

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pepsin A (Porcine) | BDBM50012623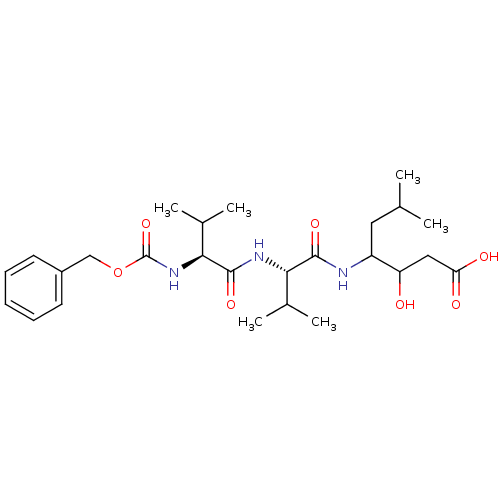 (3-hydroxy-6-methyl-4-[2-methyl-1-[2-methyl-1-benzy...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 0 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arkansas Curated by ChEMBL | Assay Description Compound was measured for the apparent inhibition constant at pepsin | J Med Chem 34: 2298-300 (1991) BindingDB Entry DOI: 10.7270/Q28G8JPJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pepsin A (Porcine) | BDBM50012626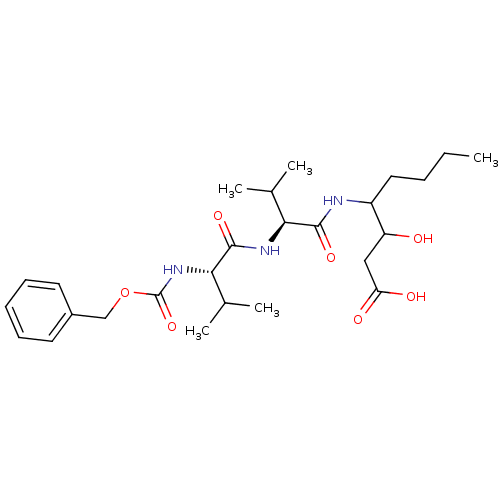 (3-hydroxy-4-[2-methyl-1-[2-methyl-1-benzyloxycarbo...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 0 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arkansas Curated by ChEMBL | Assay Description Compound was measured for the apparent inhibition constant at pepsin | J Med Chem 34: 2298-300 (1991) BindingDB Entry DOI: 10.7270/Q28G8JPJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pepsin A (Porcine) | BDBM912 ((3S,4S)-3-hydroxy-4-[(2S)-2-[(3S,4S)-3-hydroxy-6-m...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PubMed | 0 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arkansas Curated by ChEMBL | Assay Description Compound was measured for the inhibition of pepsin hydrolysis of hemoglobin. | J Med Chem 34: 2298-300 (1991) BindingDB Entry DOI: 10.7270/Q28G8JPJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pepsin A (Porcine) | BDBM50012624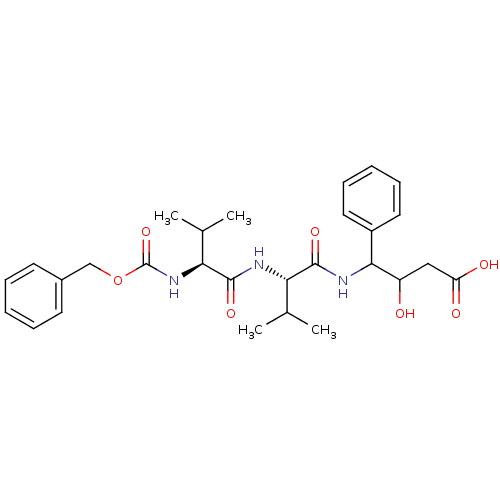 (3-hydroxy-4-[2-methyl-1-[2-methyl-1-benzyloxycarbo...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 0 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arkansas Curated by ChEMBL | Assay Description Compound was measured for the apparent inhibition constant at pepsin | J Med Chem 34: 2298-300 (1991) BindingDB Entry DOI: 10.7270/Q28G8JPJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pepsin A (Porcine) | BDBM50012622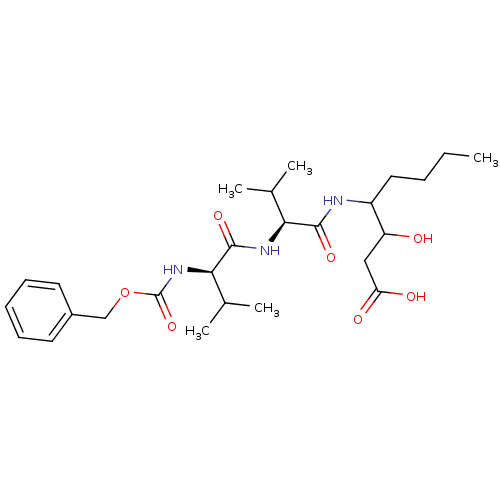 (3-hydroxy-4-[2-methyl-1-[2-methyl-1-benzyloxycarbo...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 0.0180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arkansas Curated by ChEMBL | Assay Description Compound was measured for the apparent inhibition constant at pepsin | J Med Chem 34: 2298-300 (1991) BindingDB Entry DOI: 10.7270/Q28G8JPJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pepsin A (Porcine) | BDBM50012618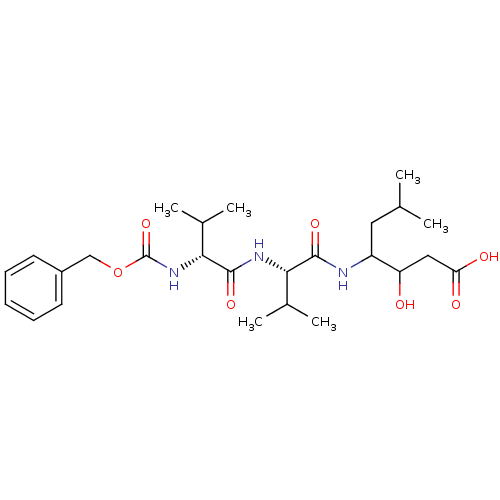 (3-hydroxy-6-methyl-4-[2-methyl-1-[2-methyl-1-benzy...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 0.0390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arkansas Curated by ChEMBL | Assay Description Compound was measured for the apparent inhibition constant at pepsin | J Med Chem 34: 2298-300 (1991) BindingDB Entry DOI: 10.7270/Q28G8JPJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50018229 (2-(4-Hydroxy-phenyl)-2-phenyl-propionic acid 2-die...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.165 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Walter Reed Army Institute of Research Curated by ChEMBL | Assay Description Displacement of [3H] N-methylscopolamine from human muscarinic M2 receptor expressed in CHOK1 cells after 30 mins by scintillation counting analysis | Bioorg Med Chem 21: 2651-62 (2013) Article DOI: 10.1016/j.bmc.2013.01.072 BindingDB Entry DOI: 10.7270/Q2X92F6X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urotensin-2 receptor (Homo sapiens (Human)) | BDBM50244020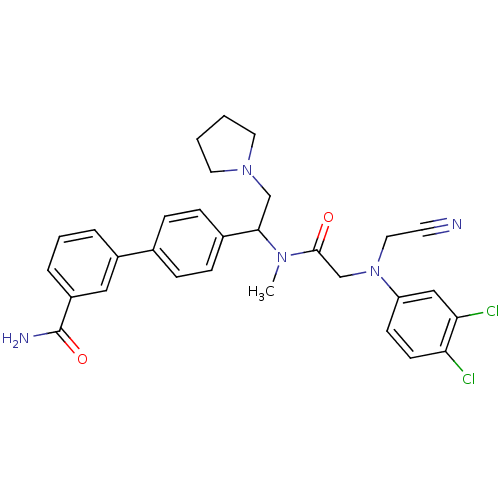 (4'-[1-({2-[Cyanomethyl-(3,4-dichloro-phenyl)-amino...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity to human urotensin2 receptor | Bioorg Med Chem Lett 18: 3716-9 (2008) Article DOI: 10.1016/j.bmcl.2008.05.058 BindingDB Entry DOI: 10.7270/Q2RN37NJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pepsin A (Porcine) | BDBM50012621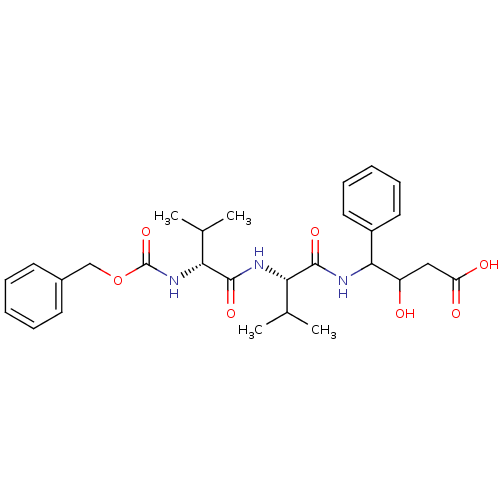 (3-hydroxy-4-[2-methyl-1-[2-methyl-1-benzyloxycarbo...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 0.346 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arkansas Curated by ChEMBL | Assay Description Compound was measured for the apparent inhibition constant at pepsin | J Med Chem 34: 2298-300 (1991) BindingDB Entry DOI: 10.7270/Q28G8JPJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urotensin-2 receptor (Homo sapiens (Human)) | BDBM50377220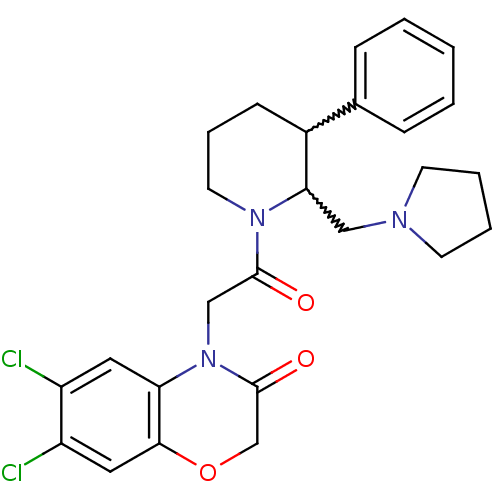 (CHEMBL255509) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [125I]hU-2 from human recombinant Urotensin 2 receptor expressed in HEK293 cells | Bioorg Med Chem Lett 18: 3500-3 (2008) Article DOI: 10.1016/j.bmcl.2008.05.027 BindingDB Entry DOI: 10.7270/Q29024Q7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urotensin-2 receptor (Homo sapiens (Human)) | BDBM50377218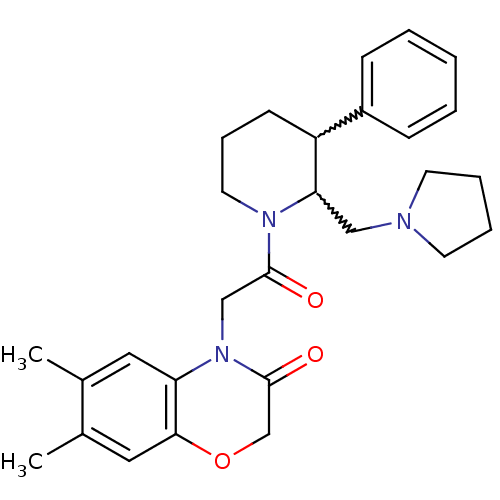 (CHEMBL257171) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [125I]hU-2 from human recombinant Urotensin 2 receptor expressed in HEK293 cells | Bioorg Med Chem Lett 18: 3500-3 (2008) Article DOI: 10.1016/j.bmcl.2008.05.027 BindingDB Entry DOI: 10.7270/Q29024Q7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50491127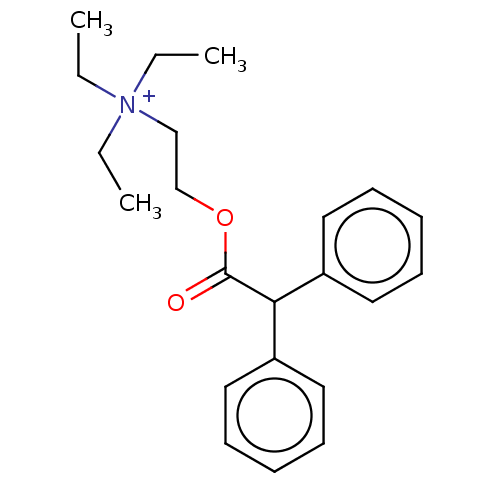 (CHEMBL2377383) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.543 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Walter Reed Army Institute of Research Curated by ChEMBL | Assay Description Displacement of [3H] N-methylscopolamine from human muscarinic M3 receptor expressed in CHOK1 cells after 30 mins by scintillation counting analysis | Bioorg Med Chem 21: 2651-62 (2013) Article DOI: 10.1016/j.bmc.2013.01.072 BindingDB Entry DOI: 10.7270/Q2X92F6X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (Homo sapiens (Human)) | BDBM50491126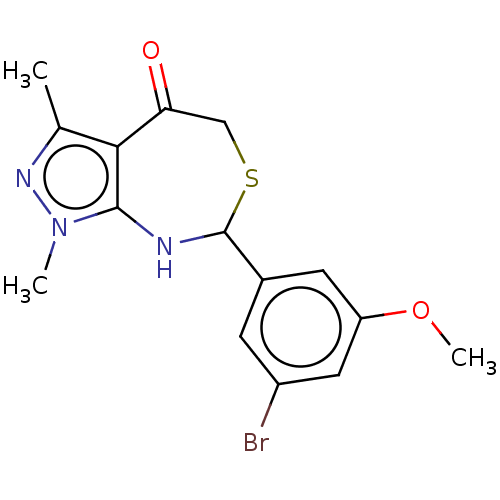 (CHEMBL2377266) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.594 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Walter Reed Army Institute of Research Curated by ChEMBL | Assay Description Displacement of [3H] N-methylscopolamine from human muscarinic M1 receptor expressed in CHOK1 cells after 30 mins by scintillation counting analysis | Bioorg Med Chem 21: 2651-62 (2013) Article DOI: 10.1016/j.bmc.2013.01.072 BindingDB Entry DOI: 10.7270/Q2X92F6X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urotensin-2 receptor (Homo sapiens (Human)) | BDBM50377217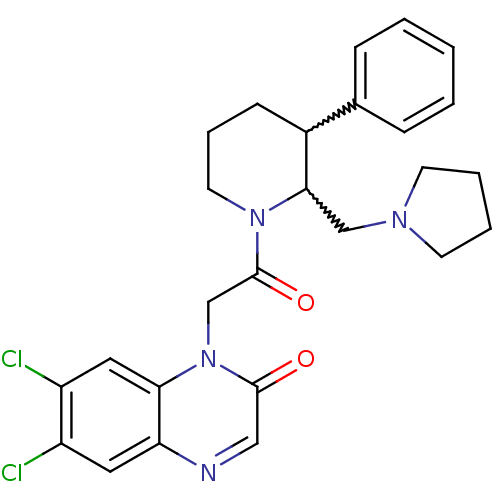 (CHEMBL256989) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [125I]hU-2 from human recombinant Urotensin 2 receptor expressed in HEK293 cells | Bioorg Med Chem Lett 18: 3500-3 (2008) Article DOI: 10.1016/j.bmcl.2008.05.027 BindingDB Entry DOI: 10.7270/Q29024Q7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (Homo sapiens (Human)) | BDBM50018229 (2-(4-Hydroxy-phenyl)-2-phenyl-propionic acid 2-die...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.881 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Walter Reed Army Institute of Research Curated by ChEMBL | Assay Description Displacement of [3H] N-methylscopolamine from human muscarinic M1 receptor expressed in CHOK1 cells after 30 mins by scintillation counting analysis | Bioorg Med Chem 21: 2651-62 (2013) Article DOI: 10.1016/j.bmc.2013.01.072 BindingDB Entry DOI: 10.7270/Q2X92F6X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (Homo sapiens (Human)) | BDBM50403547 (ATROPEN | ATROPINE) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | 0.922 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Walter Reed Army Institute of Research Curated by ChEMBL | Assay Description Displacement of [3H] N-methylscopolamine from human muscarinic M1 receptor expressed in CHOK1 cells after 30 mins by scintillation counting analysis | Bioorg Med Chem 21: 2651-62 (2013) Article DOI: 10.1016/j.bmc.2013.01.072 BindingDB Entry DOI: 10.7270/Q2X92F6X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urotensin-2 receptor (Homo sapiens (Human)) | BDBM50377215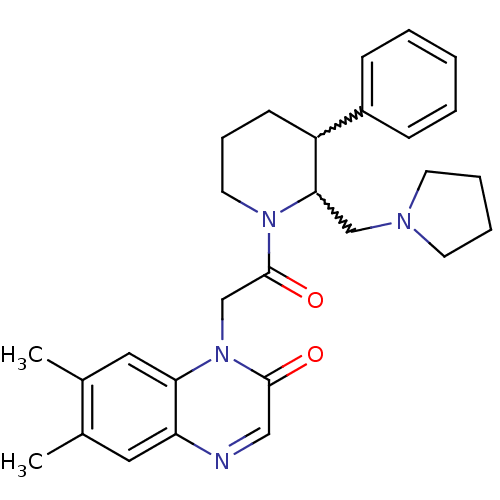 (CHEMBL257415) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [125I]hU-2 from human recombinant Urotensin 2 receptor expressed in HEK293 cells | Bioorg Med Chem Lett 18: 3500-3 (2008) Article DOI: 10.1016/j.bmcl.2008.05.027 BindingDB Entry DOI: 10.7270/Q29024Q7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pepsin A (Porcine) | BDBM50012620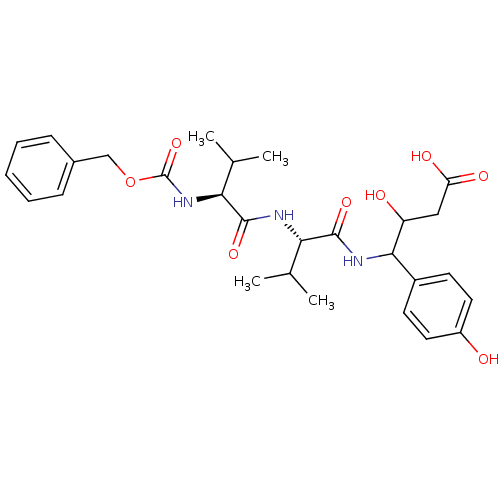 (3-hydroxy-4-(4-hydroxyphenyl)-4-[2-methyl-1-[2-met...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 1.13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arkansas Curated by ChEMBL | Assay Description Compound was measured for the inhibition of pepsin hydrolysis of hemoglobin. | J Med Chem 34: 2298-300 (1991) BindingDB Entry DOI: 10.7270/Q28G8JPJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urotensin-2 receptor (Homo sapiens (Human)) | BDBM50377227 (CHEMBL255462) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [125I]hU-2 from human recombinant Urotensin 2 receptor expressed in HEK293 cells | Bioorg Med Chem Lett 18: 3500-3 (2008) Article DOI: 10.1016/j.bmcl.2008.05.027 BindingDB Entry DOI: 10.7270/Q29024Q7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (Homo sapiens (Human)) | BDBM50491119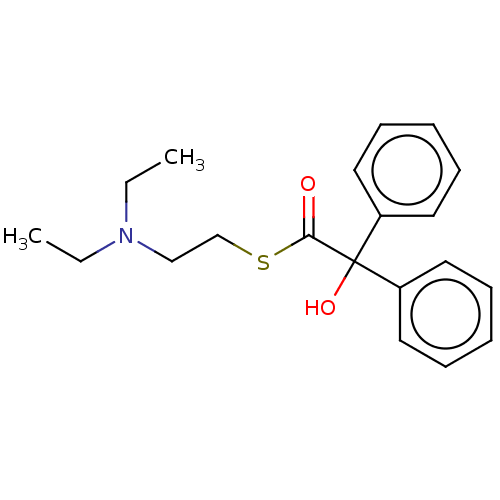 (CHEMBL2377387) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Walter Reed Army Institute of Research Curated by ChEMBL | Assay Description Displacement of [3H] N-methylscopolamine from human muscarinic M1 receptor expressed in CHOK1 cells after 30 mins by scintillation counting analysis | Bioorg Med Chem 21: 2651-62 (2013) Article DOI: 10.1016/j.bmc.2013.01.072 BindingDB Entry DOI: 10.7270/Q2X92F6X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (Homo sapiens (Human)) | BDBM50491124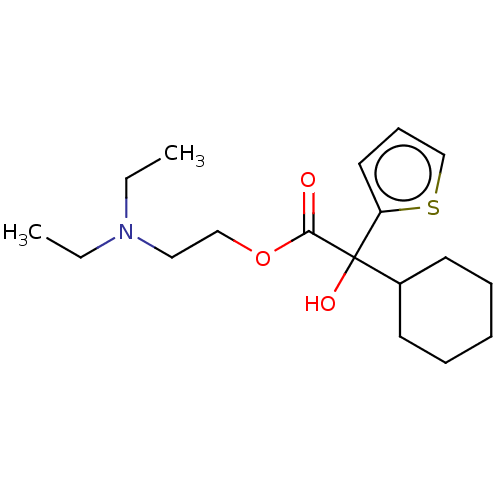 (CHEMBL2377267) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Walter Reed Army Institute of Research Curated by ChEMBL | Assay Description Displacement of [3H] N-methylscopolamine from human muscarinic M1 receptor expressed in CHOK1 cells after 30 mins by scintillation counting analysis | Bioorg Med Chem 21: 2651-62 (2013) Article DOI: 10.1016/j.bmc.2013.01.072 BindingDB Entry DOI: 10.7270/Q2X92F6X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M4 (Homo sapiens (Human)) | BDBM50403547 (ATROPEN | ATROPINE) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid UniChem Similars | DrugBank Article PubMed | 1.39 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Walter Reed Army Institute of Research Curated by ChEMBL | Assay Description Displacement of [3H] N-methylscopolamine from human muscarinic M4 receptor expressed in CHOK1 cells after 30 mins by scintillation counting analysis | Bioorg Med Chem 21: 2651-62 (2013) Article DOI: 10.1016/j.bmc.2013.01.072 BindingDB Entry DOI: 10.7270/Q2X92F6X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50403547 (ATROPEN | ATROPINE) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | 1.44 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Walter Reed Army Institute of Research Curated by ChEMBL | Assay Description Displacement of [3H] N-methylscopolamine from human muscarinic M2 receptor expressed in CHOK1 cells after 30 mins by scintillation counting analysis | Bioorg Med Chem 21: 2651-62 (2013) Article DOI: 10.1016/j.bmc.2013.01.072 BindingDB Entry DOI: 10.7270/Q2X92F6X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M4 (Homo sapiens (Human)) | BDBM50491124 (CHEMBL2377267) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.49 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Walter Reed Army Institute of Research Curated by ChEMBL | Assay Description Displacement of [3H] N-methylscopolamine from human muscarinic M4 receptor expressed in CHOK1 cells after 30 mins by scintillation counting analysis | Bioorg Med Chem 21: 2651-62 (2013) Article DOI: 10.1016/j.bmc.2013.01.072 BindingDB Entry DOI: 10.7270/Q2X92F6X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pepsin A (Porcine) | BDBM50012619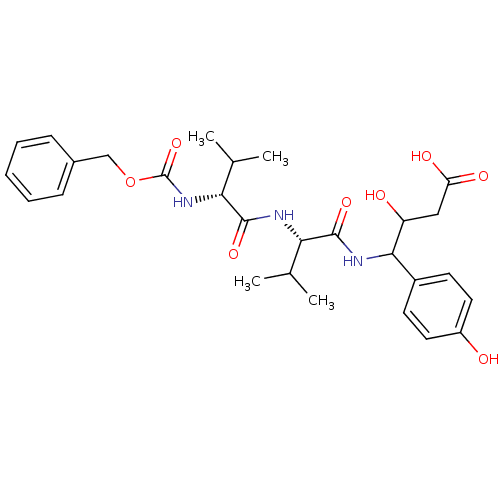 (3-hydroxy-4-(4-hydroxyphenyl)-4-[2-methyl-1-[2-met...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 1.54 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arkansas Curated by ChEMBL | Assay Description Compound was measured for the inhibition of pepsin hydrolysis of hemoglobin. | J Med Chem 34: 2298-300 (1991) BindingDB Entry DOI: 10.7270/Q28G8JPJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50491133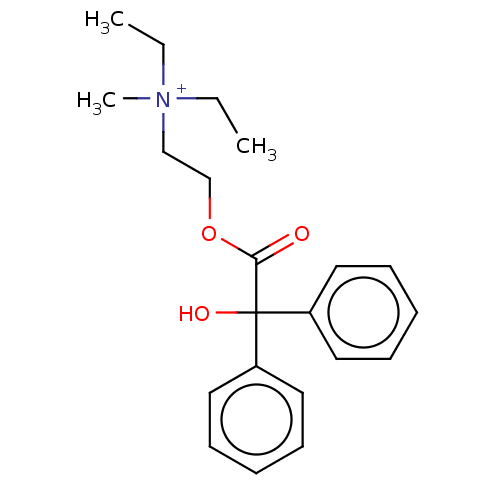 (Methylbenactyzium Bromide) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 1.54 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Walter Reed Army Institute of Research Curated by ChEMBL | Assay Description Displacement of [3H] N-methylscopolamine from human muscarinic M2 receptor expressed in CHOK1 cells after 30 mins by scintillation counting analysis | Bioorg Med Chem 21: 2651-62 (2013) Article DOI: 10.1016/j.bmc.2013.01.072 BindingDB Entry DOI: 10.7270/Q2X92F6X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (Homo sapiens (Human)) | BDBM50491133 (Methylbenactyzium Bromide) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 1.59 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Walter Reed Army Institute of Research Curated by ChEMBL | Assay Description Displacement of [3H] N-methylscopolamine from human muscarinic M1 receptor expressed in CHOK1 cells after 30 mins by scintillation counting analysis | Bioorg Med Chem 21: 2651-62 (2013) Article DOI: 10.1016/j.bmc.2013.01.072 BindingDB Entry DOI: 10.7270/Q2X92F6X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urotensin-2 receptor (Homo sapiens (Human)) | BDBM50377224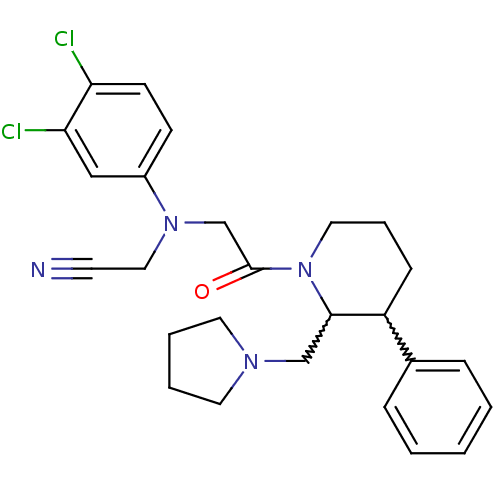 (CHEMBL257767) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [125I]hU-2 from human recombinant Urotensin 2 receptor expressed in HEK293 cells | Bioorg Med Chem Lett 18: 3500-3 (2008) Article DOI: 10.1016/j.bmcl.2008.05.027 BindingDB Entry DOI: 10.7270/Q29024Q7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urotensin-2 receptor (Homo sapiens (Human)) | BDBM50377219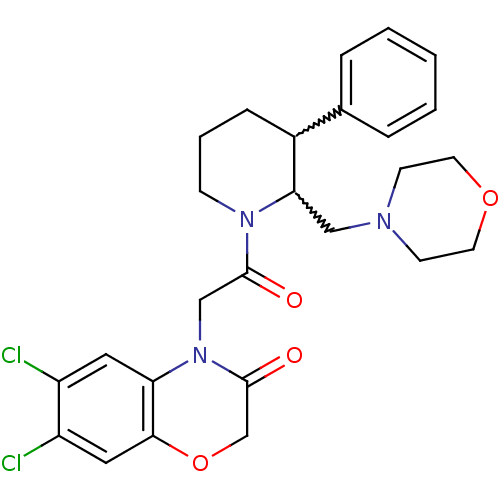 (CHEMBL402520) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [125I]hU-2 from human recombinant Urotensin 2 receptor expressed in HEK293 cells | Bioorg Med Chem Lett 18: 3500-3 (2008) Article DOI: 10.1016/j.bmcl.2008.05.027 BindingDB Entry DOI: 10.7270/Q29024Q7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50403547 (ATROPEN | ATROPINE) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid UniChem Similars | DrugBank Article PubMed | 2.18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Walter Reed Army Institute of Research Curated by ChEMBL | Assay Description Displacement of [3H] N-methylscopolamine from human muscarinic M3 receptor expressed in CHOK1 cells after 30 mins by scintillation counting analysis | Bioorg Med Chem 21: 2651-62 (2013) Article DOI: 10.1016/j.bmc.2013.01.072 BindingDB Entry DOI: 10.7270/Q2X92F6X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (Homo sapiens (Human)) | BDBM50240680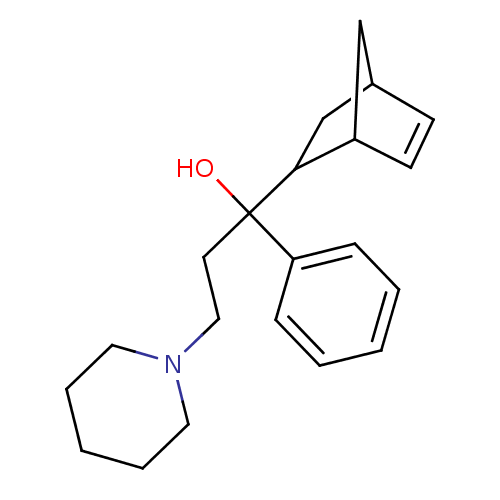 (1-(bicyclo[2.2.1]hept-5-en-2-yl)-1-phenyl-3-(piper...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents | Article PubMed | 2.36 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Walter Reed Army Institute of Research Curated by ChEMBL | Assay Description Displacement of [3H] N-methylscopolamine from human muscarinic M1 receptor expressed in CHOK1 cells after 30 mins by scintillation counting analysis | Bioorg Med Chem 21: 2651-62 (2013) Article DOI: 10.1016/j.bmc.2013.01.072 BindingDB Entry DOI: 10.7270/Q2X92F6X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urotensin-2 receptor (Homo sapiens (Human)) | BDBM50244019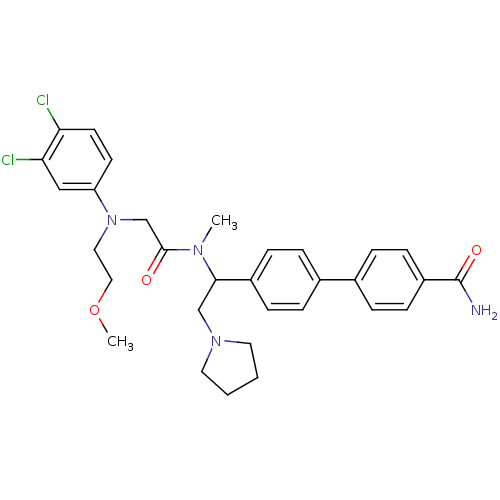 (4'-[1-({2-[(3,4-Dichloro-phenyl)-(2-methoxy-ethyl)...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity to human urotensin2 receptor | Bioorg Med Chem Lett 18: 3716-9 (2008) Article DOI: 10.1016/j.bmcl.2008.05.058 BindingDB Entry DOI: 10.7270/Q2RN37NJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urotensin-2 receptor (Homo sapiens (Human)) | BDBM50377216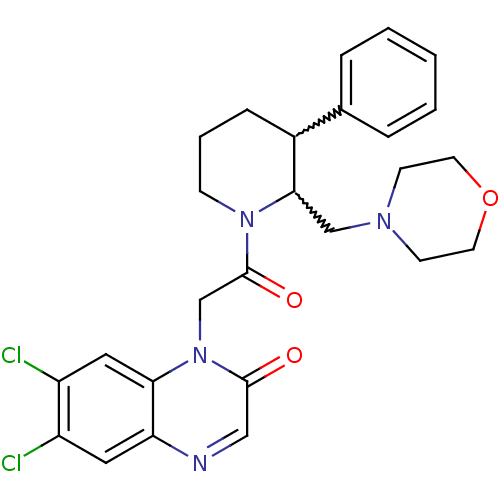 (CHEMBL256988) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [125I]hU-2 from human recombinant Urotensin 2 receptor expressed in HEK293 cells | Bioorg Med Chem Lett 18: 3500-3 (2008) Article DOI: 10.1016/j.bmcl.2008.05.027 BindingDB Entry DOI: 10.7270/Q29024Q7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urotensin-2 receptor (Homo sapiens (Human)) | BDBM50244022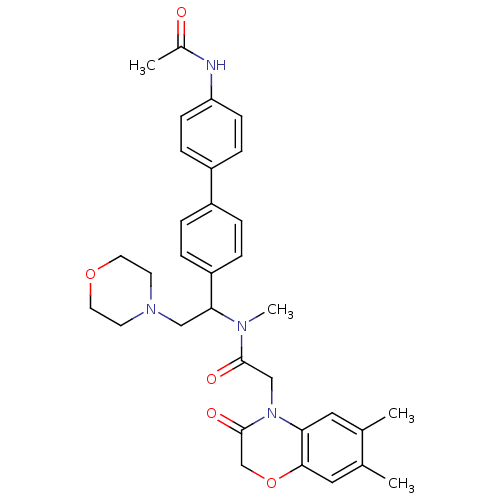 (CHEMBL449192 | N-[1-(4'-Acetylamino-biphenyl-4-yl)...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity to human urotensin2 receptor | Bioorg Med Chem Lett 18: 3716-9 (2008) Article DOI: 10.1016/j.bmcl.2008.05.058 BindingDB Entry DOI: 10.7270/Q2RN37NJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50491119 (CHEMBL2377387) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Walter Reed Army Institute of Research Curated by ChEMBL | Assay Description Displacement of [3H] N-methylscopolamine from human muscarinic M3 receptor expressed in CHOK1 cells after 30 mins by scintillation counting analysis | Bioorg Med Chem 21: 2651-62 (2013) Article DOI: 10.1016/j.bmc.2013.01.072 BindingDB Entry DOI: 10.7270/Q2X92F6X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urotensin-2 receptor (Homo sapiens (Human)) | BDBM50244021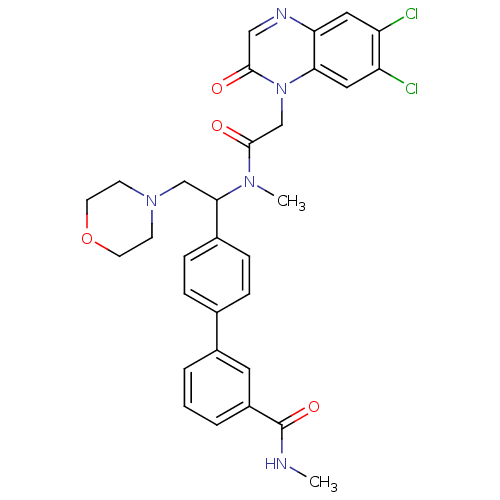 (4'-(1-{[2-(6,7-Dichloro-2-oxo-2H-quinoxalin-1-yl)-...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity to human urotensin2 receptor | Bioorg Med Chem Lett 18: 3716-9 (2008) Article DOI: 10.1016/j.bmcl.2008.05.058 BindingDB Entry DOI: 10.7270/Q2RN37NJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50491133 (Methylbenactyzium Bromide) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 4.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Walter Reed Army Institute of Research Curated by ChEMBL | Assay Description Displacement of [3H] N-methylscopolamine from human muscarinic M3 receptor expressed in CHOK1 cells after 30 mins by scintillation counting analysis | Bioorg Med Chem 21: 2651-62 (2013) Article DOI: 10.1016/j.bmc.2013.01.072 BindingDB Entry DOI: 10.7270/Q2X92F6X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urotensin-2 receptor (Homo sapiens (Human)) | BDBM50377229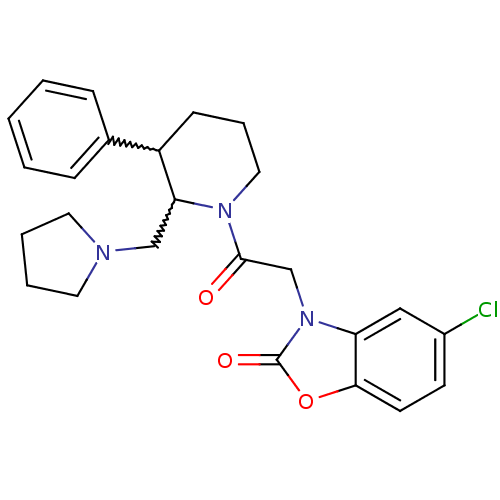 (CHEMBL257150) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [125I]hU-2 from human recombinant Urotensin 2 receptor expressed in HEK293 cells | Bioorg Med Chem Lett 18: 3500-3 (2008) Article DOI: 10.1016/j.bmcl.2008.05.027 BindingDB Entry DOI: 10.7270/Q29024Q7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urotensin-2 receptor (Homo sapiens (Human)) | BDBM50244065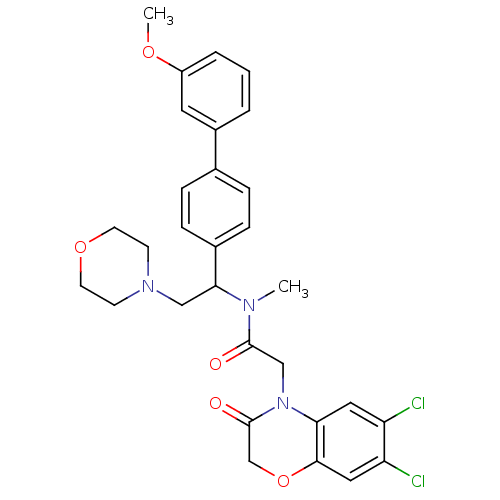 (2-(6,7-Dichloro-3-oxo-2,3-dihydro-benzo[1,4]oxazin...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity to human urotensin2 receptor | Bioorg Med Chem Lett 18: 3716-9 (2008) Article DOI: 10.1016/j.bmcl.2008.05.058 BindingDB Entry DOI: 10.7270/Q2RN37NJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urotensin-2 receptor (Homo sapiens (Human)) | BDBM50377214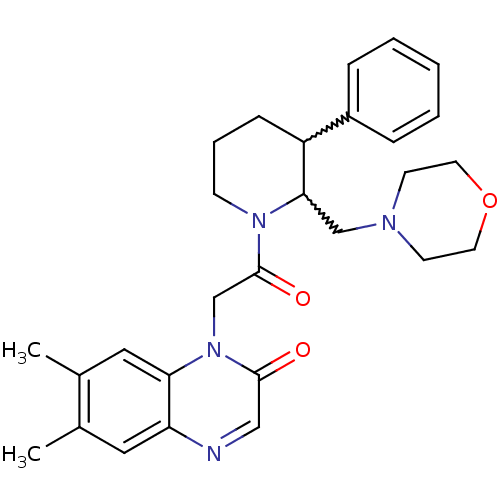 (CHEMBL256721) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [125I]hU-2 from human recombinant Urotensin 2 receptor expressed in HEK293 cells | Bioorg Med Chem Lett 18: 3500-3 (2008) Article DOI: 10.1016/j.bmcl.2008.05.027 BindingDB Entry DOI: 10.7270/Q29024Q7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50491119 (CHEMBL2377387) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 5.38 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Walter Reed Army Institute of Research Curated by ChEMBL | Assay Description Displacement of [3H] N-methylscopolamine from human muscarinic M2 receptor expressed in CHOK1 cells after 30 mins by scintillation counting analysis | Bioorg Med Chem 21: 2651-62 (2013) Article DOI: 10.1016/j.bmc.2013.01.072 BindingDB Entry DOI: 10.7270/Q2X92F6X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (Homo sapiens (Human)) | BDBM50491127 (CHEMBL2377383) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 5.75 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Walter Reed Army Institute of Research Curated by ChEMBL | Assay Description Displacement of [3H] N-methylscopolamine from human muscarinic M1 receptor expressed in CHOK1 cells after 30 mins by scintillation counting analysis | Bioorg Med Chem 21: 2651-62 (2013) Article DOI: 10.1016/j.bmc.2013.01.072 BindingDB Entry DOI: 10.7270/Q2X92F6X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (Homo sapiens (Human)) | BDBM50491121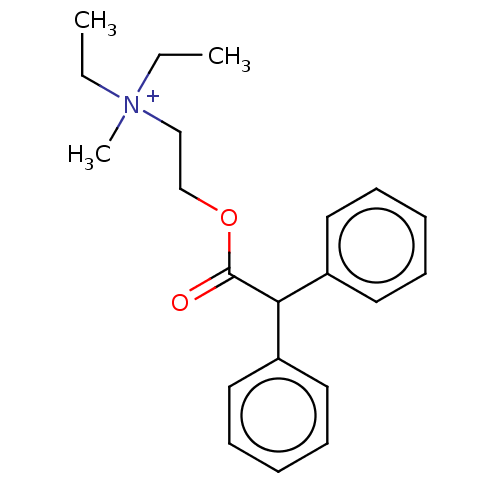 (CHEMBL2377385) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 5.78 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Walter Reed Army Institute of Research Curated by ChEMBL | Assay Description Displacement of [3H] N-methylscopolamine from human muscarinic M1 receptor expressed in CHOK1 cells after 30 mins by scintillation counting analysis | Bioorg Med Chem 21: 2651-62 (2013) Article DOI: 10.1016/j.bmc.2013.01.072 BindingDB Entry DOI: 10.7270/Q2X92F6X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50491132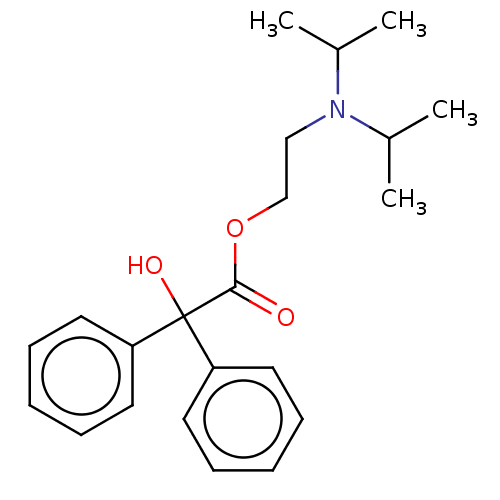 (CHEMBL2377261) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 5.85 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Walter Reed Army Institute of Research Curated by ChEMBL | Assay Description Displacement of [3H] N-methylscopolamine from human muscarinic M2 receptor expressed in CHOK1 cells after 30 mins by scintillation counting analysis | Bioorg Med Chem 21: 2651-62 (2013) Article DOI: 10.1016/j.bmc.2013.01.072 BindingDB Entry DOI: 10.7270/Q2X92F6X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50240680 (1-(bicyclo[2.2.1]hept-5-en-2-yl)-1-phenyl-3-(piper...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents | Article PubMed | 5.95 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Walter Reed Army Institute of Research Curated by ChEMBL | Assay Description Displacement of [3H] N-methylscopolamine from human muscarinic M3 receptor expressed in CHOK1 cells after 30 mins by scintillation counting analysis | Bioorg Med Chem 21: 2651-62 (2013) Article DOI: 10.1016/j.bmc.2013.01.072 BindingDB Entry DOI: 10.7270/Q2X92F6X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50491128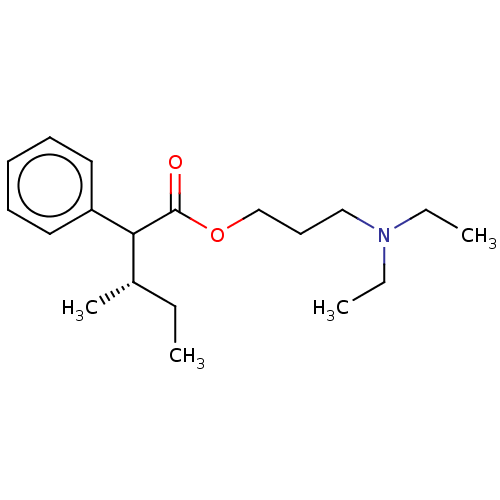 (CHEMBL2377392) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 5.97 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Walter Reed Army Institute of Research Curated by ChEMBL | Assay Description Displacement of [3H] N-methylscopolamine from human muscarinic M3 receptor expressed in CHOK1 cells after 30 mins by scintillation counting analysis | Bioorg Med Chem 21: 2651-62 (2013) Article DOI: 10.1016/j.bmc.2013.01.072 BindingDB Entry DOI: 10.7270/Q2X92F6X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urotensin-2 receptor (Homo sapiens (Human)) | BDBM50244018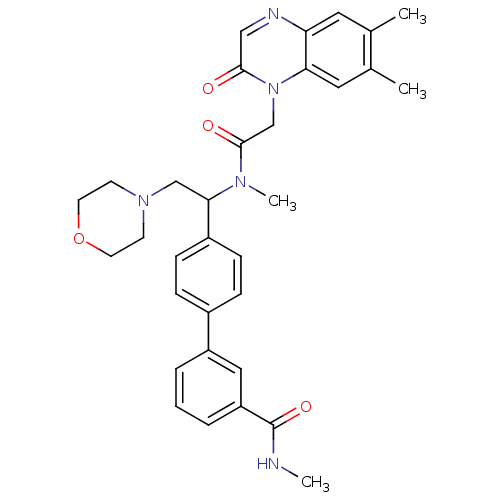 (4'-(1-{[2-(6,7-Dimethyl-2-oxo-2H-quinoxalin-1-yl)-...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity to human urotensin2 receptor | Bioorg Med Chem Lett 18: 3716-9 (2008) Article DOI: 10.1016/j.bmcl.2008.05.058 BindingDB Entry DOI: 10.7270/Q2RN37NJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urotensin-2 receptor (Homo sapiens (Human)) | BDBM50239135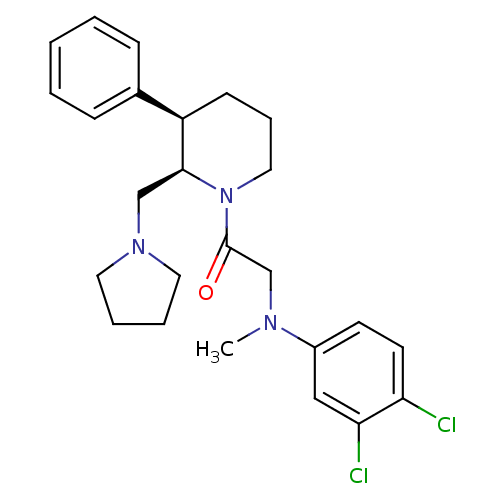 (2-((3,4-dichlorophenyl)(methyl)amino)-1-((2R,3R)-3...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity to human urotensin2 receptor | Bioorg Med Chem Lett 18: 3716-9 (2008) Article DOI: 10.1016/j.bmcl.2008.05.058 BindingDB Entry DOI: 10.7270/Q2RN37NJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urotensin-2 receptor (Homo sapiens (Human)) | BDBM50377226 (CHEMBL255460) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [125I]hU-2 from human recombinant Urotensin 2 receptor expressed in HEK293 cells | Bioorg Med Chem Lett 18: 3500-3 (2008) Article DOI: 10.1016/j.bmcl.2008.05.027 BindingDB Entry DOI: 10.7270/Q29024Q7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urotensin-2 receptor (Homo sapiens (Human)) | BDBM50377223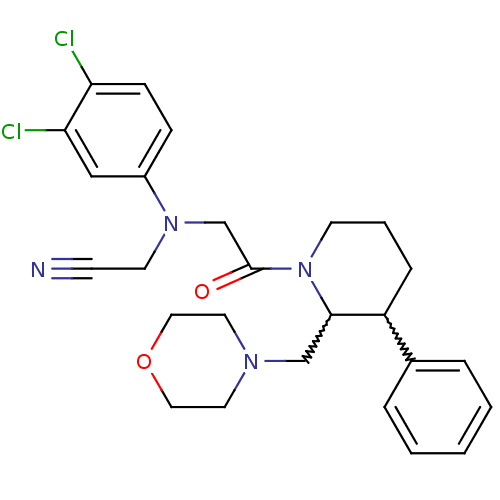 (CHEMBL258251) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [125I]hU-2 from human recombinant Urotensin 2 receptor expressed in HEK293 cells | Bioorg Med Chem Lett 18: 3500-3 (2008) Article DOI: 10.1016/j.bmcl.2008.05.027 BindingDB Entry DOI: 10.7270/Q29024Q7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 3750 total ) | Next | Last >> |