

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Non-lysosomal glucosylceramidase (Homo sapiens (Human)) | BDBM50335390 (CHEMBL1651630 | N-Hexoxypentyl-1-deoxynojirimycin) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Napoli Federico II Curated by ChEMBL | Assay Description Inhibition of NLGase in human SH-SY5Y cells using MUG-Gluc as fluorogenic substrate preincubated for 30 mins followed by substrate addition and measu... | Eur J Med Chem 175: 63-71 (2019) Article DOI: 10.1016/j.ejmech.2019.04.061 BindingDB Entry DOI: 10.7270/Q2TB1BBH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Non-lysosomal glucosylceramidase (Homo sapiens (Human)) | BDBM50524479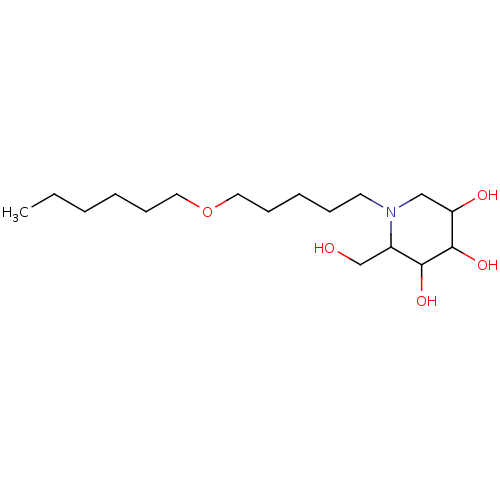 (CHEMBL4459011) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Napoli Federico II Curated by ChEMBL | Assay Description Inhibition of NLGase in human SH-SY5Y cells using MUG-Gluc as fluorogenic substrate preincubated for 30 mins followed by substrate addition and measu... | Eur J Med Chem 175: 63-71 (2019) Article DOI: 10.1016/j.ejmech.2019.04.061 BindingDB Entry DOI: 10.7270/Q2TB1BBH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Non-lysosomal glucosylceramidase (Homo sapiens (Human)) | BDBM50299749 ((2R,3R,4R,5S)-1-[5-(Adamantan-1-ylmethoxy)-pentyl]...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Napoli Federico II Curated by ChEMBL | Assay Description Inhibition of NLGase in human SH-SY5Y cells using MUG-Gluc as fluorogenic substrate preincubated for 30 mins followed by substrate addition and measu... | Eur J Med Chem 175: 63-71 (2019) Article DOI: 10.1016/j.ejmech.2019.04.061 BindingDB Entry DOI: 10.7270/Q2TB1BBH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Non-lysosomal glucosylceramidase (Homo sapiens (Human)) | BDBM18358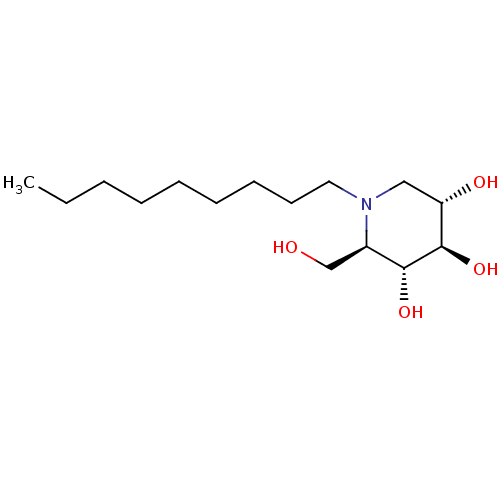 ((2R,3R,4R,5S)-2-(hydroxymethyl)-1-nonylpiperidine-...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Napoli Federico II Curated by ChEMBL | Assay Description Inhibition of NLGase in human SH-SY5Y cells using MUG-Gluc as fluorogenic substrate preincubated for 30 mins followed by substrate addition and measu... | Eur J Med Chem 175: 63-71 (2019) Article DOI: 10.1016/j.ejmech.2019.04.061 BindingDB Entry DOI: 10.7270/Q2TB1BBH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Non-lysosomal glucosylceramidase (Homo sapiens (Human)) | BDBM50524480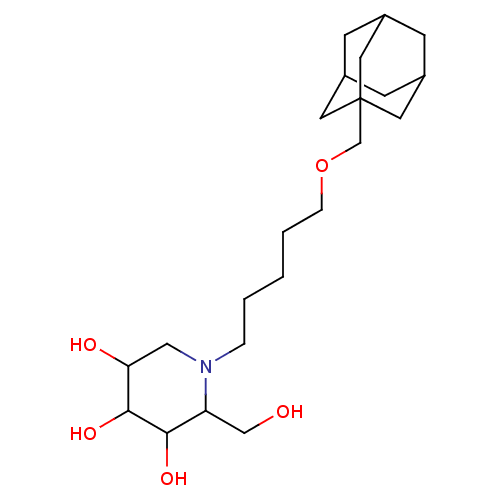 (CHEMBL4559665) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Napoli Federico II Curated by ChEMBL | Assay Description Inhibition of NLGase in human SH-SY5Y cells using MUG-Gluc as fluorogenic substrate preincubated for 30 mins followed by substrate addition and measu... | Eur J Med Chem 175: 63-71 (2019) Article DOI: 10.1016/j.ejmech.2019.04.061 BindingDB Entry DOI: 10.7270/Q2TB1BBH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Non-lysosomal glucosylceramidase (Homo sapiens (Human)) | BDBM18355 ((2R,3R,4R,5S)-1-butyl-2-(hydroxymethyl)piperidine-...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Napoli Federico II Curated by ChEMBL | Assay Description Inhibition of NLGase in human SH-SY5Y cells using MUG-Gluc as fluorogenic substrate preincubated for 30 mins followed by substrate addition and measu... | Eur J Med Chem 175: 63-71 (2019) Article DOI: 10.1016/j.ejmech.2019.04.061 BindingDB Entry DOI: 10.7270/Q2TB1BBH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| GTPase HRas (Homo sapiens (Human)) | BDBM50089962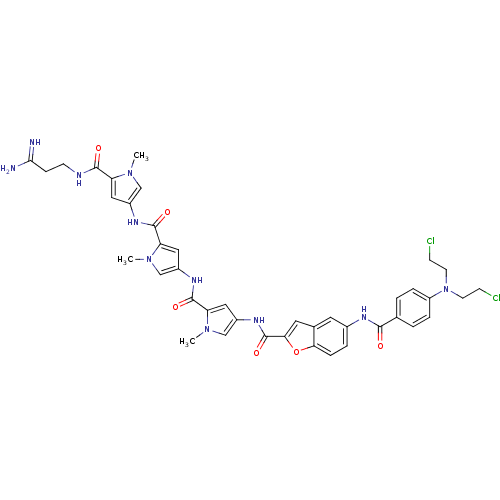 (5-{4-[Bis-(2-chloro-ethyl)-amino]-benzoylamino}-be...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Ferrara Curated by ChEMBL | Assay Description Tested for 50% inhibition of generation of Human Ha-ras polymerase chain reaction(PCR) products | J Med Chem 43: 2675-84 (2000) BindingDB Entry DOI: 10.7270/Q2959J79 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| GTPase HRas (Homo sapiens (Human)) | BDBM50089960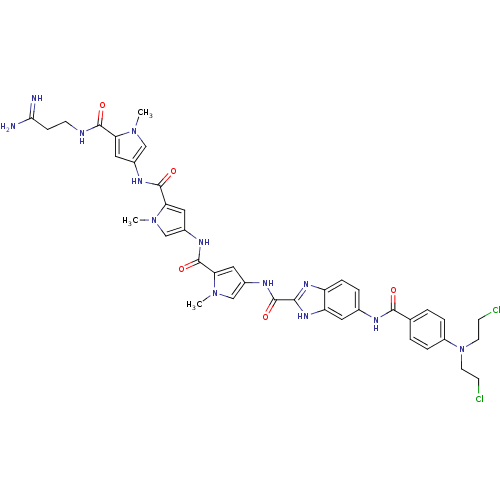 (5-{4-[Bis-(2-chloro-ethyl)-amino]-benzoylamino}-1H...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Ferrara Curated by ChEMBL | Assay Description Tested for 50% inhibition of generation of Human Ha-ras polymerase chain reaction(PCR) products | J Med Chem 43: 2675-84 (2000) BindingDB Entry DOI: 10.7270/Q2959J79 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| GTPase HRas (Homo sapiens (Human)) | BDBM50089961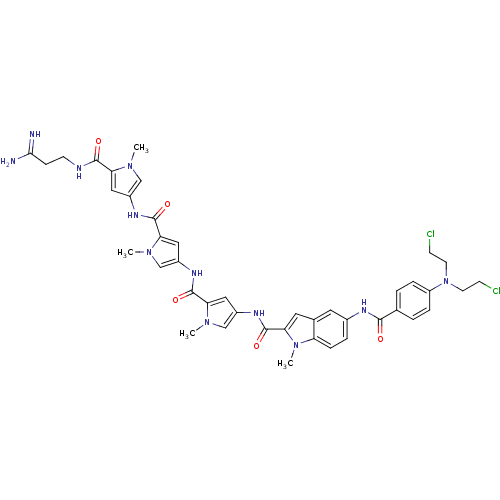 (5-{4-[Bis-(2-chloro-ethyl)-amino]-benzoylamino}-1-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Ferrara Curated by ChEMBL | Assay Description Tested for 50% inhibition of generation of Human Ha-ras polymerase chain reaction(PCR) products | J Med Chem 43: 2675-84 (2000) BindingDB Entry DOI: 10.7270/Q2959J79 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| GTPase HRas (Homo sapiens (Human)) | BDBM50089967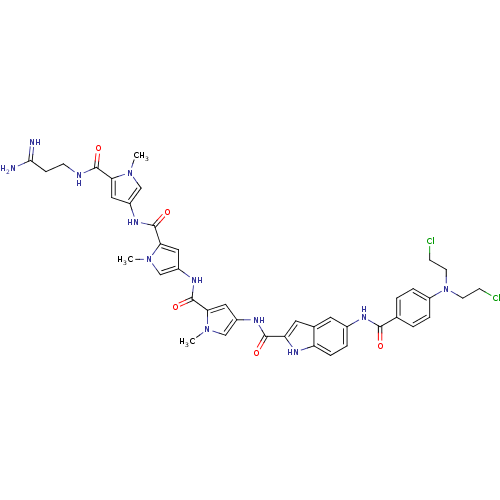 (5-{4-[Bis-(2-chloro-ethyl)-amino]-benzoylamino}-1H...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Ferrara Curated by ChEMBL | Assay Description Tested for 50% inhibition of generation of Human Ha-ras polymerase chain reaction(PCR) products | J Med Chem 43: 2675-84 (2000) BindingDB Entry DOI: 10.7270/Q2959J79 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| GTPase HRas (Homo sapiens (Human)) | BDBM50089969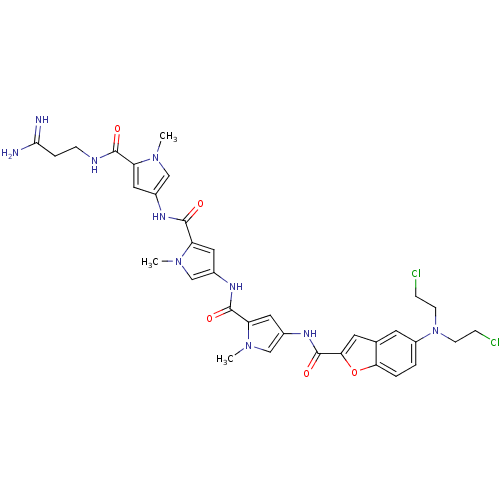 (5-[Bis-(2-chloro-ethyl)-amino]-benzofuran-2-carbox...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Ferrara Curated by ChEMBL | Assay Description Tested for 50% inhibition of generation of Human Ha-ras polymerase chain reaction(PCR) products | J Med Chem 43: 2675-84 (2000) BindingDB Entry DOI: 10.7270/Q2959J79 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| GTPase HRas (Homo sapiens (Human)) | BDBM50089978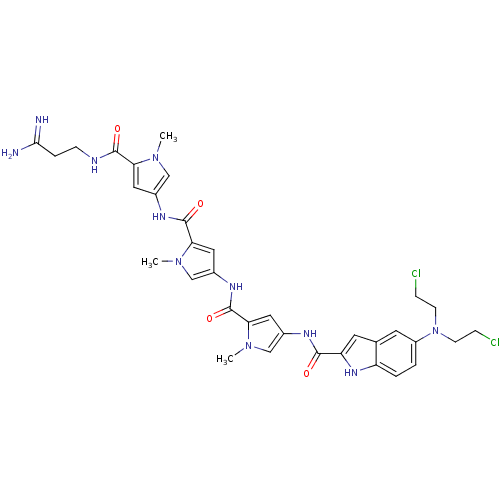 (5-[Bis-(2-chloro-ethyl)-amino]-1H-indole-2-carboxy...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Ferrara Curated by ChEMBL | Assay Description Tested for 50% inhibition of generation of Human Ha-ras polymerase chain reaction(PCR) products | J Med Chem 43: 2675-84 (2000) BindingDB Entry DOI: 10.7270/Q2959J79 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| GTPase HRas (Homo sapiens (Human)) | BDBM50089971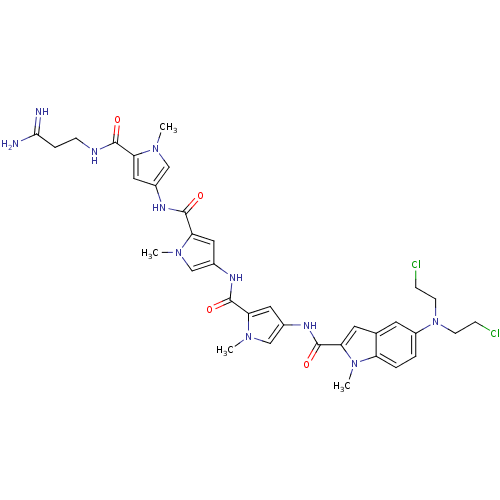 (5-[Bis-(2-chloro-ethyl)-amino]-1-methyl-1H-indole-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Ferrara Curated by ChEMBL | Assay Description Tested for 50% inhibition of generation of Human Ha-ras polymerase chain reaction(PCR) products | J Med Chem 43: 2675-84 (2000) BindingDB Entry DOI: 10.7270/Q2959J79 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Non-lysosomal glucosylceramidase (Homo sapiens (Human)) | BDBM50335387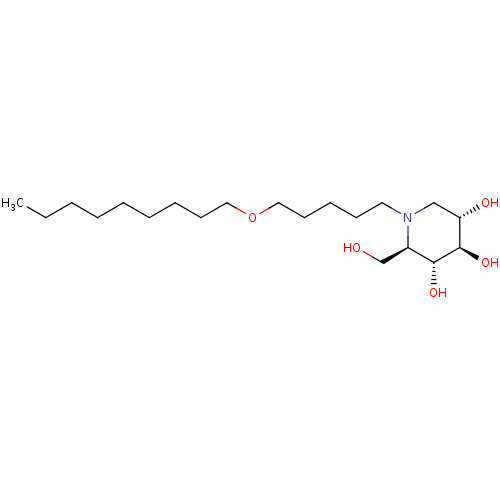 (CHEMBL1651633 | N-Nonoxypentyl-1-deoxynojirimycin) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Napoli Federico II Curated by ChEMBL | Assay Description Inhibition of NLGase in human SH-SY5Y cells using MUG-Gluc as fluorogenic substrate preincubated for 30 mins followed by substrate addition and measu... | Eur J Med Chem 175: 63-71 (2019) Article DOI: 10.1016/j.ejmech.2019.04.061 BindingDB Entry DOI: 10.7270/Q2TB1BBH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Non-lysosomal glucosylceramidase (Homo sapiens (Human)) | BDBM50156357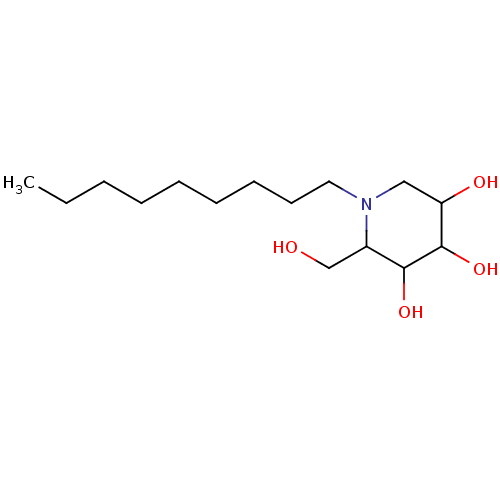 (2-Hydroxymethyl-1-nonyl-piperidine-3,4,5-triol | C...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Napoli Federico II Curated by ChEMBL | Assay Description Inhibition of NLGase in human SH-SY5Y cells using MUG-Gluc as fluorogenic substrate preincubated for 30 mins followed by substrate addition and measu... | Eur J Med Chem 175: 63-71 (2019) Article DOI: 10.1016/j.ejmech.2019.04.061 BindingDB Entry DOI: 10.7270/Q2TB1BBH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Non-lysosomal glucosylceramidase (Homo sapiens (Human)) | BDBM50524486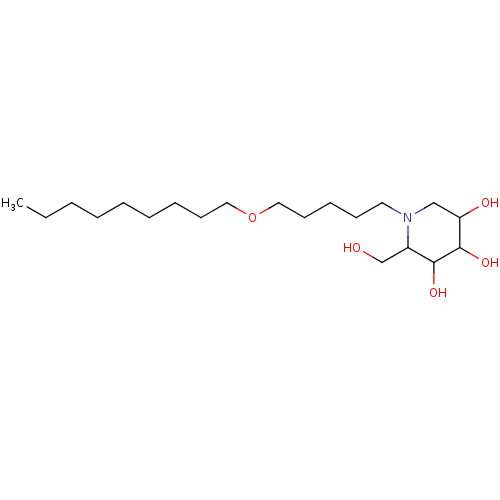 (CHEMBL4577406) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 44 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Napoli Federico II Curated by ChEMBL | Assay Description Inhibition of NLGase in human SH-SY5Y cells using MUG-Gluc as fluorogenic substrate preincubated for 30 mins followed by substrate addition and measu... | Eur J Med Chem 175: 63-71 (2019) Article DOI: 10.1016/j.ejmech.2019.04.061 BindingDB Entry DOI: 10.7270/Q2TB1BBH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Non-lysosomal glucosylceramidase (Homo sapiens (Human)) | BDBM50524485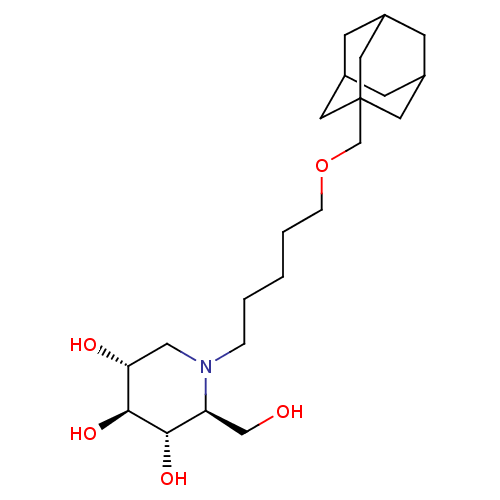 (CHEMBL4435506) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 65 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Napoli Federico II Curated by ChEMBL | Assay Description Inhibition of NLGase in human SH-SY5Y cells using MUG-Gluc as fluorogenic substrate preincubated for 30 mins followed by substrate addition and measu... | Eur J Med Chem 175: 63-71 (2019) Article DOI: 10.1016/j.ejmech.2019.04.061 BindingDB Entry DOI: 10.7270/Q2TB1BBH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Non-lysosomal glucosylceramidase (Homo sapiens (Human)) | BDBM50524481 (CHEMBL4459494) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 137 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Napoli Federico II Curated by ChEMBL | Assay Description Inhibition of NLGase in human SH-SY5Y cells using MUG-Gluc as fluorogenic substrate preincubated for 30 mins followed by substrate addition and measu... | Eur J Med Chem 175: 63-71 (2019) Article DOI: 10.1016/j.ejmech.2019.04.061 BindingDB Entry DOI: 10.7270/Q2TB1BBH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| GTPase HRas (Homo sapiens (Human)) | BDBM50055659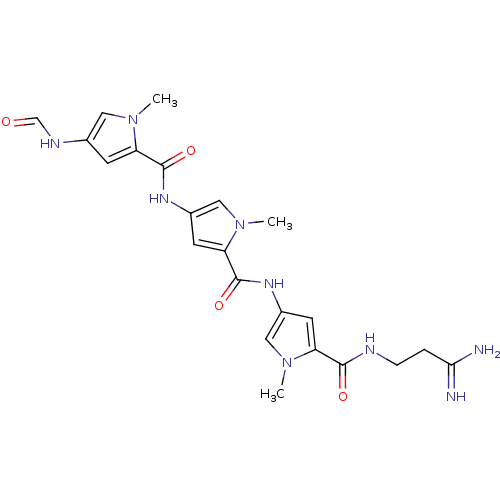 (2N-(3,3-aminoiminopropyl)-4-[4-(4-formamido-1-meth...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Ferrara Curated by ChEMBL | Assay Description Tested for 50% inhibition of generation of Human Ha-ras polymerase chain reaction(PCR) products | J Med Chem 43: 2675-84 (2000) BindingDB Entry DOI: 10.7270/Q2959J79 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Non-lysosomal glucosylceramidase (Homo sapiens (Human)) | BDBM50524484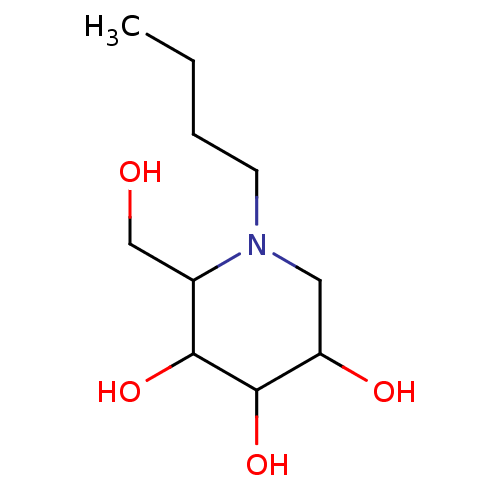 (CHEMBL187262) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 260 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Napoli Federico II Curated by ChEMBL | Assay Description Inhibition of NLGase in human SH-SY5Y cells using MUG-Gluc as fluorogenic substrate preincubated for 30 mins followed by substrate addition and measu... | Eur J Med Chem 175: 63-71 (2019) Article DOI: 10.1016/j.ejmech.2019.04.061 BindingDB Entry DOI: 10.7270/Q2TB1BBH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Similar to alpha-tubulin isoform 1 (Bos taurus) | BDBM50475850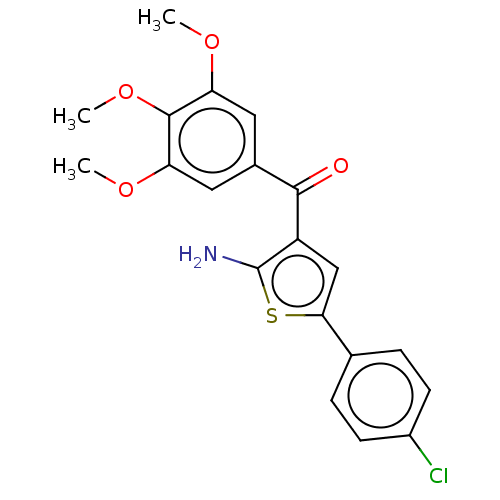 (CHEMBL378632) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 980 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Ferrara Curated by ChEMBL | Assay Description Inhibition of tubulin polymerization in bovine brain by tubulin assembly assay | J Med Chem 49: 3906-15 (2006) Article DOI: 10.1021/jm060355e BindingDB Entry DOI: 10.7270/Q20004VG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Similar to alpha-tubulin isoform 1 (Bos taurus) | BDBM50475848 (CHEMBL380060) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Ferrara Curated by ChEMBL | Assay Description Inhibition of tubulin polymerization in bovine brain by tubulin assembly assay | J Med Chem 49: 3906-15 (2006) Article DOI: 10.1021/jm060355e BindingDB Entry DOI: 10.7270/Q20004VG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Similar to alpha-tubulin isoform 1 (Bos taurus) | BDBM50475851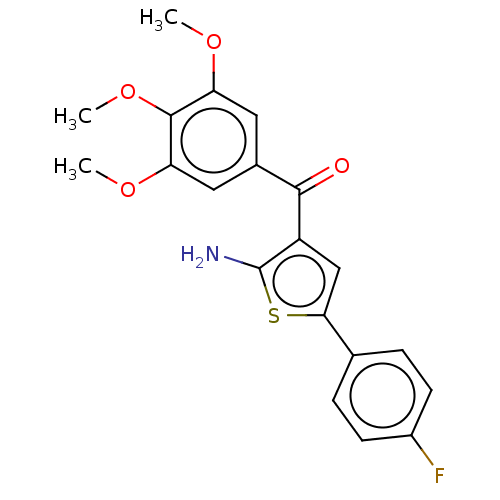 (CHEMBL212515) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Ferrara Curated by ChEMBL | Assay Description Inhibition of tubulin polymerization in bovine brain by tubulin assembly assay | J Med Chem 49: 3906-15 (2006) Article DOI: 10.1021/jm060355e BindingDB Entry DOI: 10.7270/Q20004VG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Similar to alpha-tubulin isoform 1 (Bos taurus) | BDBM50475847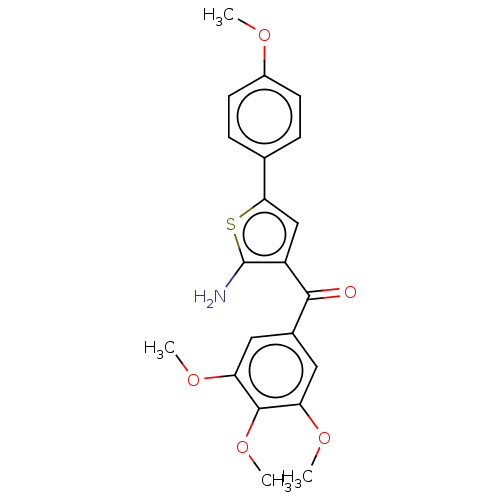 (CHEMBL210239) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Ferrara Curated by ChEMBL | Assay Description Inhibition of tubulin polymerization in bovine brain by tubulin assembly assay | J Med Chem 49: 3906-15 (2006) Article DOI: 10.1021/jm060355e BindingDB Entry DOI: 10.7270/Q20004VG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Similar to alpha-tubulin isoform 1 (Bos taurus) | BDBM50005480 ((-)-combretastatin | (Z)-3'-hydroxy-3,4,4',5-tetra...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Ferrara Curated by ChEMBL | Assay Description Inhibition of tubulin polymerization in bovine brain by tubulin assembly assay | J Med Chem 49: 3906-15 (2006) Article DOI: 10.1021/jm060355e BindingDB Entry DOI: 10.7270/Q20004VG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Similar to alpha-tubulin isoform 1 (Bos taurus) | BDBM50035218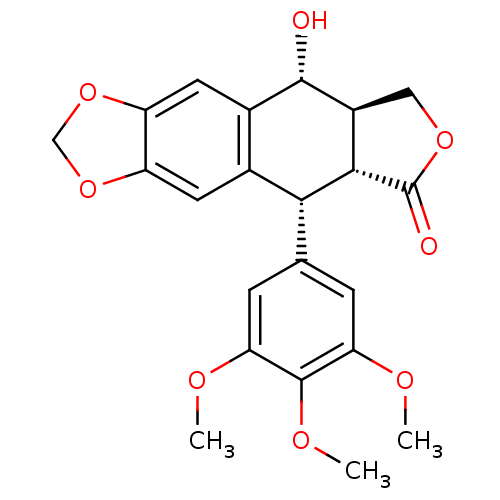 (CHEMBL61 | PODOFILOX | Podophyllinic acid lactone ...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Ferrara Curated by ChEMBL | Assay Description Inhibition of tubulin polymerization in bovine brain by tubulin assembly assay | J Med Chem 49: 3906-15 (2006) Article DOI: 10.1021/jm060355e BindingDB Entry DOI: 10.7270/Q20004VG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Similar to alpha-tubulin isoform 1 (Bos taurus) | BDBM50475852 (CHEMBL209011) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Ferrara Curated by ChEMBL | Assay Description Inhibition of tubulin polymerization in bovine brain by tubulin assembly assay | J Med Chem 49: 3906-15 (2006) Article DOI: 10.1021/jm060355e BindingDB Entry DOI: 10.7270/Q20004VG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Similar to alpha-tubulin isoform 1 (Bos taurus) | BDBM50475853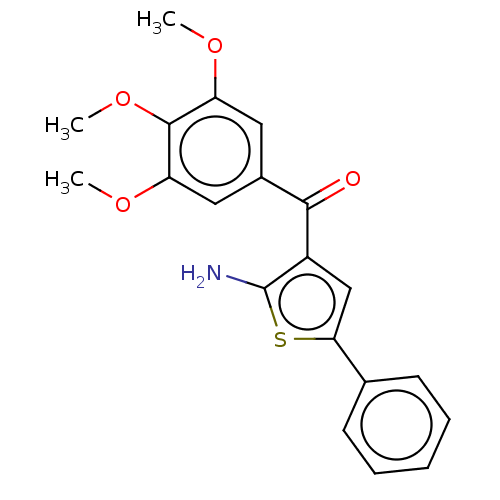 (CHEMBL379867) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Ferrara Curated by ChEMBL | Assay Description Inhibition of tubulin polymerization in bovine brain by tubulin assembly assay | J Med Chem 49: 3906-15 (2006) Article DOI: 10.1021/jm060355e BindingDB Entry DOI: 10.7270/Q20004VG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Similar to alpha-tubulin isoform 1 (Bos taurus) | BDBM50475849 (CHEMBL212734) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Ferrara Curated by ChEMBL | Assay Description Inhibition of tubulin polymerization in bovine brain by tubulin assembly assay | J Med Chem 49: 3906-15 (2006) Article DOI: 10.1021/jm060355e BindingDB Entry DOI: 10.7270/Q20004VG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear factor NF-kappa-B p105 subunit (Homo sapiens (Human)) | BDBM50331545 (2H-furo[2,3-h]chromen-2-one | CHEMBL53569 | Furo[2...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
BioPharmaNet Curated by ChEMBL | Assay Description Inhibition of NF-KB p50 subunit/DNA interaction after 20 mins by EMSA | Bioorg Med Chem 18: 8341-9 (2010) Article DOI: 10.1016/j.bmc.2010.09.063 BindingDB Entry DOI: 10.7270/Q27W6CFD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear factor NF-kappa-B p105 subunit (Homo sapiens (Human)) | BDBM50331544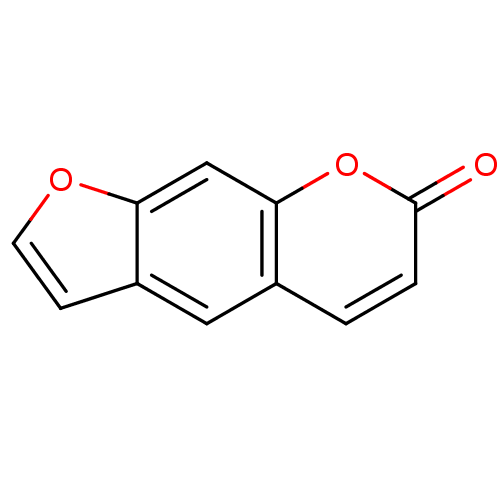 (7H-furo[3,2-g]chromen-7-one | CHEMBL164660 | Furo[...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
BioPharmaNet Curated by ChEMBL | Assay Description Inhibition of NF-KB p50 subunit/DNA interaction after 20 mins by EMSA | Bioorg Med Chem 18: 8341-9 (2010) Article DOI: 10.1016/j.bmc.2010.09.063 BindingDB Entry DOI: 10.7270/Q27W6CFD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Non-lysosomal glucosylceramidase (Homo sapiens (Human)) | BDBM50524482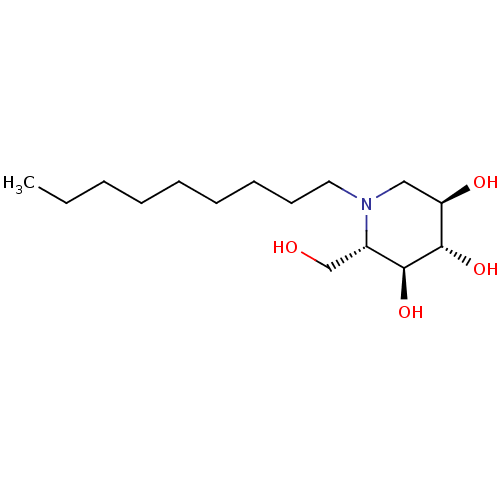 (CHEMBL4460711) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Napoli Federico II Curated by ChEMBL | Assay Description Inhibition of NLGase in human SH-SY5Y cells using MUG-Gluc as fluorogenic substrate preincubated for 30 mins followed by substrate addition and measu... | Eur J Med Chem 175: 63-71 (2019) Article DOI: 10.1016/j.ejmech.2019.04.061 BindingDB Entry DOI: 10.7270/Q2TB1BBH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear factor NF-kappa-B p105 subunit (Homo sapiens (Human)) | BDBM50331548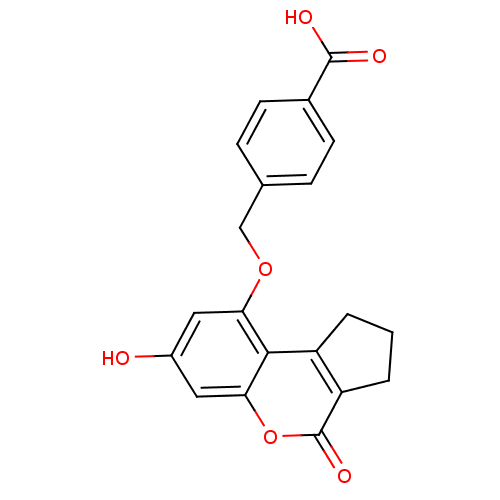 (4-((7-hydroxy-4-oxo-1,2,3,4-tetrahydrocyclopenta[c...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
BioPharmaNet Curated by ChEMBL | Assay Description Inhibition of NF-KB p50 subunit/DNA interaction after 20 mins by EMSA | Bioorg Med Chem 18: 8341-9 (2010) Article DOI: 10.1016/j.bmc.2010.09.063 BindingDB Entry DOI: 10.7270/Q27W6CFD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM50335390 (CHEMBL1651630 | N-Hexoxypentyl-1-deoxynojirimycin) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Napoli Federico II Curated by ChEMBL | Assay Description Inhibition of GCase in human SH-SY5Y cells using MUG-Gluc as fluorogenic substrate preincubated for 30 mins followed by substrate addition and measur... | Eur J Med Chem 175: 63-71 (2019) Article DOI: 10.1016/j.ejmech.2019.04.061 BindingDB Entry DOI: 10.7270/Q2TB1BBH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM18358 ((2R,3R,4R,5S)-2-(hydroxymethyl)-1-nonylpiperidine-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | n/a | n/a | 5.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Napoli Federico II Curated by ChEMBL | Assay Description Inhibition of GCase in human SH-SY5Y cells using MUG-Gluc as fluorogenic substrate preincubated for 30 mins followed by substrate addition and measur... | Eur J Med Chem 175: 63-71 (2019) Article DOI: 10.1016/j.ejmech.2019.04.061 BindingDB Entry DOI: 10.7270/Q2TB1BBH | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Non-lysosomal glucosylceramidase (Homo sapiens (Human)) | BDBM50243651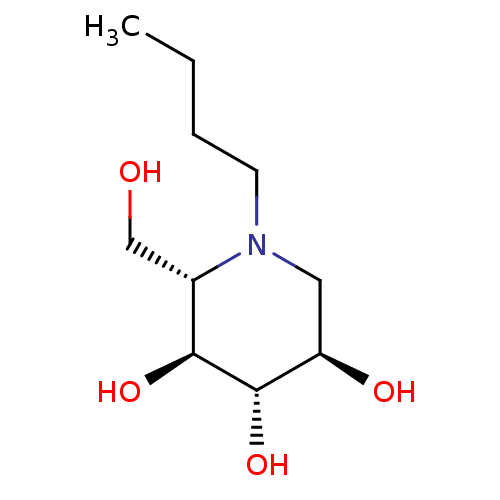 (CHEMBL4061367) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Napoli Federico II Curated by ChEMBL | Assay Description Inhibition of NLGase in human SH-SY5Y cells using MUG-Gluc as fluorogenic substrate preincubated for 30 mins followed by substrate addition and measu... | Eur J Med Chem 175: 63-71 (2019) Article DOI: 10.1016/j.ejmech.2019.04.061 BindingDB Entry DOI: 10.7270/Q2TB1BBH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| GTPase HRas (Homo sapiens (Human)) | BDBM50072339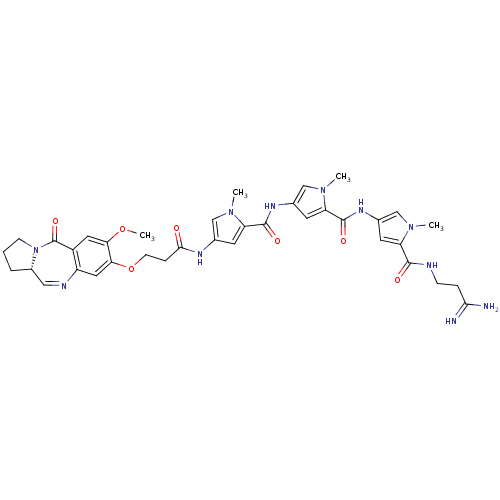 (Benzodiazepine-Distamycin Hybrid | CHEMBL102341) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Ferrara Curated by ChEMBL | Assay Description Inhibition of Ha-ras polymerase-chain reaction product | Bioorg Med Chem Lett 8: 3019-24 (1999) Article DOI: 10.1016/S0960-894X(98)00544-7 BindingDB Entry DOI: 10.7270/Q2057GFM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear factor NF-kappa-B p105 subunit (Homo sapiens (Human)) | BDBM50331546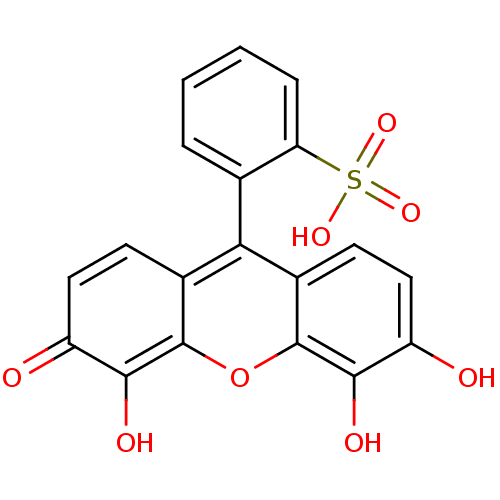 (2-(4,5,6-trihydroxy-3-oxo-3H-xanthen-9-yl)benzenes...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
BioPharmaNet Curated by ChEMBL | Assay Description Inhibition of NF-KB p50 subunit/DNA interaction after 20 mins by EMSA | Bioorg Med Chem 18: 8341-9 (2010) Article DOI: 10.1016/j.bmc.2010.09.063 BindingDB Entry DOI: 10.7270/Q27W6CFD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear factor NF-kappa-B p105 subunit (Homo sapiens (Human)) | BDBM50331542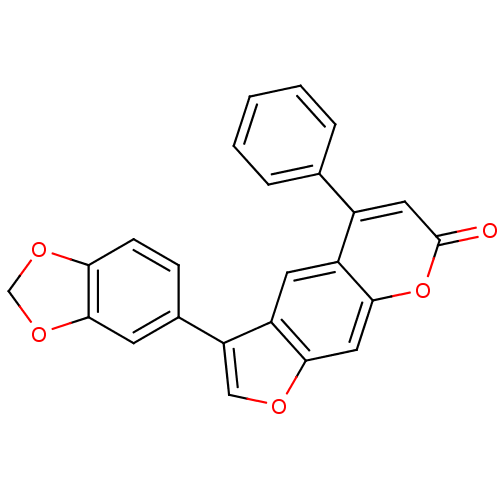 (3-(benzo[d][1,3]dioxol-5-yl)-5-phenyl-7H-furo[3,2-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
BioPharmaNet Curated by ChEMBL | Assay Description Inhibition of NF-KB p50 subunit/DNA interaction after 20 mins by EMSA | Bioorg Med Chem 18: 8341-9 (2010) Article DOI: 10.1016/j.bmc.2010.09.063 BindingDB Entry DOI: 10.7270/Q27W6CFD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear factor NF-kappa-B p105 subunit (Homo sapiens (Human)) | BDBM50331543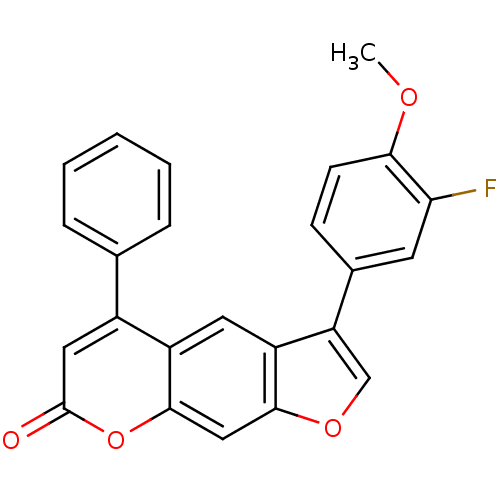 (3-(3-fluoro-4-methoxyphenyl)-5-phenyl-7H-furo[3,2-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
BioPharmaNet Curated by ChEMBL | Assay Description Inhibition of NF-KB p50 subunit/DNA interaction after 20 mins by EMSA | Bioorg Med Chem 18: 8341-9 (2010) Article DOI: 10.1016/j.bmc.2010.09.063 BindingDB Entry DOI: 10.7270/Q27W6CFD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear factor NF-kappa-B p105 subunit (Homo sapiens (Human)) | BDBM50331547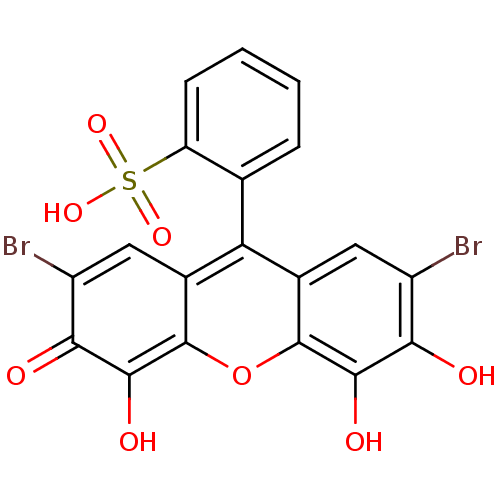 (CHEMBL210287 | bromopyrogallol red) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
BioPharmaNet Curated by ChEMBL | Assay Description Inhibition of NF-KB p50 subunit/DNA interaction after 20 mins by EMSA | Bioorg Med Chem 18: 8341-9 (2010) Article DOI: 10.1016/j.bmc.2010.09.063 BindingDB Entry DOI: 10.7270/Q27W6CFD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Non-lysosomal glucosylceramidase (Homo sapiens (Human)) | BDBM50524483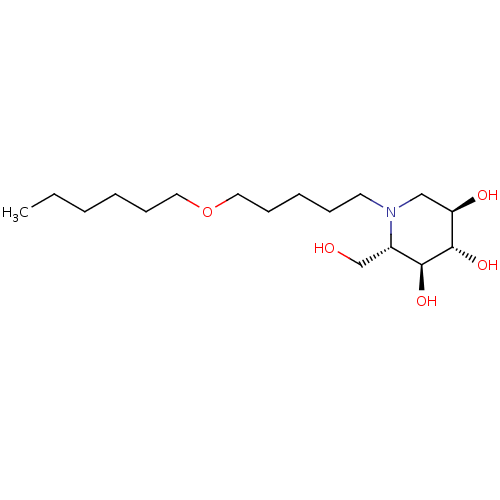 (CHEMBL4435540) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.67E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Napoli Federico II Curated by ChEMBL | Assay Description Inhibition of NLGase in human SH-SY5Y cells using MUG-Gluc as fluorogenic substrate preincubated for 30 mins followed by substrate addition and measu... | Eur J Med Chem 175: 63-71 (2019) Article DOI: 10.1016/j.ejmech.2019.04.061 BindingDB Entry DOI: 10.7270/Q2TB1BBH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear factor NF-kappa-B p105 subunit (Homo sapiens (Human)) | BDBM50331541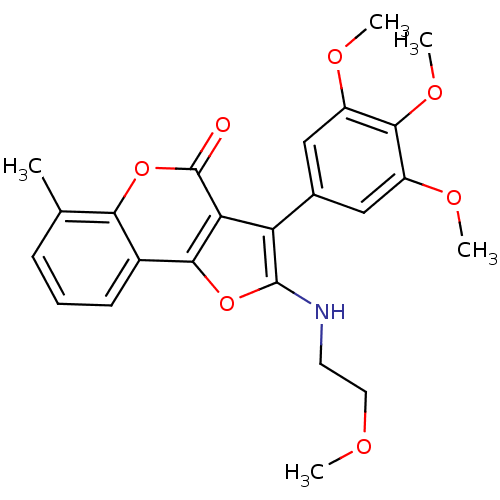 (2-(2-methoxyethylamino)-6-methyl-3-(3,4,5-trimetho...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
BioPharmaNet Curated by ChEMBL | Assay Description Inhibition of NF-KB p50 subunit/DNA interaction after 20 mins by EMSA | Bioorg Med Chem 18: 8341-9 (2010) Article DOI: 10.1016/j.bmc.2010.09.063 BindingDB Entry DOI: 10.7270/Q27W6CFD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear factor NF-kappa-B p105 subunit (Homo sapiens (Human)) | BDBM50146462 ((2E)-3-(2-hydroxyphenyl)-2-propenoic acid | (2E)-3...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
BioPharmaNet Curated by ChEMBL | Assay Description Inhibition of NF-KB p50 subunit/DNA interaction after 20 mins by EMSA | Bioorg Med Chem 18: 8341-9 (2010) Article DOI: 10.1016/j.bmc.2010.09.063 BindingDB Entry DOI: 10.7270/Q27W6CFD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear factor NF-kappa-B p105 subunit (Homo sapiens (Human)) | BDBM50331540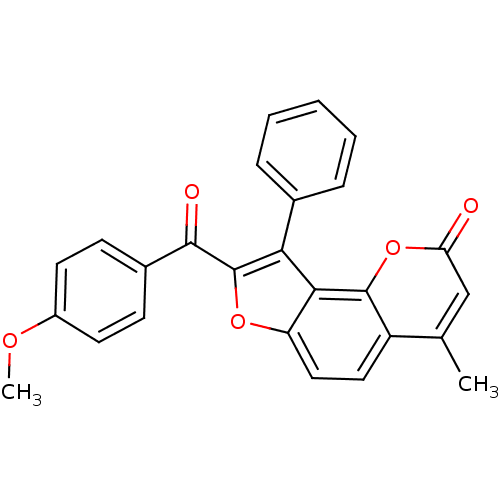 (8-(4-Methoxy-benzoyl)-4-methyl-9-phenyl-furo[2,3-h...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 6.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
BioPharmaNet Curated by ChEMBL | Assay Description Inhibition of NF-KB p50 subunit/DNA interaction after 20 mins by EMSA | Bioorg Med Chem 18: 8341-9 (2010) Article DOI: 10.1016/j.bmc.2010.09.063 BindingDB Entry DOI: 10.7270/Q27W6CFD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM50243651 (CHEMBL4061367) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Napoli Federico II Curated by ChEMBL | Assay Description Inhibition of GCase in human SH-SY5Y cells using MUG-Gluc as fluorogenic substrate preincubated for 30 mins followed by substrate addition and measur... | Eur J Med Chem 175: 63-71 (2019) Article DOI: 10.1016/j.ejmech.2019.04.061 BindingDB Entry DOI: 10.7270/Q2TB1BBH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM18355 ((2R,3R,4R,5S)-1-butyl-2-(hydroxymethyl)piperidine-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | n/a | n/a | 1.18E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Napoli Federico II Curated by ChEMBL | Assay Description Inhibition of GCase in human SH-SY5Y cells using MUG-Gluc as fluorogenic substrate preincubated for 30 mins followed by substrate addition and measur... | Eur J Med Chem 175: 63-71 (2019) Article DOI: 10.1016/j.ejmech.2019.04.061 BindingDB Entry DOI: 10.7270/Q2TB1BBH | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM50524482 (CHEMBL4460711) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.36E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Napoli Federico II Curated by ChEMBL | Assay Description Inhibition of GCase in human SH-SY5Y cells using MUG-Gluc as fluorogenic substrate preincubated for 30 mins followed by substrate addition and measur... | Eur J Med Chem 175: 63-71 (2019) Article DOI: 10.1016/j.ejmech.2019.04.061 BindingDB Entry DOI: 10.7270/Q2TB1BBH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| GTPase HRas (Homo sapiens (Human)) | BDBM50055659 (2N-(3,3-aminoiminopropyl)-4-[4-(4-formamido-1-meth...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Ferrara Curated by ChEMBL | Assay Description Inhibition of Ha-ras polymerase-chain reaction product | Bioorg Med Chem Lett 8: 3019-24 (1999) Article DOI: 10.1016/S0960-894X(98)00544-7 BindingDB Entry DOI: 10.7270/Q2057GFM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear factor NF-kappa-B p105 subunit (Homo sapiens (Human)) | BDBM50022178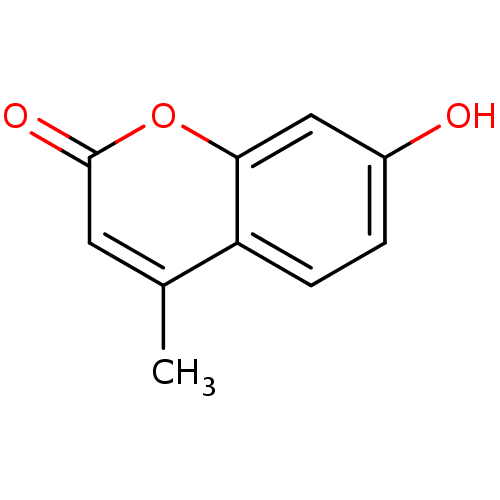 (4-Methyl-7-hydroxycoumarin | 4-methylumbelliferone...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
BioPharmaNet Curated by ChEMBL | Assay Description Inhibition of NF-KB p50 subunit/DNA interaction after 20 mins by EMSA | Bioorg Med Chem 18: 8341-9 (2010) Article DOI: 10.1016/j.bmc.2010.09.063 BindingDB Entry DOI: 10.7270/Q27W6CFD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 111 total ) | Next | Last >> |