

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50072775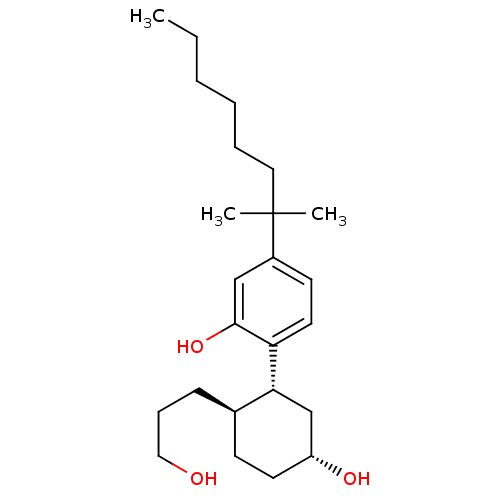 (2-((1R,2R,5R)-5-hydroxy-2-(3-hydroxypropyl)cyclohe...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 0.770 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Displacement of [3H]CP-55940 from human CB1 receptor expressed in HEK293 cells | J Med Chem 50: 3851-6 (2007) Article DOI: 10.1021/jm070317a BindingDB Entry DOI: 10.7270/Q2K64JWR | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50072775 (2-((1R,2R,5R)-5-hydroxy-2-(3-hydroxypropyl)cyclohe...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Displacement of [3H]CP-55940 human CB2 receptor expressed in CHOK1 cells | J Med Chem 50: 3851-6 (2007) Article DOI: 10.1021/jm070317a BindingDB Entry DOI: 10.7270/Q2K64JWR | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50263609 (2-cyano-4-((1-(2-hydroxyethyl)piperidin-4-yl)metho...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of human recombinant cathepsin S by fluorometric assay | Bioorg Med Chem Lett 18: 5280-4 (2008) Article DOI: 10.1016/j.bmcl.2008.08.067 BindingDB Entry DOI: 10.7270/Q2736QQ6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50263557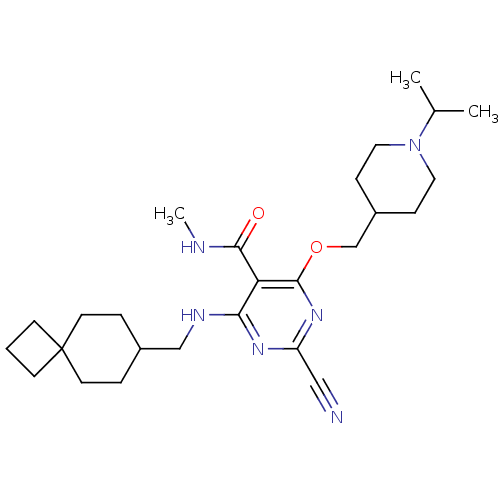 (2-cyano-4-((1-isopropylpiperidin-4-yl)methoxy)-N-m...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of human recombinant cathepsin S by fluorometric assay | Bioorg Med Chem Lett 18: 5280-4 (2008) Article DOI: 10.1016/j.bmcl.2008.08.067 BindingDB Entry DOI: 10.7270/Q2736QQ6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50263555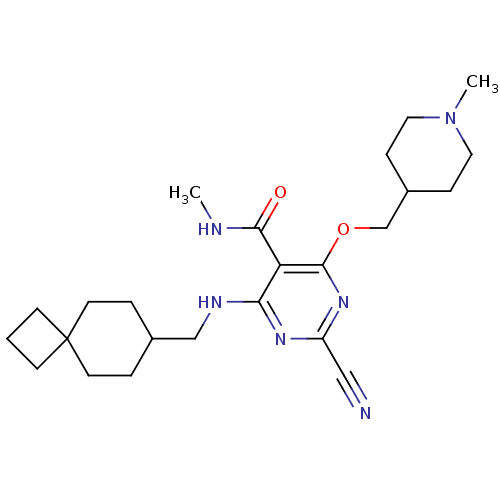 (2-cyano-N-methyl-4-((1-methylpiperidin-4-yl)methox...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of human recombinant cathepsin S by fluorometric assay | Bioorg Med Chem Lett 18: 5280-4 (2008) Article DOI: 10.1016/j.bmcl.2008.08.067 BindingDB Entry DOI: 10.7270/Q2736QQ6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50263554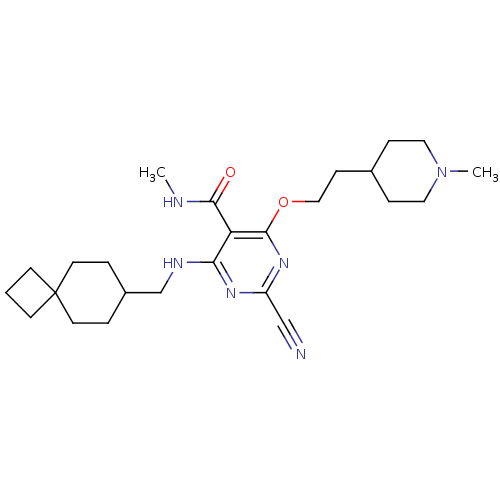 (2-cyano-N-methyl-4-(2-(1-methylpiperidin-4-yl)etho...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of human recombinant cathepsin S by fluorometric assay | Bioorg Med Chem Lett 18: 5280-4 (2008) Article DOI: 10.1016/j.bmcl.2008.08.067 BindingDB Entry DOI: 10.7270/Q2736QQ6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM25138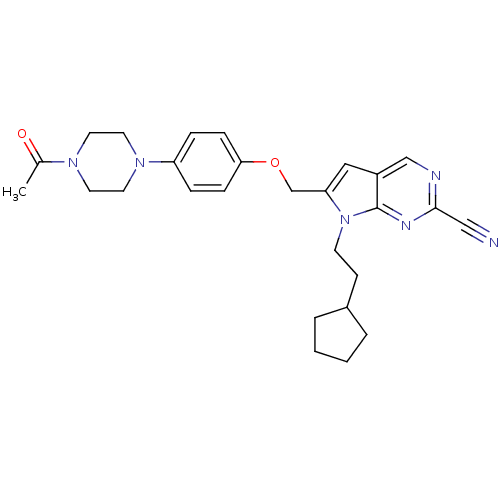 (2-cyano-pyrropyrimidine, 7d | 7-(2-cyclopentylethy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | 7.0 | 22 |
Novartis | Assay Description The substrate hydrolysis with or without inhibitor was monitored at an excitation wavelength of 360nm and an emission wavelength of 460 nm on a fluor... | J Med Chem 51: 5502-5 (2008) Article DOI: 10.1021/jm800839j BindingDB Entry DOI: 10.7270/Q2125QZR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50263611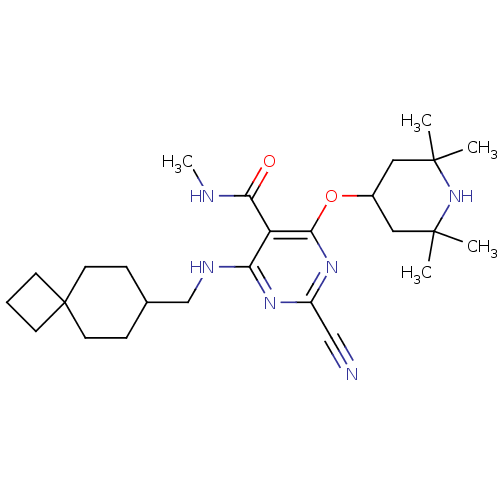 (2-cyano-N-methyl-4-(spiro[3.5]nonan-7-ylmethylamin...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of human recombinant cathepsin S by fluorometric assay | Bioorg Med Chem Lett 18: 5280-4 (2008) Article DOI: 10.1016/j.bmcl.2008.08.067 BindingDB Entry DOI: 10.7270/Q2736QQ6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50263610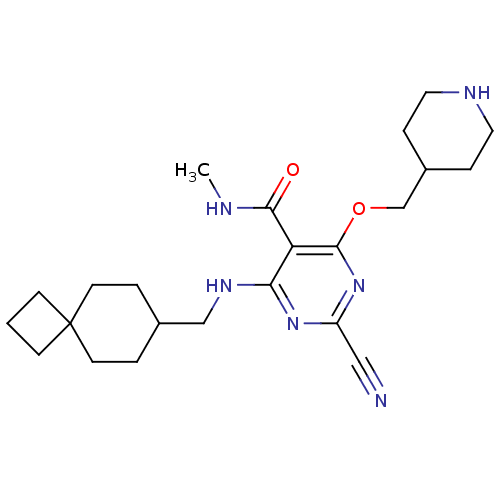 (2-cyano-N-methyl-4-(piperidin-4-ylmethoxy)-6-(spir...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of human recombinant cathepsin S by fluorometric assay | Bioorg Med Chem Lett 18: 5280-4 (2008) Article DOI: 10.1016/j.bmcl.2008.08.067 BindingDB Entry DOI: 10.7270/Q2736QQ6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50263667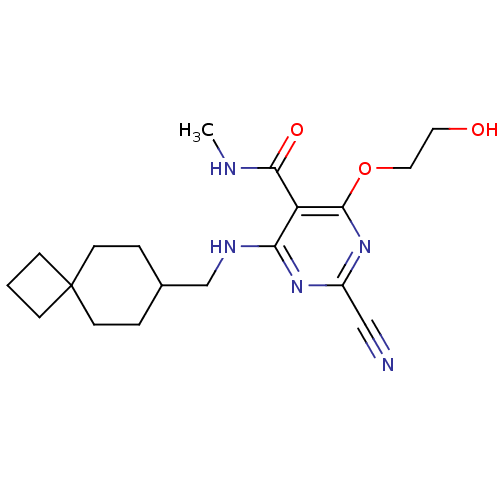 (2-cyano-4-(2-hydroxyethoxy)-N-methyl-6-(spiro[3.5]...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of human recombinant cathepsin S by fluorometric assay | Bioorg Med Chem Lett 18: 5280-4 (2008) Article DOI: 10.1016/j.bmcl.2008.08.067 BindingDB Entry DOI: 10.7270/Q2736QQ6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM21281 ((11R)-2-methyl-11-(morpholin-4-ylmethyl)-3-(naphth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 8.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Displacement of [3H]CP-55940 human CB2 receptor expressed in CHOK1 cells | J Med Chem 50: 3851-6 (2007) Article DOI: 10.1021/jm070317a BindingDB Entry DOI: 10.7270/Q2K64JWR | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM25136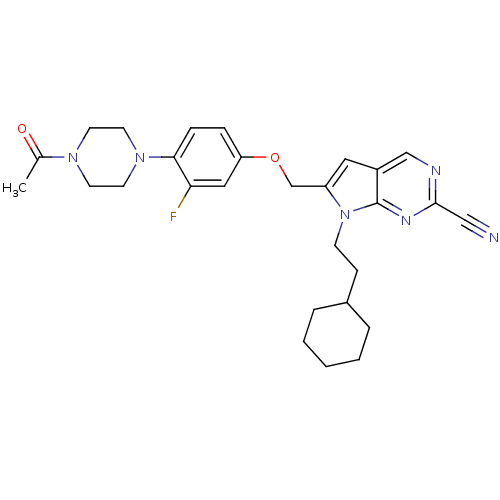 (2-cyano-pyrropyrimidine, 7b | 7-(2-cyclohexylethyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | 7.0 | 22 |
Novartis | Assay Description The substrate hydrolysis with or without inhibitor was monitored at an excitation wavelength of 360nm and an emission wavelength of 460 nm on a fluor... | J Med Chem 51: 5502-5 (2008) Article DOI: 10.1021/jm800839j BindingDB Entry DOI: 10.7270/Q2125QZR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM25134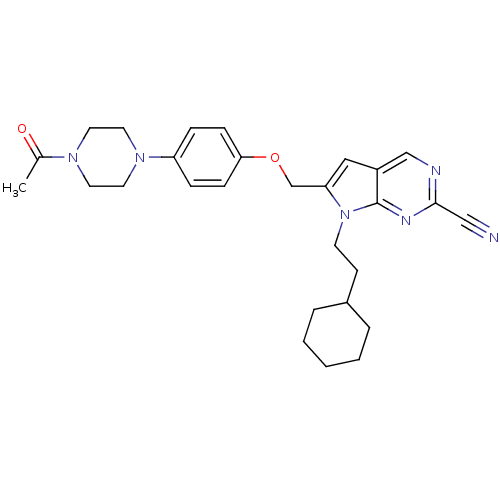 (2-cyano-pyrropyrimidine, 2 | 7-(2-cyclohexylethyl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | 7.0 | 22 |
Novartis | Assay Description The substrate hydrolysis with or without inhibitor was monitored at an excitation wavelength of 360nm and an emission wavelength of 460 nm on a fluor... | J Med Chem 51: 5502-5 (2008) Article DOI: 10.1021/jm800839j BindingDB Entry DOI: 10.7270/Q2125QZR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Mus musculus (Mouse)) | BDBM25134 (2-cyano-pyrropyrimidine, 2 | 7-(2-cyclohexylethyl)...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | 7.0 | 22 |
Novartis | Assay Description The substrate hydrolysis with or without inhibitor was monitored at an excitation wavelength of 360nm and an emission wavelength of 460 nm on a fluor... | J Med Chem 51: 5502-5 (2008) Article DOI: 10.1021/jm800839j BindingDB Entry DOI: 10.7270/Q2125QZR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM25141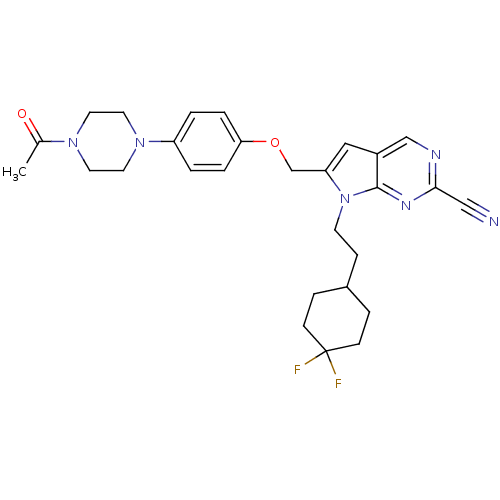 (2-cyano-pyrropyrimidine, 7g | 7-[2-(4,4-difluorocy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | 7.0 | 22 |
Novartis | Assay Description The substrate hydrolysis with or without inhibitor was monitored at an excitation wavelength of 360nm and an emission wavelength of 460 nm on a fluor... | J Med Chem 51: 5502-5 (2008) Article DOI: 10.1021/jm800839j BindingDB Entry DOI: 10.7270/Q2125QZR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50263612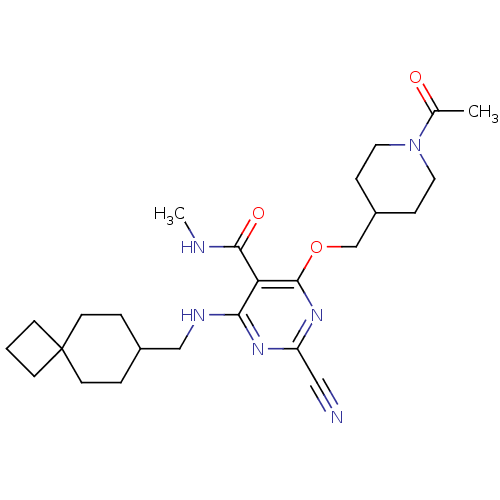 (4-((1-acetylpiperidin-4-yl)methoxy)-2-cyano-N-meth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of human recombinant cathepsin S by fluorometric assay | Bioorg Med Chem Lett 18: 5280-4 (2008) Article DOI: 10.1016/j.bmcl.2008.08.067 BindingDB Entry DOI: 10.7270/Q2736QQ6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM25137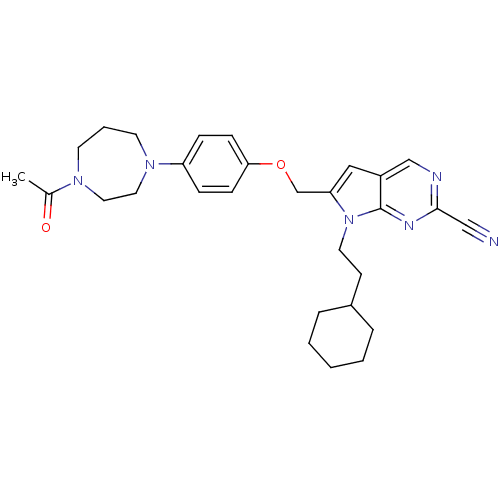 (2-cyano-pyrropyrimidine, 7c | 7-(2-cyclohexylethyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | 7.0 | 22 |
Novartis | Assay Description The substrate hydrolysis with or without inhibitor was monitored at an excitation wavelength of 360nm and an emission wavelength of 460 nm on a fluor... | J Med Chem 51: 5502-5 (2008) Article DOI: 10.1021/jm800839j BindingDB Entry DOI: 10.7270/Q2125QZR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50263511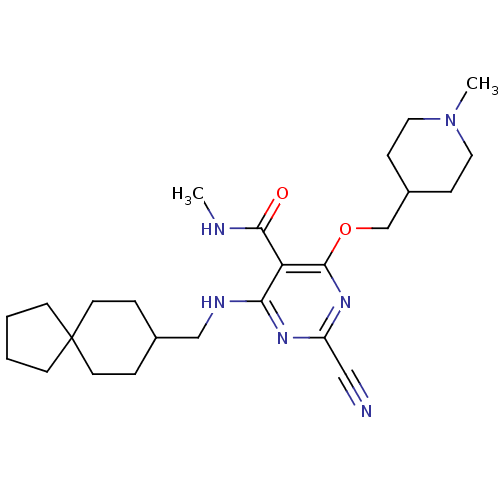 (2-cyano-N-methyl-4-((1-methylpiperidin-4-yl)methox...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of human recombinant cathepsin S by fluorometric assay | Bioorg Med Chem Lett 18: 5280-4 (2008) Article DOI: 10.1016/j.bmcl.2008.08.067 BindingDB Entry DOI: 10.7270/Q2736QQ6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Mus musculus (Mouse)) | BDBM25135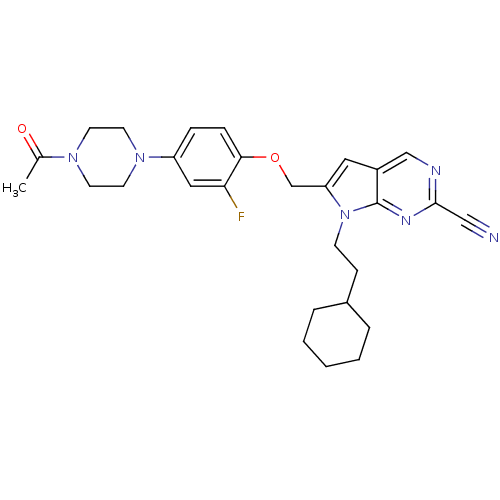 (2-cyano-pyrropyrimidine, 7a | 7-(2-cyclohexylethyl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | 7.0 | 22 |
Novartis | Assay Description The substrate hydrolysis with or without inhibitor was monitored at an excitation wavelength of 360nm and an emission wavelength of 460 nm on a fluor... | J Med Chem 51: 5502-5 (2008) Article DOI: 10.1021/jm800839j BindingDB Entry DOI: 10.7270/Q2125QZR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50218116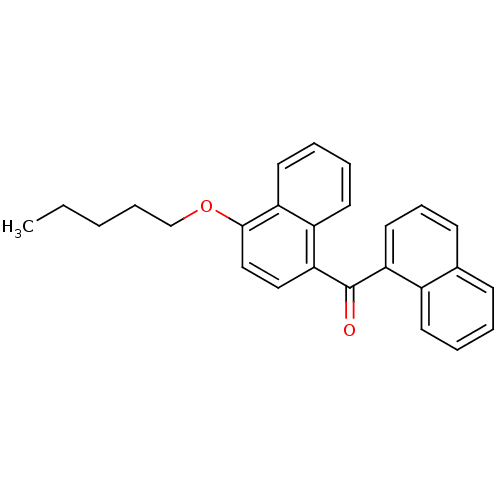 (CHEMBL244403 | naphthalen-1-yl-(4-pentyloxynaphtha...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Displacement of [3H]CP-55940 from human CB1 receptor expressed in HEK293 cells | J Med Chem 50: 3851-6 (2007) Article DOI: 10.1021/jm070317a BindingDB Entry DOI: 10.7270/Q2K64JWR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM25139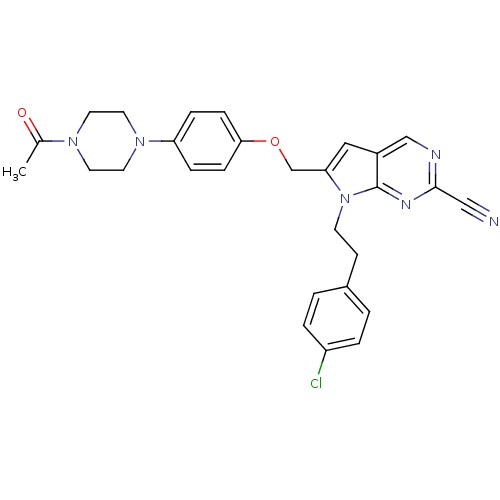 (2-cyano-pyrropyrimidine, 7e | 7-[2-(4-chlorophenyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | 7.0 | 22 |
Novartis | Assay Description The substrate hydrolysis with or without inhibitor was monitored at an excitation wavelength of 360nm and an emission wavelength of 460 nm on a fluor... | J Med Chem 51: 5502-5 (2008) Article DOI: 10.1021/jm800839j BindingDB Entry DOI: 10.7270/Q2125QZR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM25135 (2-cyano-pyrropyrimidine, 7a | 7-(2-cyclohexylethyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | 7.0 | 22 |
Novartis | Assay Description The substrate hydrolysis with or without inhibitor was monitored at an excitation wavelength of 360nm and an emission wavelength of 460 nm on a fluor... | J Med Chem 51: 5502-5 (2008) Article DOI: 10.1021/jm800839j BindingDB Entry DOI: 10.7270/Q2125QZR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Mus musculus (Mouse)) | BDBM25141 (2-cyano-pyrropyrimidine, 7g | 7-[2-(4,4-difluorocy...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | 7.0 | 22 |
Novartis | Assay Description The substrate hydrolysis with or without inhibitor was monitored at an excitation wavelength of 360nm and an emission wavelength of 460 nm on a fluor... | J Med Chem 51: 5502-5 (2008) Article DOI: 10.1021/jm800839j BindingDB Entry DOI: 10.7270/Q2125QZR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50218116 (CHEMBL244403 | naphthalen-1-yl-(4-pentyloxynaphtha...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of rat CB1 receptor | J Med Chem 50: 3851-6 (2007) Article DOI: 10.1021/jm070317a BindingDB Entry DOI: 10.7270/Q2K64JWR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50218121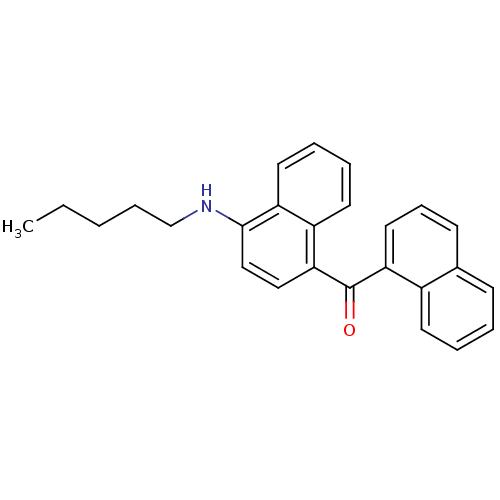 (CHEMBL390675 | naphthalen-1-yl-(4-pentylaminonapht...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Displacement of [3H]CP-55940 from human CB1 receptor expressed in HEK293 cells | J Med Chem 50: 3851-6 (2007) Article DOI: 10.1021/jm070317a BindingDB Entry DOI: 10.7270/Q2K64JWR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50263556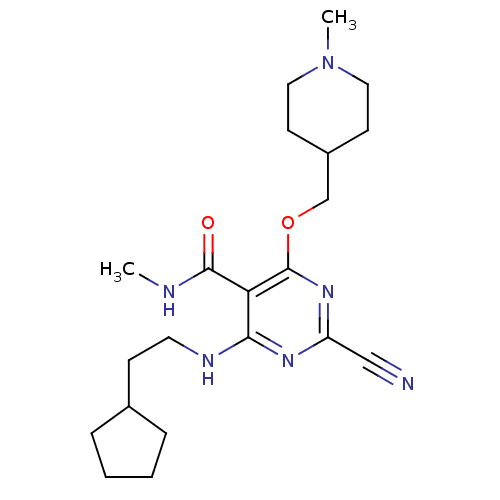 (2-cyano-4-(2-cyclopentylethylamino)-N-methyl-6-((1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of human recombinant cathepsin S by fluorometric assay | Bioorg Med Chem Lett 18: 5280-4 (2008) Article DOI: 10.1016/j.bmcl.2008.08.067 BindingDB Entry DOI: 10.7270/Q2736QQ6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50263510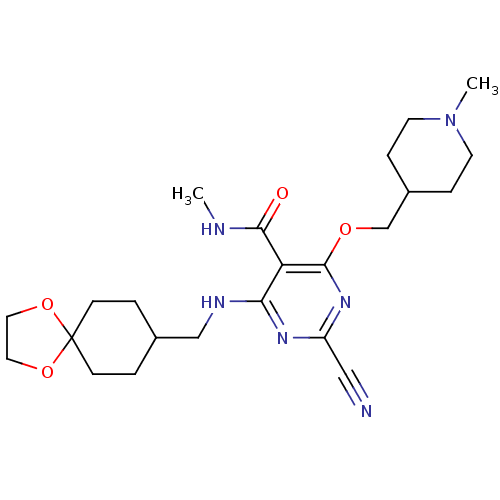 (4-(1,4-dioxaspiro[4.5]decan-8-ylmethylamino)-2-cya...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of human recombinant cathepsin S by fluorometric assay | Bioorg Med Chem Lett 18: 5280-4 (2008) Article DOI: 10.1016/j.bmcl.2008.08.067 BindingDB Entry DOI: 10.7270/Q2736QQ6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50218123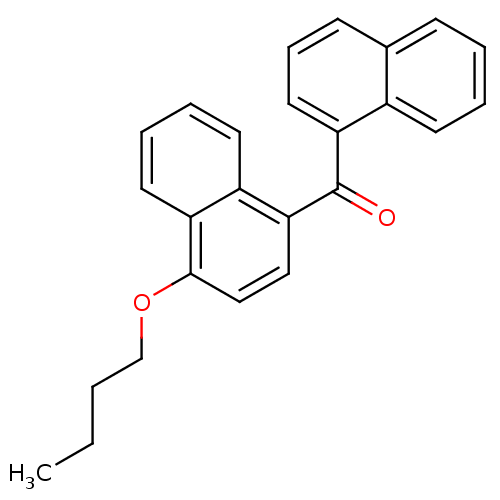 (CHEMBL244402 | naphthalen-1-yl-(4-butoxynaphthalen...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 48 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Displacement of [3H]CP-55940 from human CB1 receptor expressed in HEK293 cells | J Med Chem 50: 3851-6 (2007) Article DOI: 10.1021/jm070317a BindingDB Entry DOI: 10.7270/Q2K64JWR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM25140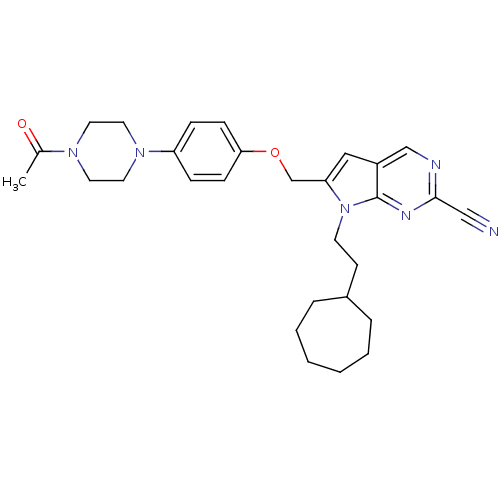 (2-cyano-pyrropyrimidine, 7f | 7-(2-cycloheptylethy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | 7.0 | 22 |
Novartis | Assay Description The substrate hydrolysis with or without inhibitor was monitored at an excitation wavelength of 360nm and an emission wavelength of 460 nm on a fluor... | J Med Chem 51: 5502-5 (2008) Article DOI: 10.1021/jm800839j BindingDB Entry DOI: 10.7270/Q2125QZR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM25138 (2-cyano-pyrropyrimidine, 7d | 7-(2-cyclopentylethy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 79 | n/a | n/a | n/a | n/a | 7.0 | 22 |
Novartis | Assay Description The substrate hydrolysis with or without inhibitor was monitored at an excitation wavelength of 360nm and an emission wavelength of 460 nm on a fluor... | J Med Chem 51: 5502-5 (2008) Article DOI: 10.1021/jm800839j BindingDB Entry DOI: 10.7270/Q2125QZR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50218123 (CHEMBL244402 | naphthalen-1-yl-(4-butoxynaphthalen...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 85 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Displacement of [3H]CP-55940 human CB2 receptor expressed in CHOK1 cells | J Med Chem 50: 3851-6 (2007) Article DOI: 10.1021/jm070317a BindingDB Entry DOI: 10.7270/Q2K64JWR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50218120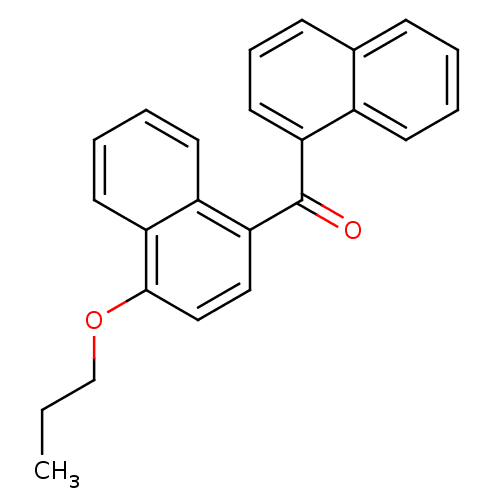 (CHEMBL243334 | naphthalen-1-yl-(4-propoxynaphthale...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Displacement of [3H]CP-55940 human CB2 receptor expressed in CHOK1 cells | J Med Chem 50: 3851-6 (2007) Article DOI: 10.1021/jm070317a BindingDB Entry DOI: 10.7270/Q2K64JWR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50218116 (CHEMBL244403 | naphthalen-1-yl-(4-pentyloxynaphtha...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 98 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Displacement of [3H]CP-55940 human CB2 receptor expressed in CHOK1 cells | J Med Chem 50: 3851-6 (2007) Article DOI: 10.1021/jm070317a BindingDB Entry DOI: 10.7270/Q2K64JWR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50218121 (CHEMBL390675 | naphthalen-1-yl-(4-pentylaminonapht...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Displacement of [3H]CP-55940 human CB2 receptor expressed in CHOK1 cells | J Med Chem 50: 3851-6 (2007) Article DOI: 10.1021/jm070317a BindingDB Entry DOI: 10.7270/Q2K64JWR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM21281 ((11R)-2-methyl-11-(morpholin-4-ylmethyl)-3-(naphth...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Displacement of [3H]CP-55940 from human CB1 receptor expressed in HEK293 cells | J Med Chem 50: 3851-6 (2007) Article DOI: 10.1021/jm070317a BindingDB Entry DOI: 10.7270/Q2K64JWR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50218119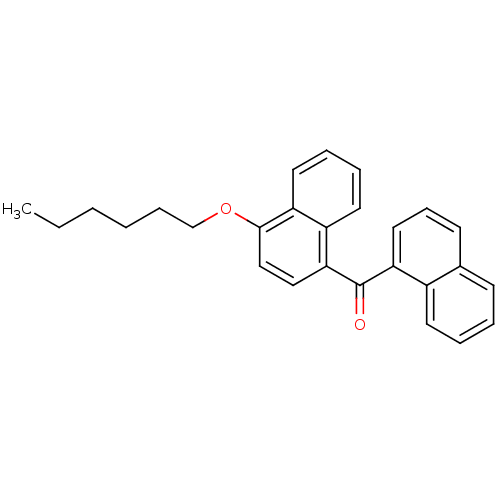 ((4-hexyloxynaphthalen-1-yl)naphthalen-1-ylmethanon...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Displacement of [3H]CP-55940 from human CB1 receptor expressed in HEK293 cells | J Med Chem 50: 3851-6 (2007) Article DOI: 10.1021/jm070317a BindingDB Entry DOI: 10.7270/Q2K64JWR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM50263511 (2-cyano-N-methyl-4-((1-methylpiperidin-4-yl)methox...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 276 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of human recombinant cathepsin L by fluorometric assay | Bioorg Med Chem Lett 18: 5280-4 (2008) Article DOI: 10.1016/j.bmcl.2008.08.067 BindingDB Entry DOI: 10.7270/Q2736QQ6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM25137 (2-cyano-pyrropyrimidine, 7c | 7-(2-cyclohexylethyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 290 | n/a | n/a | n/a | n/a | 7.0 | 22 |
Novartis | Assay Description The substrate hydrolysis with or without inhibitor was monitored at an excitation wavelength of 360nm and an emission wavelength of 460 nm on a fluor... | J Med Chem 51: 5502-5 (2008) Article DOI: 10.1021/jm800839j BindingDB Entry DOI: 10.7270/Q2125QZR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM50263609 (2-cyano-4-((1-(2-hydroxyethyl)piperidin-4-yl)metho...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of human recombinant cathepsin L by fluorometric assay | Bioorg Med Chem Lett 18: 5280-4 (2008) Article DOI: 10.1016/j.bmcl.2008.08.067 BindingDB Entry DOI: 10.7270/Q2736QQ6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50218120 (CHEMBL243334 | naphthalen-1-yl-(4-propoxynaphthale...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Displacement of [3H]CP-55940 from human CB1 receptor expressed in HEK293 cells | J Med Chem 50: 3851-6 (2007) Article DOI: 10.1021/jm070317a BindingDB Entry DOI: 10.7270/Q2K64JWR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM50263557 (2-cyano-4-((1-isopropylpiperidin-4-yl)methoxy)-N-m...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 330 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of human recombinant cathepsin L by fluorometric assay | Bioorg Med Chem Lett 18: 5280-4 (2008) Article DOI: 10.1016/j.bmcl.2008.08.067 BindingDB Entry DOI: 10.7270/Q2736QQ6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50263556 (2-cyano-4-(2-cyclopentylethylamino)-N-methyl-6-((1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 360 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Displacement of [3H]dofetilide from human ERG in HEK293 cells | Bioorg Med Chem Lett 18: 5280-4 (2008) Article DOI: 10.1016/j.bmcl.2008.08.067 BindingDB Entry DOI: 10.7270/Q2736QQ6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM50263555 (2-cyano-N-methyl-4-((1-methylpiperidin-4-yl)methox...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 360 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of human recombinant cathepsin L by fluorometric assay | Bioorg Med Chem Lett 18: 5280-4 (2008) Article DOI: 10.1016/j.bmcl.2008.08.067 BindingDB Entry DOI: 10.7270/Q2736QQ6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM50263556 (2-cyano-4-(2-cyclopentylethylamino)-N-methyl-6-((1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of human recombinant cathepsin L by fluorometric assay | Bioorg Med Chem Lett 18: 5280-4 (2008) Article DOI: 10.1016/j.bmcl.2008.08.067 BindingDB Entry DOI: 10.7270/Q2736QQ6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50263554 (2-cyano-N-methyl-4-(2-(1-methylpiperidin-4-yl)etho...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 420 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Displacement of [3H]dofetilide from human ERG in HEK293 cells | Bioorg Med Chem Lett 18: 5280-4 (2008) Article DOI: 10.1016/j.bmcl.2008.08.067 BindingDB Entry DOI: 10.7270/Q2736QQ6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM50263510 (4-(1,4-dioxaspiro[4.5]decan-8-ylmethylamino)-2-cya...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 426 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of human recombinant cathepsin L by fluorometric assay | Bioorg Med Chem Lett 18: 5280-4 (2008) Article DOI: 10.1016/j.bmcl.2008.08.067 BindingDB Entry DOI: 10.7270/Q2736QQ6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM50263554 (2-cyano-N-methyl-4-(2-(1-methylpiperidin-4-yl)etho...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 440 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of human recombinant cathepsin L by fluorometric assay | Bioorg Med Chem Lett 18: 5280-4 (2008) Article DOI: 10.1016/j.bmcl.2008.08.067 BindingDB Entry DOI: 10.7270/Q2736QQ6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM50263610 (2-cyano-N-methyl-4-(piperidin-4-ylmethoxy)-6-(spir...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 460 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of human recombinant cathepsin L by fluorometric assay | Bioorg Med Chem Lett 18: 5280-4 (2008) Article DOI: 10.1016/j.bmcl.2008.08.067 BindingDB Entry DOI: 10.7270/Q2736QQ6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50263557 (2-cyano-4-((1-isopropylpiperidin-4-yl)methoxy)-N-m...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 540 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Displacement of [3H]dofetilide from human ERG in HEK293 cells | Bioorg Med Chem Lett 18: 5280-4 (2008) Article DOI: 10.1016/j.bmcl.2008.08.067 BindingDB Entry DOI: 10.7270/Q2736QQ6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50218119 ((4-hexyloxynaphthalen-1-yl)naphthalen-1-ylmethanon...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 660 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Displacement of [3H]CP-55940 human CB2 receptor expressed in CHOK1 cells | J Med Chem 50: 3851-6 (2007) Article DOI: 10.1021/jm070317a BindingDB Entry DOI: 10.7270/Q2K64JWR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 100 total ) | Next | Last >> |