 Found 68 hits with Last Name = 'ferrara' and Initial = 'fn'
Found 68 hits with Last Name = 'ferrara' and Initial = 'fn' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Type-1 angiotensin II receptor B
(RAT) | BDBM50004154

(2-Butyl-4-chloro-6-methyl-1-[2'-(1H-tetrazol-5-yl)...)Show SMILES CCCCC1=NC(Cl)=C(C(C)N1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1)C(O)=O |c:7,t:4| Show InChI InChI=1S/C24H25ClN6O2/c1-3-4-9-20-26-22(25)21(24(32)33)15(2)31(20)14-16-10-12-17(13-11-16)18-7-5-6-8-19(18)23-27-29-30-28-23/h5-8,10-13,15H,3-4,9,14H2,1-2H3,(H,32,33)(H,27,28,29,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of [125I]- Sar,Ile8-angiotensin II binding to rat adrenal corticcal membrane angiotensin II receptor |
J Med Chem 35: 4751-63 (1993)
BindingDB Entry DOI: 10.7270/Q2VH5PFJ |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor B
(RAT) | BDBM50004155
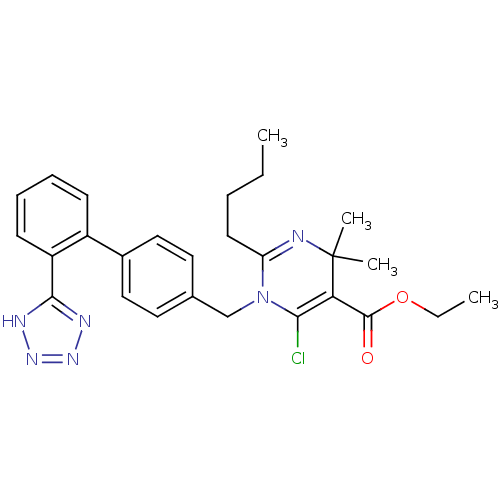
(2-Butyl-6-chloro-4,4-dimethyl-1-[2'-(1H-tetrazol-5...)Show SMILES CCCCC1=NC(C)(C)C(C(=O)OCC)=C(Cl)N1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1 |t:4,14| Show InChI InChI=1S/C27H31ClN6O2/c1-5-7-12-22-29-27(3,4)23(26(35)36-6-2)24(28)34(22)17-18-13-15-19(16-14-18)20-10-8-9-11-21(20)25-30-32-33-31-25/h8-11,13-16H,5-7,12,17H2,1-4H3,(H,30,31,32,33) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of [125I]- Sar,Ile8-angiotensin II binding to rat adrenal corticcal membrane angiotensin II receptor |
J Med Chem 35: 4751-63 (1993)
BindingDB Entry DOI: 10.7270/Q2VH5PFJ |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor B
(RAT) | BDBM50004164
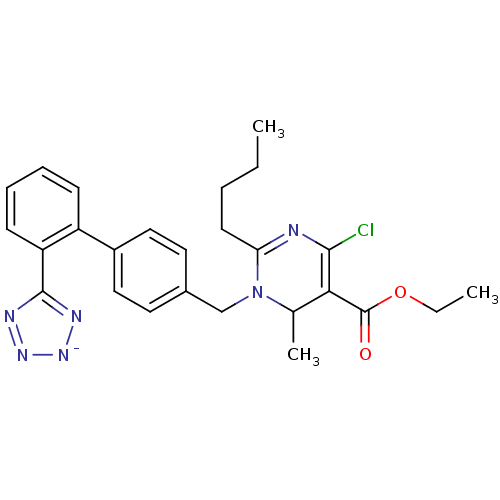
(2-Butyl-4-chloro-6-methyl-1-[2'-(1H-tetrazol-5-yl)...)Show SMILES CCCCC1=NC(Cl)=C(C(C)N1Cc1ccc(cc1)-c1ccccc1-c1nn[n-]n1)C(=O)OCC |c:7,t:4| Show InChI InChI=1S/C26H28ClN6O2/c1-4-6-11-22-28-24(27)23(26(34)35-5-2)17(3)33(22)16-18-12-14-19(15-13-18)20-9-7-8-10-21(20)25-29-31-32-30-25/h7-10,12-15,17H,4-6,11,16H2,1-3H3/q-1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 8.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of [125I]- Sar,Ile8-angiotensin II binding to rat adrenal corticcal membrane angiotensin II receptor |
J Med Chem 35: 4751-63 (1993)
BindingDB Entry DOI: 10.7270/Q2VH5PFJ |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor B
(RAT) | BDBM50406795
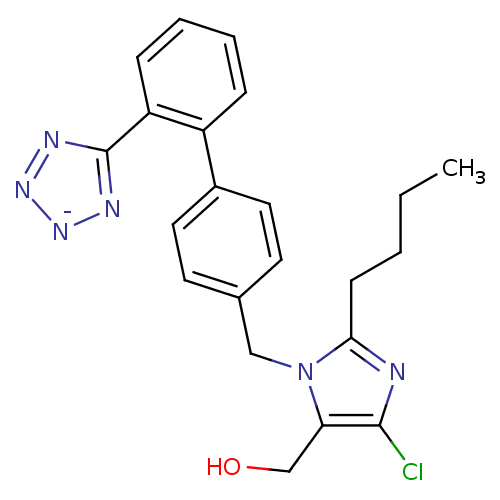
(Cozaar | LOSARTAN POTASSIUM)Show SMILES CCCCc1nc(Cl)c(CO)n1Cc1ccc(cc1)-c1ccccc1-c1nn[n-]n1 Show InChI InChI=1S/C22H22ClN6O/c1-2-3-8-20-24-21(23)19(14-30)29(20)13-15-9-11-16(12-10-15)17-6-4-5-7-18(17)22-25-27-28-26-22/h4-7,9-12,30H,2-3,8,13-14H2,1H3/q-1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| PubMed
| 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
In vitro antagonistic potency against angiotensin II receptor using [125I]- Sar,Ile8-angiotensin II as the radioligand in rat adrenal cortical membra... |
J Med Chem 35: 4751-63 (1993)
BindingDB Entry DOI: 10.7270/Q2VH5PFJ |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor B
(RAT) | BDBM50004153
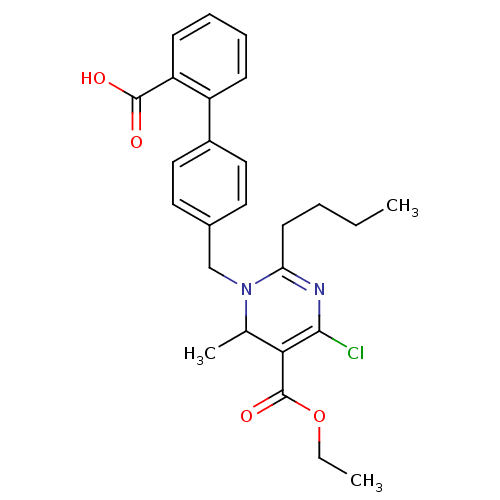
(2-Butyl-1-(2'-carboxy-biphenyl-4-ylmethyl)-4-chlor...)Show SMILES CCCCC1=NC(Cl)=C(C(C)N1Cc1ccc(cc1)-c1ccccc1C(O)=O)C(=O)OCC |c:7,t:4| Show InChI InChI=1S/C26H29ClN2O4/c1-4-6-11-22-28-24(27)23(26(32)33-5-2)17(3)29(22)16-18-12-14-19(15-13-18)20-9-7-8-10-21(20)25(30)31/h7-10,12-15,17H,4-6,11,16H2,1-3H3,(H,30,31) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 41 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of [125I]- Sar,Ile8-angiotensin II binding to rat adrenal corticcal membrane angiotensin II receptor |
J Med Chem 35: 4751-63 (1993)
BindingDB Entry DOI: 10.7270/Q2VH5PFJ |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor B
(RAT) | BDBM50004160
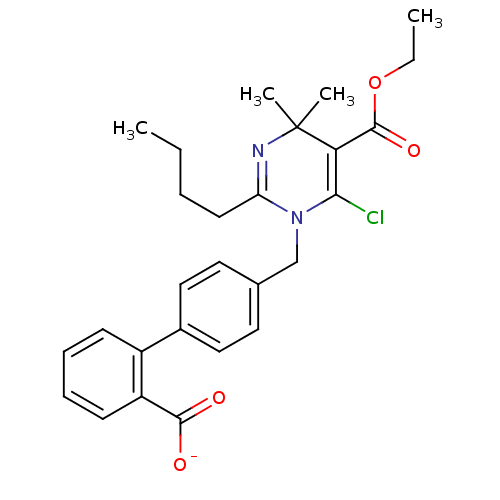
(CHEMBL145036 | Sodium; 4'-(2-butyl-6-chloro-5-etho...)Show SMILES CCCCC1=NC(C)(C)C(C(=O)OCC)=C(Cl)N1Cc1ccc(cc1)-c1ccccc1C([O-])=O |t:4,14| Show InChI InChI=1S/C27H31ClN2O4/c1-5-7-12-22-29-27(3,4)23(26(33)34-6-2)24(28)30(22)17-18-13-15-19(16-14-18)20-10-8-9-11-21(20)25(31)32/h8-11,13-16H,5-7,12,17H2,1-4H3,(H,31,32)/p-1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 58 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of [125I]- Sar,Ile8-angiotensin II binding to rat adrenal corticcal membrane angiotensin II receptor |
J Med Chem 35: 4751-63 (1993)
BindingDB Entry DOI: 10.7270/Q2VH5PFJ |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor B
(RAT) | BDBM50004163
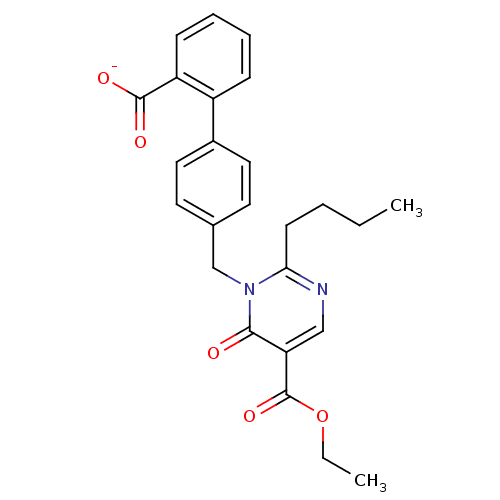
(CHEMBL342287 | Sodium; 4'-(2-butyl-5-ethoxycarbony...)Show SMILES CCCCc1ncc(C(=O)OCC)c(=O)n1Cc1ccc(cc1)-c1ccccc1C([O-])=O Show InChI InChI=1S/C25H26N2O5/c1-3-5-10-22-26-15-21(25(31)32-4-2)23(28)27(22)16-17-11-13-18(14-12-17)19-8-6-7-9-20(19)24(29)30/h6-9,11-15H,3-5,10,16H2,1-2H3,(H,29,30)/p-1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 82 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of [125I]- Sar,Ile8-angiotensin II binding to rat adrenal corticcal membrane angiotensin II receptor |
J Med Chem 35: 4751-63 (1993)
BindingDB Entry DOI: 10.7270/Q2VH5PFJ |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor B
(RAT) | BDBM82258

(CAS_114798-26-4 | Losartan | NSC_3961)Show SMILES CCCCc1nc(Cl)c(CO)n1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1 Show InChI InChI=1S/C22H23ClN6O/c1-2-3-8-20-24-21(23)19(14-30)29(20)13-15-9-11-16(12-10-15)17-6-4-5-7-18(17)22-25-27-28-26-22/h4-7,9-12,30H,2-3,8,13-14H2,1H3,(H,25,26,27,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 95 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
In vitro antagonistic potency against angiotensin II receptor using [125I]- Sar,Ile8-angiotensin II as the radioligand in rat adrenal cortical membra... |
J Med Chem 35: 4751-63 (1993)
BindingDB Entry DOI: 10.7270/Q2VH5PFJ |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor B
(RAT) | BDBM50004162
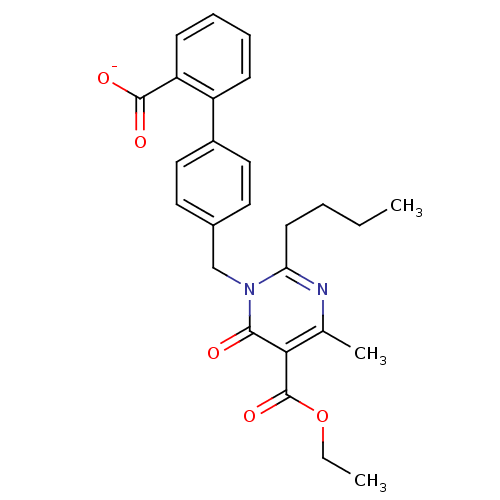
(CHEMBL145276 | Sodium; 4'-(2-butyl-5-ethoxycarbony...)Show SMILES CCCCc1nc(C)c(C(=O)OCC)c(=O)n1Cc1ccc(cc1)-c1ccccc1C([O-])=O Show InChI InChI=1S/C26H28N2O5/c1-4-6-11-22-27-17(3)23(26(32)33-5-2)24(29)28(22)16-18-12-14-19(15-13-18)20-9-7-8-10-21(20)25(30)31/h7-10,12-15H,4-6,11,16H2,1-3H3,(H,30,31)/p-1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of [125I]- Sar,Ile8-angiotensin II binding to rat adrenal corticcal membrane angiotensin II receptor |
J Med Chem 35: 4751-63 (1993)
BindingDB Entry DOI: 10.7270/Q2VH5PFJ |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor B
(RAT) | BDBM50004156

(2-Butyl-1-(2'-carboxy-biphenyl-4-ylmethyl)-4-(4-ch...)Show SMILES CCCCC1=NC(=C(C(C)N1Cc1ccc(cc1)-c1ccccc1C(O)=O)C(=O)OCC)c1ccc(Cl)cc1 |c:6,t:4| Show InChI InChI=1S/C32H33ClN2O4/c1-4-6-11-28-34-30(24-16-18-25(33)19-17-24)29(32(38)39-5-2)21(3)35(28)20-22-12-14-23(15-13-22)26-9-7-8-10-27(26)31(36)37/h7-10,12-19,21H,4-6,11,20H2,1-3H3,(H,36,37) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of [125I]- Sar,Ile8-angiotensin II binding to rat adrenal corticcal membrane angiotensin II receptor |
J Med Chem 35: 4751-63 (1993)
BindingDB Entry DOI: 10.7270/Q2VH5PFJ |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor B
(RAT) | BDBM50004157

(2-Butyl-1-(2'-carboxy-biphenyl-4-ylmethyl)-6-methy...)Show SMILES CCCCC1=NC(=C(C(C)N1Cc1ccc(cc1)-c1ccccc1C(O)=O)C(=O)OCC)c1ccccc1 |c:6,t:4| Show InChI InChI=1S/C32H34N2O4/c1-4-6-16-28-33-30(25-12-8-7-9-13-25)29(32(37)38-5-2)22(3)34(28)21-23-17-19-24(20-18-23)26-14-10-11-15-27(26)31(35)36/h7-15,17-20,22H,4-6,16,21H2,1-3H3,(H,35,36) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 850 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of [125I]- Sar,Ile8-angiotensin II binding to rat adrenal corticcal membrane angiotensin II receptor |
J Med Chem 35: 4751-63 (1993)
BindingDB Entry DOI: 10.7270/Q2VH5PFJ |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor B
(RAT) | BDBM50004158
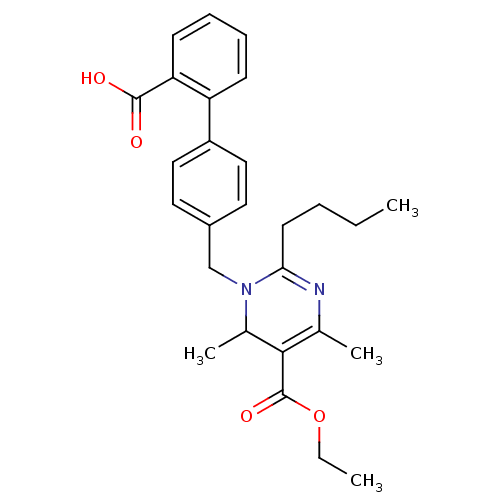
(2-Butyl-1-(2'-carboxy-biphenyl-4-ylmethyl)-4,6-dim...)Show SMILES CCCCC1=NC(C)=C(C(C)N1Cc1ccc(cc1)-c1ccccc1C(O)=O)C(=O)OCC |c:7,t:4| Show InChI InChI=1S/C27H32N2O4/c1-5-7-12-24-28-18(3)25(27(32)33-6-2)19(4)29(24)17-20-13-15-21(16-14-20)22-10-8-9-11-23(22)26(30)31/h8-11,13-16,19H,5-7,12,17H2,1-4H3,(H,30,31) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 990 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of [125I]- Sar,Ile8-angiotensin II binding to rat adrenal corticcal membrane angiotensin II receptor |
J Med Chem 35: 4751-63 (1993)
BindingDB Entry DOI: 10.7270/Q2VH5PFJ |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor B
(RAT) | BDBM50004159
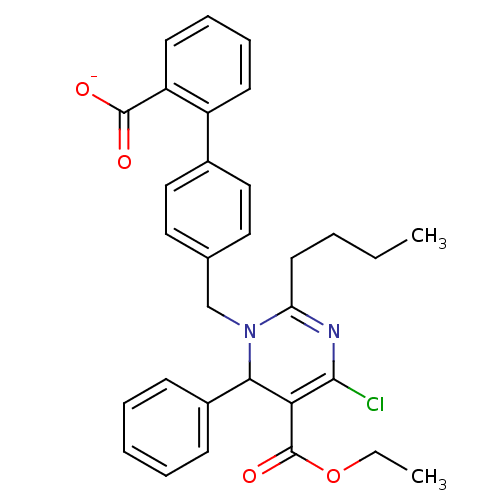
(CHEMBL343622 | Sodium; 4'-(2-butyl-4-chloro-5-etho...)Show SMILES CCCCC1=NC(Cl)=C(C(N1Cc1ccc(cc1)-c1ccccc1C([O-])=O)c1ccccc1)C(=O)OCC |c:7,t:4| Show InChI InChI=1S/C31H31ClN2O4/c1-3-5-15-26-33-29(32)27(31(37)38-4-2)28(23-11-7-6-8-12-23)34(26)20-21-16-18-22(19-17-21)24-13-9-10-14-25(24)30(35)36/h6-14,16-19,28H,3-5,15,20H2,1-2H3,(H,35,36)/p-1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.43E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of [125I]- Sar,Ile8-angiotensin II binding to rat adrenal corticcal membrane angiotensin II receptor |
J Med Chem 35: 4751-63 (1993)
BindingDB Entry DOI: 10.7270/Q2VH5PFJ |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor B
(RAT) | BDBM50004165

(CHEMBL144952 | Sodium; 4'-(2-butyl-5-ethoxycarbony...)Show SMILES CCCCc1nc(-c2ccccc2)c(C(=O)OCC)c(=O)n1Cc1ccc(cc1)-c1ccccc1C([O-])=O Show InChI InChI=1S/C31H30N2O5/c1-3-5-15-26-32-28(23-11-7-6-8-12-23)27(31(37)38-4-2)29(34)33(26)20-21-16-18-22(19-17-21)24-13-9-10-14-25(24)30(35)36/h6-14,16-19H,3-5,15,20H2,1-2H3,(H,35,36)/p-1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of [125I]- Sar,Ile8-angiotensin II binding to rat adrenal corticcal membrane angiotensin II receptor |
J Med Chem 35: 4751-63 (1993)
BindingDB Entry DOI: 10.7270/Q2VH5PFJ |
More data for this
Ligand-Target Pair | |
ATP synthase subunit beta,/delta,/epsilon,/gamma, mitochondrial
(Bos taurus) | BDBM50404297
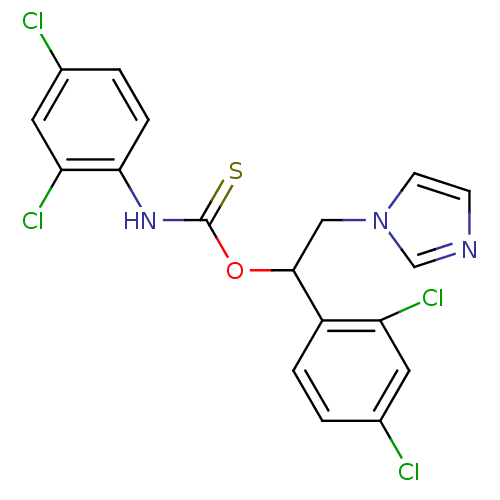
(CHEMBL269080)Show SMILES Clc1ccc(NC(=S)OC(Cn2ccnc2)c2ccc(Cl)cc2Cl)c(Cl)c1 Show InChI InChI=1S/C18H13Cl4N3OS/c19-11-1-3-13(14(21)7-11)17(9-25-6-5-23-10-25)26-18(27)24-16-4-2-12(20)8-15(16)22/h1-8,10,17H,9H2,(H,24,27) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory activity against bovine mitochondrial F1F0 ATP hydrolase |
Bioorg Med Chem Lett 14: 1027-30 (2004)
Article DOI: 10.1016/j.bmcl.2003.11.077
BindingDB Entry DOI: 10.7270/Q2VX0HQ9 |
More data for this
Ligand-Target Pair | |
ATP synthase subunit beta,/delta,/epsilon,/gamma, mitochondrial
(Bos taurus) | BDBM50404288
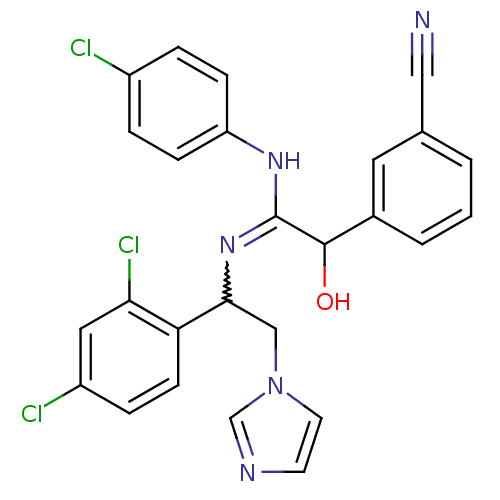
(CHEMBL269369)Show SMILES OC(C(Nc1ccc(Cl)cc1)=NC(Cn1ccnc1)c1ccc(Cl)cc1Cl)c1cccc(c1)C#N |w:11.12| Show InChI InChI=1S/C26H20Cl3N5O/c27-19-4-7-21(8-5-19)32-26(25(35)18-3-1-2-17(12-18)14-30)33-24(15-34-11-10-31-16-34)22-9-6-20(28)13-23(22)29/h1-13,16,24-25,35H,15H2,(H,32,33) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory activity against bovine mitochondrial F1F0-ATP synthase |
Bioorg Med Chem Lett 14: 1027-30 (2004)
Article DOI: 10.1016/j.bmcl.2003.11.077
BindingDB Entry DOI: 10.7270/Q2VX0HQ9 |
More data for this
Ligand-Target Pair | |
ATP synthase subunit beta,/delta,/epsilon,/gamma, mitochondrial
(Bos taurus) | BDBM50404288

(CHEMBL269369)Show SMILES OC(C(Nc1ccc(Cl)cc1)=NC(Cn1ccnc1)c1ccc(Cl)cc1Cl)c1cccc(c1)C#N |w:11.12| Show InChI InChI=1S/C26H20Cl3N5O/c27-19-4-7-21(8-5-19)32-26(25(35)18-3-1-2-17(12-18)14-30)33-24(15-34-11-10-31-16-34)22-9-6-20(28)13-23(22)29/h1-13,16,24-25,35H,15H2,(H,32,33) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory activity against bovine mitochondrial F1F0 ATP hydrolase |
Bioorg Med Chem Lett 14: 1027-30 (2004)
Article DOI: 10.1016/j.bmcl.2003.11.077
BindingDB Entry DOI: 10.7270/Q2VX0HQ9 |
More data for this
Ligand-Target Pair | |
ATP synthase subunit beta,/delta,/epsilon,/gamma, mitochondrial
(Bos taurus) | BDBM50404293
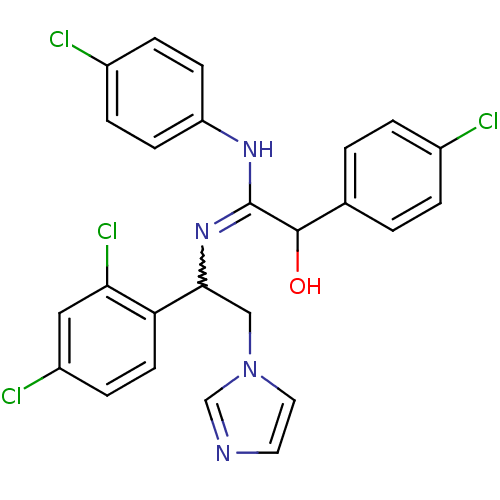
(CHEMBL8024)Show SMILES OC(C(Nc1ccc(Cl)cc1)=NC(Cn1ccnc1)c1ccc(Cl)cc1Cl)c1ccc(Cl)cc1 |w:11.12| Show InChI InChI=1S/C25H20Cl4N4O/c26-17-3-1-16(2-4-17)24(34)25(31-20-8-5-18(27)6-9-20)32-23(14-33-12-11-30-15-33)21-10-7-19(28)13-22(21)29/h1-13,15,23-24,34H,14H2,(H,31,32) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 82 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory activity against bovine mitochondrial F1F0 ATP hydrolase |
Bioorg Med Chem Lett 14: 1027-30 (2004)
Article DOI: 10.1016/j.bmcl.2003.11.077
BindingDB Entry DOI: 10.7270/Q2VX0HQ9 |
More data for this
Ligand-Target Pair | |
ATP synthase subunit beta,/delta,/epsilon,/gamma, mitochondrial
(Bos taurus) | BDBM50404286

(CHEMBL7982)Show SMILES Cc1ccc(NC(=S)OC(Cn2ccnc2)c2ccc(Cl)cc2Cl)c(C)c1 Show InChI InChI=1S/C20H19Cl2N3OS/c1-13-3-6-18(14(2)9-13)24-20(27)26-19(11-25-8-7-23-12-25)16-5-4-15(21)10-17(16)22/h3-10,12,19H,11H2,1-2H3,(H,24,27) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 230 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory activity against bovine mitochondrial F1F0-ATP synthase |
Bioorg Med Chem Lett 14: 1027-30 (2004)
Article DOI: 10.1016/j.bmcl.2003.11.077
BindingDB Entry DOI: 10.7270/Q2VX0HQ9 |
More data for this
Ligand-Target Pair | |
ATP synthase subunit gamma, mitochondrial
(Bos taurus) | BDBM50409907

(CHEMBL11859)Show SMILES CC1(C)Oc2ccc(cc2[C@H]([C@@H]1O)N(Cc1ncc[nH]1)c1ccc(Cl)cc1)C#N Show InChI InChI=1S/C22H21ClN4O2/c1-22(2)21(28)20(17-11-14(12-24)3-8-18(17)29-22)27(13-19-25-9-10-26-19)16-6-4-15(23)5-7-16/h3-11,20-21,28H,13H2,1-2H3,(H,25,26)/t20-,21+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 240 | n/a | n/a | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory concentration towards rat mitochondrial F1F0 ATP hydrolase using a pyruvate kinase / lactate dehydrogenase system |
J Med Chem 47: 1081-4 (2004)
Article DOI: 10.1021/jm030291x
BindingDB Entry DOI: 10.7270/Q23779XF |
More data for this
Ligand-Target Pair | |
ATP synthase subunit beta,/delta,/epsilon,/gamma, mitochondrial
(Bos taurus) | BDBM50404287

(CHEMBL8116)Show SMILES OC(C(Nc1ccc(Cl)cc1)=NC(Cn1ccnc1)c1ccc(Cl)cc1Cl)c1ccc(cc1)C#N |w:11.12| Show InChI InChI=1S/C26H20Cl3N5O/c27-19-5-8-21(9-6-19)32-26(25(35)18-3-1-17(14-30)2-4-18)33-24(15-34-12-11-31-16-34)22-10-7-20(28)13-23(22)29/h1-13,16,24-25,35H,15H2,(H,32,33) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 280 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory activity against bovine mitochondrial F1F0-ATP synthase |
Bioorg Med Chem Lett 14: 1027-30 (2004)
Article DOI: 10.1016/j.bmcl.2003.11.077
BindingDB Entry DOI: 10.7270/Q2VX0HQ9 |
More data for this
Ligand-Target Pair | |
ATP synthase subunit beta,/delta,/epsilon,/gamma, mitochondrial
(Bos taurus) | BDBM50404289

(CHEMBL8376)Show SMILES Clc1ccc(NC(=S)OC(Cn2ccnc2)c2ccc(Cl)cc2Cl)cc1 Show InChI InChI=1S/C18H14Cl3N3OS/c19-12-1-4-14(5-2-12)23-18(26)25-17(10-24-8-7-22-11-24)15-6-3-13(20)9-16(15)21/h1-9,11,17H,10H2,(H,23,26) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 430 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory activity against bovine mitochondrial F1F0 ATP hydrolase |
Bioorg Med Chem Lett 14: 1027-30 (2004)
Article DOI: 10.1016/j.bmcl.2003.11.077
BindingDB Entry DOI: 10.7270/Q2VX0HQ9 |
More data for this
Ligand-Target Pair | |
ATP synthase subunit gamma, mitochondrial
(Bos taurus) | BDBM50409912

(CHEMBL11502)Show SMILES CC1(C)Oc2ccc(cc2[C@@H]([C@H]1O)N(Cc1ncc[nH]1)c1ccc(Cl)cc1)S(=O)(=O)N1CCCCC1 Show InChI InChI=1S/C26H31ClN4O4S/c1-26(2)25(32)24(31(17-23-28-12-13-29-23)19-8-6-18(27)7-9-19)21-16-20(10-11-22(21)35-26)36(33,34)30-14-4-3-5-15-30/h6-13,16,24-25,32H,3-5,14-15,17H2,1-2H3,(H,28,29)/t24-,25+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 480 | n/a | n/a | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory concentration towards rat mitochondrial F1F0 ATP hydrolase using a pyruvate kinase / lactate dehydrogenase system |
J Med Chem 47: 1081-4 (2004)
Article DOI: 10.1021/jm030291x
BindingDB Entry DOI: 10.7270/Q23779XF |
More data for this
Ligand-Target Pair | |
ATP synthase subunit gamma, mitochondrial
(Bos taurus) | BDBM50409914

(BMS-191095)Show SMILES CC1(C)Oc2ccc(cc2[C@@H]([C@H]1O)N(Cc1ncc[nH]1)c1ccc(Cl)cc1)C#N Show InChI InChI=1S/C22H21ClN4O2/c1-22(2)21(28)20(17-11-14(12-24)3-8-18(17)29-22)27(13-19-25-9-10-26-19)16-6-4-15(23)5-7-16/h3-11,20-21,28H,13H2,1-2H3,(H,25,26)/t20-,21+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 480 | n/a | n/a | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory concentration towards rat mitochondrial F1F0 ATP hydrolase using a pyruvate kinase / lactate dehydrogenase system |
J Med Chem 47: 1081-4 (2004)
Article DOI: 10.1021/jm030291x
BindingDB Entry DOI: 10.7270/Q23779XF |
More data for this
Ligand-Target Pair | |
ATP synthase subunit beta,/delta,/epsilon,/gamma, mitochondrial
(Bos taurus) | BDBM50404301

(CHEMBL7871)Show SMILES Clc1ccc(NC(NC(Cn2ccnc2)c2ccc(Cl)cc2Cl)=NC#N)c(Cl)c1 |w:23.25| Show InChI InChI=1S/C19H14Cl4N6/c20-12-1-3-14(15(22)7-12)18(9-29-6-5-25-11-29)28-19(26-10-24)27-17-4-2-13(21)8-16(17)23/h1-8,11,18H,9H2,(H2,26,27,28) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory activity against bovine mitochondrial F1F0-ATP synthase |
Bioorg Med Chem Lett 14: 1027-30 (2004)
Article DOI: 10.1016/j.bmcl.2003.11.077
BindingDB Entry DOI: 10.7270/Q2VX0HQ9 |
More data for this
Ligand-Target Pair | |
ATP synthase subunit beta,/delta,/epsilon,/gamma, mitochondrial
(Bos taurus) | BDBM50404284

(CHEMBL8064)Show SMILES Cc1ccc(NC(=S)OC(Cn2ccnc2)c2ccc(Cl)cc2Cl)cc1 Show InChI InChI=1S/C19H17Cl2N3OS/c1-13-2-5-15(6-3-13)23-19(26)25-18(11-24-9-8-22-12-24)16-7-4-14(20)10-17(16)21/h2-10,12,18H,11H2,1H3,(H,23,26) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 660 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory activity against bovine mitochondrial F1F0 ATP hydrolase |
Bioorg Med Chem Lett 14: 1027-30 (2004)
Article DOI: 10.1016/j.bmcl.2003.11.077
BindingDB Entry DOI: 10.7270/Q2VX0HQ9 |
More data for this
Ligand-Target Pair | |
ATP synthase subunit beta,/delta,/epsilon,/gamma, mitochondrial
(Bos taurus) | BDBM50404285
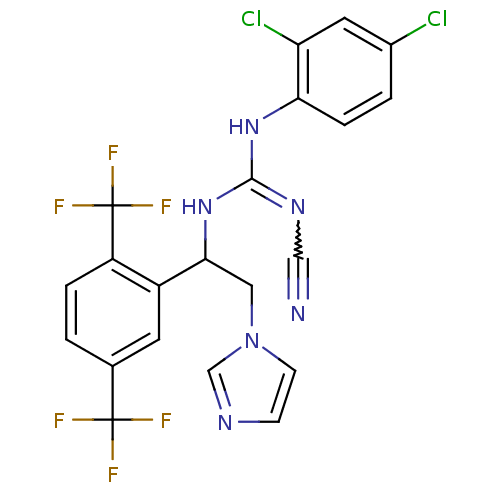
(CHEMBL8025)Show SMILES FC(F)(F)c1ccc(c(c1)C(Cn1ccnc1)NC(Nc1ccc(Cl)cc1Cl)=NC#N)C(F)(F)F |w:28.31| Show InChI InChI=1S/C21H14Cl2F6N6/c22-13-2-4-17(16(23)8-13)33-19(32-10-30)34-18(9-35-6-5-31-11-35)14-7-12(20(24,25)26)1-3-15(14)21(27,28)29/h1-8,11,18H,9H2,(H2,32,33,34) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 710 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory activity against bovine mitochondrial F1F0 ATP hydrolase |
Bioorg Med Chem Lett 14: 1027-30 (2004)
Article DOI: 10.1016/j.bmcl.2003.11.077
BindingDB Entry DOI: 10.7270/Q2VX0HQ9 |
More data for this
Ligand-Target Pair | |
ATP synthase subunit gamma, mitochondrial
(Bos taurus) | BDBM50409917

(CHEMBL273271)Show SMILES CC1(C)Oc2ccc(cc2[C@H]([C@@H]1O)N(Cc1ncc[nH]1)c1ccc(Cl)cc1)S(=O)(=O)N1CCCCC1 Show InChI InChI=1S/C26H31ClN4O4S/c1-26(2)25(32)24(31(17-23-28-12-13-29-23)19-8-6-18(27)7-9-19)21-16-20(10-11-22(21)35-26)36(33,34)30-14-4-3-5-15-30/h6-13,16,24-25,32H,3-5,14-15,17H2,1-2H3,(H,28,29)/t24-,25+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory concentration towards rat mitochondrial F1F0 ATP hydrolase using a pyruvate kinase / lactate dehydrogenase system |
J Med Chem 47: 1081-4 (2004)
Article DOI: 10.1021/jm030291x
BindingDB Entry DOI: 10.7270/Q23779XF |
More data for this
Ligand-Target Pair | |
ATP synthase subunit gamma, mitochondrial
(Bos taurus) | BDBM50409915

(CHEMBL428891)Show SMILES CC1(C)Oc2ccc(cc2[C@@H]([C@H]1O)N(Cc1ncc[nH]1)c1ccccc1)C#N Show InChI InChI=1S/C22H22N4O2/c1-22(2)21(27)20(17-12-15(13-23)8-9-18(17)28-22)26(14-19-24-10-11-25-19)16-6-4-3-5-7-16/h3-12,20-21,27H,14H2,1-2H3,(H,24,25)/t20-,21+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory concentration towards rat mitochondrial F1F0 ATP hydrolase using a pyruvate kinase / lactate dehydrogenase system |
J Med Chem 47: 1081-4 (2004)
Article DOI: 10.1021/jm030291x
BindingDB Entry DOI: 10.7270/Q23779XF |
More data for this
Ligand-Target Pair | |
ATP synthase subunit beta,/delta,/epsilon,/gamma, mitochondrial
(Bos taurus) | BDBM50404290

(CHEMBL8473)Show SMILES Clc1ccc(C(Cn2ccnc2)NC(Nc2ccccc2Cl)=NC#N)c(Cl)c1 |w:22.24| Show InChI InChI=1S/C19H15Cl3N6/c20-13-5-6-14(16(22)9-13)18(10-28-8-7-24-12-28)27-19(25-11-23)26-17-4-2-1-3-15(17)21/h1-9,12,18H,10H2,(H2,25,26,27) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.23E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory activity against bovine mitochondrial F1F0 ATP hydrolase |
Bioorg Med Chem Lett 14: 1027-30 (2004)
Article DOI: 10.1016/j.bmcl.2003.11.077
BindingDB Entry DOI: 10.7270/Q2VX0HQ9 |
More data for this
Ligand-Target Pair | |
ATP synthase subunit beta,/delta,/epsilon,/gamma, mitochondrial
(Bos taurus) | BDBM50404300
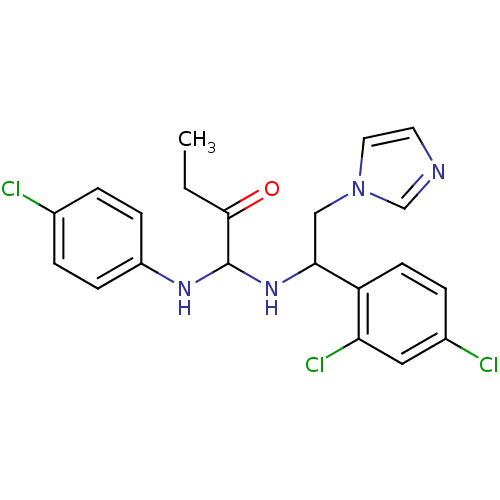
(CHEMBL7894)Show SMILES CCC(=O)C(NC(Cn1ccnc1)c1ccc(Cl)cc1Cl)Nc1ccc(Cl)cc1 Show InChI InChI=1S/C21H21Cl3N4O/c1-2-20(29)21(26-16-6-3-14(22)4-7-16)27-19(12-28-10-9-25-13-28)17-8-5-15(23)11-18(17)24/h3-11,13,19,21,26-27H,2,12H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.27E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory activity against bovine mitochondrial F1F0 ATP hydrolase |
Bioorg Med Chem Lett 14: 1027-30 (2004)
Article DOI: 10.1016/j.bmcl.2003.11.077
BindingDB Entry DOI: 10.7270/Q2VX0HQ9 |
More data for this
Ligand-Target Pair | |
ATP synthase subunit beta,/delta,/epsilon,/gamma, mitochondrial
(Bos taurus) | BDBM50404299

(CHEMBL8199)Show SMILES Clc1ccc(NC(=S)NC(Cn2ccnc2)c2ccc(Cl)cc2Cl)c(Cl)c1 Show InChI InChI=1S/C18H14Cl4N4S/c19-11-1-3-13(14(21)7-11)17(9-26-6-5-23-10-26)25-18(27)24-16-4-2-12(20)8-15(16)22/h1-8,10,17H,9H2,(H2,24,25,27) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.41E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory activity against bovine mitochondrial F1F0-ATP synthase |
Bioorg Med Chem Lett 14: 1027-30 (2004)
Article DOI: 10.1016/j.bmcl.2003.11.077
BindingDB Entry DOI: 10.7270/Q2VX0HQ9 |
More data for this
Ligand-Target Pair | |
ATP synthase subunit beta,/delta,/epsilon,/gamma, mitochondrial
(Bos taurus) | BDBM50404294
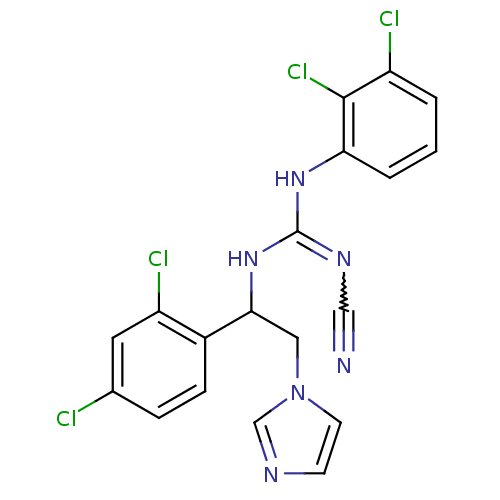
(CHEMBL269307)Show SMILES Clc1ccc(C(Cn2ccnc2)NC(Nc2cccc(Cl)c2Cl)=NC#N)c(Cl)c1 |w:23.25| Show InChI InChI=1S/C19H14Cl4N6/c20-12-4-5-13(15(22)8-12)17(9-29-7-6-25-11-29)28-19(26-10-24)27-16-3-1-2-14(21)18(16)23/h1-8,11,17H,9H2,(H2,26,27,28) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.49E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory activity against bovine mitochondrial F1F0 ATP hydrolase |
Bioorg Med Chem Lett 14: 1027-30 (2004)
Article DOI: 10.1016/j.bmcl.2003.11.077
BindingDB Entry DOI: 10.7270/Q2VX0HQ9 |
More data for this
Ligand-Target Pair | |
ATP synthase subunit beta,/delta,/epsilon,/gamma, mitochondrial
(Bos taurus) | BDBM50404295

(CHEMBL8244)Show InChI InChI=1S/C18H15Cl2N3OS/c19-13-6-7-15(16(20)10-13)17(11-23-9-8-21-12-23)24-18(25)22-14-4-2-1-3-5-14/h1-10,12,17H,11H2,(H,22,25) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory activity against bovine mitochondrial F1F0-ATP synthase |
Bioorg Med Chem Lett 14: 1027-30 (2004)
Article DOI: 10.1016/j.bmcl.2003.11.077
BindingDB Entry DOI: 10.7270/Q2VX0HQ9 |
More data for this
Ligand-Target Pair | |
ATP synthase subunit gamma, mitochondrial
(Bos taurus) | BDBM50409909
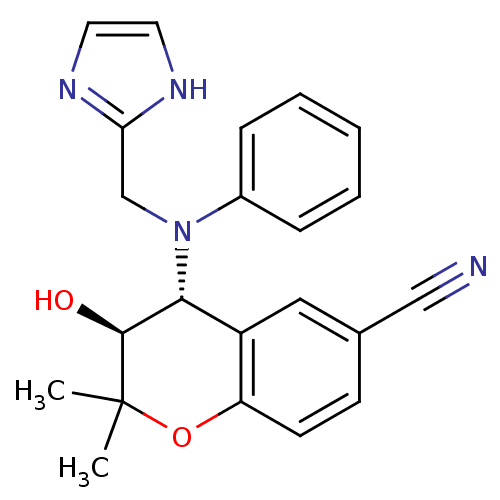
(CHEMBL273900)Show SMILES CC1(C)Oc2ccc(cc2[C@H]([C@@H]1O)N(Cc1ncc[nH]1)c1ccccc1)C#N Show InChI InChI=1S/C22H22N4O2/c1-22(2)21(27)20(17-12-15(13-23)8-9-18(17)28-22)26(14-19-24-10-11-25-19)16-6-4-3-5-7-16/h3-12,20-21,27H,14H2,1-2H3,(H,24,25)/t20-,21+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory concentration towards rat mitochondrial F1F0 ATP hydrolase using a pyruvate kinase / lactate dehydrogenase system |
J Med Chem 47: 1081-4 (2004)
Article DOI: 10.1021/jm030291x
BindingDB Entry DOI: 10.7270/Q23779XF |
More data for this
Ligand-Target Pair | |
ATP synthase subunit beta,/delta,/epsilon,/gamma, mitochondrial
(Bos taurus) | BDBM50404298
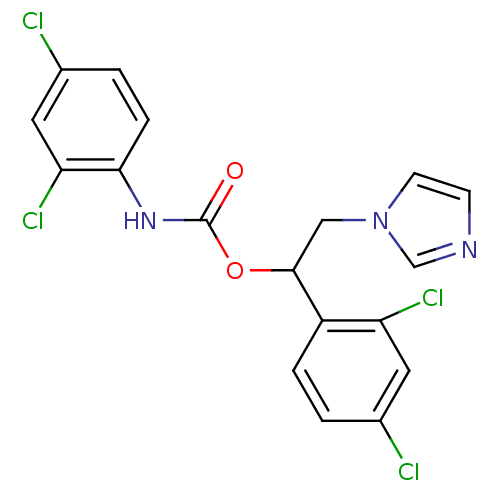
(CHEMBL416142)Show SMILES Clc1ccc(NC(=O)OC(Cn2ccnc2)c2ccc(Cl)cc2Cl)c(Cl)c1 Show InChI InChI=1S/C18H13Cl4N3O2/c19-11-1-3-13(14(21)7-11)17(9-25-6-5-23-10-25)27-18(26)24-16-4-2-12(20)8-15(16)22/h1-8,10,17H,9H2,(H,24,26) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.77E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory activity against bovine mitochondrial F1F0 ATP hydrolase |
Bioorg Med Chem Lett 14: 1027-30 (2004)
Article DOI: 10.1016/j.bmcl.2003.11.077
BindingDB Entry DOI: 10.7270/Q2VX0HQ9 |
More data for this
Ligand-Target Pair | |
ATP synthase subunit beta,/delta,/epsilon,/gamma, mitochondrial
(Bos taurus) | BDBM50404292

(CHEMBL8124)Show SMILES Clc1ccc(NC(NC(Cn2ccnc2)c2ccc(Cl)cc2Cl)=NC#N)cc1 |w:23.25| Show InChI InChI=1S/C19H15Cl3N6/c20-13-1-4-15(5-2-13)26-19(25-11-23)27-18(10-28-8-7-24-12-28)16-6-3-14(21)9-17(16)22/h1-9,12,18H,10H2,(H2,25,26,27) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory activity against bovine mitochondrial F1F0 ATP hydrolase |
Bioorg Med Chem Lett 14: 1027-30 (2004)
Article DOI: 10.1016/j.bmcl.2003.11.077
BindingDB Entry DOI: 10.7270/Q2VX0HQ9 |
More data for this
Ligand-Target Pair | |
ATP synthase subunit beta,/delta,/epsilon,/gamma, mitochondrial
(Bos taurus) | BDBM50404296

(CHEMBL8029)Show SMILES Clc1cccc(NC(NC(Cn2ccnc2)c2ccc(Cl)cc2Cl)=NC#N)c1 |w:24.26| Show InChI InChI=1S/C19H15Cl3N6/c20-13-2-1-3-15(8-13)26-19(25-11-23)27-18(10-28-7-6-24-12-28)16-5-4-14(21)9-17(16)22/h1-9,12,18H,10H2,(H2,25,26,27) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9.17E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory activity against bovine mitochondrial F1F0-ATP synthase |
Bioorg Med Chem Lett 14: 1027-30 (2004)
Article DOI: 10.1016/j.bmcl.2003.11.077
BindingDB Entry DOI: 10.7270/Q2VX0HQ9 |
More data for this
Ligand-Target Pair | |
ATP synthase subunit gamma, mitochondrial
(Bos taurus) | BDBM50409916

(CHEMBL11392)Show SMILES CCOC(=O)N(C1C(O)C(C)(C)Oc2ccc(cc12)C#N)c1ccccc1 Show InChI InChI=1S/C21H22N2O4/c1-4-26-20(25)23(15-8-6-5-7-9-15)18-16-12-14(13-22)10-11-17(16)27-21(2,3)19(18)24/h5-12,18-19,24H,4H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory concentration towards rat mitochondrial F1F0 ATP hydrolase using a pyruvate kinase / lactate dehydrogenase system |
J Med Chem 47: 1081-4 (2004)
Article DOI: 10.1021/jm030291x
BindingDB Entry DOI: 10.7270/Q23779XF |
More data for this
Ligand-Target Pair | |
ATP synthase subunit beta,/delta,/epsilon,/gamma, mitochondrial
(Bos taurus) | BDBM50404291

(CHEMBL268210)Show SMILES Cc1ccc(C(Cn2ccnc2)OC(=S)Nc2ccc(Cl)cc2)c(C)c1 Show InChI InChI=1S/C20H20ClN3OS/c1-14-3-8-18(15(2)11-14)19(12-24-10-9-22-13-24)25-20(26)23-17-6-4-16(21)5-7-17/h3-11,13,19H,12H2,1-2H3,(H,23,26) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.14E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory activity against bovine mitochondrial F1F0-ATP synthase |
Bioorg Med Chem Lett 14: 1027-30 (2004)
Article DOI: 10.1016/j.bmcl.2003.11.077
BindingDB Entry DOI: 10.7270/Q2VX0HQ9 |
More data for this
Ligand-Target Pair | |
ATP synthase subunit gamma, mitochondrial
(Bos taurus) | BDBM50409911
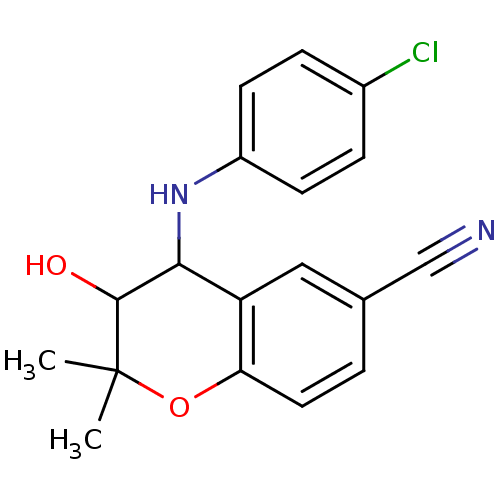
(CHEMBL274443)Show InChI InChI=1S/C18H17ClN2O2/c1-18(2)17(22)16(21-13-6-4-12(19)5-7-13)14-9-11(10-20)3-8-15(14)23-18/h3-9,16-17,21-22H,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory concentration towards rat mitochondrial F1F0 ATP hydrolase using a pyruvate kinase / lactate dehydrogenase system |
J Med Chem 47: 1081-4 (2004)
Article DOI: 10.1021/jm030291x
BindingDB Entry DOI: 10.7270/Q23779XF |
More data for this
Ligand-Target Pair | |
ATP synthase subunit beta,/delta,/epsilon,/gamma, mitochondrial
(Bos taurus) | BDBM50404290

(CHEMBL8473)Show SMILES Clc1ccc(C(Cn2ccnc2)NC(Nc2ccccc2Cl)=NC#N)c(Cl)c1 |w:22.24| Show InChI InChI=1S/C19H15Cl3N6/c20-13-5-6-14(16(22)9-13)18(10-28-8-7-24-12-28)27-19(25-11-23)26-17-4-2-1-3-15(17)21/h1-9,12,18H,10H2,(H2,25,26,27) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory activity against bovine mitochondrial F1F0-ATP synthase |
Bioorg Med Chem Lett 14: 1027-30 (2004)
Article DOI: 10.1016/j.bmcl.2003.11.077
BindingDB Entry DOI: 10.7270/Q2VX0HQ9 |
More data for this
Ligand-Target Pair | |
ATP synthase subunit beta,/delta,/epsilon,/gamma, mitochondrial
(Bos taurus) | BDBM50404288

(CHEMBL269369)Show SMILES OC(C(Nc1ccc(Cl)cc1)=NC(Cn1ccnc1)c1ccc(Cl)cc1Cl)c1cccc(c1)C#N |w:11.12| Show InChI InChI=1S/C26H20Cl3N5O/c27-19-4-7-21(8-5-19)32-26(25(35)18-3-1-2-17(12-18)14-30)33-24(15-34-11-10-31-16-34)22-9-6-20(28)13-23(22)29/h1-13,16,24-25,35H,15H2,(H,32,33) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory activity against bovine mitochondrial F1F0 ATP hydrolase |
Bioorg Med Chem Lett 14: 1027-30 (2004)
Article DOI: 10.1016/j.bmcl.2003.11.077
BindingDB Entry DOI: 10.7270/Q2VX0HQ9 |
More data for this
Ligand-Target Pair | |
ATP synthase subunit beta,/delta,/epsilon,/gamma, mitochondrial
(Bos taurus) | BDBM50404289

(CHEMBL8376)Show SMILES Clc1ccc(NC(=S)OC(Cn2ccnc2)c2ccc(Cl)cc2Cl)cc1 Show InChI InChI=1S/C18H14Cl3N3OS/c19-12-1-4-14(5-2-12)23-18(26)25-17(10-24-8-7-22-11-24)15-6-3-13(20)9-16(15)21/h1-9,11,17H,10H2,(H,23,26) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory activity against bovine mitochondrial F1F0-ATP synthase |
Bioorg Med Chem Lett 14: 1027-30 (2004)
Article DOI: 10.1016/j.bmcl.2003.11.077
BindingDB Entry DOI: 10.7270/Q2VX0HQ9 |
More data for this
Ligand-Target Pair | |
ATP synthase subunit beta,/delta,/epsilon,/gamma, mitochondrial
(Bos taurus) | BDBM50404293

(CHEMBL8024)Show SMILES OC(C(Nc1ccc(Cl)cc1)=NC(Cn1ccnc1)c1ccc(Cl)cc1Cl)c1ccc(Cl)cc1 |w:11.12| Show InChI InChI=1S/C25H20Cl4N4O/c26-17-3-1-16(2-4-17)24(34)25(31-20-8-5-18(27)6-9-20)32-23(14-33-12-11-30-15-33)21-10-7-19(28)13-22(21)29/h1-13,15,23-24,34H,14H2,(H,31,32) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory activity against bovine mitochondrial F1F0 ATP hydrolase |
Bioorg Med Chem Lett 14: 1027-30 (2004)
Article DOI: 10.1016/j.bmcl.2003.11.077
BindingDB Entry DOI: 10.7270/Q2VX0HQ9 |
More data for this
Ligand-Target Pair | |
ATP synthase subunit beta,/delta,/epsilon,/gamma, mitochondrial
(Bos taurus) | BDBM50404296

(CHEMBL8029)Show SMILES Clc1cccc(NC(NC(Cn2ccnc2)c2ccc(Cl)cc2Cl)=NC#N)c1 |w:24.26| Show InChI InChI=1S/C19H15Cl3N6/c20-13-2-1-3-15(8-13)26-19(25-11-23)27-18(10-28-7-6-24-12-28)16-5-4-14(21)9-17(16)22/h1-9,12,18H,10H2,(H2,25,26,27) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory activity against bovine mitochondrial F1F0 ATP hydrolase |
Bioorg Med Chem Lett 14: 1027-30 (2004)
Article DOI: 10.1016/j.bmcl.2003.11.077
BindingDB Entry DOI: 10.7270/Q2VX0HQ9 |
More data for this
Ligand-Target Pair | |
ATP synthase subunit beta,/delta,/epsilon,/gamma, mitochondrial
(Bos taurus) | BDBM50404299

(CHEMBL8199)Show SMILES Clc1ccc(NC(=S)NC(Cn2ccnc2)c2ccc(Cl)cc2Cl)c(Cl)c1 Show InChI InChI=1S/C18H14Cl4N4S/c19-11-1-3-13(14(21)7-11)17(9-26-6-5-23-10-26)25-18(27)24-16-4-2-12(20)8-15(16)22/h1-8,10,17H,9H2,(H2,24,25,27) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory activity against bovine mitochondrial F1F0 ATP hydrolase |
Bioorg Med Chem Lett 14: 1027-30 (2004)
Article DOI: 10.1016/j.bmcl.2003.11.077
BindingDB Entry DOI: 10.7270/Q2VX0HQ9 |
More data for this
Ligand-Target Pair | |
ATP synthase subunit beta,/delta,/epsilon,/gamma, mitochondrial
(Bos taurus) | BDBM50404295

(CHEMBL8244)Show InChI InChI=1S/C18H15Cl2N3OS/c19-13-6-7-15(16(20)10-13)17(11-23-9-8-21-12-23)24-18(25)22-14-4-2-1-3-5-14/h1-10,12,17H,11H2,(H,22,25) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory activity against bovine mitochondrial F1F0 ATP hydrolase |
Bioorg Med Chem Lett 14: 1027-30 (2004)
Article DOI: 10.1016/j.bmcl.2003.11.077
BindingDB Entry DOI: 10.7270/Q2VX0HQ9 |
More data for this
Ligand-Target Pair | |
ATP synthase subunit beta,/delta,/epsilon,/gamma, mitochondrial
(Bos taurus) | BDBM50404288

(CHEMBL269369)Show SMILES OC(C(Nc1ccc(Cl)cc1)=NC(Cn1ccnc1)c1ccc(Cl)cc1Cl)c1cccc(c1)C#N |w:11.12| Show InChI InChI=1S/C26H20Cl3N5O/c27-19-4-7-21(8-5-19)32-26(25(35)18-3-1-2-17(12-18)14-30)33-24(15-34-11-10-31-16-34)22-9-6-20(28)13-23(22)29/h1-13,16,24-25,35H,15H2,(H,32,33) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory activity against bovine mitochondrial F1F0-ATP synthase |
Bioorg Med Chem Lett 14: 1027-30 (2004)
Article DOI: 10.1016/j.bmcl.2003.11.077
BindingDB Entry DOI: 10.7270/Q2VX0HQ9 |
More data for this
Ligand-Target Pair | |
ATP synthase subunit beta,/delta,/epsilon,/gamma, mitochondrial
(Bos taurus) | BDBM50404294

(CHEMBL269307)Show SMILES Clc1ccc(C(Cn2ccnc2)NC(Nc2cccc(Cl)c2Cl)=NC#N)c(Cl)c1 |w:23.25| Show InChI InChI=1S/C19H14Cl4N6/c20-12-4-5-13(15(22)8-12)17(9-29-7-6-25-11-29)28-19(26-10-24)27-16-3-1-2-14(21)18(16)23/h1-8,11,17H,9H2,(H2,26,27,28) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory activity against bovine mitochondrial F1F0-ATP synthase |
Bioorg Med Chem Lett 14: 1027-30 (2004)
Article DOI: 10.1016/j.bmcl.2003.11.077
BindingDB Entry DOI: 10.7270/Q2VX0HQ9 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data