 Found 262 hits with Last Name = 'fernandes' and Initial = 'e'
Found 262 hits with Last Name = 'fernandes' and Initial = 'e' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Polycomb protein EED
(Homo sapiens (Human)) | BDBM223987
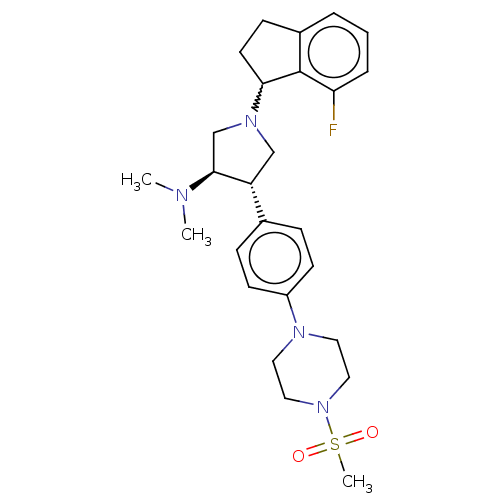
(A-395 (5) | rac-(3R,4S)-1-(7-fluoro-2,3-dihydro-1H...)Show SMILES CN(C)[C@H]1CN(C[C@@H]1c1ccc(cc1)N1CCN(CC1)S(C)(=O)=O)C1CCc2cccc(F)c12 |r,w:24.26| Show InChI InChI=1S/C26H35FN4O2S/c1-28(2)25-18-30(24-12-9-20-5-4-6-23(27)26(20)24)17-22(25)19-7-10-21(11-8-19)29-13-15-31(16-14-29)34(3,32)33/h4-8,10-11,22,24-25H,9,12-18H2,1-3H3/t22-,24?,25+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 0.400 | -53.6 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
AbbVie Inc.
| Assay Description
For the assay, compounds were dispensed in assay-ready plates using a three-fold serial dilution from 50 μM to ~850 pM using an Echo 550 Acousti... |
Nat Chem Biol 13: 389-395 (2017)
Article DOI: 10.1038/nchembio.2306
BindingDB Entry DOI: 10.7270/Q2NG4PGD |
More data for this
Ligand-Target Pair | |
Polycomb protein EED
(Homo sapiens (Human)) | BDBM223987

(A-395 (5) | rac-(3R,4S)-1-(7-fluoro-2,3-dihydro-1H...)Show SMILES CN(C)[C@H]1CN(C[C@@H]1c1ccc(cc1)N1CCN(CC1)S(C)(=O)=O)C1CCc2cccc(F)c12 |r,w:24.26| Show InChI InChI=1S/C26H35FN4O2S/c1-28(2)25-18-30(24-12-9-20-5-4-6-23(27)26(20)24)17-22(25)19-7-10-21(11-8-19)29-13-15-31(16-14-29)34(3,32)33/h4-8,10-11,22,24-25H,9,12-18H2,1-3H3/t22-,24?,25+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 0.5 | -53.1 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
AbbVie Inc.
| Assay Description
For the assay, compounds were dispensed in assay-ready plates using a three-fold serial dilution from 50 μM to ~850 pM using an Echo 550 Acousti... |
Nat Chem Biol 13: 389-395 (2017)
Article DOI: 10.1038/nchembio.2306
BindingDB Entry DOI: 10.7270/Q2NG4PGD |
More data for this
Ligand-Target Pair | |
Polycomb protein EED
(Homo sapiens (Human)) | BDBM223986
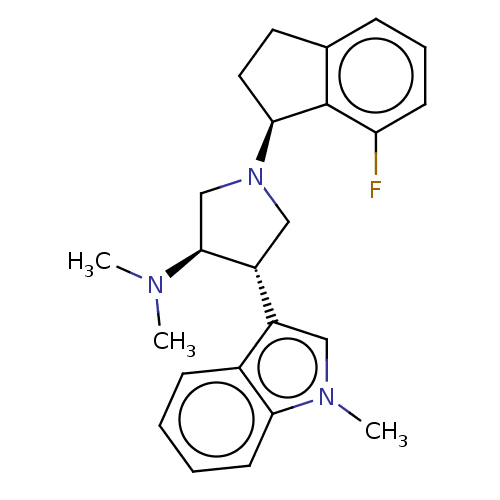
((3R,4S)-1-[(1S)-7-fluoroindan-1-yl]-N,N-dimethyl-4...)Show SMILES CN(C)[C@H]1CN(C[C@@H]1c1cn(C)c2ccccc12)[C@H]1CCc2cccc(F)c12 |r| Show InChI InChI=1S/C24H28FN3/c1-26(2)23-15-28(22-12-11-16-7-6-9-20(25)24(16)22)14-19(23)18-13-27(3)21-10-5-4-8-17(18)21/h4-10,13,19,22-23H,11-12,14-15H2,1-3H3/t19-,22+,23+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 1 | -51.4 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
AbbVie Inc.
| Assay Description
For the assay, compounds were dispensed in assay-ready plates using a three-fold serial dilution from 50 μM to ~850 pM using an Echo 550 Acousti... |
Nat Chem Biol 13: 389-395 (2017)
Article DOI: 10.1038/nchembio.2306
BindingDB Entry DOI: 10.7270/Q2NG4PGD |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Polycomb protein EED
(Homo sapiens (Human)) | BDBM223985
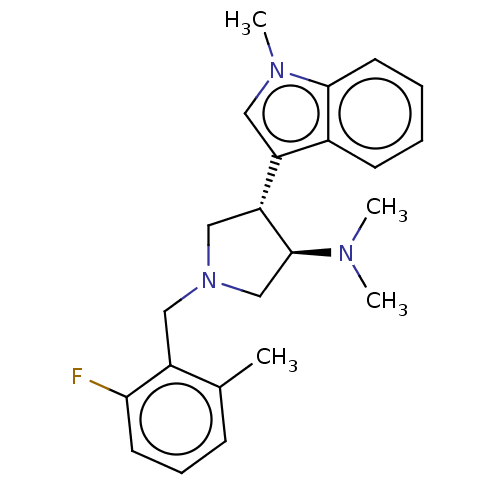
(rac-(3R,4S)-1-(2-fluoro-6-methylbenzyl)-N,N-dimeth...)Show SMILES CN(C)[C@H]1CN(Cc2c(C)cccc2F)C[C@@H]1c1cn(C)c2ccccc12 |r| Show InChI InChI=1S/C23H28FN3/c1-16-8-7-10-21(24)18(16)13-27-14-20(23(15-27)25(2)3)19-12-26(4)22-11-6-5-9-17(19)22/h5-12,20,23H,13-15H2,1-4H3/t20-,23+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 4 | -47.9 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
AbbVie Inc.
| Assay Description
For the assay, compounds were dispensed in assay-ready plates using a three-fold serial dilution from 50 μM to ~850 pM using an Echo 550 Acousti... |
Nat Chem Biol 13: 389-395 (2017)
Article DOI: 10.1038/nchembio.2306
BindingDB Entry DOI: 10.7270/Q2NG4PGD |
More data for this
Ligand-Target Pair | |
Syntenin-1
(Homo sapiens) | BDBM50557298
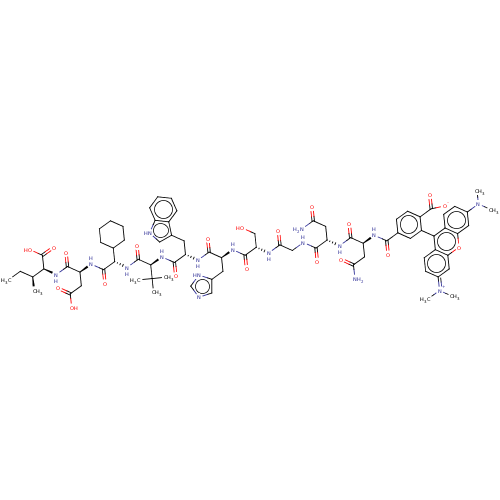
(CHEMBL4750927)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](CC(O)=O)NC(=O)[C@@H](NC(=O)[C@@H](NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@H](CO)NC(=O)CNC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CC(N)=O)NC(=O)c1ccc(C([O-])=O)c(c1)-c1c2ccc(cc2oc2cc(ccc12)=[N+](C)C)N(C)C)C(C)(C)C)C1CCCCC1)C(O)=O |r,wU:58.65,24.36,16.16,48.53,wD:8.12,20.20,66.73,2.2,38.47,4.4,(53.52,-22.4,;53.51,-23.94,;52.17,-24.71,;52.15,-26.25,;50.84,-23.93,;49.49,-24.68,;48.17,-23.9,;48.18,-22.36,;46.83,-24.66,;46.81,-26.2,;48.14,-26.98,;48.13,-28.52,;49.49,-26.22,;45.5,-23.88,;44.16,-24.64,;44.15,-26.18,;42.84,-23.85,;41.5,-24.61,;40.17,-23.83,;40.18,-22.29,;38.83,-24.59,;37.5,-23.8,;36.16,-24.56,;36.15,-26.1,;34.84,-23.78,;34.85,-22.24,;36.19,-21.48,;37.59,-22.13,;38.63,-20.99,;37.88,-19.65,;38.36,-18.19,;37.34,-17.03,;35.83,-17.34,;35.34,-18.8,;36.37,-19.95,;33.5,-24.54,;32.17,-23.76,;32.19,-22.22,;30.83,-24.51,;30.82,-26.05,;32.14,-26.84,;33.55,-26.22,;34.57,-27.37,;33.79,-28.71,;32.29,-28.37,;29.5,-23.73,;28.16,-24.5,;28.14,-26.04,;26.83,-23.71,;26.85,-22.17,;28.19,-21.42,;25.49,-24.47,;24.17,-23.68,;24.18,-22.14,;22.83,-24.45,;21.5,-23.67,;20.16,-24.42,;20.15,-25.96,;18.83,-23.64,;18.85,-22.1,;20.19,-21.34,;21.51,-22.13,;20.2,-19.8,;17.49,-24.4,;16.17,-23.62,;16.18,-22.08,;14.83,-24.37,;14.81,-25.91,;16.14,-26.7,;16.12,-28.24,;17.48,-25.94,;13.5,-23.59,;12.16,-24.35,;12.15,-25.89,;10.83,-23.57,;10.84,-22.03,;9.52,-21.25,;8.18,-22,;6.85,-21.22,;5.32,-21.05,;6.87,-19.68,;8.16,-23.54,;9.49,-24.33,;6.82,-24.3,;5.5,-23.52,;5.51,-21.98,;4.19,-21.2,;2.85,-21.96,;2.83,-23.5,;4.16,-24.28,;4.14,-25.82,;5.47,-26.6,;5.46,-28.14,;6.78,-28.92,;8.13,-28.16,;8.13,-26.62,;6.81,-25.84,;6.77,-30.46,;5.43,-31.22,;8.1,-31.24,;1.52,-21.17,;.18,-21.93,;1.53,-19.63,;38.81,-26.13,;37.48,-26.88,;40.13,-26.91,;38.8,-27.66,;42.85,-22.31,;41.52,-21.53,;41.53,-19.99,;42.87,-19.23,;44.21,-20.02,;44.19,-21.56,;50.85,-22.39,;49.52,-21.6,;52.2,-21.63,)| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of syntenin-PDZ1-2 domain (unknown origin) expressed in Escherichia coli BL21 (DE3) incubated for 5 mins by fluorescence polarization assa... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00382
BindingDB Entry DOI: 10.7270/Q2V98CRX |
More data for this
Ligand-Target Pair | |
Syntenin-1
(Homo sapiens) | BDBM50557293
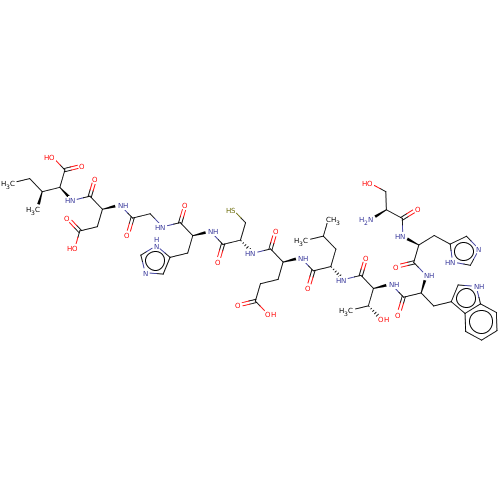
(CHEMBL4781637)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](CC(O)=O)NC(=O)CNC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@H](CS)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@@H](N)CO)[C@@H](C)O)C(O)=O |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of syntenin-PDZ1-2 domain (unknown origin) expressed in Escherichia coli BL21 (DE3) incubated for 5 mins by fluorescence polarization assa... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00382
BindingDB Entry DOI: 10.7270/Q2V98CRX |
More data for this
Ligand-Target Pair | |
Syntenin-1
(Homo sapiens) | BDBM50557299
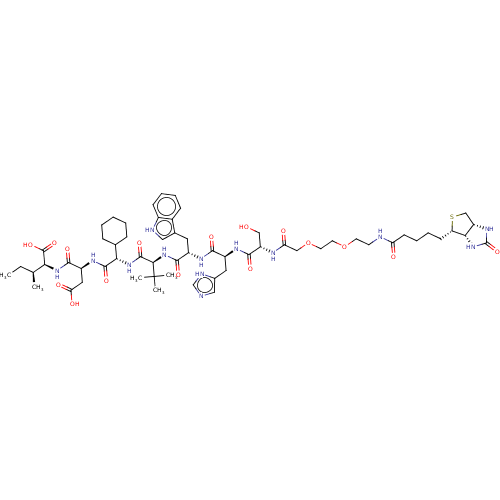
(CHEMBL4799812)Show SMILES [H][C@]12CS[C@@H](CCCCC(=O)NCCOCCOCC(=O)N[C@@H](CO)C(=O)N[C@@H](Cc3cnc[nH]3)C(=O)N[C@@H](Cc3c[nH]c4ccccc34)C(=O)N[C@H](C(=O)N[C@@H](C3CCCCC3)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H]([C@@H](C)CC)C(O)=O)C(C)(C)C)[C@@]1([H])NC(=O)N2 |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to syntenin-PDZ1-2 domain (unknown origin) expressed in Escherichia coli BL21 (DE3) by SPR analysis |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00382
BindingDB Entry DOI: 10.7270/Q2V98CRX |
More data for this
Ligand-Target Pair | |
Syntenin-1
(Homo sapiens) | BDBM50557299

(CHEMBL4799812)Show SMILES [H][C@]12CS[C@@H](CCCCC(=O)NCCOCCOCC(=O)N[C@@H](CO)C(=O)N[C@@H](Cc3cnc[nH]3)C(=O)N[C@@H](Cc3c[nH]c4ccccc34)C(=O)N[C@H](C(=O)N[C@@H](C3CCCCC3)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H]([C@@H](C)CC)C(O)=O)C(C)(C)C)[C@@]1([H])NC(=O)N2 |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of syntenin-PDZ1-2 domain (unknown origin) expressed in Escherichia coli BL21 (DE3) incubated for 5 mins by fluorescence polarization assa... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00382
BindingDB Entry DOI: 10.7270/Q2V98CRX |
More data for this
Ligand-Target Pair | |
Syntenin-1
(Homo sapiens) | BDBM50557296

(CHEMBL4792861)Show SMILES CC[C@H](C)[C@@H](NC(=O)[C@H](CC(O)=O)NC(=O)[C@@H](NC(=O)[C@@H](NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@H](CO)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CCCNC(N)=N)NC(=O)CNC(=O)[C@H](Cc1ccc(O)cc1)NC(C)=O)C(C)(C)C)C1CCCCC1)C(O)=O |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of syntenin-PDZ1-2 domain (unknown origin) expressed in Escherichia coli BL21 (DE3) incubated for 5 mins by fluorescence polarization assa... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00382
BindingDB Entry DOI: 10.7270/Q2V98CRX |
More data for this
Ligand-Target Pair | |
Syntenin-1
(Homo sapiens) | BDBM50557297
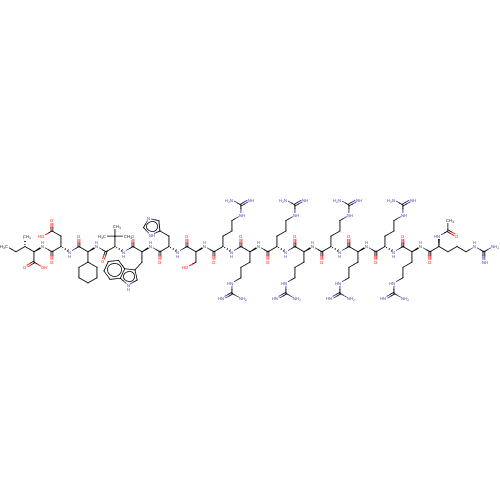
(CHEMBL4747962)Show SMILES CC[C@H](C)[C@@H](NC(=O)[C@H](CC(O)=O)NC(=O)[C@@H](NC(=O)[C@@H](NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@H](CO)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CCCNC(N)=N)NC(C)=O)C(C)(C)C)C1CCCCC1)C(O)=O |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of syntenin-PDZ1-2 domain (unknown origin) expressed in Escherichia coli BL21 (DE3) incubated for 5 mins by fluorescence polarization assa... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00382
BindingDB Entry DOI: 10.7270/Q2V98CRX |
More data for this
Ligand-Target Pair | |
Syntenin-1
(Homo sapiens) | BDBM50557295

(CHEMBL4758599)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](CC(O)=O)NC(=O)[C@@H](NC(=O)[C@@H](NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@H](CO)NC(=O)[C@H](CCC=O)NC(=O)[C@@H](CCCNC(N)=N)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H](CCCCN)NC(=O)[C@H](CCCCN)NC(=O)[C@@H](CCCNC(N)=N)NC(=O)CNC(=O)[C@@H](CCCNC(N)=N)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H](CCCNC(N)=N)NC(=O)[C@H](CCCCN)NC(C)=O)C(C)(C)C)C1CCCCC1)C(O)=O |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| 250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of syntenin-PDZ1-2 domain (unknown origin) expressed in Escherichia coli BL21 (DE3) incubated for 5 mins by fluorescence polarization assa... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00382
BindingDB Entry DOI: 10.7270/Q2V98CRX |
More data for this
Ligand-Target Pair | |
Syntenin-1
(Homo sapiens) | BDBM50557294

(CHEMBL4749490)Show SMILES CC[C@H](C)[C@@H](NC(=O)[C@H](CC(O)=O)NC(=O)[C@@H](NC(=O)[C@@H](NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@H](CO)NC(=O)[C@@H]1CCC(=O)NCCCC[C@H](NC(C)=O)C(=O)N[C@H](CCCNC(N)=N)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@H](CCCNC(N)=N)C(=O)NCC(=O)N[C@H](CCCNC(N)=N)C(=O)N[C@@H](CCCCN)C(=O)N[C@H](CCCCN)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@H](CCCNC(N)=N)C(=O)N1)C(C)(C)C)C1CCCCC1)C(O)=O |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of syntenin-PDZ1-2 domain (unknown origin) expressed in Escherichia coli BL21 (DE3) incubated for 5 mins by fluorescence polarization assa... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00382
BindingDB Entry DOI: 10.7270/Q2V98CRX |
More data for this
Ligand-Target Pair | |
Polycomb protein EED
(Homo sapiens (Human)) | BDBM223984
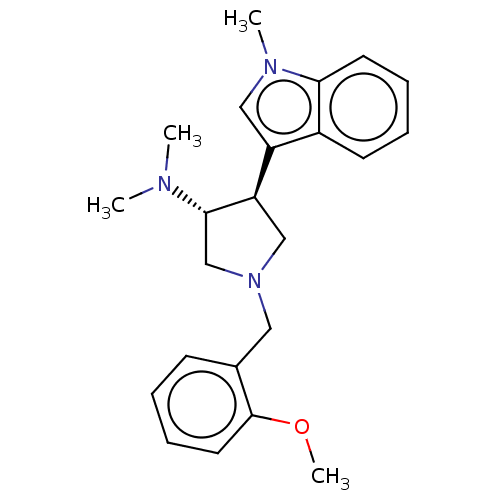
(rac-(3R,4S)-1-(2-methoxybenzyl)-N,N-dimethyl-4-(1-...)Show SMILES COc1ccccc1CN1C[C@@H]([C@H](C1)c1cn(C)c2ccccc12)N(C)C |r| Show InChI InChI=1S/C23H29N3O/c1-24(2)22-16-26(13-17-9-5-8-12-23(17)27-4)15-20(22)19-14-25(3)21-11-7-6-10-18(19)21/h5-12,14,20,22H,13,15-16H2,1-4H3/t20-,22+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 1.20E+3 | -33.8 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
AbbVie Inc.
| Assay Description
For the assay, compounds were dispensed in assay-ready plates using a three-fold serial dilution from 50 μM to ~850 pM using an Echo 550 Acousti... |
Nat Chem Biol 13: 389-395 (2017)
Article DOI: 10.1038/nchembio.2306
BindingDB Entry DOI: 10.7270/Q2NG4PGD |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Platelet-derived growth factor receptor alpha/beta
(Mus musculus (mouse)) | BDBM50611873
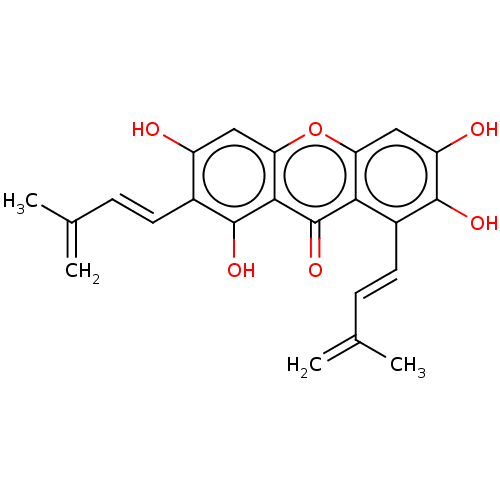
(CHEMBL5274020)Show SMILES CC(=C)\C=C\c1c(O)cc2oc3cc(O)c(O)c(\C=C\C(C)=C)c3c(=O)c2c1O | MMDB
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| UniChem
| | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Polycomb protein EED
(Homo sapiens (Human)) | BDBM223989

(A-395N (6))Show SMILES CN(C)[C@@H]1CN(Cc2ccccc2)C[C@H]1c1ccc(cc1)N1CCN(CC1)S(C)(=O)=O |r| Show InChI InChI=1S/C24H34N4O2S/c1-25(2)24-19-26(17-20-7-5-4-6-8-20)18-23(24)21-9-11-22(12-10-21)27-13-15-28(16-14-27)31(3,29)30/h4-12,23-24H,13-19H2,1-3H3/t23-,24+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.83E+3 | -32.7 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
AbbVie Inc.
| Assay Description
For the assay, compounds were dispensed in assay-ready plates using a three-fold serial dilution from 50 μM to ~850 pM using an Echo 550 Acousti... |
Nat Chem Biol 13: 389-395 (2017)
Article DOI: 10.1038/nchembio.2306
BindingDB Entry DOI: 10.7270/Q2NG4PGD |
More data for this
Ligand-Target Pair | |
Syntenin-1
(Homo sapiens) | BDBM50557286
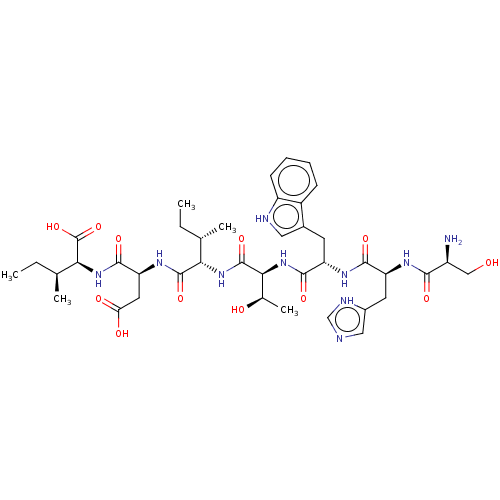
(CHEMBL4798461)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](CC(O)=O)NC(=O)[C@@H](NC(=O)[C@@H](NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@@H](N)CO)[C@@H](C)O)[C@@H](C)CC)C(O)=O |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of syntenin-PDZ1-2 domain (unknown origin) expressed in Escherichia coli BL21 (DE3) incubated for 5 mins by fluorescence polarization assa... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00382
BindingDB Entry DOI: 10.7270/Q2V98CRX |
More data for this
Ligand-Target Pair | |
Syntenin-1
(Homo sapiens) | BDBM50557287

(CHEMBL4752622)Show SMILES C[C@@H](O)[C@H](N)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](C)C(O)=O |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of syntenin-PDZ1-2 domain (unknown origin) expressed in Escherichia coli BL21 (DE3) incubated for 5 mins by fluorescence polarization assa... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00382
BindingDB Entry DOI: 10.7270/Q2V98CRX |
More data for this
Ligand-Target Pair | |
Platelet-derived growth factor receptor alpha/beta
(Mus musculus (mouse)) | BDBM50611878

(CHEMBL5273304)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]\[#6](-[#6])=[#6]\[#6]-c1c(-[#8])cc2oc3c(-[#8])ccc(-[#8])c3c(=O)c2c1-[#8] | MMDB
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| UniChem
| | 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Platelet-derived growth factor receptor alpha/beta
(Mus musculus (mouse)) | BDBM50611869
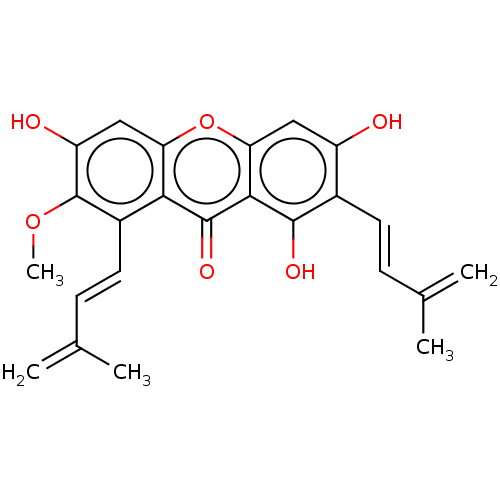
(CHEMBL5268563)Show SMILES COc1c(O)cc2oc3cc(O)c(\C=C\C(C)=C)c(O)c3c(=O)c2c1\C=C\C(C)=C | MMDB
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| UniChem
| | 7.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Platelet-derived growth factor receptor alpha/beta
(Mus musculus (mouse)) | BDBM50611871
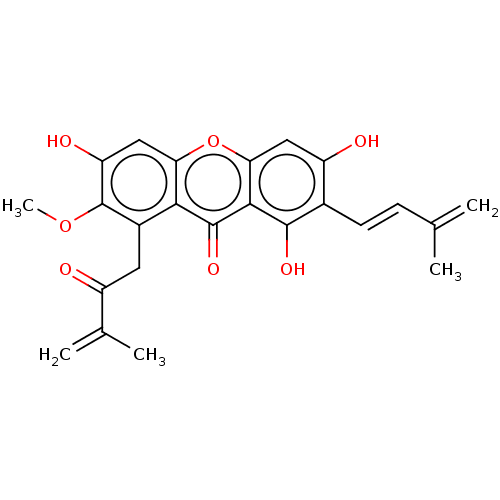
(CHEMBL5271556)Show SMILES COc1c(O)cc2oc3cc(O)c(\C=C\C(C)=C)c(O)c3c(=O)c2c1CC(=O)C(C)=C | MMDB
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| UniChem
| | 8.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Platelet-derived growth factor receptor alpha/beta
(Mus musculus (mouse)) | BDBM50611877

(CHEMBL5287666)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-c1c(-[#8])c(\[#6]=[#6]\[#6](-[#6])=[#6])c(-[#8])c2c1oc1c(-[#8])ccc(-[#8])c1c2=O | MMDB
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| UniChem
| | 8.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Syntenin-1
(Homo sapiens) | BDBM50557288

(CHEMBL4792781)Show SMILES CC(C)[C@H](NC(=O)[C@H](C)NC(=O)[C@@H](NC(=O)[C@H](CCC(O)=O)NC(=O)CNC(=O)[C@H](CCCCN)NC(=O)[C@@H](N)CO)[C@@H](C)O)C(O)=O |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of syntenin-PDZ1-2 domain (unknown origin) expressed in Escherichia coli BL21 (DE3) incubated for 5 mins by fluorescence polarization assa... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00382
BindingDB Entry DOI: 10.7270/Q2V98CRX |
More data for this
Ligand-Target Pair | |
Platelet-derived growth factor receptor alpha/beta
(Mus musculus (mouse)) | BDBM50611876
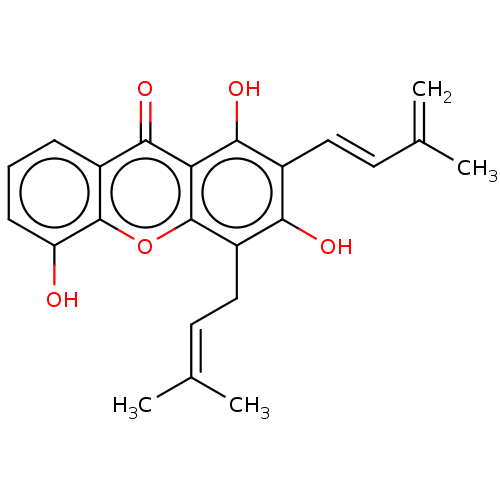
(CHEMBL5275750)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-c1c(-[#8])c(\[#6]=[#6]\[#6](-[#6])=[#6])c(-[#8])c2c1oc1c(-[#8])cccc1c2=O | MMDB
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| UniChem
| | 1.23E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Platelet-derived growth factor receptor alpha/beta
(Mus musculus (mouse)) | BDBM50611870
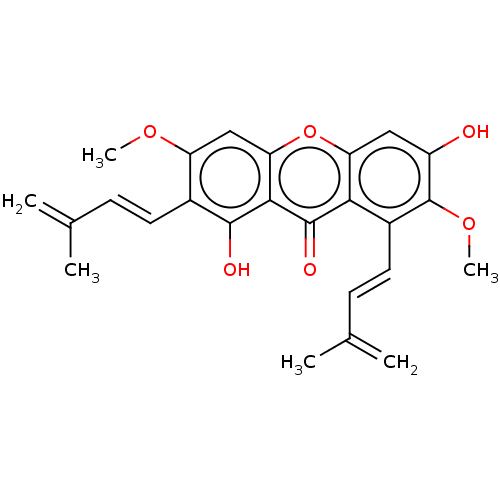
(CHEMBL5289397)Show SMILES COc1cc2oc3cc(O)c(OC)c(\C=C\C(C)=C)c3c(=O)c2c(O)c1\C=C\C(C)=C | MMDB
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| UniChem
| | 1.48E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Seed linoleate 13S-lipoxygenase-1
(Glycine max (soybean)) | BDBM50445691
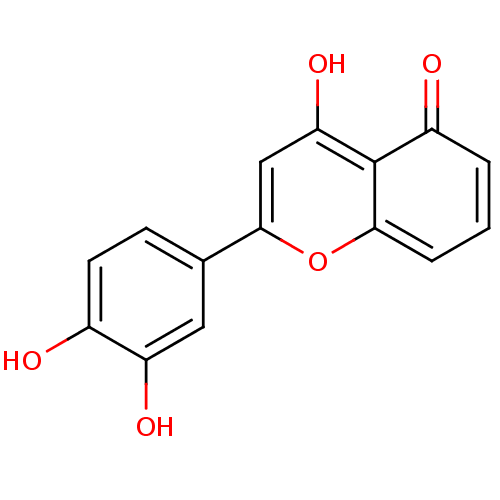
(CHEMBL243677)Show InChI InChI=1S/C15H10O5/c16-9-5-4-8(6-11(9)18)14-7-12(19)15-10(17)2-1-3-13(15)20-14/h1-7,16,18-19H | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade do Porto
Curated by ChEMBL
| Assay Description
Uncompetitive inhibition of soybean LOX-1 using linoleic acid as substrate preincubated for 5 mins followed by substrate addition by Lineweaver-Burk ... |
Eur J Med Chem 72: 137-45 (2014)
Article DOI: 10.1016/j.ejmech.2013.11.030
BindingDB Entry DOI: 10.7270/Q27H1M2P |
More data for this
Ligand-Target Pair | |
Platelet-derived growth factor receptor alpha/beta
(Mus musculus (mouse)) | BDBM50325677
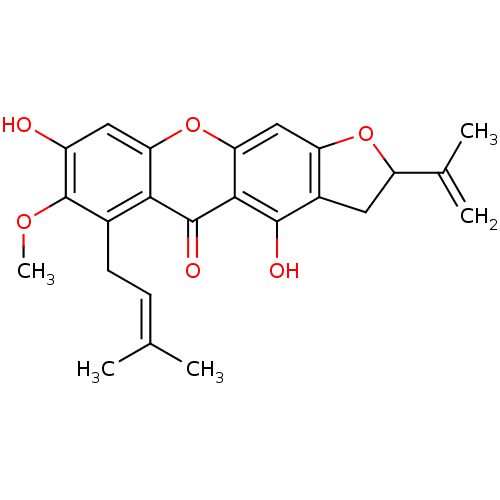
(4,8-dihydroxy-7-methoxy-6-(3-methylbut-2-enyl)-2-(...)Show SMILES [#6]-[#8]-c1c(-[#8])cc2oc3cc4-[#8]-[#6](-[#6]-c4c(-[#8])c3c(=O)c2c1-[#6]\[#6]=[#6](\[#6])-[#6])-[#6](-[#6])=[#6] Show InChI InChI=1S/C24H24O6/c1-11(2)6-7-13-20-18(9-15(25)24(13)28-5)30-19-10-17-14(8-16(29-17)12(3)4)22(26)21(19)23(20)27/h6,9-10,16,25-26H,3,7-8H2,1-2,4-5H3 | MMDB
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | 1.81E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Seed linoleate 13S-lipoxygenase-1
(Glycine max (soybean)) | BDBM7461
(5,7-dihydroxy-2-phenyl-4H-chromen-4-one | 5,7-dihy...)Show InChI InChI=1S/C15H10O4/c16-10-6-11(17)15-12(18)8-13(19-14(15)7-10)9-4-2-1-3-5-9/h1-8,16-17H | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 1.94E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade do Porto
Curated by ChEMBL
| Assay Description
Mixed noncompetitive type inhibition of soybean LOX-1 using linoleic acid as substrate preincubated for 5 mins followed by substrate addition by Line... |
Eur J Med Chem 72: 137-45 (2014)
Article DOI: 10.1016/j.ejmech.2013.11.030
BindingDB Entry DOI: 10.7270/Q27H1M2P |
More data for this
Ligand-Target Pair | |
Platelet-derived growth factor receptor alpha/beta
(Mus musculus (mouse)) | BDBM50611881
(CHEMBL5273562)Show SMILES COc1c(O)cc2oc3cc4OC(C)(C)C(O)Cc4c(O)c3c(=O)c2c1\C=C\C(C)=C | MMDB
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| UniChem
| | 2.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Syntenin-1
(Homo sapiens) | BDBM50557289
(CHEMBL4740875)Show SMILES CSCC[C@H](NC(=O)[C@@H](NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)CNC(=O)[C@H](CO)NC(=O)[C@@H](N)CCCNC(N)=N)C(C)C)C(O)=O |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of syntenin-PDZ1-2 domain (unknown origin) expressed in Escherichia coli BL21 (DE3) incubated for 5 mins by fluorescence polarization assa... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00382
BindingDB Entry DOI: 10.7270/Q2V98CRX |
More data for this
Ligand-Target Pair | |
Syntenin-1
(Homo sapiens) | BDBM50557290

(CHEMBL4798840)Show SMILES CC(C)C[C@H](NC(=O)[C@@H](N)[C@@H](C)O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CO)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](Cc1ccccc1)C(O)=O |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 4.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of syntenin-PDZ1-2 domain (unknown origin) expressed in Escherichia coli BL21 (DE3) incubated for 5 mins by fluorescence polarization assa... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00382
BindingDB Entry DOI: 10.7270/Q2V98CRX |
More data for this
Ligand-Target Pair | |
Seed linoleate 13S-lipoxygenase-1
(Glycine max (soybean)) | BDBM50157555
(2-(3,4-dihydroxyphenyl)-4H-chromen-4-one | 3',4'-d...)Show InChI InChI=1S/C15H10O4/c16-11-6-5-9(7-13(11)18)15-8-12(17)10-3-1-2-4-14(10)19-15/h1-8,16,18H | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 8.45E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade do Porto
Curated by ChEMBL
| Assay Description
Mixed noncompetitive type inhibition of soybean LOX-1 using linoleic acid as substrate preincubated for 5 mins followed by substrate addition by Line... |
Eur J Med Chem 72: 137-45 (2014)
Article DOI: 10.1016/j.ejmech.2013.11.030
BindingDB Entry DOI: 10.7270/Q27H1M2P |
More data for this
Ligand-Target Pair | |
Seed linoleate 13S-lipoxygenase-1
(Glycine max (soybean)) | BDBM50445690
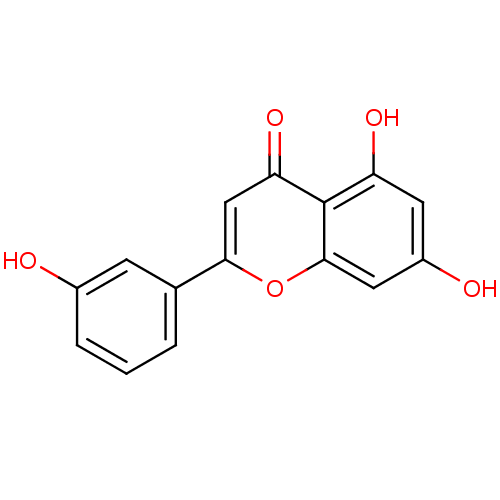
(CHEMBL457821)Show InChI InChI=1S/C15H10O5/c16-9-3-1-2-8(4-9)13-7-12(19)15-11(18)5-10(17)6-14(15)20-13/h1-7,16-18H | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 9.54E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade do Porto
Curated by ChEMBL
| Assay Description
Uncompetitive inhibition of soybean LOX-1 using linoleic acid as substrate preincubated for 5 mins followed by substrate addition by Lineweaver-Burk ... |
Eur J Med Chem 72: 137-45 (2014)
Article DOI: 10.1016/j.ejmech.2013.11.030
BindingDB Entry DOI: 10.7270/Q27H1M2P |
More data for this
Ligand-Target Pair | |
Syntenin-1
(Homo sapiens) | BDBM50557291

(CHEMBL4785814)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](C)NC(=O)[C@@H](N)CC(N)=O)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](CO)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](C)C(O)=O |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of syntenin-PDZ1-2 domain (unknown origin) expressed in Escherichia coli BL21 (DE3) incubated for 5 mins by fluorescence polarization assa... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00382
BindingDB Entry DOI: 10.7270/Q2V98CRX |
More data for this
Ligand-Target Pair | |
Seed linoleate 13S-lipoxygenase-1
(Glycine max (soybean)) | BDBM50312650
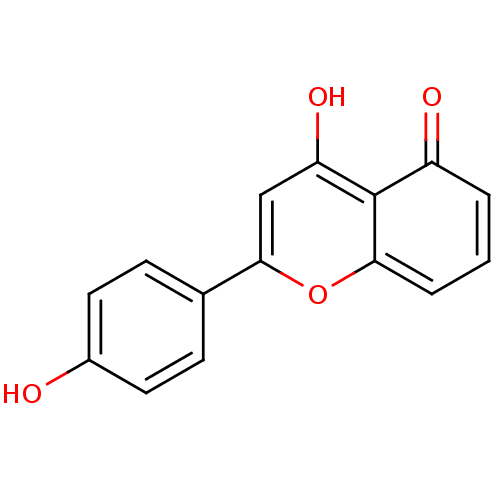
(5,4'-dihydroxyflavone | 5-hydroxy-2-(4-hydroxyphen...)Show InChI InChI=1S/C15H10O4/c16-10-6-4-9(5-7-10)14-8-12(18)15-11(17)2-1-3-13(15)19-14/h1-8,16,18H | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.03E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade do Porto
Curated by ChEMBL
| Assay Description
Mixed noncompetitive type inhibition of soybean LOX-1 using linoleic acid as substrate preincubated for 5 mins followed by substrate addition by Line... |
Eur J Med Chem 72: 137-45 (2014)
Article DOI: 10.1016/j.ejmech.2013.11.030
BindingDB Entry DOI: 10.7270/Q27H1M2P |
More data for this
Ligand-Target Pair | |
Seed linoleate 13S-lipoxygenase-1
(Glycine max (soybean)) | BDBM7460

(2-(3,4-dihydroxyphenyl)-3,5,7-trihydroxy-4H-chrome...)Show InChI InChI=1S/C15H10O7/c16-7-4-10(19)12-11(5-7)22-15(14(21)13(12)20)6-1-2-8(17)9(18)3-6/h1-5,16-19,21H | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 1.07E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade do Porto
Curated by ChEMBL
| Assay Description
Uncompetitive inhibition of soybean LOX-1 using linoleic acid as substrate preincubated for 5 mins followed by substrate addition by Lineweaver-Burk ... |
Eur J Med Chem 72: 137-45 (2014)
Article DOI: 10.1016/j.ejmech.2013.11.030
BindingDB Entry DOI: 10.7270/Q27H1M2P |
More data for this
Ligand-Target Pair | |
Syntenin-1
(Homo sapiens) | BDBM50557292

(CHEMBL4779632)Show SMILES CC(C)[C@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CC(O)=O)NC(=O)[C@@H](N)CCCCN)C(O)=O |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.10E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of syntenin-PDZ1-2 domain (unknown origin) expressed in Escherichia coli BL21 (DE3) incubated for 5 mins by fluorescence polarization assa... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00382
BindingDB Entry DOI: 10.7270/Q2V98CRX |
More data for this
Ligand-Target Pair | |
Seed linoleate 13S-lipoxygenase-1
(Glycine max (soybean)) | BDBM7459

(2-(3,4-dihydroxyphenyl)-5,7-dihydroxy-4H-chromen-4...)Show InChI InChI=1S/C15H10O6/c16-8-4-11(19)15-12(20)6-13(21-14(15)5-8)7-1-2-9(17)10(18)3-7/h1-6,16-19H | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 1.22E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade do Porto
Curated by ChEMBL
| Assay Description
Uncompetitive inhibition of soybean LOX-1 using linoleic acid as substrate preincubated for 5 mins followed by substrate addition by Lineweaver-Burk ... |
Eur J Med Chem 72: 137-45 (2014)
Article DOI: 10.1016/j.ejmech.2013.11.030
BindingDB Entry DOI: 10.7270/Q27H1M2P |
More data for this
Ligand-Target Pair | |
Seed linoleate 13S-lipoxygenase-1
(Glycine max (soybean)) | BDBM7458

(5,7-dihydroxy-2-(4-hydroxyphenyl)-4H-chromen-4-one...)Show InChI InChI=1S/C15H10O5/c16-9-3-1-8(2-4-9)13-7-12(19)15-11(18)5-10(17)6-14(15)20-13/h1-7,16-18H | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 1.78E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade do Porto
Curated by ChEMBL
| Assay Description
Mixed noncompetitive type inhibition of soybean LOX-1 using linoleic acid as substrate preincubated for 5 mins followed by substrate addition by Line... |
Eur J Med Chem 72: 137-45 (2014)
Article DOI: 10.1016/j.ejmech.2013.11.030
BindingDB Entry DOI: 10.7270/Q27H1M2P |
More data for this
Ligand-Target Pair | |
Syntenin-1
(Homo sapiens) | BDBM50557285
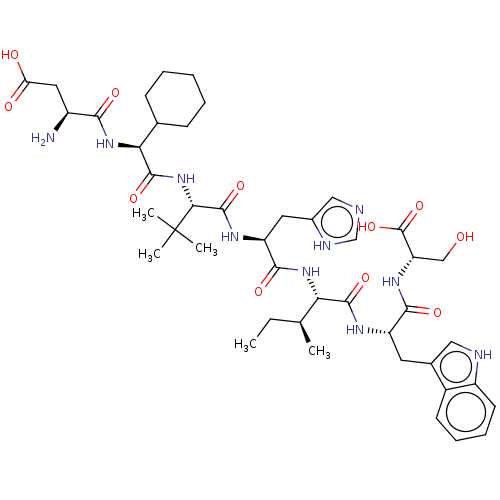
(CHEMBL4788058)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@@H](NC(=O)[C@@H](NC(=O)[C@@H](N)CC(O)=O)C1CCCCC1)C(C)(C)C)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CO)C(O)=O |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| >5.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of syntenin-PDZ1-2 domain (unknown origin) expressed in Escherichia coli BL21 (DE3) incubated for 5 mins by fluorescence polarization assa... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00382
BindingDB Entry DOI: 10.7270/Q2V98CRX |
More data for this
Ligand-Target Pair | |
Platelet-derived growth factor receptor alpha/beta
(Mus musculus (mouse)) | BDBM50155446
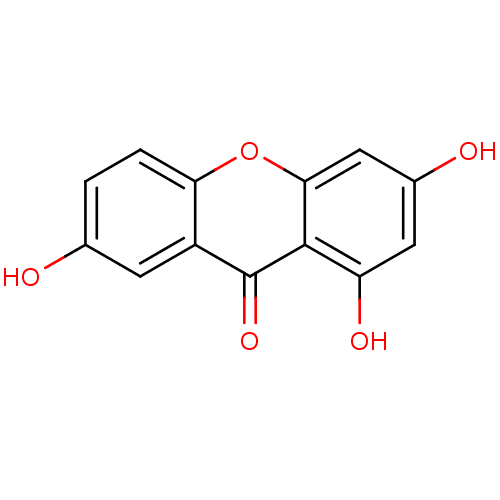
(1,3,7-Trihydroxy xanthone (3) | 1,3,7-Trihydroxy-x...)Show InChI InChI=1S/C13H8O5/c14-6-1-2-10-8(3-6)13(17)12-9(16)4-7(15)5-11(12)18-10/h1-5,14-16H | MMDB
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| | n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Polycomb protein EED
(Homo sapiens (Human)) | BDBM223987

(A-395 (5) | rac-(3R,4S)-1-(7-fluoro-2,3-dihydro-1H...)Show SMILES CN(C)[C@H]1CN(C[C@@H]1c1ccc(cc1)N1CCN(CC1)S(C)(=O)=O)C1CCc2cccc(F)c12 |r,w:24.26| Show InChI InChI=1S/C26H35FN4O2S/c1-28(2)25-18-30(24-12-9-20-5-4-6-23(27)26(20)24)17-22(25)19-7-10-21(11-8-19)29-13-15-31(16-14-29)34(3,32)33/h4-8,10-11,22,24-25H,9,12-18H2,1-3H3/t22-,24?,25+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7.51 | n/a | n/a | n/a | n/a | 7.5 | 25 |
AbbVie Inc.
| Assay Description
For the assay, compounds or nonbiotinylated H3(23-34) peptide were dispensed in assay-ready plates using a three-fold serial dilution from 50 μM... |
Nat Chem Biol 13: 389-395 (2017)
Article DOI: 10.1038/nchembio.2306
BindingDB Entry DOI: 10.7270/Q2NG4PGD |
More data for this
Ligand-Target Pair | |
Platelet-derived growth factor receptor alpha/beta
(Mus musculus (mouse)) | BDBM50611917

(CHEMBL2436928) | MMDB
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 7.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Platelet-derived growth factor receptor alpha/beta
(Mus musculus (mouse)) | BDBM50611920
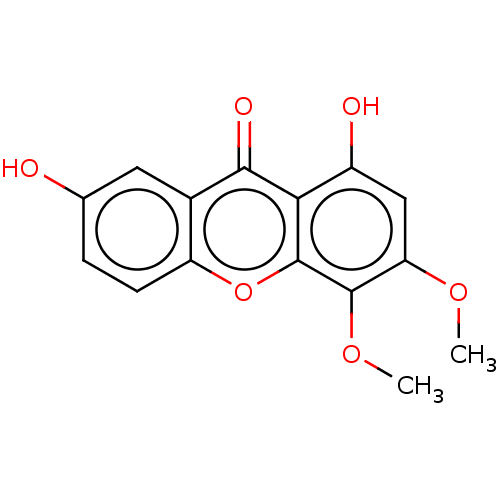
(CHEMBL4100829) | MMDB
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Platelet-derived growth factor receptor alpha/beta
(Mus musculus (mouse)) | BDBM50611919
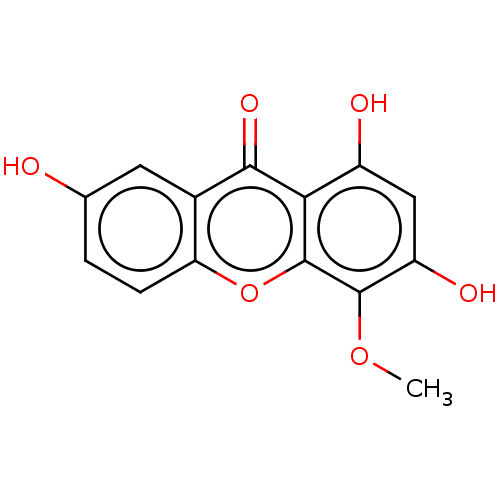
(CHEMBL5274472) | MMDB
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Polycomb protein EED
(Homo sapiens (Human)) | BDBM223987

(A-395 (5) | rac-(3R,4S)-1-(7-fluoro-2,3-dihydro-1H...)Show SMILES CN(C)[C@H]1CN(C[C@@H]1c1ccc(cc1)N1CCN(CC1)S(C)(=O)=O)C1CCc2cccc(F)c12 |r,w:24.26| Show InChI InChI=1S/C26H35FN4O2S/c1-28(2)25-18-30(24-12-9-20-5-4-6-23(27)26(20)24)17-22(25)19-7-10-21(11-8-19)29-13-15-31(16-14-29)34(3,32)33/h4-8,10-11,22,24-25H,9,12-18H2,1-3H3/t22-,24?,25+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | 23 |
AbbVie Inc.
| Assay Description
IC50 values were determined for compounds A-395 and A-395N at 75 nM of EZH2 trimeric complex (EZH2-EED-SUZ12), 3 μM of human nucleosome and 1 &#... |
Nat Chem Biol 13: 389-395 (2017)
Article DOI: 10.1038/nchembio.2306
BindingDB Entry DOI: 10.7270/Q2NG4PGD |
More data for this
Ligand-Target Pair | |
Platelet-derived growth factor receptor alpha/beta
(Mus musculus (mouse)) | BDBM50611832
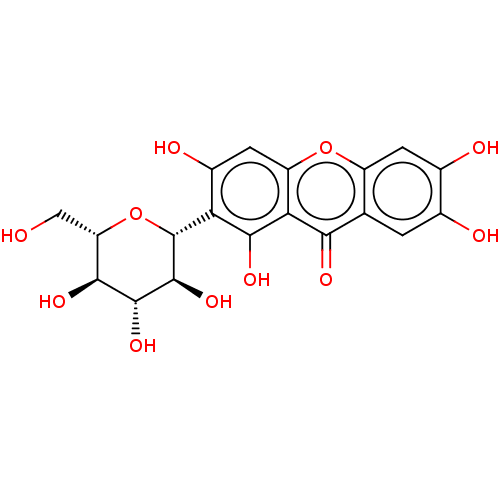
(CHEMBL4856778)Show SMILES OC[C@@H]1O[C@@H]([C@@H](O)[C@H](O)[C@H]1O)c1c(O)cc2oc3cc(O)c(O)cc3c(=O)c2c1O |r| | MMDB
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Platelet-derived growth factor receptor alpha/beta
(Mus musculus (mouse)) | BDBM50485132
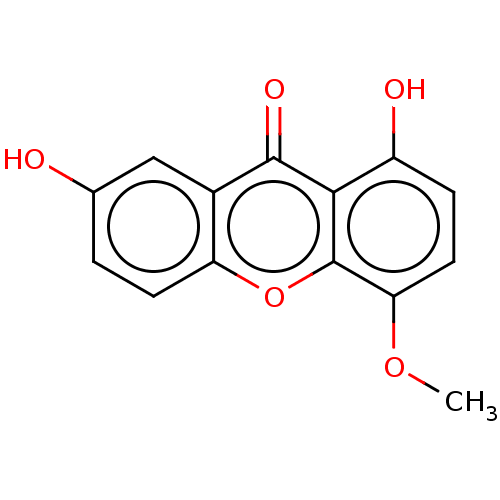
(CHEBI:67553 | CHEMBL484030)Show InChI InChI=1S/C14H10O5/c1-18-11-5-3-9(16)12-13(17)8-6-7(15)2-4-10(8)19-14(11)12/h2-6,15-16H,1H3 | MMDB
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| | n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Platelet-derived growth factor receptor alpha/beta
(Mus musculus (mouse)) | BDBM50268290
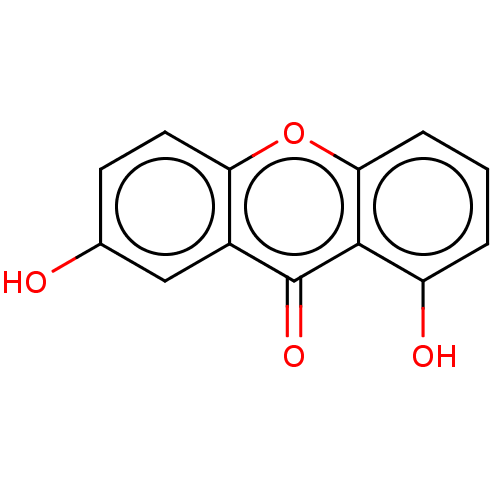
(1,7-Dihydroxyxanthone | CHEBI:4946 | CHEMBL389166)Show InChI InChI=1S/C13H8O4/c14-7-4-5-10-8(6-7)13(16)12-9(15)2-1-3-11(12)17-10/h1-6,14-15H | MMDB
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Platelet-derived growth factor receptor alpha/beta
(Mus musculus (mouse)) | BDBM50611923
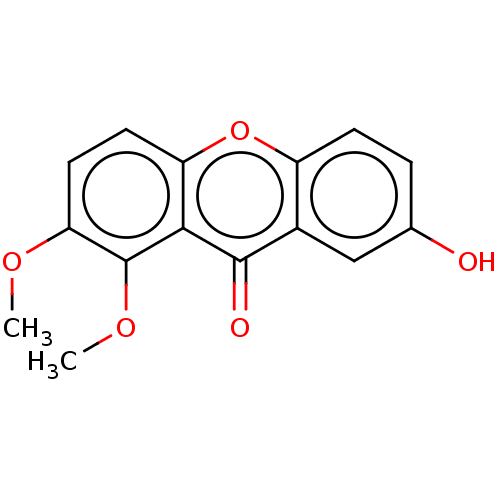
(CHEMBL4083803) | MMDB
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 49 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Platelet-derived growth factor receptor alpha/beta
(Mus musculus (mouse)) | BDBM23406

((3R,4R,5S,6R)-5-{[(2R,3R,4R,5S,6R)-5-{[(2R,3R,4S,5...)Show SMILES C[C@H]1O[C@H](O[C@@H]2[C@@H](CO)O[C@H](O[C@@H]3[C@@H](CO)OC(O)[C@H](O)[C@H]3O)[C@H](O)[C@H]2O)[C@H](O)[C@@H](O)[C@@H]1N[C@H]1C=C(CO)[C@@H](O)[C@H](O)[C@H]1O |t:37| Show InChI InChI=1S/C25H43NO18/c1-6-11(26-8-2-7(3-27)12(30)15(33)13(8)31)14(32)19(37)24(40-6)43-22-10(5-29)42-25(20(38)17(22)35)44-21-9(4-28)41-23(39)18(36)16(21)34/h2,6,8-39H,3-5H2,1H3/t6-,8+,9-,10-,11-,12-,13+,14+,15+,16-,17-,18-,19-,20-,21-,22-,23?,24-,25-/m1/s1 | MMDB
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
PDB
UniChem
Similars
| | n/a | n/a | 54 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data