

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dihydrofolate reductase (Escherichia coli) | BDBM50026300 (6-[5-(2,4-Diamino-pyrimidin-5-ylmethyl)-2,3-dimeth...) | MMDB NCI pathway Reactome pathway KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.0240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity for E. coli Dihydrofolate reductase | J Med Chem 25: 1120-2 (1983) BindingDB Entry DOI: 10.7270/Q28G8JRF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Escherichia coli) | BDBM50026308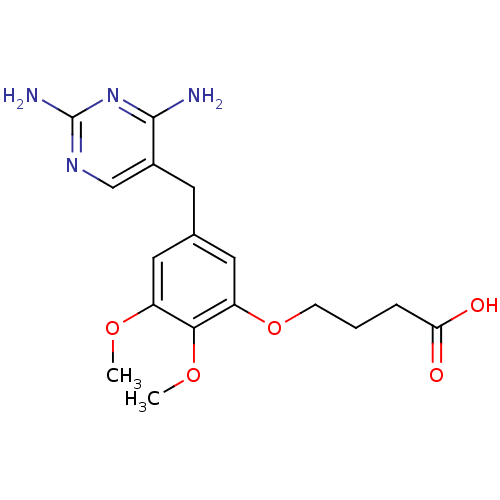 (4-[5-(2,4-Diamino-pyrimidin-5-ylmethyl)-2,3-dimeth...) | MMDB NCI pathway Reactome pathway KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity for E. coli Dihydrofolate reductase | J Med Chem 25: 1120-2 (1983) BindingDB Entry DOI: 10.7270/Q28G8JRF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Escherichia coli) | BDBM50026318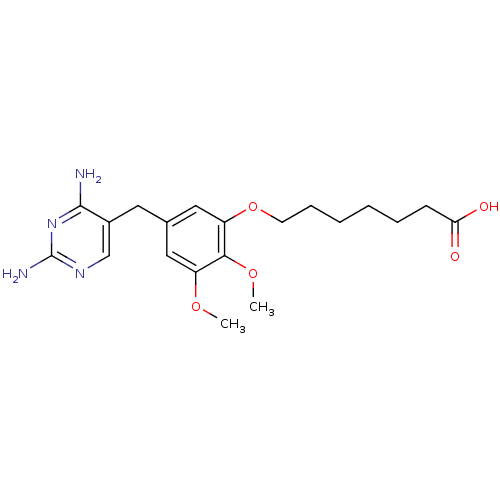 (7-[5-(2,4-Diamino-pyrimidin-5-ylmethyl)-2,3-dimeth...) | MMDB NCI pathway Reactome pathway KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity for E. coli Dihydrofolate reductase | J Med Chem 25: 1120-2 (1983) BindingDB Entry DOI: 10.7270/Q28G8JRF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Escherichia coli) | BDBM50026314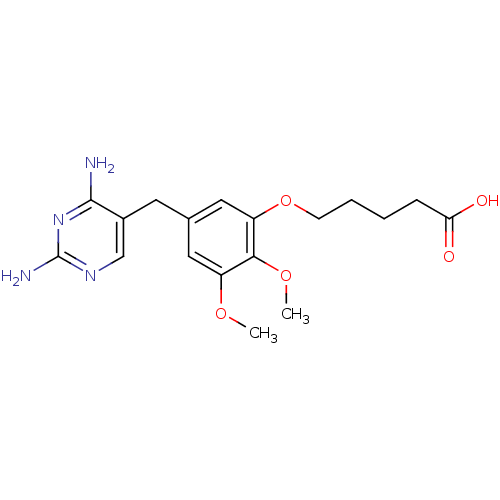 (5-[5-(2,4-Diamino-pyrimidin-5-ylmethyl)-2,3-dimeth...) | MMDB NCI pathway Reactome pathway KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0660 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity for E. coli Dihydrofolate reductase | J Med Chem 25: 1120-2 (1983) BindingDB Entry DOI: 10.7270/Q28G8JRF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Escherichia coli) | BDBM50026307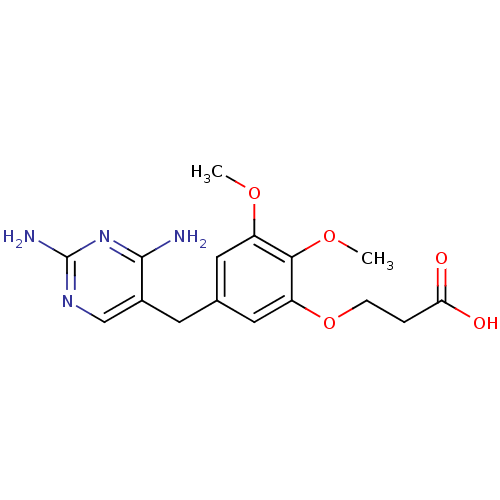 (3-[5-(2,4-Diamino-pyrimidin-5-ylmethyl)-2,3-dimeth...) | MMDB NCI pathway Reactome pathway KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity for E. coli Dihydrofolate reductase | J Med Chem 25: 1120-2 (1983) BindingDB Entry DOI: 10.7270/Q28G8JRF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Escherichia coli) | BDBM50026317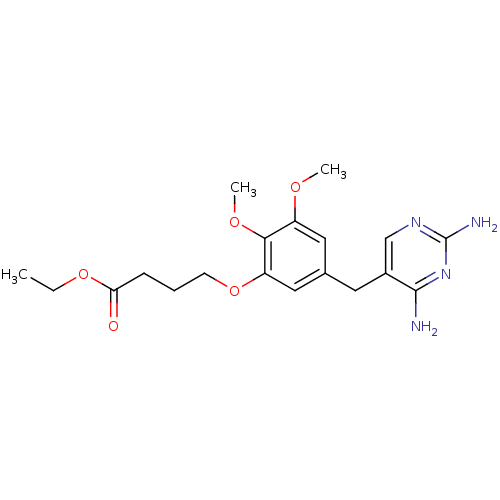 (4-[5-(2,4-Diamino-pyrimidin-5-ylmethyl)-2,3-dimeth...) | MMDB NCI pathway Reactome pathway KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity for E. coli Dihydrofolate reductase | J Med Chem 25: 1120-2 (1983) BindingDB Entry DOI: 10.7270/Q28G8JRF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM335426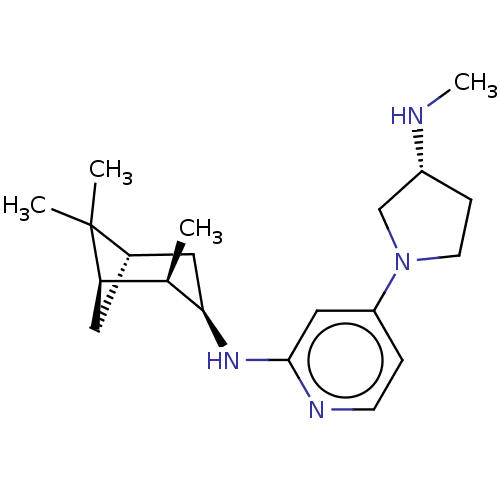 (4-[(3R)-3-(Methylamino)pyrrolidin-1-yl]-N-[(1R,2R,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
JANSSEN PHARMACEUTICA N.V. US Patent | Assay Description Cell pellets from SK-N-MC cells stably or transiently transfected with human H4 receptor (NCBI accession No. AF312230) were used for the binding assa... | US Patent US9732087 (2017) BindingDB Entry DOI: 10.7270/Q2251M9T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Escherichia coli) | BDBM50026316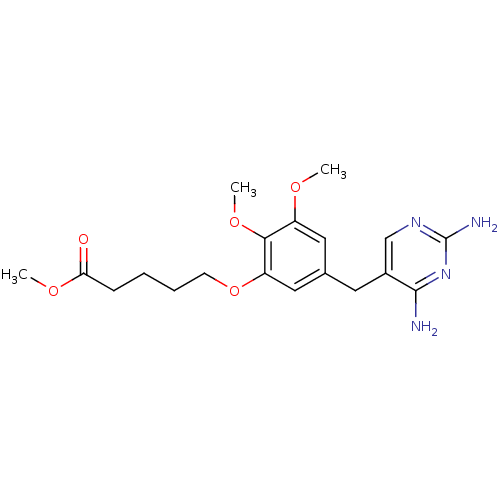 (5-[5-(2,4-Diamino-pyrimidin-5-ylmethyl)-2,3-dimeth...) | MMDB NCI pathway Reactome pathway KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.760 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity for E. coli Dihydrofolate reductase | J Med Chem 25: 1120-2 (1983) BindingDB Entry DOI: 10.7270/Q28G8JRF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Escherichia coli) | BDBM50026306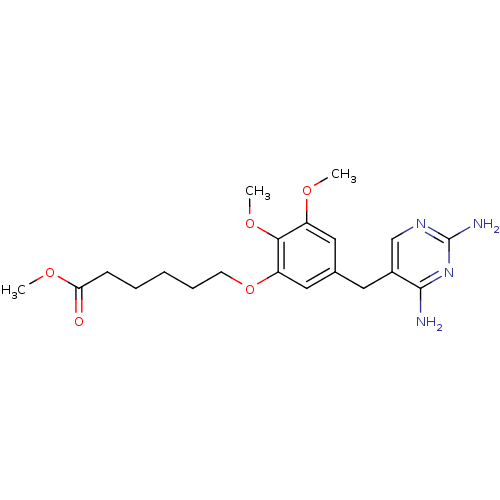 (6-[5-(2,4-Diamino-pyrimidin-5-ylmethyl)-2,3-dimeth...) | MMDB NCI pathway Reactome pathway KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.860 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity for E. coli Dihydrofolate reductase | J Med Chem 25: 1120-2 (1983) BindingDB Entry DOI: 10.7270/Q28G8JRF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50006745 (CHEMBL3236556) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.920 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research& Development, LLC Curated by ChEMBL | Assay Description Displacement of [3H]histamine from human recombinant histamine H4 receptor expressed in SK-N-MC cells after 45 mins by competition binding analysis | J Med Chem 57: 2429-39 (2014) Article DOI: 10.1021/jm401727m BindingDB Entry DOI: 10.7270/Q25H7HSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50061098 (CHEMBL3393534 | US9732087, 1) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
JANSSEN PHARMACEUTICA N.V. US Patent | Assay Description Cell pellets from SK-N-MC cells stably or transiently transfected with human H4 receptor (NCBI accession No. AF312230) were used for the binding assa... | US Patent US9732087 (2017) BindingDB Entry DOI: 10.7270/Q2251M9T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM335425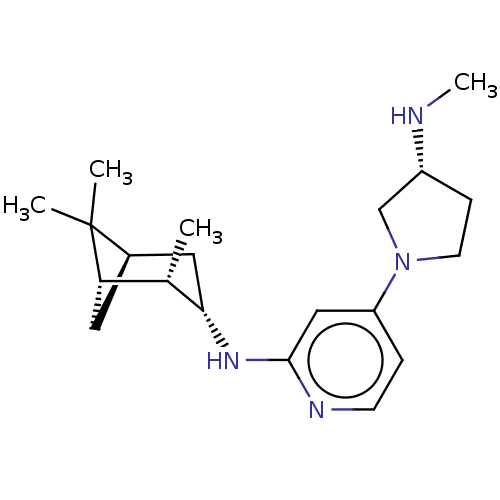 (4-[(3R)-3-(Methylamino)pyrrolidin-1-yl]-N-[(1S,2S,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
JANSSEN PHARMACEUTICA N.V. US Patent | Assay Description Cell pellets from SK-N-MC cells stably or transiently transfected with human H4 receptor (NCBI accession No. AF312230) were used for the binding assa... | US Patent US9732087 (2017) BindingDB Entry DOI: 10.7270/Q2251M9T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50061008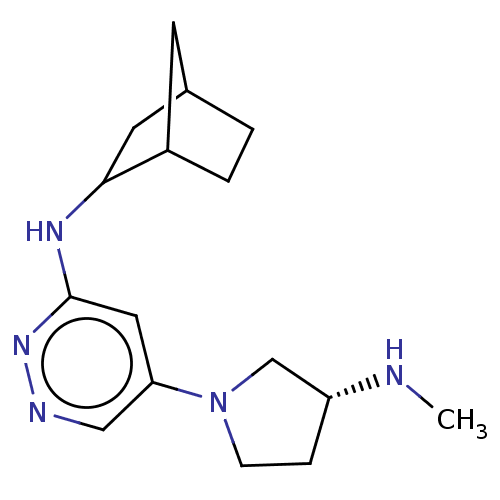 (CHEMBL3393556 | US9732087, 96) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
JANSSEN PHARMACEUTICA N.V. US Patent | Assay Description Cell pellets from SK-N-MC cells stably or transiently transfected with human H4 receptor (NCBI accession No. AF312230) were used for the binding assa... | US Patent US9732087 (2017) BindingDB Entry DOI: 10.7270/Q2251M9T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50006743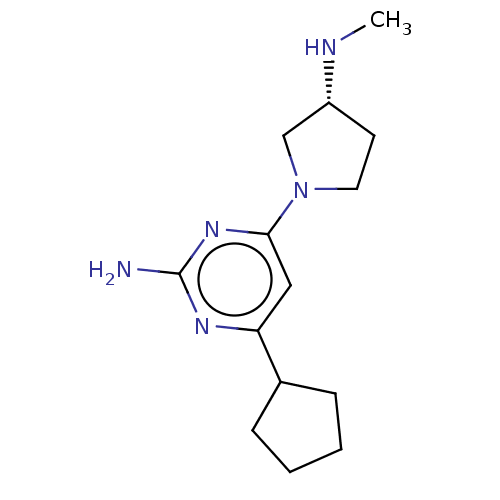 (CHEMBL3236554) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research& Development, LLC Curated by ChEMBL | Assay Description Displacement of [3H]histamine from human recombinant histamine H4 receptor expressed in SK-N-MC cells after 45 mins by competition binding analysis | J Med Chem 57: 2429-39 (2014) Article DOI: 10.1021/jm401727m BindingDB Entry DOI: 10.7270/Q25H7HSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50006742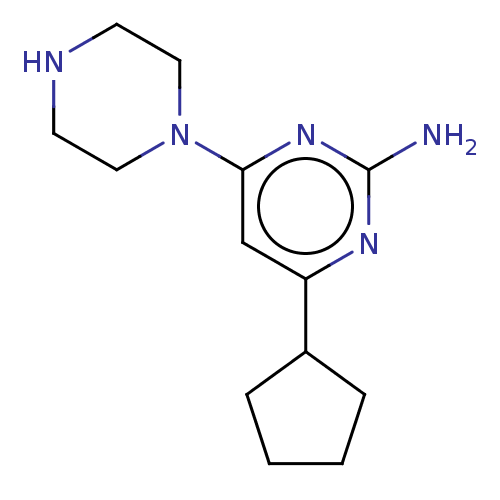 (CHEMBL3236553) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research& Development, LLC Curated by ChEMBL | Assay Description Displacement of [3H]histamine from human recombinant histamine H4 receptor expressed in SK-N-MC cells after 45 mins by competition binding analysis | J Med Chem 57: 2429-39 (2014) Article DOI: 10.1021/jm401727m BindingDB Entry DOI: 10.7270/Q25H7HSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Escherichia coli) | BDBM18069 (5-[(3,4,5-trimethoxyphenyl)methyl]pyrimidine-2,4-d...) | MMDB NCI pathway Reactome pathway KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity for E. coli Dihydrofolate reductase | J Med Chem 25: 1120-2 (1983) BindingDB Entry DOI: 10.7270/Q28G8JRF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50006739 (CHEMBL3236552) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research& Development, LLC Curated by ChEMBL | Assay Description Displacement of [3H]histamine from human recombinant histamine H4 receptor expressed in SK-N-MC cells after 45 mins by competition binding analysis | J Med Chem 57: 2429-39 (2014) Article DOI: 10.1021/jm401727m BindingDB Entry DOI: 10.7270/Q25H7HSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50006761 (CHEMBL3236571) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research& Development, LLC Curated by ChEMBL | Assay Description Displacement of [3H]histamine from human recombinant histamine H4 receptor expressed in SK-N-MC cells after 45 mins by competition binding analysis | J Med Chem 57: 2429-39 (2014) Article DOI: 10.1021/jm401727m BindingDB Entry DOI: 10.7270/Q25H7HSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50006769 (CHEMBL3236579) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research& Development, LLC Curated by ChEMBL | Assay Description Displacement of [3H]histamine from human recombinant histamine H4 receptor expressed in SK-N-MC cells after 45 mins by competition binding analysis | J Med Chem 57: 2429-39 (2014) Article DOI: 10.1021/jm401727m BindingDB Entry DOI: 10.7270/Q25H7HSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50061143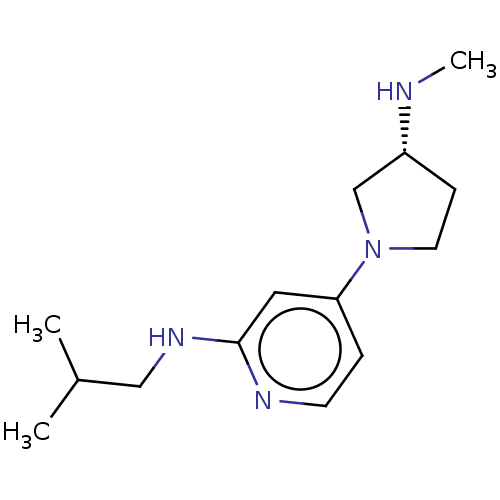 (CHEMBL3393526 | US9732087, 22) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
JANSSEN PHARMACEUTICA N.V. US Patent | Assay Description Cell pellets from SK-N-MC cells stably or transiently transfected with human H4 receptor (NCBI accession No. AF312230) were used for the binding assa... | US Patent US9732087 (2017) BindingDB Entry DOI: 10.7270/Q2251M9T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50006749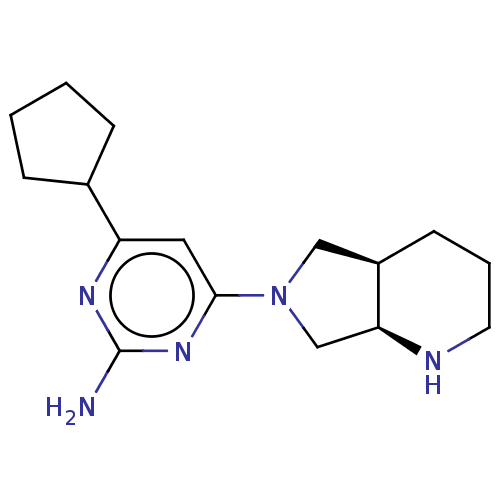 (CHEMBL3236560) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research& Development, LLC Curated by ChEMBL | Assay Description Displacement of [3H]histamine from human recombinant histamine H4 receptor expressed in SK-N-MC cells after 45 mins by competition binding analysis | J Med Chem 57: 2429-39 (2014) Article DOI: 10.1021/jm401727m BindingDB Entry DOI: 10.7270/Q25H7HSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Escherichia coli) | BDBM50026304 (7-[5-(2,4-Diamino-pyrimidin-5-ylmethyl)-2,3-dimeth...) | MMDB NCI pathway Reactome pathway KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity for E. coli Dihydrofolate reductase | J Med Chem 25: 1120-2 (1983) BindingDB Entry DOI: 10.7270/Q28G8JRF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50061056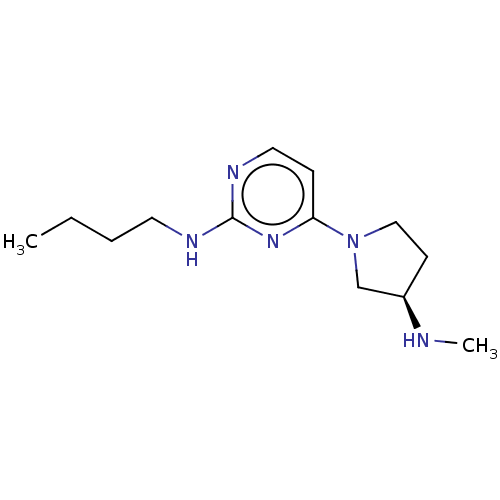 (CHEMBL3393542 | US9732087, 73) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
JANSSEN PHARMACEUTICA N.V. US Patent | Assay Description Cell pellets from SK-N-MC cells stably or transiently transfected with human H4 receptor (NCBI accession No. AF312230) were used for the binding assa... | US Patent US9732087 (2017) BindingDB Entry DOI: 10.7270/Q2251M9T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 1 (Homo sapiens (Human)) | BDBM35650 (pyrimidylpyrrole, 10c) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | <2 | <-50.5 | n/a | n/a | n/a | n/a | n/a | 7.5 | 30 |
Vertex | Assay Description A coupled spectrophotometric assay was used in which ADP generated by kinase was converted to ATP with the concomitant production of pyruvate from PE... | J Med Chem 52: 6362-8 (2009) Article DOI: 10.1021/jm900630q BindingDB Entry DOI: 10.7270/Q2D798SS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM35653 (pyrimidylpyrrole, 11a) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | <2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex | Assay Description A coupled spectrophotometric assay was used in which ADP generated by kinase was converted to ATP with the concomitant production of pyruvate from PE... | J Med Chem 52: 6362-8 (2009) Article DOI: 10.1021/jm900630q BindingDB Entry DOI: 10.7270/Q2D798SS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM35647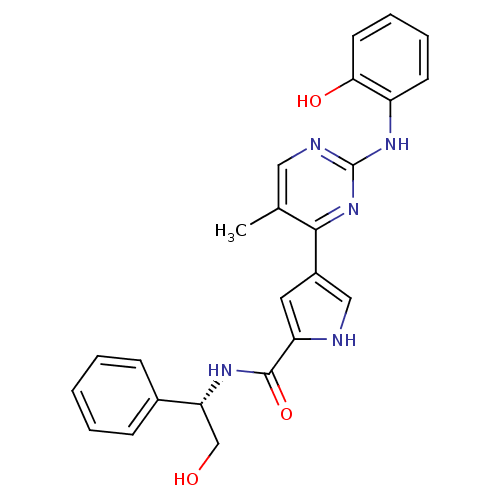 (erk000636 | pyrimidylpyrrole, 9f) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | <2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex | Assay Description A coupled spectrophotometric assay was used in which ADP generated by kinase was converted to ATP with the concomitant production of pyruvate from PE... | J Med Chem 52: 6362-8 (2009) Article DOI: 10.1021/jm900630q BindingDB Entry DOI: 10.7270/Q2D798SS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 1 (Homo sapiens (Human)) | BDBM35663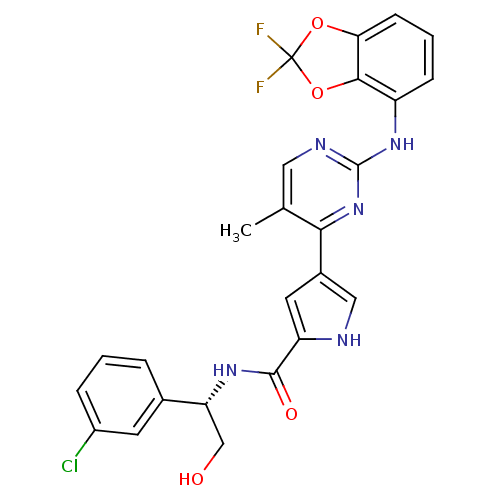 (pyrimidylpyrrole, 11k) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | <2 | <-50.5 | n/a | n/a | n/a | n/a | n/a | 7.5 | 30 |
Vertex | Assay Description A coupled spectrophotometric assay was used in which ADP generated by kinase was converted to ATP with the concomitant production of pyruvate from PE... | J Med Chem 52: 6362-8 (2009) Article DOI: 10.1021/jm900630q BindingDB Entry DOI: 10.7270/Q2D798SS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 1 (Homo sapiens (Human)) | BDBM35662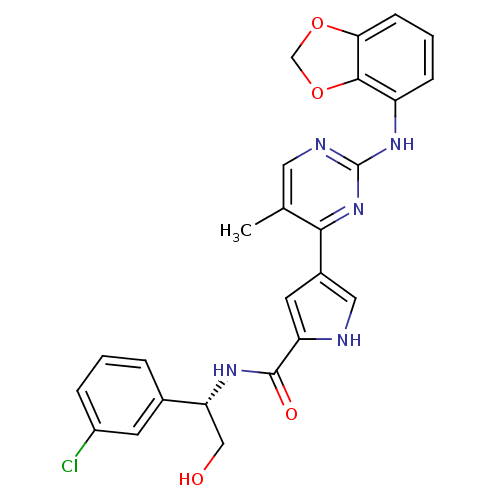 (pyrimidylpyrrole, 11j) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | <2 | <-50.5 | n/a | n/a | n/a | n/a | n/a | 7.5 | 30 |
Vertex | Assay Description A coupled spectrophotometric assay was used in which ADP generated by kinase was converted to ATP with the concomitant production of pyruvate from PE... | J Med Chem 52: 6362-8 (2009) Article DOI: 10.1021/jm900630q BindingDB Entry DOI: 10.7270/Q2D798SS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 1 (Homo sapiens (Human)) | BDBM15645 (N-((S)-1-(3-Chloro-4-fluorophenyl)-2-hydroxyethyl)...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex | Assay Description A coupled spectrophotometric assay was used in which ADP generated by ERK2 was converted to ATP by pyruvate kinase with the production of pyruvate fr... | J Med Chem 50: 1280-7 (2007) Article DOI: 10.1021/jm061381f BindingDB Entry DOI: 10.7270/Q2BR8QFV | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Mitogen-activated protein kinase 1 (Homo sapiens (Human)) | BDBM35641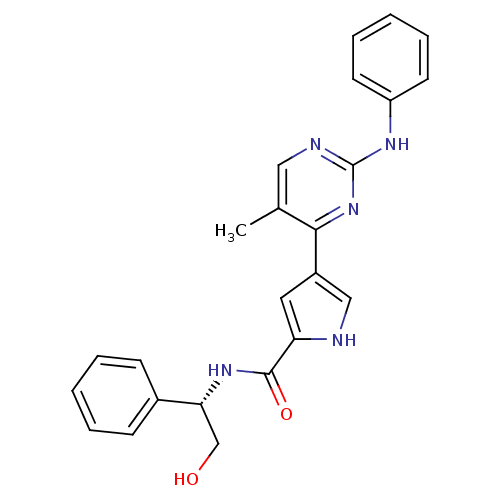 (erk000040 | pyrimidylpyrrole, 2) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | <2 | <-50.5 | n/a | n/a | n/a | n/a | n/a | 7.5 | 30 |
Vertex | Assay Description A coupled spectrophotometric assay was used in which ADP generated by kinase was converted to ATP with the concomitant production of pyruvate from PE... | J Med Chem 52: 6362-8 (2009) Article DOI: 10.1021/jm900630q BindingDB Entry DOI: 10.7270/Q2D798SS | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Mitogen-activated protein kinase 1 (Homo sapiens (Human)) | BDBM35642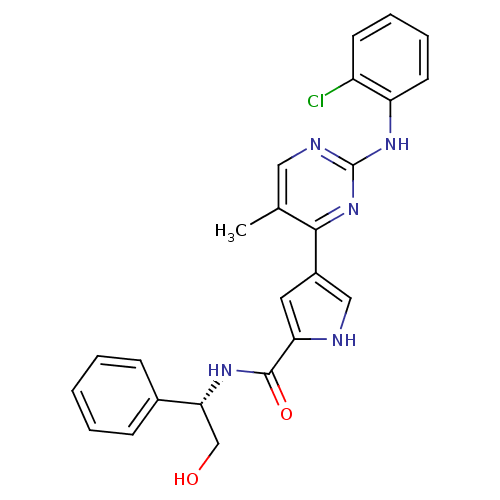 (pyrimidylpyrrole, 9a) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | <2 | <-50.5 | n/a | n/a | n/a | n/a | n/a | 7.5 | 30 |
Vertex | Assay Description A coupled spectrophotometric assay was used in which ADP generated by kinase was converted to ATP with the concomitant production of pyruvate from PE... | J Med Chem 52: 6362-8 (2009) Article DOI: 10.1021/jm900630q BindingDB Entry DOI: 10.7270/Q2D798SS | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Mitogen-activated protein kinase 1 (Homo sapiens (Human)) | BDBM35643 (pyrimidylpyrrole, 9b) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | <2 | <-50.5 | n/a | n/a | n/a | n/a | n/a | 7.5 | 30 |
Vertex | Assay Description A coupled spectrophotometric assay was used in which ADP generated by kinase was converted to ATP with the concomitant production of pyruvate from PE... | J Med Chem 52: 6362-8 (2009) Article DOI: 10.1021/jm900630q BindingDB Entry DOI: 10.7270/Q2D798SS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 1 (Homo sapiens (Human)) | BDBM35645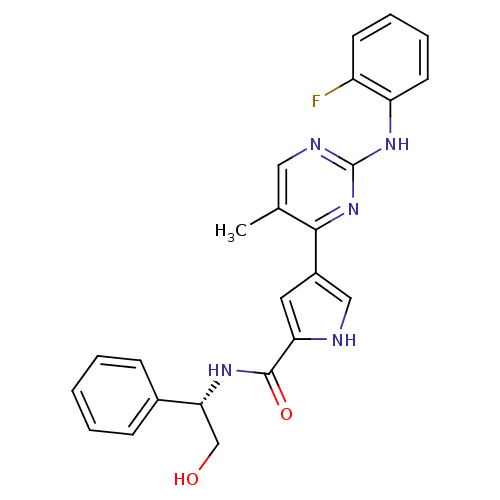 (pyrimidylpyrrole, 9d) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | <2 | <-50.5 | n/a | n/a | n/a | n/a | n/a | 7.5 | 30 |
Vertex | Assay Description A coupled spectrophotometric assay was used in which ADP generated by kinase was converted to ATP with the concomitant production of pyruvate from PE... | J Med Chem 52: 6362-8 (2009) Article DOI: 10.1021/jm900630q BindingDB Entry DOI: 10.7270/Q2D798SS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 1 (Homo sapiens (Human)) | BDBM35649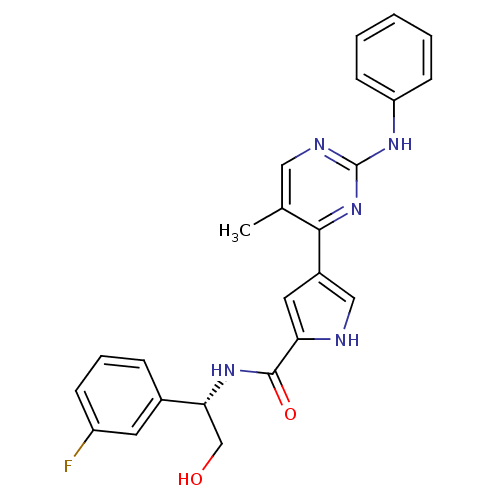 (erk000537 | pyrimidylpyrrole, 10b) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | <2 | <-50.5 | n/a | n/a | n/a | n/a | n/a | 7.5 | 30 |
Vertex | Assay Description A coupled spectrophotometric assay was used in which ADP generated by kinase was converted to ATP with the concomitant production of pyruvate from PE... | J Med Chem 52: 6362-8 (2009) Article DOI: 10.1021/jm900630q BindingDB Entry DOI: 10.7270/Q2D798SS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 1 (Homo sapiens (Human)) | BDBM35651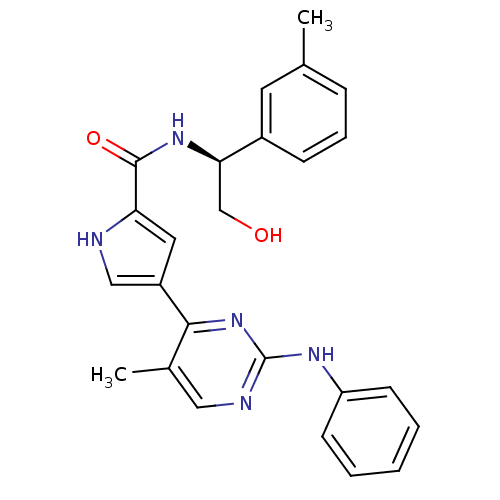 (pyrimidylpyrrole, 10d) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | <2 | <-50.5 | n/a | n/a | n/a | n/a | n/a | 7.5 | 30 |
Vertex | Assay Description A coupled spectrophotometric assay was used in which ADP generated by kinase was converted to ATP with the concomitant production of pyruvate from PE... | J Med Chem 52: 6362-8 (2009) Article DOI: 10.1021/jm900630q BindingDB Entry DOI: 10.7270/Q2D798SS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 1 (Homo sapiens (Human)) | BDBM35653 (pyrimidylpyrrole, 11a) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | <2 | <-50.5 | n/a | n/a | n/a | n/a | n/a | 7.5 | 30 |
Vertex | Assay Description A coupled spectrophotometric assay was used in which ADP generated by kinase was converted to ATP with the concomitant production of pyruvate from PE... | J Med Chem 52: 6362-8 (2009) Article DOI: 10.1021/jm900630q BindingDB Entry DOI: 10.7270/Q2D798SS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 1 (Homo sapiens (Human)) | BDBM35654 (erk000526 | pyrimidylpyrrole, 11b) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | <2 | <-50.5 | n/a | n/a | n/a | n/a | n/a | 7.5 | 30 |
Vertex | Assay Description A coupled spectrophotometric assay was used in which ADP generated by kinase was converted to ATP with the concomitant production of pyruvate from PE... | J Med Chem 52: 6362-8 (2009) Article DOI: 10.1021/jm900630q BindingDB Entry DOI: 10.7270/Q2D798SS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 1 (Homo sapiens (Human)) | BDBM35657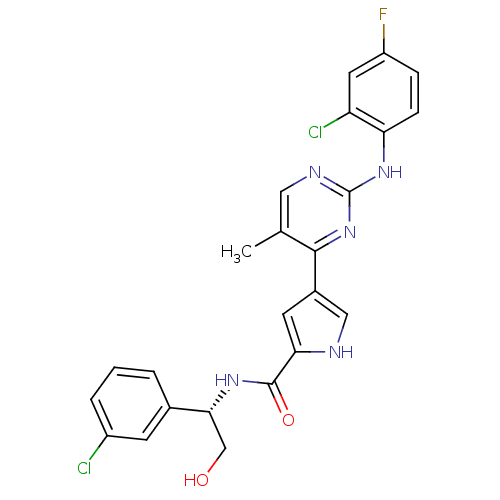 (pyrimidylpyrrole, 11e) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | <2 | <-50.5 | n/a | n/a | n/a | n/a | n/a | 7.5 | 30 |
Vertex | Assay Description A coupled spectrophotometric assay was used in which ADP generated by kinase was converted to ATP with the concomitant production of pyruvate from PE... | J Med Chem 52: 6362-8 (2009) Article DOI: 10.1021/jm900630q BindingDB Entry DOI: 10.7270/Q2D798SS | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Mitogen-activated protein kinase 1 (Homo sapiens (Human)) | BDBM35659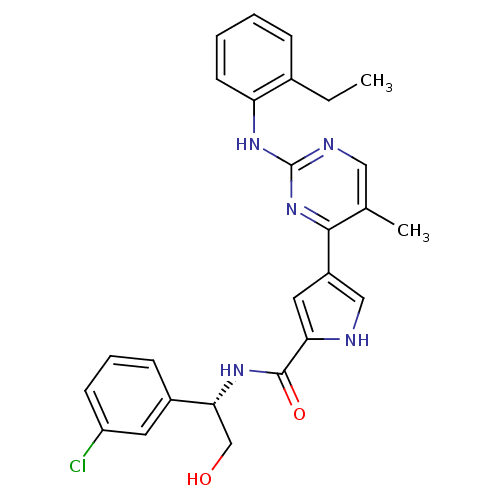 (pyrimidylpyrrole, 11g) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | <2 | <-50.5 | n/a | n/a | n/a | n/a | n/a | 7.5 | 30 |
Vertex | Assay Description A coupled spectrophotometric assay was used in which ADP generated by kinase was converted to ATP with the concomitant production of pyruvate from PE... | J Med Chem 52: 6362-8 (2009) Article DOI: 10.1021/jm900630q BindingDB Entry DOI: 10.7270/Q2D798SS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 1 (Homo sapiens (Human)) | BDBM35660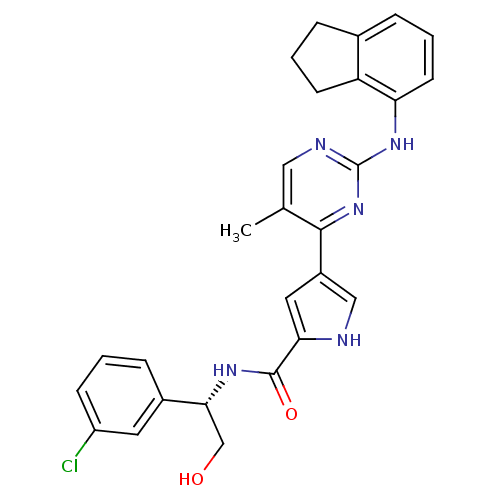 (pyrimidylpyrrole, 11h) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | <2 | <-50.5 | n/a | n/a | n/a | n/a | n/a | 7.5 | 30 |
Vertex | Assay Description A coupled spectrophotometric assay was used in which ADP generated by kinase was converted to ATP with the concomitant production of pyruvate from PE... | J Med Chem 52: 6362-8 (2009) Article DOI: 10.1021/jm900630q BindingDB Entry DOI: 10.7270/Q2D798SS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50006766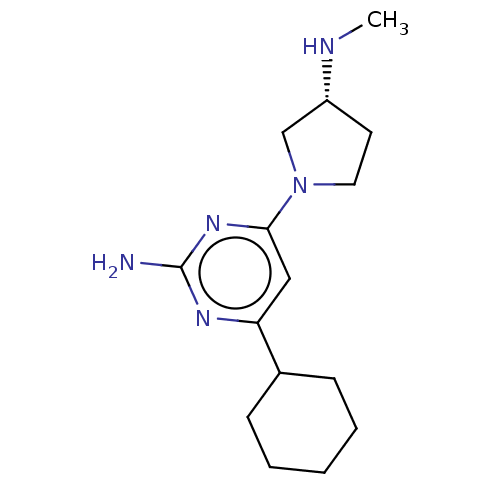 (CHEMBL3236576) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research& Development, LLC Curated by ChEMBL | Assay Description Displacement of [3H]histamine from human recombinant histamine H4 receptor expressed in SK-N-MC cells after 45 mins by competition binding analysis | J Med Chem 57: 2429-39 (2014) Article DOI: 10.1021/jm401727m BindingDB Entry DOI: 10.7270/Q25H7HSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50006765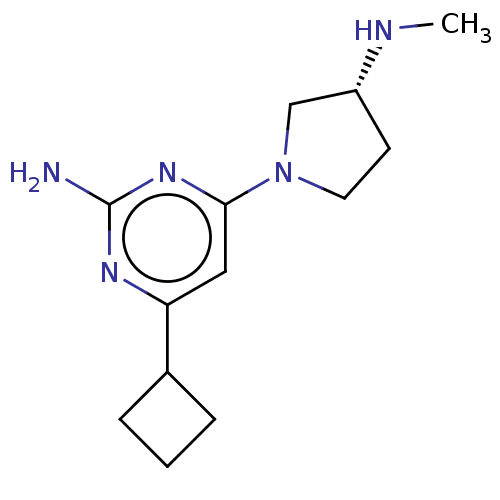 (CHEMBL3236575) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research& Development, LLC Curated by ChEMBL | Assay Description Displacement of [3H]histamine from human recombinant histamine H4 receptor expressed in SK-N-MC cells after 45 mins by competition binding analysis | J Med Chem 57: 2429-39 (2014) Article DOI: 10.1021/jm401727m BindingDB Entry DOI: 10.7270/Q25H7HSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Rattus norvegicus (rat)) | BDBM22566 (5-chloro-2-[(4-methylpiperazin-1-yl)carbonyl]-1H-i...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 2.60 | -49.0 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Johnson & Johnson Pharmaceutical | Assay Description The Ki values were calculated based on an experimentally determined appropriate Kd value according to Cheng and Prusoff. | J Pharmacol Exp Ther 309: 404-13 (2004) Article DOI: 10.1124/jpet.103.061754 BindingDB Entry DOI: 10.7270/Q2VX0DTD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Escherichia coli) | BDBM50026302 (CHEMBL14201 | [5-(2,4-Diamino-pyrimidin-5-ylmethyl...) | MMDB NCI pathway Reactome pathway KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity for E. coli Dihydrofolate reductase | J Med Chem 25: 1120-2 (1983) BindingDB Entry DOI: 10.7270/Q28G8JRF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50006750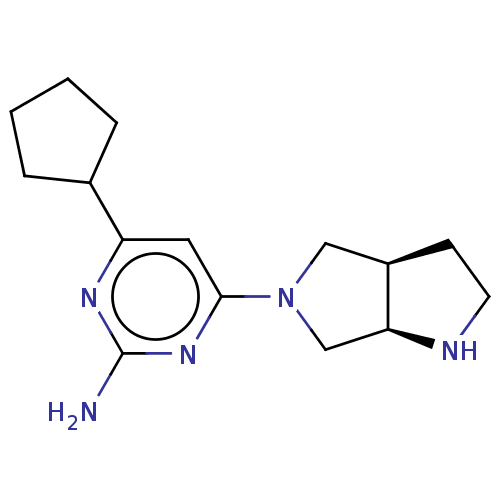 (CHEMBL3236561) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research& Development, LLC Curated by ChEMBL | Assay Description Displacement of [3H]histamine from human recombinant histamine H4 receptor expressed in SK-N-MC cells after 45 mins by competition binding analysis | J Med Chem 57: 2429-39 (2014) Article DOI: 10.1021/jm401727m BindingDB Entry DOI: 10.7270/Q25H7HSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50179351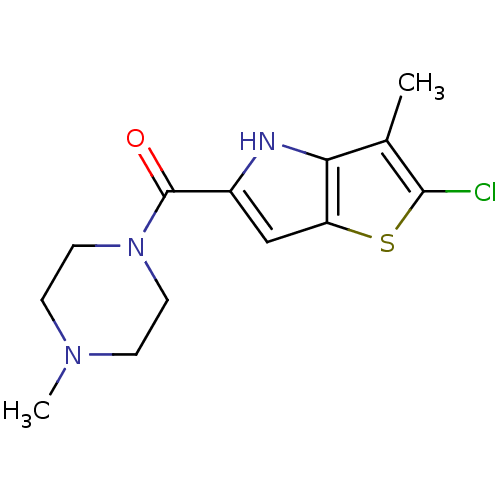 ((2-chloro-3-methyl-4H-thieno[3,2-b]pyrrol-5-yl)(4-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]histamine from recombinant human histamine H4 receptor in SK-N-MC cells | J Med Chem 48: 8289-98 (2005) Article DOI: 10.1021/jm0502081 BindingDB Entry DOI: 10.7270/Q2C24W00 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 1 (Homo sapiens (Human)) | BDBM35644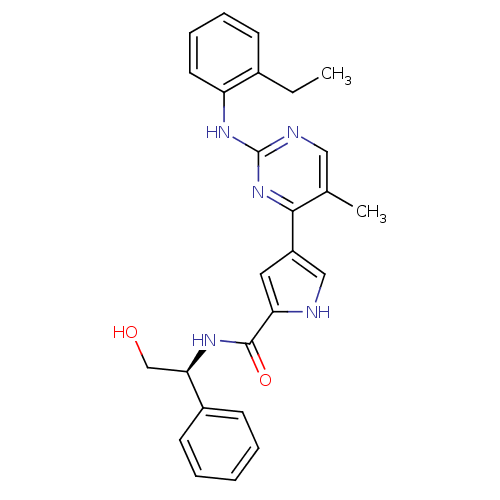 (erk000524 | pyrimidylpyrrole, 9c) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 3 | -49.5 | n/a | n/a | n/a | n/a | n/a | 7.5 | 30 |
Vertex | Assay Description A coupled spectrophotometric assay was used in which ADP generated by kinase was converted to ATP with the concomitant production of pyruvate from PE... | J Med Chem 52: 6362-8 (2009) Article DOI: 10.1021/jm900630q BindingDB Entry DOI: 10.7270/Q2D798SS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 1 (Homo sapiens (Human)) | BDBM35665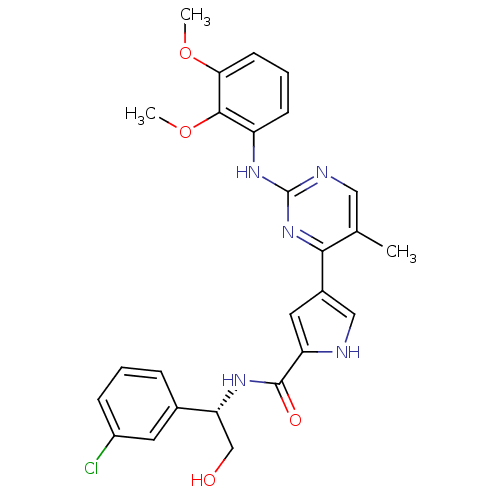 (erk000617 | pyrimidylpyrrole, 11m) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 3 | -49.5 | n/a | n/a | n/a | n/a | n/a | 7.5 | 30 |
Vertex | Assay Description A coupled spectrophotometric assay was used in which ADP generated by kinase was converted to ATP with the concomitant production of pyruvate from PE... | J Med Chem 52: 6362-8 (2009) Article DOI: 10.1021/jm900630q BindingDB Entry DOI: 10.7270/Q2D798SS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50061060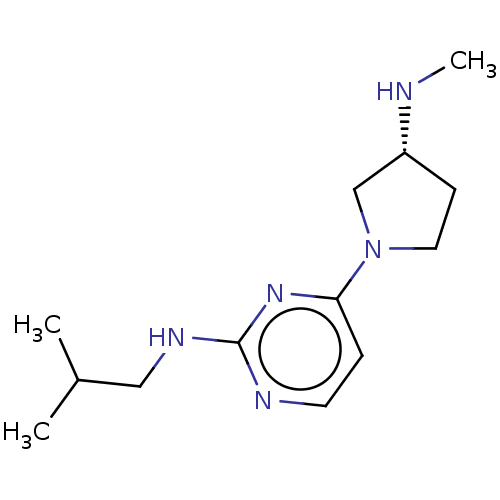 (CHEMBL3393539 | US9732087, 32) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | 3.08 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
JANSSEN PHARMACEUTICA N.V. US Patent | Assay Description Cell pellets from SK-N-MC cells stably or transiently transfected with human H4 receptor (NCBI accession No. AF312230) were used for the binding assa... | US Patent US9732087 (2017) BindingDB Entry DOI: 10.7270/Q2251M9T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM335429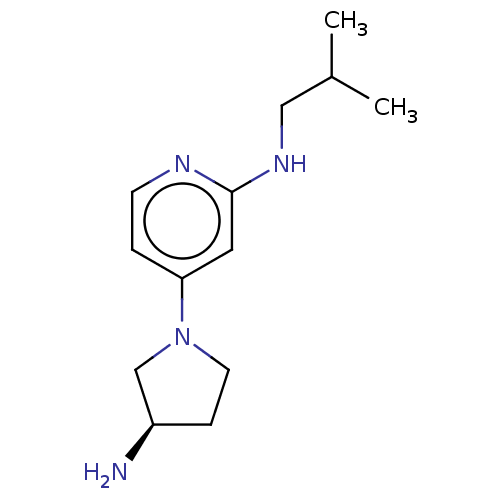 (4-[(3R)-3-Aminopyrrolidin-1-yl]-N-(2-methylpropyl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
JANSSEN PHARMACEUTICA N.V. US Patent | Assay Description Cell pellets from SK-N-MC cells stably or transiently transfected with human H4 receptor (NCBI accession No. AF312230) were used for the binding assa... | US Patent US9732087 (2017) BindingDB Entry DOI: 10.7270/Q2251M9T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 544 total ) | Next | Last >> |