

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Prothrombin (Homo sapiens (Human)) | BDBM50123490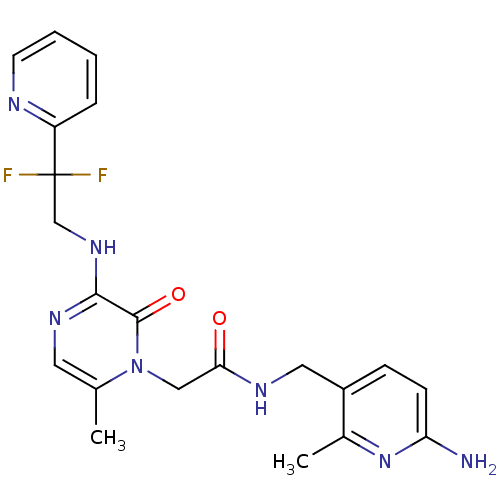 (CHEMBL143418 | N-(6-Amino-2-methyl-pyridin-3-ylmet...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory constant against thrombin (IIa) | J Med Chem 46: 461-73 (2003) Article DOI: 10.1021/jm020311f BindingDB Entry DOI: 10.7270/Q2W958J5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50123504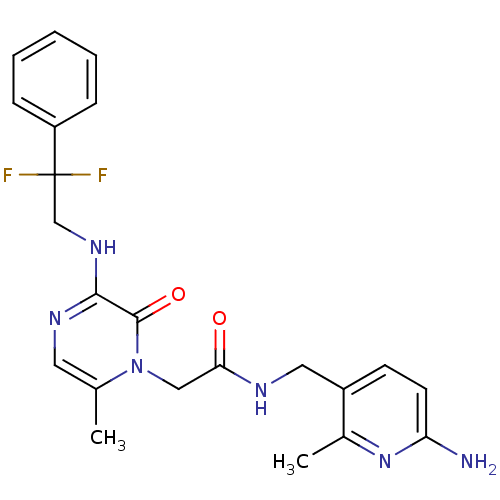 (CHEMBL142546 | N-((6-amino-2-methylpyridin-3-yl)me...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory constant against thrombin (IIa) | J Med Chem 46: 461-73 (2003) Article DOI: 10.1021/jm020311f BindingDB Entry DOI: 10.7270/Q2W958J5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50230322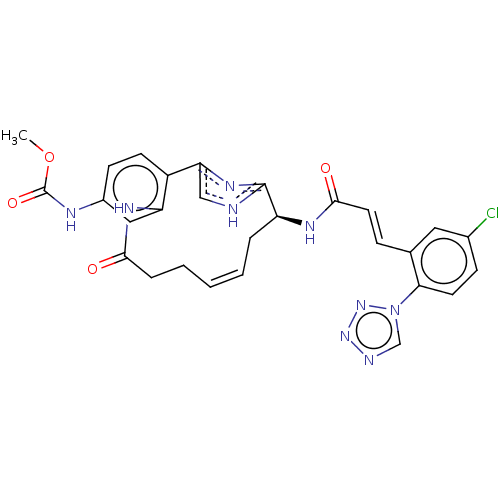 (CHEMBL4071545) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of activated human coagulation factor 11 using pyroGlu-Pro-Arg-pNA as substrate incubated for 10 to 120 mins by spectrofluorometric method | Bioorg Med Chem Lett 29: (2019) Article DOI: 10.1016/j.bmcl.2019.08.008 BindingDB Entry DOI: 10.7270/Q23F4T2C | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50230322 (CHEMBL4071545) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of recombinant human activated coagulation factor XI using pyro-Glu-Pro-Arg-pNA as substrate by spectrophotometry | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2020.126949 BindingDB Entry DOI: 10.7270/Q24Q7ZJH | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Alpha-1A adrenergic receptor (Homo sapiens (Human)) | BDBM50090647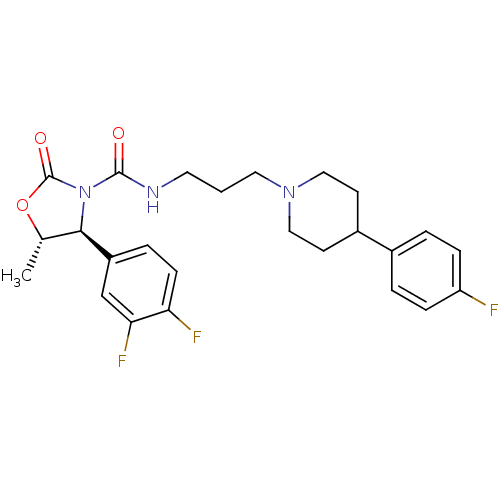 ((4S,5S)-4-(3,4-Difluoro-phenyl)-5-methyl-2-oxo-oxa...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Synaptic Pharmaceutical Corporation Curated by ChEMBL | Assay Description Binding affinity of the compound towards Alpha1A human adrenergic receptors, using [125I]-HEAT as radioligand. | J Med Chem 43: 2775-8 (2000) BindingDB Entry DOI: 10.7270/Q2PZ582K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM142872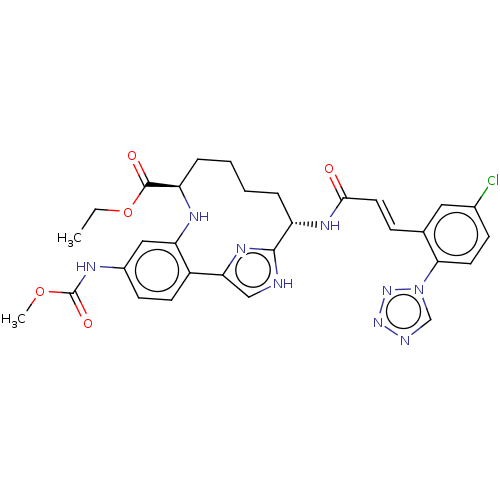 (US8940720, I-67) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of recombinant human activated coagulation factor XI using pyro-Glu-Pro-Arg-pNA as substrate by spectrophotometry | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2020.126949 BindingDB Entry DOI: 10.7270/Q24Q7ZJH | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Alpha-1A adrenergic receptor (Homo sapiens (Human)) | BDBM50090647 ((4S,5S)-4-(3,4-Difluoro-phenyl)-5-methyl-2-oxo-oxa...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Synaptic Pharmaceutical Corporation Curated by ChEMBL | Assay Description Binding affinity towards Alpha1A dog adrenergic receptors, using [125I]HEAT as radioligand. | J Med Chem 43: 2775-8 (2000) BindingDB Entry DOI: 10.7270/Q2PZ582K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50541586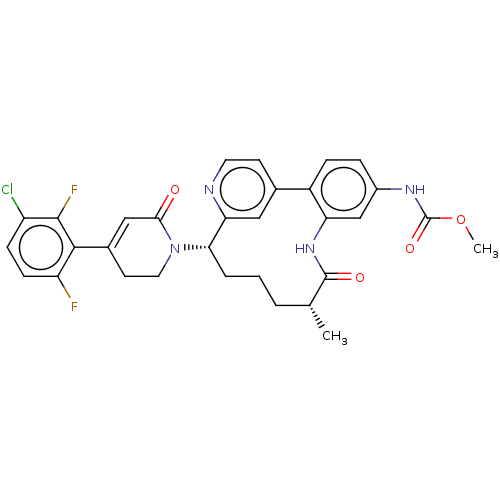 (CHEMBL4638245) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB Article PubMed | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of human factor11a using pyro-Glu-Pro-Arg-pNA(para-nitroaniline) substrate by spectrophotometry | J Med Chem 63: 7226-7242 (2020) Article DOI: 10.1021/acs.jmedchem.0c00464 BindingDB Entry DOI: 10.7270/Q2J67MHG | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50525762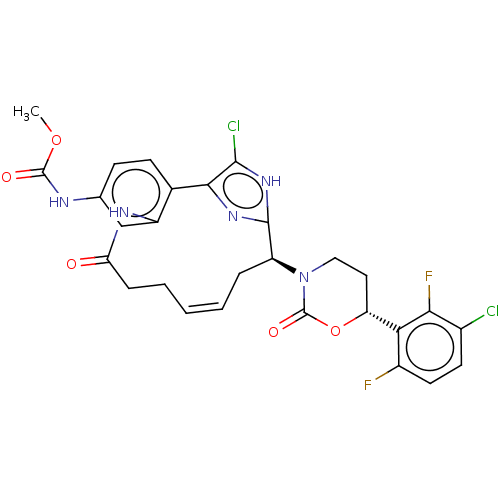 (CHEMBL4467360) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of activated human coagulation factor 11 using pyroGlu-Pro-Arg-pNA as substrate incubated for 10 to 120 mins by spectrofluorometric method | Bioorg Med Chem Lett 29: (2019) Article DOI: 10.1016/j.bmcl.2019.08.008 BindingDB Entry DOI: 10.7270/Q23F4T2C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50150298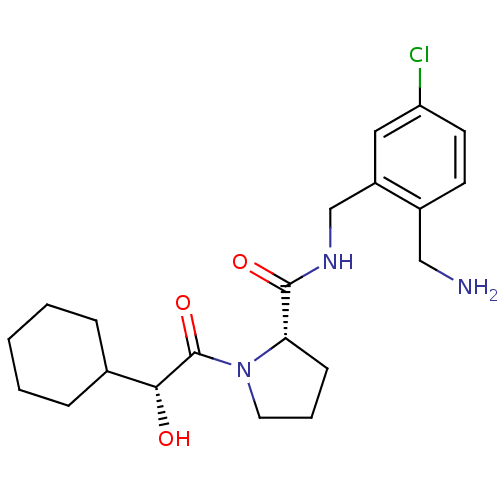 ((S)-1-((R)-2-Cyclohexyl-2-hydroxy-acetyl)-pyrrolid...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 0.270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition constant against human Thrombin | Bioorg Med Chem Lett 14: 4161-4 (2004) Article DOI: 10.1016/j.bmcl.2004.06.030 BindingDB Entry DOI: 10.7270/Q2833RH3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50123496 (CHEMBL143138 | N-(6-Amino-2-methyl-pyridin-3-ylmet...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory constant against thrombin (IIa) | J Med Chem 46: 461-73 (2003) Article DOI: 10.1021/jm020311f BindingDB Entry DOI: 10.7270/Q2W958J5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50539655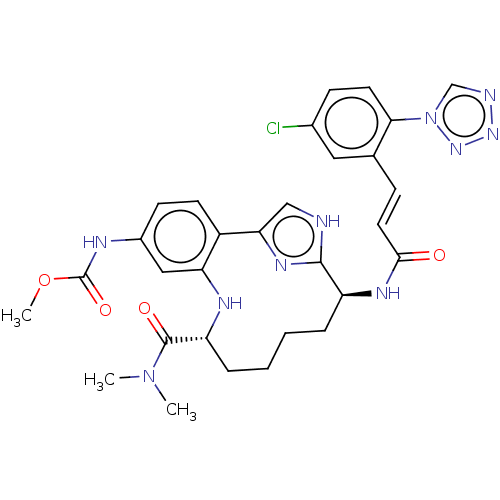 (CHEMBL4632633) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of recombinant human activated coagulation factor XI using pyro-Glu-Pro-Arg-pNA as substrate by spectrophotometry | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2020.126949 BindingDB Entry DOI: 10.7270/Q24Q7ZJH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B1 bradykinin receptor (Homo sapiens (Human)) | BDBM50371333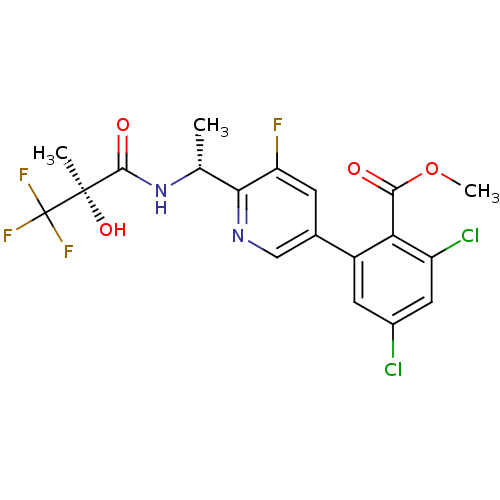 (CHEMBL256671) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity to human bradikinin B1 receptor expressed in CHO cells | Bioorg Med Chem Lett 18: 716-20 (2008) Article DOI: 10.1016/j.bmcl.2007.11.050 BindingDB Entry DOI: 10.7270/Q2GM8849 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (Rattus norvegicus (Rat)) | BDBM50090647 ((4S,5S)-4-(3,4-Difluoro-phenyl)-5-methyl-2-oxo-oxa...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Synaptic Pharmaceutical Corporation Curated by ChEMBL | Assay Description Binding affinity towards Alpha1A rat adrenergic receptors, using [125I]HEAT as radioligand. | J Med Chem 43: 2775-8 (2000) BindingDB Entry DOI: 10.7270/Q2PZ582K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM335990 (4-(2-(3-(3-chlorophenyl)- 4,5-dihydroisoxazole-5- ...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description Plasma kallikrein determinations were made in 0.1 M sodium phosphate buffer at a pH of 7.5 containing 0.1-0.2 M sodium chloride and 0.5% PEG 8000. De... | US Patent US9738655 (2017) BindingDB Entry DOI: 10.7270/Q2BG2R3R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50123486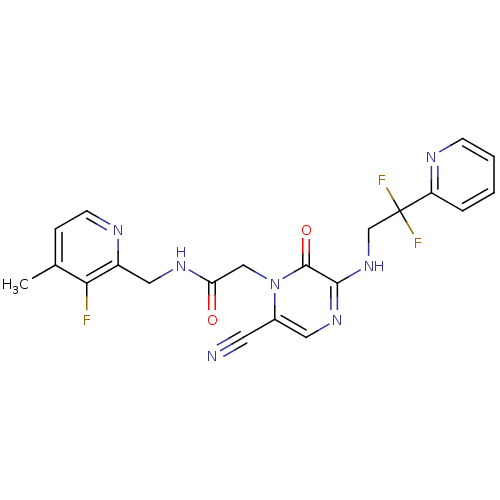 (2-[6-Cyano-3-(2,2-difluoro-2-pyridin-2-yl-ethylami...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory constant against thrombin (IIa) | J Med Chem 46: 461-73 (2003) Article DOI: 10.1021/jm020311f BindingDB Entry DOI: 10.7270/Q2W958J5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B1 bradykinin receptor (Homo sapiens (Human)) | BDBM50371320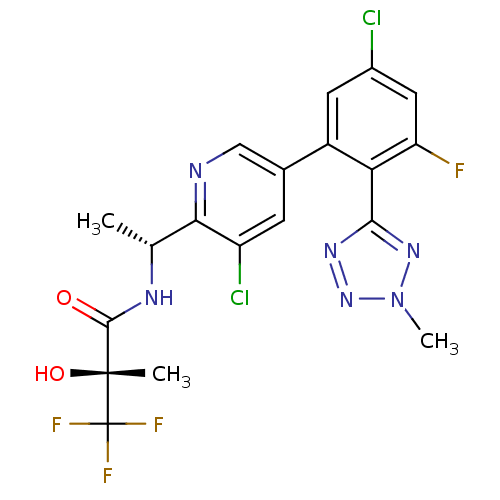 (CHEMBL271283) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity to human bradikinin B1 receptor expressed in CHO cells | Bioorg Med Chem Lett 18: 716-20 (2008) Article DOI: 10.1016/j.bmcl.2007.11.050 BindingDB Entry DOI: 10.7270/Q2GM8849 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50539654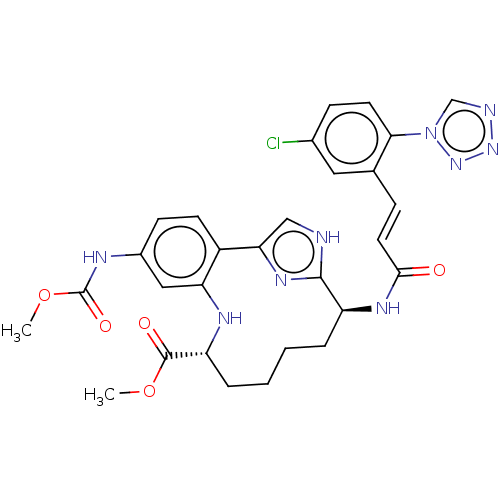 (CHEMBL4640111) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of recombinant human activated coagulation factor XI using pyro-Glu-Pro-Arg-pNA as substrate by spectrophotometry | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2020.126949 BindingDB Entry DOI: 10.7270/Q24Q7ZJH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM335779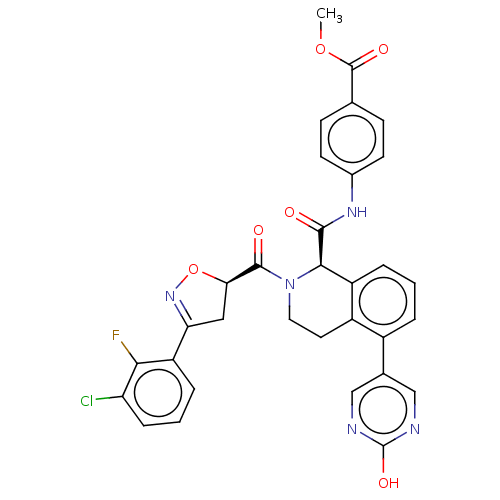 (4-(2-(1-(3-chloro-2- fluorophenyl)-1H-1,2,3- triaz...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description Plasma kallikrein determinations were made in 0.1 M sodium phosphate buffer at a pH of 7.5 containing 0.1-0.2 M sodium chloride and 0.5% PEG 8000. De... | US Patent US9738655 (2017) BindingDB Entry DOI: 10.7270/Q2BG2R3R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50164264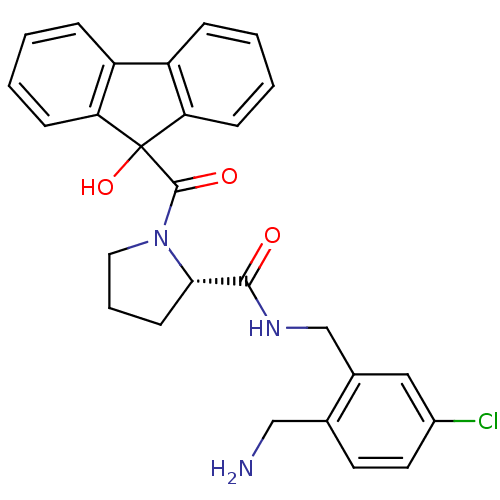 ((S)-1-(9-Hydroxy-9H-fluorene-9-carbonyl)-pyrrolidi...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.490 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of coagulation factor II (thrombin) of human | J Med Chem 48: 2282-93 (2005) Article DOI: 10.1021/jm049423s BindingDB Entry DOI: 10.7270/Q2BR8RPD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50541581 (CHEMBL4646341) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of human factor11a using pyro-Glu-Pro-Arg-pNA(para-nitroaniline) substrate by spectrophotometry | J Med Chem 63: 7226-7242 (2020) Article DOI: 10.1021/acs.jmedchem.0c00464 BindingDB Entry DOI: 10.7270/Q2J67MHG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50150297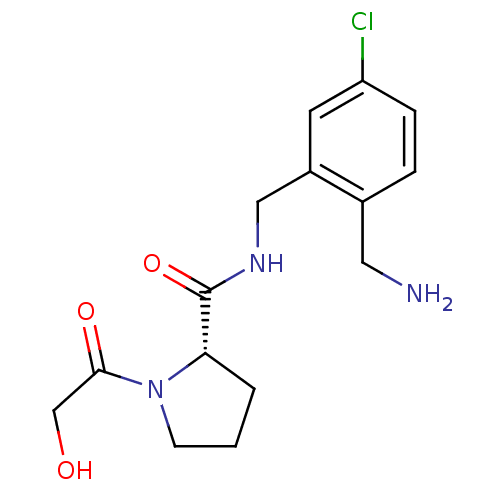 ((S)-1-(2-Hydroxy-acetyl)-pyrrolidine-2-carboxylic ...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition constant against human Thrombin | Bioorg Med Chem Lett 14: 4161-4 (2004) Article DOI: 10.1016/j.bmcl.2004.06.030 BindingDB Entry DOI: 10.7270/Q2833RH3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM335984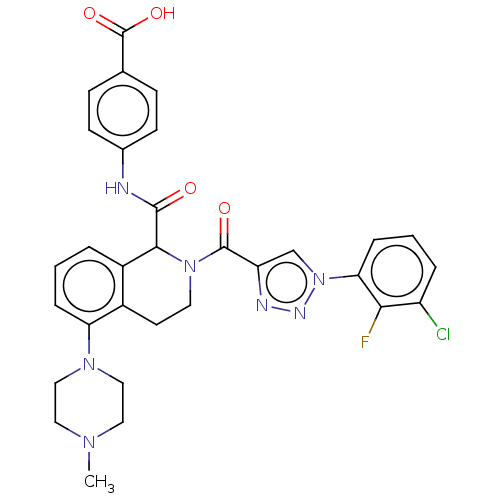 (4-(2-(1-(3-chloro-2- fluorophenyl)-1H-1,2,3- triaz...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.520 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description Plasma kallikrein determinations were made in 0.1 M sodium phosphate buffer at a pH of 7.5 containing 0.1-0.2 M sodium chloride and 0.5% PEG 8000. De... | US Patent US9738655 (2017) BindingDB Entry DOI: 10.7270/Q2BG2R3R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50123479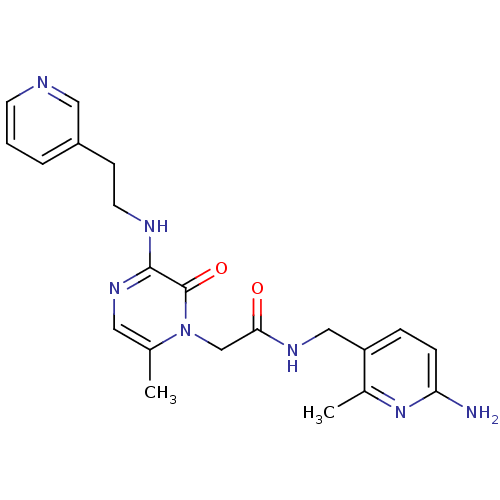 (CHEMBL143008 | N-(6-Amino-2-methyl-pyridin-3-ylmet...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.540 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory constant against thrombin (IIa) | J Med Chem 46: 461-73 (2003) Article DOI: 10.1021/jm020311f BindingDB Entry DOI: 10.7270/Q2W958J5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50164256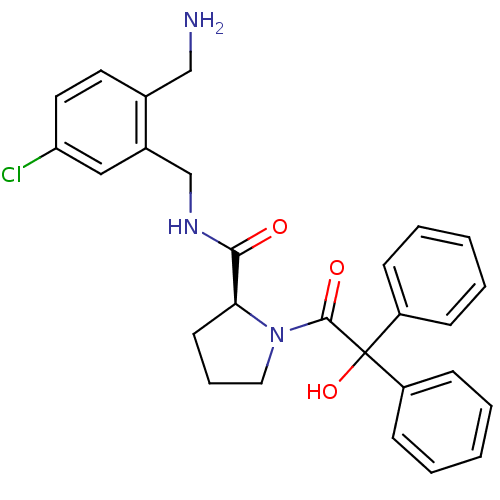 ((S)-1-(2-Hydroxy-2,2-diphenyl-acetyl)-pyrrolidine-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.550 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of coagulation factor II (thrombin) of human | J Med Chem 48: 2282-93 (2005) Article DOI: 10.1021/jm049423s BindingDB Entry DOI: 10.7270/Q2BR8RPD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B1 bradykinin receptor (Homo sapiens (Human)) | BDBM50371332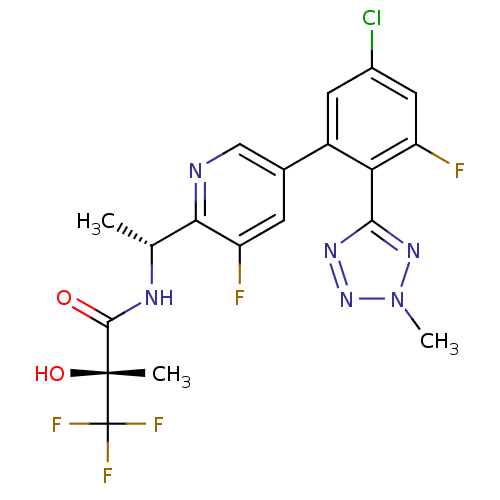 (CHEMBL258324) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.590 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity to human bradikinin B1 receptor expressed in CHO cells | Bioorg Med Chem Lett 18: 716-20 (2008) Article DOI: 10.1016/j.bmcl.2007.11.050 BindingDB Entry DOI: 10.7270/Q2GM8849 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50541577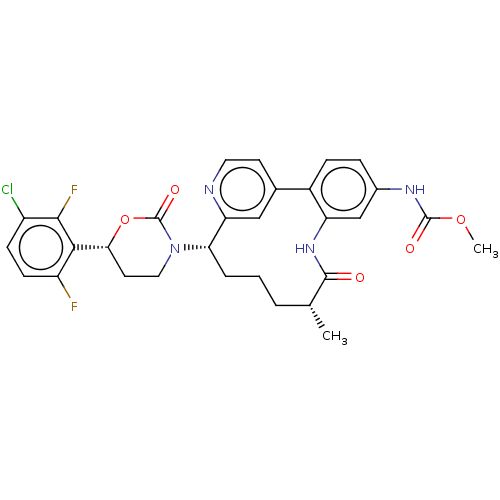 (CHEMBL4646441) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of human factor11a using pyro-Glu-Pro-Arg-pNA(para-nitroaniline) substrate by spectrophotometry | J Med Chem 63: 7226-7242 (2020) Article DOI: 10.1021/acs.jmedchem.0c00464 BindingDB Entry DOI: 10.7270/Q2J67MHG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM142873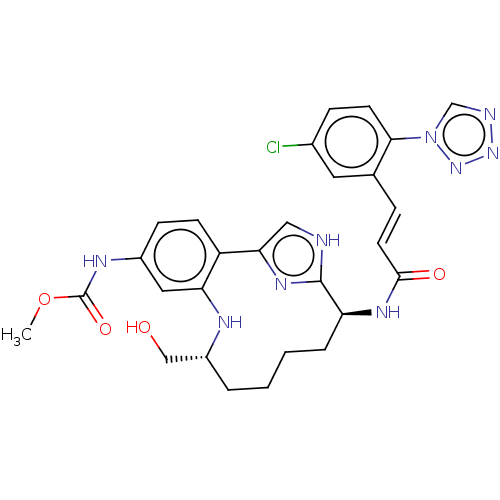 (US10487086, Example I-77 | US11136327, Example I-7...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.610 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of recombinant human activated coagulation factor XI using pyro-Glu-Pro-Arg-pNA as substrate by spectrophotometry | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2020.126949 BindingDB Entry DOI: 10.7270/Q24Q7ZJH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM335977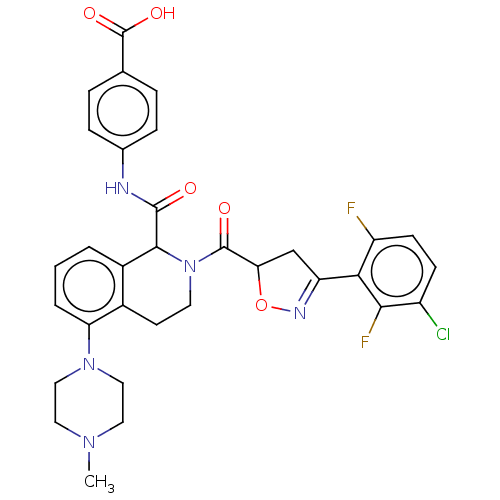 (4-(2-(3-(3-chloro-2,6- difluorophenyl)-4,5- dihydr...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.640 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description Plasma kallikrein determinations were made in 0.1 M sodium phosphate buffer at a pH of 7.5 containing 0.1-0.2 M sodium chloride and 0.5% PEG 8000. De... | US Patent US9738655 (2017) BindingDB Entry DOI: 10.7270/Q2BG2R3R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B1 bradykinin receptor (Homo sapiens (Human)) | BDBM50229295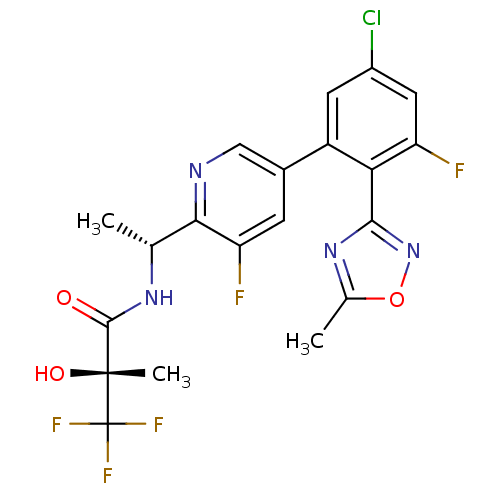 ((R)-N-((R)-1-(5-(5-chloro-3-fluoro-2-(5-methyl-1,2...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.660 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity to human bradikinin B1 receptor expressed in CHO cells | Bioorg Med Chem Lett 18: 716-20 (2008) Article DOI: 10.1016/j.bmcl.2007.11.050 BindingDB Entry DOI: 10.7270/Q2GM8849 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B1 bradykinin receptor (Homo sapiens (Human)) | BDBM50371321 (CHEMBL258326) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.660 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity to human bradikinin B1 receptor expressed in CHO cells | Bioorg Med Chem Lett 18: 716-20 (2008) Article DOI: 10.1016/j.bmcl.2007.11.050 BindingDB Entry DOI: 10.7270/Q2GM8849 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM420081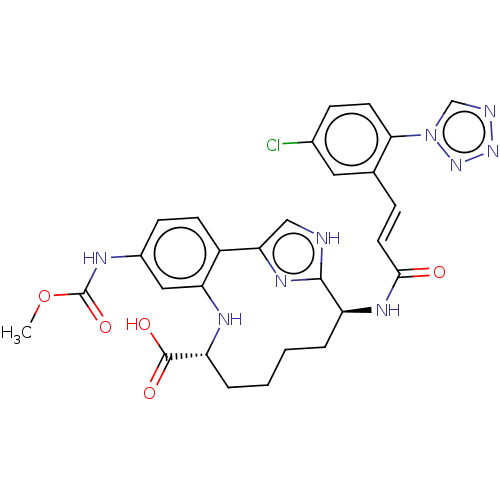 (US10487086, Example I-84) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | UniChem | Article PubMed | 0.670 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of recombinant human activated coagulation factor XI using pyro-Glu-Pro-Arg-pNA as substrate by spectrophotometry | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2020.126949 BindingDB Entry DOI: 10.7270/Q24Q7ZJH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50541576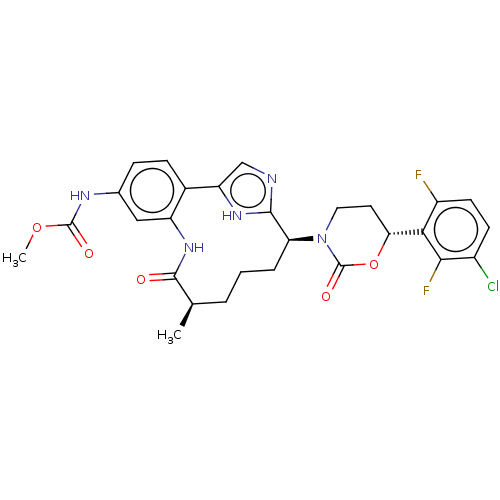 (CHEMBL4644510) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of human factor11a using pyro-Glu-Pro-Arg-pNA(para-nitroaniline) substrate by spectrophotometry | J Med Chem 63: 7226-7242 (2020) Article DOI: 10.1021/acs.jmedchem.0c00464 BindingDB Entry DOI: 10.7270/Q2J67MHG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B1 bradykinin receptor (Homo sapiens (Human)) | BDBM50371322 (CHEMBL257729) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.790 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity to human bradikinin B1 receptor expressed in CHO cells | Bioorg Med Chem Lett 18: 716-20 (2008) Article DOI: 10.1016/j.bmcl.2007.11.050 BindingDB Entry DOI: 10.7270/Q2GM8849 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50067797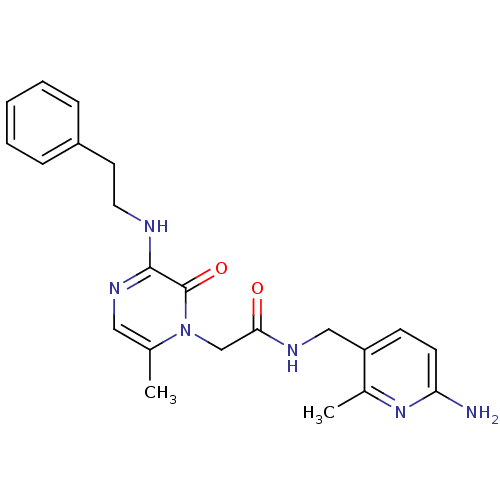 (CHEMBL19080 | L-37378 | N-(6-Amino-2-methyl-pyridi...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory constant against thrombin (IIa) | J Med Chem 46: 461-73 (2003) Article DOI: 10.1021/jm020311f BindingDB Entry DOI: 10.7270/Q2W958J5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50123497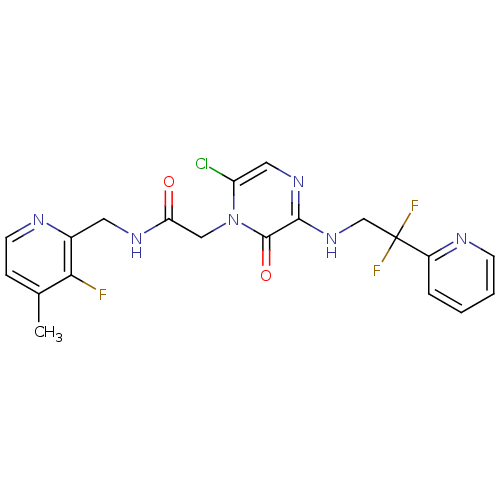 (2-[6-Chloro-3-(2,2-difluoro-2-pyridin-2-yl-ethylam...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory constant against thrombin (IIa) | J Med Chem 46: 461-73 (2003) Article DOI: 10.1021/jm020311f BindingDB Entry DOI: 10.7270/Q2W958J5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50150299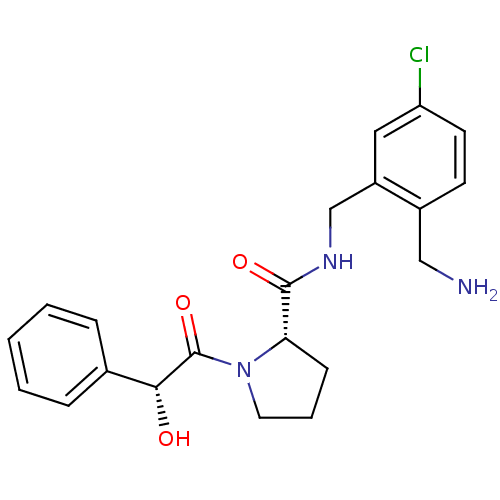 ((S)-1-((R)-2-Hydroxy-2-phenyl-acetyl)-pyrrolidine-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition constant against human Thrombin | Bioorg Med Chem Lett 14: 4161-4 (2004) Article DOI: 10.1016/j.bmcl.2004.06.030 BindingDB Entry DOI: 10.7270/Q2833RH3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50541582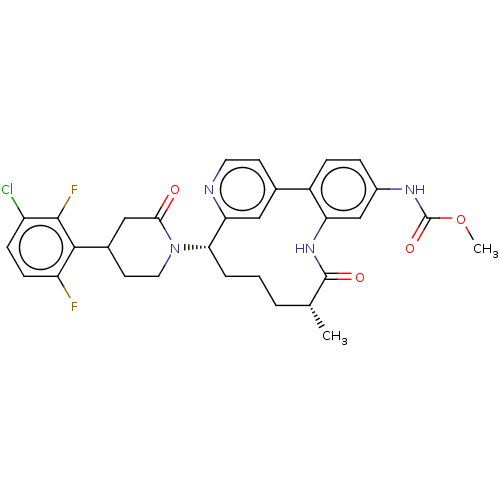 (CHEMBL4636247) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of human factor11a using pyro-Glu-Pro-Arg-pNA(para-nitroaniline) substrate by spectrophotometry | J Med Chem 63: 7226-7242 (2020) Article DOI: 10.1021/acs.jmedchem.0c00464 BindingDB Entry DOI: 10.7270/Q2J67MHG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM335801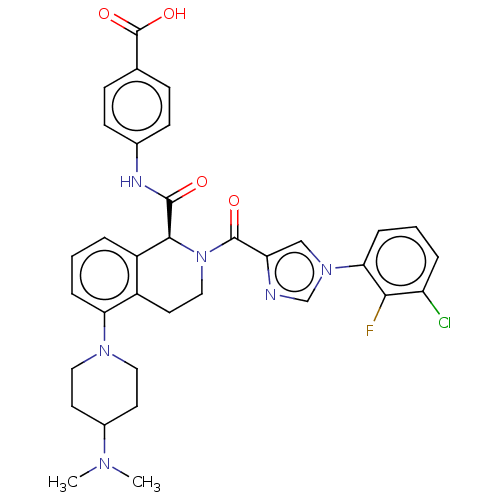 ((S)-4-(2-(1-(3-chloro-2- fluorophenyl)-1H-imidazol...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.920 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description Plasma kallikrein determinations were made in 0.1 M sodium phosphate buffer at a pH of 7.5 containing 0.1-0.2 M sodium chloride and 0.5% PEG 8000. De... | US Patent US9738655 (2017) BindingDB Entry DOI: 10.7270/Q2BG2R3R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50525762 (CHEMBL4467360) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.950 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of activated human coagulation factor 11 using pyroGlu-Pro-Arg-pNA as substrate incubated for 10 to 120 mins by spectrofluorometric method | Bioorg Med Chem Lett 29: (2019) Article DOI: 10.1016/j.bmcl.2019.08.008 BindingDB Entry DOI: 10.7270/Q23F4T2C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM335873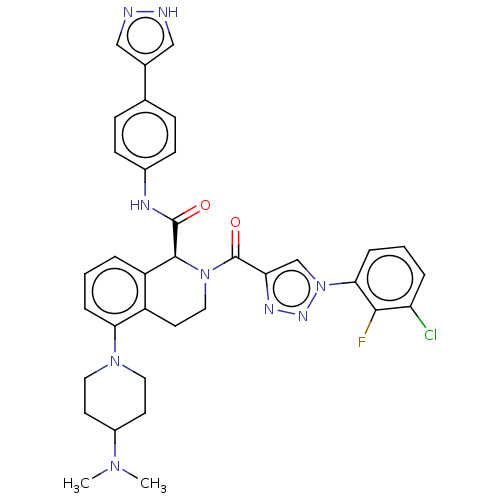 ((S)-N-(4-(1H-Pyrazol-4-yl)phenyl)-2-(1-(3-chloro-2...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.960 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description Plasma kallikrein determinations were made in 0.1 M sodium phosphate buffer at a pH of 7.5 containing 0.1-0.2 M sodium chloride and 0.5% PEG 8000. De... | US Patent US9738655 (2017) BindingDB Entry DOI: 10.7270/Q2BG2R3R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM47108 (US10487086, Example 10 | US11136327, Example 10 | ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of recombinant human activated coagulation factor XI using pyro-Glu-Pro-Arg-pNA as substrate by spectrophotometry | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2020.126949 BindingDB Entry DOI: 10.7270/Q24Q7ZJH | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50123503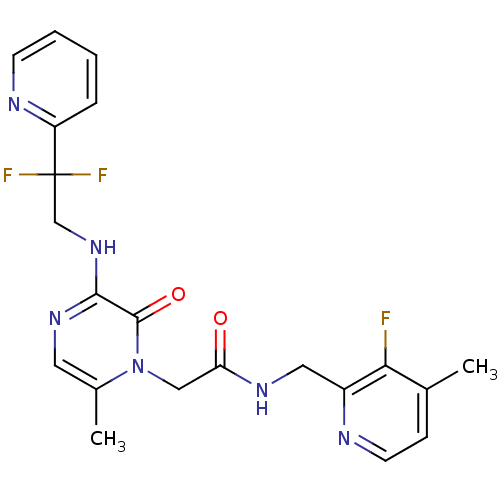 (2-[3-(2,2-Difluoro-2-pyridin-2-yl-ethylamino)-6-me...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid UniChem Patents Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory constant against thrombin (IIa) | J Med Chem 46: 461-73 (2003) Article DOI: 10.1021/jm020311f BindingDB Entry DOI: 10.7270/Q2W958J5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM335755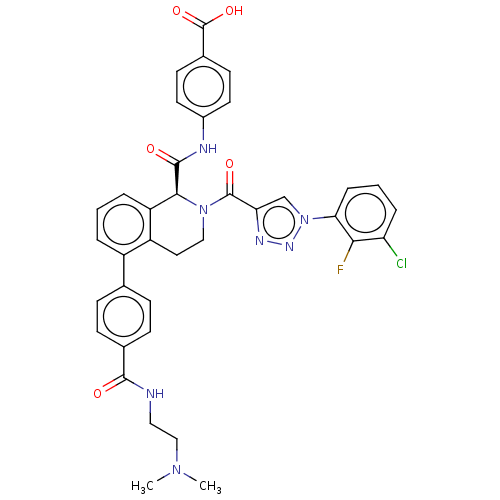 ((S)-4-(2-(1-(3-Chloro-2-fluorophenyl)-1H-1,2,3-tri...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1.08 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description Plasma kallikrein determinations were made in 0.1 M sodium phosphate buffer at a pH of 7.5 containing 0.1-0.2 M sodium chloride and 0.5% PEG 8000. De... | US Patent US9738655 (2017) BindingDB Entry DOI: 10.7270/Q2BG2R3R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50164260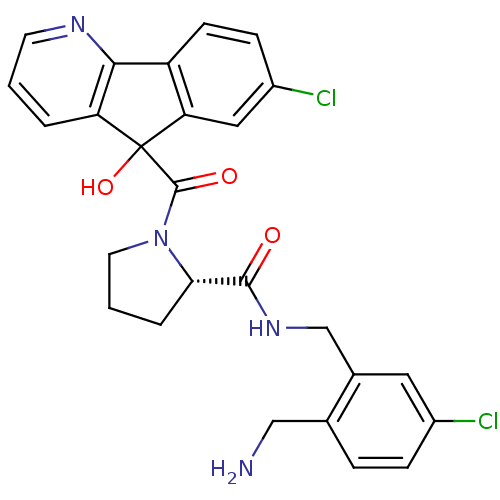 ((S)-1-(7-Chloro-5-hydroxy-5H-indeno[1,2-b]pyridine...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of coagulation factor II (thrombin) of human | J Med Chem 48: 2282-93 (2005) Article DOI: 10.1021/jm049423s BindingDB Entry DOI: 10.7270/Q2BR8RPD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50539651 (CHEMBL4647704) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of recombinant human activated coagulation factor XI using pyro-Glu-Pro-Arg-pNA as substrate by spectrophotometry | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2020.126949 BindingDB Entry DOI: 10.7270/Q24Q7ZJH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50123500 (CHEMBL143139 | N-(6-Amino-2-methyl-pyridin-3-ylmet...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory constant against thrombin (IIa) | J Med Chem 46: 461-73 (2003) Article DOI: 10.1021/jm020311f BindingDB Entry DOI: 10.7270/Q2W958J5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50123493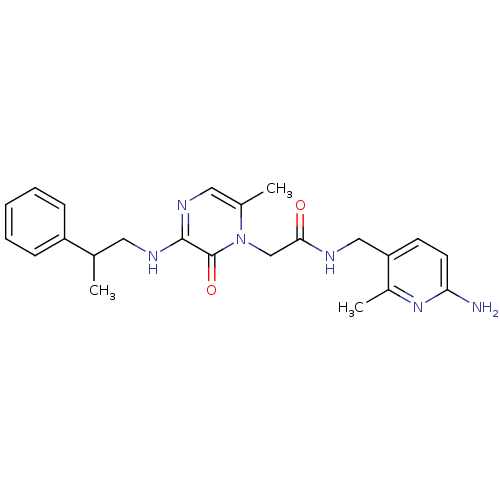 (CHEMBL142566 | N-(6-Amino-2-methyl-pyridin-3-ylmet...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory constant against thrombin (IIa) | J Med Chem 46: 461-73 (2003) Article DOI: 10.1021/jm020311f BindingDB Entry DOI: 10.7270/Q2W958J5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50164260 ((S)-1-(7-Chloro-5-hydroxy-5H-indeno[1,2-b]pyridine...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of coagulation factor II (thrombin) of human | J Med Chem 48: 2282-93 (2005) Article DOI: 10.1021/jm049423s BindingDB Entry DOI: 10.7270/Q2BR8RPD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM335974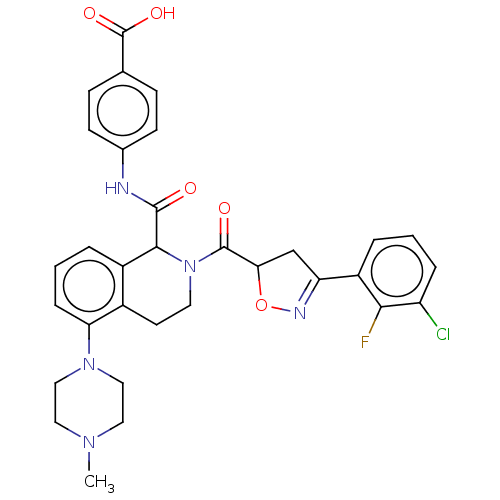 (4-(2-(3-(3-chloro-2- fluorophenyl)-4,5- dihydroiso...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1.11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description Plasma kallikrein determinations were made in 0.1 M sodium phosphate buffer at a pH of 7.5 containing 0.1-0.2 M sodium chloride and 0.5% PEG 8000. De... | US Patent US9738655 (2017) BindingDB Entry DOI: 10.7270/Q2BG2R3R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 6525 total ) | Next | Last >> |