 Found 117 hits with Last Name = 'enroth' and Initial = 'c'
Found 117 hits with Last Name = 'enroth' and Initial = 'c' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50365480
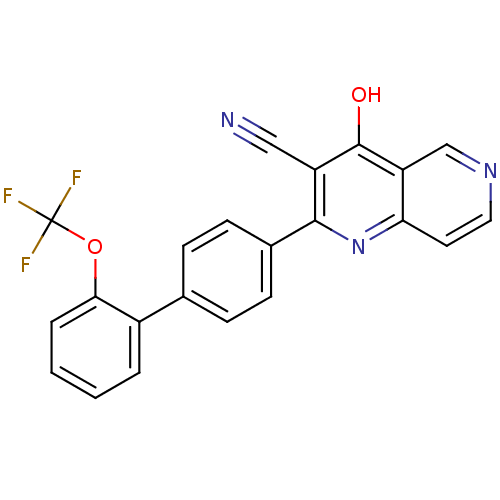
(CHEMBL1957366)Show SMILES Oc1c(C#N)c(nc2ccncc12)-c1ccc(cc1)-c1ccccc1OC(F)(F)F Show InChI InChI=1S/C22H12F3N3O2/c23-22(24,25)30-19-4-2-1-3-15(19)13-5-7-14(8-6-13)20-16(11-26)21(29)17-12-27-10-9-18(17)28-20/h1-10,12H,(H,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant full length PDE10A using cAMP as substrate preincubated for 20 mins measured after 4 hrs |
Bioorg Med Chem Lett 22: 1944-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.046
BindingDB Entry DOI: 10.7270/Q2KH0NTP |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50365495
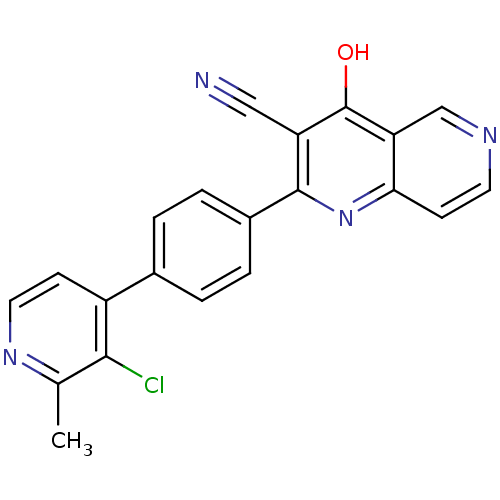
(CHEMBL1957460)Show SMILES Cc1nccc(-c2ccc(cc2)-c2nc3ccncc3c(O)c2C#N)c1Cl Show InChI InChI=1S/C21H13ClN4O/c1-12-19(22)15(6-9-25-12)13-2-4-14(5-3-13)20-16(10-23)21(27)17-11-24-8-7-18(17)26-20/h2-9,11H,1H3,(H,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant full length PDE10A using cAMP as substrate preincubated for 20 mins measured after 4 hrs |
Bioorg Med Chem Lett 22: 1944-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.046
BindingDB Entry DOI: 10.7270/Q2KH0NTP |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-beta
(Homo sapiens (Human)) | BDBM50317735
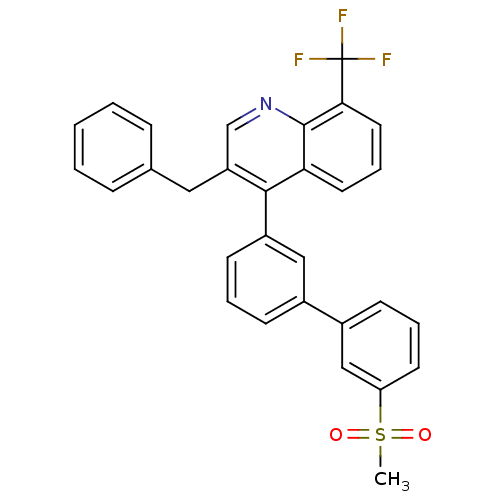
(3-benzyl-4-(3'-(methylsulfonyl)biphenyl-3-yl)-8-(t...)Show SMILES CS(=O)(=O)c1cccc(c1)-c1cccc(c1)-c1c(Cc2ccccc2)cnc2c(cccc12)C(F)(F)F Show InChI InChI=1S/C30H22F3NO2S/c1-37(35,36)25-13-6-11-22(18-25)21-10-5-12-23(17-21)28-24(16-20-8-3-2-4-9-20)19-34-29-26(28)14-7-15-27(29)30(31,32)33/h2-15,17-19H,16H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [3H]T0901317 from LXRbeta ligand binding domain |
Bioorg Med Chem Lett 20: 2903-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.031
BindingDB Entry DOI: 10.7270/Q2TD9XJZ |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-beta
(Homo sapiens (Human)) | BDBM50317744
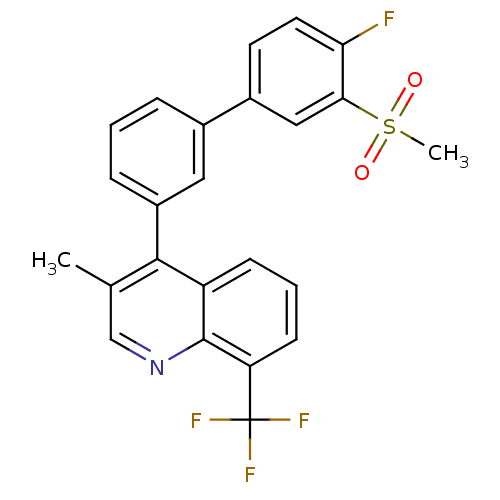
(4-(4'-fluoro-3'-(methylsulfonyl)biphenyl-3-yl)-3-m...)Show SMILES Cc1cnc2c(cccc2c1-c1cccc(c1)-c1ccc(F)c(c1)S(C)(=O)=O)C(F)(F)F Show InChI InChI=1S/C24H17F4NO2S/c1-14-13-29-23-18(7-4-8-19(23)24(26,27)28)22(14)17-6-3-5-15(11-17)16-9-10-20(25)21(12-16)32(2,30)31/h3-13H,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [3H]T0901317 from LXRbeta ligand binding domain |
Bioorg Med Chem Lett 20: 2903-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.031
BindingDB Entry DOI: 10.7270/Q2TD9XJZ |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-beta
(Homo sapiens (Human)) | BDBM50317733
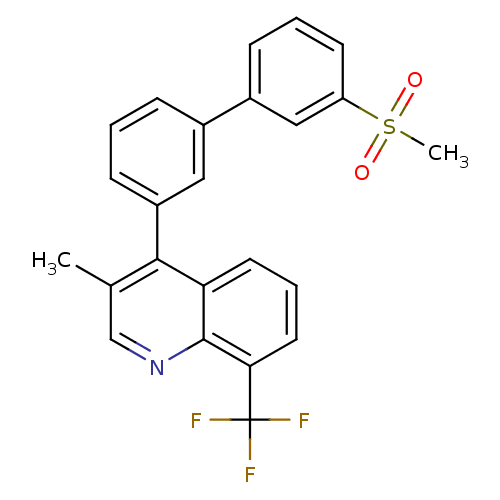
(3-methyl-4-(3'-(methylsulfonyl)biphenyl-3-yl)-8-(t...)Show SMILES Cc1cnc2c(cccc2c1-c1cccc(c1)-c1cccc(c1)S(C)(=O)=O)C(F)(F)F Show InChI InChI=1S/C24H18F3NO2S/c1-15-14-28-23-20(10-5-11-21(23)24(25,26)27)22(15)18-8-3-6-16(12-18)17-7-4-9-19(13-17)31(2,29)30/h3-14H,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [3H]T0901317 from LXRbeta ligand binding domain |
Bioorg Med Chem Lett 20: 2903-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.031
BindingDB Entry DOI: 10.7270/Q2TD9XJZ |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-beta [154-461]
(Homo sapiens (Human)) | BDBM20001
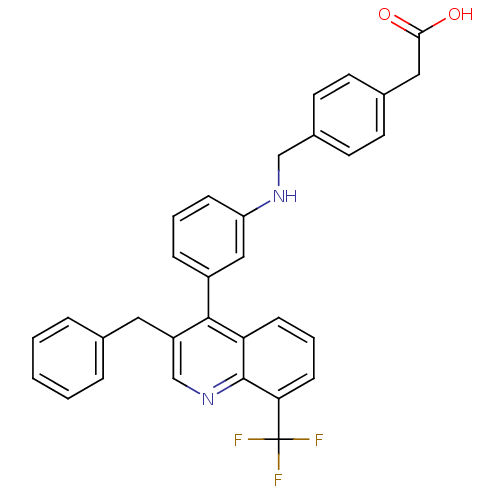
(2-{4-[({3-[3-benzyl-8-(trifluoromethyl)quinolin-4-...)Show SMILES OC(=O)Cc1ccc(CNc2cccc(c2)-c2c(Cc3ccccc3)cnc3c(cccc23)C(F)(F)F)cc1 Show InChI InChI=1S/C32H25F3N2O2/c33-32(34,35)28-11-5-10-27-30(25(20-37-31(27)28)16-21-6-2-1-3-7-21)24-8-4-9-26(18-24)36-19-23-14-12-22(13-15-23)17-29(38)39/h1-15,18,20,36H,16-17,19H2,(H,38,39) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.90 | n/a | 33 | n/a | n/a | 7.4 | 4 |
Wyeth Research
| Assay Description
Binding reaction was initiated by adding a dilution series of test compound in tracer solution to LXR-coated flash plates. Each concentration of test... |
J Med Chem 49: 6151-4 (2006)
Article DOI: 10.1021/jm0609566
BindingDB Entry DOI: 10.7270/Q20863KH |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50365481

(CHEMBL1957367)Show SMILES Oc1c(C#N)c(nc2ccncc12)-c1ccc(cc1)-c1ccccc1OCC#N Show InChI InChI=1S/C23H14N4O2/c24-10-12-29-21-4-2-1-3-17(21)15-5-7-16(8-6-15)22-18(13-25)23(28)19-14-26-11-9-20(19)27-22/h1-9,11,14H,12H2,(H,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant full length PDE10A using cAMP as substrate preincubated for 20 mins measured after 4 hrs |
Bioorg Med Chem Lett 22: 1944-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.046
BindingDB Entry DOI: 10.7270/Q2KH0NTP |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-beta [154-461]
(Homo sapiens (Human)) | BDBM20000

(2-(4-{3-[3-benzyl-8-(trifluoromethyl)quinolin-4-yl...)Show SMILES OC(=O)Cc1ccc(COc2cccc(c2)-c2c(Cc3ccccc3)cnc3c(cccc23)C(F)(F)F)cc1 Show InChI InChI=1S/C32H24F3NO3/c33-32(34,35)28-11-5-10-27-30(25(19-36-31(27)28)16-21-6-2-1-3-7-21)24-8-4-9-26(18-24)39-20-23-14-12-22(13-15-23)17-29(37)38/h1-15,18-19H,16-17,20H2,(H,37,38) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 2.10 | n/a | 71 | n/a | n/a | 7.4 | 4 |
Wyeth Research
| Assay Description
Binding reaction was initiated by adding a dilution series of test compound in tracer solution to LXR-coated flash plates. Each concentration of test... |
J Med Chem 49: 6151-4 (2006)
Article DOI: 10.1021/jm0609566
BindingDB Entry DOI: 10.7270/Q20863KH |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Oxysterols receptor LXR-beta
(Homo sapiens (Human)) | BDBM50317737
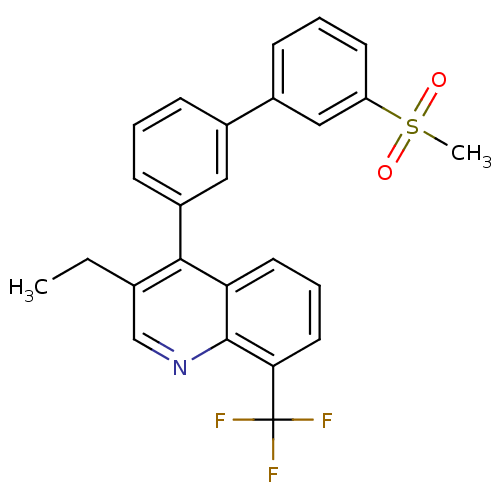
(3-ethyl-4-(3'-(methylsulfonyl)biphenyl-3-yl)-8-(tr...)Show SMILES CCc1cnc2c(cccc2c1-c1cccc(c1)-c1cccc(c1)S(C)(=O)=O)C(F)(F)F Show InChI InChI=1S/C25H20F3NO2S/c1-3-16-15-29-24-21(11-6-12-22(24)25(26,27)28)23(16)19-9-4-7-17(13-19)18-8-5-10-20(14-18)32(2,30)31/h4-15H,3H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [3H]T0901317 from LXRbeta ligand binding domain |
Bioorg Med Chem Lett 20: 2903-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.031
BindingDB Entry DOI: 10.7270/Q2TD9XJZ |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-beta
(Homo sapiens (Human)) | BDBM50317746
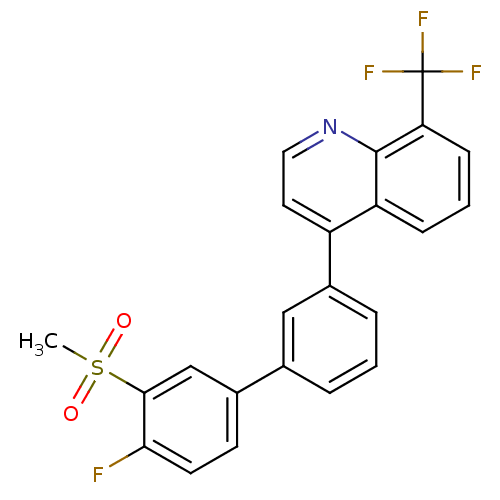
(4-(4'-fluoro-3'-(methylsulfonyl)biphenyl-3-yl)-8-(...)Show SMILES CS(=O)(=O)c1cc(ccc1F)-c1cccc(c1)-c1ccnc2c(cccc12)C(F)(F)F Show InChI InChI=1S/C23H15F4NO2S/c1-31(29,30)21-13-15(8-9-20(21)24)14-4-2-5-16(12-14)17-10-11-28-22-18(17)6-3-7-19(22)23(25,26)27/h2-13H,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [3H]T0901317 from LXRbeta ligand binding domain |
Bioorg Med Chem Lett 20: 2903-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.031
BindingDB Entry DOI: 10.7270/Q2TD9XJZ |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50365487
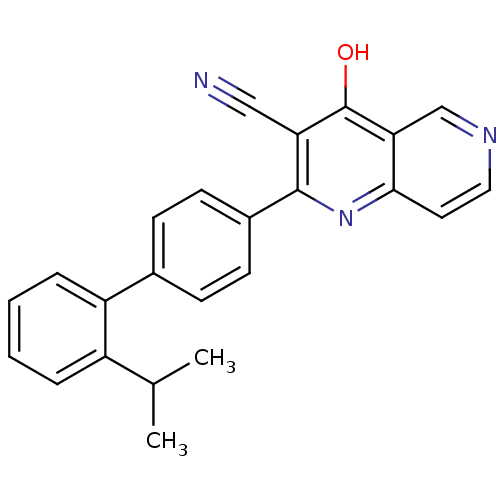
(CHEMBL1957375)Show SMILES CC(C)c1ccccc1-c1ccc(cc1)-c1nc2ccncc2c(O)c1C#N Show InChI InChI=1S/C24H19N3O/c1-15(2)18-5-3-4-6-19(18)16-7-9-17(10-8-16)23-20(13-25)24(28)21-14-26-12-11-22(21)27-23/h3-12,14-15H,1-2H3,(H,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant full length PDE10A using cAMP as substrate preincubated for 20 mins measured after 4 hrs |
Bioorg Med Chem Lett 22: 1944-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.046
BindingDB Entry DOI: 10.7270/Q2KH0NTP |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50365484

(CHEMBL1955881)Show SMILES Oc1c(C#N)c(nc2ccncc12)-c1ccc(cc1)-c1ccccc1C(F)(F)F Show InChI InChI=1S/C22H12F3N3O/c23-22(24,25)18-4-2-1-3-15(18)13-5-7-14(8-6-13)20-16(11-26)21(29)17-12-27-10-9-19(17)28-20/h1-10,12H,(H,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant full length PDE10A using cAMP as substrate preincubated for 20 mins measured after 4 hrs |
Bioorg Med Chem Lett 22: 1944-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.046
BindingDB Entry DOI: 10.7270/Q2KH0NTP |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-beta
(Homo sapiens (Human)) | BDBM50317742
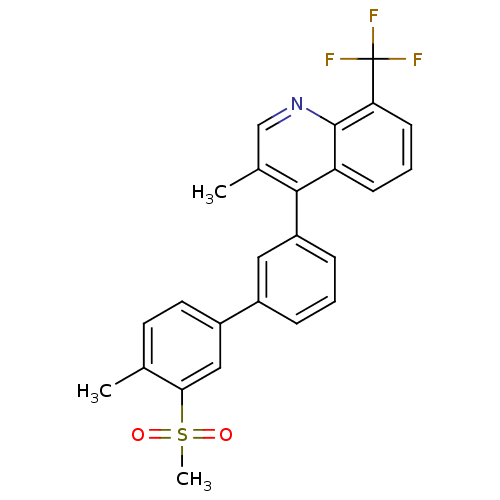
(3-methyl-4-(4'-methyl-3'-(methylsulfonyl)biphenyl-...)Show SMILES Cc1ccc(cc1S(C)(=O)=O)-c1cccc(c1)-c1c(C)cnc2c(cccc12)C(F)(F)F Show InChI InChI=1S/C25H20F3NO2S/c1-15-10-11-18(13-22(15)32(3,30)31)17-6-4-7-19(12-17)23-16(2)14-29-24-20(23)8-5-9-21(24)25(26,27)28/h4-14H,1-3H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [3H]T0901317 from LXRbeta ligand binding domain |
Bioorg Med Chem Lett 20: 2903-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.031
BindingDB Entry DOI: 10.7270/Q2TD9XJZ |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-beta
(Homo sapiens (Human)) | BDBM50317745
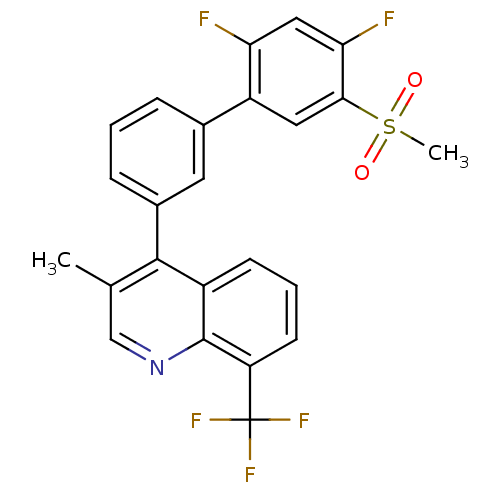
(4-(2',4'-difluoro-5'-(methylsulfonyl)biphenyl-3-yl...)Show SMILES Cc1cnc2c(cccc2c1-c1cccc(c1)-c1cc(c(F)cc1F)S(C)(=O)=O)C(F)(F)F Show InChI InChI=1S/C24H16F5NO2S/c1-13-12-30-23-16(7-4-8-18(23)24(27,28)29)22(13)15-6-3-5-14(9-15)17-10-21(33(2,31)32)20(26)11-19(17)25/h3-12H,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [3H]T0901317 from LXRbeta ligand binding domain |
Bioorg Med Chem Lett 20: 2903-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.031
BindingDB Entry DOI: 10.7270/Q2TD9XJZ |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-beta
(Homo sapiens (Human)) | BDBM50317738
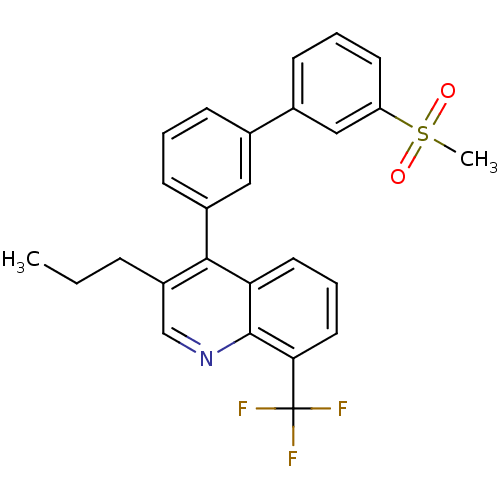
(4-(3'-(methylsulfonyl)biphenyl-3-yl)-3-propyl-8-(t...)Show SMILES CCCc1cnc2c(cccc2c1-c1cccc(c1)-c1cccc(c1)S(C)(=O)=O)C(F)(F)F Show InChI InChI=1S/C26H22F3NO2S/c1-3-7-20-16-30-25-22(12-6-13-23(25)26(27,28)29)24(20)19-10-4-8-17(14-19)18-9-5-11-21(15-18)33(2,31)32/h4-6,8-16H,3,7H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [3H]T0901317 from LXRbeta ligand binding domain |
Bioorg Med Chem Lett 20: 2903-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.031
BindingDB Entry DOI: 10.7270/Q2TD9XJZ |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-beta
(Homo sapiens (Human)) | BDBM50317739
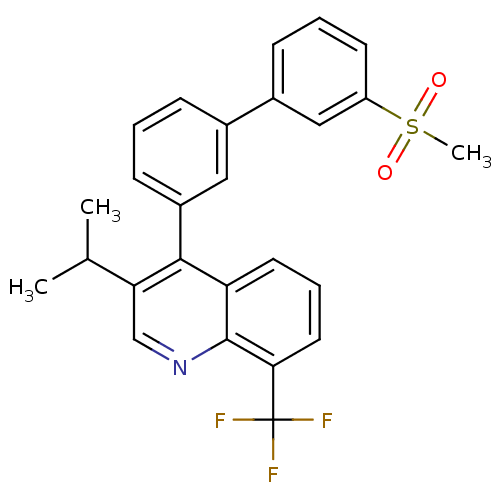
(3-isopropyl-4-(3'-(methylsulfonyl)biphenyl-3-yl)-8...)Show SMILES CC(C)c1cnc2c(cccc2c1-c1cccc(c1)-c1cccc(c1)S(C)(=O)=O)C(F)(F)F Show InChI InChI=1S/C26H22F3NO2S/c1-16(2)22-15-30-25-21(11-6-12-23(25)26(27,28)29)24(22)19-9-4-7-17(13-19)18-8-5-10-20(14-18)33(3,31)32/h4-16H,1-3H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [3H]T0901317 from LXRbeta ligand binding domain |
Bioorg Med Chem Lett 20: 2903-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.031
BindingDB Entry DOI: 10.7270/Q2TD9XJZ |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-beta
(Homo sapiens (Human)) | BDBM50317731
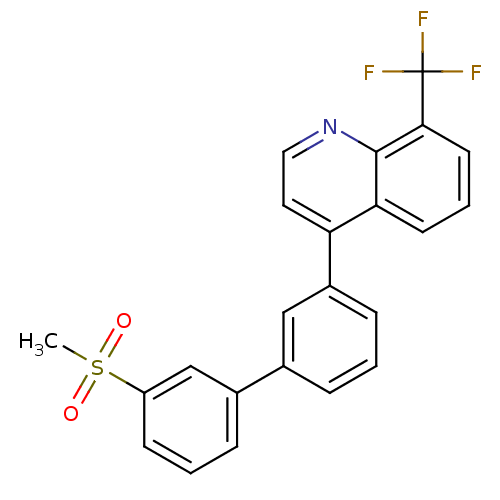
(4-(3'-(methylsulfonyl)biphenyl-3-yl)-8-(trifluorom...)Show SMILES CS(=O)(=O)c1cccc(c1)-c1cccc(c1)-c1ccnc2c(cccc12)C(F)(F)F Show InChI InChI=1S/C23H16F3NO2S/c1-30(28,29)18-8-3-6-16(14-18)15-5-2-7-17(13-15)19-11-12-27-22-20(19)9-4-10-21(22)23(24,25)26/h2-14H,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [3H]T0901317 from LXRbeta ligand binding domain |
Bioorg Med Chem Lett 20: 2903-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.031
BindingDB Entry DOI: 10.7270/Q2TD9XJZ |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-alpha
(Homo sapiens (Human)) | BDBM50317737

(3-ethyl-4-(3'-(methylsulfonyl)biphenyl-3-yl)-8-(tr...)Show SMILES CCc1cnc2c(cccc2c1-c1cccc(c1)-c1cccc(c1)S(C)(=O)=O)C(F)(F)F Show InChI InChI=1S/C25H20F3NO2S/c1-3-16-15-29-24-21(11-6-12-22(24)25(26,27)28)23(16)19-9-4-7-17(13-19)18-8-5-10-20(14-18)32(2,30)31/h4-15H,3H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [3H]T0901317 from LXRalpha ligand binding domain |
Bioorg Med Chem Lett 20: 2903-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.031
BindingDB Entry DOI: 10.7270/Q2TD9XJZ |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-beta
(Homo sapiens (Human)) | BDBM50317747
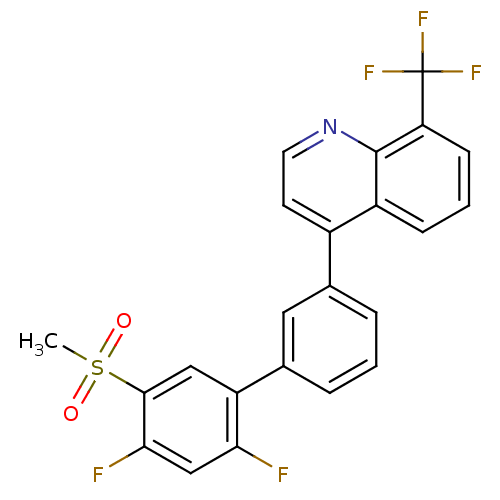
(4-(2',4'-difluoro-5'-(methylsulfonyl)biphenyl-3-yl...)Show SMILES CS(=O)(=O)c1cc(c(F)cc1F)-c1cccc(c1)-c1ccnc2c(cccc12)C(F)(F)F Show InChI InChI=1S/C23H14F5NO2S/c1-32(30,31)21-11-17(19(24)12-20(21)25)14-5-2-4-13(10-14)15-8-9-29-22-16(15)6-3-7-18(22)23(26,27)28/h2-12H,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [3H]T0901317 from LXRbeta ligand binding domain |
Bioorg Med Chem Lett 20: 2903-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.031
BindingDB Entry DOI: 10.7270/Q2TD9XJZ |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-beta [154-461]
(Homo sapiens (Human)) | BDBM19999
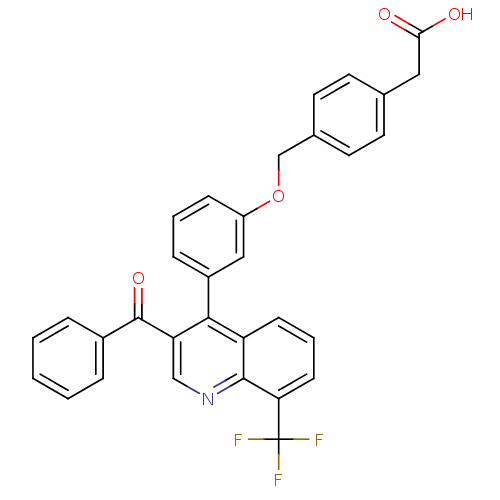
(2-(4-{3-[3-benzoyl-8-(trifluoromethyl)quinolin-4-y...)Show SMILES OC(=O)Cc1ccc(COc2cccc(c2)-c2c(cnc3c(cccc23)C(F)(F)F)C(=O)c2ccccc2)cc1 Show InChI InChI=1S/C32H22F3NO4/c33-32(34,35)27-11-5-10-25-29(26(18-36-30(25)27)31(39)22-6-2-1-3-7-22)23-8-4-9-24(17-23)40-19-21-14-12-20(13-15-21)16-28(37)38/h1-15,17-18H,16,19H2,(H,37,38) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | 143 | n/a | n/a | 7.4 | 4 |
Wyeth Research
| Assay Description
Binding reaction was initiated by adding a dilution series of test compound in tracer solution to LXR-coated flash plates. Each concentration of test... |
J Med Chem 49: 6151-4 (2006)
Article DOI: 10.1021/jm0609566
BindingDB Entry DOI: 10.7270/Q20863KH |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-beta
(Homo sapiens (Human)) | BDBM50317741

(4-(3'-(ethylsulfonyl)biphenyl-3-yl)-3-methyl-8-(tr...)Show SMILES CCS(=O)(=O)c1cccc(c1)-c1cccc(c1)-c1c(C)cnc2c(cccc12)C(F)(F)F Show InChI InChI=1S/C25H20F3NO2S/c1-3-32(30,31)20-10-5-8-18(14-20)17-7-4-9-19(13-17)23-16(2)15-29-24-21(23)11-6-12-22(24)25(26,27)28/h4-15H,3H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [3H]T0901317 from LXRbeta ligand binding domain |
Bioorg Med Chem Lett 20: 2903-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.031
BindingDB Entry DOI: 10.7270/Q2TD9XJZ |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-beta [154-461]
(Homo sapiens (Human)) | BDBM20002
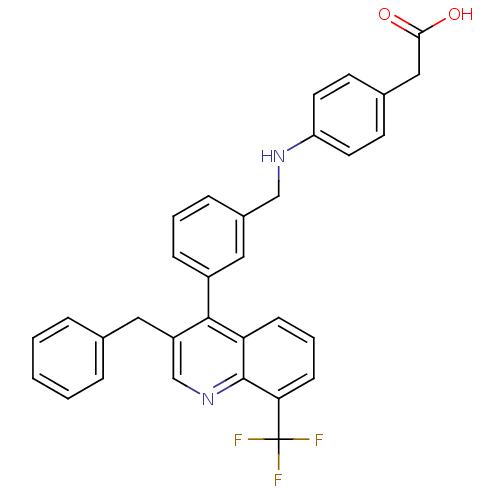
(2-{4-[({3-[3-benzyl-8-(trifluoromethyl)quinolin-4-...)Show SMILES OC(=O)Cc1ccc(NCc2cccc(c2)-c2c(Cc3ccccc3)cnc3c(cccc23)C(F)(F)F)cc1 Show InChI InChI=1S/C32H25F3N2O2/c33-32(34,35)28-11-5-10-27-30(25(20-37-31(27)28)16-21-6-2-1-3-7-21)24-9-4-8-23(17-24)19-36-26-14-12-22(13-15-26)18-29(38)39/h1-15,17,20,36H,16,18-19H2,(H,38,39) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | 7.4 | 4 |
Wyeth Research
| Assay Description
Binding reaction was initiated by adding a dilution series of test compound in tracer solution to LXR-coated flash plates. Each concentration of test... |
J Med Chem 49: 6151-4 (2006)
Article DOI: 10.1021/jm0609566
BindingDB Entry DOI: 10.7270/Q20863KH |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-alpha
(Homo sapiens (Human)) | BDBM50317735

(3-benzyl-4-(3'-(methylsulfonyl)biphenyl-3-yl)-8-(t...)Show SMILES CS(=O)(=O)c1cccc(c1)-c1cccc(c1)-c1c(Cc2ccccc2)cnc2c(cccc12)C(F)(F)F Show InChI InChI=1S/C30H22F3NO2S/c1-37(35,36)25-13-6-11-22(18-25)21-10-5-12-23(17-21)28-24(16-20-8-3-2-4-9-20)19-34-29-26(28)14-7-15-27(29)30(31,32)33/h2-15,17-19H,16H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [3H]T0901317 from LXRalpha ligand binding domain |
Bioorg Med Chem Lett 20: 2903-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.031
BindingDB Entry DOI: 10.7270/Q2TD9XJZ |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-alpha
(Homo sapiens (Human)) | BDBM50317738

(4-(3'-(methylsulfonyl)biphenyl-3-yl)-3-propyl-8-(t...)Show SMILES CCCc1cnc2c(cccc2c1-c1cccc(c1)-c1cccc(c1)S(C)(=O)=O)C(F)(F)F Show InChI InChI=1S/C26H22F3NO2S/c1-3-7-20-16-30-25-22(12-6-13-23(25)26(27,28)29)24(20)19-10-4-8-17(14-19)18-9-5-11-21(15-18)33(2,31)32/h4-6,8-16H,3,7H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [3H]T0901317 from LXRalpha ligand binding domain |
Bioorg Med Chem Lett 20: 2903-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.031
BindingDB Entry DOI: 10.7270/Q2TD9XJZ |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-alpha [197-447]
(Homo sapiens (Human)) | BDBM20001

(2-{4-[({3-[3-benzyl-8-(trifluoromethyl)quinolin-4-...)Show SMILES OC(=O)Cc1ccc(CNc2cccc(c2)-c2c(Cc3ccccc3)cnc3c(cccc23)C(F)(F)F)cc1 Show InChI InChI=1S/C32H25F3N2O2/c33-32(34,35)28-11-5-10-27-30(25(20-37-31(27)28)16-21-6-2-1-3-7-21)24-8-4-9-26(18-24)36-19-23-14-12-22(13-15-23)17-29(38)39/h1-15,18,20,36H,16-17,19H2,(H,38,39) | PDB
MMDB
Reactome pathway
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7.60 | n/a | n/a | n/a | n/a | 7.4 | 4 |
Wyeth Research
| Assay Description
Binding reaction was initiated by adding a dilution series of test compound in tracer solution to LXR-coated flash plates. Each concentration of test... |
J Med Chem 49: 6151-4 (2006)
Article DOI: 10.1021/jm0609566
BindingDB Entry DOI: 10.7270/Q20863KH |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50209531
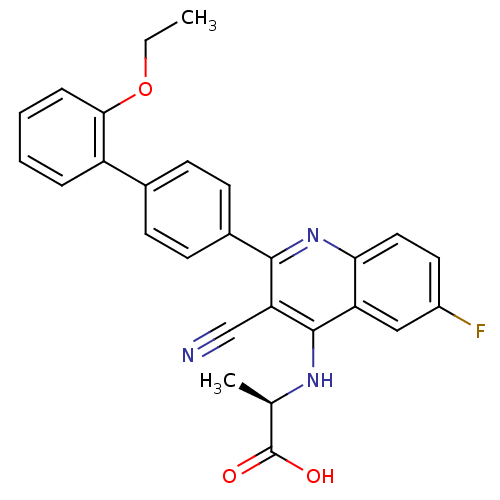
((R)-2-[3-cyano-2-(2'-ethoxy-biphenyl-4-yl)-6-fluor...)Show SMILES CCOc1ccccc1-c1ccc(cc1)-c1nc2ccc(F)cc2c(N[C@H](C)C(O)=O)c1C#N Show InChI InChI=1S/C27H22FN3O3/c1-3-34-24-7-5-4-6-20(24)17-8-10-18(11-9-17)25-22(15-29)26(30-16(2)27(32)33)21-14-19(28)12-13-23(21)31-25/h4-14,16H,3H2,1-2H3,(H,30,31)(H,32,33)/t16-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant full length PDE10A using cAMP as substrate preincubated for 20 mins measured after 4 hrs |
Bioorg Med Chem Lett 22: 1944-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.046
BindingDB Entry DOI: 10.7270/Q2KH0NTP |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50365483

(CHEMBL1957369)Show SMILES Oc1c(C#N)c(nc2ccncc12)-c1ccc(cc1)-c1ccccc1Cl Show InChI InChI=1S/C21H12ClN3O/c22-18-4-2-1-3-15(18)13-5-7-14(8-6-13)20-16(11-23)21(26)17-12-24-10-9-19(17)25-20/h1-10,12H,(H,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant full length PDE10A using cAMP as substrate preincubated for 20 mins measured after 4 hrs |
Bioorg Med Chem Lett 22: 1944-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.046
BindingDB Entry DOI: 10.7270/Q2KH0NTP |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-alpha
(Homo sapiens (Human)) | BDBM50317739

(3-isopropyl-4-(3'-(methylsulfonyl)biphenyl-3-yl)-8...)Show SMILES CC(C)c1cnc2c(cccc2c1-c1cccc(c1)-c1cccc(c1)S(C)(=O)=O)C(F)(F)F Show InChI InChI=1S/C26H22F3NO2S/c1-16(2)22-15-30-25-21(11-6-12-23(25)26(27,28)29)24(22)19-9-4-7-17(13-19)18-8-5-10-20(14-18)33(3,31)32/h4-16H,1-3H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [3H]T0901317 from LXRalpha ligand binding domain |
Bioorg Med Chem Lett 20: 2903-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.031
BindingDB Entry DOI: 10.7270/Q2TD9XJZ |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-alpha
(Homo sapiens (Human)) | BDBM50317733

(3-methyl-4-(3'-(methylsulfonyl)biphenyl-3-yl)-8-(t...)Show SMILES Cc1cnc2c(cccc2c1-c1cccc(c1)-c1cccc(c1)S(C)(=O)=O)C(F)(F)F Show InChI InChI=1S/C24H18F3NO2S/c1-15-14-28-23-20(10-5-11-21(23)24(25,26)27)22(15)18-8-3-6-16(12-18)17-7-4-9-19(13-17)31(2,29)30/h3-14H,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [3H]T0901317 from LXRalpha ligand binding domain |
Bioorg Med Chem Lett 20: 2903-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.031
BindingDB Entry DOI: 10.7270/Q2TD9XJZ |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-beta
(Homo sapiens (Human)) | BDBM19993
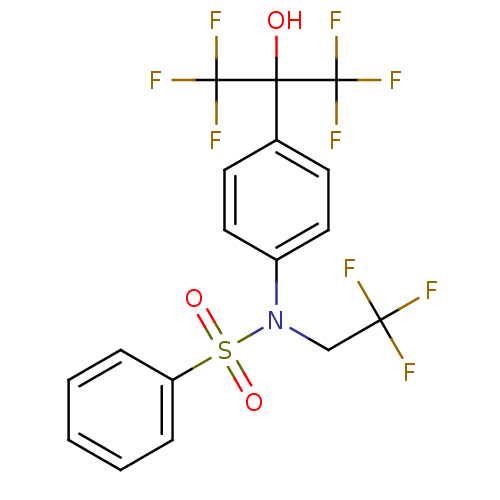
(CHEMBL62136 | N-[4-(1,1,1,3,3,3-hexafluoro-2-hydro...)Show SMILES OC(c1ccc(cc1)N(CC(F)(F)F)S(=O)(=O)c1ccccc1)(C(F)(F)F)C(F)(F)F Show InChI InChI=1S/C17H12F9NO3S/c18-14(19,20)10-27(31(29,30)13-4-2-1-3-5-13)12-8-6-11(7-9-12)15(28,16(21,22)23)17(24,25)26/h1-9,28H,10H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [3H]T0901317 from LXRbeta ligand binding domain |
Bioorg Med Chem Lett 20: 2903-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.031
BindingDB Entry DOI: 10.7270/Q2TD9XJZ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50365486
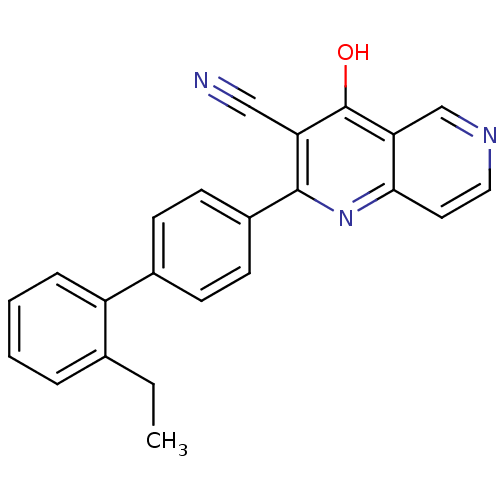
(CHEMBL1957374)Show InChI InChI=1S/C23H17N3O/c1-2-15-5-3-4-6-18(15)16-7-9-17(10-8-16)22-19(13-24)23(27)20-14-25-12-11-21(20)26-22/h3-12,14H,2H2,1H3,(H,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant full length PDE10A using cAMP as substrate preincubated for 20 mins measured after 4 hrs |
Bioorg Med Chem Lett 22: 1944-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.046
BindingDB Entry DOI: 10.7270/Q2KH0NTP |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-alpha [197-447]
(Homo sapiens (Human)) | BDBM20000

(2-(4-{3-[3-benzyl-8-(trifluoromethyl)quinolin-4-yl...)Show SMILES OC(=O)Cc1ccc(COc2cccc(c2)-c2c(Cc3ccccc3)cnc3c(cccc23)C(F)(F)F)cc1 Show InChI InChI=1S/C32H24F3NO3/c33-32(34,35)28-11-5-10-27-30(25(19-36-31(27)28)16-21-6-2-1-3-7-21)24-8-4-9-26(18-24)39-20-23-14-12-22(13-15-23)17-29(37)38/h1-15,18-19H,16-17,20H2,(H,37,38) | PDB
MMDB
Reactome pathway
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 9.5 | n/a | n/a | n/a | n/a | 7.4 | 4 |
Wyeth Research
| Assay Description
Binding reaction was initiated by adding a dilution series of test compound in tracer solution to LXR-coated flash plates. Each concentration of test... |
J Med Chem 49: 6151-4 (2006)
Article DOI: 10.1021/jm0609566
BindingDB Entry DOI: 10.7270/Q20863KH |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-beta [154-461]
(Homo sapiens (Human)) | BDBM19993

(CHEMBL62136 | N-[4-(1,1,1,3,3,3-hexafluoro-2-hydro...)Show SMILES OC(c1ccc(cc1)N(CC(F)(F)F)S(=O)(=O)c1ccccc1)(C(F)(F)F)C(F)(F)F Show InChI InChI=1S/C17H12F9NO3S/c18-14(19,20)10-27(31(29,30)13-4-2-1-3-5-13)12-8-6-11(7-9-12)15(28,16(21,22)23)17(24,25)26/h1-9,28H,10H2 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| n/a | n/a | 10 | n/a | 16 | n/a | n/a | 7.4 | 4 |
Wyeth Research
| Assay Description
Binding reaction was initiated by adding a dilution series of test compound in tracer solution to LXR-coated flash plates. Each concentration of test... |
J Med Chem 49: 6151-4 (2006)
Article DOI: 10.1021/jm0609566
BindingDB Entry DOI: 10.7270/Q20863KH |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Oxysterols receptor LXR-alpha [197-447]
(Homo sapiens (Human)) | BDBM19993

(CHEMBL62136 | N-[4-(1,1,1,3,3,3-hexafluoro-2-hydro...)Show SMILES OC(c1ccc(cc1)N(CC(F)(F)F)S(=O)(=O)c1ccccc1)(C(F)(F)F)C(F)(F)F Show InChI InChI=1S/C17H12F9NO3S/c18-14(19,20)10-27(31(29,30)13-4-2-1-3-5-13)12-8-6-11(7-9-12)15(28,16(21,22)23)17(24,25)26/h1-9,28H,10H2 | PDB
MMDB
Reactome pathway
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | 7.4 | 4 |
Wyeth Research
| Assay Description
Binding reaction was initiated by adding a dilution series of test compound in tracer solution to LXR-coated flash plates. Each concentration of test... |
J Med Chem 49: 6151-4 (2006)
Article DOI: 10.1021/jm0609566
BindingDB Entry DOI: 10.7270/Q20863KH |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Oxysterols receptor LXR-alpha
(Homo sapiens (Human)) | BDBM50317745

(4-(2',4'-difluoro-5'-(methylsulfonyl)biphenyl-3-yl...)Show SMILES Cc1cnc2c(cccc2c1-c1cccc(c1)-c1cc(c(F)cc1F)S(C)(=O)=O)C(F)(F)F Show InChI InChI=1S/C24H16F5NO2S/c1-13-12-30-23-16(7-4-8-18(23)24(27,28)29)22(13)15-6-3-5-14(9-15)17-10-21(33(2,31)32)20(26)11-19(17)25/h3-12H,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [3H]T0901317 from LXRalpha ligand binding domain |
Bioorg Med Chem Lett 20: 2903-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.031
BindingDB Entry DOI: 10.7270/Q2TD9XJZ |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-alpha
(Homo sapiens (Human)) | BDBM50317744

(4-(4'-fluoro-3'-(methylsulfonyl)biphenyl-3-yl)-3-m...)Show SMILES Cc1cnc2c(cccc2c1-c1cccc(c1)-c1ccc(F)c(c1)S(C)(=O)=O)C(F)(F)F Show InChI InChI=1S/C24H17F4NO2S/c1-14-13-29-23-18(7-4-8-19(23)24(26,27)28)22(14)17-6-3-5-15(11-17)16-9-10-20(25)21(12-16)32(2,30)31/h3-13H,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [3H]T0901317 from LXRalpha ligand binding domain |
Bioorg Med Chem Lett 20: 2903-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.031
BindingDB Entry DOI: 10.7270/Q2TD9XJZ |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-alpha
(Homo sapiens (Human)) | BDBM50317742

(3-methyl-4-(4'-methyl-3'-(methylsulfonyl)biphenyl-...)Show SMILES Cc1ccc(cc1S(C)(=O)=O)-c1cccc(c1)-c1c(C)cnc2c(cccc12)C(F)(F)F Show InChI InChI=1S/C25H20F3NO2S/c1-15-10-11-18(13-22(15)32(3,30)31)17-6-4-7-19(12-17)23-16(2)14-29-24-20(23)8-5-9-21(24)25(26,27)28/h4-14H,1-3H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [3H]T0901317 from LXRalpha ligand binding domain |
Bioorg Med Chem Lett 20: 2903-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.031
BindingDB Entry DOI: 10.7270/Q2TD9XJZ |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-beta [154-461]
(Homo sapiens (Human)) | BDBM19992

(2-[3-[3-[[2-chloro-3-(trifluoromethyl)phenyl]methy...)Show SMILES OC(=O)Cc1cccc(OCCCN(CC(c2ccccc2)c2ccccc2)Cc2cccc(c2Cl)C(F)(F)F)c1 Show InChI InChI=1S/C33H31ClF3NO3/c34-32-27(15-8-17-30(32)33(35,36)37)22-38(18-9-19-41-28-16-7-10-24(20-28)21-31(39)40)23-29(25-11-3-1-4-12-25)26-13-5-2-6-14-26/h1-8,10-17,20,29H,9,18-19,21-23H2,(H,39,40) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 12 | n/a | 410 | n/a | n/a | 7.4 | 4 |
Wyeth Research
| Assay Description
Binding reaction was initiated by adding a dilution series of test compound in tracer solution to LXR-coated flash plates. Each concentration of test... |
J Med Chem 49: 6151-4 (2006)
Article DOI: 10.1021/jm0609566
BindingDB Entry DOI: 10.7270/Q20863KH |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50365476
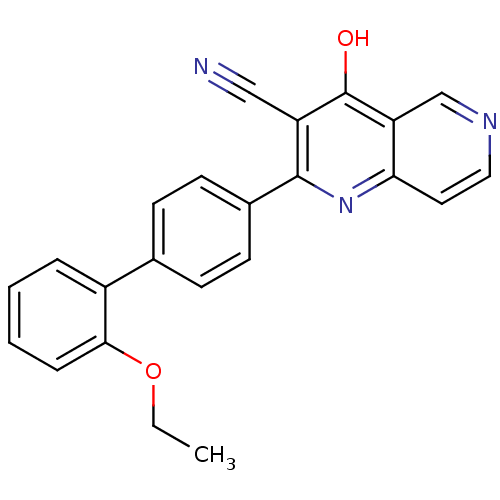
(CHEMBL1957361)Show SMILES CCOc1ccccc1-c1ccc(cc1)-c1nc2ccncc2c(O)c1C#N Show InChI InChI=1S/C23H17N3O2/c1-2-28-21-6-4-3-5-17(21)15-7-9-16(10-8-15)22-18(13-24)23(27)19-14-25-12-11-20(19)26-22/h3-12,14H,2H2,1H3,(H,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant full length PDE10A using cAMP as substrate preincubated for 20 mins measured after 4 hrs |
Bioorg Med Chem Lett 22: 1944-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.046
BindingDB Entry DOI: 10.7270/Q2KH0NTP |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50365479
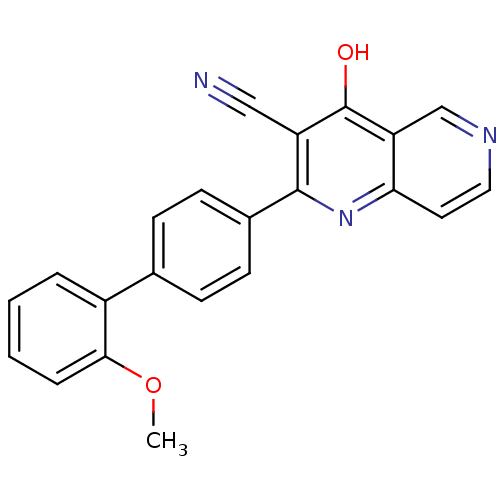
(CHEMBL1957364)Show InChI InChI=1S/C22H15N3O2/c1-27-20-5-3-2-4-16(20)14-6-8-15(9-7-14)21-17(12-23)22(26)18-13-24-11-10-19(18)25-21/h2-11,13H,1H3,(H,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant full length PDE10A using cAMP as substrate preincubated for 20 mins measured after 4 hrs |
Bioorg Med Chem Lett 22: 1944-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.046
BindingDB Entry DOI: 10.7270/Q2KH0NTP |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50365500
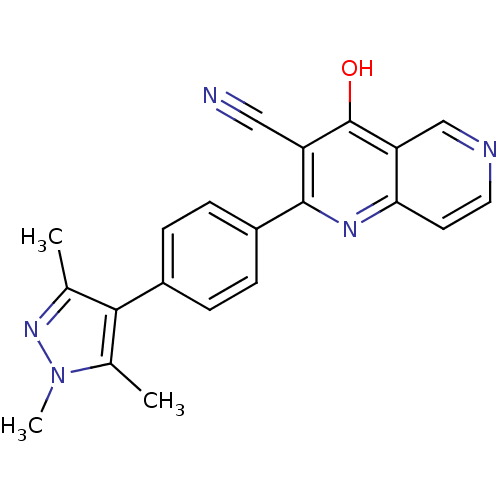
(CHEMBL1957462)Show SMILES Cc1nn(C)c(C)c1-c1ccc(cc1)-c1nc2ccncc2c(O)c1C#N Show InChI InChI=1S/C21H17N5O/c1-12-19(13(2)26(3)25-12)14-4-6-15(7-5-14)20-16(10-22)21(27)17-11-23-9-8-18(17)24-20/h4-9,11H,1-3H3,(H,24,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant full length PDE10A using cAMP as substrate preincubated for 20 mins measured after 4 hrs |
Bioorg Med Chem Lett 22: 1944-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.046
BindingDB Entry DOI: 10.7270/Q2KH0NTP |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-alpha
(Homo sapiens (Human)) | BDBM19993

(CHEMBL62136 | N-[4-(1,1,1,3,3,3-hexafluoro-2-hydro...)Show SMILES OC(c1ccc(cc1)N(CC(F)(F)F)S(=O)(=O)c1ccccc1)(C(F)(F)F)C(F)(F)F Show InChI InChI=1S/C17H12F9NO3S/c18-14(19,20)10-27(31(29,30)13-4-2-1-3-5-13)12-8-6-11(7-9-12)15(28,16(21,22)23)17(24,25)26/h1-9,28H,10H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [3H]T0901317 from LXRalpha ligand binding domain |
Bioorg Med Chem Lett 20: 2903-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.031
BindingDB Entry DOI: 10.7270/Q2TD9XJZ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Oxysterols receptor LXR-alpha [197-447]
(Homo sapiens (Human)) | BDBM19999

(2-(4-{3-[3-benzoyl-8-(trifluoromethyl)quinolin-4-y...)Show SMILES OC(=O)Cc1ccc(COc2cccc(c2)-c2c(cnc3c(cccc23)C(F)(F)F)C(=O)c2ccccc2)cc1 Show InChI InChI=1S/C32H22F3NO4/c33-32(34,35)27-11-5-10-25-29(26(18-36-30(25)27)31(39)22-6-2-1-3-7-22)23-8-4-9-24(17-23)40-19-21-14-12-20(13-15-21)16-28(37)38/h1-15,17-18H,16,19H2,(H,37,38) | PDB
MMDB
Reactome pathway
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 16.5 | n/a | n/a | n/a | n/a | 7.4 | 4 |
Wyeth Research
| Assay Description
Binding reaction was initiated by adding a dilution series of test compound in tracer solution to LXR-coated flash plates. Each concentration of test... |
J Med Chem 49: 6151-4 (2006)
Article DOI: 10.1021/jm0609566
BindingDB Entry DOI: 10.7270/Q20863KH |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50365485

(CHEMBL1957373)Show SMILES Oc1c(C#N)c(nc2ccncc12)-c1ccc(cc1)-c1ccccc1C#N Show InChI InChI=1S/C22H12N4O/c23-11-16-3-1-2-4-17(16)14-5-7-15(8-6-14)21-18(12-24)22(27)19-13-25-10-9-20(19)26-21/h1-10,13H,(H,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant full length PDE10A using cAMP as substrate preincubated for 20 mins measured after 4 hrs |
Bioorg Med Chem Lett 22: 1944-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.046
BindingDB Entry DOI: 10.7270/Q2KH0NTP |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50365482
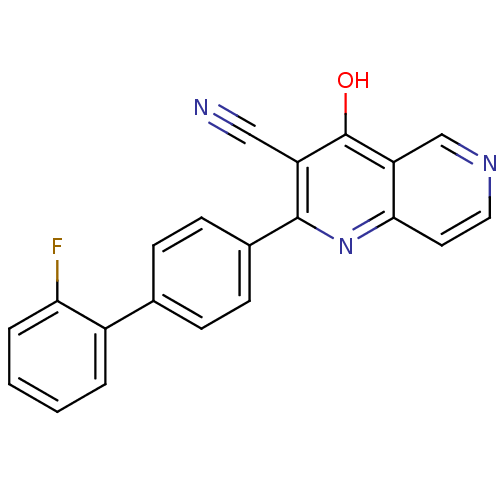
(CHEMBL1957368)Show SMILES Oc1c(C#N)c(nc2ccncc12)-c1ccc(cc1)-c1ccccc1F Show InChI InChI=1S/C21H12FN3O/c22-18-4-2-1-3-15(18)13-5-7-14(8-6-13)20-16(11-23)21(26)17-12-24-10-9-19(17)25-20/h1-10,12H,(H,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant full length PDE10A using cAMP as substrate preincubated for 20 mins measured after 4 hrs |
Bioorg Med Chem Lett 22: 1944-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.046
BindingDB Entry DOI: 10.7270/Q2KH0NTP |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-alpha
(Homo sapiens (Human)) | BDBM50317747

(4-(2',4'-difluoro-5'-(methylsulfonyl)biphenyl-3-yl...)Show SMILES CS(=O)(=O)c1cc(c(F)cc1F)-c1cccc(c1)-c1ccnc2c(cccc12)C(F)(F)F Show InChI InChI=1S/C23H14F5NO2S/c1-32(30,31)21-11-17(19(24)12-20(21)25)14-5-2-4-13(10-14)15-8-9-29-22-16(15)6-3-7-18(22)23(26,27)28/h2-12H,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [3H]T0901317 from LXRalpha ligand binding domain |
Bioorg Med Chem Lett 20: 2903-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.031
BindingDB Entry DOI: 10.7270/Q2TD9XJZ |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50365494
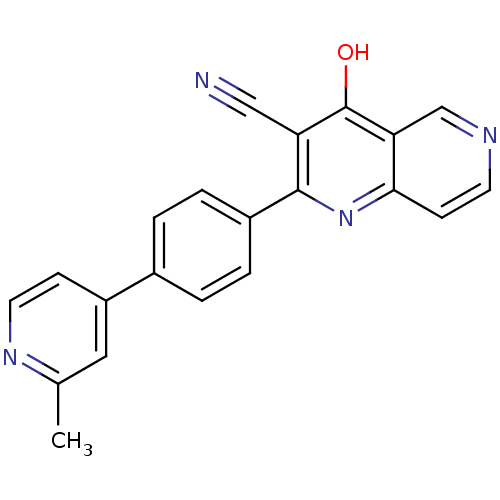
(CHEMBL1957381)Show SMILES Cc1cc(ccn1)-c1ccc(cc1)-c1nc2ccncc2c(O)c1C#N Show InChI InChI=1S/C21H14N4O/c1-13-10-16(6-9-24-13)14-2-4-15(5-3-14)20-17(11-22)21(26)18-12-23-8-7-19(18)25-20/h2-10,12H,1H3,(H,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant full length PDE10A using cAMP as substrate preincubated for 20 mins measured after 4 hrs |
Bioorg Med Chem Lett 22: 1944-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.046
BindingDB Entry DOI: 10.7270/Q2KH0NTP |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50365496

(CHEMBL1957370)Show SMILES Oc1c(C#N)c(nc2ccncc12)-c1ccc(cc1)-c1ccncc1Cl Show InChI InChI=1S/C20H11ClN4O/c21-17-11-24-7-5-14(17)12-1-3-13(4-2-12)19-15(9-22)20(26)16-10-23-8-6-18(16)25-19/h1-8,10-11H,(H,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant full length PDE10A using cAMP as substrate preincubated for 20 mins measured after 4 hrs |
Bioorg Med Chem Lett 22: 1944-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.046
BindingDB Entry DOI: 10.7270/Q2KH0NTP |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-alpha
(Homo sapiens (Human)) | BDBM50317746

(4-(4'-fluoro-3'-(methylsulfonyl)biphenyl-3-yl)-8-(...)Show SMILES CS(=O)(=O)c1cc(ccc1F)-c1cccc(c1)-c1ccnc2c(cccc12)C(F)(F)F Show InChI InChI=1S/C23H15F4NO2S/c1-31(29,30)21-13-15(8-9-20(21)24)14-4-2-5-16(12-14)17-10-11-28-22-18(17)6-3-7-19(22)23(25,26)27/h2-13H,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [3H]T0901317 from LXRalpha ligand binding domain |
Bioorg Med Chem Lett 20: 2903-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.031
BindingDB Entry DOI: 10.7270/Q2TD9XJZ |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-beta
(Homo sapiens (Human)) | BDBM50317740

(4-(3'-(ethylsulfonyl)biphenyl-3-yl)-8-(trifluorome...)Show SMILES CCS(=O)(=O)c1cccc(c1)-c1cccc(c1)-c1ccnc2c(cccc12)C(F)(F)F Show InChI InChI=1S/C24H18F3NO2S/c1-2-31(29,30)19-9-4-7-17(15-19)16-6-3-8-18(14-16)20-12-13-28-23-21(20)10-5-11-22(23)24(25,26)27/h3-15H,2H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [3H]T0901317 from LXRbeta ligand binding domain |
Bioorg Med Chem Lett 20: 2903-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.031
BindingDB Entry DOI: 10.7270/Q2TD9XJZ |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data