 Found 414 hits with Last Name = 'delos santos' and Initial = 'e'
Found 414 hits with Last Name = 'delos santos' and Initial = 'e' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50308782
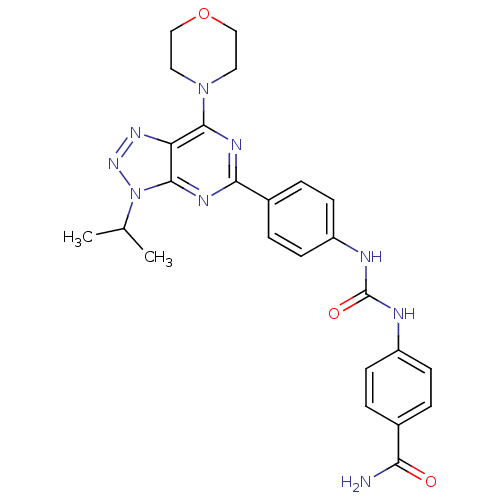
(4-{3-[4-(3-Isopropyl-7-morpholin-4-yl-3H-[1,2,3]-t...)Show SMILES CC(C)n1nnc2c(nc(nc12)-c1ccc(NC(=O)Nc2ccc(cc2)C(N)=O)cc1)N1CCOCC1 Show InChI InChI=1S/C25H27N9O3/c1-15(2)34-24-20(31-32-34)23(33-11-13-37-14-12-33)29-22(30-24)17-5-9-19(10-6-17)28-25(36)27-18-7-3-16(4-8-18)21(26)35/h3-10,15H,11-14H2,1-2H3,(H2,26,35)(H2,27,28,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.310 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of mTOR by DELFIA |
J Med Chem 53: 798-810 (2010)
Article DOI: 10.1021/jm9014982
BindingDB Entry DOI: 10.7270/Q2NZ87RZ |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50308780
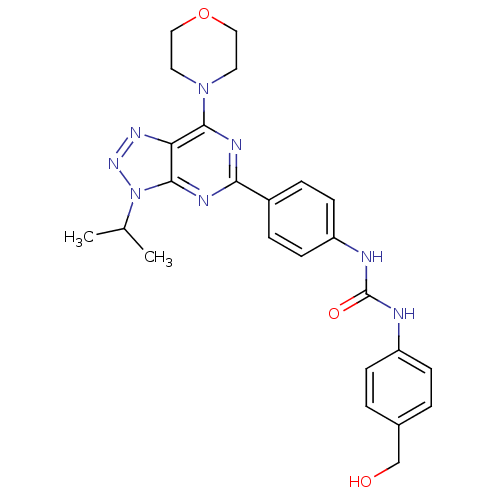
(1-(4-Hydroxymethylphenyl)-3-[4-(3-isopropyl-7-morp...)Show SMILES CC(C)n1nnc2c(nc(nc12)-c1ccc(NC(=O)Nc2ccc(CO)cc2)cc1)N1CCOCC1 Show InChI InChI=1S/C25H28N8O3/c1-16(2)33-24-21(30-31-33)23(32-11-13-36-14-12-32)28-22(29-24)18-5-9-20(10-6-18)27-25(35)26-19-7-3-17(15-34)4-8-19/h3-10,16,34H,11-15H2,1-2H3,(H2,26,27,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.320 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of mTOR by DELFIA |
J Med Chem 53: 798-810 (2010)
Article DOI: 10.1021/jm9014982
BindingDB Entry DOI: 10.7270/Q2NZ87RZ |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50308784
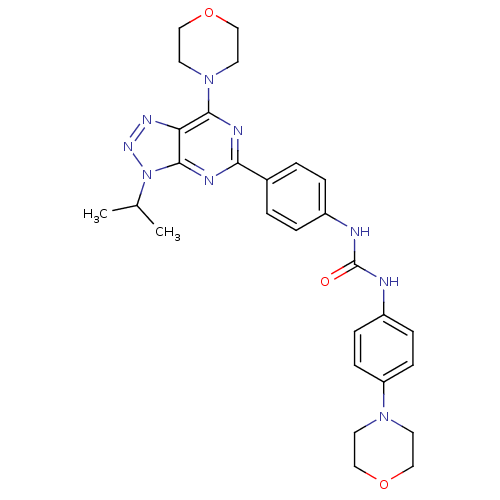
(1-[4-(3-Isopropyl-7-morpholin-4-yl-3H-[1,2,3]-tria...)Show SMILES CC(C)n1nnc2c(nc(nc12)-c1ccc(NC(=O)Nc2ccc(cc2)N2CCOCC2)cc1)N1CCOCC1 Show InChI InChI=1S/C28H33N9O3/c1-19(2)37-27-24(33-34-37)26(36-13-17-40-18-14-36)31-25(32-27)20-3-5-21(6-4-20)29-28(38)30-22-7-9-23(10-8-22)35-11-15-39-16-12-35/h3-10,19H,11-18H2,1-2H3,(H2,29,30,38) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.350 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of mTOR by DELFIA |
J Med Chem 53: 798-810 (2010)
Article DOI: 10.1021/jm9014982
BindingDB Entry DOI: 10.7270/Q2NZ87RZ |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50308781
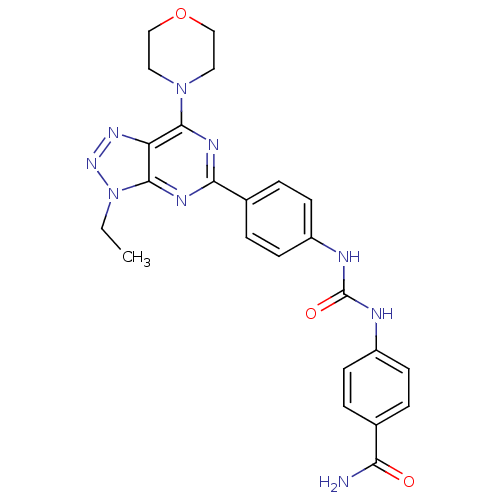
(4-(3-(4-(3-ethyl-7-morpholino-3H-[1,2,3]triazolo[4...)Show SMILES CCn1nnc2c(nc(nc12)-c1ccc(NC(=O)Nc2ccc(cc2)C(N)=O)cc1)N1CCOCC1 Show InChI InChI=1S/C24H25N9O3/c1-2-33-23-19(30-31-33)22(32-11-13-36-14-12-32)28-21(29-23)16-5-9-18(10-6-16)27-24(35)26-17-7-3-15(4-8-17)20(25)34/h3-10H,2,11-14H2,1H3,(H2,25,34)(H2,26,27,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.390 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of mTOR by DELFIA |
J Med Chem 53: 798-810 (2010)
Article DOI: 10.1021/jm9014982
BindingDB Entry DOI: 10.7270/Q2NZ87RZ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50308156
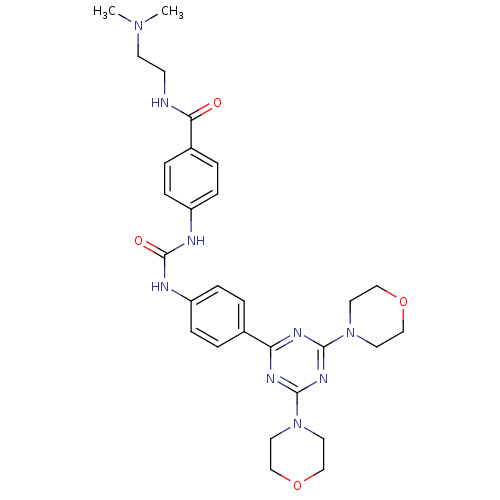
(CHEMBL592615 | N-[2-(Dimethylamino)ethyl]-4-({[4-(...)Show SMILES CN(C)CCNC(=O)c1ccc(NC(=O)Nc2ccc(cc2)-c2nc(nc(n2)N2CCOCC2)N2CCOCC2)cc1 Show InChI InChI=1S/C29H37N9O4/c1-36(2)12-11-30-26(39)22-5-9-24(10-6-22)32-29(40)31-23-7-3-21(4-8-23)25-33-27(37-13-17-41-18-14-37)35-28(34-25)38-15-19-42-20-16-38/h3-10H,11-20H2,1-2H3,(H,30,39)(H2,31,32,40) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha |
J Med Chem 53: 2636-45 (2010)
Article DOI: 10.1021/jm901830p
BindingDB Entry DOI: 10.7270/Q2862HDH |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50118985
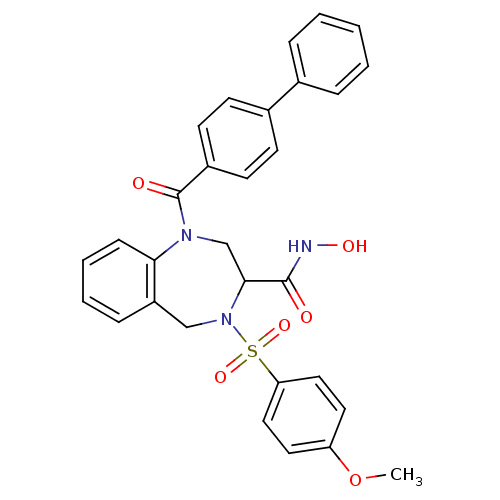
(1-(Biphenyl-4-carbonyl)-4-(4-methoxy-benzenesulfon...)Show SMILES COc1ccc(cc1)S(=O)(=O)N1Cc2ccccc2N(CC1C(=O)NO)C(=O)c1ccc(cc1)-c1ccccc1 Show InChI InChI=1S/C30H27N3O6S/c1-39-25-15-17-26(18-16-25)40(37,38)33-19-24-9-5-6-10-27(24)32(20-28(33)29(34)31-36)30(35)23-13-11-22(12-14-23)21-7-3-2-4-8-21/h2-18,28,36H,19-20H2,1H3,(H,31,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Concentration of the compound required in vitro to inhibit Matrix metalloproteinase-13 |
Bioorg Med Chem Lett 12: 2867-70 (2002)
BindingDB Entry DOI: 10.7270/Q2JS9PSH |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50118983
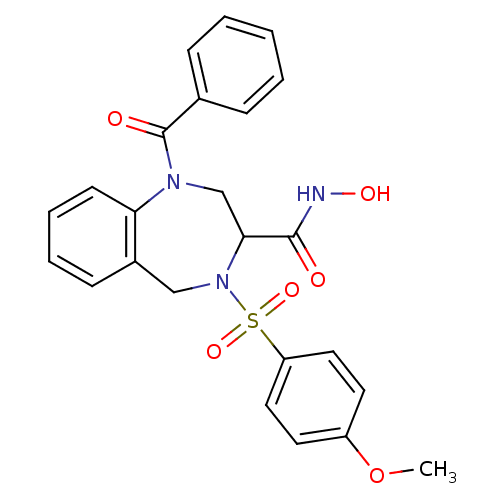
(1-Benzoyl-4-(4-methoxy-benzenesulfonyl)-2,3,4,5-te...)Show SMILES COc1ccc(cc1)S(=O)(=O)N1Cc2ccccc2N(CC1C(=O)NO)C(=O)c1ccccc1 Show InChI InChI=1S/C24H23N3O6S/c1-33-19-11-13-20(14-12-19)34(31,32)27-15-18-9-5-6-10-21(18)26(16-22(27)23(28)25-30)24(29)17-7-3-2-4-8-17/h2-14,22,30H,15-16H2,1H3,(H,25,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Concentration of the compound required in vitro to inhibit Matrix metalloproteinase-13 |
Bioorg Med Chem Lett 12: 2867-70 (2002)
BindingDB Entry DOI: 10.7270/Q2JS9PSH |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50308135
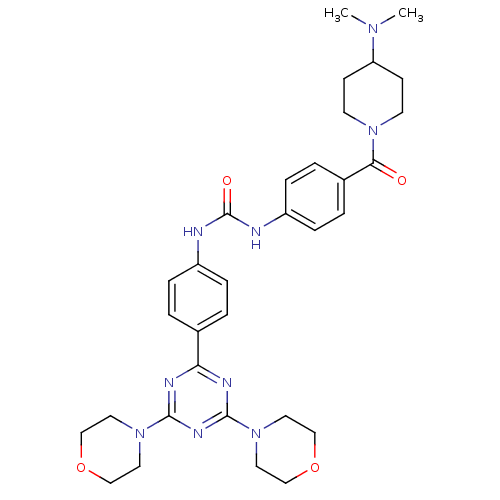
(1-(4-{[4-(Dimethylamino)piperidin-1-yl]carbonyl}ph...)Show SMILES CN(C)C1CCN(CC1)C(=O)c1ccc(NC(=O)Nc2ccc(cc2)-c2nc(nc(n2)N2CCOCC2)N2CCOCC2)cc1 Show InChI InChI=1S/C32H41N9O4/c1-38(2)27-11-13-39(14-12-27)29(42)24-5-9-26(10-6-24)34-32(43)33-25-7-3-23(4-8-25)28-35-30(40-15-19-44-20-16-40)37-31(36-28)41-17-21-45-22-18-41/h3-10,27H,11-22H2,1-2H3,(H2,33,34,43) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha |
J Med Chem 53: 2636-45 (2010)
Article DOI: 10.1021/jm901830p
BindingDB Entry DOI: 10.7270/Q2862HDH |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50378663

(CHEMBL1204015)Show SMILES CN(C)CCN(C)C(=O)c1ccc(NC(=O)Nc2ccc(cc2)-c2nc(nc(n2)N2CCOCC2)N2CCOCC2)cc1 Show InChI InChI=1S/C30H39N9O4/c1-36(2)12-13-37(3)27(40)23-6-10-25(11-7-23)32-30(41)31-24-8-4-22(5-9-24)26-33-28(38-14-18-42-19-15-38)35-29(34-26)39-16-20-43-21-17-39/h4-11H,12-21H2,1-3H3,(H2,31,32,41) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha |
J Med Chem 53: 2636-45 (2010)
Article DOI: 10.1021/jm901830p
BindingDB Entry DOI: 10.7270/Q2862HDH |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50378662
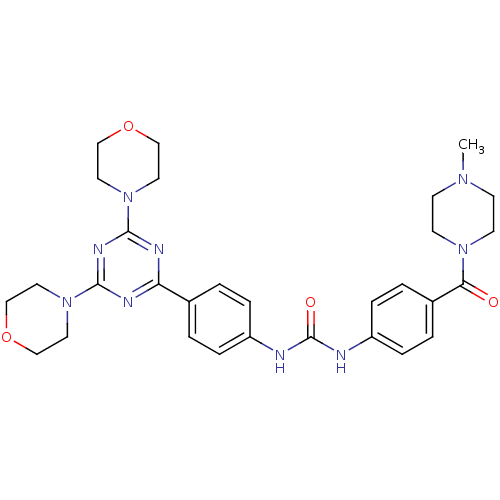
(CHEMBL1204014)Show SMILES CN1CCN(CC1)C(=O)c1ccc(NC(=O)Nc2ccc(cc2)-c2nc(nc(n2)N2CCOCC2)N2CCOCC2)cc1 Show InChI InChI=1S/C30H37N9O4/c1-36-10-12-37(13-11-36)27(40)23-4-8-25(9-5-23)32-30(41)31-24-6-2-22(3-7-24)26-33-28(38-14-18-42-19-15-38)35-29(34-26)39-16-20-43-21-17-39/h2-9H,10-21H2,1H3,(H2,31,32,41) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha |
J Med Chem 53: 2636-45 (2010)
Article DOI: 10.1021/jm901830p
BindingDB Entry DOI: 10.7270/Q2862HDH |
More data for this
Ligand-Target Pair | |
Vasopressin V2 receptor
(Rattus norvegicus (Rat)) | BDBM50065115

(3-chloro-4-(10,11-dihydro-5H-benzo[e]pyrrolo[1,2-a...)Show SMILES Cc1ccc(F)cc1C(=O)Nc1ccc(C(=O)N2Cc3cccn3Cc3ccccc23)c(Cl)c1 Show InChI InChI=1S/C27H21ClFN3O2/c1-17-8-9-19(29)13-23(17)26(33)30-20-10-11-22(24(28)14-20)27(34)32-16-21-6-4-12-31(21)15-18-5-2-3-7-25(18)32/h2-14H,15-16H2,1H3,(H,30,33) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research
Curated by ChEMBL
| Assay Description
Displacement of P[3H]-AVP from isolated rat kidney medullary V2 receptor |
Bioorg Med Chem Lett 9: 1737-40 (1999)
BindingDB Entry DOI: 10.7270/Q23B5ZBZ |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50308788

(CHEMBL590587 | N-[2-(Dimethylamino)ethyl]-4-({[4-(...)Show SMILES CCn1nnc2c(nc(nc12)-c1ccc(NC(=O)Nc2ccc(cc2)C(=O)NCCN(C)C)cc1)N1CCOCC1 Show InChI InChI=1S/C28H34N10O3/c1-4-38-26-23(34-35-38)25(37-15-17-41-18-16-37)32-24(33-26)19-5-9-21(10-6-19)30-28(40)31-22-11-7-20(8-12-22)27(39)29-13-14-36(2)3/h5-12H,4,13-18H2,1-3H3,(H,29,39)(H2,30,31,40) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of mTOR by DELFIA |
J Med Chem 53: 798-810 (2010)
Article DOI: 10.1021/jm9014982
BindingDB Entry DOI: 10.7270/Q2NZ87RZ |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50308156

(CHEMBL592615 | N-[2-(Dimethylamino)ethyl]-4-({[4-(...)Show SMILES CN(C)CCNC(=O)c1ccc(NC(=O)Nc2ccc(cc2)-c2nc(nc(n2)N2CCOCC2)N2CCOCC2)cc1 Show InChI InChI=1S/C29H37N9O4/c1-36(2)12-11-30-26(39)22-5-9-24(10-6-22)32-29(40)31-23-7-3-21(4-8-23)25-33-27(37-13-17-41-18-14-37)35-28(34-25)38-15-19-42-20-16-38/h3-10H,11-20H2,1-2H3,(H,30,39)(H2,31,32,40) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of mTOR |
J Med Chem 53: 2636-45 (2010)
Article DOI: 10.1021/jm901830p
BindingDB Entry DOI: 10.7270/Q2862HDH |
More data for this
Ligand-Target Pair | |
Vasopressin V2 receptor
(Homo sapiens (Human)) | BDBM50065118
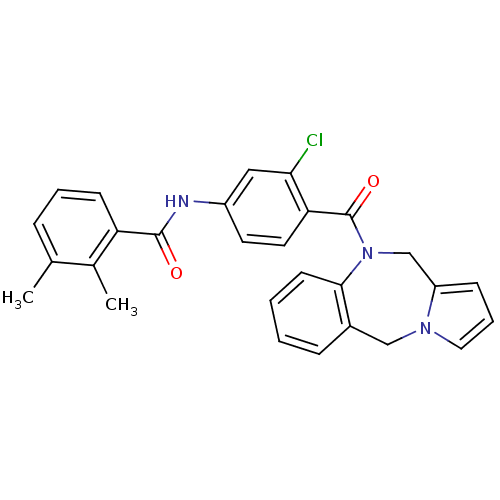
(CHEMBL311931 | N-[4-(5H,11H-Benzo[e]pyrrolo[1,2-a]...)Show SMILES Cc1cccc(C(=O)Nc2ccc(C(=O)N3Cc4cccn4Cc4ccccc34)c(Cl)c2)c1C Show InChI InChI=1S/C28H24ClN3O2/c1-18-7-5-10-23(19(18)2)27(33)30-21-12-13-24(25(29)15-21)28(34)32-17-22-9-6-14-31(22)16-20-8-3-4-11-26(20)32/h3-15H,16-17H2,1-2H3,(H,30,33) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]-AVP from human V2 receptor expressed in murine fibroblast cell line (LV2) membranes |
J Med Chem 41: 2442-4 (1998)
Article DOI: 10.1021/jm980179c
BindingDB Entry DOI: 10.7270/Q2CC0ZT5 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50308769
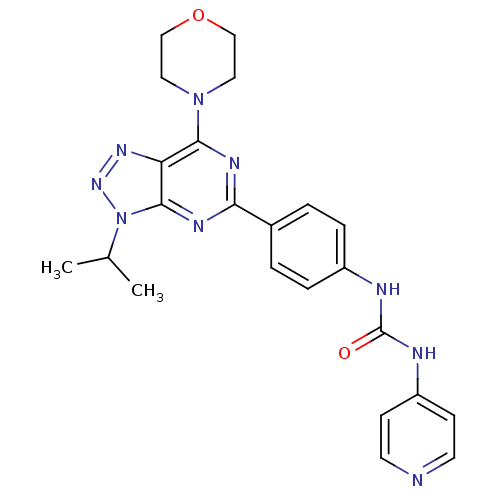
(1-[4-(3-Isopropyl-7-morpholin-4-yl-3H-[1,2,3]-tria...)Show SMILES CC(C)n1nnc2c(nc(nc12)-c1ccc(NC(=O)Nc2ccncc2)cc1)N1CCOCC1 Show InChI InChI=1S/C23H25N9O2/c1-15(2)32-22-19(29-30-32)21(31-11-13-34-14-12-31)27-20(28-22)16-3-5-17(6-4-16)25-23(33)26-18-7-9-24-10-8-18/h3-10,15H,11-14H2,1-2H3,(H2,24,25,26,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.510 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of mTOR by DELFIA |
J Med Chem 53: 798-810 (2010)
Article DOI: 10.1021/jm9014982
BindingDB Entry DOI: 10.7270/Q2NZ87RZ |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50308785
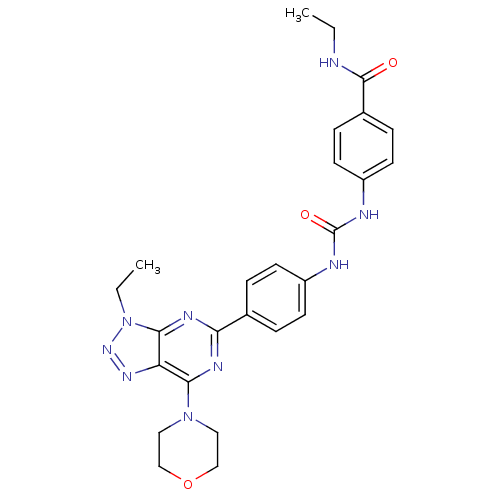
(CHEMBL591357 | N-Ethyl-4-({[4-(3-ethyl-7-morpholin...)Show SMILES CCNC(=O)c1ccc(NC(=O)Nc2ccc(cc2)-c2nc(N3CCOCC3)c3nnn(CC)c3n2)cc1 Show InChI InChI=1S/C26H29N9O3/c1-3-27-25(36)18-7-11-20(12-8-18)29-26(37)28-19-9-5-17(6-10-19)22-30-23(34-13-15-38-16-14-34)21-24(31-22)35(4-2)33-32-21/h5-12H,3-4,13-16H2,1-2H3,(H,27,36)(H2,28,29,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.560 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of mTOR by DELFIA |
J Med Chem 53: 798-810 (2010)
Article DOI: 10.1021/jm9014982
BindingDB Entry DOI: 10.7270/Q2NZ87RZ |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50308774
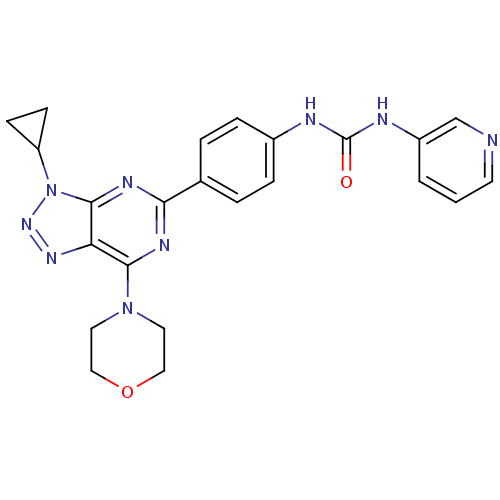
(1-(4-(3-Cyclopropyl-7-morpholino-3H-[1,2,3]-triazo...)Show SMILES O=C(Nc1ccc(cc1)-c1nc(N2CCOCC2)c2nnn(C3CC3)c2n1)Nc1cccnc1 Show InChI InChI=1S/C23H23N9O2/c33-23(26-17-2-1-9-24-14-17)25-16-5-3-15(4-6-16)20-27-21(31-10-12-34-13-11-31)19-22(28-20)32(30-29-19)18-7-8-18/h1-6,9,14,18H,7-8,10-13H2,(H2,25,26,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.570 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of mTOR by DELFIA |
J Med Chem 53: 798-810 (2010)
Article DOI: 10.1021/jm9014982
BindingDB Entry DOI: 10.7270/Q2NZ87RZ |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50308772

(1-[4-(3-Isopropyl-7-morpholin-4-yl-3H-[1,2,3]-tria...)Show SMILES CC(C)n1nnc2c(nc(nc12)-c1ccc(NC(=O)Nc2cccnc2)cc1)N1CCOCC1 Show InChI InChI=1S/C23H25N9O2/c1-15(2)32-22-19(29-30-32)21(31-10-12-34-13-11-31)27-20(28-22)16-5-7-17(8-6-16)25-23(33)26-18-4-3-9-24-14-18/h3-9,14-15H,10-13H2,1-2H3,(H2,25,26,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.570 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of mTOR by DELFIA |
J Med Chem 53: 798-810 (2010)
Article DOI: 10.1021/jm9014982
BindingDB Entry DOI: 10.7270/Q2NZ87RZ |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50118983

(1-Benzoyl-4-(4-methoxy-benzenesulfonyl)-2,3,4,5-te...)Show SMILES COc1ccc(cc1)S(=O)(=O)N1Cc2ccccc2N(CC1C(=O)NO)C(=O)c1ccccc1 Show InChI InChI=1S/C24H23N3O6S/c1-33-19-11-13-20(14-12-19)34(31,32)27-15-18-9-5-6-10-21(18)26(16-22(27)23(28)25-30)24(29)17-7-3-2-4-8-17/h2-14,22,30H,15-16H2,1H3,(H,25,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Concentration required in vitro to inhibit Matrix metalloproteinase-9 |
Bioorg Med Chem Lett 12: 2867-70 (2002)
BindingDB Entry DOI: 10.7270/Q2JS9PSH |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50308137

(1-(4-(1,4'-bipiperidine-1'-carbonyl)phenyl)-3-(4-(...)Show SMILES O=C(Nc1ccc(cc1)C(=O)N1CCC(CC1)N1CCCCC1)Nc1ccc(cc1)-c1nc(nc(n1)N1CCOCC1)N1CCOCC1 Show InChI InChI=1S/C35H45N9O4/c45-32(42-16-12-30(13-17-42)41-14-2-1-3-15-41)27-6-10-29(11-7-27)37-35(46)36-28-8-4-26(5-9-28)31-38-33(43-18-22-47-23-19-43)40-34(39-31)44-20-24-48-25-21-44/h4-11,30H,1-3,12-25H2,(H2,36,37,46) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha |
J Med Chem 53: 2636-45 (2010)
Article DOI: 10.1021/jm901830p
BindingDB Entry DOI: 10.7270/Q2862HDH |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50378663

(CHEMBL1204015)Show SMILES CN(C)CCN(C)C(=O)c1ccc(NC(=O)Nc2ccc(cc2)-c2nc(nc(n2)N2CCOCC2)N2CCOCC2)cc1 Show InChI InChI=1S/C30H39N9O4/c1-36(2)12-13-37(3)27(40)23-6-10-25(11-7-23)32-30(41)31-24-8-4-22(5-9-24)26-33-28(38-14-18-42-19-15-38)35-29(34-26)39-16-20-43-21-17-39/h4-11H,12-21H2,1-3H3,(H2,31,32,41) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of mTOR |
J Med Chem 53: 2636-45 (2010)
Article DOI: 10.1021/jm901830p
BindingDB Entry DOI: 10.7270/Q2862HDH |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50308768
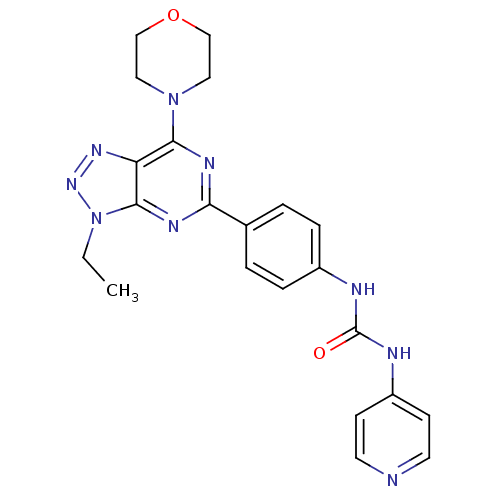
(1-[4-(3-Ethyl-7-morpholin-4-yl-3H-[1,2,3]triazolo-...)Show SMILES CCn1nnc2c(nc(nc12)-c1ccc(NC(=O)Nc2ccncc2)cc1)N1CCOCC1 Show InChI InChI=1S/C22H23N9O2/c1-2-31-21-18(28-29-31)20(30-11-13-33-14-12-30)26-19(27-21)15-3-5-16(6-4-15)24-22(32)25-17-7-9-23-10-8-17/h3-10H,2,11-14H2,1H3,(H2,23,24,25,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.630 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of mTOR by DELFIA |
J Med Chem 53: 798-810 (2010)
Article DOI: 10.1021/jm9014982
BindingDB Entry DOI: 10.7270/Q2NZ87RZ |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50308155
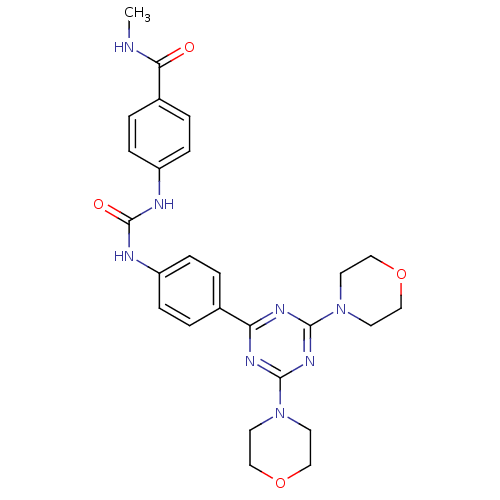
(4-(3-(4-(4,6-Dimorpholino-1,3,5-triazin-2-yl)pheny...)Show SMILES CNC(=O)c1ccc(NC(=O)Nc2ccc(cc2)-c2nc(nc(n2)N2CCOCC2)N2CCOCC2)cc1 Show InChI InChI=1S/C26H30N8O4/c1-27-23(35)19-4-8-21(9-5-19)29-26(36)28-20-6-2-18(3-7-20)22-30-24(33-10-14-37-15-11-33)32-25(31-22)34-12-16-38-17-13-34/h2-9H,10-17H2,1H3,(H,27,35)(H2,28,29,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of mTOR |
J Med Chem 53: 2636-45 (2010)
Article DOI: 10.1021/jm901830p
BindingDB Entry DOI: 10.7270/Q2862HDH |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50308137

(1-(4-(1,4'-bipiperidine-1'-carbonyl)phenyl)-3-(4-(...)Show SMILES O=C(Nc1ccc(cc1)C(=O)N1CCC(CC1)N1CCCCC1)Nc1ccc(cc1)-c1nc(nc(n1)N1CCOCC1)N1CCOCC1 Show InChI InChI=1S/C35H45N9O4/c45-32(42-16-12-30(13-17-42)41-14-2-1-3-15-41)27-6-10-29(11-7-27)37-35(46)36-28-8-4-26(5-9-28)31-38-33(43-18-22-47-23-19-43)40-34(39-31)44-20-24-48-25-21-44/h4-11,30H,1-3,12-25H2,(H2,36,37,46) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of mTOR |
J Med Chem 53: 2636-45 (2010)
Article DOI: 10.1021/jm901830p
BindingDB Entry DOI: 10.7270/Q2862HDH |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50118985

(1-(Biphenyl-4-carbonyl)-4-(4-methoxy-benzenesulfon...)Show SMILES COc1ccc(cc1)S(=O)(=O)N1Cc2ccccc2N(CC1C(=O)NO)C(=O)c1ccc(cc1)-c1ccccc1 Show InChI InChI=1S/C30H27N3O6S/c1-39-25-15-17-26(18-16-25)40(37,38)33-19-24-9-5-6-10-27(24)32(20-28(33)29(34)31-36)30(35)23-13-11-22(12-14-23)21-7-3-2-4-8-21/h2-18,28,36H,19-20H2,1H3,(H,31,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Concentration required in vitro to inhibit Matrix metalloproteinase-9 |
Bioorg Med Chem Lett 12: 2867-70 (2002)
BindingDB Entry DOI: 10.7270/Q2JS9PSH |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50308136
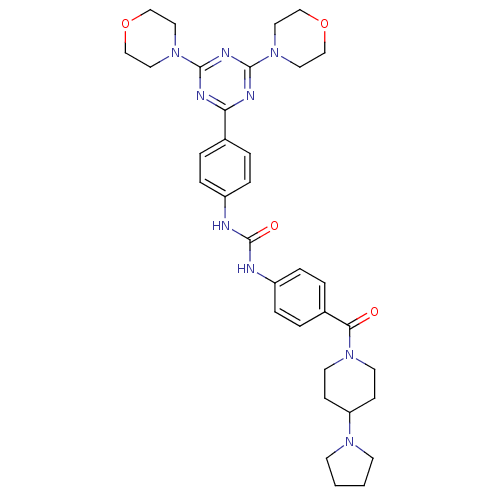
(1-[4-(4,6-Dimorpholin-4-yl-1,3,5-triazin-2-yl)-phe...)Show SMILES O=C(Nc1ccc(cc1)C(=O)N1CCC(CC1)N1CCCC1)Nc1ccc(cc1)-c1nc(nc(n1)N1CCOCC1)N1CCOCC1 Show InChI InChI=1S/C34H43N9O4/c44-31(41-15-11-29(12-16-41)40-13-1-2-14-40)26-5-9-28(10-6-26)36-34(45)35-27-7-3-25(4-8-27)30-37-32(42-17-21-46-22-18-42)39-33(38-30)43-19-23-47-24-20-43/h3-10,29H,1-2,11-24H2,(H2,35,36,45) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha |
J Med Chem 53: 2636-45 (2010)
Article DOI: 10.1021/jm901830p
BindingDB Entry DOI: 10.7270/Q2862HDH |
More data for this
Ligand-Target Pair | |
Vasopressin V2 receptor
(Homo sapiens (Human)) | BDBM50065123
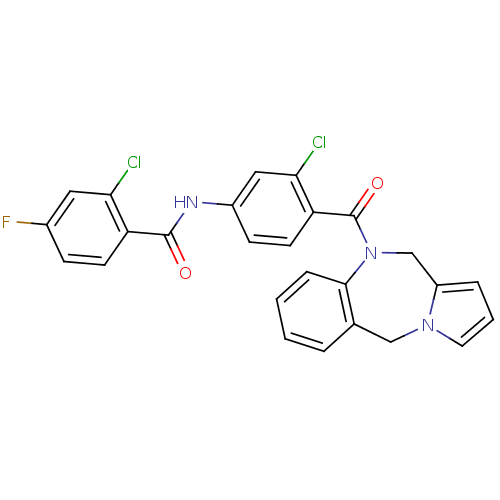
(CHEMBL420031 | N-[4-(5H,11H-Benzo[e]pyrrolo[1,2-a]...)Show SMILES Fc1ccc(C(=O)Nc2ccc(C(=O)N3Cc4cccn4Cc4ccccc34)c(Cl)c2)c(Cl)c1 Show InChI InChI=1S/C26H18Cl2FN3O2/c27-22-12-17(29)7-9-20(22)25(33)30-18-8-10-21(23(28)13-18)26(34)32-15-19-5-3-11-31(19)14-16-4-1-2-6-24(16)32/h1-13H,14-15H2,(H,30,33) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]-AVP from human V2 receptor expressed in murine fibroblast cell line (LV2) membranes |
J Med Chem 41: 2442-4 (1998)
Article DOI: 10.1021/jm980179c
BindingDB Entry DOI: 10.7270/Q2CC0ZT5 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50308786

(4-({[4-(3-Ethyl-7-morpholin-4-yl-3H-[1,2,3]-triazo...)Show SMILES CCn1nnc2c(nc(nc12)-c1ccc(NC(=O)Nc2ccc(cc2)C(=O)N(C)C)cc1)N1CCOCC1 Show InChI InChI=1S/C26H29N9O3/c1-4-35-24-21(31-32-35)23(34-13-15-38-16-14-34)29-22(30-24)17-5-9-19(10-6-17)27-26(37)28-20-11-7-18(8-12-20)25(36)33(2)3/h5-12H,4,13-16H2,1-3H3,(H2,27,28,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.75 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of mTOR by DELFIA |
J Med Chem 53: 798-810 (2010)
Article DOI: 10.1021/jm9014982
BindingDB Entry DOI: 10.7270/Q2NZ87RZ |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50308136

(1-[4-(4,6-Dimorpholin-4-yl-1,3,5-triazin-2-yl)-phe...)Show SMILES O=C(Nc1ccc(cc1)C(=O)N1CCC(CC1)N1CCCC1)Nc1ccc(cc1)-c1nc(nc(n1)N1CCOCC1)N1CCOCC1 Show InChI InChI=1S/C34H43N9O4/c44-31(41-15-11-29(12-16-41)40-13-1-2-14-40)26-5-9-28(10-6-26)36-34(45)35-27-7-3-25(4-8-27)30-37-32(42-17-21-46-22-18-42)39-33(38-30)43-19-23-47-24-20-43/h3-10,29H,1-2,11-24H2,(H2,35,36,45) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of mTOR |
J Med Chem 53: 2636-45 (2010)
Article DOI: 10.1021/jm901830p
BindingDB Entry DOI: 10.7270/Q2862HDH |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50308770

(1-(4-(3-Cyclopropyl-7-morpholino-3H-[1,2,3]-triazo...)Show SMILES O=C(Nc1ccncc1)Nc1ccc(cc1)-c1nc(N2CCOCC2)c2nnn(C3CC3)c2n1 Show InChI InChI=1S/C23H23N9O2/c33-23(26-17-7-9-24-10-8-17)25-16-3-1-15(2-4-16)20-27-21(31-11-13-34-14-12-31)19-22(28-20)32(30-29-19)18-5-6-18/h1-4,7-10,18H,5-6,11-14H2,(H2,24,25,26,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.840 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of mTOR by DELFIA |
J Med Chem 53: 798-810 (2010)
Article DOI: 10.1021/jm9014982
BindingDB Entry DOI: 10.7270/Q2NZ87RZ |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50308771
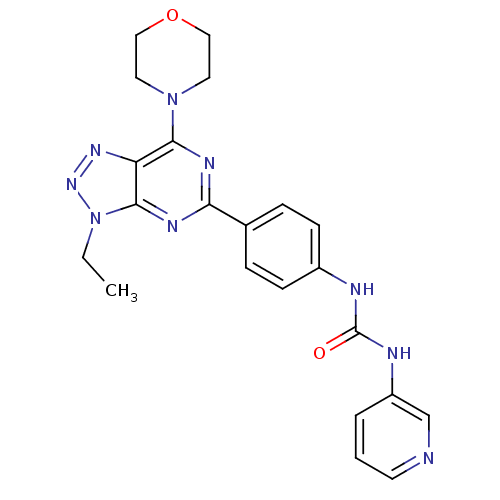
(1-[4-(3-Ethyl-7-morpholin-4-yl-3H-[1,2,3]triazolo-...)Show SMILES CCn1nnc2c(nc(nc12)-c1ccc(NC(=O)Nc2cccnc2)cc1)N1CCOCC1 Show InChI InChI=1S/C22H23N9O2/c1-2-31-21-18(28-29-31)20(30-10-12-33-13-11-30)26-19(27-21)15-5-7-16(8-6-15)24-22(32)25-17-4-3-9-23-14-17/h3-9,14H,2,10-13H2,1H3,(H2,24,25,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.890 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of mTOR by DELFIA |
J Med Chem 53: 798-810 (2010)
Article DOI: 10.1021/jm9014982
BindingDB Entry DOI: 10.7270/Q2NZ87RZ |
More data for this
Ligand-Target Pair | |
Vasopressin V2 receptor
(Rattus norvegicus (Rat)) | BDBM50078656
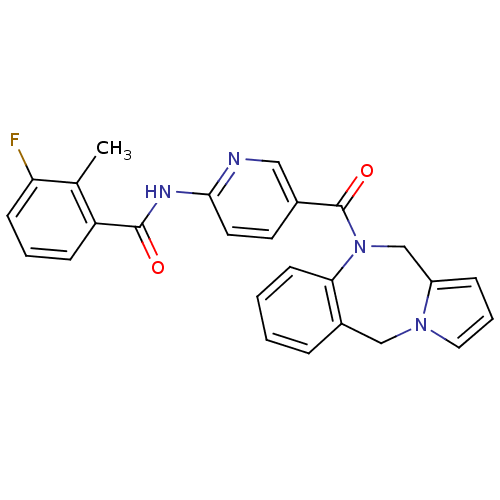
(CHEMBL46295 | N-[5-(5H,11H-Benzo[e]pyrrolo[1,2-a][...)Show SMILES Cc1c(F)cccc1C(=O)Nc1ccc(cn1)C(=O)N1Cc2cccn2Cc2ccccc12 Show InChI InChI=1S/C26H21FN4O2/c1-17-21(8-4-9-22(17)27)25(32)29-24-12-11-18(14-28-24)26(33)31-16-20-7-5-13-30(20)15-19-6-2-3-10-23(19)31/h2-14H,15-16H2,1H3,(H,28,29,32) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research
Curated by ChEMBL
| Assay Description
Displacement of P[3H]-AVP from isolated rat kidney medullary V2 receptor |
Bioorg Med Chem Lett 9: 1737-40 (1999)
BindingDB Entry DOI: 10.7270/Q23B5ZBZ |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50118975
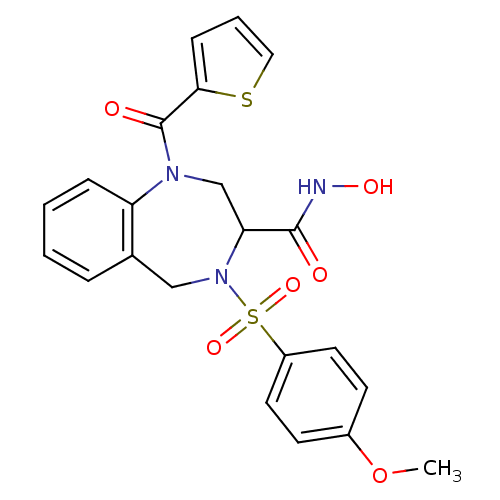
(4-(4-Methoxy-benzenesulfonyl)-1-(thiophene-2-carbo...)Show SMILES COc1ccc(cc1)S(=O)(=O)N1Cc2ccccc2N(CC1C(=O)NO)C(=O)c1cccs1 Show InChI InChI=1S/C22H21N3O6S2/c1-31-16-8-10-17(11-9-16)33(29,30)25-13-15-5-2-3-6-18(15)24(14-19(25)21(26)23-28)22(27)20-7-4-12-32-20/h2-12,19,28H,13-14H2,1H3,(H,23,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Concentration required in vitro to inhibit Matrix metalloproteinase-9 |
Bioorg Med Chem Lett 12: 2867-70 (2002)
BindingDB Entry DOI: 10.7270/Q2JS9PSH |
More data for this
Ligand-Target Pair | |
Vasopressin V2 receptor
(Homo sapiens (Human)) | BDBM50065121

(CHEMBL310416 | N-[4-(5H,11H-Benzo[e]pyrrolo[1,2-a]...)Show SMILES Cc1ccccc1C(=O)Nc1ccc(C(=O)N2Cc3cccn3Cc3ccccc23)c(Cl)c1 Show InChI InChI=1S/C27H22ClN3O2/c1-18-7-2-4-10-22(18)26(32)29-20-12-13-23(24(28)15-20)27(33)31-17-21-9-6-14-30(21)16-19-8-3-5-11-25(19)31/h2-15H,16-17H2,1H3,(H,29,32) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]-AVP from human V2 receptor expressed in murine fibroblast cell line (LV2) membranes |
J Med Chem 41: 2442-4 (1998)
Article DOI: 10.1021/jm980179c
BindingDB Entry DOI: 10.7270/Q2CC0ZT5 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50308788

(CHEMBL590587 | N-[2-(Dimethylamino)ethyl]-4-({[4-(...)Show SMILES CCn1nnc2c(nc(nc12)-c1ccc(NC(=O)Nc2ccc(cc2)C(=O)NCCN(C)C)cc1)N1CCOCC1 Show InChI InChI=1S/C28H34N10O3/c1-4-38-26-23(34-35-38)25(37-15-17-41-18-16-37)32-24(33-26)19-5-9-21(10-6-19)30-28(40)31-22-11-7-20(8-12-22)27(39)29-13-14-36(2)3/h5-12H,4,13-18H2,1-3H3,(H,29,39)(H2,30,31,40) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of human PI3Kalpha by fluorescence polarization format assay |
J Med Chem 53: 798-810 (2010)
Article DOI: 10.1021/jm9014982
BindingDB Entry DOI: 10.7270/Q2NZ87RZ |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50118974
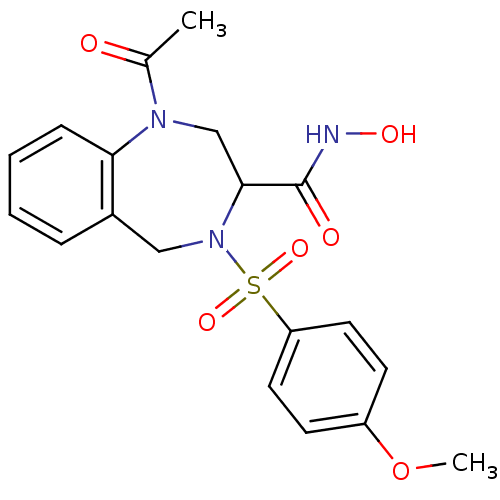
(1-Acetyl-4-(4-methoxy-benzenesulfonyl)-2,3,4,5-tet...)Show SMILES COc1ccc(cc1)S(=O)(=O)N1Cc2ccccc2N(CC1C(=O)NO)C(C)=O Show InChI InChI=1S/C19H21N3O6S/c1-13(23)21-12-18(19(24)20-25)22(11-14-5-3-4-6-17(14)21)29(26,27)16-9-7-15(28-2)8-10-16/h3-10,18,25H,11-12H2,1-2H3,(H,20,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Concentration of the compound required in vitro to inhibit Matrix metalloproteinase-13 |
Bioorg Med Chem Lett 12: 2867-70 (2002)
BindingDB Entry DOI: 10.7270/Q2JS9PSH |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50118975

(4-(4-Methoxy-benzenesulfonyl)-1-(thiophene-2-carbo...)Show SMILES COc1ccc(cc1)S(=O)(=O)N1Cc2ccccc2N(CC1C(=O)NO)C(=O)c1cccs1 Show InChI InChI=1S/C22H21N3O6S2/c1-31-16-8-10-17(11-9-16)33(29,30)25-13-15-5-2-3-6-18(15)24(14-19(25)21(26)23-28)22(27)20-7-4-12-32-20/h2-12,19,28H,13-14H2,1H3,(H,23,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Concentration of the compound required in vitro to inhibit Matrix metalloproteinase-13 |
Bioorg Med Chem Lett 12: 2867-70 (2002)
BindingDB Entry DOI: 10.7270/Q2JS9PSH |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50308147
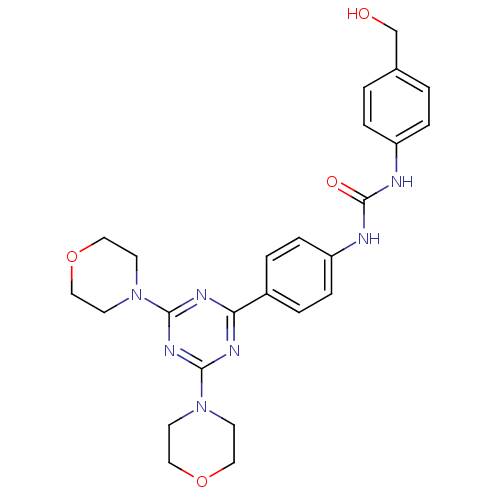
(1-[4-(4,6-Dimorpholin-4-yl-1,3,5-triazin-2-yl)-phe...)Show SMILES OCc1ccc(NC(=O)Nc2ccc(cc2)-c2nc(nc(n2)N2CCOCC2)N2CCOCC2)cc1 Show InChI InChI=1S/C25H29N7O4/c33-17-18-1-5-20(6-2-18)26-25(34)27-21-7-3-19(4-8-21)22-28-23(31-9-13-35-14-10-31)30-24(29-22)32-11-15-36-16-12-32/h1-8,33H,9-17H2,(H2,26,27,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha |
J Med Chem 53: 2636-45 (2010)
Article DOI: 10.1021/jm901830p
BindingDB Entry DOI: 10.7270/Q2862HDH |
More data for this
Ligand-Target Pair | |
Vasopressin V2 receptor
(Rattus norvegicus (Rat)) | BDBM50078652
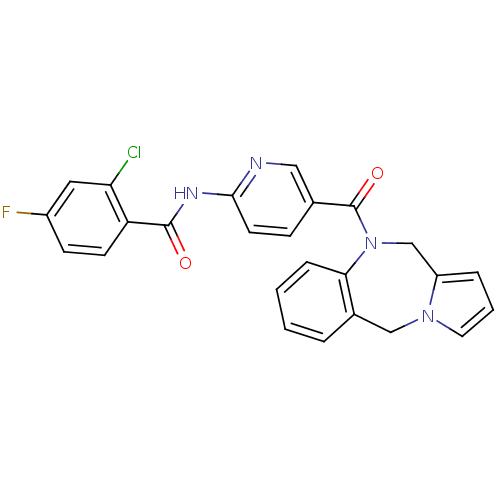
(CHEMBL301788 | N-[5-(5H,11H-Benzo[e]pyrrolo[1,2-a]...)Show SMILES Fc1ccc(C(=O)Nc2ccc(cn2)C(=O)N2Cc3cccn3Cc3ccccc23)c(Cl)c1 Show InChI InChI=1S/C25H18ClFN4O2/c26-21-12-18(27)8-9-20(21)24(32)29-23-10-7-16(13-28-23)25(33)31-15-19-5-3-11-30(19)14-17-4-1-2-6-22(17)31/h1-13H,14-15H2,(H,28,29,32) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research
Curated by ChEMBL
| Assay Description
Displacement of P[3H]-AVP from isolated rat kidney medullary V2 receptor |
Bioorg Med Chem Lett 9: 1737-40 (1999)
BindingDB Entry DOI: 10.7270/Q23B5ZBZ |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50308790
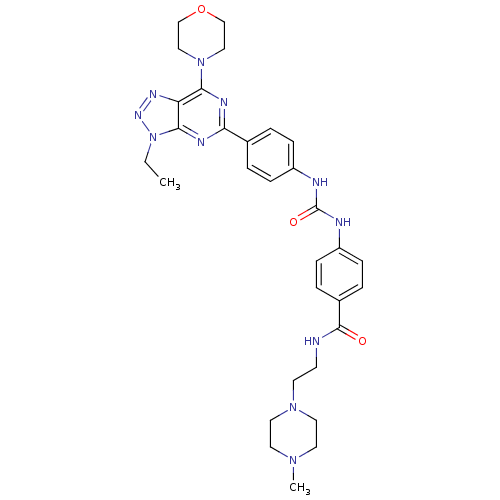
(4-({[4-(3-Ethyl-7-morpholin-4-yl-3H-[1,2,3]-triazo...)Show SMILES CCn1nnc2c(nc(nc12)-c1ccc(NC(=O)Nc2ccc(cc2)C(=O)NCCN2CCN(C)CC2)cc1)N1CCOCC1 Show InChI InChI=1S/C31H39N11O3/c1-3-42-29-26(37-38-42)28(41-18-20-45-21-19-41)35-27(36-29)22-4-8-24(9-5-22)33-31(44)34-25-10-6-23(7-11-25)30(43)32-12-13-40-16-14-39(2)15-17-40/h4-11H,3,12-21H2,1-2H3,(H,32,43)(H2,33,34,44) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of mTOR by DELFIA |
J Med Chem 53: 798-810 (2010)
Article DOI: 10.1021/jm9014982
BindingDB Entry DOI: 10.7270/Q2NZ87RZ |
More data for this
Ligand-Target Pair | |
Vasopressin V2 receptor
(Homo sapiens (Human)) | BDBM50065115

(3-chloro-4-(10,11-dihydro-5H-benzo[e]pyrrolo[1,2-a...)Show SMILES Cc1ccc(F)cc1C(=O)Nc1ccc(C(=O)N2Cc3cccn3Cc3ccccc23)c(Cl)c1 Show InChI InChI=1S/C27H21ClFN3O2/c1-17-8-9-19(29)13-23(17)26(33)30-20-10-11-22(24(28)14-20)27(34)32-16-21-6-4-12-31(21)15-18-5-2-3-7-25(18)32/h2-14H,15-16H2,1H3,(H,30,33) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research
Curated by ChEMBL
| Assay Description
In vitro binding affinity to human V2 receptor |
Bioorg Med Chem Lett 10: 695-8 (2000)
BindingDB Entry DOI: 10.7270/Q2X34WP8 |
More data for this
Ligand-Target Pair | |
Vasopressin V2 receptor
(Rattus norvegicus (Rat)) | BDBM50087550
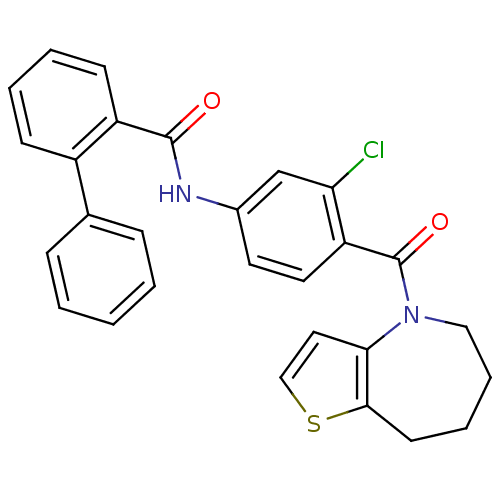
(Biphenyl-2-carboxylic acid [3-chloro-4-(5,6,7,8-te...)Show SMILES Clc1cc(NC(=O)c2ccccc2-c2ccccc2)ccc1C(=O)N1CCCCc2sccc12 Show InChI InChI=1S/C28H23ClN2O2S/c29-24-18-20(30-27(32)22-11-5-4-10-21(22)19-8-2-1-3-9-19)13-14-23(24)28(33)31-16-7-6-12-26-25(31)15-17-34-26/h1-5,8-11,13-15,17-18H,6-7,12,16H2,(H,30,32) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research
Curated by ChEMBL
| Assay Description
Binding affinity to rat V2 receptor |
Bioorg Med Chem Lett 10: 695-8 (2000)
BindingDB Entry DOI: 10.7270/Q2X34WP8 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50308150
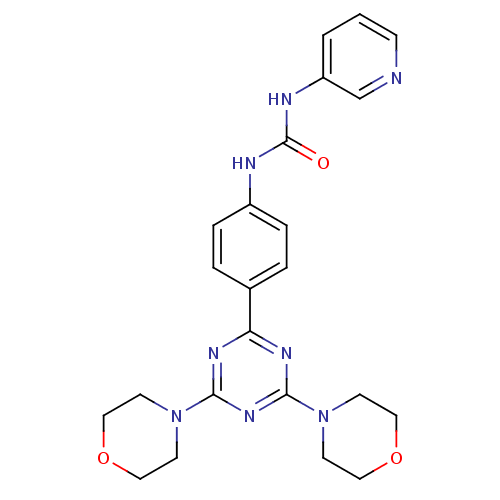
(1-[4-(4,6-Dimorpholin-4-yl-1,3,5-triazin-2-yl)-phe...)Show SMILES O=C(Nc1ccc(cc1)-c1nc(nc(n1)N1CCOCC1)N1CCOCC1)Nc1cccnc1 Show InChI InChI=1S/C23H26N8O3/c32-23(26-19-2-1-7-24-16-19)25-18-5-3-17(4-6-18)20-27-21(30-8-12-33-13-9-30)29-22(28-20)31-10-14-34-15-11-31/h1-7,16H,8-15H2,(H2,25,26,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of mTOR |
J Med Chem 53: 2636-45 (2010)
Article DOI: 10.1021/jm901830p
BindingDB Entry DOI: 10.7270/Q2862HDH |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50308151
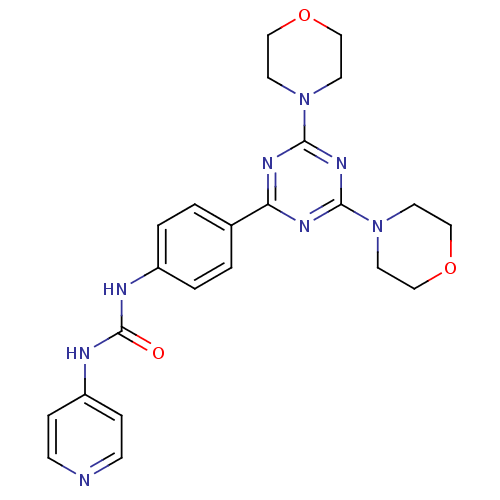
(1-[4-(4,6-Dimorpholin-4-yl-1,3,5-triazin-2-yl)-phe...)Show SMILES O=C(Nc1ccncc1)Nc1ccc(cc1)-c1nc(nc(n1)N1CCOCC1)N1CCOCC1 Show InChI InChI=1S/C23H26N8O3/c32-23(26-19-5-7-24-8-6-19)25-18-3-1-17(2-4-18)20-27-21(30-9-13-33-14-10-30)29-22(28-20)31-11-15-34-16-12-31/h1-8H,9-16H2,(H2,24,25,26,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of mTOR |
J Med Chem 53: 2636-45 (2010)
Article DOI: 10.1021/jm901830p
BindingDB Entry DOI: 10.7270/Q2862HDH |
More data for this
Ligand-Target Pair | |
Vasopressin V2 receptor
(Homo sapiens (Human)) | BDBM50065115

(3-chloro-4-(10,11-dihydro-5H-benzo[e]pyrrolo[1,2-a...)Show SMILES Cc1ccc(F)cc1C(=O)Nc1ccc(C(=O)N2Cc3cccn3Cc3ccccc23)c(Cl)c1 Show InChI InChI=1S/C27H21ClFN3O2/c1-17-8-9-19(29)13-23(17)26(33)30-20-10-11-22(24(28)14-20)27(34)32-16-21-6-4-12-31(21)15-18-5-2-3-7-25(18)32/h2-14H,15-16H2,1H3,(H,30,33) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]-AVP from human V2 receptor expressed in murine fibroblast cell line (LV2) membranes |
J Med Chem 41: 2442-4 (1998)
Article DOI: 10.1021/jm980179c
BindingDB Entry DOI: 10.7270/Q2CC0ZT5 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50308789

(4-({[4-(3-Ethyl-7-morpholin-4-yl-3H-[1,2,3]-triazo...)Show SMILES CCn1nnc2c(nc(nc12)-c1ccc(NC(=O)Nc2ccc(cc2)C(=O)NCCc2ccccn2)cc1)N1CCOCC1 Show InChI InChI=1S/C31H32N10O3/c1-2-41-29-26(38-39-41)28(40-17-19-44-20-18-40)36-27(37-29)21-6-10-24(11-7-21)34-31(43)35-25-12-8-22(9-13-25)30(42)33-16-14-23-5-3-4-15-32-23/h3-13,15H,2,14,16-20H2,1H3,(H,33,42)(H2,34,35,43) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.21 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of mTOR by DELFIA |
J Med Chem 53: 798-810 (2010)
Article DOI: 10.1021/jm9014982
BindingDB Entry DOI: 10.7270/Q2NZ87RZ |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50378662

(CHEMBL1204014)Show SMILES CN1CCN(CC1)C(=O)c1ccc(NC(=O)Nc2ccc(cc2)-c2nc(nc(n2)N2CCOCC2)N2CCOCC2)cc1 Show InChI InChI=1S/C30H37N9O4/c1-36-10-12-37(13-11-36)27(40)23-4-8-25(9-5-23)32-30(41)31-24-6-2-22(3-7-24)26-33-28(38-14-18-42-19-15-38)35-29(34-26)39-16-20-43-21-17-39/h2-9H,10-21H2,1H3,(H2,31,32,41) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of mTOR |
J Med Chem 53: 2636-45 (2010)
Article DOI: 10.1021/jm901830p
BindingDB Entry DOI: 10.7270/Q2862HDH |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50118974

(1-Acetyl-4-(4-methoxy-benzenesulfonyl)-2,3,4,5-tet...)Show SMILES COc1ccc(cc1)S(=O)(=O)N1Cc2ccccc2N(CC1C(=O)NO)C(C)=O Show InChI InChI=1S/C19H21N3O6S/c1-13(23)21-12-18(19(24)20-25)22(11-14-5-3-4-6-17(14)21)29(26,27)16-9-7-15(28-2)8-10-16/h3-10,18,25H,11-12H2,1-2H3,(H,20,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Concentration required in vitro to inhibit Matrix metalloproteinase-9 |
Bioorg Med Chem Lett 12: 2867-70 (2002)
BindingDB Entry DOI: 10.7270/Q2JS9PSH |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50308767
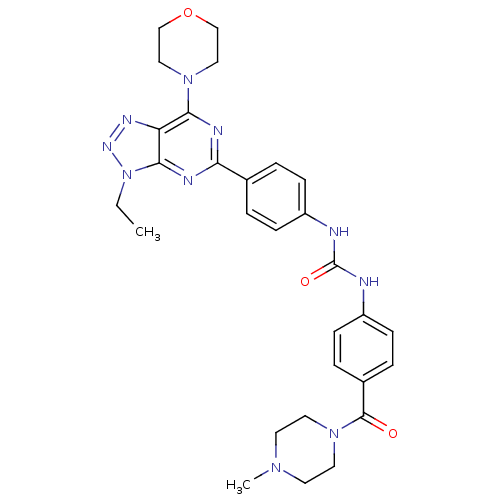
(1-[4-(3-Ethyl-7-morpholin-4-yl-3H-[1,2,3]triazolo-...)Show SMILES CCn1nnc2c(nc(nc12)-c1ccc(NC(=O)Nc2ccc(cc2)C(=O)N2CCN(C)CC2)cc1)N1CCOCC1 Show InChI InChI=1S/C29H34N10O3/c1-3-39-27-24(34-35-39)26(37-16-18-42-19-17-37)32-25(33-27)20-4-8-22(9-5-20)30-29(41)31-23-10-6-21(7-11-23)28(40)38-14-12-36(2)13-15-38/h4-11H,3,12-19H2,1-2H3,(H2,30,31,41) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of human PI3Kalpha by fluorescence polarization format assay |
J Med Chem 53: 798-810 (2010)
Article DOI: 10.1021/jm9014982
BindingDB Entry DOI: 10.7270/Q2NZ87RZ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50308786

(4-({[4-(3-Ethyl-7-morpholin-4-yl-3H-[1,2,3]-triazo...)Show SMILES CCn1nnc2c(nc(nc12)-c1ccc(NC(=O)Nc2ccc(cc2)C(=O)N(C)C)cc1)N1CCOCC1 Show InChI InChI=1S/C26H29N9O3/c1-4-35-24-21(31-32-35)23(34-13-15-38-16-14-34)29-22(30-24)17-5-9-19(10-6-17)27-26(37)28-20-11-7-18(8-12-20)25(36)33(2)3/h5-12H,4,13-16H2,1-3H3,(H2,27,28,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of human PI3Kalpha by fluorescence polarization format assay |
J Med Chem 53: 798-810 (2010)
Article DOI: 10.1021/jm9014982
BindingDB Entry DOI: 10.7270/Q2NZ87RZ |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data