

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TGF-beta receptor type-1 (Homo sapiens (Human)) | BDBM412734 (N-(3-fluoropyridin-4-yl)-2-(6-methylpyridin-2-yl)-...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase UniChem | Article PubMed | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of human His-tagged TGFbetaR1 T204D mutant expressed in Sf9 insect cells after 1 hr by HTRF assay | Bioorg Med Chem 26: 1026-1034 (2018) Article DOI: 10.1016/j.bmc.2018.01.014 BindingDB Entry DOI: 10.7270/Q27M0BHV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TGF-beta receptor type-1 (Homo sapiens (Human)) | BDBM50454871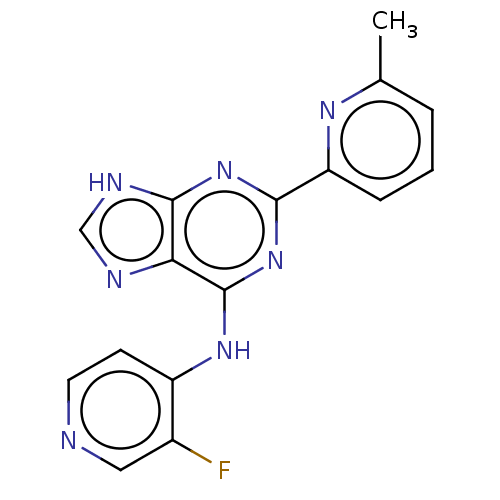 (CHEMBL4209835) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of human His-tagged TGFbetaR1 T204D mutant expressed in Sf9 insect cells after 1 hr by HTRF assay | Bioorg Med Chem 26: 1026-1034 (2018) Article DOI: 10.1016/j.bmc.2018.01.014 BindingDB Entry DOI: 10.7270/Q27M0BHV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TGF-beta receptor type-1 (Homo sapiens (Human)) | BDBM412755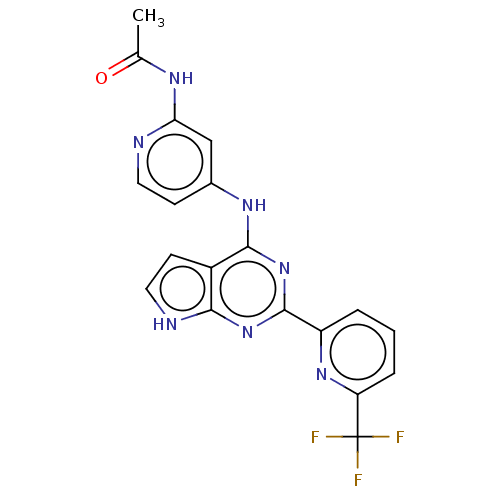 (N-(4-((2-(6-(trifluoromethyl)pyridin-2-yl)-7H-pyrr...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | UniChem | Article PubMed | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of human His-tagged TGFbetaR1 T204D mutant expressed in Sf9 insect cells after 1 hr by HTRF assay | Bioorg Med Chem 26: 1026-1034 (2018) Article DOI: 10.1016/j.bmc.2018.01.014 BindingDB Entry DOI: 10.7270/Q27M0BHV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TGF-beta receptor type-1 (Homo sapiens (Human)) | BDBM412745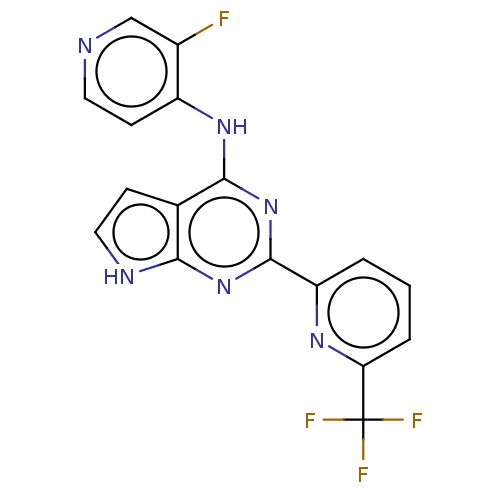 (N-(3-fluoropyridin-4-yl)-2-(6-(trifluoromethyl)pyr...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PDB UniChem | PDB Article PubMed | 0.220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of human His-tagged TGFbetaR1 T204D mutant expressed in Sf9 insect cells after 1 hr by HTRF assay | Bioorg Med Chem 26: 1026-1034 (2018) Article DOI: 10.1016/j.bmc.2018.01.014 BindingDB Entry DOI: 10.7270/Q27M0BHV | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| 17-beta-hydroxysteroid dehydrogenase type 3 (Homo sapiens (Human)) | BDBM50179969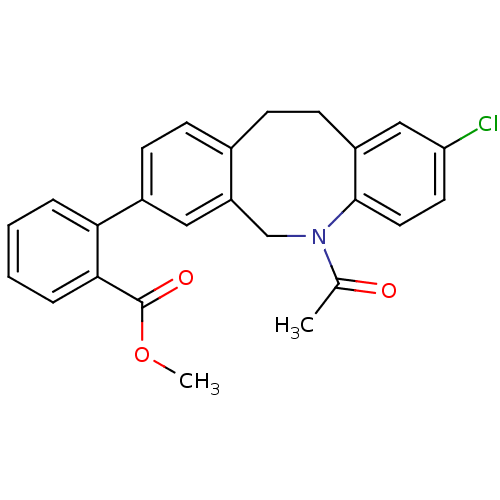 (2-(5-acetyl-2-chloro-5,6,11,12-tetrahydro-dibenzo[...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of 17beta-HSD3 | Bioorg Med Chem Lett 16: 1532-6 (2006) Article DOI: 10.1016/j.bmcl.2005.12.039 BindingDB Entry DOI: 10.7270/Q2CV4HBV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 17-beta-hydroxysteroid dehydrogenase type 3 (Homo sapiens (Human)) | BDBM50179935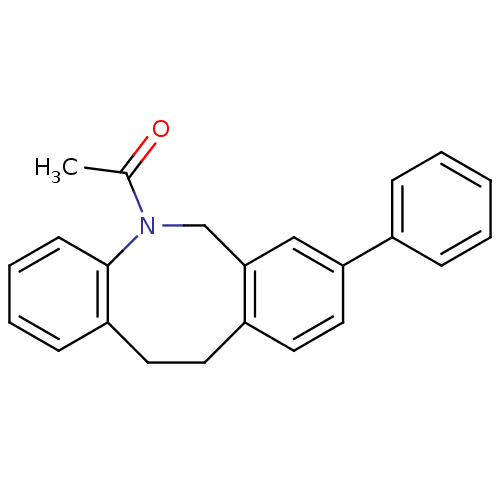 (1-(8-Phenyl-11,12-dihydro-6H-dibenzo[b,f]azocin-5-...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of 17beta-HSD3 | Bioorg Med Chem Lett 16: 1532-6 (2006) Article DOI: 10.1016/j.bmcl.2005.12.039 BindingDB Entry DOI: 10.7270/Q2CV4HBV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 17-beta-hydroxysteroid dehydrogenase type 3 (Homo sapiens (Human)) | BDBM50179960 (1-[8-(2-acetyl-phenyl)-2-chloro-11,12-dihydro-6H-d...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of 17beta-HSD3 | Bioorg Med Chem Lett 16: 1532-6 (2006) Article DOI: 10.1016/j.bmcl.2005.12.039 BindingDB Entry DOI: 10.7270/Q2CV4HBV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Casein kinase II subunit alpha' (Homo sapiens (Human)) | BDBM142829 (US8940736, 391) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.0300 | n/a | n/a | n/a | n/a | 7.4 | 30 |
Bristol-Myers Squibb Company US Patent | Assay Description The effectiveness of compounds of the present invention as inhibitors of protein kinases can be readily tested by assays known to those skilled in th... | US Patent US8940736 (2015) BindingDB Entry DOI: 10.7270/Q2QV3K72 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Casein kinase II subunit alpha' (Homo sapiens (Human)) | BDBM142775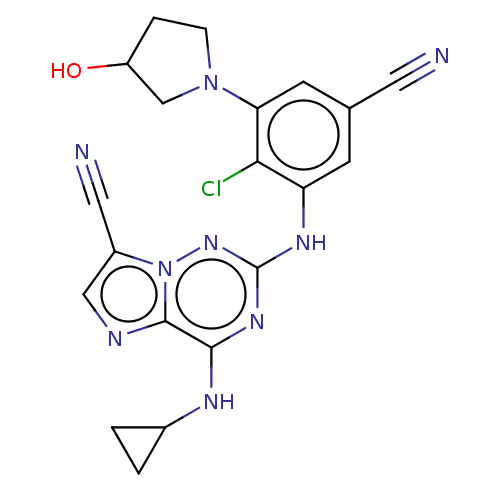 (US8940736, 7) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.0700 | n/a | n/a | n/a | n/a | 7.4 | 30 |
Bristol-Myers Squibb Company US Patent | Assay Description The effectiveness of compounds of the present invention as inhibitors of protein kinases can be readily tested by assays known to those skilled in th... | US Patent US8940736 (2015) BindingDB Entry DOI: 10.7270/Q2QV3K72 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Casein kinase II subunit alpha' (Homo sapiens (Human)) | BDBM142774 (US8940736, 6) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.0900 | n/a | n/a | n/a | n/a | 7.4 | 30 |
Bristol-Myers Squibb Company US Patent | Assay Description The effectiveness of compounds of the present invention as inhibitors of protein kinases can be readily tested by assays known to those skilled in th... | US Patent US8940736 (2015) BindingDB Entry DOI: 10.7270/Q2QV3K72 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Casein kinase II subunit alpha' (Homo sapiens (Human)) | BDBM142816 (US8940736, 281) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.0900 | n/a | n/a | n/a | n/a | 7.4 | 30 |
Bristol-Myers Squibb Company US Patent | Assay Description The effectiveness of compounds of the present invention as inhibitors of protein kinases can be readily tested by assays known to those skilled in th... | US Patent US8940736 (2015) BindingDB Entry DOI: 10.7270/Q2QV3K72 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Casein kinase II subunit alpha' (Homo sapiens (Human)) | BDBM142835 (US8940736, 402) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.110 | n/a | n/a | n/a | n/a | 7.4 | 30 |
Bristol-Myers Squibb Company US Patent | Assay Description The effectiveness of compounds of the present invention as inhibitors of protein kinases can be readily tested by assays known to those skilled in th... | US Patent US8940736 (2015) BindingDB Entry DOI: 10.7270/Q2QV3K72 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Casein kinase II subunit alpha' (Homo sapiens (Human)) | BDBM142792 (US8940736, 160) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.110 | n/a | n/a | n/a | n/a | 7.4 | 30 |
Bristol-Myers Squibb Company US Patent | Assay Description The effectiveness of compounds of the present invention as inhibitors of protein kinases can be readily tested by assays known to those skilled in th... | US Patent US8940736 (2015) BindingDB Entry DOI: 10.7270/Q2QV3K72 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Casein kinase II subunit alpha' (Homo sapiens (Human)) | BDBM142777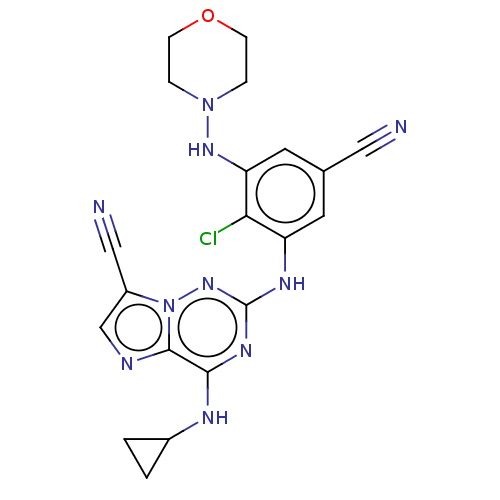 (US8940736, 49) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.110 | n/a | n/a | n/a | n/a | 7.4 | 30 |
Bristol-Myers Squibb Company US Patent | Assay Description The effectiveness of compounds of the present invention as inhibitors of protein kinases can be readily tested by assays known to those skilled in th... | US Patent US8940736 (2015) BindingDB Entry DOI: 10.7270/Q2QV3K72 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Casein kinase II subunit alpha (Homo sapiens (Human)) | BDBM142829 (US8940736, 391) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.130 | n/a | n/a | n/a | n/a | 7.4 | 30 |
Bristol-Myers Squibb Company US Patent | Assay Description The effectiveness of compounds of the present invention as inhibitors of protein kinases can be readily tested by assays known to those skilled in th... | US Patent US8940736 (2015) BindingDB Entry DOI: 10.7270/Q2QV3K72 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Casein kinase II subunit alpha' (Homo sapiens (Human)) | BDBM142822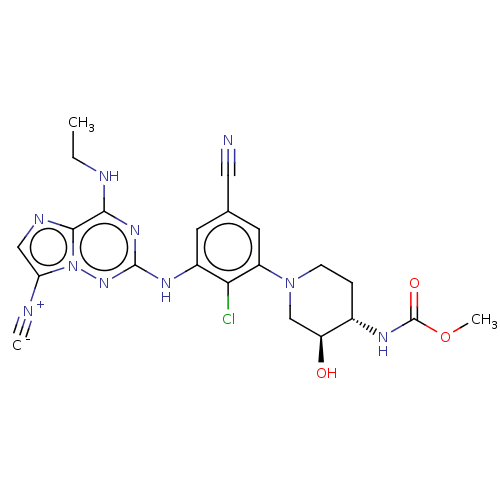 (US8940736, 328) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.130 | n/a | n/a | n/a | n/a | 7.4 | 30 |
Bristol-Myers Squibb Company US Patent | Assay Description The effectiveness of compounds of the present invention as inhibitors of protein kinases can be readily tested by assays known to those skilled in th... | US Patent US8940736 (2015) BindingDB Entry DOI: 10.7270/Q2QV3K72 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Casein kinase II subunit alpha (Homo sapiens (Human)) | BDBM142817 (US8940736, 285) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.140 | n/a | n/a | n/a | n/a | 7.4 | 30 |
Bristol-Myers Squibb Company US Patent | Assay Description The effectiveness of compounds of the present invention as inhibitors of protein kinases can be readily tested by assays known to those skilled in th... | US Patent US8940736 (2015) BindingDB Entry DOI: 10.7270/Q2QV3K72 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Casein kinase II subunit alpha' (Homo sapiens (Human)) | BDBM142784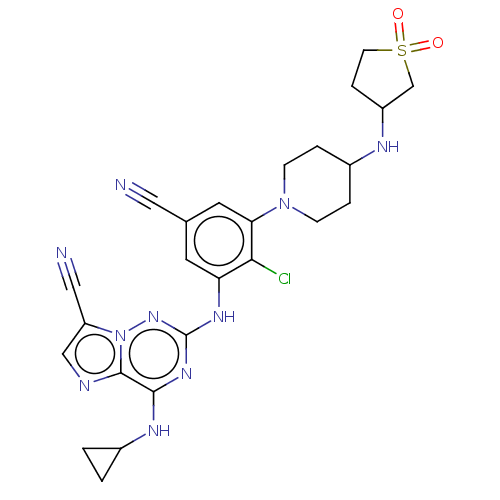 (US8940736, 117) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.150 | n/a | n/a | n/a | n/a | 7.4 | 30 |
Bristol-Myers Squibb Company US Patent | Assay Description The effectiveness of compounds of the present invention as inhibitors of protein kinases can be readily tested by assays known to those skilled in th... | US Patent US8940736 (2015) BindingDB Entry DOI: 10.7270/Q2QV3K72 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Casein kinase II subunit alpha (Homo sapiens (Human)) | BDBM142774 (US8940736, 6) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.150 | n/a | n/a | n/a | n/a | 7.4 | 30 |
Bristol-Myers Squibb Company US Patent | Assay Description The effectiveness of compounds of the present invention as inhibitors of protein kinases can be readily tested by assays known to those skilled in th... | US Patent US8940736 (2015) BindingDB Entry DOI: 10.7270/Q2QV3K72 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Casein kinase II subunit alpha' (Homo sapiens (Human)) | BDBM142806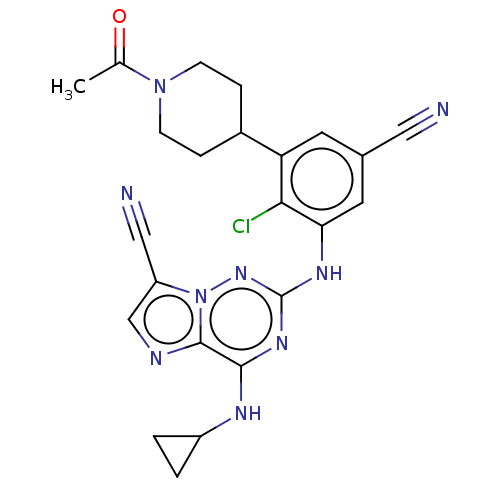 (US8940736, 229) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.160 | n/a | n/a | n/a | n/a | 7.4 | 30 |
Bristol-Myers Squibb Company US Patent | Assay Description The effectiveness of compounds of the present invention as inhibitors of protein kinases can be readily tested by assays known to those skilled in th... | US Patent US8940736 (2015) BindingDB Entry DOI: 10.7270/Q2QV3K72 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Casein kinase II subunit alpha (Homo sapiens (Human)) | BDBM142835 (US8940736, 402) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.160 | n/a | n/a | n/a | n/a | 7.4 | 30 |
Bristol-Myers Squibb Company US Patent | Assay Description The effectiveness of compounds of the present invention as inhibitors of protein kinases can be readily tested by assays known to those skilled in th... | US Patent US8940736 (2015) BindingDB Entry DOI: 10.7270/Q2QV3K72 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Casein kinase II subunit alpha' (Homo sapiens (Human)) | BDBM142834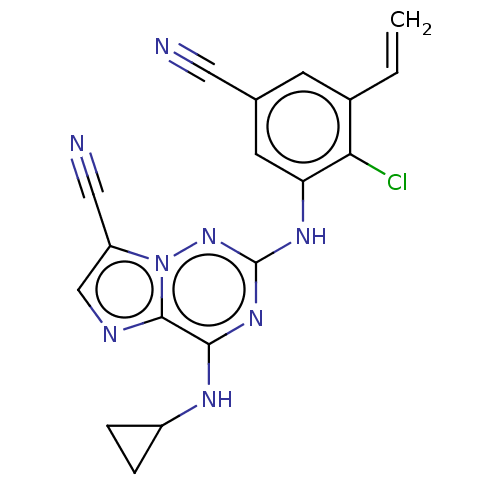 (US8940736, 399) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.160 | n/a | n/a | n/a | n/a | 7.4 | 30 |
Bristol-Myers Squibb Company US Patent | Assay Description The effectiveness of compounds of the present invention as inhibitors of protein kinases can be readily tested by assays known to those skilled in th... | US Patent US8940736 (2015) BindingDB Entry DOI: 10.7270/Q2QV3K72 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Casein kinase II subunit alpha (Homo sapiens (Human)) | BDBM142775 (US8940736, 7) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.170 | n/a | n/a | n/a | n/a | 7.4 | 30 |
Bristol-Myers Squibb Company US Patent | Assay Description The effectiveness of compounds of the present invention as inhibitors of protein kinases can be readily tested by assays known to those skilled in th... | US Patent US8940736 (2015) BindingDB Entry DOI: 10.7270/Q2QV3K72 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TGF-beta receptor type-1 (Homo sapiens (Human)) | BDBM406730 (N-[4-({2-[6-(difluoromethyl)pyridin-2-yl]pyrrolo[2...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co. US Patent | Assay Description Assays are conducted in 1536-well plates and 2 mL reactions are prepared from addition of HIS-TGFβR1 T204D or HIS-TGFβR2 WT, anti-HIS detec... | US Patent US10336761 (2019) BindingDB Entry DOI: 10.7270/Q2M90C2R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TGF-beta receptor type-1 (Homo sapiens (Human)) | BDBM406730 (N-[4-({2-[6-(difluoromethyl)pyridin-2-yl]pyrrolo[2...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co. US Patent | Assay Description Assays are conducted in 1536-well plates and 2 mL reactions are prepared from addition of HIS-TGFβR1 T204D or HIS-TGFβR2 WT, anti-HIS detec... | US Patent US10336761 (2019) BindingDB Entry DOI: 10.7270/Q2M90C2R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Casein kinase II subunit alpha' (Homo sapiens (Human)) | BDBM142778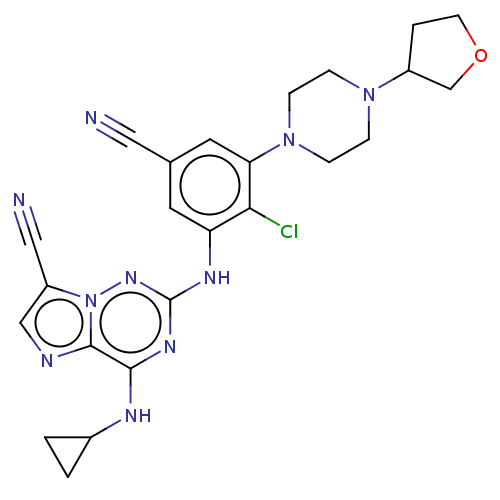 (US8940736, 55) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | 7.4 | 30 |
Bristol-Myers Squibb Company US Patent | Assay Description The effectiveness of compounds of the present invention as inhibitors of protein kinases can be readily tested by assays known to those skilled in th... | US Patent US8940736 (2015) BindingDB Entry DOI: 10.7270/Q2QV3K72 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Casein kinase II subunit alpha (Homo sapiens (Human)) | BDBM142793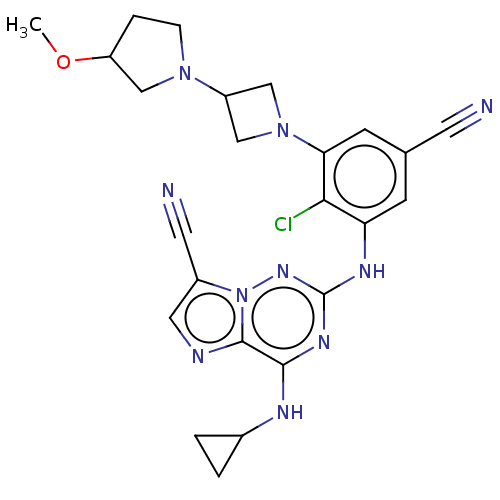 (US8940736, 161) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.210 | n/a | n/a | n/a | n/a | 7.4 | 30 |
Bristol-Myers Squibb Company US Patent | Assay Description The effectiveness of compounds of the present invention as inhibitors of protein kinases can be readily tested by assays known to those skilled in th... | US Patent US8940736 (2015) BindingDB Entry DOI: 10.7270/Q2QV3K72 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Casein kinase II subunit alpha' (Homo sapiens (Human)) | BDBM142832 (US8940736, 395) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.210 | n/a | n/a | n/a | n/a | 7.4 | 30 |
Bristol-Myers Squibb Company US Patent | Assay Description The effectiveness of compounds of the present invention as inhibitors of protein kinases can be readily tested by assays known to those skilled in th... | US Patent US8940736 (2015) BindingDB Entry DOI: 10.7270/Q2QV3K72 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Casein kinase II subunit alpha' (Homo sapiens (Human)) | BDBM142818 (US8940736, 313) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.210 | n/a | n/a | n/a | n/a | 7.4 | 30 |
Bristol-Myers Squibb Company US Patent | Assay Description The effectiveness of compounds of the present invention as inhibitors of protein kinases can be readily tested by assays known to those skilled in th... | US Patent US8940736 (2015) BindingDB Entry DOI: 10.7270/Q2QV3K72 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Casein kinase II subunit alpha (Homo sapiens (Human)) | BDBM142806 (US8940736, 229) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.210 | n/a | n/a | n/a | n/a | 7.4 | 30 |
Bristol-Myers Squibb Company US Patent | Assay Description The effectiveness of compounds of the present invention as inhibitors of protein kinases can be readily tested by assays known to those skilled in th... | US Patent US8940736 (2015) BindingDB Entry DOI: 10.7270/Q2QV3K72 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Casein kinase II subunit alpha (Homo sapiens (Human)) | BDBM142832 (US8940736, 395) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.220 | n/a | n/a | n/a | n/a | 7.4 | 30 |
Bristol-Myers Squibb Company US Patent | Assay Description The effectiveness of compounds of the present invention as inhibitors of protein kinases can be readily tested by assays known to those skilled in th... | US Patent US8940736 (2015) BindingDB Entry DOI: 10.7270/Q2QV3K72 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Casein kinase II subunit alpha (Homo sapiens (Human)) | BDBM142784 (US8940736, 117) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.220 | n/a | n/a | n/a | n/a | 7.4 | 30 |
Bristol-Myers Squibb Company US Patent | Assay Description The effectiveness of compounds of the present invention as inhibitors of protein kinases can be readily tested by assays known to those skilled in th... | US Patent US8940736 (2015) BindingDB Entry DOI: 10.7270/Q2QV3K72 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Casein kinase II subunit alpha' (Homo sapiens (Human)) | BDBM142802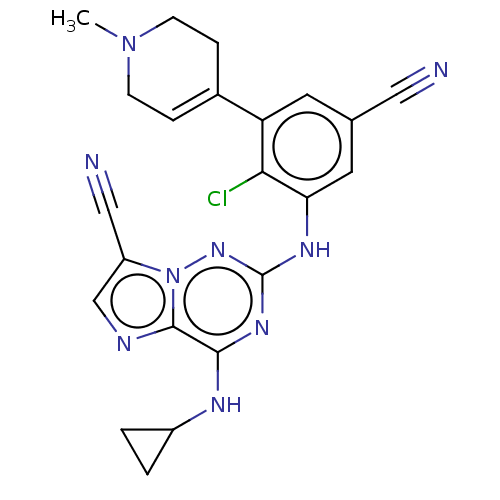 (US8940736, 207) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.230 | n/a | n/a | n/a | n/a | 7.4 | 30 |
Bristol-Myers Squibb Company US Patent | Assay Description The effectiveness of compounds of the present invention as inhibitors of protein kinases can be readily tested by assays known to those skilled in th... | US Patent US8940736 (2015) BindingDB Entry DOI: 10.7270/Q2QV3K72 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Casein kinase II subunit alpha (Homo sapiens (Human)) | BDBM142834 (US8940736, 399) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.230 | n/a | n/a | n/a | n/a | 7.4 | 30 |
Bristol-Myers Squibb Company US Patent | Assay Description The effectiveness of compounds of the present invention as inhibitors of protein kinases can be readily tested by assays known to those skilled in th... | US Patent US8940736 (2015) BindingDB Entry DOI: 10.7270/Q2QV3K72 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Casein kinase II subunit alpha' (Homo sapiens (Human)) | BDBM142836 (US8940736, 408) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.230 | n/a | n/a | n/a | n/a | 7.4 | 30 |
Bristol-Myers Squibb Company US Patent | Assay Description The effectiveness of compounds of the present invention as inhibitors of protein kinases can be readily tested by assays known to those skilled in th... | US Patent US8940736 (2015) BindingDB Entry DOI: 10.7270/Q2QV3K72 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Casein kinase II subunit alpha (Homo sapiens (Human)) | BDBM142777 (US8940736, 49) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.230 | n/a | n/a | n/a | n/a | 7.4 | 30 |
Bristol-Myers Squibb Company US Patent | Assay Description The effectiveness of compounds of the present invention as inhibitors of protein kinases can be readily tested by assays known to those skilled in th... | US Patent US8940736 (2015) BindingDB Entry DOI: 10.7270/Q2QV3K72 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Casein kinase II subunit alpha (Homo sapiens (Human)) | BDBM142792 (US8940736, 160) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.240 | n/a | n/a | n/a | n/a | 7.4 | 30 |
Bristol-Myers Squibb Company US Patent | Assay Description The effectiveness of compounds of the present invention as inhibitors of protein kinases can be readily tested by assays known to those skilled in th... | US Patent US8940736 (2015) BindingDB Entry DOI: 10.7270/Q2QV3K72 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TGF-beta receptor type-1 (Homo sapiens (Human)) | BDBM407009 (N-(4-{[5-(morpholin-4-ylmethyl)-2-(pyridin-2-yl)py...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co. US Patent | Assay Description Assays are conducted in 1536-well plates and 2 mL reactions are prepared from addition of HIS-TGFβR1 T204D or HIS-TGFβR2 WT, anti-HIS detec... | US Patent US10336761 (2019) BindingDB Entry DOI: 10.7270/Q2M90C2R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TGF-beta receptor type-1 (Homo sapiens (Human)) | BDBM407012 ((3-{[({4-[(3-fluoropyridin-4-yl)amino]-2-(pyridin-...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <0.25 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co. US Patent | Assay Description Assays are conducted in 1536-well plates and 2 mL reactions are prepared from addition of HIS-TGFβR1 T204D or HIS-TGFβR2 WT, anti-HIS detec... | US Patent US10336761 (2019) BindingDB Entry DOI: 10.7270/Q2M90C2R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Casein kinase II subunit alpha' (Homo sapiens (Human)) | BDBM142820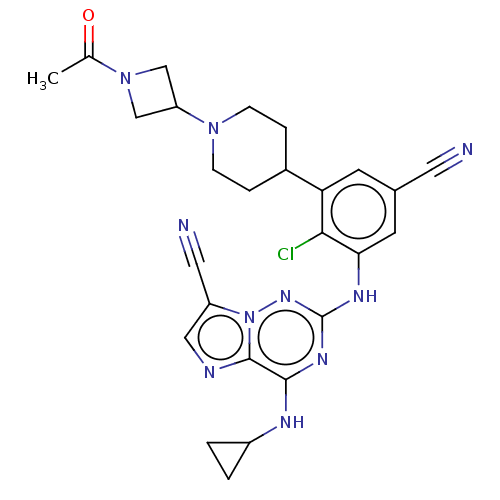 (US8940736, 319) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.25 | n/a | n/a | n/a | n/a | 7.4 | 30 |
Bristol-Myers Squibb Company US Patent | Assay Description The effectiveness of compounds of the present invention as inhibitors of protein kinases can be readily tested by assays known to those skilled in th... | US Patent US8940736 (2015) BindingDB Entry DOI: 10.7270/Q2QV3K72 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TGF-beta receptor type-1 (Homo sapiens (Human)) | BDBM406793 (N-{5-[(4,4-difluoropiperidin-1-yl)methyl]-2-(6-met...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co. US Patent | Assay Description Assays are conducted in 1536-well plates and 2 mL reactions are prepared from addition of HIS-TGFβR1 T204D or HIS-TGFβR2 WT, anti-HIS detec... | US Patent US10336761 (2019) BindingDB Entry DOI: 10.7270/Q2M90C2R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TGF-beta receptor type-1 [4-503,T204D] (Homo sapiens (Human)) | BDBM412735 (2-(6-(difluoromethyl)pyridin-2-yl)-N-(3-fluoropyri...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | UniChem | US Patent | n/a | n/a | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myer Squibb Company US Patent | Assay Description Assays are conducted in 1536-well plates and 2 mL reactions are prepared from addition of HIS-TGFβR1 T204D or HIS-TGFβR2 WT, anti-HIS detec... | US Patent US10399987 (2019) BindingDB Entry DOI: 10.7270/Q2222X4N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TGF-beta receptor type-1 (Homo sapiens (Human)) | BDBM406951 (1-({[2-(5-fluoropyridin-2-yl)-4-[(3-fluoropyridin-...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <0.25 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co. US Patent | Assay Description Assays are conducted in 1536-well plates and 2 mL reactions are prepared from addition of HIS-TGFβR1 T204D or HIS-TGFβR2 WT, anti-HIS detec... | US Patent US10336761 (2019) BindingDB Entry DOI: 10.7270/Q2M90C2R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TGF-beta receptor type-1 [4-503,T204D] (Homo sapiens (Human)) | BDBM412768 (N-(3-fluoropyridin-4-yl)-5-methyl-2-(6-methylpyrid...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | UniChem | US Patent | n/a | n/a | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myer Squibb Company US Patent | Assay Description Assays are conducted in 1536-well plates and 2 mL reactions are prepared from addition of HIS-TGFβR1 T204D or HIS-TGFβR2 WT, anti-HIS detec... | US Patent US10399987 (2019) BindingDB Entry DOI: 10.7270/Q2222X4N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TGF-beta receptor type-1 [4-503,T204D] (Homo sapiens (Human)) | BDBM412743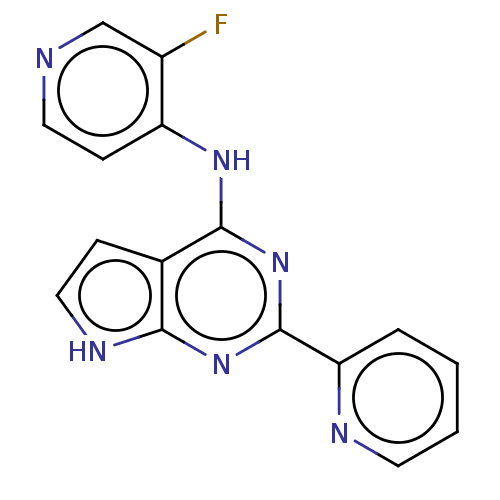 (N-(3-fluoropyridin-4-yl)-2-(pyridin-2-yl)-7H-pyrro...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | UniChem | US Patent | n/a | n/a | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myer Squibb Company US Patent | Assay Description Assays are conducted in 1536-well plates and 2 mL reactions are prepared from addition of HIS-TGFβR1 T204D or HIS-TGFβR2 WT, anti-HIS detec... | US Patent US10399987 (2019) BindingDB Entry DOI: 10.7270/Q2222X4N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Casein kinase II subunit alpha' (Homo sapiens (Human)) | BDBM142799 (US8940736, 179) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.260 | n/a | n/a | n/a | n/a | 7.4 | 30 |
Bristol-Myers Squibb Company US Patent | Assay Description The effectiveness of compounds of the present invention as inhibitors of protein kinases can be readily tested by assays known to those skilled in th... | US Patent US8940736 (2015) BindingDB Entry DOI: 10.7270/Q2QV3K72 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Casein kinase II subunit alpha' (Homo sapiens (Human)) | BDBM142807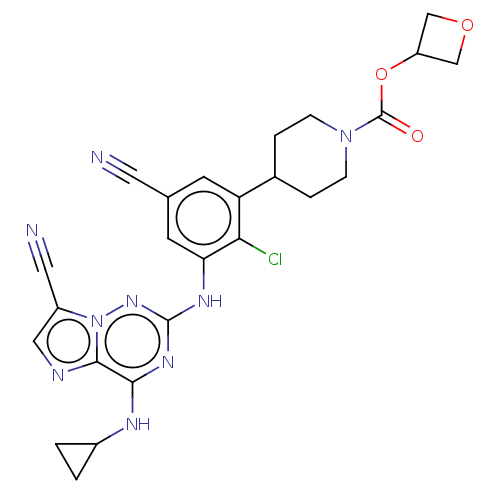 (US8940736, 238) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.270 | n/a | n/a | n/a | n/a | 7.4 | 30 |
Bristol-Myers Squibb Company US Patent | Assay Description The effectiveness of compounds of the present invention as inhibitors of protein kinases can be readily tested by assays known to those skilled in th... | US Patent US8940736 (2015) BindingDB Entry DOI: 10.7270/Q2QV3K72 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Casein kinase II subunit alpha (Homo sapiens (Human)) | BDBM142794 (US8940736, 163) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.270 | n/a | n/a | n/a | n/a | 7.4 | 30 |
Bristol-Myers Squibb Company US Patent | Assay Description The effectiveness of compounds of the present invention as inhibitors of protein kinases can be readily tested by assays known to those skilled in th... | US Patent US8940736 (2015) BindingDB Entry DOI: 10.7270/Q2QV3K72 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Casein kinase II subunit alpha (Homo sapiens (Human)) | BDBM142816 (US8940736, 281) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.270 | n/a | n/a | n/a | n/a | 7.4 | 30 |
Bristol-Myers Squibb Company US Patent | Assay Description The effectiveness of compounds of the present invention as inhibitors of protein kinases can be readily tested by assays known to those skilled in th... | US Patent US8940736 (2015) BindingDB Entry DOI: 10.7270/Q2QV3K72 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TGF-beta receptor type-1 (Homo sapiens (Human)) | BDBM406957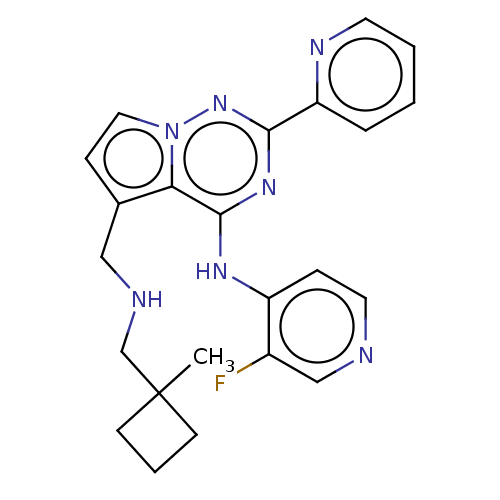 (3-fluoro-N-(5-{[(1-methylcyclobutyl)amino]methyl}-...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.270 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co. US Patent | Assay Description Assays are conducted in 1536-well plates and 2 mL reactions are prepared from addition of HIS-TGFβR1 T204D or HIS-TGFβR2 WT, anti-HIS detec... | US Patent US10336761 (2019) BindingDB Entry DOI: 10.7270/Q2M90C2R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 2116 total ) | Next | Last >> |