

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| D(1A) dopamine receptor (RAT) | BDBM60917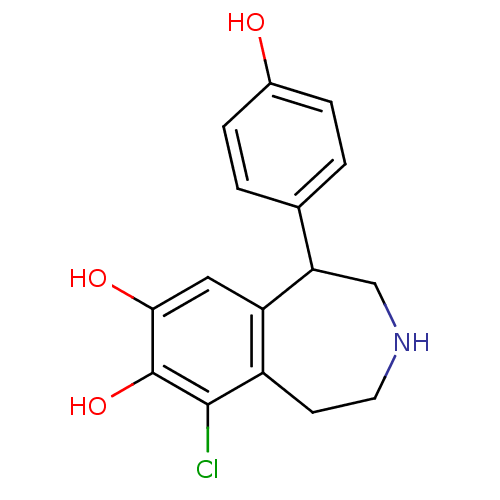 (9-chloranyl-5-(4-hydroxyphenyl)-2,3,4,5-tetrahydro...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid PDB UniChem Similars | PDB PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [3H]fenoldopam from Dopamine receptor D1 of rat striatum membranes | J Med Chem 29: 1904-12 (1986) BindingDB Entry DOI: 10.7270/Q2TQ623R | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| D(1A) dopamine receptor (RAT) | BDBM50025202 (9-Aminomethyl-9H-fluorene-2,5,6-triol | CHEMBL5767...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 43 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [3H]fenoldopam from Dopamine receptor D1 of rat striatum membranes | J Med Chem 29: 1904-12 (1986) BindingDB Entry DOI: 10.7270/Q2TQ623R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (RAT) | BDBM50001955 ((-)6-Methyl-5,6,6a,7-tetrahydro-4H-dibenzo[de,g]qu...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | 53 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [3H]fenoldopam from Dopamine receptor D1 of rat striatum membranes | J Med Chem 29: 1904-12 (1986) BindingDB Entry DOI: 10.7270/Q2TQ623R | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dopamine beta-hydroxylase (Bos taurus) | BDBM50014968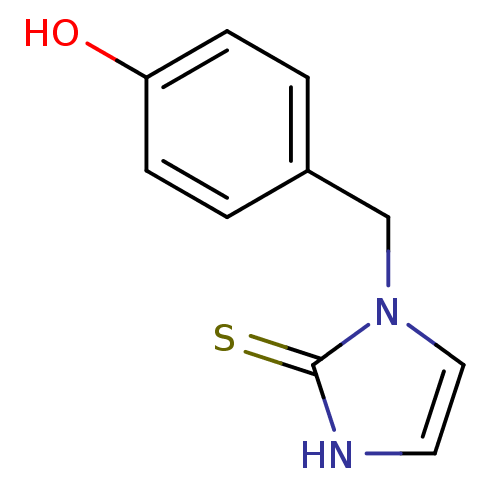 (1-(4-Hydroxy-benzyl)-1,3-dihydro-imidazole-2-thion...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 55 | n/a | n/a | n/a | n/a | n/a | n/a | 4.5 | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for the inhibition of Dopamine beta hydroxylase at pH 4.5 | J Med Chem 29: 2465-72 (1987) BindingDB Entry DOI: 10.7270/Q2BR8R5P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (RAT) | BDBM50025206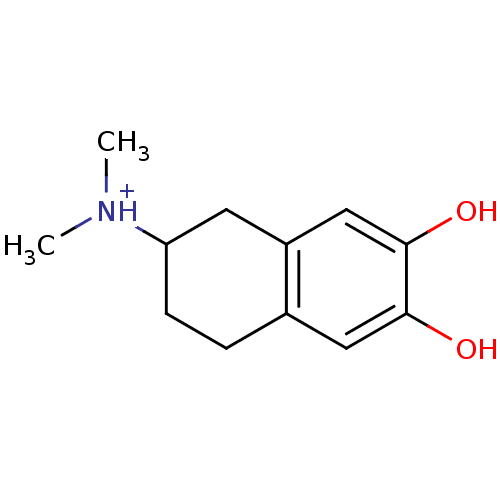 ((6,7-Dihydroxy-1,2,3,4-tetrahydro-naphthalen-2-yl)...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | PubMed | 57 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [3H]fenoldopam from Dopamine receptor D1 of rat striatum membranes | J Med Chem 29: 1904-12 (1986) BindingDB Entry DOI: 10.7270/Q2TQ623R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dopamine beta-hydroxylase (Bos taurus) | BDBM50000439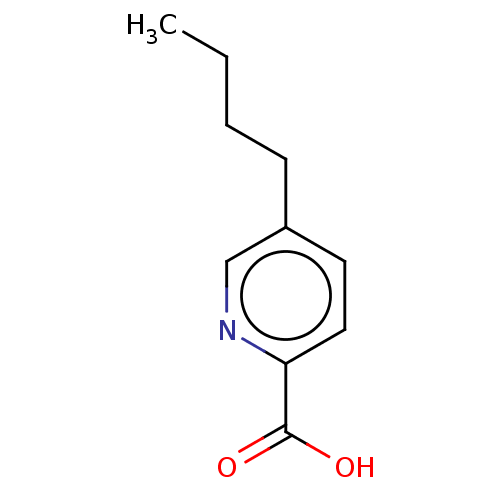 (5-Butyl-pyridine-2-carboxylic acid | 5-Butyl-pyrid...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents | PubMed | 149 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for the inhibition of Dopamine beta hydroxylase at pH 4.5 | J Med Chem 29: 2465-72 (1987) BindingDB Entry DOI: 10.7270/Q2BR8R5P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (RAT) | BDBM55121 (3-HYDROXYTYRAMINE HYDROCHLORIDE | 4-(2-aminoethyl)...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase DrugBank KEGG PC cid PC sid PDB UniChem Similars | PDB PubMed | 150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [3H]fenoldopam from Dopamine receptor D1 of rat striatum membranes | J Med Chem 29: 1904-12 (1986) BindingDB Entry DOI: 10.7270/Q2TQ623R | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| D(2) dopamine receptor (BOVINE) | BDBM50001955 ((-)6-Methyl-5,6,6a,7-tetrahydro-4H-dibenzo[de,g]qu...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank PC cid PC sid PDB UniChem Patents Similars | PubMed | 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for inhibition of [3H]spiroperidol binding against Dopamine receptor D2 | J Med Chem 29: 1904-12 (1986) BindingDB Entry DOI: 10.7270/Q2TQ623R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (BOVINE) | BDBM50025206 ((6,7-Dihydroxy-1,2,3,4-tetrahydro-naphthalen-2-yl)...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | PubMed | 255 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for inhibition of [3H]spiroperidol binding against Dopamine receptor D2 | J Med Chem 29: 1904-12 (1986) BindingDB Entry DOI: 10.7270/Q2TQ623R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dopamine beta-hydroxylase (Bos taurus) | BDBM50014968 (1-(4-Hydroxy-benzyl)-1,3-dihydro-imidazole-2-thion...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 344 | n/a | n/a | n/a | n/a | n/a | n/a | 6.6 | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for the inhibition of Dopamine beta hydroxylase at pH 6.6 | J Med Chem 29: 2465-72 (1987) BindingDB Entry DOI: 10.7270/Q2BR8R5P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (RAT) | BDBM50025201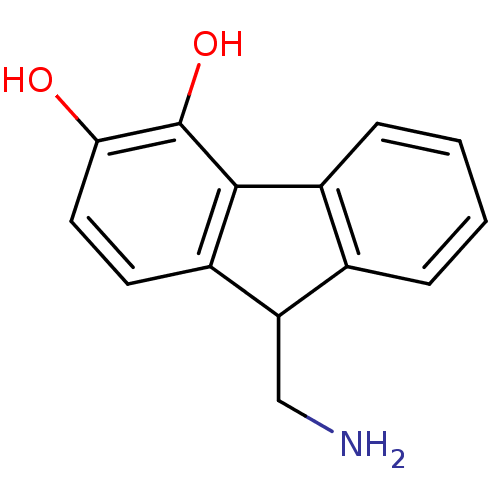 (9-Aminomethyl-9H-fluorene-3,4-diol | CHEMBL55693) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [3H]fenoldopam from Dopamine receptor D1 of rat striatum membranes | J Med Chem 29: 1904-12 (1986) BindingDB Entry DOI: 10.7270/Q2TQ623R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (RAT) | BDBM50025208 (6-Chloro-9-phenyl-2,3,4,5-tetrahydro-1H-benzo[d]az...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [3H]fenoldopam from Dopamine receptor D1 of rat striatum membranes | J Med Chem 29: 1904-12 (1986) BindingDB Entry DOI: 10.7270/Q2TQ623R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (BOVINE) | BDBM60917 (9-chloranyl-5-(4-hydroxyphenyl)-2,3,4,5-tetrahydro...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid PDB UniChem Similars | PubMed | 790 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for inhibition of [3H]spiroperidol binding against Dopamine receptor D2 | J Med Chem 29: 1904-12 (1986) BindingDB Entry DOI: 10.7270/Q2TQ623R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (RAT) | BDBM50004821 (2,3,4,5-Tetrahydro-1H-benzo[d]azepine-7,8-diol | C...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 970 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for binding affinity against dDopamine receptor D1 using [3H]fenoldopam as a radioligand | J Med Chem 29: 1904-12 (1986) BindingDB Entry DOI: 10.7270/Q2TQ623R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (RAT) | BDBM50025204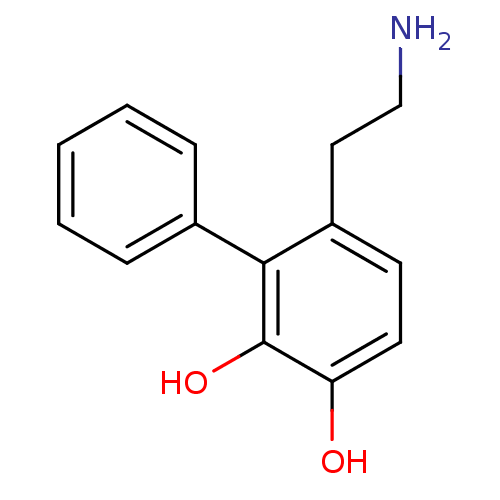 (6-(2-Amino-ethyl)-biphenyl-2,3-diol | CHEMBL299511) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [3H]fenoldopam from Dopamine receptor D1 of rat striatum membranes | J Med Chem 29: 1904-12 (1986) BindingDB Entry DOI: 10.7270/Q2TQ623R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (RAT) | BDBM50025209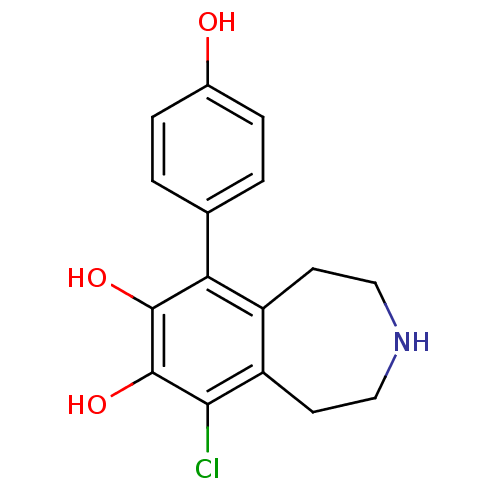 (6-Chloro-9-(4-hydroxy-phenyl)-2,3,4,5-tetrahydro-1...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.63E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [3H]fenoldopam from Dopamine receptor D1 of rat striatum membranes | J Med Chem 29: 1904-12 (1986) BindingDB Entry DOI: 10.7270/Q2TQ623R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (RAT) | BDBM50025207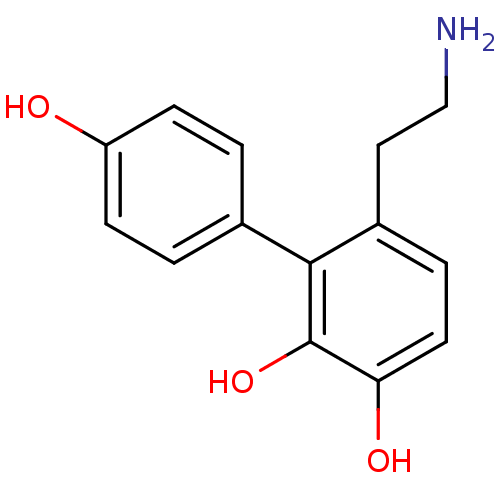 (6-(2-Amino-ethyl)-biphenyl-2,3,4'-triol | CHEMBL57...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.65E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [3H]fenoldopam from Dopamine receptor D1 of rat striatum membranes | J Med Chem 29: 1904-12 (1986) BindingDB Entry DOI: 10.7270/Q2TQ623R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (RAT) | BDBM50025203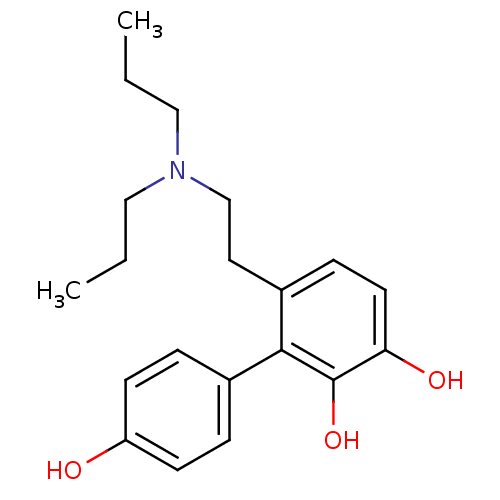 (6-(2-Dipropylamino-ethyl)-biphenyl-2,3,4'-triol | ...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.74E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [3H]fenoldopam from Dopamine receptor D1 of rat striatum membranes | J Med Chem 29: 1904-12 (1986) BindingDB Entry DOI: 10.7270/Q2TQ623R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (RAT) | BDBM50025210 (6-Phenyl-2,3,4,5-tetrahydro-1H-benzo[d]azepine-7,8...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.79E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [3H]fenoldopam from Dopamine receptor D1 of rat striatum membranes | J Med Chem 29: 1904-12 (1986) BindingDB Entry DOI: 10.7270/Q2TQ623R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (BOVINE) | BDBM50025203 (6-(2-Dipropylamino-ethyl)-biphenyl-2,3,4'-triol | ...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for inhibition of [3H]spiroperidol binding against Dopamine receptor D2 | J Med Chem 29: 1904-12 (1986) BindingDB Entry DOI: 10.7270/Q2TQ623R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (RAT) | BDBM50025205 (6-(2-Dipropylamino-ethyl)-biphenyl-2,3-diol | CHEM...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.94E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [3H]fenoldopam from Dopamine receptor D1 of rat striatum membranes | J Med Chem 29: 1904-12 (1986) BindingDB Entry DOI: 10.7270/Q2TQ623R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (BOVINE) | BDBM55121 (3-HYDROXYTYRAMINE HYDROCHLORIDE | 4-(2-aminoethyl)...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase DrugBank KEGG PC cid PC sid PDB UniChem Similars | PubMed | 2.35E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for inhibition of [3H]spiroperidol binding against Dopamine receptor D2 | J Med Chem 29: 1904-12 (1986) BindingDB Entry DOI: 10.7270/Q2TQ623R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (RAT) | BDBM50025211 (6-(4-Hydroxy-phenyl)-2,3,4,5-tetrahydro-1H-benzo[d...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [3H]fenoldopam from Dopamine receptor D1 of rat striatum membranes | J Med Chem 29: 1904-12 (1986) BindingDB Entry DOI: 10.7270/Q2TQ623R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (BOVINE) | BDBM50025202 (9-Aminomethyl-9H-fluorene-2,5,6-triol | CHEMBL5767...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 5.74E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for inhibition of [3H]spiroperidol binding against Dopamine receptor D2 | J Med Chem 29: 1904-12 (1986) BindingDB Entry DOI: 10.7270/Q2TQ623R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dopamine beta-hydroxylase (Bos taurus) | BDBM50241361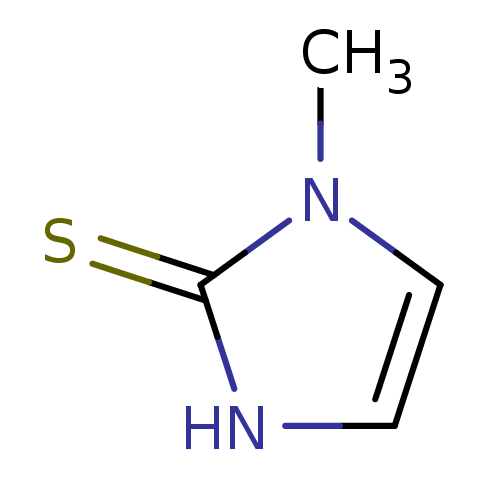 (CHEMBL1515 | METHIMAZOLE | US9138393, Methimazole ...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents | PubMed | 4.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | 4.5 | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for the inhibition of Dopamine beta hydroxylase at pH 6.6 | J Med Chem 29: 2465-72 (1987) BindingDB Entry DOI: 10.7270/Q2BR8R5P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dopamine beta-hydroxylase (Bos taurus) | BDBM50241361 (CHEMBL1515 | METHIMAZOLE | US9138393, Methimazole ...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents | PubMed | 7.16E+5 | n/a | n/a | n/a | n/a | n/a | n/a | 6.6 | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for the inhibition of Dopamine beta hydroxylase at pH 4.5 | J Med Chem 29: 2465-72 (1987) BindingDB Entry DOI: 10.7270/Q2BR8R5P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dopamine beta-hydroxylase (Bos taurus) | BDBM50014970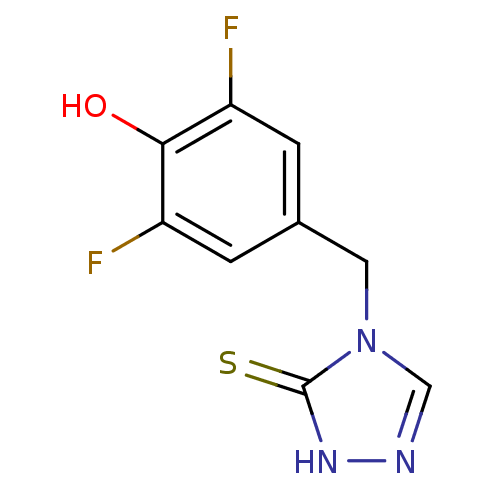 (4-(3,5-Difluoro-4-hydroxy-benzyl)-2,4-dihydro-[1,2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline & French Laboratories Curated by ChEMBL | Assay Description Inhibitory activity was determined against bovine dopamine beta-hydroxylase(DBH) | J Med Chem 33: 781-9 (1990) BindingDB Entry DOI: 10.7270/Q2DZ078G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (BOVINE) | BDBM50020682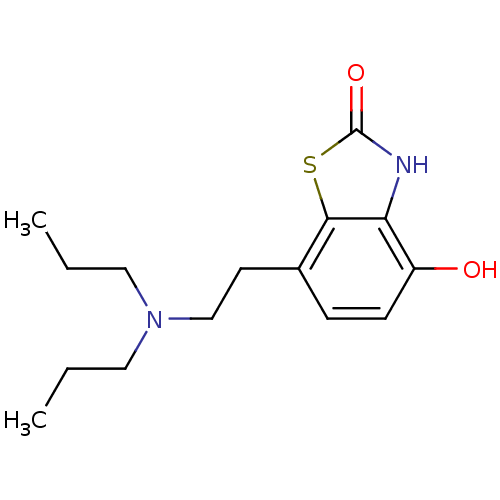 (7-(2-Dipropylamino-ethyl)-4-hydroxy-3H-benzothiazo...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 49 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [3H]-spiroperidol from homogenized bovine pituitary Dopamine receptor D2 | J Med Chem 30: 1166-76 (1987) BindingDB Entry DOI: 10.7270/Q2028QJ6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dopamine beta-hydroxylase (Bos taurus) | BDBM50014978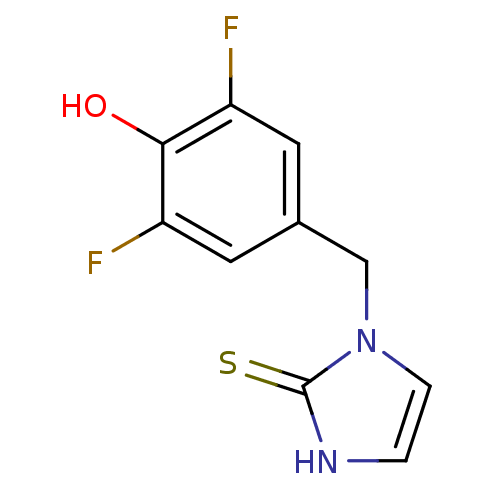 (1-(3,5-Difluoro-4-hydroxy-benzyl)-1,3-dihydro-imid...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 74 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory Concentration against bovine Dopamine beta hydroxylase (DBH); Range is between (0.060-0.0877). | J Med Chem 30: 486-94 (1987) BindingDB Entry DOI: 10.7270/Q2FJ2FSM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dopamine beta-hydroxylase (Bos taurus) | BDBM50014978 (1-(3,5-Difluoro-4-hydroxy-benzyl)-1,3-dihydro-imid...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 74 | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline & French Laboratories Curated by ChEMBL | Assay Description Inhibitory activity was determined against bovine dopamine beta-hydroxylase(DBH) | J Med Chem 33: 781-9 (1990) BindingDB Entry DOI: 10.7270/Q2DZ078G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (BOVINE) | BDBM50019393 (4-(2-Dipropylamino-ethyl)-7-hydroxy-1,3-dihydro-in...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 530 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [3H]-spiroperidol from homogenized bovine pituitary Dopamine receptor D2 | J Med Chem 30: 1166-76 (1987) BindingDB Entry DOI: 10.7270/Q2028QJ6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (BOVINE) | BDBM50020677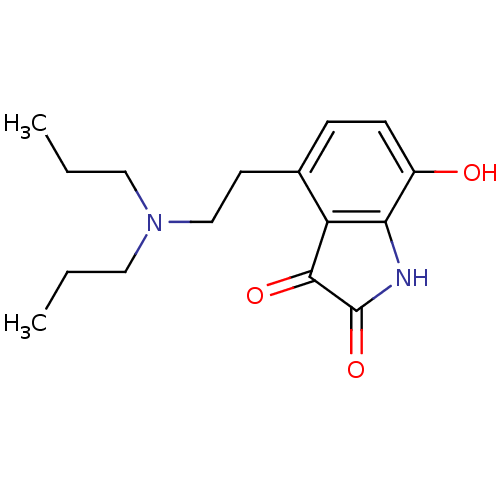 (4-(2-Dipropylamino-ethyl)-7-hydroxy-1H-indole-2,3-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 560 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [3H]-spiroperidol from homogenized bovine pituitary Dopamine receptor D2 | J Med Chem 30: 1166-76 (1987) BindingDB Entry DOI: 10.7270/Q2028QJ6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dopamine beta-hydroxylase (Bos taurus) | BDBM50014963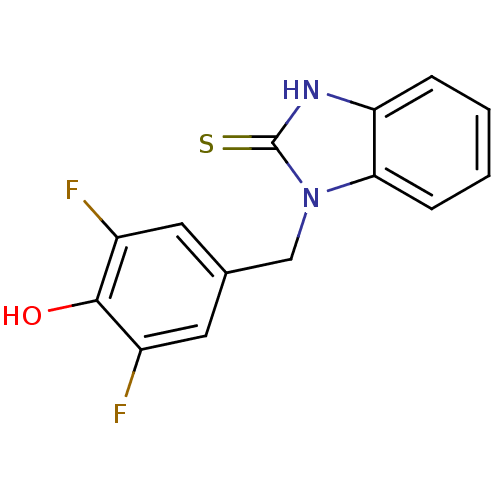 (1-(3,5-Difluoro-4-hydroxy-benzyl)-1,3-dihydro-benz...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 660 | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline & French Laboratories Curated by ChEMBL | Assay Description Inhibitory activity was determined against bovine dopamine beta-hydroxylase(DBH) | J Med Chem 33: 781-9 (1990) BindingDB Entry DOI: 10.7270/Q2DZ078G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dopamine beta-hydroxylase (Bos taurus) | BDBM50025811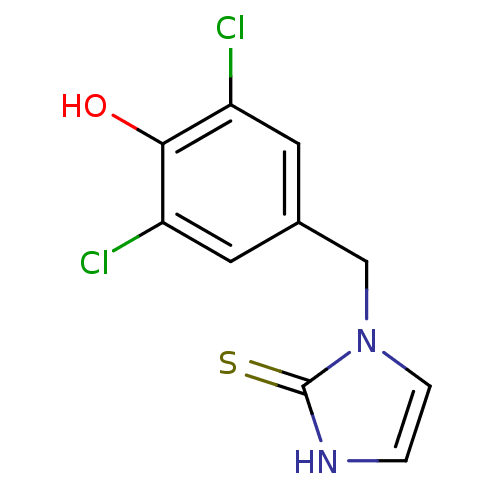 (1-(3,5-Dichloro-4-hydroxy-benzyl)-1,3-dihydro-imid...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 680 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory Concentration against bovine Dopamine beta hydroxylase (DBH); Range is between (0.42-1.1). | J Med Chem 30: 486-94 (1987) BindingDB Entry DOI: 10.7270/Q2FJ2FSM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dopamine beta-hydroxylase (Rattus norvegicus) | BDBM50000439 (5-Butyl-pyridine-2-carboxylic acid | 5-Butyl-pyrid...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents | PubMed | n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of Dopamine beta hydroxylase in spontaneously hypertensive rats; Value ranges from 0.4-1.1 | J Med Chem 30: 1309-13 (1987) BindingDB Entry DOI: 10.7270/Q2MC8Z0M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dopamine beta-hydroxylase (Bos taurus) | BDBM50014984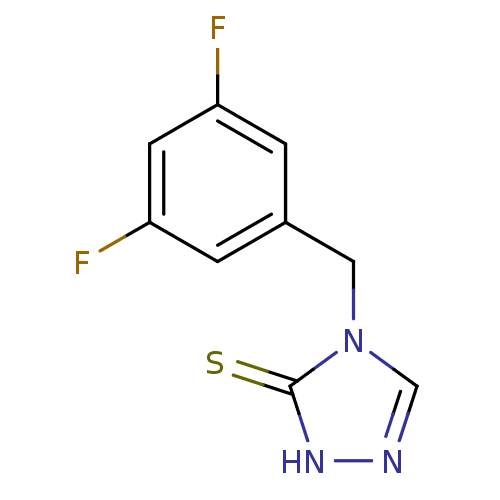 (4-(3,5-Difluoro-benzyl)-2,4-dihydro-[1,2,4]triazol...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline & French Laboratories Curated by ChEMBL | Assay Description Inhibitory activity was determined against bovine dopamine beta-hydroxylase(DBH) | J Med Chem 33: 781-9 (1990) BindingDB Entry DOI: 10.7270/Q2DZ078G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (BOVINE) | BDBM50020684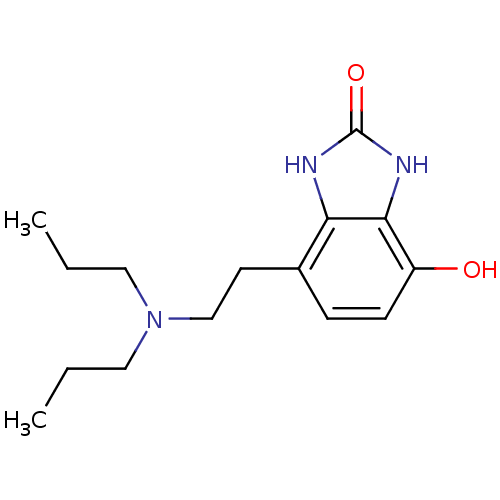 (4-(2-Dipropylamino-ethyl)-7-hydroxy-1,3-dihydro-be...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [3H]-spiroperidol from homogenized bovine pituitary Dopamine receptor D2 | J Med Chem 30: 1166-76 (1987) BindingDB Entry DOI: 10.7270/Q2028QJ6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (BOVINE) | BDBM50020678 (7-(2-Dipropylamino-ethyl)-3H-benzothiazol-2-one | ...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [3H]-spiroperidol from homogenized bovine pituitary Dopamine receptor D2 | J Med Chem 30: 1166-76 (1987) BindingDB Entry DOI: 10.7270/Q2028QJ6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dopamine beta-hydroxylase (Bos taurus) | BDBM50014983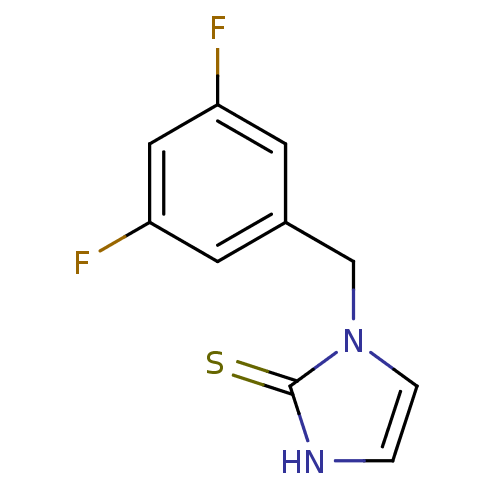 (1-(3,5-Difluoro-benzyl)-1,3-dihydro-imidazole-2-th...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline & French Laboratories Curated by ChEMBL | Assay Description Inhibitory activity was determined against bovine dopamine beta-hydroxylase(DBH) | J Med Chem 33: 781-9 (1990) BindingDB Entry DOI: 10.7270/Q2DZ078G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (BOVINE) | BDBM50020685 (7-(2-Dipropylamino-ethyl)-4-hydroxy-3H-benzooxazol...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [3H]-spiroperidol from homogenized bovine pituitary Dopamine receptor D2 | J Med Chem 30: 1166-76 (1987) BindingDB Entry DOI: 10.7270/Q2028QJ6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dopamine beta-hydroxylase (Rattus norvegicus) | BDBM50014983 (1-(3,5-Difluoro-benzyl)-1,3-dihydro-imidazole-2-th...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of Dopamine beta hydroxylase in spontaneously hypertensive rats; Value ranges from 1.1-1.4 | J Med Chem 30: 1309-13 (1987) BindingDB Entry DOI: 10.7270/Q2MC8Z0M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dopamine beta-hydroxylase (Bos taurus) | BDBM50014983 (1-(3,5-Difluoro-benzyl)-1,3-dihydro-imidazole-2-th...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory Concentration against bovine Dopamine beta hydroxylase (DBH); Range is between (1.0-1.9) | J Med Chem 30: 486-94 (1987) BindingDB Entry DOI: 10.7270/Q2FJ2FSM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dopamine beta-hydroxylase (Bos taurus) | BDBM50014971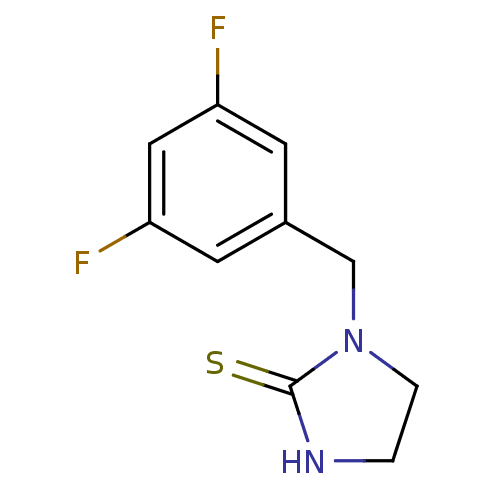 (1-(3,5-Difluoro-benzyl)-imidazolidine-2-thione | C...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline & French Laboratories Curated by ChEMBL | Assay Description Inhibitory activity was determined against bovine dopamine beta-hydroxylase(DBH) | J Med Chem 33: 781-9 (1990) BindingDB Entry DOI: 10.7270/Q2DZ078G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dopamine beta-hydroxylase (Bos taurus) | BDBM50025795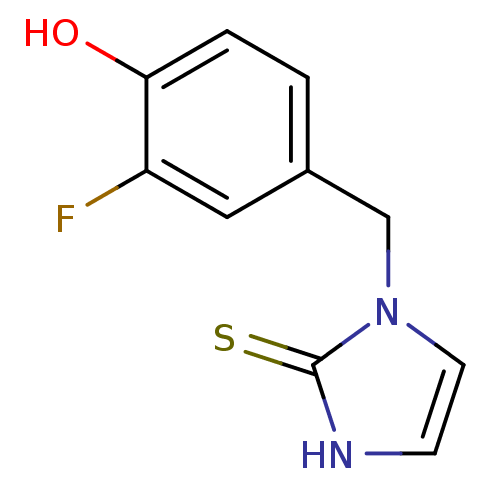 (1-(3-Fluoro-4-hydroxy-benzyl)-1,3-dihydro-imidazol...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory Concentration against bovine Dopamine beta hydroxylase (DBH); Range is between (0.9-2.2) | J Med Chem 30: 486-94 (1987) BindingDB Entry DOI: 10.7270/Q2FJ2FSM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dopamine beta-hydroxylase (Bos taurus) | BDBM50014967 (1-(3,5-Difluoro-benzyl)-1H-tetrazole-5-thiol | CHE...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | n/a | n/a | 1.54E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline & French Laboratories Curated by ChEMBL | Assay Description Inhibitory activity was determined against bovine dopamine beta-hydroxylase(DBH) | J Med Chem 33: 781-9 (1990) BindingDB Entry DOI: 10.7270/Q2DZ078G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (BOVINE) | BDBM60917 (9-chloranyl-5-(4-hydroxyphenyl)-2,3,4,5-tetrahydro...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid PDB UniChem Similars | PubMed | n/a | n/a | 1.58E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [3H]-spiroperidol from homogenized bovine pituitary Dopamine receptor D2 | J Med Chem 30: 1166-76 (1987) BindingDB Entry DOI: 10.7270/Q2028QJ6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dopamine beta-hydroxylase (Bos taurus) | BDBM50014979 (4-(3-Fluoro-benzyl)-2,4-dihydro-[1,2,4]triazole-3-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline & French Laboratories Curated by ChEMBL | Assay Description Inhibitory activity was determined against bovine dopamine beta-hydroxylase(DBH) | J Med Chem 33: 781-9 (1990) BindingDB Entry DOI: 10.7270/Q2DZ078G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (BOVINE) | BDBM50020679 (7-(2-Amino-ethyl)-4-hydroxy-3H-benzothiazol-2-one ...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [3H]-spiroperidol from homogenized bovine pituitary Dopamine receptor D2 | J Med Chem 30: 1166-76 (1987) BindingDB Entry DOI: 10.7270/Q2028QJ6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dopamine beta-hydroxylase (Bos taurus) | BDBM50025806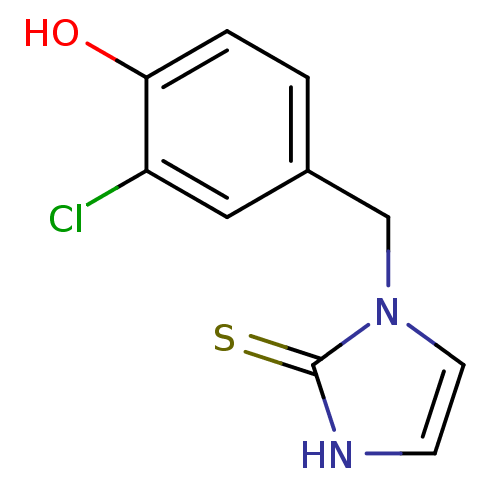 (1-(3-Chloro-4-hydroxy-benzyl)-1,3-dihydro-imidazol...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory Concentration against bovine Dopamine beta hydroxylase (DBH); Range is between (1.7-2.4) | J Med Chem 30: 486-94 (1987) BindingDB Entry DOI: 10.7270/Q2FJ2FSM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dopamine beta-hydroxylase (Bos taurus) | BDBM50025798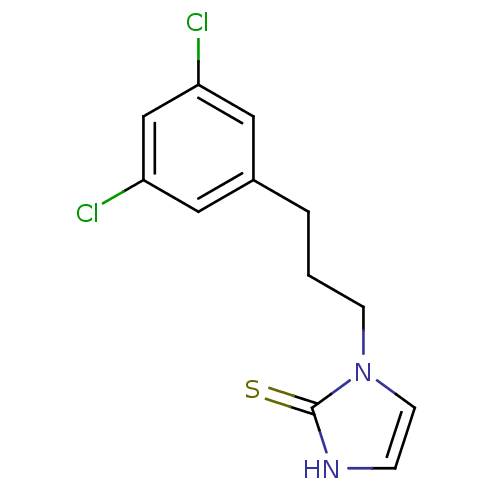 (1-[3-(3,5-Dichloro-phenyl)-propyl]-1,3-dihydro-imi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | n/a | n/a | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Percent inhibition against bovine Dopamine beta hydroxylase at 10E-4 M concentration of the compound; Range is between (1.8-2.6). | J Med Chem 30: 486-94 (1987) BindingDB Entry DOI: 10.7270/Q2FJ2FSM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 130 total ) | Next | Last >> |