

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Androgen receptor (Homo sapiens (Human)) | BDBM50253371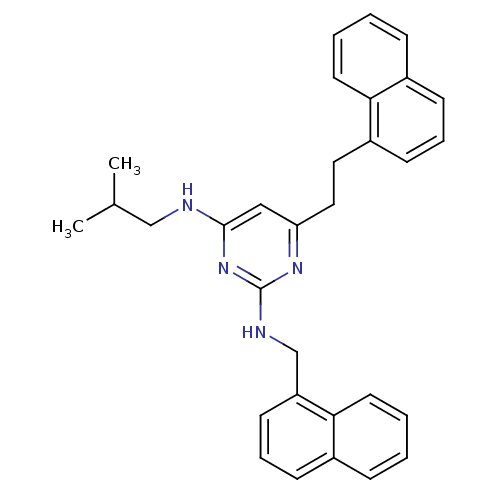 (CHEMBL522172 | N4-Isobutyl-6-(2-naphthalen-1-yl-et...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Tokyo Curated by ChEMBL | Assay Description Inhibition of AR | Bioorg Med Chem Lett 20: 1712-7 (2010) Article DOI: 10.1016/j.bmcl.2010.01.079 BindingDB Entry DOI: 10.7270/Q2TM7B76 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50313149 (6-(2-benzylpyridin-4-yl)-3-hydroxy-5-isobutyl-1-(n...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 4.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Tokyo Curated by ChEMBL | Assay Description Inhibition of ER | Bioorg Med Chem Lett 20: 1712-7 (2010) Article DOI: 10.1016/j.bmcl.2010.01.079 BindingDB Entry DOI: 10.7270/Q2TM7B76 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50313148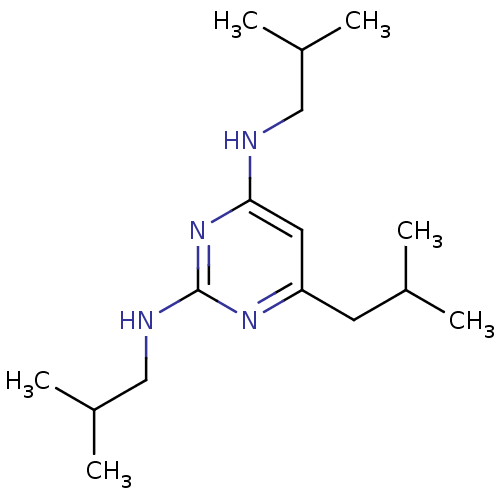 (CHEMBL1087884 | N2,N4,6-triisobutylpyrimidine-2,4-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Tokyo Curated by ChEMBL | Assay Description Inhibition of ER | Bioorg Med Chem Lett 20: 1712-7 (2010) Article DOI: 10.1016/j.bmcl.2010.01.079 BindingDB Entry DOI: 10.7270/Q2TM7B76 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vitamin D3 receptor (Homo sapiens (Human)) | BDBM50313152 ((3R,5S)-5-((R)-2-((1R,3aS,7aR,E)-4-((Z)-2-((3S,5R)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 220 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Tokyo Curated by ChEMBL | Assay Description Antagonist activity at human recombinant VDR LBD expressed in HEK293 cells assessed as inhibition of 3 nM 1,25-(OH)2D3-induced transcriptional activa... | Bioorg Med Chem Lett 20: 1712-7 (2010) Article DOI: 10.1016/j.bmcl.2010.01.079 BindingDB Entry DOI: 10.7270/Q2TM7B76 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| M-phase inducer phosphatase 1 (Homo sapiens (Human)) | BDBM50147586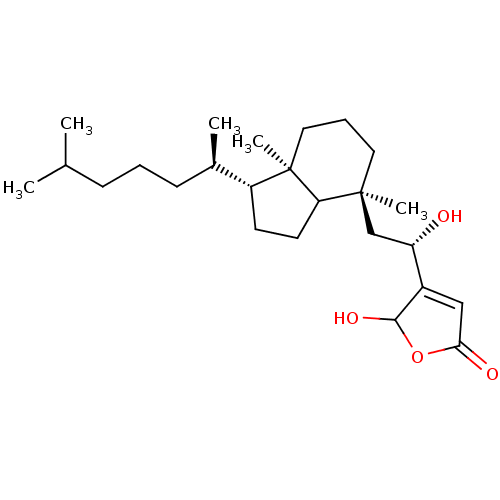 ((S)-4-{2-[(3R,7S)-1-((2R,3R)-1,5-Dimethyl-hexyl)-4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 440 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Tokyo Curated by ChEMBL | Assay Description Concentration required to inhibit human Cell division cycle 25A activity | Bioorg Med Chem Lett 14: 3291-4 (2004) Article DOI: 10.1016/j.bmcl.2004.03.100 BindingDB Entry DOI: 10.7270/Q23T9GNJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| M-phase inducer phosphatase 1 (Homo sapiens (Human)) | BDBM50147585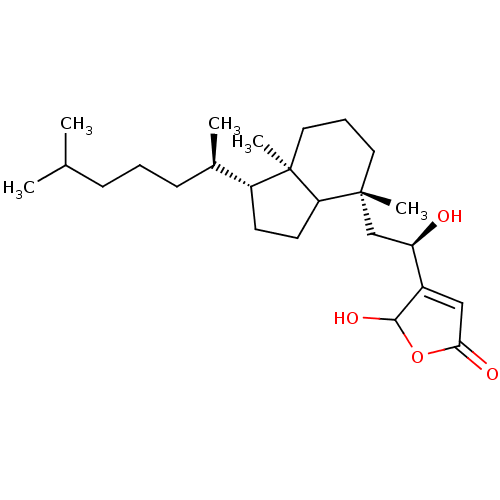 ((R)-4-{2-[(3R,7R)-1-((2R,3R)-1,5-Dimethyl-hexyl)-4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 480 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Tokyo Curated by ChEMBL | Assay Description Concentration required to inhibit human Cell division cycle 25A activity | Bioorg Med Chem Lett 14: 3291-4 (2004) Article DOI: 10.1016/j.bmcl.2004.03.100 BindingDB Entry DOI: 10.7270/Q23T9GNJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| M-phase inducer phosphatase 1 (Homo sapiens (Human)) | BDBM50147582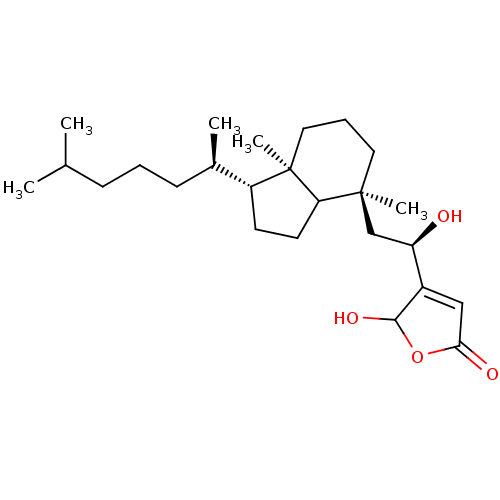 (4-{(R)-2-[(3aR,4S)-1-((1R,2R)-1,5-Dimethyl-hexyl)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 860 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Tokyo Curated by ChEMBL | Assay Description Concentration required to inhibit human Cell division cycle 25A activity | Bioorg Med Chem Lett 14: 3291-4 (2004) Article DOI: 10.1016/j.bmcl.2004.03.100 BindingDB Entry DOI: 10.7270/Q23T9GNJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| M-phase inducer phosphatase 1 (Homo sapiens (Human)) | BDBM50147583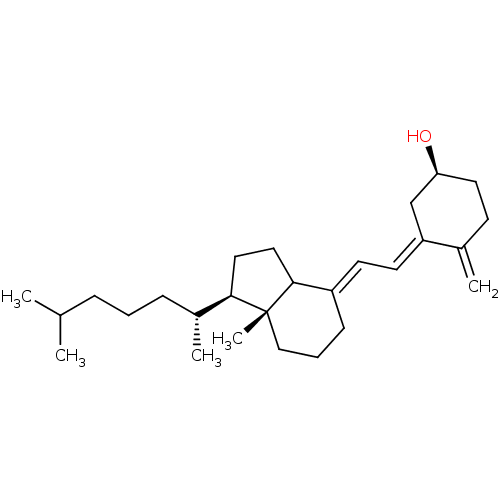 ((S)-4-Methylene-3-{2-[(R)-7a-(R)-methyl-1-((R)-5-m...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 890 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Tokyo Curated by ChEMBL | Assay Description Concentration required to inhibit human Cell division cycle 25A activity; Value ranges from 0.44 uM to 0.89 uM | Bioorg Med Chem Lett 14: 3291-4 (2004) Article DOI: 10.1016/j.bmcl.2004.03.100 BindingDB Entry DOI: 10.7270/Q23T9GNJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| M-phase inducer phosphatase 1 (Homo sapiens (Human)) | BDBM50147587 ((S)-4-{2-[(3R,7R)-1-((2R,3R)-1,5-Dimethyl-hexyl)-4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 890 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Tokyo Curated by ChEMBL | Assay Description Concentration required to inhibit human Cell division cycle 25A activity | Bioorg Med Chem Lett 14: 3291-4 (2004) Article DOI: 10.1016/j.bmcl.2004.03.100 BindingDB Entry DOI: 10.7270/Q23T9GNJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| M-phase inducer phosphatase 2 (Homo sapiens (Human)) | BDBM50147585 ((R)-4-{2-[(3R,7R)-1-((2R,3R)-1,5-Dimethyl-hexyl)-4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.44E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Tokyo Curated by ChEMBL | Assay Description Concentration required to inhibit human Cell division cycle 25B activity | Bioorg Med Chem Lett 14: 3291-4 (2004) Article DOI: 10.1016/j.bmcl.2004.03.100 BindingDB Entry DOI: 10.7270/Q23T9GNJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| M-phase inducer phosphatase 2 (Homo sapiens (Human)) | BDBM50147587 ((S)-4-{2-[(3R,7R)-1-((2R,3R)-1,5-Dimethyl-hexyl)-4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.62E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Tokyo Curated by ChEMBL | Assay Description Concentration required to inhibit human Cell division cycle 25B activity | Bioorg Med Chem Lett 14: 3291-4 (2004) Article DOI: 10.1016/j.bmcl.2004.03.100 BindingDB Entry DOI: 10.7270/Q23T9GNJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| M-phase inducer phosphatase 2 (Homo sapiens (Human)) | BDBM50147582 (4-{(R)-2-[(3aR,4S)-1-((1R,2R)-1,5-Dimethyl-hexyl)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.88E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Tokyo Curated by ChEMBL | Assay Description Concentration required to inhibit human Cell division cycle 25B activity | Bioorg Med Chem Lett 14: 3291-4 (2004) Article DOI: 10.1016/j.bmcl.2004.03.100 BindingDB Entry DOI: 10.7270/Q23T9GNJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| M-phase inducer phosphatase 2 (Homo sapiens (Human)) | BDBM50147586 ((S)-4-{2-[(3R,7S)-1-((2R,3R)-1,5-Dimethyl-hexyl)-4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Tokyo Curated by ChEMBL | Assay Description Concentration required to inhibit human Cell division cycle 25B activity | Bioorg Med Chem Lett 14: 3291-4 (2004) Article DOI: 10.1016/j.bmcl.2004.03.100 BindingDB Entry DOI: 10.7270/Q23T9GNJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM23843 (5,16,25-trimethoxy-14-oxapentacyclo[20.2.2.2^{10,1...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | 37 |
University of Tokyo | Assay Description Human embryonic kidney (HEK) 293 cells were cultured in D-MEM medium. Transfections were performed by the calcium phosphate coprecipitation method. T... | Bioorg Med Chem 16: 4272-85 (2008) Article DOI: 10.1016/j.bmc.2008.02.078 BindingDB Entry DOI: 10.7270/Q28S4N7J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM23844 (16,25-dimethoxy-14-oxapentacyclo[20.2.2.2^{10,13}....) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.20E+3 | n/a | n/a | n/a | n/a | n/a | 37 |
University of Tokyo | Assay Description Human embryonic kidney (HEK) 293 cells were cultured in D-MEM medium. Transfections were performed by the calcium phosphate coprecipitation method. T... | Bioorg Med Chem 16: 4272-85 (2008) Article DOI: 10.1016/j.bmc.2008.02.078 BindingDB Entry DOI: 10.7270/Q28S4N7J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EHMT2 (Homo sapiens (Human)) | BDBM50315537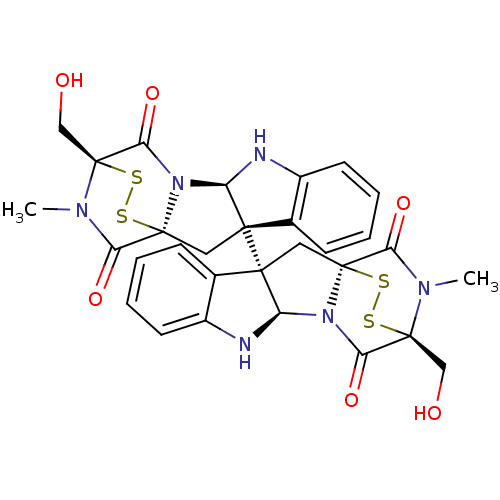 (CHEMBL1089316 | chaetocin) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
RIKEN Advanced Science Institute Curated by ChEMBL | Assay Description Inhibition of protein lysine methyltransferase G9a (unknown origin) by ELISA assay | Bioorg Med Chem Lett 23: 733-6 (2013) Article DOI: 10.1016/j.bmcl.2012.11.087 BindingDB Entry DOI: 10.7270/Q22V2HFN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM23848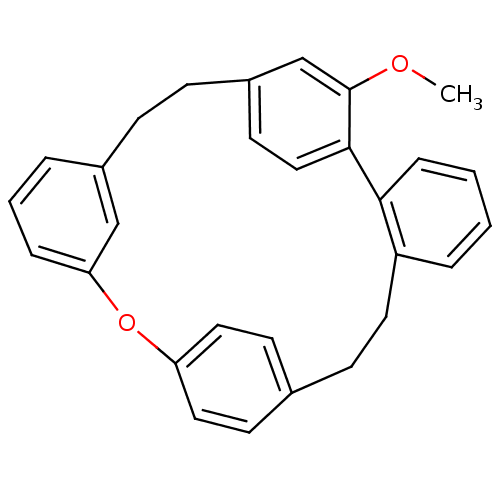 (25-methoxy-14-oxapentacyclo[20.2.2.2^{10,13}.1^{15...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tokyo | Assay Description Human embryonic kidney (HEK) 293 cells were cultured in D-MEM medium. Transfections were performed by the calcium phosphate coprecipitation method. T... | Bioorg Med Chem 16: 4272-85 (2008) Article DOI: 10.1016/j.bmc.2008.02.078 BindingDB Entry DOI: 10.7270/Q28S4N7J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM23842 (2-[2,6-bis(propan-2-yl)phenyl]-3-sulfanylidene-2,3...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.10E+3 | n/a | n/a | n/a | n/a | n/a | 37 |
University of Tokyo | Assay Description Human embryonic kidney (HEK) 293 cells were cultured in D-MEM medium. Transfections were performed by the calcium phosphate coprecipitation method. T... | Bioorg Med Chem 16: 4272-85 (2008) Article DOI: 10.1016/j.bmc.2008.02.078 BindingDB Entry DOI: 10.7270/Q28S4N7J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM23853 (14-oxapentacyclo[20.2.2.2^{10,13}.1^{15,19}.0^{2,7...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tokyo | Assay Description Human embryonic kidney (HEK) 293 cells were cultured in D-MEM medium. Transfections were performed by the calcium phosphate coprecipitation method. T... | Bioorg Med Chem 16: 4272-85 (2008) Article DOI: 10.1016/j.bmc.2008.02.078 BindingDB Entry DOI: 10.7270/Q28S4N7J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EHMT2 (Homo sapiens (Human)) | BDBM50425308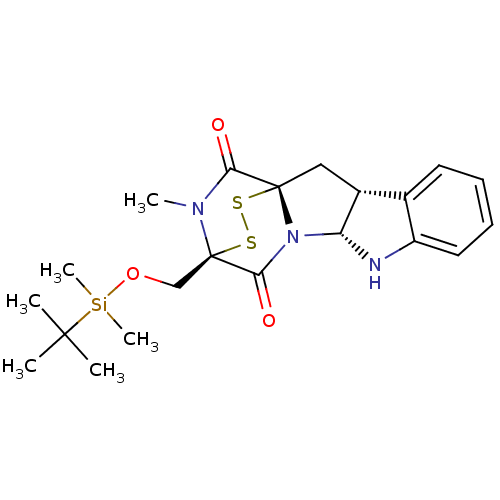 (CHEMBL2311579) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
RIKEN Advanced Science Institute Curated by ChEMBL | Assay Description Inhibition of protein lysine methyltransferase G9a (unknown origin) by modified ELISA assay | Bioorg Med Chem Lett 23: 733-6 (2013) Article DOI: 10.1016/j.bmcl.2012.11.087 BindingDB Entry DOI: 10.7270/Q22V2HFN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vitamin D3 receptor (Homo sapiens (Human)) | BDBM50313152 ((3R,5S)-5-((R)-2-((1R,3aS,7aR,E)-4-((Z)-2-((3S,5R)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Tokyo Curated by ChEMBL | Assay Description Antagonist activity at human recombinant VDR LBD expressed in HEK293 cells assessed as inhibition of 300 nM 1,25-(OH)2D3-induced transcriptional acti... | Bioorg Med Chem Lett 20: 1712-7 (2010) Article DOI: 10.1016/j.bmcl.2010.01.079 BindingDB Entry DOI: 10.7270/Q2TM7B76 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM23845 (5,16-dimethoxy-14-oxapentacyclo[20.2.2.2^{10,13}.1...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.60E+3 | n/a | n/a | n/a | n/a | n/a | 37 |
University of Tokyo | Assay Description Human embryonic kidney (HEK) 293 cells were cultured in D-MEM medium. Transfections were performed by the calcium phosphate coprecipitation method. T... | Bioorg Med Chem 16: 4272-85 (2008) Article DOI: 10.1016/j.bmc.2008.02.078 BindingDB Entry DOI: 10.7270/Q28S4N7J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM23850 (14-oxapentacyclo[20.2.2.2^{10,13}.1^{15,19}.0^{2,7...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tokyo | Assay Description Human embryonic kidney (HEK) 293 cells were cultured in D-MEM medium. Transfections were performed by the calcium phosphate coprecipitation method. T... | Bioorg Med Chem 16: 4272-85 (2008) Article DOI: 10.1016/j.bmc.2008.02.078 BindingDB Entry DOI: 10.7270/Q28S4N7J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-glucosidase MAL32 (Saccharomyces cerevisiae) | BDBM19992 (2-[3-[3-[[2-chloro-3-(trifluoromethyl)phenyl]methy...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 4.80E+3 | n/a | n/a | n/a | n/a | 7.0 | 37 |
University of Tokyo | Assay Description The alpha-glucosidase inhibitory activity of test compounds was determined in a 96-well plate format. The reaction mixture containing enzyme and chro... | Bioorg Med Chem 16: 4272-85 (2008) Article DOI: 10.1016/j.bmc.2008.02.078 BindingDB Entry DOI: 10.7270/Q28S4N7J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-glucosidase MAL32 (Saccharomyces cerevisiae) | BDBM23850 (14-oxapentacyclo[20.2.2.2^{10,13}.1^{15,19}.0^{2,7...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.90E+3 | n/a | n/a | n/a | n/a | 7.0 | 37 |
University of Tokyo | Assay Description The alpha-glucosidase inhibitory activity of test compounds was determined in a 96-well plate format. The reaction mixture containing enzyme and chro... | Bioorg Med Chem 16: 4272-85 (2008) Article DOI: 10.1016/j.bmc.2008.02.078 BindingDB Entry DOI: 10.7270/Q28S4N7J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM23839 (14-oxapentacyclo[20.2.2.2^{10,13}.1^{15,19}.0^{2,7...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.90E+3 | n/a | n/a | n/a | n/a | n/a | 37 |
University of Tokyo | Assay Description Human embryonic kidney (HEK) 293 cells were cultured in D-MEM medium. Transfections were performed by the calcium phosphate coprecipitation method. T... | Bioorg Med Chem 16: 4272-85 (2008) Article DOI: 10.1016/j.bmc.2008.02.078 BindingDB Entry DOI: 10.7270/Q28S4N7J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-glucosidase MAL32 (Saccharomyces cerevisiae) | BDBM23851 (14-oxapentacyclo[20.2.2.2^{10,13}.1^{15,19}.0^{2,7...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.10E+3 | n/a | n/a | n/a | n/a | 7.0 | 37 |
University of Tokyo | Assay Description The alpha-glucosidase inhibitory activity of test compounds was determined in a 96-well plate format. The reaction mixture containing enzyme and chro... | Bioorg Med Chem 16: 4272-85 (2008) Article DOI: 10.1016/j.bmc.2008.02.078 BindingDB Entry DOI: 10.7270/Q28S4N7J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EHMT2 (Homo sapiens (Human)) | BDBM50425305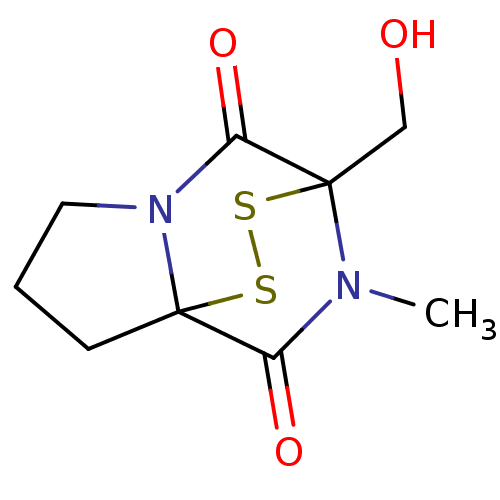 (CHEMBL2315521) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
RIKEN Advanced Science Institute Curated by ChEMBL | Assay Description Inhibition of protein lysine methyltransferase G9a (unknown origin) by modified ELISA assay | Bioorg Med Chem Lett 23: 733-6 (2013) Article DOI: 10.1016/j.bmcl.2012.11.087 BindingDB Entry DOI: 10.7270/Q22V2HFN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EHMT2 (Homo sapiens (Human)) | BDBM50425304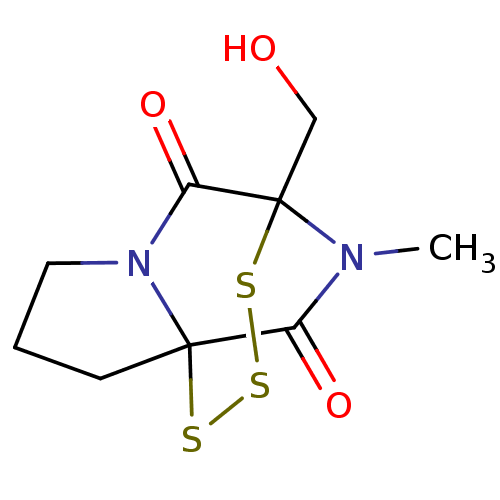 (CHEMBL2315522) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
RIKEN Advanced Science Institute Curated by ChEMBL | Assay Description Inhibition of protein lysine methyltransferase G9a (unknown origin) by modified ELISA assay | Bioorg Med Chem Lett 23: 733-6 (2013) Article DOI: 10.1016/j.bmcl.2012.11.087 BindingDB Entry DOI: 10.7270/Q22V2HFN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| M-phase inducer phosphatase 1 (Homo sapiens (Human)) | BDBM50087141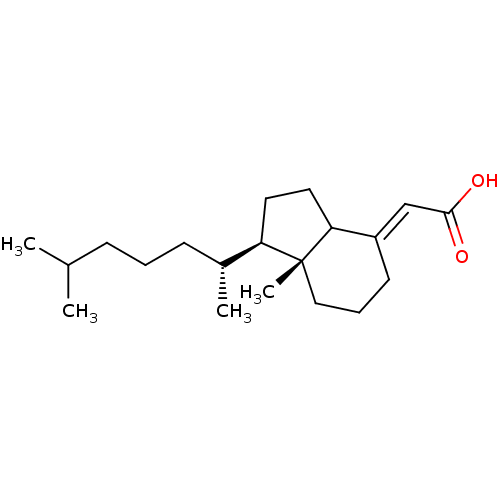 ((E)-2-((1R,7aR)-7a-methyl-1-((R)-6-methylheptan-2-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Tokyo Curated by ChEMBL | Assay Description Concentration required to inhibit human Cell division cycle 25A activity | Bioorg Med Chem Lett 14: 3291-4 (2004) Article DOI: 10.1016/j.bmcl.2004.03.100 BindingDB Entry DOI: 10.7270/Q23T9GNJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM23852 (14-oxapentacyclo[20.2.2.2^{10,13}.1^{15,19}.0^{2,7...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tokyo | Assay Description Human embryonic kidney (HEK) 293 cells were cultured in D-MEM medium. Transfections were performed by the calcium phosphate coprecipitation method. T... | Bioorg Med Chem 16: 4272-85 (2008) Article DOI: 10.1016/j.bmc.2008.02.078 BindingDB Entry DOI: 10.7270/Q28S4N7J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EHMT2 (Homo sapiens (Human)) | BDBM50396029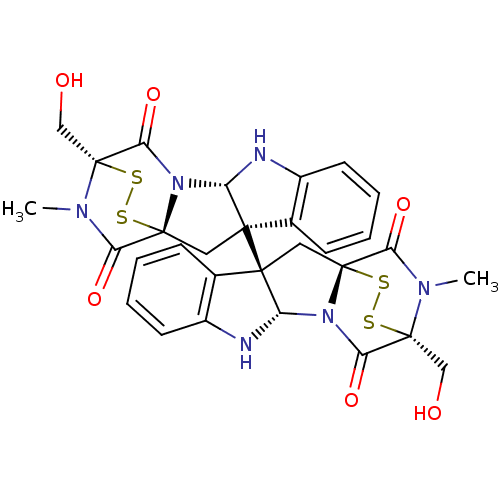 (CHEMBL1222849) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
RIKEN Advanced Science Institute Curated by ChEMBL | Assay Description Inhibition of protein lysine methyltransferase G9a (unknown origin) by modified ELISA assay | Bioorg Med Chem Lett 23: 733-6 (2013) Article DOI: 10.1016/j.bmcl.2012.11.087 BindingDB Entry DOI: 10.7270/Q22V2HFN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM23839 (14-oxapentacyclo[20.2.2.2^{10,13}.1^{15,19}.0^{2,7...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tokyo | Assay Description Human embryonic kidney (HEK) 293 cells were cultured in D-MEM medium. Transfections were performed by the calcium phosphate coprecipitation method. T... | Bioorg Med Chem 16: 4272-85 (2008) Article DOI: 10.1016/j.bmc.2008.02.078 BindingDB Entry DOI: 10.7270/Q28S4N7J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM23849 (14-oxapentacyclo[20.2.2.2^{10,13}.1^{15,19}.0^{2,7...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tokyo | Assay Description Human embryonic kidney (HEK) 293 cells were cultured in D-MEM medium. Transfections were performed by the calcium phosphate coprecipitation method. T... | Bioorg Med Chem 16: 4272-85 (2008) Article DOI: 10.1016/j.bmc.2008.02.078 BindingDB Entry DOI: 10.7270/Q28S4N7J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM23854 (14-oxapentacyclo[20.2.2.2^{10,13}.1^{15,19}.0^{2,7...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tokyo | Assay Description Human embryonic kidney (HEK) 293 cells were cultured in D-MEM medium. Transfections were performed by the calcium phosphate coprecipitation method. T... | Bioorg Med Chem 16: 4272-85 (2008) Article DOI: 10.1016/j.bmc.2008.02.078 BindingDB Entry DOI: 10.7270/Q28S4N7J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EHMT2 (Homo sapiens (Human)) | BDBM50315537 (CHEMBL1089316 | chaetocin) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
RIKEN Advanced Science Institute Curated by ChEMBL | Assay Description Inhibition of protein lysine methyltransferase G9a (unknown origin) by modified ELISA assay | Bioorg Med Chem Lett 23: 733-6 (2013) Article DOI: 10.1016/j.bmcl.2012.11.087 BindingDB Entry DOI: 10.7270/Q22V2HFN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EHMT2 (Homo sapiens (Human)) | BDBM50425311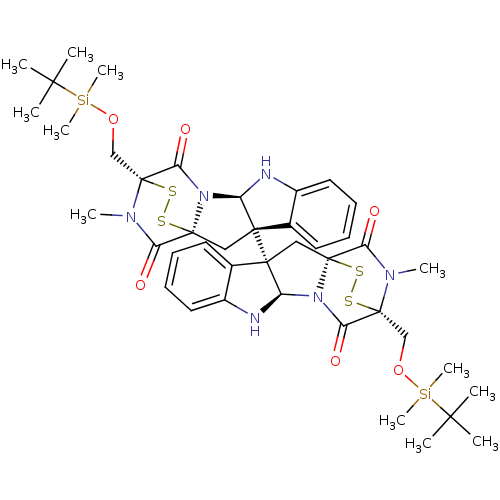 (CHEMBL2315526) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
RIKEN Advanced Science Institute Curated by ChEMBL | Assay Description Inhibition of protein lysine methyltransferase G9a (unknown origin) by modified ELISA assay | Bioorg Med Chem Lett 23: 733-6 (2013) Article DOI: 10.1016/j.bmcl.2012.11.087 BindingDB Entry DOI: 10.7270/Q22V2HFN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM23853 (14-oxapentacyclo[20.2.2.2^{10,13}.1^{15,19}.0^{2,7...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tokyo | Assay Description Human embryonic kidney (HEK) 293 cells were cultured in D-MEM medium. Transfections were performed by the calcium phosphate coprecipitation method. T... | Bioorg Med Chem 16: 4272-85 (2008) Article DOI: 10.1016/j.bmc.2008.02.078 BindingDB Entry DOI: 10.7270/Q28S4N7J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM23847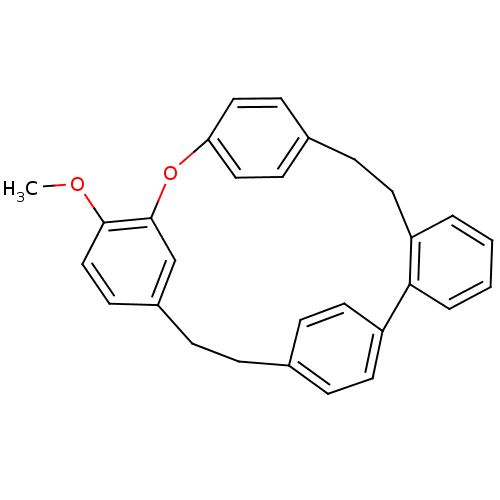 (16-methoxy-14-oxapentacyclo[20.2.2.2^{10,13}.1^{15...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tokyo | Assay Description Human embryonic kidney (HEK) 293 cells were cultured in D-MEM medium. Transfections were performed by the calcium phosphate coprecipitation method. T... | Bioorg Med Chem 16: 4272-85 (2008) Article DOI: 10.1016/j.bmc.2008.02.078 BindingDB Entry DOI: 10.7270/Q28S4N7J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM23850 (14-oxapentacyclo[20.2.2.2^{10,13}.1^{15,19}.0^{2,7...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tokyo | Assay Description Human embryonic kidney (HEK) 293 cells were cultured in D-MEM medium. Transfections were performed by the calcium phosphate coprecipitation method. T... | Bioorg Med Chem 16: 4272-85 (2008) Article DOI: 10.1016/j.bmc.2008.02.078 BindingDB Entry DOI: 10.7270/Q28S4N7J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| M-phase inducer phosphatase 1 (Homo sapiens (Human)) | BDBM50087141 ((E)-2-((1R,7aR)-7a-methyl-1-((R)-6-methylheptan-2-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 7.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Tokyo Curated by ChEMBL | Assay Description Concentration required for the inhibitory activity against human Cell division cycle 25A | Bioorg Med Chem Lett 10: 615-7 (2000) BindingDB Entry DOI: 10.7270/Q27H1HT9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| M-phase inducer phosphatase 2 (Homo sapiens (Human)) | BDBM50087141 ((E)-2-((1R,7aR)-7a-methyl-1-((R)-6-methylheptan-2-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Tokyo Curated by ChEMBL | Assay Description Concentration required to inhibit human Cell division cycle 25B activity | Bioorg Med Chem Lett 14: 3291-4 (2004) Article DOI: 10.1016/j.bmcl.2004.03.100 BindingDB Entry DOI: 10.7270/Q23T9GNJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-glucosidase MAL32 (Saccharomyces cerevisiae) | BDBM23842 (2-[2,6-bis(propan-2-yl)phenyl]-3-sulfanylidene-2,3...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8.00E+3 | n/a | n/a | n/a | n/a | 7.0 | 37 |
University of Tokyo | Assay Description The alpha-glucosidase inhibitory activity of test compounds was determined in a 96-well plate format. The reaction mixture containing enzyme and chro... | Bioorg Med Chem 16: 4272-85 (2008) Article DOI: 10.1016/j.bmc.2008.02.078 BindingDB Entry DOI: 10.7270/Q28S4N7J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM23851 (14-oxapentacyclo[20.2.2.2^{10,13}.1^{15,19}.0^{2,7...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tokyo | Assay Description Human embryonic kidney (HEK) 293 cells were cultured in D-MEM medium. Transfections were performed by the calcium phosphate coprecipitation method. T... | Bioorg Med Chem 16: 4272-85 (2008) Article DOI: 10.1016/j.bmc.2008.02.078 BindingDB Entry DOI: 10.7270/Q28S4N7J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-glucosidase MAL32 (Saccharomyces cerevisiae) | BDBM23849 (14-oxapentacyclo[20.2.2.2^{10,13}.1^{15,19}.0^{2,7...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.60E+3 | n/a | n/a | n/a | n/a | 7.0 | 37 |
University of Tokyo | Assay Description The alpha-glucosidase inhibitory activity of test compounds was determined in a 96-well plate format. The reaction mixture containing enzyme and chro... | Bioorg Med Chem 16: 4272-85 (2008) Article DOI: 10.1016/j.bmc.2008.02.078 BindingDB Entry DOI: 10.7270/Q28S4N7J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| M-phase inducer phosphatase 1 (Homo sapiens (Human)) | BDBM50087143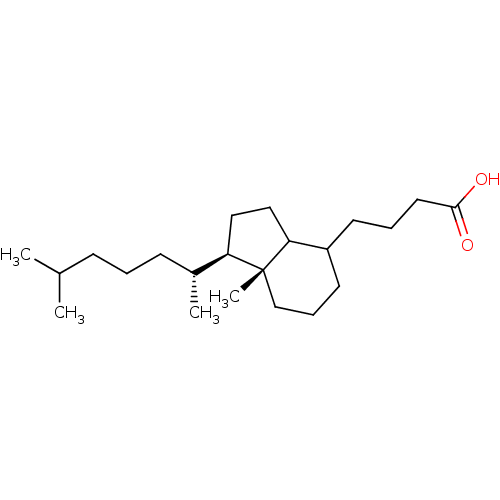 (4-[(1R,7aR)-1-((R)-1,5-Dimethyl-hexyl)-7a-methyl-o...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 9.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Tokyo Curated by ChEMBL | Assay Description Concentration required for the inhibitory activity against human Cell division cycle 25A | Bioorg Med Chem Lett 10: 615-7 (2000) BindingDB Entry DOI: 10.7270/Q27H1HT9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| M-phase inducer phosphatase 1 (Homo sapiens (Human)) | BDBM50341997 (CHEMBL1765353 | Dysidiolide) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Tokyo Curated by ChEMBL | Assay Description Concentration required to inhibit human Cell division cycle 25A activity | Bioorg Med Chem Lett 14: 3291-4 (2004) Article DOI: 10.1016/j.bmcl.2004.03.100 BindingDB Entry DOI: 10.7270/Q23T9GNJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM23840 (2-[2-(2-phenylethyl)phenyl]-2,3-dihydro-1H-isoindo...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.80E+3 | n/a | n/a | n/a | n/a | n/a | 37 |
University of Tokyo | Assay Description Human embryonic kidney (HEK) 293 cells were cultured in D-MEM medium. Transfections were performed by the calcium phosphate coprecipitation method. T... | Bioorg Med Chem 16: 4272-85 (2008) Article DOI: 10.1016/j.bmc.2008.02.078 BindingDB Entry DOI: 10.7270/Q28S4N7J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-glucosidase MAL32 (Saccharomyces cerevisiae) | BDBM23839 (14-oxapentacyclo[20.2.2.2^{10,13}.1^{15,19}.0^{2,7...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.90E+3 | n/a | n/a | n/a | n/a | 7.0 | 37 |
University of Tokyo | Assay Description The alpha-glucosidase inhibitory activity of test compounds was determined in a 96-well plate format. The reaction mixture containing enzyme and chro... | Bioorg Med Chem 16: 4272-85 (2008) Article DOI: 10.1016/j.bmc.2008.02.078 BindingDB Entry DOI: 10.7270/Q28S4N7J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM23851 (14-oxapentacyclo[20.2.2.2^{10,13}.1^{15,19}.0^{2,7...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tokyo | Assay Description Human embryonic kidney (HEK) 293 cells were cultured in D-MEM medium. Transfections were performed by the calcium phosphate coprecipitation method. T... | Bioorg Med Chem 16: 4272-85 (2008) Article DOI: 10.1016/j.bmc.2008.02.078 BindingDB Entry DOI: 10.7270/Q28S4N7J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 192 total ) | Next | Last >> |