

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cannabinoid receptor 1 (Mus musculus (Mouse)) | BDBM239073 (US9416103, CP-55,940) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | US Patent | 0.370 | -53.8 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
The Board of Trustees of the University of Arkansas; The University of Kansas US Patent | Assay Description Competition receptor binding was performed as previously described [Shoemaker et al., J. Pharmacol. Exp. Ther., 314:868-75]. Briefly, 50 μg of mou... | US Patent US9416103 (2016) BindingDB Entry DOI: 10.7270/Q2HX1BKQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM239078 (US9416103, TV-5-157) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.810 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Kansas Curated by ChEMBL | Assay Description Agonist activity at human CB2 receptor transfected in CHO cells assessed as inhibition of forskolin-stimulated adenylyl cyclase activity after 15 min... | J Med Chem 56: 4537-50 (2013) Article DOI: 10.1021/jm400268b BindingDB Entry DOI: 10.7270/Q25M68M1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM239078 (US9416103, TV-5-157) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.810 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Board of Trustees of the University of Arkansas; The University of Kansas US Patent | Assay Description A functional assay screen for the inhibition of adenylate cyclase (AC) activity was chosen as the subsequent assay. This screen would allow us to gai... | US Patent US9416103 (2016) BindingDB Entry DOI: 10.7270/Q2HX1BKQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Mus musculus (Mouse)) | BDBM239078 (US9416103, TV-5-157) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Board of Trustees of the University of Arkansas; The University of Kansas US Patent | Assay Description A functional assay screen for the inhibition of adenylate cyclase (AC) activity was chosen as the subsequent assay. This screen would allow us to gai... | US Patent US9416103 (2016) BindingDB Entry DOI: 10.7270/Q2HX1BKQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Mus musculus (Mouse)) | BDBM239078 (US9416103, TV-5-157) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Kansas Curated by ChEMBL | Assay Description Agonist activity at CB1 receptor in mouse Neuro2a cells assessed as inhibition of forskolin-stimulated adenylyl cyclase activity after 15 mins by liq... | J Med Chem 56: 4537-50 (2013) Article DOI: 10.1021/jm400268b BindingDB Entry DOI: 10.7270/Q25M68M1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (Homo sapiens (Human)) | BDBM50160160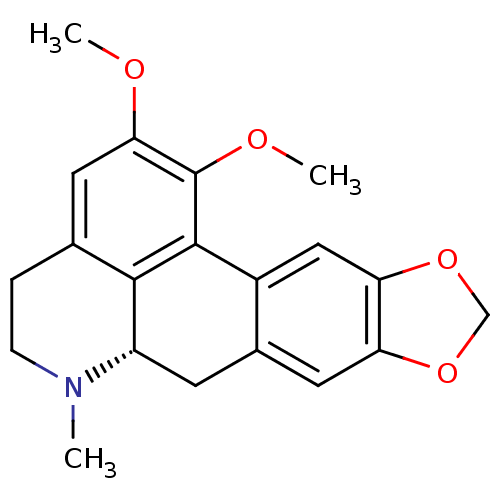 ((S)-1,2-Dimethoxy-6-methyl-5,6,6a,7-tetrahydro-4H-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hunter College and the Graduate Center of the City University of New York Curated by ChEMBL | Assay Description Displacement of [3H]Prazosin from human adrenergic alpha1A receptor | Bioorg Med Chem Lett 20: 628-31 (2010) Article DOI: 10.1016/j.bmcl.2009.11.053 BindingDB Entry DOI: 10.7270/Q2C53MT3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM85804 (1-Naphthyl(1-butyl-1H-indole-3-yl)methanone | JWH-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 9.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Kansas Curated by ChEMBL | Assay Description Agonist activity at human CB2 receptor transfected in CHO cells assessed as inhibition of forskolin-stimulated adenylyl cyclase activity after 15 min... | J Med Chem 56: 4537-50 (2013) Article DOI: 10.1021/jm400268b BindingDB Entry DOI: 10.7270/Q25M68M1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM85804 (1-Naphthyl(1-butyl-1H-indole-3-yl)methanone | JWH-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | US Patent | 9.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Board of Trustees of the University of Arkansas; The University of Kansas US Patent | Assay Description A functional assay screen for the inhibition of adenylate cyclase (AC) activity was chosen as the subsequent assay. This screen would allow us to gai... | US Patent US9416103 (2016) BindingDB Entry DOI: 10.7270/Q2HX1BKQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM239077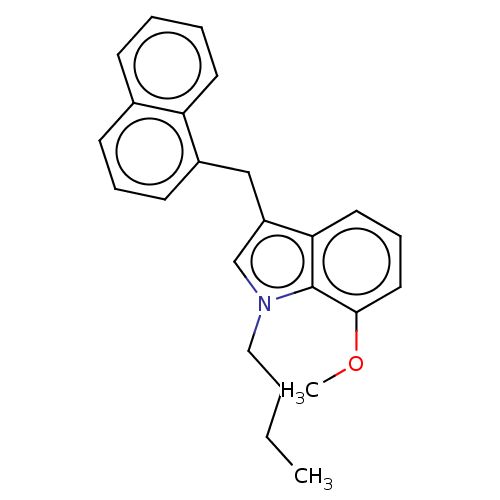 (US9416103, TV-5-249) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 10.9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Board of Trustees of the University of Arkansas; The University of Kansas US Patent | Assay Description A functional assay screen for the inhibition of adenylate cyclase (AC) activity was chosen as the subsequent assay. This screen would allow us to gai... | US Patent US9416103 (2016) BindingDB Entry DOI: 10.7270/Q2HX1BKQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM239077 (US9416103, TV-5-249) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Kansas Curated by ChEMBL | Assay Description Agonist activity at human CB2 receptor transfected in CHO cells assessed as inhibition of forskolin-stimulated adenylyl cyclase activity after 15 min... | J Med Chem 56: 4537-50 (2013) Article DOI: 10.1021/jm400268b BindingDB Entry DOI: 10.7270/Q25M68M1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Mus musculus (Mouse)) | BDBM85804 (1-Naphthyl(1-butyl-1H-indole-3-yl)methanone | JWH-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | US Patent | 12.9 | -45.0 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
The Board of Trustees of the University of Arkansas; The University of Kansas US Patent | Assay Description Competition receptor binding was performed as previously described [Shoemaker et al., J. Pharmacol. Exp. Ther., 314:868-75]. Briefly, 50 μg of mou... | US Patent US9416103 (2016) BindingDB Entry DOI: 10.7270/Q2HX1BKQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Mus musculus (Mouse)) | BDBM85804 (1-Naphthyl(1-butyl-1H-indole-3-yl)methanone | JWH-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | US Patent | 12.9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Board of Trustees of the University of Arkansas; The University of Kansas US Patent | Assay Description A functional assay screen for the inhibition of adenylate cyclase (AC) activity was chosen as the subsequent assay. This screen would allow us to gai... | US Patent US9416103 (2016) BindingDB Entry DOI: 10.7270/Q2HX1BKQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Mus musculus (Mouse)) | BDBM85804 (1-Naphthyl(1-butyl-1H-indole-3-yl)methanone | JWH-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Kansas Curated by ChEMBL | Assay Description Agonist activity at CB1 receptor in mouse Neuro2a cells assessed as inhibition of forskolin-stimulated adenylyl cyclase activity after 15 mins by liq... | J Med Chem 56: 4537-50 (2013) Article DOI: 10.1021/jm400268b BindingDB Entry DOI: 10.7270/Q25M68M1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Mus musculus (Mouse)) | BDBM239074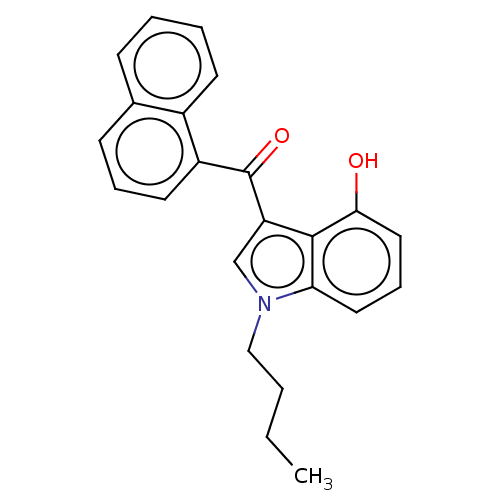 (US9416103, JWH-073-M1) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | US Patent | 14.1 | -44.8 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
The Board of Trustees of the University of Arkansas; The University of Kansas US Patent | Assay Description Competition receptor binding was performed as previously described [Shoemaker et al., J. Pharmacol. Exp. Ther., 314:868-75]. Briefly, 50 μg of mou... | US Patent US9416103 (2016) BindingDB Entry DOI: 10.7270/Q2HX1BKQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Mus musculus (Mouse)) | BDBM239077 (US9416103, TV-5-249) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Kansas Curated by ChEMBL | Assay Description Agonist activity at CB1 receptor in mouse Neuro2a cells assessed as inhibition of forskolin-stimulated adenylyl cyclase activity after 15 mins by liq... | J Med Chem 56: 4537-50 (2013) Article DOI: 10.1021/jm400268b BindingDB Entry DOI: 10.7270/Q25M68M1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Mus musculus (Mouse)) | BDBM239077 (US9416103, TV-5-249) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 15.4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Board of Trustees of the University of Arkansas; The University of Kansas US Patent | Assay Description A functional assay screen for the inhibition of adenylate cyclase (AC) activity was chosen as the subsequent assay. This screen would allow us to gai... | US Patent US9416103 (2016) BindingDB Entry DOI: 10.7270/Q2HX1BKQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Mus musculus (Mouse)) | BDBM239075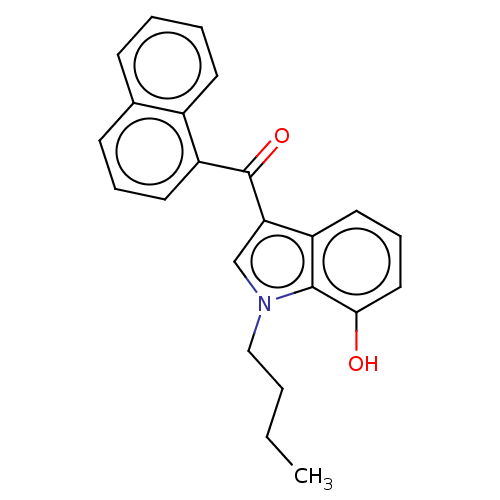 (US9416103, JWH-073-M4) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Kansas Curated by ChEMBL | Assay Description Agonist activity at CB1 receptor in mouse Neuro2a cells assessed as inhibition of forskolin-stimulated adenylyl cyclase activity after 15 mins by liq... | J Med Chem 56: 4537-50 (2013) Article DOI: 10.1021/jm400268b BindingDB Entry DOI: 10.7270/Q25M68M1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Mus musculus (Mouse)) | BDBM239075 (US9416103, JWH-073-M4) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | US Patent | 24.2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Board of Trustees of the University of Arkansas; The University of Kansas US Patent | Assay Description A functional assay screen for the inhibition of adenylate cyclase (AC) activity was chosen as the subsequent assay. This screen would allow us to gai... | US Patent US9416103 (2016) BindingDB Entry DOI: 10.7270/Q2HX1BKQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM239079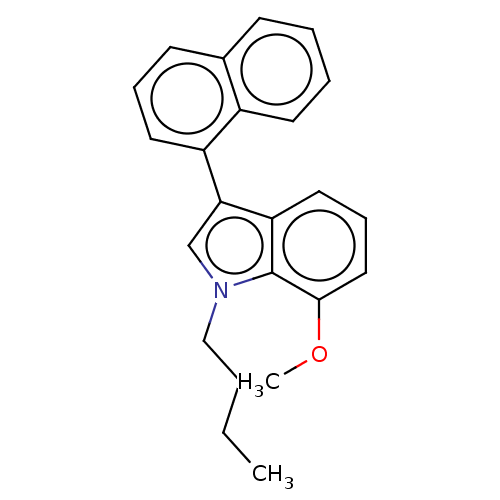 (US9416103, TV-6-41) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 26.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Board of Trustees of the University of Arkansas; The University of Kansas US Patent | Assay Description A functional assay screen for the inhibition of adenylate cyclase (AC) activity was chosen as the subsequent assay. This screen would allow us to gai... | US Patent US9416103 (2016) BindingDB Entry DOI: 10.7270/Q2HX1BKQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50491525 (CHEMBL2380408) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Kansas Curated by ChEMBL | Assay Description Agonist activity at human CB2 receptor transfected in CHO cells assessed as inhibition of forskolin-stimulated adenylyl cyclase activity after 15 min... | J Med Chem 56: 4537-50 (2013) Article DOI: 10.1021/jm400268b BindingDB Entry DOI: 10.7270/Q25M68M1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Mus musculus (Mouse)) | BDBM50491525 (CHEMBL2380408) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 37 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Kansas Curated by ChEMBL | Assay Description Agonist activity at CB1 receptor in mouse Neuro2a cells assessed as inhibition of forskolin-stimulated adenylyl cyclase activity after 15 mins by liq... | J Med Chem 56: 4537-50 (2013) Article DOI: 10.1021/jm400268b BindingDB Entry DOI: 10.7270/Q25M68M1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Mus musculus (Mouse)) | BDBM239079 (US9416103, TV-6-41) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 37.3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Board of Trustees of the University of Arkansas; The University of Kansas US Patent | Assay Description A functional assay screen for the inhibition of adenylate cyclase (AC) activity was chosen as the subsequent assay. This screen would allow us to gai... | US Patent US9416103 (2016) BindingDB Entry DOI: 10.7270/Q2HX1BKQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1D (Homo sapiens (Human)) | BDBM50160160 ((S)-1,2-Dimethoxy-6-methyl-5,6,6a,7-tetrahydro-4H-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 49 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hunter College and the Graduate Center of the City University of New York Curated by ChEMBL | Assay Description Displacement of [3H]GR127543 from human 5HT1D receptor | Bioorg Med Chem Lett 20: 628-31 (2010) Article DOI: 10.1016/j.bmcl.2009.11.053 BindingDB Entry DOI: 10.7270/Q2C53MT3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 7 (Homo sapiens (Human)) | BDBM50160160 ((S)-1,2-Dimethoxy-6-methyl-5,6,6a,7-tetrahydro-4H-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 67 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hunter College and the Graduate Center of the City University of New York Curated by ChEMBL | Assay Description Displacement of [3H]LSD from human 5HT7 receptor | Bioorg Med Chem Lett 20: 628-31 (2010) Article DOI: 10.1016/j.bmcl.2009.11.053 BindingDB Entry DOI: 10.7270/Q2C53MT3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM239075 (US9416103, JWH-073-M4) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 78 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Kansas Curated by ChEMBL | Assay Description Agonist activity at human CB2 receptor transfected in CHO cells assessed as inhibition of forskolin-stimulated adenylyl cyclase activity after 15 min... | J Med Chem 56: 4537-50 (2013) Article DOI: 10.1021/jm400268b BindingDB Entry DOI: 10.7270/Q25M68M1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM239075 (US9416103, JWH-073-M4) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | US Patent | 78.3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Board of Trustees of the University of Arkansas; The University of Kansas US Patent | Assay Description A functional assay screen for the inhibition of adenylate cyclase (AC) activity was chosen as the subsequent assay. This screen would allow us to gai... | US Patent US9416103 (2016) BindingDB Entry DOI: 10.7270/Q2HX1BKQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1B (Homo sapiens (Human)) | BDBM50160160 ((S)-1,2-Dimethoxy-6-methyl-5,6,6a,7-tetrahydro-4H-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hunter College and the Graduate Center of the City University of New York Curated by ChEMBL | Assay Description Displacement of [3H]GR127543 from human 5HT1B receptor | Bioorg Med Chem Lett 20: 628-31 (2010) Article DOI: 10.1016/j.bmcl.2009.11.053 BindingDB Entry DOI: 10.7270/Q2C53MT3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Mus musculus (Mouse)) | BDBM239075 (US9416103, JWH-073-M4) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | US Patent | 122 | -39.5 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
The Board of Trustees of the University of Arkansas; The University of Kansas US Patent | Assay Description Competition receptor binding was performed as previously described [Shoemaker et al., J. Pharmacol. Exp. Ther., 314:868-75]. Briefly, 50 μg of mou... | US Patent US9416103 (2016) BindingDB Entry DOI: 10.7270/Q2HX1BKQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2C adrenergic receptor (Homo sapiens (Human)) | BDBM50160160 ((S)-1,2-Dimethoxy-6-methyl-5,6,6a,7-tetrahydro-4H-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 181 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hunter College and the Graduate Center of the City University of New York Curated by ChEMBL | Assay Description Displacement of [3H]Clonidine from human adrenergic Alpha-2C receptor | Bioorg Med Chem Lett 20: 628-31 (2010) Article DOI: 10.1016/j.bmcl.2009.11.053 BindingDB Entry DOI: 10.7270/Q2C53MT3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Mus musculus (Mouse)) | BDBM60993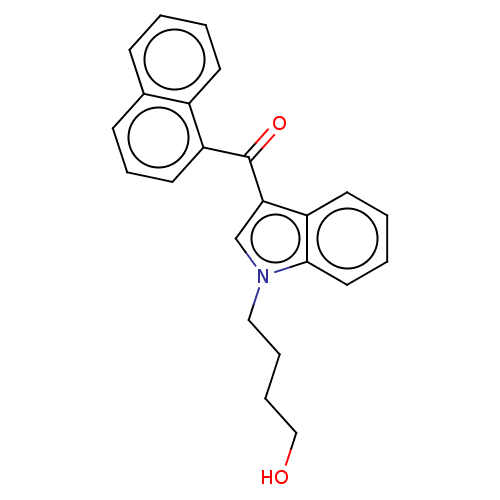 (US9416103, JWH-073-M5) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | US Patent | 224 | -38.0 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
The Board of Trustees of the University of Arkansas; The University of Kansas US Patent | Assay Description Competition receptor binding was performed as previously described [Shoemaker et al., J. Pharmacol. Exp. Ther., 314:868-75]. Briefly, 50 μg of mou... | US Patent US9416103 (2016) BindingDB Entry DOI: 10.7270/Q2HX1BKQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Homo sapiens (Human)) | BDBM50160160 ((S)-1,2-Dimethoxy-6-methyl-5,6,6a,7-tetrahydro-4H-...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 244 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hunter College and the Graduate Center of the City University of New York Curated by ChEMBL | Assay Description Displacement of [3H]Citalopram from human SERT | Bioorg Med Chem Lett 20: 628-31 (2010) Article DOI: 10.1016/j.bmcl.2009.11.053 BindingDB Entry DOI: 10.7270/Q2C53MT3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM239075 (US9416103, JWH-073-M4) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | US Patent | 251 | -37.7 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
The Board of Trustees of the University of Arkansas; The University of Kansas US Patent | Assay Description Competition receptor binding was performed as previously described [Shoemaker et al., J. Pharmacol. Exp. Ther., 314:868-75]. Briefly, 50 μg of mou... | US Patent US9416103 (2016) BindingDB Entry DOI: 10.7270/Q2HX1BKQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2B adrenergic receptor (Homo sapiens (Human)) | BDBM50160160 ((S)-1,2-Dimethoxy-6-methyl-5,6,6a,7-tetrahydro-4H-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 252 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hunter College and the Graduate Center of the City University of New York Curated by ChEMBL | Assay Description Displacement of [3H]Clonidine from human adrenergic alpha2B receptor | Bioorg Med Chem Lett 20: 628-31 (2010) Article DOI: 10.1016/j.bmcl.2009.11.053 BindingDB Entry DOI: 10.7270/Q2C53MT3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50160160 ((S)-1,2-Dimethoxy-6-methyl-5,6,6a,7-tetrahydro-4H-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 257 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hunter College and the Graduate Center of the City University of New York Curated by ChEMBL | Assay Description Displacement of [3H]LSD from human 5HT6 receptor | Bioorg Med Chem Lett 20: 628-31 (2010) Article DOI: 10.1016/j.bmcl.2009.11.053 BindingDB Entry DOI: 10.7270/Q2C53MT3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(4) dopamine receptor (Homo sapiens (Human)) | BDBM50160160 ((S)-1,2-Dimethoxy-6-methyl-5,6,6a,7-tetrahydro-4H-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 262 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hunter College and the Graduate Center of the City University of New York Curated by ChEMBL | Assay Description Displacement of [3H]N-methylspiperone from human dopamine D4 receptor | Bioorg Med Chem Lett 20: 628-31 (2010) Article DOI: 10.1016/j.bmcl.2009.11.053 BindingDB Entry DOI: 10.7270/Q2C53MT3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM239076 (US9416103, TV-5-129) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 281 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Kansas Curated by ChEMBL | Assay Description Agonist activity at human CB2 receptor transfected in CHO cells assessed as inhibition of forskolin-stimulated adenylyl cyclase activity after 15 min... | J Med Chem 56: 4537-50 (2013) Article DOI: 10.1021/jm400268b BindingDB Entry DOI: 10.7270/Q25M68M1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM239076 (US9416103, TV-5-129) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 281 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Board of Trustees of the University of Arkansas; The University of Kansas US Patent | Assay Description A functional assay screen for the inhibition of adenylate cyclase (AC) activity was chosen as the subsequent assay. This screen would allow us to gai... | US Patent US9416103 (2016) BindingDB Entry DOI: 10.7270/Q2HX1BKQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50160160 ((S)-1,2-Dimethoxy-6-methyl-5,6,6a,7-tetrahydro-4H-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 309 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hunter College and the Graduate Center of the City University of New York Curated by ChEMBL | Assay Description Displacement of [3H]N-methylspiperone from human dopamine D3 receptor | Bioorg Med Chem Lett 20: 628-31 (2010) Article DOI: 10.1016/j.bmcl.2009.11.053 BindingDB Entry DOI: 10.7270/Q2C53MT3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1D adrenergic receptor (Homo sapiens (Human)) | BDBM50160160 ((S)-1,2-Dimethoxy-6-methyl-5,6,6a,7-tetrahydro-4H-...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hunter College and the Graduate Center of the City University of New York Curated by ChEMBL | Assay Description Binding affinity to human adrenergic Alpha-1D receptor | Bioorg Med Chem Lett 20: 628-31 (2010) Article DOI: 10.1016/j.bmcl.2009.11.053 BindingDB Entry DOI: 10.7270/Q2C53MT3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Mus musculus (Mouse)) | BDBM239076 (US9416103, TV-5-129) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 387 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Board of Trustees of the University of Arkansas; The University of Kansas US Patent | Assay Description A functional assay screen for the inhibition of adenylate cyclase (AC) activity was chosen as the subsequent assay. This screen would allow us to gai... | US Patent US9416103 (2016) BindingDB Entry DOI: 10.7270/Q2HX1BKQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Mus musculus (Mouse)) | BDBM239076 (US9416103, TV-5-129) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 387 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Kansas Curated by ChEMBL | Assay Description Agonist activity at CB1 receptor in mouse Neuro2a cells assessed as inhibition of forskolin-stimulated adenylyl cyclase activity after 15 mins by liq... | J Med Chem 56: 4537-50 (2013) Article DOI: 10.1021/jm400268b BindingDB Entry DOI: 10.7270/Q25M68M1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2B (Homo sapiens (Human)) | BDBM50160160 ((S)-1,2-Dimethoxy-6-methyl-5,6,6a,7-tetrahydro-4H-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 543 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hunter College and the Graduate Center of the City University of New York Curated by ChEMBL | Assay Description Displacement of [3H]LSD from human 5HT2B receptor | Bioorg Med Chem Lett 20: 628-31 (2010) Article DOI: 10.1016/j.bmcl.2009.11.053 BindingDB Entry DOI: 10.7270/Q2C53MT3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H2 receptor (Homo sapiens (Human)) | BDBM50160160 ((S)-1,2-Dimethoxy-6-methyl-5,6,6a,7-tetrahydro-4H-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 672 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hunter College and the Graduate Center of the City University of New York Curated by ChEMBL | Assay Description Displacement of [3H]Tiotidine from human histamine H2 receptor | Bioorg Med Chem Lett 20: 628-31 (2010) Article DOI: 10.1016/j.bmcl.2009.11.053 BindingDB Entry DOI: 10.7270/Q2C53MT3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50160160 ((S)-1,2-Dimethoxy-6-methyl-5,6,6a,7-tetrahydro-4H-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 832 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hunter College and the Graduate Center of the City University of New York Curated by ChEMBL | Assay Description Displacement of [3H]Ketanserin from human 5HT2A receptor | Bioorg Med Chem Lett 20: 628-31 (2010) Article DOI: 10.1016/j.bmcl.2009.11.053 BindingDB Entry DOI: 10.7270/Q2C53MT3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50160160 ((S)-1,2-Dimethoxy-6-methyl-5,6,6a,7-tetrahydro-4H-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 858 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hunter College and the Graduate Center of the City University of New York Curated by ChEMBL | Assay Description Displacement of [3H]N-methylspiperone from human dopamine D2 receptor | Bioorg Med Chem Lett 20: 628-31 (2010) Article DOI: 10.1016/j.bmcl.2009.11.053 BindingDB Entry DOI: 10.7270/Q2C53MT3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (Homo sapiens (Human)) | BDBM50160160 ((S)-1,2-Dimethoxy-6-methyl-5,6,6a,7-tetrahydro-4H-...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 895 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hunter College and the Graduate Center of the City University of New York Curated by ChEMBL | Assay Description Displacement of [3H]SCH233930 from human dopamine D1 receptor | Bioorg Med Chem Lett 20: 628-31 (2010) Article DOI: 10.1016/j.bmcl.2009.11.053 BindingDB Entry DOI: 10.7270/Q2C53MT3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Homo sapiens (Human)) | BDBM50160160 ((S)-1,2-Dimethoxy-6-methyl-5,6,6a,7-tetrahydro-4H-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 1.07E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hunter College and the Graduate Center of the City University of New York Curated by ChEMBL | Assay Description Displacement of [3H]Mesulergine from human 5HT2C receptor | Bioorg Med Chem Lett 20: 628-31 (2010) Article DOI: 10.1016/j.bmcl.2009.11.053 BindingDB Entry DOI: 10.7270/Q2C53MT3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1B adrenergic receptor (Homo sapiens (Human)) | BDBM50160160 ((S)-1,2-Dimethoxy-6-methyl-5,6,6a,7-tetrahydro-4H-...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 1.19E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hunter College and the Graduate Center of the City University of New York Curated by ChEMBL | Assay Description Displacement of [3H]Prazosin from human adrenergic Alpha-1B receptor | Bioorg Med Chem Lett 20: 628-31 (2010) Article DOI: 10.1016/j.bmcl.2009.11.053 BindingDB Entry DOI: 10.7270/Q2C53MT3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Mus musculus (Mouse)) | BDBM60992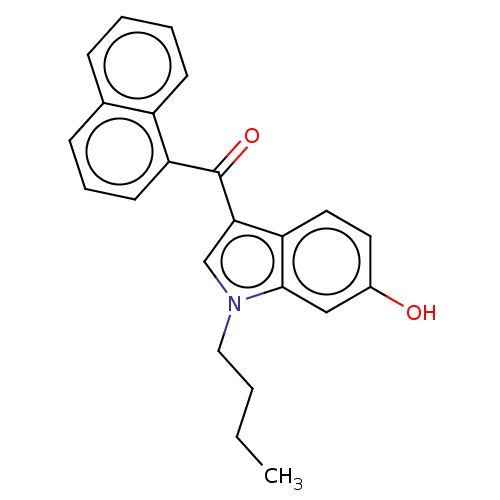 (US9416103, JWH-073-M3) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | US Patent | 1.28E+3 | -33.6 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
The Board of Trustees of the University of Arkansas; The University of Kansas US Patent | Assay Description Competition receptor binding was performed as previously described [Shoemaker et al., J. Pharmacol. Exp. Ther., 314:868-75]. Briefly, 50 μg of mou... | US Patent US9416103 (2016) BindingDB Entry DOI: 10.7270/Q2HX1BKQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2A adrenergic receptor (Homo sapiens (Human)) | BDBM50160160 ((S)-1,2-Dimethoxy-6-methyl-5,6,6a,7-tetrahydro-4H-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 1.29E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hunter College and the Graduate Center of the City University of New York Curated by ChEMBL | Assay Description Displacement of [3H]Clonidine from human adrenergic alpha2A receptor | Bioorg Med Chem Lett 20: 628-31 (2010) Article DOI: 10.1016/j.bmcl.2009.11.053 BindingDB Entry DOI: 10.7270/Q2C53MT3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 77 total ) | Next | Last >> |