2cccc(c2)C(N)=N)C1=O Show InChI InChI=1S/C23H24N4O4S/c1-31-19-7-5-16-6-8-20(13-18(16)12-19)32(29,30)26-21-9-10-27(23(21)28)14-15-3-2-4-17(11-15)22(24)25/h2-8,11-13,21,26H,9-10,14H2,1H3,(H3,24,25)/t21-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rh£ne-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Compound was evaluated for the inhibition of human Coagulation factor Xa |
J Med Chem 42: 3557-71 (1999)
Article DOI: 10.1021/jm990040h
BindingDB Entry DOI: 10.7270/Q2M04640 |
More data for this
Ligand-Target Pair | |
Dimer of Gag-Pol polyprotein [501-599,Q508K,L534I,L564I,C568A,C596A]
(Human immunodeficiency virus type 1) | BDBM9197
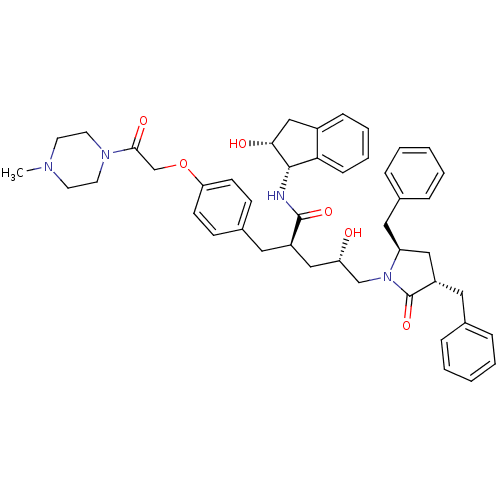
((2R,4S)-5-[(3S,5R)-3,5-dibenzyl-2-oxopyrrolidin-1-...)Show SMILES CN1CCN(CC1)C(=O)COc1ccc(C[C@H](C[C@H](O)CN2[C@@H](Cc3ccccc3)C[C@H](Cc3ccccc3)C2=O)C(=O)N[C@@H]2[C@H](O)Cc3ccccc23)cc1 |r| Show InChI InChI=1S/C46H54N4O6/c1-48-20-22-49(23-21-48)43(53)31-56-40-18-16-34(17-19-40)24-36(45(54)47-44-41-15-9-8-14-35(41)29-42(44)52)28-39(51)30-50-38(26-33-12-6-3-7-13-33)27-37(46(50)55)25-32-10-4-2-5-11-32/h2-19,36-39,42,44,51-52H,20-31H2,1H3,(H,47,54)/t36-,37+,38+,39+,42-,44+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0500 | -58.8 | 130 | n/a | n/a | n/a | n/a | 6.4 | 25 |
GlaxoSmithKline
| Assay Description
The Ki values were determined using fluorogenic substrate, 2-(aminobenzoyl)-Thr-Ile-Nle-Phe(p-NO2)-Gln-ArgNH2. A standard curve relating changes in f... |
Bioorg Med Chem Lett 15: 81-4 (2005)
Article DOI: 10.1016/j.bmcl.2004.10.029
BindingDB Entry DOI: 10.7270/Q29G5K1M |
More data for this
Ligand-Target Pair | |
Dimer of Gag-Pol polyprotein [501-599,Q508K,L534I,L564I,C568A,C596A]
(Human immunodeficiency virus type 1) | BDBM9196

(2-{4-[(2R)-2-[(2S)-3-[(3S,5R)-3,5-dibenzyl-2-oxopy...)Show SMILES O[C@@H](C[C@@H](Cc1ccc(OCCOC(=O)N2CCOCC2)cc1)C(=O)N[C@@H]1[C@H](O)Cc2ccccc12)CN1[C@@H](Cc2ccccc2)C[C@H](Cc2ccccc2)C1=O |r| Show InChI InChI=1S/C46H53N3O8/c50-39(31-49-38(27-33-11-5-2-6-12-33)28-37(45(49)53)26-32-9-3-1-4-10-32)29-36(44(52)47-43-41-14-8-7-13-35(41)30-42(43)51)25-34-15-17-40(18-16-34)56-23-24-57-46(54)48-19-21-55-22-20-48/h1-18,36-39,42-43,50-51H,19-31H2,(H,47,52)/t36-,37+,38+,39+,42-,43+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0500 | -58.8 | 240 | n/a | n/a | n/a | n/a | 6.4 | 25 |
GlaxoSmithKline
| Assay Description
The Ki values were determined using fluorogenic substrate, 2-(aminobenzoyl)-Thr-Ile-Nle-Phe(p-NO2)-Gln-ArgNH2. A standard curve relating changes in f... |
Bioorg Med Chem Lett 15: 81-4 (2005)
Article DOI: 10.1016/j.bmcl.2004.10.029
BindingDB Entry DOI: 10.7270/Q29G5K1M |
More data for this
Ligand-Target Pair | |
Dimer of Gag-Pol polyprotein [501-599,Q508K,L534I,L564I,C568A,C596A]
(Human immunodeficiency virus type 1) | BDBM9183
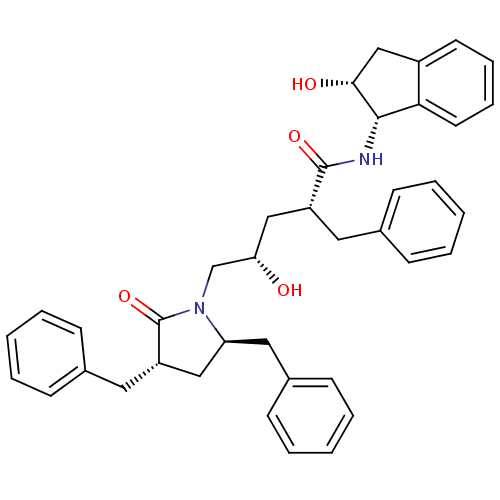
((2R,4S)-2-benzyl-5-[(3S,5R)-3,5-dibenzyl-2-oxopyrr...)Show SMILES O[C@@H](C[C@@H](Cc1ccccc1)C(=O)N[C@@H]1[C@H](O)Cc2ccccc12)CN1[C@@H](Cc2ccccc2)C[C@H](Cc2ccccc2)C1=O |r| Show InChI InChI=1S/C39H42N2O4/c42-34(26-41-33(22-29-16-8-3-9-17-29)23-32(39(41)45)21-28-14-6-2-7-15-28)24-31(20-27-12-4-1-5-13-27)38(44)40-37-35-19-11-10-18-30(35)25-36(37)43/h1-19,31-34,36-37,42-43H,20-26H2,(H,40,44)/t31-,32+,33+,34+,36-,37+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0500 | -58.8 | 720 | n/a | n/a | n/a | n/a | 6.4 | 25 |
GlaxoSmithKline
| Assay Description
The Ki values were determined using fluorogenic substrate, 2-(aminobenzoyl)-Thr-Ile-Nle-Phe(p-NO2)-Gln-ArgNH2. A standard curve relating changes in f... |
Bioorg Med Chem Lett 15: 81-4 (2005)
Article DOI: 10.1016/j.bmcl.2004.10.029
BindingDB Entry DOI: 10.7270/Q29G5K1M |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(Homo sapiens (Human)) | BDBM50345693
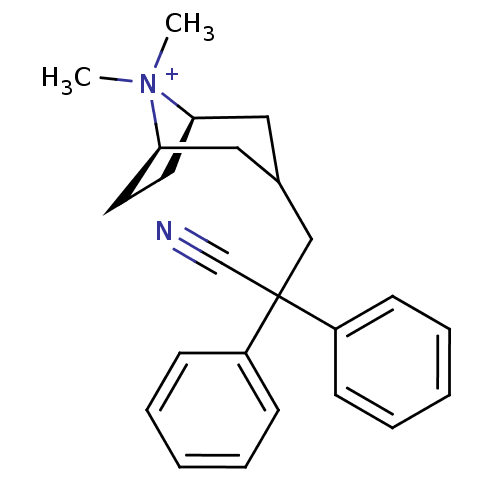
((3-endo)-3-(2-Cyano-2,2-diphenylethyl)-8,8-dimethy...)Show SMILES C[N+]1(C)[C@@H]2CC[C@@H]1CC(CC(C#N)(c1ccccc1)c1ccccc1)C2 |r,THB:9:8:1:4.5| Show InChI InChI=1S/C24H29N2/c1-26(2)22-13-14-23(26)16-19(15-22)17-24(18-25,20-9-5-3-6-10-20)21-11-7-4-8-12-21/h3-12,19,22-23H,13-17H2,1-2H3/q+1/t22-,23-/m1/s1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-methyl Scopolamine from human muscarinic M1 receptor expressed in CHO cells by scintillation proximity assay |
J Med Chem 52: 5241-52 (2010)
Article DOI: 10.1021/jm900736e
BindingDB Entry DOI: 10.7270/Q2PK0H54 |
More data for this
Ligand-Target Pair | |
Dimer of Gag-Pol polyprotein [501-599,Q508K,L534I,L564I,C568A,C596A]
(Human immunodeficiency virus type 1) | BDBM9186

((2R,4S)-5-[(3S,5R)-3,5-dibenzyl-2-oxopyrrolidin-1-...)Show SMILES O[C@@H](C[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H]1[C@H](O)Cc2ccccc12)CN1[C@@H](Cc2ccccc2)C[C@H](Cc2ccccc2)C1=O |r| Show InChI InChI=1S/C39H42N2O5/c42-33-17-15-28(16-18-33)19-30(38(45)40-37-35-14-8-7-13-29(35)24-36(37)44)23-34(43)25-41-32(21-27-11-5-2-6-12-27)22-31(39(41)46)20-26-9-3-1-4-10-26/h1-18,30-32,34,36-37,42-44H,19-25H2,(H,40,45)/t30-,31+,32+,34+,36-,37+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0600 | -58.3 | 320 | n/a | n/a | n/a | n/a | 6.4 | 25 |
GlaxoSmithKline
| Assay Description
The Ki values were determined using fluorogenic substrate, 2-(aminobenzoyl)-Thr-Ile-Nle-Phe(p-NO2)-Gln-ArgNH2. A standard curve relating changes in f... |
Bioorg Med Chem Lett 15: 81-4 (2005)
Article DOI: 10.1016/j.bmcl.2004.10.029
BindingDB Entry DOI: 10.7270/Q29G5K1M |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50345693

((3-endo)-3-(2-Cyano-2,2-diphenylethyl)-8,8-dimethy...)Show SMILES C[N+]1(C)[C@@H]2CC[C@@H]1CC(CC(C#N)(c1ccccc1)c1ccccc1)C2 |r,THB:9:8:1:4.5| Show InChI InChI=1S/C24H29N2/c1-26(2)22-13-14-23(26)16-19(15-22)17-24(18-25,20-9-5-3-6-10-20)21-11-7-4-8-12-21/h3-12,19,22-23H,13-17H2,1-2H3/q+1/t22-,23-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-methyl Scopolamine from human muscarinic M3 receptor expressed in CHO cells by scintillation proximity assay |
J Med Chem 52: 5241-52 (2010)
Article DOI: 10.1021/jm900736e
BindingDB Entry DOI: 10.7270/Q2PK0H54 |
More data for this
Ligand-Target Pair | |
Dimer of Gag-Pol polyprotein [501-599,Q508K,L534I,L564I,C568A,C596A]
(Human immunodeficiency virus type 1) | BDBM9198

((2R,4S)-2-{[4-(carbamoylmethoxy)phenyl]methyl}-5-[...)Show SMILES NC(=O)COc1ccc(C[C@H](C[C@H](O)CN2[C@@H](Cc3ccccc3)C[C@H](Cc3ccccc3)C2=O)C(=O)N[C@@H]2[C@H](O)Cc3ccccc23)cc1 |r| Show InChI InChI=1S/C41H45N3O6/c42-38(47)26-50-35-17-15-29(16-18-35)19-31(40(48)43-39-36-14-8-7-13-30(36)24-37(39)46)23-34(45)25-44-33(21-28-11-5-2-6-12-28)22-32(41(44)49)20-27-9-3-1-4-10-27/h1-18,31-34,37,39,45-46H,19-26H2,(H2,42,47)(H,43,48)/t31-,32+,33+,34+,37-,39+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0700 | -58.0 | 360 | n/a | n/a | n/a | n/a | 6.4 | 25 |
GlaxoSmithKline
| Assay Description
The Ki values were determined using fluorogenic substrate, 2-(aminobenzoyl)-Thr-Ile-Nle-Phe(p-NO2)-Gln-ArgNH2. A standard curve relating changes in f... |
Bioorg Med Chem Lett 15: 81-4 (2005)
Article DOI: 10.1016/j.bmcl.2004.10.029
BindingDB Entry DOI: 10.7270/Q29G5K1M |
More data for this
Ligand-Target Pair | |
Dimer of Gag-Pol polyprotein [501-599,Q508K,L534I,L564I,C568A,C596A]
(Human immunodeficiency virus type 1) | BDBM9194

((2R,4S)-5-[(3S,5R)-3,5-dibenzyl-2-oxopyrrolidin-1-...)Show SMILES O[C@@H](C[C@@H](Cc1ccc(OCc2ccccn2)cc1)C(=O)N[C@@H]1[C@H](O)Cc2ccccc12)CN1[C@@H](Cc2ccccc2)C[C@H](Cc2ccccc2)C1=O |r| Show InChI InChI=1S/C45H47N3O5/c49-39(29-48-38(25-32-13-5-2-6-14-32)26-36(45(48)52)24-31-11-3-1-4-12-31)27-35(44(51)47-43-41-17-8-7-15-34(41)28-42(43)50)23-33-18-20-40(21-19-33)53-30-37-16-9-10-22-46-37/h1-22,35-36,38-39,42-43,49-50H,23-30H2,(H,47,51)/t35-,36+,38+,39+,42-,43+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0700 | -58.0 | 380 | n/a | n/a | n/a | n/a | 6.4 | 25 |
GlaxoSmithKline
| Assay Description
The Ki values were determined using fluorogenic substrate, 2-(aminobenzoyl)-Thr-Ile-Nle-Phe(p-NO2)-Gln-ArgNH2. A standard curve relating changes in f... |
Bioorg Med Chem Lett 15: 81-4 (2005)
Article DOI: 10.1016/j.bmcl.2004.10.029
BindingDB Entry DOI: 10.7270/Q29G5K1M |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50001775
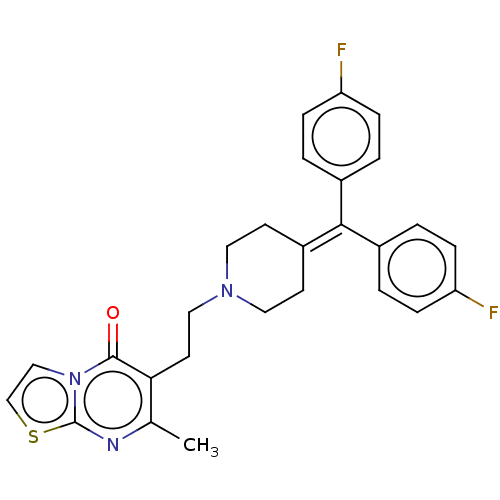
((ritanserin)6-(2-{4-[Bis-(4-fluoro-phenyl)-methyle...)Show SMILES [#6]-c1nc2sccn2c(=O)c1-[#6]-[#6]-[#7]-1-[#6]-[#6]\[#6](-[#6]-[#6]-1)=[#6](\c1ccc(F)cc1)-c1ccc(F)cc1 Show InChI InChI=1S/C27H25F2N3OS/c1-18-24(26(33)32-16-17-34-27(32)30-18)12-15-31-13-10-21(11-14-31)25(19-2-6-22(28)7-3-19)20-4-8-23(29)9-5-20/h2-9,16-17H,10-15H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.0830 | -53.5 | n/a | n/a | n/a | n/a | n/a | n/a | 4 |
ACADIA Pharmaceuticals Inc.
| Assay Description
For the membrane binding, NIH-3T3 cells were grown to 70% confluence in 15-cm2 dishes and transfected with 10 ug of receptor plasmid DNA using Polyfe... |
J Pharmacol Exp Ther 317: 910-8 (2006)
Article DOI: 10.1124/jpet.105.097006
BindingDB Entry DOI: 10.7270/Q269728N |
More data for this
Ligand-Target Pair | |
Dimer of Gag-Pol polyprotein [501-599,Q508K,L534I,L564I,C568A,C596A]
(Human immunodeficiency virus type 1) | BDBM9193
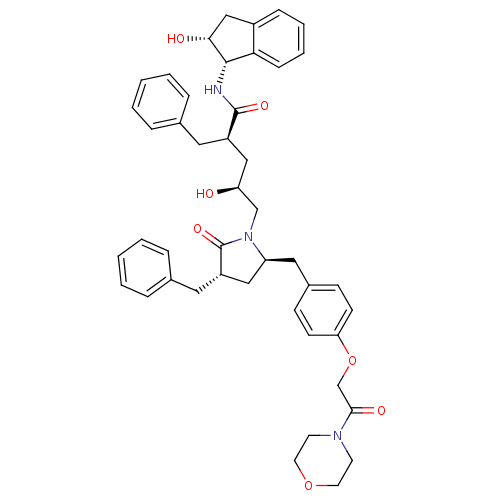
(pyrrolidinone based inhibitor 1k)Show SMILES O[C@@H](C[C@@H](Cc1ccccc1)C(=O)N[C@@H]1[C@H](O)Cc2ccccc12)CN1[C@@H](Cc2ccc(OCC(=O)N3CCOCC3)cc2)C[C@H](Cc2ccccc2)C1=O |r| Show InChI InChI=1S/C45H51N3O7/c49-38(27-35(23-31-9-3-1-4-10-31)44(52)46-43-40-14-8-7-13-34(40)28-41(43)50)29-48-37(26-36(45(48)53)24-32-11-5-2-6-12-32)25-33-15-17-39(18-16-33)55-30-42(51)47-19-21-54-22-20-47/h1-18,35-38,41,43,49-50H,19-30H2,(H,46,52)/t35-,36+,37+,38+,41-,43+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.100 | -57.1 | 200 | n/a | n/a | n/a | n/a | 6.4 | 25 |
GlaxoSmithKline
| Assay Description
The Ki values were determined using fluorogenic substrate, 2-(aminobenzoyl)-Thr-Ile-Nle-Phe(p-NO2)-Gln-ArgNH2. A standard curve relating changes in f... |
Bioorg Med Chem Lett 15: 81-4 (2005)
Article DOI: 10.1016/j.bmcl.2004.10.029
BindingDB Entry DOI: 10.7270/Q29G5K1M |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM81486
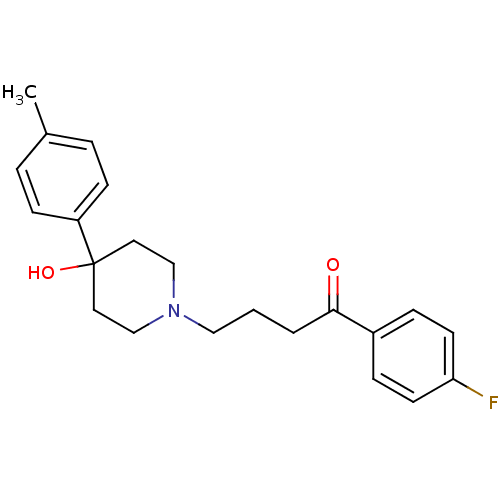
(CAS_1050-79-9 | MOPERONE | NSC_4249)Show SMILES Cc1ccc(cc1)C1(O)CCN(CCCC(=O)c2ccc(F)cc2)CC1 Show InChI InChI=1S/C22H26FNO2/c1-17-4-8-19(9-5-17)22(26)12-15-24(16-13-22)14-2-3-21(25)18-6-10-20(23)11-7-18/h4-11,26H,2-3,12-16H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ACADIA Pharmaceuticals
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 315: 1278-87 (2005)
Article DOI: 10.1124/jpet.105.092155
BindingDB Entry DOI: 10.7270/Q2Z60MNB |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM50252513
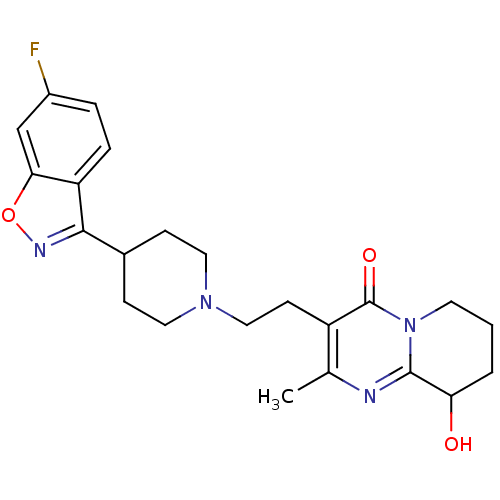
(3-(2-(4-(6-fluorobenzo[d]isoxazol-3-yl)piperidin-1...)Show SMILES Cc1nc2C(O)CCCn2c(=O)c1CCN1CCC(CC1)c1noc2cc(F)ccc12 Show InChI InChI=1S/C23H27FN4O3/c1-14-17(23(30)28-9-2-3-19(29)22(28)25-14)8-12-27-10-6-15(7-11-27)21-18-5-4-16(24)13-20(18)31-26-21/h4-5,13,15,19,29H,2-3,6-12H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ACADIA Pharmaceuticals
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 315: 1278-87 (2005)
Article DOI: 10.1124/jpet.105.092155
BindingDB Entry DOI: 10.7270/Q2Z60MNB |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM84734
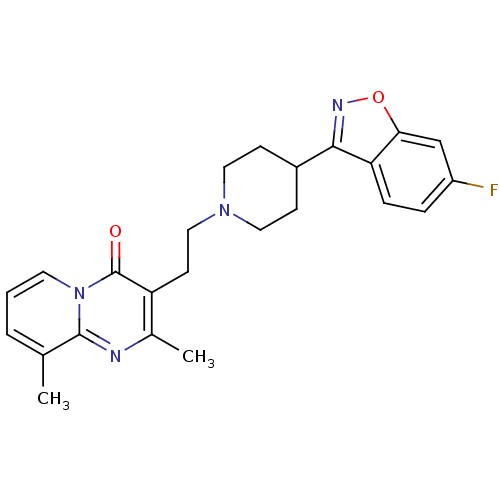
(CAS_129029-23-8 | NSC_71351 | Ocaperidone)Show SMILES Cc1cccn2c1nc(C)c(CCN1CCC(CC1)c1noc3cc(F)ccc13)c2=O Show InChI InChI=1S/C24H25FN4O2/c1-15-4-3-10-29-23(15)26-16(2)19(24(29)30)9-13-28-11-7-17(8-12-28)22-20-6-5-18(25)14-21(20)31-27-22/h3-6,10,14,17H,7-9,11-13H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ACADIA Pharmaceuticals
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 315: 1278-87 (2005)
Article DOI: 10.1124/jpet.105.092155
BindingDB Entry DOI: 10.7270/Q2Z60MNB |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(Homo sapiens (Human)) | BDBM50381654
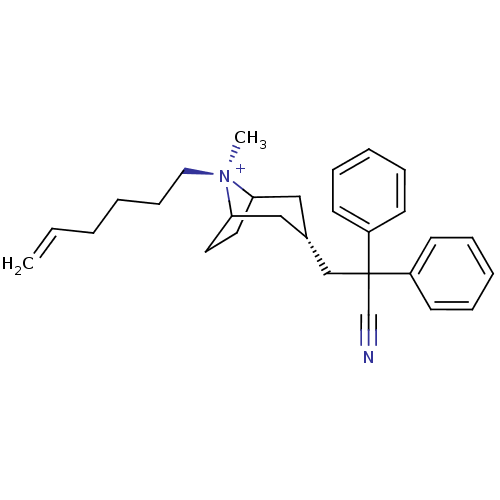
(CHEMBL2023764)Show SMILES C[N@+]1(CCCCC=C)C2CCC1C[C@@H](CC(C#N)(c1ccccc1)c1ccccc1)C2 |r,wU:1.1,wD:13.14,1.0,TLB:0:1:13.12.30:10.9,THB:2:1:13.12.30:10.9,14:13:1:10.9,(20.22,-1.4,;21.72,-1.81,;22.48,-.47,;21.7,.86,;22.46,2.2,;21.68,3.53,;22.44,4.87,;21.66,6.2,;22.77,-2.64,;22.17,-3.98,;20.99,-4.69,;21.98,-3.34,;23.82,-3.34,;24.8,-4.15,;25.57,-5.48,;27.11,-5.48,;27.5,-3.98,;27.89,-2.48,;27.88,-6.81,;27.11,-8.14,;27.89,-9.47,;29.43,-9.47,;30.19,-8.12,;29.42,-6.8,;28.59,-5.07,;29.68,-6.15,;31.16,-5.75,;31.55,-4.26,;30.45,-3.18,;28.97,-3.59,;24.53,-2.61,)| Show InChI InChI=1S/C29H37N2/c1-3-4-5-12-19-31(2)27-17-18-28(31)21-24(20-27)22-29(23-30,25-13-8-6-9-14-25)26-15-10-7-11-16-26/h3,6-11,13-16,24,27-28H,1,4-5,12,17-22H2,2H3/q+1/t24-,27?,28?,31- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]-N-methyl scopolamine from muscarinic acetylcholine M2 receptor expressed in CHO cell membrane |
Bioorg Med Chem Lett 22: 3366-9 (2012)
Article DOI: 10.1016/j.bmcl.2012.02.015
BindingDB Entry DOI: 10.7270/Q2C82B9Q |
More data for this
Ligand-Target Pair | |
Dimer of Gag-Pol polyprotein [501-599,Q508K,L534I,L564I,C568A,C596A]
(Human immunodeficiency virus type 1) | BDBM9184

((2R,4S)-2-[(4-benzylphenyl)methyl]-5-[(3S,5R)-3,5-...)Show SMILES O[C@@H](C[C@@H](Cc1ccc(Cc2ccccc2)cc1)C(=O)N[C@@H]1[C@H](O)Cc2ccccc12)CN1[C@@H](Cc2ccccc2)C[C@H](Cc2ccccc2)C1=O |r| Show InChI InChI=1S/C46H48N2O4/c49-41(31-48-40(27-34-16-8-3-9-17-34)28-39(46(48)52)26-33-14-6-2-7-15-33)29-38(45(51)47-44-42-19-11-10-18-37(42)30-43(44)50)25-36-22-20-35(21-23-36)24-32-12-4-1-5-13-32/h1-23,38-41,43-44,49-50H,24-31H2,(H,47,51)/t38-,39+,40+,41+,43-,44+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.110 | -56.8 | 920 | n/a | n/a | n/a | n/a | 6.4 | 25 |
GlaxoSmithKline
| Assay Description
The Ki values were determined using fluorogenic substrate, 2-(aminobenzoyl)-Thr-Ile-Nle-Phe(p-NO2)-Gln-ArgNH2. A standard curve relating changes in f... |
Bioorg Med Chem Lett 15: 81-4 (2005)
Article DOI: 10.1016/j.bmcl.2004.10.029
BindingDB Entry DOI: 10.7270/Q29G5K1M |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(Homo sapiens (Human)) | BDBM50381654

(CHEMBL2023764)Show SMILES C[N@+]1(CCCCC=C)C2CCC1C[C@@H](CC(C#N)(c1ccccc1)c1ccccc1)C2 |r,wU:1.1,wD:13.14,1.0,TLB:0:1:13.12.30:10.9,THB:2:1:13.12.30:10.9,14:13:1:10.9,(20.22,-1.4,;21.72,-1.81,;22.48,-.47,;21.7,.86,;22.46,2.2,;21.68,3.53,;22.44,4.87,;21.66,6.2,;22.77,-2.64,;22.17,-3.98,;20.99,-4.69,;21.98,-3.34,;23.82,-3.34,;24.8,-4.15,;25.57,-5.48,;27.11,-5.48,;27.5,-3.98,;27.89,-2.48,;27.88,-6.81,;27.11,-8.14,;27.89,-9.47,;29.43,-9.47,;30.19,-8.12,;29.42,-6.8,;28.59,-5.07,;29.68,-6.15,;31.16,-5.75,;31.55,-4.26,;30.45,-3.18,;28.97,-3.59,;24.53,-2.61,)| Show InChI InChI=1S/C29H37N2/c1-3-4-5-12-19-31(2)27-17-18-28(31)21-24(20-27)22-29(23-30,25-13-8-6-9-14-25)26-15-10-7-11-16-26/h3,6-11,13-16,24,27-28H,1,4-5,12,17-22H2,2H3/q+1/t24-,27?,28?,31- | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]-N-methyl scopolamine from muscarinic acetylcholine M1 receptor expressed in CHO cell membrane |
Bioorg Med Chem Lett 22: 3366-9 (2012)
Article DOI: 10.1016/j.bmcl.2012.02.015
BindingDB Entry DOI: 10.7270/Q2C82B9Q |
More data for this
Ligand-Target Pair | |
Dimer of Gag-Pol polyprotein [501-599,Q508K,L534I,L564I,C568A,C596A]
(Human immunodeficiency virus type 1) | BDBM9187
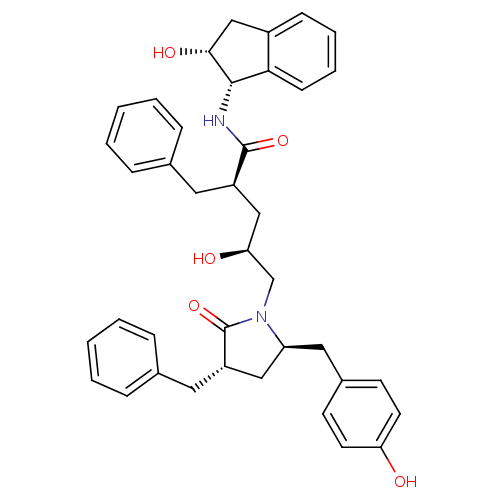
((2R,4S)-2-benzyl-5-[(3S,5R)-3-benzyl-5-[(4-hydroxy...)Show SMILES O[C@@H](C[C@@H](Cc1ccccc1)C(=O)N[C@@H]1[C@H](O)Cc2ccccc12)CN1[C@@H](Cc2ccc(O)cc2)C[C@H](Cc2ccccc2)C1=O |r| Show InChI InChI=1S/C39H42N2O5/c42-33-17-15-28(16-18-33)21-32-22-31(20-27-11-5-2-6-12-27)39(46)41(32)25-34(43)23-30(19-26-9-3-1-4-10-26)38(45)40-37-35-14-8-7-13-29(35)24-36(37)44/h1-18,30-32,34,36-37,42-44H,19-25H2,(H,40,45)/t30-,31+,32+,34+,36-,37+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.130 | -56.4 | 200 | n/a | n/a | n/a | n/a | 6.4 | 25 |
GlaxoSmithKline
| Assay Description
The Ki values were determined using fluorogenic substrate, 2-(aminobenzoyl)-Thr-Ile-Nle-Phe(p-NO2)-Gln-ArgNH2. A standard curve relating changes in f... |
Bioorg Med Chem Lett 15: 81-4 (2005)
Article DOI: 10.1016/j.bmcl.2004.10.029
BindingDB Entry DOI: 10.7270/Q29G5K1M |
More data for this
Ligand-Target Pair | |
Dimer of Gag-Pol polyprotein [501-599,Q508K,L534I,L564I,C568A,C596A]
(Human immunodeficiency virus type 1) | BDBM9185

((2R,4S)-2-benzyl-5-[(3S,5R)-3-benzyl-5-[(4-benzylp...)Show SMILES O[C@@H](C[C@@H](Cc1ccccc1)C(=O)N[C@@H]1[C@H](O)Cc2ccccc12)CN1[C@@H](Cc2ccc(Cc3ccccc3)cc2)C[C@H](Cc2ccccc2)C1=O |r| Show InChI InChI=1S/C46H48N2O4/c49-41(29-38(25-33-14-6-2-7-15-33)45(51)47-44-42-19-11-10-18-37(42)30-43(44)50)31-48-40(28-39(46(48)52)26-34-16-8-3-9-17-34)27-36-22-20-35(21-23-36)24-32-12-4-1-5-13-32/h1-23,38-41,43-44,49-50H,24-31H2,(H,47,51)/t38-,39+,40+,41+,43-,44+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.130 | -56.4 | 550 | n/a | n/a | n/a | n/a | 6.4 | 25 |
GlaxoSmithKline
| Assay Description
The Ki values were determined using fluorogenic substrate, 2-(aminobenzoyl)-Thr-Ile-Nle-Phe(p-NO2)-Gln-ArgNH2. A standard curve relating changes in f... |
Bioorg Med Chem Lett 15: 81-4 (2005)
Article DOI: 10.1016/j.bmcl.2004.10.029
BindingDB Entry DOI: 10.7270/Q29G5K1M |
More data for this
Ligand-Target Pair | |
Dimer of Gag-Pol polyprotein [501-599,Q508K,L534I,L564I,C568A,C596A]
(Human immunodeficiency virus type 1) | BDBM9188
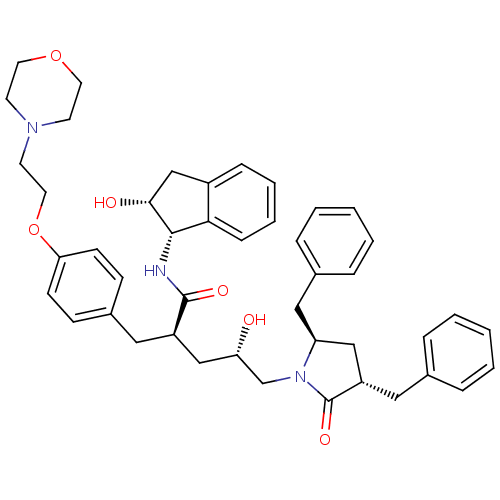
((2R,4S)-5-[(3S,5R)-3,5-dibenzyl-2-oxopyrrolidin-1-...)Show SMILES O[C@@H](C[C@@H](Cc1ccc(OCCN2CCOCC2)cc1)C(=O)N[C@@H]1[C@H](O)Cc2ccccc12)CN1[C@@H](Cc2ccccc2)C[C@H](Cc2ccccc2)C1=O |r| Show InChI InChI=1S/C45H53N3O6/c49-39(31-48-38(27-33-11-5-2-6-12-33)28-37(45(48)52)26-32-9-3-1-4-10-32)29-36(44(51)46-43-41-14-8-7-13-35(41)30-42(43)50)25-34-15-17-40(18-16-34)54-24-21-47-19-22-53-23-20-47/h1-18,36-39,42-43,49-50H,19-31H2,(H,46,51)/t36-,37+,38+,39+,42-,43+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.140 | -56.2 | 83 | n/a | n/a | n/a | n/a | 6.4 | 25 |
GlaxoSmithKline
| Assay Description
The Ki values were determined using fluorogenic substrate, 2-(aminobenzoyl)-Thr-Ile-Nle-Phe(p-NO2)-Gln-ArgNH2. A standard curve relating changes in f... |
Bioorg Med Chem Lett 15: 81-4 (2005)
Article DOI: 10.1016/j.bmcl.2004.10.029
BindingDB Entry DOI: 10.7270/Q29G5K1M |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM50444496
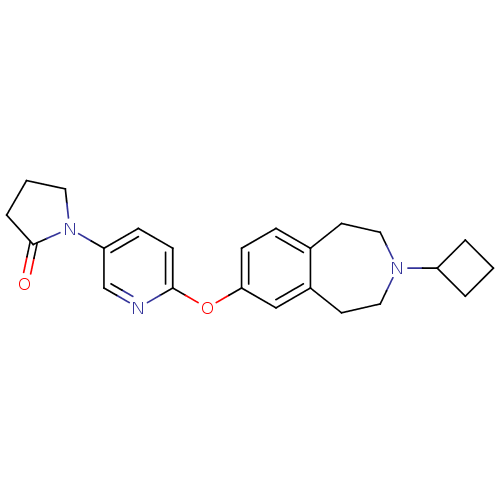
(CHEMBL3092650)Show SMILES O=C1CCCN1c1ccc(Oc2ccc3CCN(CCc3c2)C2CCC2)nc1 Show InChI InChI=1S/C23H27N3O2/c27-23-5-2-12-26(23)20-7-9-22(24-16-20)28-21-8-6-17-10-13-25(19-3-1-4-19)14-11-18(17)15-21/h6-9,15-16,19H,1-5,10-14H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.148 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D
Curated by ChEMBL
| Assay Description
Displacement of [3H]-R-alpha-ethylhistamine from rat histamine H3 receptor expressed in HEK293 cells after 45 mins by liquid scintillation spectromet... |
Bioorg Med Chem Lett 23: 6890-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.09.090
BindingDB Entry DOI: 10.7270/Q2PZ5B83 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM50444496

(CHEMBL3092650)Show SMILES O=C1CCCN1c1ccc(Oc2ccc3CCN(CCc3c2)C2CCC2)nc1 Show InChI InChI=1S/C23H27N3O2/c27-23-5-2-12-26(23)20-7-9-22(24-16-20)28-21-8-6-17-10-13-25(19-3-1-4-19)14-11-18(17)15-21/h6-9,15-16,19H,1-5,10-14H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.148 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D
Curated by ChEMBL
| Assay Description
Displacement of [3H]-R-alpha-ethylhistamine from rat histamine H3 receptor expressed in HEK293 cells after 45 mins by liquid scintillation spectromet... |
Bioorg Med Chem Lett 23: 6890-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.09.090
BindingDB Entry DOI: 10.7270/Q2PZ5B83 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50381654

(CHEMBL2023764)Show SMILES C[N@+]1(CCCCC=C)C2CCC1C[C@@H](CC(C#N)(c1ccccc1)c1ccccc1)C2 |r,wU:1.1,wD:13.14,1.0,TLB:0:1:13.12.30:10.9,THB:2:1:13.12.30:10.9,14:13:1:10.9,(20.22,-1.4,;21.72,-1.81,;22.48,-.47,;21.7,.86,;22.46,2.2,;21.68,3.53,;22.44,4.87,;21.66,6.2,;22.77,-2.64,;22.17,-3.98,;20.99,-4.69,;21.98,-3.34,;23.82,-3.34,;24.8,-4.15,;25.57,-5.48,;27.11,-5.48,;27.5,-3.98,;27.89,-2.48,;27.88,-6.81,;27.11,-8.14,;27.89,-9.47,;29.43,-9.47,;30.19,-8.12,;29.42,-6.8,;28.59,-5.07,;29.68,-6.15,;31.16,-5.75,;31.55,-4.26,;30.45,-3.18,;28.97,-3.59,;24.53,-2.61,)| Show InChI InChI=1S/C29H37N2/c1-3-4-5-12-19-31(2)27-17-18-28(31)21-24(20-27)22-29(23-30,25-13-8-6-9-14-25)26-15-10-7-11-16-26/h3,6-11,13-16,24,27-28H,1,4-5,12,17-22H2,2H3/q+1/t24-,27?,28?,31- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]-N-methyl scopolamine from muscarinic acetylcholine M3 receptor expressed in CHO cell membrane |
Bioorg Med Chem Lett 22: 3366-9 (2012)
Article DOI: 10.1016/j.bmcl.2012.02.015
BindingDB Entry DOI: 10.7270/Q2C82B9Q |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(Homo sapiens (Human)) | BDBM50345693

((3-endo)-3-(2-Cyano-2,2-diphenylethyl)-8,8-dimethy...)Show SMILES C[N+]1(C)[C@@H]2CC[C@@H]1CC(CC(C#N)(c1ccccc1)c1ccccc1)C2 |r,THB:9:8:1:4.5| Show InChI InChI=1S/C24H29N2/c1-26(2)22-13-14-23(26)16-19(15-22)17-24(18-25,20-9-5-3-6-10-20)21-11-7-4-8-12-21/h3-12,19,22-23H,13-17H2,1-2H3/q+1/t22-,23-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-methyl Scopolamine from human muscarinic M2 receptor expressed in CHO cells by scintillation proximity assay |
J Med Chem 52: 5241-52 (2010)
Article DOI: 10.1021/jm900736e
BindingDB Entry DOI: 10.7270/Q2PK0H54 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM50444491

(CHEMBL3092823)Show InChI InChI=1S/C22H25N3O2/c26-22-10-13-25(22)19-5-7-21(23-15-19)27-20-6-4-16-8-11-24(18-2-1-3-18)12-9-17(16)14-20/h4-7,14-15,18H,1-3,8-13H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D
Curated by ChEMBL
| Assay Description
Displacement of [3H]-R-alpha-ethylhistamine from histamine H3 receptor in rat cerebral cortical tissue membranes after 45 mins by liquid scintillatio... |
Bioorg Med Chem Lett 23: 6890-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.09.090
BindingDB Entry DOI: 10.7270/Q2PZ5B83 |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM21397

(8-[4-(4-fluorophenyl)-4-keto-butyl]-1-phenyl-1,3,8...)Show SMILES Fc1ccc(cc1)C(=O)CCCN1CCC2(CC1)N(CNC2=O)c1ccccc1 Show InChI InChI=1S/C23H26FN3O2/c24-19-10-8-18(9-11-19)21(28)7-4-14-26-15-12-23(13-16-26)22(29)25-17-27(23)20-5-2-1-3-6-20/h1-3,5-6,8-11H,4,7,12-17H2,(H,25,29) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ACADIA Pharmaceuticals
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 315: 1278-87 (2005)
Article DOI: 10.1124/jpet.105.092155
BindingDB Entry DOI: 10.7270/Q2Z60MNB |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM86722
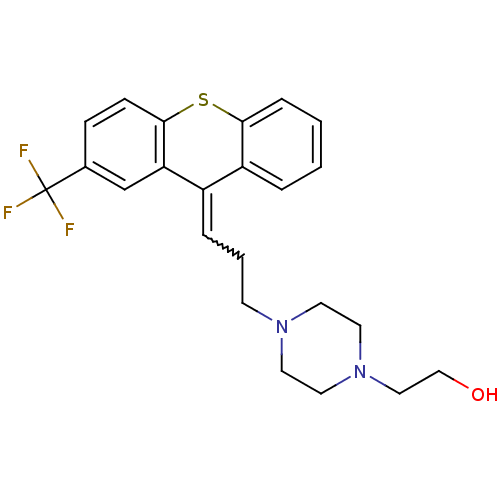
(CAS_53772-82-0 | CAS_53772-85-3 | FLUPENTHIXOL, Al...)Show SMILES OCCN1CCN(CCC=C2c3ccccc3Sc3ccc(cc23)C(F)(F)F)CC1 |w:9.8| Show InChI InChI=1S/C23H25F3N2OS/c24-23(25,26)17-7-8-22-20(16-17)18(19-4-1-2-6-21(19)30-22)5-3-9-27-10-12-28(13-11-27)14-15-29/h1-2,4-8,16,29H,3,9-15H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ACADIA Pharmaceuticals
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 315: 1278-87 (2005)
Article DOI: 10.1124/jpet.105.092155
BindingDB Entry DOI: 10.7270/Q2Z60MNB |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM78433
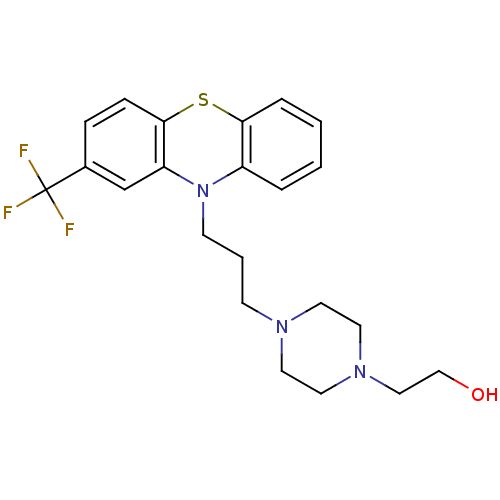
(2-[4-[3-[2-(trifluoromethyl)-10-phenothiazinyl]pro...)Show SMILES OCCN1CCN(CCCN2c3ccccc3Sc3ccc(cc23)C(F)(F)F)CC1 Show InChI InChI=1S/C22H26F3N3OS/c23-22(24,25)17-6-7-21-19(16-17)28(18-4-1-2-5-20(18)30-21)9-3-8-26-10-12-27(13-11-26)14-15-29/h1-2,4-7,16,29H,3,8-15H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| DrugBank
Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ACADIA Pharmaceuticals
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 315: 1278-87 (2005)
Article DOI: 10.1124/jpet.105.092155
BindingDB Entry DOI: 10.7270/Q2Z60MNB |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM26948
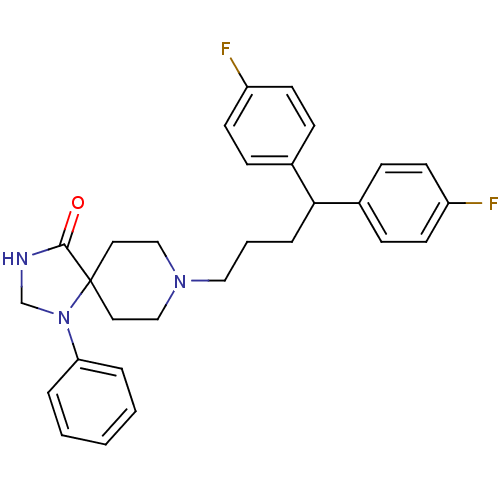
(8-[4,4-bis(4-fluorophenyl)butyl]-1-phenyl-1,3,8-tr...)Show SMILES Fc1ccc(cc1)C(CCCN1CCC2(CC1)N(CNC2=O)c1ccccc1)c1ccc(F)cc1 Show InChI InChI=1S/C29H31F2N3O/c30-24-12-8-22(9-13-24)27(23-10-14-25(31)15-11-23)7-4-18-33-19-16-29(17-20-33)28(35)32-21-34(29)26-5-2-1-3-6-26/h1-3,5-6,8-15,27H,4,7,16-21H2,(H,32,35) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ACADIA Pharmaceuticals
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 315: 1278-87 (2005)
Article DOI: 10.1124/jpet.105.092155
BindingDB Entry DOI: 10.7270/Q2Z60MNB |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM26948

(8-[4,4-bis(4-fluorophenyl)butyl]-1-phenyl-1,3,8-tr...)Show SMILES Fc1ccc(cc1)C(CCCN1CCC2(CC1)N(CNC2=O)c1ccccc1)c1ccc(F)cc1 Show InChI InChI=1S/C29H31F2N3O/c30-24-12-8-22(9-13-24)27(23-10-14-25(31)15-11-23)7-4-18-33-19-16-29(17-20-33)28(35)32-21-34(29)26-5-2-1-3-6-26/h1-3,5-6,8-15,27H,4,7,16-21H2,(H,32,35) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ACADIA Pharmaceuticals
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 315: 1278-87 (2005)
Article DOI: 10.1124/jpet.105.092155
BindingDB Entry DOI: 10.7270/Q2Z60MNB |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM22872

(1-(3-chloro-5,6-dihydrobenzo[b][1]benzothiepin-5-y...)Show InChI InChI=1S/C19H21ClN2S/c1-21-8-10-22(11-9-21)17-12-14-4-2-3-5-18(14)23-19-7-6-15(20)13-16(17)19/h2-7,13,17H,8-12H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ACADIA Pharmaceuticals
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 315: 1278-87 (2005)
Article DOI: 10.1124/jpet.105.092155
BindingDB Entry DOI: 10.7270/Q2Z60MNB |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM78434
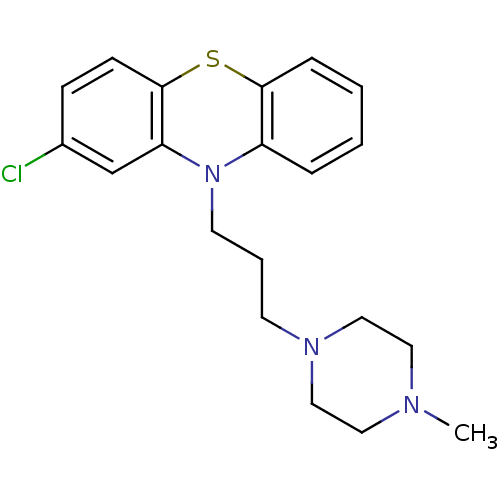
(2-chloranyl-10-[3-(4-methylpiperazin-1-yl)propyl]p...)Show InChI InChI=1S/C20H24ClN3S/c1-22-11-13-23(14-12-22)9-4-10-24-17-5-2-3-6-19(17)25-20-8-7-16(21)15-18(20)24/h2-3,5-8,15H,4,9-14H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| DrugBank
Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ACADIA Pharmaceuticals
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 315: 1278-87 (2005)
Article DOI: 10.1124/jpet.105.092155
BindingDB Entry DOI: 10.7270/Q2Z60MNB |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM86723
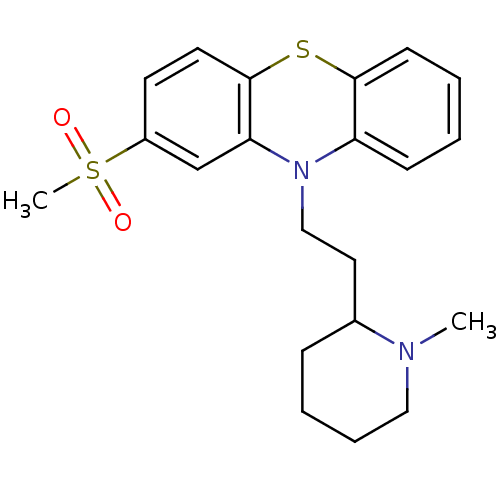
(CAS_14759-06-9 | NSC_31765 | sulforidazine)Show SMILES CN1CCCCC1CCN1c2ccccc2Sc2ccc(cc12)S(C)(=O)=O Show InChI InChI=1S/C21H26N2O2S2/c1-22-13-6-5-7-16(22)12-14-23-18-8-3-4-9-20(18)26-21-11-10-17(15-19(21)23)27(2,24)25/h3-4,8-11,15-16H,5-7,12-14H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ACADIA Pharmaceuticals
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 315: 1278-87 (2005)
Article DOI: 10.1124/jpet.105.092155
BindingDB Entry DOI: 10.7270/Q2Z60MNB |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM86187

(CAS_22189-31-7 | NSC_5454 | THIOTHIXENE)Show SMILES CN(C)S(=O)(=O)c1ccc2Sc3ccccc3C(=CCCN3CCN(C)CC3)c2c1 |w:18.19| Show InChI InChI=1S/C23H29N3O2S2/c1-24(2)30(27,28)18-10-11-23-21(17-18)19(20-7-4-5-9-22(20)29-23)8-6-12-26-15-13-25(3)14-16-26/h4-5,7-11,17H,6,12-16H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ACADIA Pharmaceuticals
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 315: 1278-87 (2005)
Article DOI: 10.1124/jpet.105.092155
BindingDB Entry DOI: 10.7270/Q2Z60MNB |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM81493
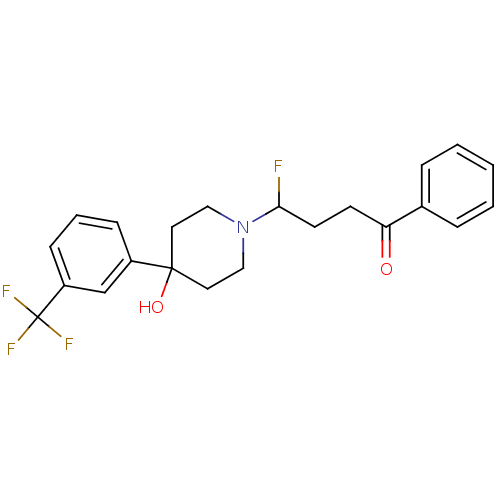
(CAS_749-13-3 | TRIFLUORPERIDOL)Show SMILES OC1(CCN(CC1)C(F)CCC(=O)c1ccccc1)c1cccc(c1)C(F)(F)F Show InChI InChI=1S/C22H23F4NO2/c23-20(10-9-19(28)16-5-2-1-3-6-16)27-13-11-21(29,12-14-27)17-7-4-8-18(15-17)22(24,25)26/h1-8,15,20,29H,9-14H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ACADIA Pharmaceuticals
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 315: 1278-87 (2005)
Article DOI: 10.1124/jpet.105.092155
BindingDB Entry DOI: 10.7270/Q2Z60MNB |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM81484
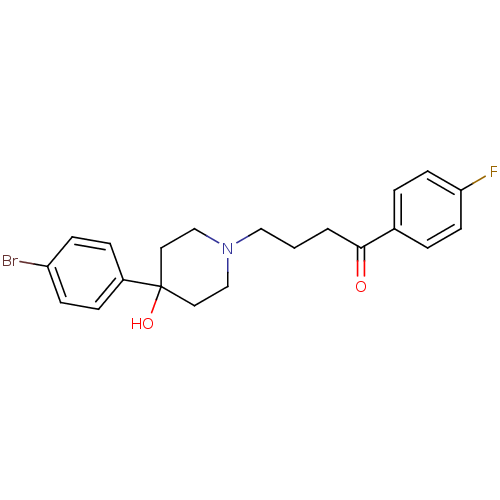
(BROMPERIDOL | Bromoperidol | CAS_2448 | NSC_2448)Show SMILES OC1(CCN(CCCC(=O)c2ccc(F)cc2)CC1)c1ccc(Br)cc1 Show InChI InChI=1S/C21H23BrFNO2/c22-18-7-5-17(6-8-18)21(26)11-14-24(15-12-21)13-1-2-20(25)16-3-9-19(23)10-4-16/h3-10,26H,1-2,11-15H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ACADIA Pharmaceuticals
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 315: 1278-87 (2005)
Article DOI: 10.1124/jpet.105.092155
BindingDB Entry DOI: 10.7270/Q2Z60MNB |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM21398

(4-[4-(4-Chloro-phenyl)-4-hydroxy-piperidin-1-yl]-1...)Show SMILES OC1(CCN(CCCC(=O)c2ccc(F)cc2)CC1)c1ccc(Cl)cc1 Show InChI InChI=1S/C21H23ClFNO2/c22-18-7-5-17(6-8-18)21(26)11-14-24(15-12-21)13-1-2-20(25)16-3-9-19(23)10-4-16/h3-10,26H,1-2,11-15H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ACADIA Pharmaceuticals
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 315: 1278-87 (2005)
Article DOI: 10.1124/jpet.105.092155
BindingDB Entry DOI: 10.7270/Q2Z60MNB |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM22872

(1-(3-chloro-5,6-dihydrobenzo[b][1]benzothiepin-5-y...)Show InChI InChI=1S/C19H21ClN2S/c1-21-8-10-22(11-9-21)17-12-14-4-2-3-5-18(14)23-19-7-6-15(20)13-16(17)19/h2-7,13,17H,8-12H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ACADIA Pharmaceuticals
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 315: 1278-87 (2005)
Article DOI: 10.1124/jpet.105.092155
BindingDB Entry DOI: 10.7270/Q2Z60MNB |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM50334150
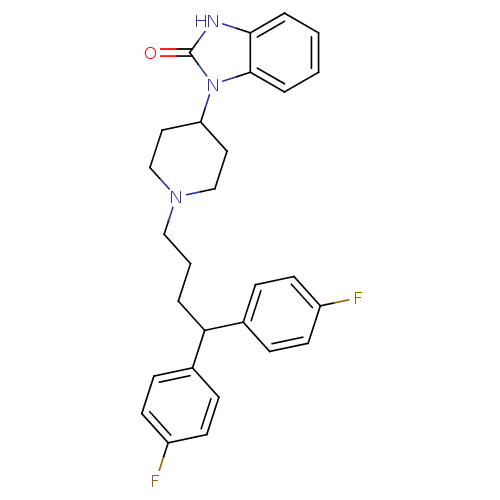
(1-(1-(4,4-bis(4-fluorophenyl)butyl)piperidin-4-yl)...)Show SMILES Fc1ccc(cc1)C(CCCN1CCC(CC1)n1c2ccccc2[nH]c1=O)c1ccc(F)cc1 Show InChI InChI=1S/C28H29F2N3O/c29-22-11-7-20(8-12-22)25(21-9-13-23(30)14-10-21)4-3-17-32-18-15-24(16-19-32)33-27-6-2-1-5-26(27)31-28(33)34/h1-2,5-14,24-25H,3-4,15-19H2,(H,31,34) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ACADIA Pharmaceuticals
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 315: 1278-87 (2005)
Article DOI: 10.1124/jpet.105.092155
BindingDB Entry DOI: 10.7270/Q2Z60MNB |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM50444491

(CHEMBL3092823)Show InChI InChI=1S/C22H25N3O2/c26-22-10-13-25(22)19-5-7-21(23-15-19)27-20-6-4-16-8-11-24(18-2-1-3-18)12-9-17(16)14-20/h4-7,14-15,18H,1-3,8-13H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D
Curated by ChEMBL
| Assay Description
Displacement of [3H]-R-alpha-ethylhistamine from histamine H3 receptor in rat cerebral cortical tissue membranes after 45 mins by liquid scintillatio... |
Bioorg Med Chem Lett 23: 6890-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.09.090
BindingDB Entry DOI: 10.7270/Q2PZ5B83 |
More data for this
Ligand-Target Pair | |
Dimer of Gag-Pol polyprotein [501-599,Q508K,L534I,L564I,C568A,C596A]
(Human immunodeficiency virus type 1) | BDBM9191
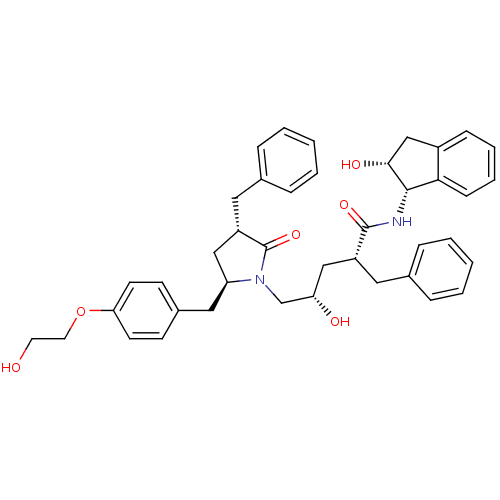
((2R,4S)-2-benzyl-5-[(3S,5R)-3-benzyl-5-{[4-(2-hydr...)Show SMILES OCCOc1ccc(C[C@H]2C[C@H](Cc3ccccc3)C(=O)N2C[C@@H](O)C[C@@H](Cc2ccccc2)C(=O)N[C@@H]2[C@H](O)Cc3ccccc23)cc1 |r| Show InChI InChI=1S/C41H46N2O6/c44-19-20-49-36-17-15-30(16-18-36)23-34-24-33(22-29-11-5-2-6-12-29)41(48)43(34)27-35(45)25-32(21-28-9-3-1-4-10-28)40(47)42-39-37-14-8-7-13-31(37)26-38(39)46/h1-18,32-35,38-39,44-46H,19-27H2,(H,42,47)/t32-,33+,34+,35+,38-,39+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.300 | -54.4 | 480 | n/a | n/a | n/a | n/a | 6.4 | 25 |
GlaxoSmithKline
| Assay Description
The Ki values were determined using fluorogenic substrate, 2-(aminobenzoyl)-Thr-Ile-Nle-Phe(p-NO2)-Gln-ArgNH2. A standard curve relating changes in f... |
Bioorg Med Chem Lett 15: 81-4 (2005)
Article DOI: 10.1016/j.bmcl.2004.10.029
BindingDB Entry DOI: 10.7270/Q29G5K1M |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM86721
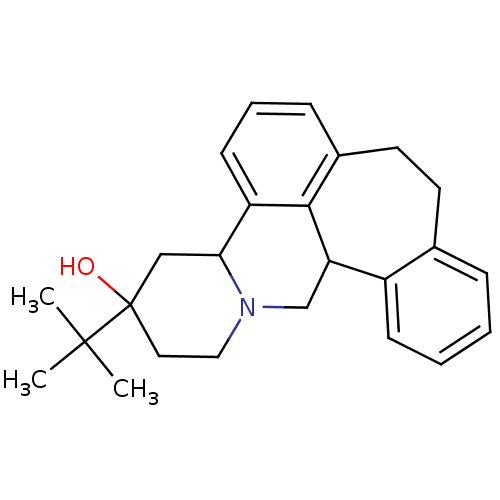
(BUTACLAMOL | CAS_51152-91-1 | NSC_37459 | US111478...)Show SMILES CC(C)(C)C1(O)CCN2CC3c4ccccc4CCc4cccc(C2C1)c34 Show InChI InChI=1S/C25H31NO/c1-24(2,3)25(27)13-14-26-16-21-19-9-5-4-7-17(19)11-12-18-8-6-10-20(23(18)21)22(26)15-25/h4-10,21-22,27H,11-16H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ACADIA Pharmaceuticals
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 315: 1278-87 (2005)
Article DOI: 10.1124/jpet.105.092155
BindingDB Entry DOI: 10.7270/Q2Z60MNB |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data