 Found 79 hits with Last Name = 'elowe' and Initial = 'nh'
Found 79 hits with Last Name = 'elowe' and Initial = 'nh' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Cystathionine beta-lyase MetC
(Escherichia coli K-12) | BDBM50370860

(CHEMBL219268)Show InChI InChI=1S/C10H10F3N3O2/c11-10(12,13)7-4-2-1-3-6(7)9(18)15-5-8(17)16-14/h1-4H,5,14H2,(H,15,18)(H,16,17) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 79 | n/a | n/a | n/a | n/a | n/a | n/a |
McMaster University
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli CBL |
J Med Chem 50: 755-64 (2007)
Article DOI: 10.1021/jm061132r
BindingDB Entry DOI: 10.7270/Q2KS6SCJ |
More data for this
Ligand-Target Pair | |
Cystathionine beta-lyase MetC
(Escherichia coli K-12) | BDBM50370870

(CHEMBL218090)Show InChI InChI=1S/C11H9F6NO/c1-6-4-2-3-5-7(6)18-9(19)8(10(12,13)14)11(15,16)17/h2-5,8H,1H3,(H,18,19) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 280 | n/a | n/a | n/a | n/a | n/a | n/a |
McMaster University
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli CBL |
J Med Chem 50: 755-64 (2007)
Article DOI: 10.1021/jm061132r
BindingDB Entry DOI: 10.7270/Q2KS6SCJ |
More data for this
Ligand-Target Pair | |
Cystathionine beta-lyase MetC
(Escherichia coli K-12) | BDBM50370869
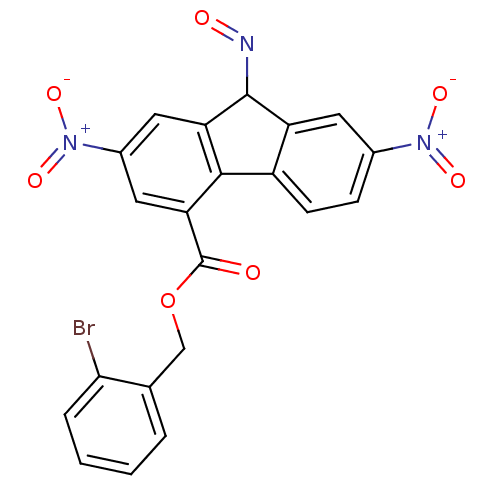
(CHEMBL220764)Show SMILES [O-][N+](=O)c1ccc2c(c1)[c-](N=[OH+])c1cc(cc(C(=O)OCc3ccccc3Br)c21)[N+]([O-])=O Show InChI InChI=1S/C21H11BrN3O7/c22-18-4-2-1-3-11(18)10-32-21(26)17-9-13(25(30)31)8-16-19(17)14-6-5-12(24(28)29)7-15(14)20(16)23-27/h1-9H,10H2/q-1/p+1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 530 | n/a | n/a | n/a | n/a | n/a | n/a |
McMaster University
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli CBL |
J Med Chem 50: 755-64 (2007)
Article DOI: 10.1021/jm061132r
BindingDB Entry DOI: 10.7270/Q2KS6SCJ |
More data for this
Ligand-Target Pair | |
Cystathionine beta-lyase MetC
(Escherichia coli K-12) | BDBM50370861
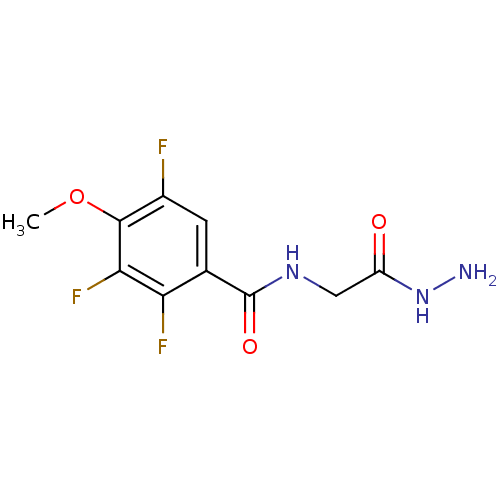
(CHEMBL221848)Show InChI InChI=1S/C10H10F3N3O3/c1-19-9-5(11)2-4(7(12)8(9)13)10(18)15-3-6(17)16-14/h2H,3,14H2,1H3,(H,15,18)(H,16,17) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 650 | n/a | n/a | n/a | n/a | n/a | n/a |
McMaster University
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli CBL |
J Med Chem 50: 755-64 (2007)
Article DOI: 10.1021/jm061132r
BindingDB Entry DOI: 10.7270/Q2KS6SCJ |
More data for this
Ligand-Target Pair | |
Cystathionine beta-lyase MetC
(Escherichia coli K-12) | BDBM50370865

(CHEMBL221706)Show InChI InChI=1S/C6H6Cl2N2O2S/c7-5-2-1-4(3-6(5)8)13(11,12)10-9/h1-3,10H,9H2 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
McMaster University
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli CBL |
J Med Chem 50: 755-64 (2007)
Article DOI: 10.1021/jm061132r
BindingDB Entry DOI: 10.7270/Q2KS6SCJ |
More data for this
Ligand-Target Pair | |
Cystathionine beta-lyase MetC
(Escherichia coli K-12) | BDBM50370853
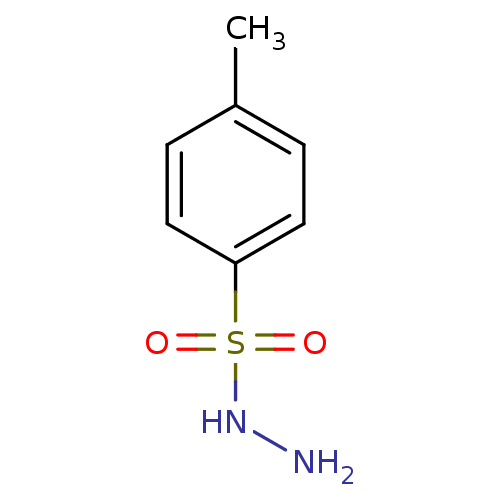
(CHEMBL221182)Show InChI InChI=1S/C7H10N2O2S/c1-6-2-4-7(5-3-6)12(10,11)9-8/h2-5,9H,8H2,1H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
McMaster University
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli CBL |
J Med Chem 50: 755-64 (2007)
Article DOI: 10.1021/jm061132r
BindingDB Entry DOI: 10.7270/Q2KS6SCJ |
More data for this
Ligand-Target Pair | |
Cystathionine beta-lyase MetC
(Escherichia coli K-12) | BDBM50370850
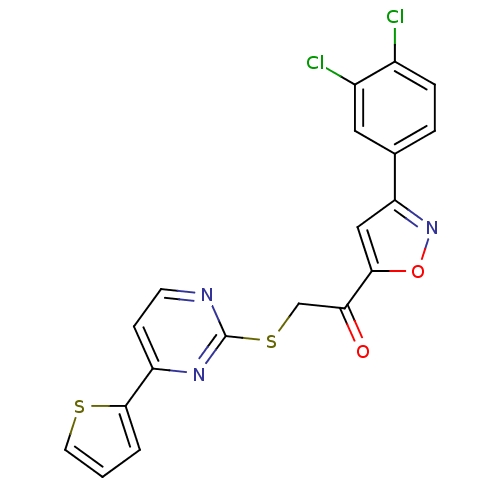
(CHEMBL373750)Show SMILES Clc1ccc(cc1Cl)-c1cc(on1)C(=O)CSc1nccc(n1)-c1cccs1 Show InChI InChI=1S/C19H11Cl2N3O2S2/c20-12-4-3-11(8-13(12)21)15-9-17(26-24-15)16(25)10-28-19-22-6-5-14(23-19)18-2-1-7-27-18/h1-9H,10H2 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
McMaster University
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli CBL |
J Med Chem 50: 755-64 (2007)
Article DOI: 10.1021/jm061132r
BindingDB Entry DOI: 10.7270/Q2KS6SCJ |
More data for this
Ligand-Target Pair | |
Cystathionine beta-lyase MetC
(Escherichia coli K-12) | BDBM50370867
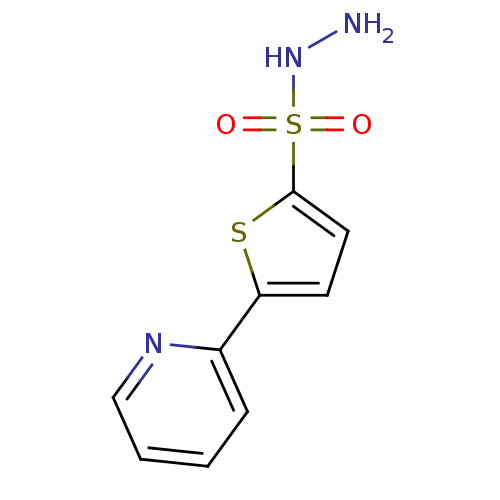
(CHEMBL221705)Show InChI InChI=1S/C9H9N3O2S2/c10-12-16(13,14)9-5-4-8(15-9)7-3-1-2-6-11-7/h1-6,12H,10H2 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
McMaster University
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli CBL |
J Med Chem 50: 755-64 (2007)
Article DOI: 10.1021/jm061132r
BindingDB Entry DOI: 10.7270/Q2KS6SCJ |
More data for this
Ligand-Target Pair | |
Cystathionine beta-lyase MetC
(Escherichia coli K-12) | BDBM50370855

(CHEMBL218737)Show InChI InChI=1S/C9H10N4O4/c10-12-8(14)5-11-9(15)6-3-1-2-4-7(6)13(16)17/h1-4H,5,10H2,(H,11,15)(H,12,14) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
McMaster University
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli CBL |
J Med Chem 50: 755-64 (2007)
Article DOI: 10.1021/jm061132r
BindingDB Entry DOI: 10.7270/Q2KS6SCJ |
More data for this
Ligand-Target Pair | |
Cystathionine beta-lyase MetC
(Escherichia coli K-12) | BDBM50370857
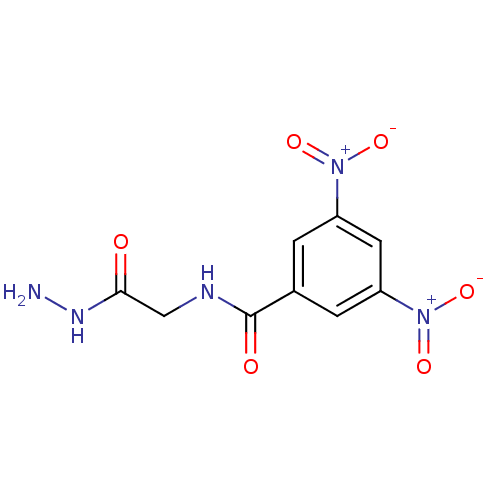
(CHEMBL220531)Show SMILES NNC(=O)CNC(=O)c1cc(cc(c1)[N+]([O-])=O)[N+]([O-])=O Show InChI InChI=1S/C9H9N5O6/c10-12-8(15)4-11-9(16)5-1-6(13(17)18)3-7(2-5)14(19)20/h1-3H,4,10H2,(H,11,16)(H,12,15) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
McMaster University
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli CBL |
J Med Chem 50: 755-64 (2007)
Article DOI: 10.1021/jm061132r
BindingDB Entry DOI: 10.7270/Q2KS6SCJ |
More data for this
Ligand-Target Pair | |
Cystathionine beta-lyase MetC
(Escherichia coli K-12) | BDBM50370854

(CHEMBL220494)Show InChI InChI=1S/C10H11N3O2S/c11-9-5-1-4-8-7(9)3-2-6-10(8)16(14,15)13-12/h1-6,13H,11-12H2 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
McMaster University
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli CBL |
J Med Chem 50: 755-64 (2007)
Article DOI: 10.1021/jm061132r
BindingDB Entry DOI: 10.7270/Q2KS6SCJ |
More data for this
Ligand-Target Pair | |
Cystathionine beta-lyase MetC
(Escherichia coli K-12) | BDBM50370864

(CHEMBL451558)Show InChI InChI=1S/C4H5ClN2O2S2/c5-3-1-2-4(10-3)11(8,9)7-6/h1-2,7H,6H2 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
McMaster University
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli CBL |
J Med Chem 50: 755-64 (2007)
Article DOI: 10.1021/jm061132r
BindingDB Entry DOI: 10.7270/Q2KS6SCJ |
More data for this
Ligand-Target Pair | |
Cystathionine beta-lyase MetC
(Escherichia coli K-12) | BDBM50370856
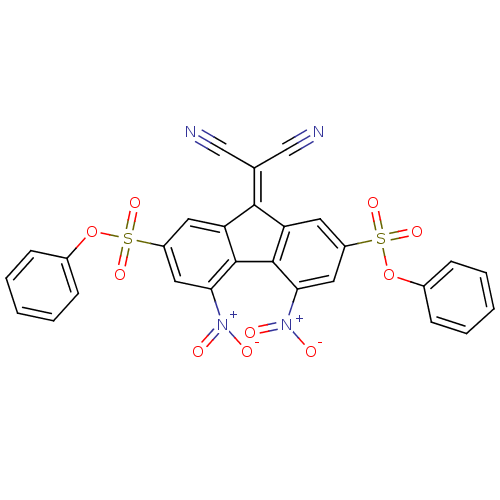
(CHEMBL376005)Show SMILES [#8-]-[#7+](=O)-c1cc(cc2\[#6](=[#6](/C#N)C#N)-c3cc(cc(c3-c12)-[#7+](-[#8-])=O)S(=O)(=O)[#8]-c1ccccc1)S(=O)(=O)[#8]-c1ccccc1 Show InChI InChI=1S/C28H14N4O10S2/c29-15-17(16-30)26-22-11-20(43(37,38)41-18-7-3-1-4-8-18)13-24(31(33)34)27(22)28-23(26)12-21(14-25(28)32(35)36)44(39,40)42-19-9-5-2-6-10-19/h1-14H | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 9.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
McMaster University
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli CBL |
J Med Chem 50: 755-64 (2007)
Article DOI: 10.1021/jm061132r
BindingDB Entry DOI: 10.7270/Q2KS6SCJ |
More data for this
Ligand-Target Pair | |
Cystathionine beta-lyase MetC
(Escherichia coli K-12) | BDBM50370866

(CHEMBL219582)Show InChI InChI=1S/C10H10F3N3O2/c11-10(12,13)7-3-1-6(2-4-7)9(18)15-5-8(17)16-14/h1-4H,5,14H2,(H,15,18)(H,16,17) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
McMaster University
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli CBL |
J Med Chem 50: 755-64 (2007)
Article DOI: 10.1021/jm061132r
BindingDB Entry DOI: 10.7270/Q2KS6SCJ |
More data for this
Ligand-Target Pair | |
Cystathionine beta-lyase MetC
(Escherichia coli K-12) | BDBM50370848
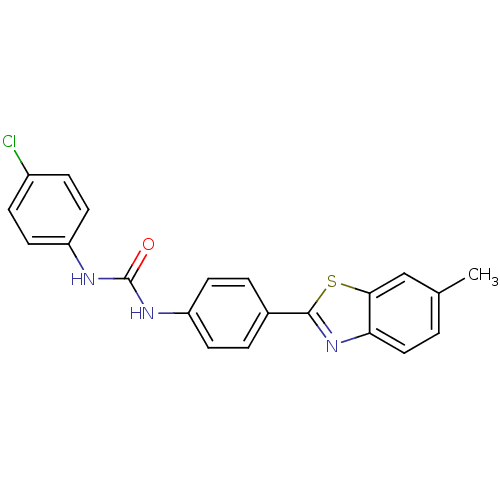
(CHEMBL218058)Show SMILES Cc1ccc2nc(sc2c1)-c1ccc(NC(=O)Nc2ccc(Cl)cc2)cc1 Show InChI InChI=1S/C21H16ClN3OS/c1-13-2-11-18-19(12-13)27-20(25-18)14-3-7-16(8-4-14)23-21(26)24-17-9-5-15(22)6-10-17/h2-12H,1H3,(H2,23,24,26) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
McMaster University
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli CBL |
J Med Chem 50: 755-64 (2007)
Article DOI: 10.1021/jm061132r
BindingDB Entry DOI: 10.7270/Q2KS6SCJ |
More data for this
Ligand-Target Pair | |
Cystathionine beta-lyase MetC
(Escherichia coli K-12) | BDBM50370858
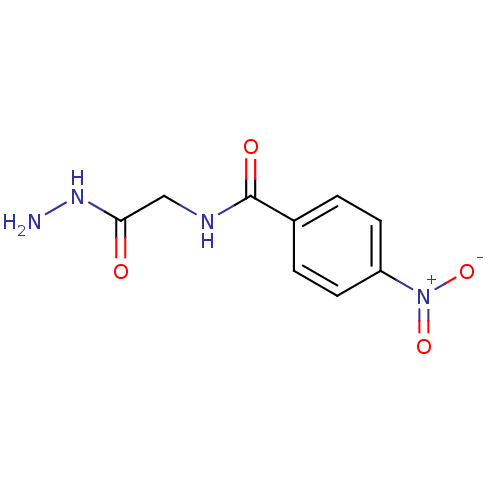
(CHEMBL220530)Show InChI InChI=1S/C9H10N4O4/c10-12-8(14)5-11-9(15)6-1-3-7(4-2-6)13(16)17/h1-4H,5,10H2,(H,11,15)(H,12,14) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
McMaster University
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli CBL |
J Med Chem 50: 755-64 (2007)
Article DOI: 10.1021/jm061132r
BindingDB Entry DOI: 10.7270/Q2KS6SCJ |
More data for this
Ligand-Target Pair | |
Cystathionine beta-lyase MetC
(Escherichia coli K-12) | BDBM50370859
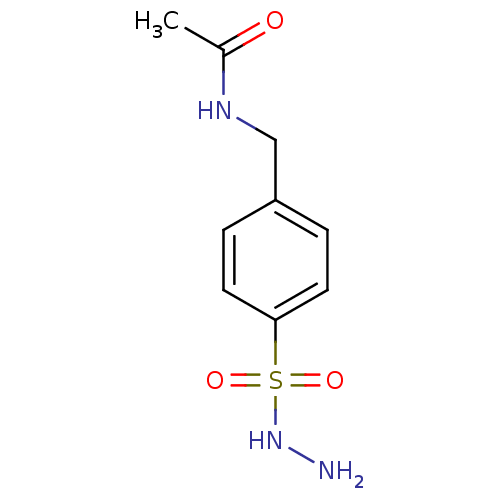
(CHEMBL383877)Show InChI InChI=1S/C9H13N3O3S/c1-7(13)11-6-8-2-4-9(5-3-8)16(14,15)12-10/h2-5,12H,6,10H2,1H3,(H,11,13) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
McMaster University
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli CBL |
J Med Chem 50: 755-64 (2007)
Article DOI: 10.1021/jm061132r
BindingDB Entry DOI: 10.7270/Q2KS6SCJ |
More data for this
Ligand-Target Pair | |
Cystathionine beta-lyase MetC
(Escherichia coli K-12) | BDBM50370852
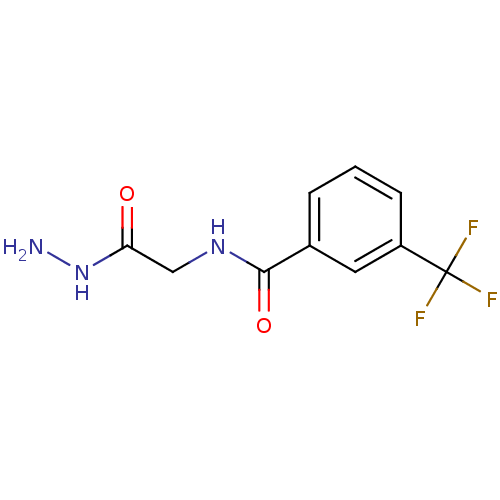
(CHEMBL219322)Show InChI InChI=1S/C10H10F3N3O2/c11-10(12,13)7-3-1-2-6(4-7)9(18)15-5-8(17)16-14/h1-4H,5,14H2,(H,15,18)(H,16,17) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
McMaster University
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli CBL |
J Med Chem 50: 755-64 (2007)
Article DOI: 10.1021/jm061132r
BindingDB Entry DOI: 10.7270/Q2KS6SCJ |
More data for this
Ligand-Target Pair | |
Cystathionine beta-lyase MetC
(Escherichia coli K-12) | BDBM50370851

(CHEMBL375564)Show InChI InChI=1S/C11H15N3O4/c1-17-7-4-3-5-8(18-2)10(7)11(16)13-6-9(15)14-12/h3-5H,6,12H2,1-2H3,(H,13,16)(H,14,15) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
McMaster University
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli CBL |
J Med Chem 50: 755-64 (2007)
Article DOI: 10.1021/jm061132r
BindingDB Entry DOI: 10.7270/Q2KS6SCJ |
More data for this
Ligand-Target Pair | |
Cystathionine beta-lyase MetC
(Escherichia coli K-12) | BDBM50370863
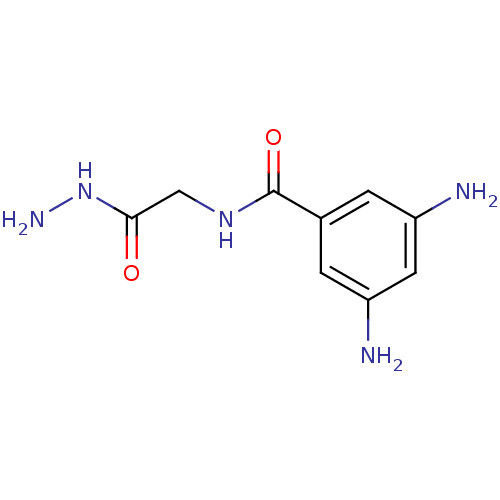
(CHEMBL222116)Show InChI InChI=1S/C9H13N5O2/c10-6-1-5(2-7(11)3-6)9(16)13-4-8(15)14-12/h1-3H,4,10-12H2,(H,13,16)(H,14,15) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
McMaster University
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli CBL |
J Med Chem 50: 755-64 (2007)
Article DOI: 10.1021/jm061132r
BindingDB Entry DOI: 10.7270/Q2KS6SCJ |
More data for this
Ligand-Target Pair | |
Cystathionine beta-lyase MetC
(Escherichia coli K-12) | BDBM50370868

(CHEMBL218485)Show SMILES Cc1ccc2nc(sc2c1)-c1ccc(NC(=O)c2ccc(Cl)cc2)cc1 Show InChI InChI=1S/C21H15ClN2OS/c1-13-2-11-18-19(12-13)26-21(24-18)15-5-9-17(10-6-15)23-20(25)14-3-7-16(22)8-4-14/h2-12H,1H3,(H,23,25) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
McMaster University
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli CBL |
J Med Chem 50: 755-64 (2007)
Article DOI: 10.1021/jm061132r
BindingDB Entry DOI: 10.7270/Q2KS6SCJ |
More data for this
Ligand-Target Pair | |
Cystathionine beta-lyase MetC
(Escherichia coli K-12) | BDBM50370849

(CHEMBL385118)Show InChI InChI=1S/C14H15N3O2/c15-17-14(19)9-16-13(18)8-11-6-3-5-10-4-1-2-7-12(10)11/h1-7H,8-9,15H2,(H,16,18)(H,17,19) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
McMaster University
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli CBL |
J Med Chem 50: 755-64 (2007)
Article DOI: 10.1021/jm061132r
BindingDB Entry DOI: 10.7270/Q2KS6SCJ |
More data for this
Ligand-Target Pair | |
Cystathionine beta-lyase MetC
(Escherichia coli K-12) | BDBM50370862
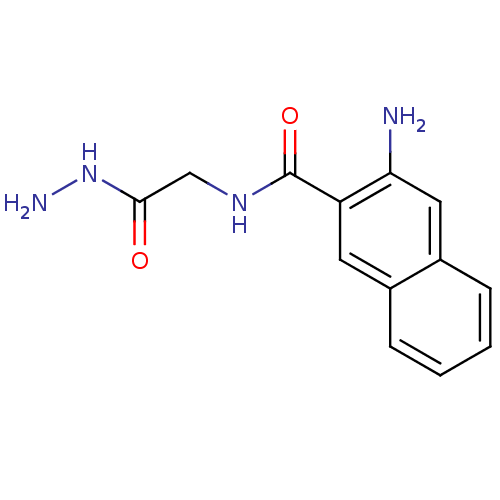
(CHEMBL221962)Show InChI InChI=1S/C13H14N4O2/c14-11-6-9-4-2-1-3-8(9)5-10(11)13(19)16-7-12(18)17-15/h1-6H,7,14-15H2,(H,16,19)(H,17,18) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.60E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
McMaster University
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli CBL |
J Med Chem 50: 755-64 (2007)
Article DOI: 10.1021/jm061132r
BindingDB Entry DOI: 10.7270/Q2KS6SCJ |
More data for this
Ligand-Target Pair | |
Hexokinase-4
(Homo sapiens (Human)) | BDBM50239040

(CHEMBL4086771)Show SMILES CN1C(CCC1=O)c1cc2[nH]c(cc2cc1Oc1ccc(nc1)S(C)(=O)=O)-c1ncc(C)s1 Show InChI InChI=1S/C23H22N4O4S2/c1-13-11-25-23(32-13)18-8-14-9-20(31-15-4-6-21(24-12-15)33(3,29)30)16(10-17(14)26-18)19-5-7-22(28)27(19)2/h4,6,8-12,19,26H,5,7H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 5.5 | n/a | n/a | n/a | n/a |
Merck& Co., Inc.
Curated by ChEMBL
| Assay Description
Activation of recombinant human glucokinase assessed as conversion of D-glucose to D-glucose-6-phosphate in presence of 2.5 mM glucose by G6PDH coupl... |
Bioorg Med Chem Lett 27: 2069-2073 (2017)
Article DOI: 10.1016/j.bmcl.2016.10.085
BindingDB Entry DOI: 10.7270/Q2DZ0BM6 |
More data for this
Ligand-Target Pair | |
Hexokinase-4
(Homo sapiens (Human)) | BDBM50239042
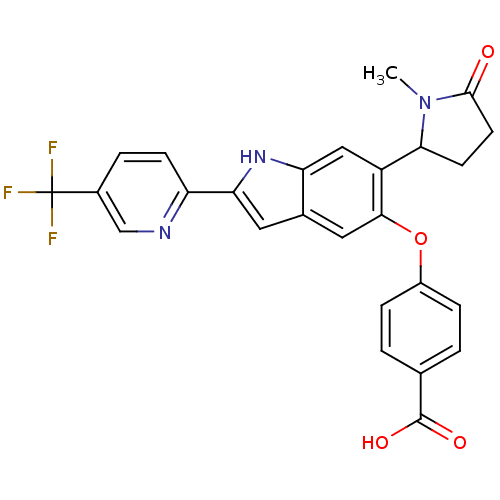
(CHEMBL4094511)Show SMILES CN1C(CCC1=O)c1cc2[nH]c(cc2cc1Oc1ccc(cc1)C(O)=O)-c1ccc(cn1)C(F)(F)F Show InChI InChI=1S/C26H20F3N3O4/c1-32-22(8-9-24(32)33)18-12-20-15(11-23(18)36-17-5-2-14(3-6-17)25(34)35)10-21(31-20)19-7-4-16(13-30-19)26(27,28)29/h2-7,10-13,22,31H,8-9H2,1H3,(H,34,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 24 | n/a | n/a | n/a | n/a |
Merck& Co., Inc.
Curated by ChEMBL
| Assay Description
Ability to displace [3H]spiperone binding from anterior pituitary Dopamine receptor D2 in the presence of 100 uM GTP |
Bioorg Med Chem Lett 27: 2069-2073 (2017)
Article DOI: 10.1016/j.bmcl.2016.10.085
BindingDB Entry DOI: 10.7270/Q2DZ0BM6 |
More data for this
Ligand-Target Pair | |
Hexokinase-4
(Homo sapiens (Human)) | BDBM50239043
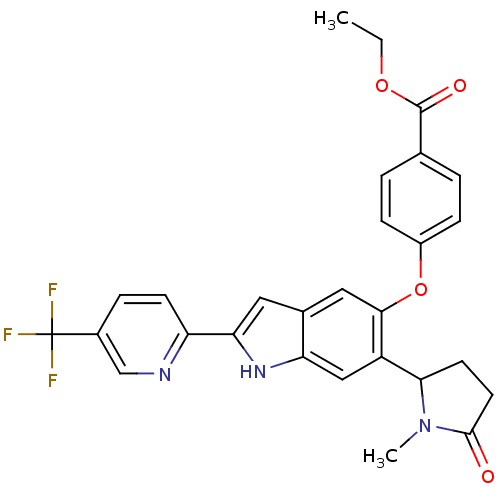
(CHEMBL4067037)Show SMILES CCOC(=O)c1ccc(Oc2cc3cc([nH]c3cc2C2CCC(=O)N2C)-c2ccc(cn2)C(F)(F)F)cc1 Show InChI InChI=1S/C28H24F3N3O4/c1-3-37-27(36)16-4-7-19(8-5-16)38-25-13-17-12-23(21-9-6-18(15-32-21)28(29,30)31)33-22(17)14-20(25)24-10-11-26(35)34(24)2/h4-9,12-15,24,33H,3,10-11H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 4.80 | n/a | n/a | n/a | n/a |
Merck& Co., Inc.
Curated by ChEMBL
| Assay Description
Activation of recombinant human glucokinase assessed as conversion of D-glucose to D-glucose-6-phosphate in presence of 2.5 mM glucose by G6PDH coupl... |
Bioorg Med Chem Lett 27: 2069-2073 (2017)
Article DOI: 10.1016/j.bmcl.2016.10.085
BindingDB Entry DOI: 10.7270/Q2DZ0BM6 |
More data for this
Ligand-Target Pair | |
Hexokinase-4
(Homo sapiens (Human)) | BDBM50239044
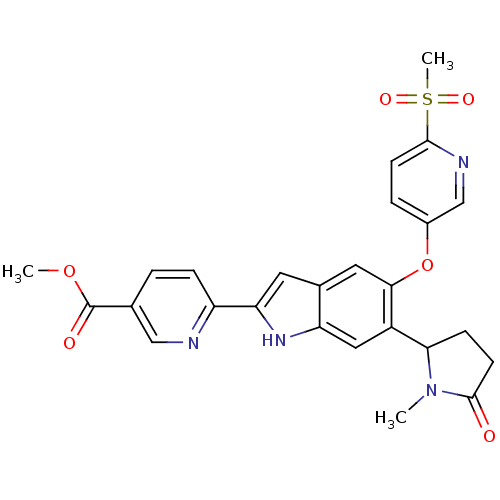
(CHEMBL4104680)Show SMILES COC(=O)c1ccc(nc1)-c1cc2cc(Oc3ccc(nc3)S(C)(=O)=O)c(cc2[nH]1)C1CCC(=O)N1C Show InChI InChI=1S/C26H24N4O6S/c1-30-22(7-9-25(30)31)18-12-20-16(10-21(29-20)19-6-4-15(13-27-19)26(32)35-2)11-23(18)36-17-5-8-24(28-14-17)37(3,33)34/h4-6,8,10-14,22,29H,7,9H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | n/a | n/a | 8.20 | n/a | n/a | n/a | n/a |
Merck& Co., Inc.
Curated by ChEMBL
| Assay Description
Activation of recombinant human glucokinase assessed as conversion of D-glucose to D-glucose-6-phosphate in presence of 2.5 mM glucose by G6PDH coupl... |
Bioorg Med Chem Lett 27: 2069-2073 (2017)
Article DOI: 10.1016/j.bmcl.2016.10.085
BindingDB Entry DOI: 10.7270/Q2DZ0BM6 |
More data for this
Ligand-Target Pair | |
Hexokinase-4
(Homo sapiens (Human)) | BDBM50239045
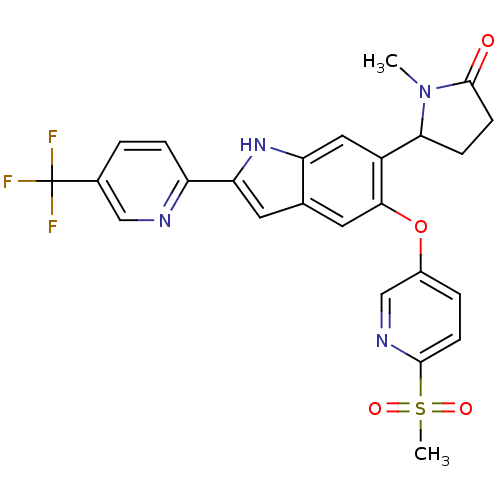
(CHEMBL4083339)Show SMILES CN1C(CCC1=O)c1cc2[nH]c(cc2cc1Oc1ccc(nc1)S(C)(=O)=O)-c1ccc(cn1)C(F)(F)F Show InChI InChI=1S/C25H21F3N4O4S/c1-32-21(6-8-24(32)33)17-11-19-14(9-20(31-19)18-5-3-15(12-29-18)25(26,27)28)10-22(17)36-16-4-7-23(30-13-16)37(2,34)35/h3-5,7,9-13,21,31H,6,8H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 86 | n/a | n/a | n/a | n/a |
Merck& Co., Inc.
Curated by ChEMBL
| Assay Description
Activation of recombinant human glucokinase assessed as conversion of D-glucose to D-glucose-6-phosphate in presence of 2.5 mM glucose by G6PDH coupl... |
Bioorg Med Chem Lett 27: 2069-2073 (2017)
Article DOI: 10.1016/j.bmcl.2016.10.085
BindingDB Entry DOI: 10.7270/Q2DZ0BM6 |
More data for this
Ligand-Target Pair | |
Hexokinase-4
(Homo sapiens (Human)) | BDBM50239045

(CHEMBL4083339)Show SMILES CN1C(CCC1=O)c1cc2[nH]c(cc2cc1Oc1ccc(nc1)S(C)(=O)=O)-c1ccc(cn1)C(F)(F)F Show InChI InChI=1S/C25H21F3N4O4S/c1-32-21(6-8-24(32)33)17-11-19-14(9-20(31-19)18-5-3-15(12-29-18)25(26,27)28)10-22(17)36-16-4-7-23(30-13-16)37(2,34)35/h3-5,7,9-13,21,31H,6,8H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 86 | n/a | n/a | n/a | n/a |
Merck& Co., Inc.
Curated by ChEMBL
| Assay Description
Activation of recombinant human glucokinase assessed as conversion of D-glucose to D-glucose-6-phosphate in presence of 2.5 mM glucose by G6PDH coupl... |
Bioorg Med Chem Lett 27: 2069-2073 (2017)
Article DOI: 10.1016/j.bmcl.2016.10.085
BindingDB Entry DOI: 10.7270/Q2DZ0BM6 |
More data for this
Ligand-Target Pair | |
Hexokinase-4
(Homo sapiens (Human)) | BDBM50239046
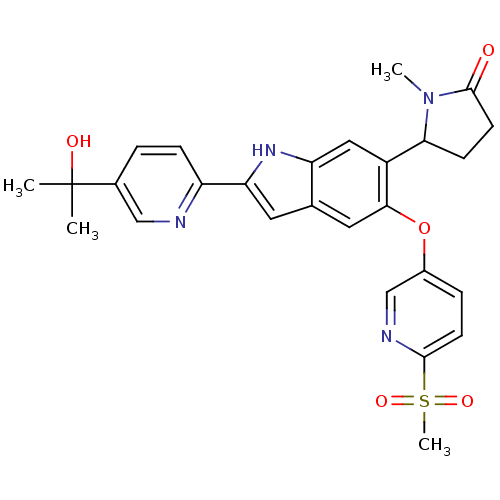
(CHEMBL4068048)Show SMILES CN1C(CCC1=O)c1cc2[nH]c(cc2cc1Oc1ccc(nc1)S(C)(=O)=O)-c1ccc(cn1)C(C)(C)O Show InChI InChI=1S/C27H28N4O5S/c1-27(2,33)17-5-7-20(28-14-17)22-11-16-12-24(36-18-6-9-25(29-15-18)37(4,34)35)19(13-21(16)30-22)23-8-10-26(32)31(23)3/h5-7,9,11-15,23,30,33H,8,10H2,1-4H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 31 | n/a | n/a | n/a | n/a |
Merck& Co., Inc.
Curated by ChEMBL
| Assay Description
Activation of recombinant human glucokinase assessed as conversion of D-glucose to D-glucose-6-phosphate in presence of 2.5 mM glucose by G6PDH coupl... |
Bioorg Med Chem Lett 27: 2069-2073 (2017)
Article DOI: 10.1016/j.bmcl.2016.10.085
BindingDB Entry DOI: 10.7270/Q2DZ0BM6 |
More data for this
Ligand-Target Pair | |
Hexokinase-4
(Homo sapiens (Human)) | BDBM50239047
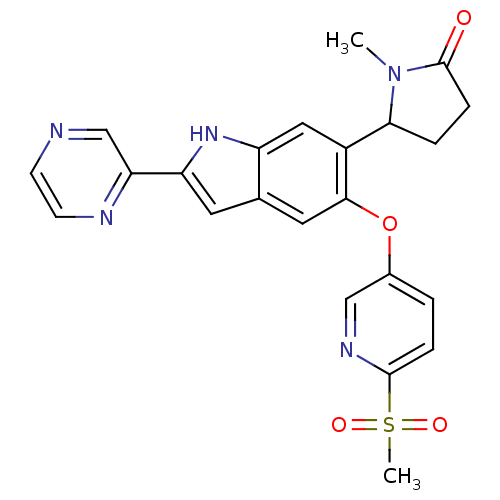
(CHEMBL4078915)Show SMILES CN1C(CCC1=O)c1cc2[nH]c(cc2cc1Oc1ccc(nc1)S(C)(=O)=O)-c1cnccn1 Show InChI InChI=1S/C23H21N5O4S/c1-28-20(4-6-23(28)29)16-11-17-14(9-18(27-17)19-13-24-7-8-25-19)10-21(16)32-15-3-5-22(26-12-15)33(2,30)31/h3,5,7-13,20,27H,4,6H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 48 | n/a | n/a | n/a | n/a |
Merck& Co., Inc.
Curated by ChEMBL
| Assay Description
Activation of recombinant human glucokinase assessed as conversion of D-glucose to D-glucose-6-phosphate in presence of 2.5 mM glucose by G6PDH coupl... |
Bioorg Med Chem Lett 27: 2069-2073 (2017)
Article DOI: 10.1016/j.bmcl.2016.10.085
BindingDB Entry DOI: 10.7270/Q2DZ0BM6 |
More data for this
Ligand-Target Pair | |
Hexokinase-4
(Homo sapiens (Human)) | BDBM50239040

(CHEMBL4086771)Show SMILES CN1C(CCC1=O)c1cc2[nH]c(cc2cc1Oc1ccc(nc1)S(C)(=O)=O)-c1ncc(C)s1 Show InChI InChI=1S/C23H22N4O4S2/c1-13-11-25-23(32-13)18-8-14-9-20(31-15-4-6-21(24-12-15)33(3,29)30)16(10-17(14)26-18)19-5-7-22(28)27(19)2/h4,6,8-12,19,26H,5,7H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 5.5 | n/a | n/a | n/a | n/a |
Merck& Co., Inc.
Curated by ChEMBL
| Assay Description
Activation of recombinant human glucokinase assessed as conversion of D-glucose to D-glucose-6-phosphate in presence of 2.5 mM glucose by G6PDH coupl... |
Bioorg Med Chem Lett 27: 2069-2073 (2017)
Article DOI: 10.1016/j.bmcl.2016.10.085
BindingDB Entry DOI: 10.7270/Q2DZ0BM6 |
More data for this
Ligand-Target Pair | |
Hexokinase-4
(Homo sapiens (Human)) | BDBM50239048
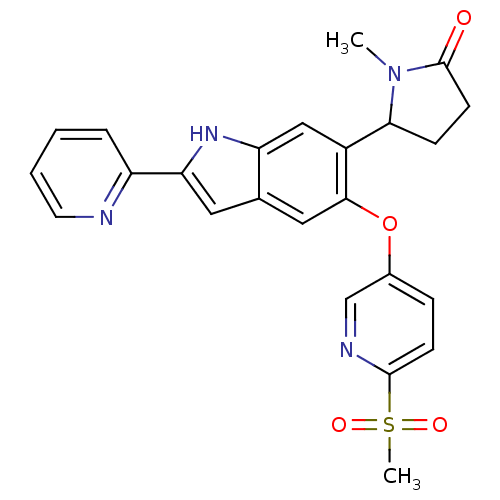
(CHEMBL4067028)Show SMILES CN1C(CCC1=O)c1cc2[nH]c(cc2cc1Oc1ccc(nc1)S(C)(=O)=O)-c1ccccn1 Show InChI InChI=1S/C24H22N4O4S/c1-28-21(7-9-24(28)29)17-13-19-15(11-20(27-19)18-5-3-4-10-25-18)12-22(17)32-16-6-8-23(26-14-16)33(2,30)31/h3-6,8,10-14,21,27H,7,9H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 4.20 | n/a | n/a | n/a | n/a |
Merck& Co., Inc.
Curated by ChEMBL
| Assay Description
Activation of recombinant human glucokinase assessed as conversion of D-glucose to D-glucose-6-phosphate in presence of 2.5 mM glucose by G6PDH coupl... |
Bioorg Med Chem Lett 27: 2069-2073 (2017)
Article DOI: 10.1016/j.bmcl.2016.10.085
BindingDB Entry DOI: 10.7270/Q2DZ0BM6 |
More data for this
Ligand-Target Pair | |
Hexokinase-4
(Homo sapiens (Human)) | BDBM50239048

(CHEMBL4067028)Show SMILES CN1C(CCC1=O)c1cc2[nH]c(cc2cc1Oc1ccc(nc1)S(C)(=O)=O)-c1ccccn1 Show InChI InChI=1S/C24H22N4O4S/c1-28-21(7-9-24(28)29)17-13-19-15(11-20(27-19)18-5-3-4-10-25-18)12-22(17)32-16-6-8-23(26-14-16)33(2,30)31/h3-6,8,10-14,21,27H,7,9H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 4.20 | n/a | n/a | n/a | n/a |
Merck& Co., Inc.
Curated by ChEMBL
| Assay Description
Activation of recombinant human glucokinase assessed as conversion of D-glucose to D-glucose-6-phosphate in presence of 2.5 mM glucose by G6PDH coupl... |
Bioorg Med Chem Lett 27: 2069-2073 (2017)
Article DOI: 10.1016/j.bmcl.2016.10.085
BindingDB Entry DOI: 10.7270/Q2DZ0BM6 |
More data for this
Ligand-Target Pair | |
Hexokinase-4
(Rattus norvegicus) | BDBM50239048

(CHEMBL4067028)Show SMILES CN1C(CCC1=O)c1cc2[nH]c(cc2cc1Oc1ccc(nc1)S(C)(=O)=O)-c1ccccn1 Show InChI InChI=1S/C24H22N4O4S/c1-28-21(7-9-24(28)29)17-13-19-15(11-20(27-19)18-5-3-4-10-25-18)12-22(17)32-16-6-8-23(26-14-16)33(2,30)31/h3-6,8,10-14,21,27H,7,9H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a |
Merck& Co., Inc.
Curated by ChEMBL
| Assay Description
Activation of glucokinase in rat INS-1 cells assessed as increase in D-2-deoxyglucose-6-phosphate level in presence of D-2-deoxyglucose by HILIC LC-M... |
Bioorg Med Chem Lett 27: 2069-2073 (2017)
Article DOI: 10.1016/j.bmcl.2016.10.085
BindingDB Entry DOI: 10.7270/Q2DZ0BM6 |
More data for this
Ligand-Target Pair | |
Hexokinase-4
(Rattus norvegicus) | BDBM50239048

(CHEMBL4067028)Show SMILES CN1C(CCC1=O)c1cc2[nH]c(cc2cc1Oc1ccc(nc1)S(C)(=O)=O)-c1ccccn1 Show InChI InChI=1S/C24H22N4O4S/c1-28-21(7-9-24(28)29)17-13-19-15(11-20(27-19)18-5-3-4-10-25-18)12-22(17)32-16-6-8-23(26-14-16)33(2,30)31/h3-6,8,10-14,21,27H,7,9H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a |
Merck& Co., Inc.
Curated by ChEMBL
| Assay Description
Activation of glucokinase in rat INS-1 cells assessed as increase in D-2-deoxyglucose-6-phosphate level in presence of D-2-deoxyglucose by HILIC LC-M... |
Bioorg Med Chem Lett 27: 2069-2073 (2017)
Article DOI: 10.1016/j.bmcl.2016.10.085
BindingDB Entry DOI: 10.7270/Q2DZ0BM6 |
More data for this
Ligand-Target Pair | |
Hexokinase-4
(Rattus norvegicus) | BDBM50239040

(CHEMBL4086771)Show SMILES CN1C(CCC1=O)c1cc2[nH]c(cc2cc1Oc1ccc(nc1)S(C)(=O)=O)-c1ncc(C)s1 Show InChI InChI=1S/C23H22N4O4S2/c1-13-11-25-23(32-13)18-8-14-9-20(31-15-4-6-21(24-12-15)33(3,29)30)16(10-17(14)26-18)19-5-7-22(28)27(19)2/h4,6,8-12,19,26H,5,7H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 21 | n/a | n/a | n/a | n/a |
Merck& Co., Inc.
Curated by ChEMBL
| Assay Description
Activation of glucokinase in rat INS-1 cells assessed as increase in D-2-deoxyglucose-6-phosphate level in presence of D-2-deoxyglucose by HILIC LC-M... |
Bioorg Med Chem Lett 27: 2069-2073 (2017)
Article DOI: 10.1016/j.bmcl.2016.10.085
BindingDB Entry DOI: 10.7270/Q2DZ0BM6 |
More data for this
Ligand-Target Pair | |
Hexokinase-4
(Rattus norvegicus) | BDBM50239040

(CHEMBL4086771)Show SMILES CN1C(CCC1=O)c1cc2[nH]c(cc2cc1Oc1ccc(nc1)S(C)(=O)=O)-c1ncc(C)s1 Show InChI InChI=1S/C23H22N4O4S2/c1-13-11-25-23(32-13)18-8-14-9-20(31-15-4-6-21(24-12-15)33(3,29)30)16(10-17(14)26-18)19-5-7-22(28)27(19)2/h4,6,8-12,19,26H,5,7H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 21 | n/a | n/a | n/a | n/a |
Merck& Co., Inc.
Curated by ChEMBL
| Assay Description
Activation of glucokinase in rat INS-1 cells assessed as increase in D-2-deoxyglucose-6-phosphate level in presence of D-2-deoxyglucose by HILIC LC-M... |
Bioorg Med Chem Lett 27: 2069-2073 (2017)
Article DOI: 10.1016/j.bmcl.2016.10.085
BindingDB Entry DOI: 10.7270/Q2DZ0BM6 |
More data for this
Ligand-Target Pair | |
Hexokinase-4
(Rattus norvegicus) | BDBM50239047

(CHEMBL4078915)Show SMILES CN1C(CCC1=O)c1cc2[nH]c(cc2cc1Oc1ccc(nc1)S(C)(=O)=O)-c1cnccn1 Show InChI InChI=1S/C23H21N5O4S/c1-28-20(4-6-23(28)29)16-11-17-14(9-18(27-17)19-13-24-7-8-25-19)10-21(16)32-15-3-5-22(26-12-15)33(2,30)31/h3,5,7-13,20,27H,4,6H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 7.20 | n/a | n/a | n/a | n/a |
Merck& Co., Inc.
Curated by ChEMBL
| Assay Description
Activation of glucokinase in rat INS-1 cells assessed as increase in D-2-deoxyglucose-6-phosphate level in presence of D-2-deoxyglucose by HILIC LC-M... |
Bioorg Med Chem Lett 27: 2069-2073 (2017)
Article DOI: 10.1016/j.bmcl.2016.10.085
BindingDB Entry DOI: 10.7270/Q2DZ0BM6 |
More data for this
Ligand-Target Pair | |
Hexokinase-4
(Rattus norvegicus) | BDBM50239046

(CHEMBL4068048)Show SMILES CN1C(CCC1=O)c1cc2[nH]c(cc2cc1Oc1ccc(nc1)S(C)(=O)=O)-c1ccc(cn1)C(C)(C)O Show InChI InChI=1S/C27H28N4O5S/c1-27(2,33)17-5-7-20(28-14-17)22-11-16-12-24(36-18-6-9-25(29-15-18)37(4,34)35)19(13-21(16)30-22)23-8-10-26(32)31(23)3/h5-7,9,11-15,23,30,33H,8,10H2,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 3.90 | n/a | n/a | n/a | n/a |
Merck& Co., Inc.
Curated by ChEMBL
| Assay Description
Activation of glucokinase in rat INS-1 cells assessed as increase in D-2-deoxyglucose-6-phosphate level in presence of D-2-deoxyglucose by HILIC LC-M... |
Bioorg Med Chem Lett 27: 2069-2073 (2017)
Article DOI: 10.1016/j.bmcl.2016.10.085
BindingDB Entry DOI: 10.7270/Q2DZ0BM6 |
More data for this
Ligand-Target Pair | |
Hexokinase-4
(Rattus norvegicus) | BDBM50239046

(CHEMBL4068048)Show SMILES CN1C(CCC1=O)c1cc2[nH]c(cc2cc1Oc1ccc(nc1)S(C)(=O)=O)-c1ccc(cn1)C(C)(C)O Show InChI InChI=1S/C27H28N4O5S/c1-27(2,33)17-5-7-20(28-14-17)22-11-16-12-24(36-18-6-9-25(29-15-18)37(4,34)35)19(13-21(16)30-22)23-8-10-26(32)31(23)3/h5-7,9,11-15,23,30,33H,8,10H2,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 3.90 | n/a | n/a | n/a | n/a |
Merck& Co., Inc.
Curated by ChEMBL
| Assay Description
Activation of glucokinase in rat INS-1 cells assessed as increase in D-2-deoxyglucose-6-phosphate level in presence of D-2-deoxyglucose by HILIC LC-M... |
Bioorg Med Chem Lett 27: 2069-2073 (2017)
Article DOI: 10.1016/j.bmcl.2016.10.085
BindingDB Entry DOI: 10.7270/Q2DZ0BM6 |
More data for this
Ligand-Target Pair | |
Hexokinase-4
(Rattus norvegicus) | BDBM50239045

(CHEMBL4083339)Show SMILES CN1C(CCC1=O)c1cc2[nH]c(cc2cc1Oc1ccc(nc1)S(C)(=O)=O)-c1ccc(cn1)C(F)(F)F Show InChI InChI=1S/C25H21F3N4O4S/c1-32-21(6-8-24(32)33)17-11-19-14(9-20(31-19)18-5-3-15(12-29-18)25(26,27)28)10-22(17)36-16-4-7-23(30-13-16)37(2,34)35/h3-5,7,9-13,21,31H,6,8H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a |
Merck& Co., Inc.
Curated by ChEMBL
| Assay Description
Activation of glucokinase in rat INS-1 cells assessed as increase in D-2-deoxyglucose-6-phosphate level in presence of D-2-deoxyglucose by HILIC LC-M... |
Bioorg Med Chem Lett 27: 2069-2073 (2017)
Article DOI: 10.1016/j.bmcl.2016.10.085
BindingDB Entry DOI: 10.7270/Q2DZ0BM6 |
More data for this
Ligand-Target Pair | |
Hexokinase-4
(Rattus norvegicus) | BDBM50239044

(CHEMBL4104680)Show SMILES COC(=O)c1ccc(nc1)-c1cc2cc(Oc3ccc(nc3)S(C)(=O)=O)c(cc2[nH]1)C1CCC(=O)N1C Show InChI InChI=1S/C26H24N4O6S/c1-30-22(7-9-25(30)31)18-12-20-16(10-21(29-20)19-6-4-15(13-27-19)26(32)35-2)11-23(18)36-17-5-8-24(28-14-17)37(3,33)34/h4-6,8,10-14,22,29H,7,9H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | n/a | n/a | 7.80 | n/a | n/a | n/a | n/a |
Merck& Co., Inc.
Curated by ChEMBL
| Assay Description
Activation of glucokinase in rat INS-1 cells assessed as increase in D-2-deoxyglucose-6-phosphate level in presence of D-2-deoxyglucose by HILIC LC-M... |
Bioorg Med Chem Lett 27: 2069-2073 (2017)
Article DOI: 10.1016/j.bmcl.2016.10.085
BindingDB Entry DOI: 10.7270/Q2DZ0BM6 |
More data for this
Ligand-Target Pair | |
Hexokinase-4
(Rattus norvegicus) | BDBM50239049
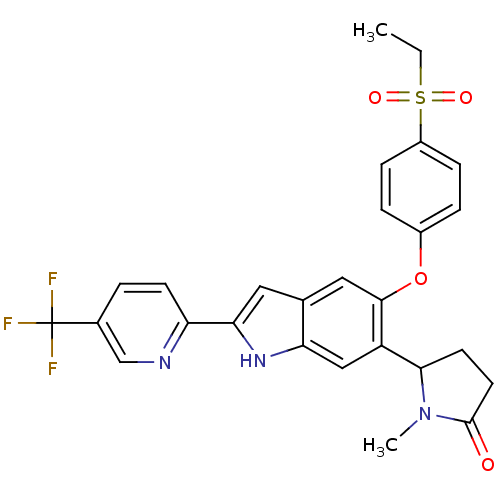
(CHEMBL4086782)Show SMILES CCS(=O)(=O)c1ccc(Oc2cc3cc([nH]c3cc2C2CCC(=O)N2C)-c2ccc(cn2)C(F)(F)F)cc1 Show InChI InChI=1S/C27H24F3N3O4S/c1-3-38(35,36)19-7-5-18(6-8-19)37-25-13-16-12-23(21-9-4-17(15-31-21)27(28,29)30)32-22(16)14-20(25)24-10-11-26(34)33(24)2/h4-9,12-15,24,32H,3,10-11H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a |
Merck& Co., Inc.
Curated by ChEMBL
| Assay Description
Activation of glucokinase in rat INS-1 cells assessed as increase in D-2-deoxyglucose-6-phosphate level in presence of D-2-deoxyglucose by HILIC LC-M... |
Bioorg Med Chem Lett 27: 2069-2073 (2017)
Article DOI: 10.1016/j.bmcl.2016.10.085
BindingDB Entry DOI: 10.7270/Q2DZ0BM6 |
More data for this
Ligand-Target Pair | |
Hexokinase-4
(Rattus norvegicus) | BDBM50239043

(CHEMBL4067037)Show SMILES CCOC(=O)c1ccc(Oc2cc3cc([nH]c3cc2C2CCC(=O)N2C)-c2ccc(cn2)C(F)(F)F)cc1 Show InChI InChI=1S/C28H24F3N3O4/c1-3-37-27(36)16-4-7-19(8-5-16)38-25-13-17-12-23(21-9-6-18(15-32-21)28(29,30)31)33-22(17)14-20(25)24-10-11-26(35)34(24)2/h4-9,12-15,24,33H,3,10-11H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 22 | n/a | n/a | n/a | n/a |
Merck& Co., Inc.
Curated by ChEMBL
| Assay Description
Activation of glucokinase in rat INS-1 cells assessed as increase in D-2-deoxyglucose-6-phosphate level in presence of D-2-deoxyglucose by HILIC LC-M... |
Bioorg Med Chem Lett 27: 2069-2073 (2017)
Article DOI: 10.1016/j.bmcl.2016.10.085
BindingDB Entry DOI: 10.7270/Q2DZ0BM6 |
More data for this
Ligand-Target Pair | |
Hexokinase-4
(Rattus norvegicus) | BDBM50239048

(CHEMBL4067028)Show SMILES CN1C(CCC1=O)c1cc2[nH]c(cc2cc1Oc1ccc(nc1)S(C)(=O)=O)-c1ccccn1 Show InChI InChI=1S/C24H22N4O4S/c1-28-21(7-9-24(28)29)17-13-19-15(11-20(27-19)18-5-3-4-10-25-18)12-22(17)32-16-6-8-23(26-14-16)33(2,30)31/h3-6,8,10-14,21,27H,7,9H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 7.5 | n/a | n/a | n/a | n/a |
Merck& Co., Inc.
Curated by ChEMBL
| Assay Description
Activation of glucokinase in primary rat hepatocytes assessed as increase in D-2-deoxyglucose-6-phosphate level in presence of D-2-deoxyglucose by HI... |
Bioorg Med Chem Lett 27: 2069-2073 (2017)
Article DOI: 10.1016/j.bmcl.2016.10.085
BindingDB Entry DOI: 10.7270/Q2DZ0BM6 |
More data for this
Ligand-Target Pair | |
Hexokinase-4
(Rattus norvegicus) | BDBM50239048

(CHEMBL4067028)Show SMILES CN1C(CCC1=O)c1cc2[nH]c(cc2cc1Oc1ccc(nc1)S(C)(=O)=O)-c1ccccn1 Show InChI InChI=1S/C24H22N4O4S/c1-28-21(7-9-24(28)29)17-13-19-15(11-20(27-19)18-5-3-4-10-25-18)12-22(17)32-16-6-8-23(26-14-16)33(2,30)31/h3-6,8,10-14,21,27H,7,9H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 7.5 | n/a | n/a | n/a | n/a |
Merck& Co., Inc.
Curated by ChEMBL
| Assay Description
Activation of glucokinase in primary rat hepatocytes assessed as increase in D-2-deoxyglucose-6-phosphate level in presence of D-2-deoxyglucose by HI... |
Bioorg Med Chem Lett 27: 2069-2073 (2017)
Article DOI: 10.1016/j.bmcl.2016.10.085
BindingDB Entry DOI: 10.7270/Q2DZ0BM6 |
More data for this
Ligand-Target Pair | |
Hexokinase-4
(Rattus norvegicus) | BDBM50239040

(CHEMBL4086771)Show SMILES CN1C(CCC1=O)c1cc2[nH]c(cc2cc1Oc1ccc(nc1)S(C)(=O)=O)-c1ncc(C)s1 Show InChI InChI=1S/C23H22N4O4S2/c1-13-11-25-23(32-13)18-8-14-9-20(31-15-4-6-21(24-12-15)33(3,29)30)16(10-17(14)26-18)19-5-7-22(28)27(19)2/h4,6,8-12,19,26H,5,7H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 16 | n/a | n/a | n/a | n/a |
Merck& Co., Inc.
Curated by ChEMBL
| Assay Description
Activation of glucokinase in primary rat hepatocytes assessed as increase in D-2-deoxyglucose-6-phosphate level in presence of D-2-deoxyglucose by HI... |
Bioorg Med Chem Lett 27: 2069-2073 (2017)
Article DOI: 10.1016/j.bmcl.2016.10.085
BindingDB Entry DOI: 10.7270/Q2DZ0BM6 |
More data for this
Ligand-Target Pair | |
Hexokinase-4
(Rattus norvegicus) | BDBM50239047

(CHEMBL4078915)Show SMILES CN1C(CCC1=O)c1cc2[nH]c(cc2cc1Oc1ccc(nc1)S(C)(=O)=O)-c1cnccn1 Show InChI InChI=1S/C23H21N5O4S/c1-28-20(4-6-23(28)29)16-11-17-14(9-18(27-17)19-13-24-7-8-25-19)10-21(16)32-15-3-5-22(26-12-15)33(2,30)31/h3,5,7-13,20,27H,4,6H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 23 | n/a | n/a | n/a | n/a |
Merck& Co., Inc.
Curated by ChEMBL
| Assay Description
Activation of glucokinase in primary rat hepatocytes assessed as increase in D-2-deoxyglucose-6-phosphate level in presence of D-2-deoxyglucose by HI... |
Bioorg Med Chem Lett 27: 2069-2073 (2017)
Article DOI: 10.1016/j.bmcl.2016.10.085
BindingDB Entry DOI: 10.7270/Q2DZ0BM6 |
More data for this
Ligand-Target Pair | |
Hexokinase-4
(Rattus norvegicus) | BDBM50239047

(CHEMBL4078915)Show SMILES CN1C(CCC1=O)c1cc2[nH]c(cc2cc1Oc1ccc(nc1)S(C)(=O)=O)-c1cnccn1 Show InChI InChI=1S/C23H21N5O4S/c1-28-20(4-6-23(28)29)16-11-17-14(9-18(27-17)19-13-24-7-8-25-19)10-21(16)32-15-3-5-22(26-12-15)33(2,30)31/h3,5,7-13,20,27H,4,6H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 23 | n/a | n/a | n/a | n/a |
Merck& Co., Inc.
Curated by ChEMBL
| Assay Description
Activation of glucokinase in primary rat hepatocytes assessed as increase in D-2-deoxyglucose-6-phosphate level in presence of D-2-deoxyglucose by HI... |
Bioorg Med Chem Lett 27: 2069-2073 (2017)
Article DOI: 10.1016/j.bmcl.2016.10.085
BindingDB Entry DOI: 10.7270/Q2DZ0BM6 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data