 Found 137 hits with Last Name = 'gant' and Initial = 't'
Found 137 hits with Last Name = 'gant' and Initial = 't' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Calcitonin gene-related peptide type 1 receptor
(Homo sapiens (Human)) | BDBM50440788
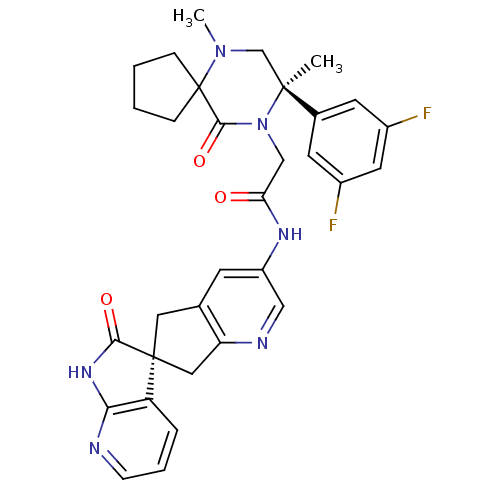
(CHEMBL2431249)Show SMILES CN1C[C@](C)(N(CC(=O)Nc2cnc3C[C@]4(Cc3c2)C(=O)Nc2ncccc42)C(=O)C11CCCC1)c1cc(F)cc(F)c1 |r| Show InChI InChI=1S/C32H32F2N6O3/c1-30(20-11-21(33)13-22(34)12-20)18-39(2)32(7-3-4-8-32)29(43)40(30)17-26(41)37-23-10-19-14-31(15-25(19)36-16-23)24-6-5-9-35-27(24)38-28(31)42/h5-6,9-13,16H,3-4,7-8,14-15,17-18H2,1-2H3,(H,37,41)(H,35,38,42)/t30-,31-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Labortories
Curated by ChEMBL
| Assay Description
Displacement of [125I]-hCGRP from human CGRP receptor expressed in HEK293 cells |
ACS Med Chem Lett 4: 863-8 (2013)
Article DOI: 10.1021/ml400199p
BindingDB Entry DOI: 10.7270/Q25D8T8V |
More data for this
Ligand-Target Pair | |
Calcitonin gene-related peptide type 1 receptor
(Homo sapiens (Human)) | BDBM50440782
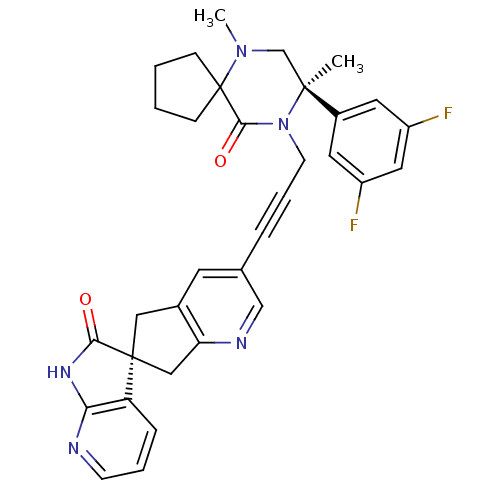
(CHEMBL2431246)Show SMILES CN1C[C@](C)(N(CC#Cc2cnc3C[C@]4(Cc3c2)C(=O)Nc2ncccc42)C(=O)C11CCCC1)c1cc(F)cc(F)c1 |r| Show InChI InChI=1S/C33H31F2N5O2/c1-31(23-14-24(34)16-25(35)15-23)20-39(2)33(9-3-4-10-33)30(42)40(31)12-6-7-21-13-22-17-32(18-27(22)37-19-21)26-8-5-11-36-28(26)38-29(32)41/h5,8,11,13-16,19H,3-4,9-10,12,17-18,20H2,1-2H3,(H,36,38,41)/t31-,32-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Labortories
Curated by ChEMBL
| Assay Description
Displacement of [125I]-hCGRP from human CGRP receptor expressed in HEK293 cells |
ACS Med Chem Lett 4: 863-8 (2013)
Article DOI: 10.1021/ml400199p
BindingDB Entry DOI: 10.7270/Q25D8T8V |
More data for this
Ligand-Target Pair | |
Calcitonin gene-related peptide type 1 receptor
(Homo sapiens (Human)) | BDBM50356282
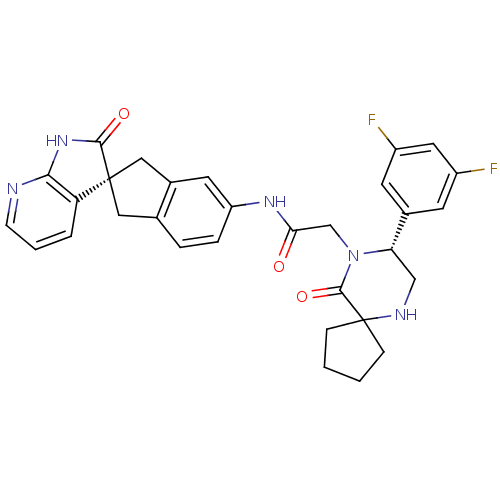
(CHEMBL1910936)Show SMILES Fc1cc(F)cc(c1)[C@@H]1CNC2(CCCC2)C(=O)N1CC(=O)Nc1ccc2C[C@]3(Cc2c1)C(=O)Nc1ncccc31 |r| Show InChI InChI=1S/C31H29F2N5O3/c32-21-10-19(11-22(33)13-21)25-16-35-31(7-1-2-8-31)29(41)38(25)17-26(39)36-23-6-5-18-14-30(15-20(18)12-23)24-4-3-9-34-27(24)37-28(30)40/h3-6,9-13,25,35H,1-2,7-8,14-17H2,(H,36,39)(H,34,37,40)/t25-,30+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Labortories
Curated by ChEMBL
| Assay Description
Displacement of [125I]-hCGRP from human CGRP receptor expressed in HEK293 cells |
ACS Med Chem Lett 4: 863-8 (2013)
Article DOI: 10.1021/ml400199p
BindingDB Entry DOI: 10.7270/Q25D8T8V |
More data for this
Ligand-Target Pair | |
Calcitonin gene-related peptide type 1 receptor
(Homo sapiens (Human)) | BDBM50440784
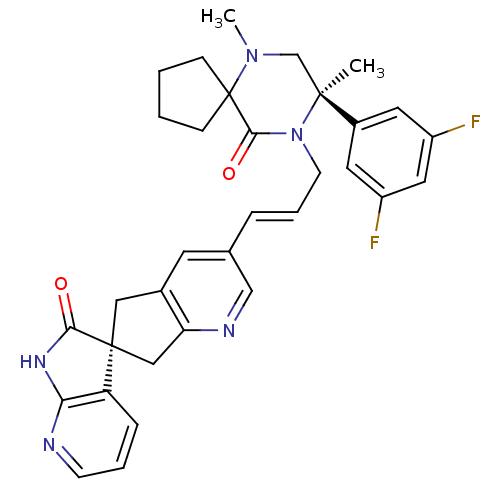
(CHEMBL2431253)Show SMILES CN1C[C@](C)(N(C\C=C\c2cnc3C[C@]4(Cc3c2)C(=O)Nc2ncccc42)C(=O)C11CCCC1)c1cc(F)cc(F)c1 |r| Show InChI InChI=1S/C33H33F2N5O2/c1-31(23-14-24(34)16-25(35)15-23)20-39(2)33(9-3-4-10-33)30(42)40(31)12-6-7-21-13-22-17-32(18-27(22)37-19-21)26-8-5-11-36-28(26)38-29(32)41/h5-8,11,13-16,19H,3-4,9-10,12,17-18,20H2,1-2H3,(H,36,38,41)/b7-6+/t31-,32-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Labortories
Curated by ChEMBL
| Assay Description
Displacement of [125I]-hCGRP from human CGRP receptor expressed in HEK293 cells |
ACS Med Chem Lett 4: 863-8 (2013)
Article DOI: 10.1021/ml400199p
BindingDB Entry DOI: 10.7270/Q25D8T8V |
More data for this
Ligand-Target Pair | |
Calcitonin gene-related peptide type 1 receptor
(Homo sapiens (Human)) | BDBM50440791
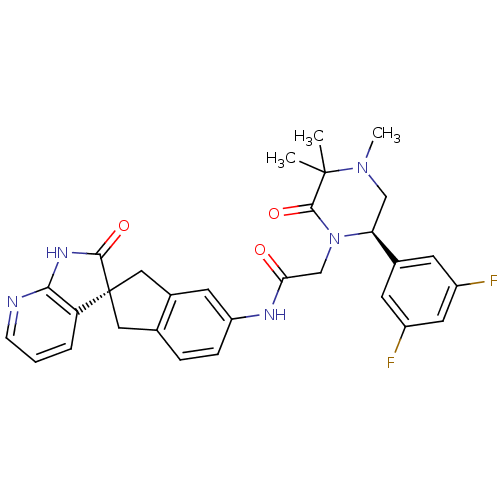
(CHEMBL2431256)Show SMILES CN1C[C@H](N(CC(=O)Nc2ccc3C[C@]4(Cc3c2)C(=O)Nc2ncccc42)C(=O)C1(C)C)c1cc(F)cc(F)c1 |r| Show InChI InChI=1S/C30H29F2N5O3/c1-29(2)28(40)37(24(15-36(29)3)18-9-20(31)12-21(32)10-18)16-25(38)34-22-7-6-17-13-30(14-19(17)11-22)23-5-4-8-33-26(23)35-27(30)39/h4-12,24H,13-16H2,1-3H3,(H,34,38)(H,33,35,39)/t24-,30+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Labortories
Curated by ChEMBL
| Assay Description
Displacement of [125I]-hCGRP from human CGRP receptor expressed in HEK293 cells |
ACS Med Chem Lett 4: 863-8 (2013)
Article DOI: 10.1021/ml400199p
BindingDB Entry DOI: 10.7270/Q25D8T8V |
More data for this
Ligand-Target Pair | |
Calcitonin gene-related peptide type 1 receptor
(Homo sapiens (Human)) | BDBM50440793
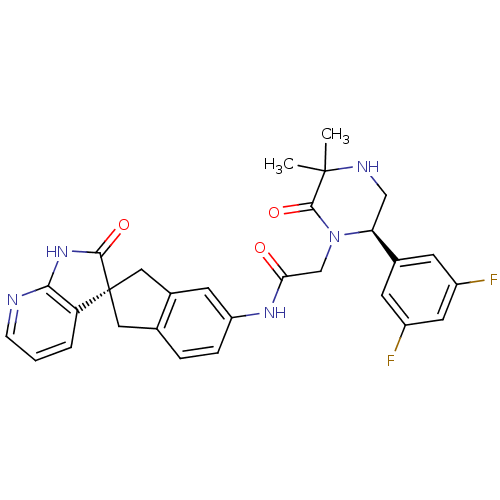
(CHEMBL2431254)Show SMILES CC1(C)NC[C@H](N(CC(=O)Nc2ccc3C[C@]4(Cc3c2)C(=O)Nc2ncccc42)C1=O)c1cc(F)cc(F)c1 |r| Show InChI InChI=1S/C29H27F2N5O3/c1-28(2)27(39)36(23(14-33-28)17-8-19(30)11-20(31)9-17)15-24(37)34-21-6-5-16-12-29(13-18(16)10-21)22-4-3-7-32-25(22)35-26(29)38/h3-11,23,33H,12-15H2,1-2H3,(H,34,37)(H,32,35,38)/t23-,29+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Labortories
Curated by ChEMBL
| Assay Description
Displacement of [125I]-hCGRP from human CGRP receptor expressed in HEK293 cells |
ACS Med Chem Lett 4: 863-8 (2013)
Article DOI: 10.1021/ml400199p
BindingDB Entry DOI: 10.7270/Q25D8T8V |
More data for this
Ligand-Target Pair | |
Calcitonin gene-related peptide type 1 receptor
(Homo sapiens (Human)) | BDBM50385314
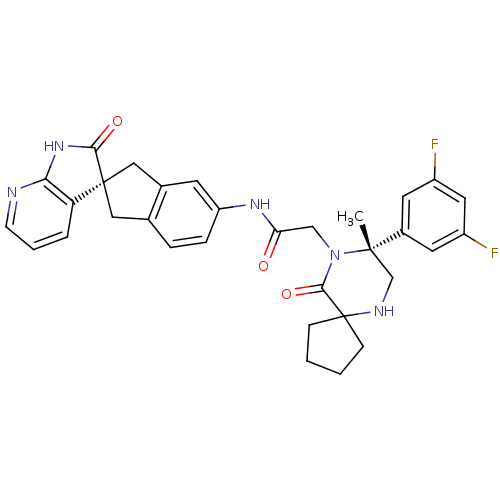
(CHEMBL2035981)Show SMILES C[C@]1(CNC2(CCCC2)C(=O)N1CC(=O)Nc1ccc2C[C@]3(Cc2c1)C(=O)Nc1ncccc31)c1cc(F)cc(F)c1 |r| Show InChI InChI=1S/C32H31F2N5O3/c1-30(21-12-22(33)14-23(34)13-21)18-36-32(8-2-3-9-32)29(42)39(30)17-26(40)37-24-7-6-19-15-31(16-20(19)11-24)25-5-4-10-35-27(25)38-28(31)41/h4-7,10-14,36H,2-3,8-9,15-18H2,1H3,(H,37,40)(H,35,38,41)/t30-,31+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Labortories
Curated by ChEMBL
| Assay Description
Displacement of [125I]-hCGRP from human CGRP receptor expressed in HEK293 cells |
ACS Med Chem Lett 4: 863-8 (2013)
Article DOI: 10.1021/ml400199p
BindingDB Entry DOI: 10.7270/Q25D8T8V |
More data for this
Ligand-Target Pair | |
Calcitonin gene-related peptide type 1 receptor
(Homo sapiens (Human)) | BDBM50440786
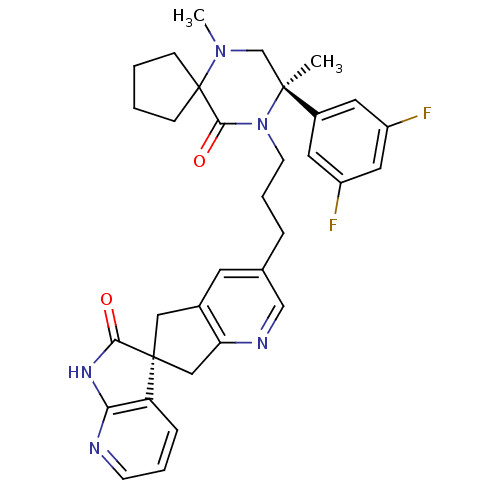
(CHEMBL2431251)Show SMILES CN1C[C@](C)(N(CCCc2cnc3C[C@]4(Cc3c2)C(=O)Nc2ncccc42)C(=O)C11CCCC1)c1cc(F)cc(F)c1 |r| Show InChI InChI=1S/C33H35F2N5O2/c1-31(23-14-24(34)16-25(35)15-23)20-39(2)33(9-3-4-10-33)30(42)40(31)12-6-7-21-13-22-17-32(18-27(22)37-19-21)26-8-5-11-36-28(26)38-29(32)41/h5,8,11,13-16,19H,3-4,6-7,9-10,12,17-18,20H2,1-2H3,(H,36,38,41)/t31-,32-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Labortories
Curated by ChEMBL
| Assay Description
Displacement of [125I]-hCGRP from human CGRP receptor expressed in HEK293 cells |
ACS Med Chem Lett 4: 863-8 (2013)
Article DOI: 10.1021/ml400199p
BindingDB Entry DOI: 10.7270/Q25D8T8V |
More data for this
Ligand-Target Pair | |
Calcitonin gene-related peptide type 1 receptor
(Homo sapiens (Human)) | BDBM50440789

(CHEMBL2431248)Show SMILES CN1C[C@](C)(N(CC(=O)Nc2ccc3C[C@]4(Cc3c2)C(=O)Nc2ncccc42)C(=O)C11CCCC1)c1cc(F)cc(F)c1 |r| Show InChI InChI=1S/C33H33F2N5O3/c1-31(22-13-23(34)15-24(35)14-22)19-39(2)33(9-3-4-10-33)30(43)40(31)18-27(41)37-25-8-7-20-16-32(17-21(20)12-25)26-6-5-11-36-28(26)38-29(32)42/h5-8,11-15H,3-4,9-10,16-19H2,1-2H3,(H,37,41)(H,36,38,42)/t31-,32+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Labortories
Curated by ChEMBL
| Assay Description
Displacement of [125I]-hCGRP from human CGRP receptor expressed in HEK293 cells |
ACS Med Chem Lett 4: 863-8 (2013)
Article DOI: 10.1021/ml400199p
BindingDB Entry DOI: 10.7270/Q25D8T8V |
More data for this
Ligand-Target Pair | |
Calcitonin gene-related peptide type 1 receptor
(Homo sapiens (Human)) | BDBM50440790
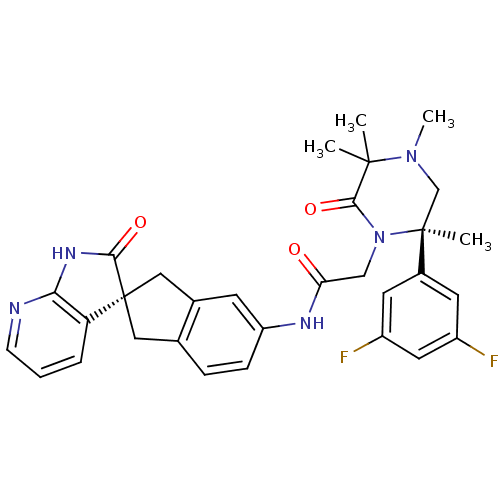
(CHEMBL2431247)Show SMILES CN1C[C@](C)(N(CC(=O)Nc2ccc3C[C@]4(Cc3c2)C(=O)Nc2ncccc42)C(=O)C1(C)C)c1cc(F)cc(F)c1 |r| Show InChI InChI=1S/C31H31F2N5O3/c1-29(2)28(41)38(30(3,17-37(29)4)20-11-21(32)13-22(33)12-20)16-25(39)35-23-8-7-18-14-31(15-19(18)10-23)24-6-5-9-34-26(24)36-27(31)40/h5-13H,14-17H2,1-4H3,(H,35,39)(H,34,36,40)/t30-,31+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0560 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Labortories
Curated by ChEMBL
| Assay Description
Displacement of [125I]-hCGRP from human CGRP receptor expressed in HEK293 cells |
ACS Med Chem Lett 4: 863-8 (2013)
Article DOI: 10.1021/ml400199p
BindingDB Entry DOI: 10.7270/Q25D8T8V |
More data for this
Ligand-Target Pair | |
Calcitonin gene-related peptide type 1 receptor
(Homo sapiens (Human)) | BDBM50440785
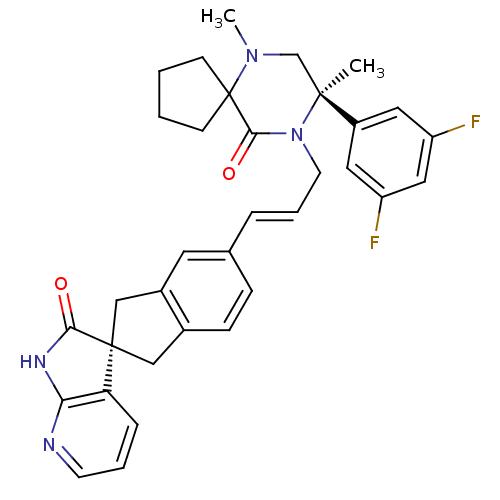
(CHEMBL2431252)Show SMILES CN1C[C@](C)(N(C\C=C\c2ccc3C[C@]4(Cc3c2)C(=O)Nc2ncccc42)C(=O)C11CCCC1)c1cc(F)cc(F)c1 |r| Show InChI InChI=1S/C34H34F2N4O2/c1-32(25-16-26(35)18-27(36)17-25)21-39(2)34(11-3-4-12-34)31(42)40(32)14-6-7-22-9-10-23-19-33(20-24(23)15-22)28-8-5-13-37-29(28)38-30(33)41/h5-10,13,15-18H,3-4,11-12,14,19-21H2,1-2H3,(H,37,38,41)/b7-6+/t32-,33+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0940 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Labortories
Curated by ChEMBL
| Assay Description
Displacement of [125I]-hCGRP from human CGRP receptor expressed in HEK293 cells |
ACS Med Chem Lett 4: 863-8 (2013)
Article DOI: 10.1021/ml400199p
BindingDB Entry DOI: 10.7270/Q25D8T8V |
More data for this
Ligand-Target Pair | |
Calcitonin gene-related peptide type 1 receptor
(Homo sapiens (Human)) | BDBM50440792

(CHEMBL2431255)Show SMILES CC1(C)NC[C@](C)(N(CC(=O)Nc2ccc3C[C@]4(Cc3c2)C(=O)Nc2ncccc42)C1=O)c1cc(F)cc(F)c1 |r| Show InChI InChI=1S/C30H29F2N5O3/c1-28(2)27(40)37(29(3,16-34-28)19-10-20(31)12-21(32)11-19)15-24(38)35-22-7-6-17-13-30(14-18(17)9-22)23-5-4-8-33-25(23)36-26(30)39/h4-12,34H,13-16H2,1-3H3,(H,35,38)(H,33,36,39)/t29-,30+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Labortories
Curated by ChEMBL
| Assay Description
Displacement of [125I]-hCGRP from human CGRP receptor expressed in HEK293 cells |
ACS Med Chem Lett 4: 863-8 (2013)
Article DOI: 10.1021/ml400199p
BindingDB Entry DOI: 10.7270/Q25D8T8V |
More data for this
Ligand-Target Pair | |
Calcitonin gene-related peptide type 1 receptor
(Homo sapiens (Human)) | BDBM50440783
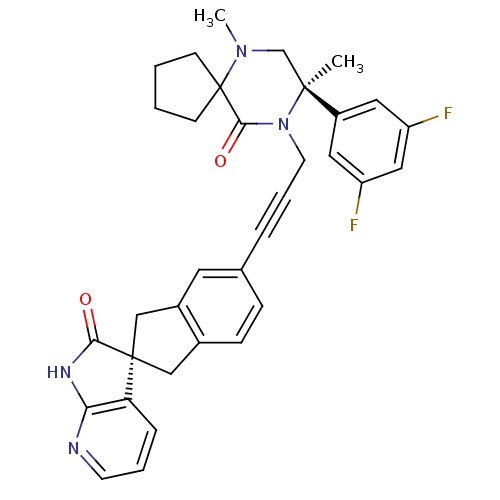
(CHEMBL2429882)Show SMILES CN1C[C@](C)(N(CC#Cc2ccc3C[C@]4(Cc3c2)C(=O)Nc2ncccc42)C(=O)C11CCCC1)c1cc(F)cc(F)c1 |r| Show InChI InChI=1S/C34H32F2N4O2/c1-32(25-16-26(35)18-27(36)17-25)21-39(2)34(11-3-4-12-34)31(42)40(32)14-6-7-22-9-10-23-19-33(20-24(23)15-22)28-8-5-13-37-29(28)38-30(33)41/h5,8-10,13,15-18H,3-4,11-12,14,19-21H2,1-2H3,(H,37,38,41)/t32-,33+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Labortories
Curated by ChEMBL
| Assay Description
Displacement of [125I]-hCGRP from human CGRP receptor expressed in HEK293 cells |
ACS Med Chem Lett 4: 863-8 (2013)
Article DOI: 10.1021/ml400199p
BindingDB Entry DOI: 10.7270/Q25D8T8V |
More data for this
Ligand-Target Pair | |
Calcitonin gene-related peptide type 1 receptor
(Homo sapiens (Human)) | BDBM50440787
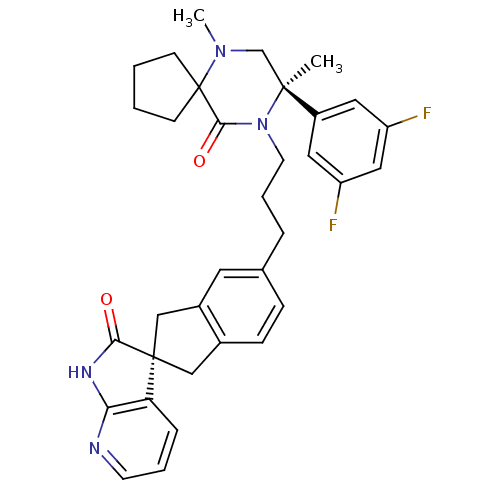
(CHEMBL2431250)Show SMILES CN1C[C@](C)(N(CCCc2ccc3C[C@]4(Cc3c2)C(=O)Nc2ncccc42)C(=O)C11CCCC1)c1cc(F)cc(F)c1 |r| Show InChI InChI=1S/C34H36F2N4O2/c1-32(25-16-26(35)18-27(36)17-25)21-39(2)34(11-3-4-12-34)31(42)40(32)14-6-7-22-9-10-23-19-33(20-24(23)15-22)28-8-5-13-37-29(28)38-30(33)41/h5,8-10,13,15-18H,3-4,6-7,11-12,14,19-21H2,1-2H3,(H,37,38,41)/t32-,33+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Labortories
Curated by ChEMBL
| Assay Description
Displacement of [125I]-hCGRP from human CGRP receptor expressed in HEK293 cells |
ACS Med Chem Lett 4: 863-8 (2013)
Article DOI: 10.1021/ml400199p
BindingDB Entry DOI: 10.7270/Q25D8T8V |
More data for this
Ligand-Target Pair | |
Calcitonin gene-related peptide type 1 receptor
(Homo sapiens (Human)) | BDBM50224431
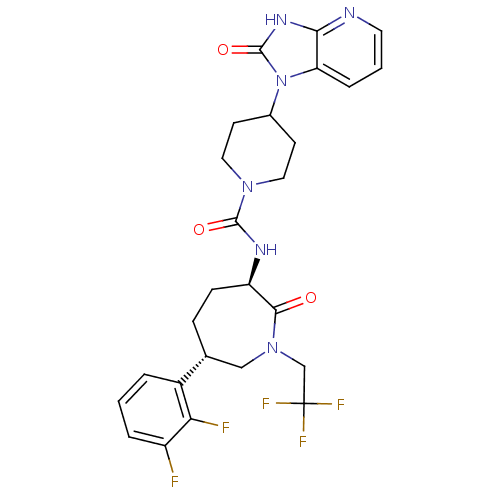
(CHEMBL236593 | MK-0974 | N-[(3R,6S)-6-(2,3-difluor...)Show SMILES Fc1cccc([C@@H]2CC[C@@H](NC(=O)N3CCC(CC3)n3c4cccnc4[nH]c3=O)C(=O)N(CC(F)(F)F)C2)c1F Show InChI InChI=1S/C26H27F5N6O3/c27-18-4-1-3-17(21(18)28)15-6-7-19(23(38)36(13-15)14-26(29,30)31)33-24(39)35-11-8-16(9-12-35)37-20-5-2-10-32-22(20)34-25(37)40/h1-5,10,15-16,19H,6-9,11-14H2,(H,33,39)(H,32,34,40)/t15-,19-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.770 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Labortories
Curated by ChEMBL
| Assay Description
Displacement of [125I]-hCGRP from human CGRP receptor expressed in HEK293 cells |
ACS Med Chem Lett 4: 863-8 (2013)
Article DOI: 10.1021/ml400199p
BindingDB Entry DOI: 10.7270/Q25D8T8V |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Calcitonin gene-related peptide type 1 receptor
(Homo sapiens (Human)) | BDBM50440789

(CHEMBL2431248)Show SMILES CN1C[C@](C)(N(CC(=O)Nc2ccc3C[C@]4(Cc3c2)C(=O)Nc2ncccc42)C(=O)C11CCCC1)c1cc(F)cc(F)c1 |r| Show InChI InChI=1S/C33H33F2N5O3/c1-31(22-13-23(34)15-24(35)14-22)19-39(2)33(9-3-4-10-33)30(43)40(31)18-27(41)37-25-8-7-20-16-32(17-21(20)12-25)26-6-5-11-36-28(26)38-29(32)42/h5-8,11-15H,3-4,9-10,16-19H2,1-2H3,(H,37,41)(H,36,38,42)/t31-,32+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0970 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Labortories
Curated by ChEMBL
| Assay Description
Antagonist activity at CGRP receptor (unknown origin) by cell based cAMP assay |
ACS Med Chem Lett 4: 863-8 (2013)
Article DOI: 10.1021/ml400199p
BindingDB Entry DOI: 10.7270/Q25D8T8V |
More data for this
Ligand-Target Pair | |
Calcitonin gene-related peptide type 1 receptor
(Homo sapiens (Human)) | BDBM50440789

(CHEMBL2431248)Show SMILES CN1C[C@](C)(N(CC(=O)Nc2ccc3C[C@]4(Cc3c2)C(=O)Nc2ncccc42)C(=O)C11CCCC1)c1cc(F)cc(F)c1 |r| Show InChI InChI=1S/C33H33F2N5O3/c1-31(22-13-23(34)15-24(35)14-22)19-39(2)33(9-3-4-10-33)30(43)40(31)18-27(41)37-25-8-7-20-16-32(17-21(20)12-25)26-6-5-11-36-28(26)38-29(32)42/h5-8,11-15H,3-4,9-10,16-19H2,1-2H3,(H,37,41)(H,36,38,42)/t31-,32+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.390 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Labortories
Curated by ChEMBL
| Assay Description
Antagonist activity at CGRP receptor (unknown origin) by cell based cAMP assay in presence of human serum |
ACS Med Chem Lett 4: 863-8 (2013)
Article DOI: 10.1021/ml400199p
BindingDB Entry DOI: 10.7270/Q25D8T8V |
More data for this
Ligand-Target Pair | |
High affinity nerve growth factor receptor
(Homo sapiens (Human)) | BDBM24922
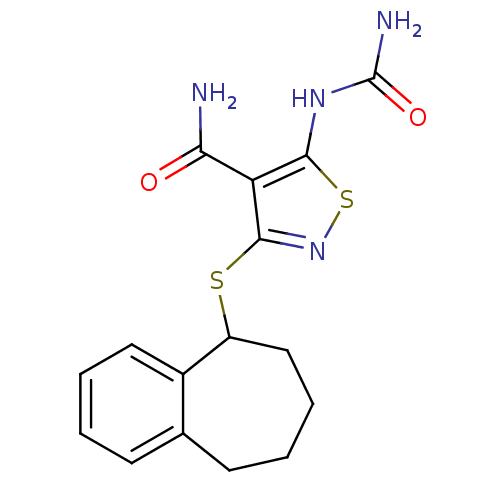
(5-(carbamoylamino)-3-{6,7,8,9-tetrahydro-5H-benzo[...)Show InChI InChI=1S/C16H18N4O2S2/c17-13(21)12-14(19-16(18)22)24-20-15(12)23-11-8-4-2-6-9-5-1-3-7-10(9)11/h1,3,5,7,11H,2,4,6,8H2,(H2,17,21)(H3,18,19,22) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <1 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Pfizer
| Assay Description
The kinase domain of the human TrkA receptor in phosphorylation buffer with 0.5 uM ATP is incubated in plates coated with PGT substrate. Compounds ar... |
Bioorg Med Chem Lett 16: 3444-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.003
BindingDB Entry DOI: 10.7270/Q26Q1VH5 |
More data for this
Ligand-Target Pair | |
High affinity nerve growth factor receptor
(Homo sapiens (Human)) | BDBM24923
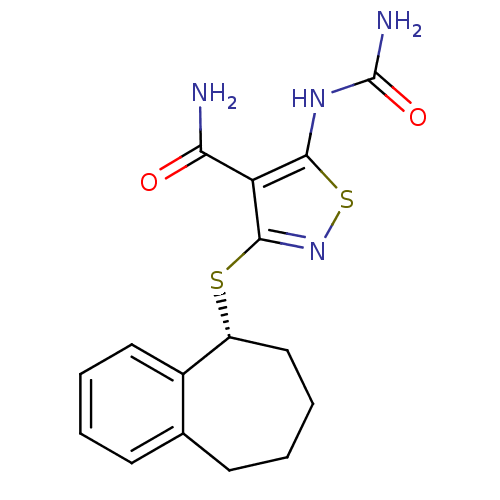
(5-(carbamoylamino)-3-[(5R)-6,7,8,9-tetrahydro-5H-b...)Show SMILES NC(=O)Nc1snc(S[C@@H]2CCCCc3ccccc23)c1C(N)=O |r| Show InChI InChI=1S/C16H18N4O2S2/c17-13(21)12-14(19-16(18)22)24-20-15(12)23-11-8-4-2-6-9-5-1-3-7-10(9)11/h1,3,5,7,11H,2,4,6,8H2,(H2,17,21)(H3,18,19,22)/t11-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MMDB
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <1 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Pfizer
| Assay Description
The kinase domain of the human TrkA receptor in phosphorylation buffer with 0.5 uM ATP is incubated in plates coated with PGT substrate. Compounds ar... |
Bioorg Med Chem Lett 16: 3444-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.003
BindingDB Entry DOI: 10.7270/Q26Q1VH5 |
More data for this
Ligand-Target Pair | |
High affinity nerve growth factor receptor
(Homo sapiens (Human)) | BDBM24899

(3-{[1-(4-chlorophenyl)ethyl]sulfanyl}-5-[(methylca...)Show InChI InChI=1S/C14H15ClN4O2S2/c1-7(8-3-5-9(15)6-4-8)22-13-10(11(16)20)12(23-19-13)18-14(21)17-2/h3-7H,1-2H3,(H2,16,20)(H2,17,18,21) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Pfizer
| Assay Description
The kinase domain of the human TrkA receptor in phosphorylation buffer with 0.5 uM ATP is incubated in plates coated with PGT substrate. Compounds ar... |
Bioorg Med Chem Lett 16: 3444-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.003
BindingDB Entry DOI: 10.7270/Q26Q1VH5 |
More data for this
Ligand-Target Pair | |
High affinity nerve growth factor receptor
(Homo sapiens (Human)) | BDBM24901
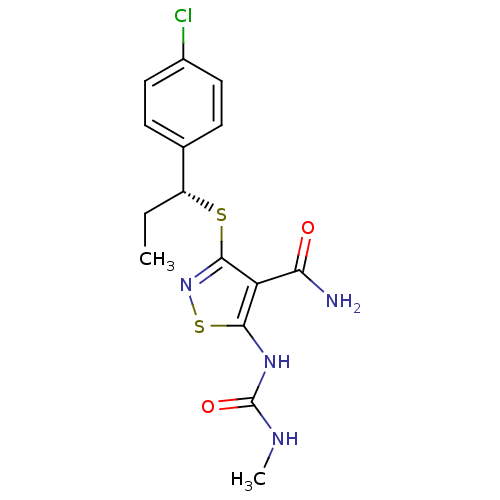
(3-{[(1R)-1-(4-chlorophenyl)propyl]sulfanyl}-5-[(me...)Show SMILES CC[C@@H](Sc1nsc(NC(=O)NC)c1C(N)=O)c1ccc(Cl)cc1 |r| Show InChI InChI=1S/C15H17ClN4O2S2/c1-3-10(8-4-6-9(16)7-5-8)23-14-11(12(17)21)13(24-20-14)19-15(22)18-2/h4-7,10H,3H2,1-2H3,(H2,17,21)(H2,18,19,22)/t10-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Pfizer
| Assay Description
The kinase domain of the human TrkA receptor in phosphorylation buffer with 0.5 uM ATP is incubated in plates coated with PGT substrate. Compounds ar... |
Bioorg Med Chem Lett 16: 3444-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.003
BindingDB Entry DOI: 10.7270/Q26Q1VH5 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 5
(Homo sapiens (Human)) | BDBM50155213
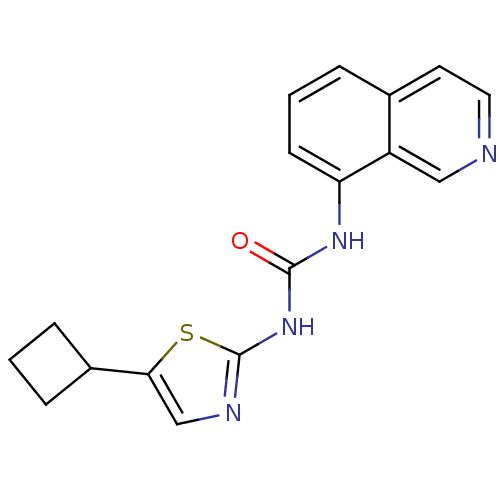
(1-(5-Cyclobutyl-thiazol-2-yl)-3-isoquinolin-8-yl-u...)Show InChI InChI=1S/C17H16N4OS/c22-16(21-17-19-10-15(23-17)12-4-1-5-12)20-14-6-2-3-11-7-8-18-9-13(11)14/h2-3,6-10,12H,1,4-5H2,(H2,19,20,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Concentration required for inhibition of cyclin-dependent kinase 5 was measured by scintillation proximity assay |
Bioorg Med Chem Lett 14: 5521-5 (2004)
Article DOI: 10.1016/j.bmcl.2004.09.006
BindingDB Entry DOI: 10.7270/Q2D50MFB |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 5 activator 1
(Homo sapiens (Human)) | BDBM50415045

(CHEMBL571782)Show SMILES Clc1cccc(n1)C(=O)N[C@H]1C[C@H](C1)n1cnc(NC(=O)Cc2cccc3ccccc23)c1 |r,wU:10.10,12.15,(-7.08,-11.69,;-7.1,-10.15,;-8.45,-9.39,;-8.47,-7.85,;-7.14,-7.07,;-5.81,-7.83,;-5.78,-9.36,;-4.48,-7.05,;-4.5,-5.51,;-3.14,-7.8,;-1.81,-7.02,;-1.13,-5.64,;.26,-6.32,;-.44,-7.71,;1.72,-5.83,;2.18,-4.37,;3.72,-4.35,;4.21,-5.81,;5.54,-6.58,;5.54,-8.13,;4.2,-8.89,;6.87,-8.9,;6.86,-10.44,;8.19,-11.21,;8.19,-12.74,;6.85,-13.52,;5.52,-12.74,;4.19,-13.5,;2.87,-12.74,;2.87,-11.2,;4.2,-10.44,;5.52,-11.21,;2.97,-6.73,)| Show InChI InChI=1S/C25H22ClN5O2/c26-22-10-4-9-21(29-22)25(33)28-18-12-19(13-18)31-14-23(27-15-31)30-24(32)11-17-7-3-6-16-5-1-2-8-20(16)17/h1-10,14-15,18-19H,11-13H2,(H,28,33)(H,30,32)/t18-,19+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of CDK5/p25 by scintillation proximity assay |
Bioorg Med Chem Lett 19: 5703-7 (2009)
Article DOI: 10.1016/j.bmcl.2009.08.019
BindingDB Entry DOI: 10.7270/Q29G5P29 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 5 activator 1
(Homo sapiens (Human)) | BDBM50415046

(CHEMBL583658)Show SMILES Cc1cccc(n1)C(=O)N[C@H]1C[C@H](C1)n1cnc(NC(=O)Cc2cccc3ccccc23)c1 |r,wU:10.10,12.15,(14.65,-10.99,;14.63,-9.45,;13.28,-8.7,;13.27,-7.16,;14.59,-6.38,;15.92,-7.14,;15.95,-8.67,;17.25,-6.35,;17.24,-4.81,;18.59,-7.11,;19.92,-6.33,;20.6,-4.95,;21.99,-5.63,;21.3,-7.01,;23.45,-5.14,;23.91,-3.67,;25.45,-3.66,;25.94,-5.12,;27.27,-5.89,;27.27,-7.43,;25.93,-8.2,;28.6,-8.21,;28.59,-9.75,;29.92,-10.51,;29.92,-12.05,;28.58,-12.82,;27.25,-12.05,;25.93,-12.81,;24.6,-12.05,;24.6,-10.51,;25.93,-9.74,;27.25,-10.51,;24.7,-6.03,)| Show InChI InChI=1S/C26H25N5O2/c1-17-6-4-11-23(28-17)26(33)29-20-13-21(14-20)31-15-24(27-16-31)30-25(32)12-19-9-5-8-18-7-2-3-10-22(18)19/h2-11,15-16,20-21H,12-14H2,1H3,(H,29,33)(H,30,32)/t20-,21+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of CDK5/p25 by scintillation proximity assay |
Bioorg Med Chem Lett 19: 5703-7 (2009)
Article DOI: 10.1016/j.bmcl.2009.08.019
BindingDB Entry DOI: 10.7270/Q29G5P29 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 5
(Homo sapiens (Human)) | BDBM50155231
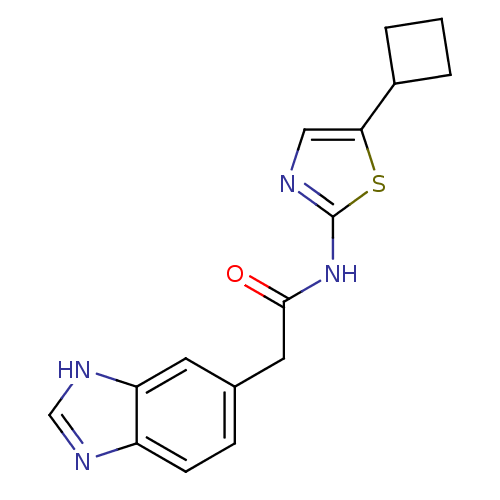
(2-(1H-Benzoimidazol-5-yl)-N-(5-cyclobutyl-thiazol-...)Show InChI InChI=1S/C16H16N4OS/c21-15(7-10-4-5-12-13(6-10)19-9-18-12)20-16-17-8-14(22-16)11-2-1-3-11/h4-6,8-9,11H,1-3,7H2,(H,18,19)(H,17,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Concentration required for inhibition of cyclin-dependent kinase 5 was measured by scintillation proximity assay |
Bioorg Med Chem Lett 14: 5521-5 (2004)
Article DOI: 10.1016/j.bmcl.2004.09.006
BindingDB Entry DOI: 10.7270/Q2D50MFB |
More data for this
Ligand-Target Pair | |
High affinity nerve growth factor receptor
(Homo sapiens (Human)) | BDBM24898
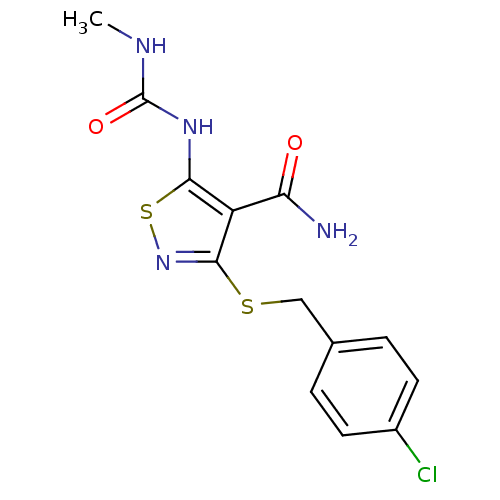
(3-{[(4-chlorophenyl)methyl]sulfanyl}-5-[(methylcar...)Show InChI InChI=1S/C13H13ClN4O2S2/c1-16-13(20)17-11-9(10(15)19)12(18-22-11)21-6-7-2-4-8(14)5-3-7/h2-5H,6H2,1H3,(H2,15,19)(H2,16,17,20) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Pfizer
| Assay Description
The kinase domain of the human TrkA receptor in phosphorylation buffer with 0.5 uM ATP is incubated in plates coated with PGT substrate. Compounds ar... |
Bioorg Med Chem Lett 16: 3444-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.003
BindingDB Entry DOI: 10.7270/Q26Q1VH5 |
More data for this
Ligand-Target Pair | |
High affinity nerve growth factor receptor
(Homo sapiens (Human)) | BDBM24919
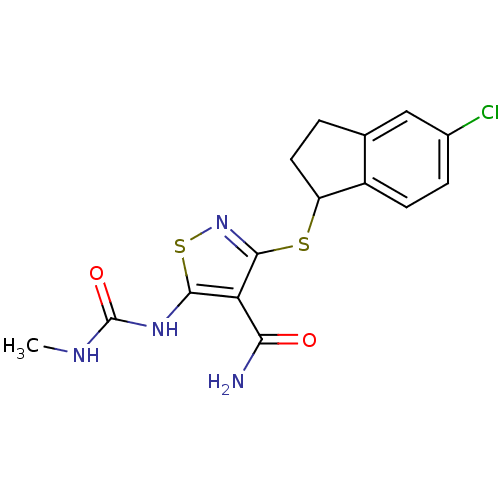
(3-[(5-chloro-2,3-dihydro-1H-inden-1-yl)sulfanyl]-5...)Show InChI InChI=1S/C15H15ClN4O2S2/c1-18-15(22)19-13-11(12(17)21)14(20-24-13)23-10-5-2-7-6-8(16)3-4-9(7)10/h3-4,6,10H,2,5H2,1H3,(H2,17,21)(H2,18,19,22) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Pfizer
| Assay Description
The kinase domain of the human TrkA receptor in phosphorylation buffer with 0.5 uM ATP is incubated in plates coated with PGT substrate. Compounds ar... |
Bioorg Med Chem Lett 16: 3444-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.003
BindingDB Entry DOI: 10.7270/Q26Q1VH5 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 5 activator 1
(Homo sapiens (Human)) | BDBM50415039

(CHEMBL571780)Show SMILES CC(=O)N[C@H]1C[C@H](C1)n1cnc(NC(=O)Cc2cccc3ccccc23)c1 |r,wU:6.8,4.3,(6.38,-30.85,;7.71,-30.06,;9.05,-30.82,;7.69,-28.52,;9.02,-27.74,;9.7,-26.36,;11.09,-27.05,;10.4,-28.43,;12.55,-26.56,;13.01,-25.09,;14.55,-25.07,;15.04,-26.53,;16.37,-27.31,;16.37,-28.85,;15.03,-29.61,;17.7,-29.62,;17.69,-31.16,;19.02,-31.93,;19.02,-33.46,;17.68,-34.24,;16.35,-33.46,;15.03,-34.22,;13.7,-33.46,;13.7,-31.92,;15.03,-31.16,;16.35,-31.93,;13.81,-27.45,)| Show InChI InChI=1S/C21H22N4O2/c1-14(26)23-17-10-18(11-17)25-12-20(22-13-25)24-21(27)9-16-7-4-6-15-5-2-3-8-19(15)16/h2-8,12-13,17-18H,9-11H2,1H3,(H,23,26)(H,24,27)/t17-,18+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of CDK5/p25 by scintillation proximity assay |
Bioorg Med Chem Lett 19: 5703-7 (2009)
Article DOI: 10.1016/j.bmcl.2009.08.019
BindingDB Entry DOI: 10.7270/Q29G5P29 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 5
(Homo sapiens (Human)) | BDBM50155209

(1-(5-Cyclobutyl-thiazol-2-yl)-3-isoquinolin-5-yl-u...)Show InChI InChI=1S/C17H16N4OS/c22-16(21-17-19-10-15(23-17)11-3-1-4-11)20-14-6-2-5-12-9-18-8-7-13(12)14/h2,5-11H,1,3-4H2,(H2,19,20,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Concentration required for inhibition of cyclin-dependent kinase 5 was measured by scintillation proximity assay |
Bioorg Med Chem Lett 14: 5521-5 (2004)
Article DOI: 10.1016/j.bmcl.2004.09.006
BindingDB Entry DOI: 10.7270/Q2D50MFB |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 5 activator 1
(Homo sapiens (Human)) | BDBM50415037

(CHEMBL576502)Show SMILES COc1ccc(CC(=O)Nc2cn(cn2)[C@@H]2C[C@@H](C2)NC(C)=O)cc1 |r,wU:15.15,17.20,(2.53,9.19,;1,9.03,;.37,7.63,;-1.16,7.46,;-1.78,6.06,;-.88,4.81,;-1.51,3.41,;-.6,2.16,;.93,2.32,;-1.23,.75,;-.32,-.49,;-.8,-1.96,;.45,-2.86,;1.69,-1.96,;1.22,-.49,;.45,-4.4,;-.64,-5.49,;.45,-6.58,;1.54,-5.49,;.45,-8.12,;-.89,-8.89,;-.89,-10.43,;-2.22,-8.12,;.65,4.97,;1.28,6.38,)| Show InChI InChI=1S/C18H22N4O3/c1-12(23)20-14-8-15(9-14)22-10-17(19-11-22)21-18(24)7-13-3-5-16(25-2)6-4-13/h3-6,10-11,14-15H,7-9H2,1-2H3,(H,20,23)(H,21,24)/t14-,15+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of CDK5/p25 by scintillation proximity assay |
Bioorg Med Chem Lett 19: 5703-7 (2009)
Article DOI: 10.1016/j.bmcl.2009.08.019
BindingDB Entry DOI: 10.7270/Q29G5P29 |
More data for this
Ligand-Target Pair | |
High affinity nerve growth factor receptor
(Homo sapiens (Human)) | BDBM24900

(3-{[1-(4-chlorophenyl)propyl]sulfanyl}-5-[(methylc...)Show InChI InChI=1S/C15H17ClN4O2S2/c1-3-10(8-4-6-9(16)7-5-8)23-14-11(12(17)21)13(24-20-14)19-15(22)18-2/h4-7,10H,3H2,1-2H3,(H2,17,21)(H2,18,19,22) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Pfizer
| Assay Description
The kinase domain of the human TrkA receptor in phosphorylation buffer with 0.5 uM ATP is incubated in plates coated with PGT substrate. Compounds ar... |
Bioorg Med Chem Lett 16: 3444-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.003
BindingDB Entry DOI: 10.7270/Q26Q1VH5 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 5
(Homo sapiens (Human)) | BDBM50155236
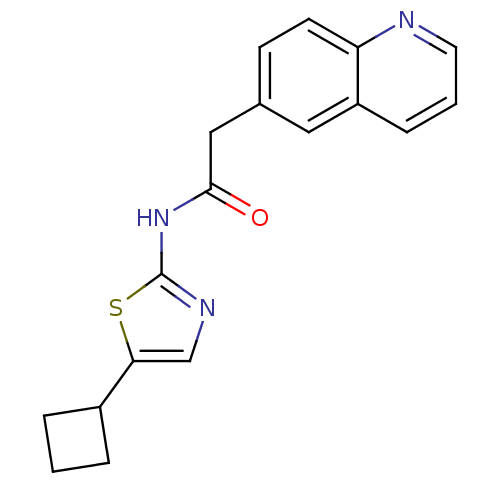
(CHEMBL363954 | N-(5-Cyclobutyl-thiazol-2-yl)-2-qui...)Show InChI InChI=1S/C18H17N3OS/c22-17(21-18-20-11-16(23-18)13-3-1-4-13)10-12-6-7-15-14(9-12)5-2-8-19-15/h2,5-9,11,13H,1,3-4,10H2,(H,20,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Concentration required for inhibition of cyclin-dependent kinase 5 was measured by scintillation proximity assay |
Bioorg Med Chem Lett 14: 5521-5 (2004)
Article DOI: 10.1016/j.bmcl.2004.09.006
BindingDB Entry DOI: 10.7270/Q2D50MFB |
More data for this
Ligand-Target Pair | |
High affinity nerve growth factor receptor
(Homo sapiens (Human)) | BDBM24918
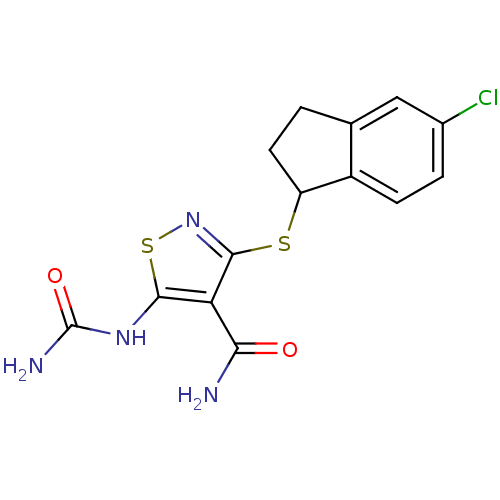
(5-(carbamoylamino)-3-[(5-chloro-2,3-dihydro-1H-ind...)Show InChI InChI=1S/C14H13ClN4O2S2/c15-7-2-3-8-6(5-7)1-4-9(8)22-13-10(11(16)20)12(23-19-13)18-14(17)21/h2-3,5,9H,1,4H2,(H2,16,20)(H3,17,18,21) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Pfizer
| Assay Description
The kinase domain of the human TrkA receptor in phosphorylation buffer with 0.5 uM ATP is incubated in plates coated with PGT substrate. Compounds ar... |
Bioorg Med Chem Lett 16: 3444-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.003
BindingDB Entry DOI: 10.7270/Q26Q1VH5 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 5
(Homo sapiens (Human)) | BDBM50155235
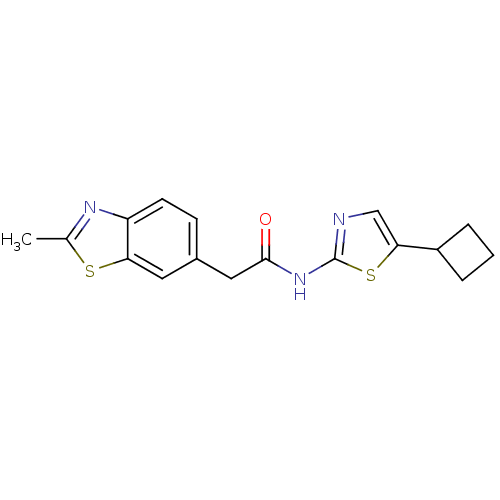
(CHEMBL186470 | N-(5-Cyclobutyl-thiazol-2-yl)-2-(2-...)Show InChI InChI=1S/C17H17N3OS2/c1-10-19-13-6-5-11(7-14(13)22-10)8-16(21)20-17-18-9-15(23-17)12-3-2-4-12/h5-7,9,12H,2-4,8H2,1H3,(H,18,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Concentration required for inhibition of cyclin-dependent kinase 5 was measured by scintillation proximity assay |
Bioorg Med Chem Lett 14: 5521-5 (2004)
Article DOI: 10.1016/j.bmcl.2004.09.006
BindingDB Entry DOI: 10.7270/Q2D50MFB |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 5
(Homo sapiens (Human)) | BDBM50155237

(CHEMBL184404 | N-(5-Cyclobutyl-thiazol-2-yl)-2-iso...)Show InChI InChI=1S/C18H17N3OS/c22-17(21-18-20-11-16(23-18)12-3-1-4-12)9-13-5-2-6-14-10-19-8-7-15(13)14/h2,5-8,10-12H,1,3-4,9H2,(H,20,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Concentration required for inhibition of cyclin-dependent kinase 5 was measured by scintillation proximity assay |
Bioorg Med Chem Lett 14: 5521-5 (2004)
Article DOI: 10.1016/j.bmcl.2004.09.006
BindingDB Entry DOI: 10.7270/Q2D50MFB |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 5
(Homo sapiens (Human)) | BDBM50155230
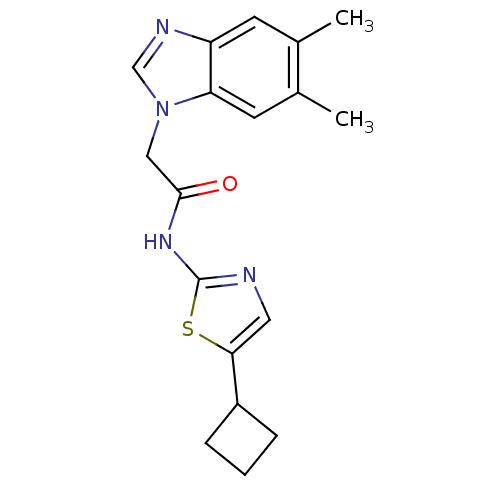
(CHEMBL187903 | N-(5-Cyclobutyl-thiazol-2-yl)-2-(5,...)Show InChI InChI=1S/C18H20N4OS/c1-11-6-14-15(7-12(11)2)22(10-20-14)9-17(23)21-18-19-8-16(24-18)13-4-3-5-13/h6-8,10,13H,3-5,9H2,1-2H3,(H,19,21,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Concentration required for inhibition of cyclin-dependent kinase 5 was measured by scintillation proximity assay |
Bioorg Med Chem Lett 14: 5521-5 (2004)
Article DOI: 10.1016/j.bmcl.2004.09.006
BindingDB Entry DOI: 10.7270/Q2D50MFB |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 5
(Homo sapiens (Human)) | BDBM50155207

(1-(5-Cyclobutyl-thiazol-2-yl)-3-quinolin-5-yl-urea...)Show InChI InChI=1S/C17H16N4OS/c22-16(21-17-19-10-15(23-17)11-4-1-5-11)20-14-8-2-7-13-12(14)6-3-9-18-13/h2-3,6-11H,1,4-5H2,(H2,19,20,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Concentration required for inhibition of cyclin-dependent kinase 5 was measured by scintillation proximity assay |
Bioorg Med Chem Lett 14: 5521-5 (2004)
Article DOI: 10.1016/j.bmcl.2004.09.006
BindingDB Entry DOI: 10.7270/Q2D50MFB |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 5 activator 1
(Homo sapiens (Human)) | BDBM50415044

(CHEMBL569587)Show SMILES O=C(Cc1cccc2ccccc12)Nc1cn(cn1)[C@@H]1C[C@@H](C1)NC(=O)c1ccccn1 |r,wU:21.26,19.21,(28.29,2.47,;29.63,3.24,;30.96,2.46,;30.96,.92,;32.28,.16,;32.28,-1.38,;30.94,-2.15,;29.61,-1.38,;28.29,-2.14,;26.96,-1.38,;26.96,.16,;28.29,.93,;29.62,.16,;29.63,4.78,;28.3,5.55,;27.07,4.63,;25.81,5.53,;26.27,7,;27.81,7.01,;24.35,5.04,;22.96,5.72,;22.28,4.34,;23.66,3.66,;20.95,3.56,;19.61,4.32,;19.6,5.86,;18.29,3.53,;16.95,4.29,;15.63,3.51,;15.64,1.97,;16.99,1.21,;18.31,2,)| Show InChI InChI=1S/C25H23N5O2/c31-24(12-18-8-5-7-17-6-1-2-9-21(17)18)29-23-15-30(16-27-23)20-13-19(14-20)28-25(32)22-10-3-4-11-26-22/h1-11,15-16,19-20H,12-14H2,(H,28,32)(H,29,31)/t19-,20+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of CDK5/p25 by scintillation proximity assay |
Bioorg Med Chem Lett 19: 5703-7 (2009)
Article DOI: 10.1016/j.bmcl.2009.08.019
BindingDB Entry DOI: 10.7270/Q29G5P29 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 2/G1/S-specific cyclin-E1
(Homo sapiens (Human)) | BDBM50155235

(CHEMBL186470 | N-(5-Cyclobutyl-thiazol-2-yl)-2-(2-...)Show InChI InChI=1S/C17H17N3OS2/c1-10-19-13-6-5-11(7-14(13)22-10)8-16(21)20-17-18-9-15(23-17)12-3-2-4-12/h5-7,9,12H,2-4,8H2,1H3,(H,18,20,21) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Concentration required for inhibition of cyclin dependant kinase 2-cyclin E was measured by scintillation proximity assay |
Bioorg Med Chem Lett 14: 5521-5 (2004)
Article DOI: 10.1016/j.bmcl.2004.09.006
BindingDB Entry DOI: 10.7270/Q2D50MFB |
More data for this
Ligand-Target Pair | |
High affinity nerve growth factor receptor
(Homo sapiens (Human)) | BDBM24903

(3-{[1-(4-chlorophenyl)-2-methylpropyl]sulfanyl}-5-...)Show SMILES CNC(=O)Nc1snc(SC(C(C)C)c2ccc(Cl)cc2)c1C(N)=O Show InChI InChI=1S/C16H19ClN4O2S2/c1-8(2)12(9-4-6-10(17)7-5-9)24-15-11(13(18)22)14(25-21-15)20-16(23)19-3/h4-8,12H,1-3H3,(H2,18,22)(H2,19,20,23) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Pfizer
| Assay Description
The kinase domain of the human TrkA receptor in phosphorylation buffer with 0.5 uM ATP is incubated in plates coated with PGT substrate. Compounds ar... |
Bioorg Med Chem Lett 16: 3444-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.003
BindingDB Entry DOI: 10.7270/Q26Q1VH5 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 5
(Homo sapiens (Human)) | BDBM50155214
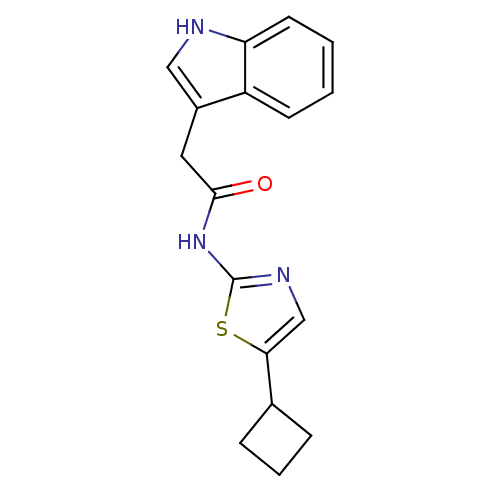
(CHEMBL186240 | N-(5-Cyclobutyl-thiazol-2-yl)-2-(1H...)Show InChI InChI=1S/C17H17N3OS/c21-16(8-12-9-18-14-7-2-1-6-13(12)14)20-17-19-10-15(22-17)11-4-3-5-11/h1-2,6-7,9-11,18H,3-5,8H2,(H,19,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Concentration required for inhibition of cyclin-dependent kinase 5 was measured by scintillation proximity assay |
Bioorg Med Chem Lett 14: 5521-5 (2004)
Article DOI: 10.1016/j.bmcl.2004.09.006
BindingDB Entry DOI: 10.7270/Q2D50MFB |
More data for this
Ligand-Target Pair | |
High affinity nerve growth factor receptor
(Homo sapiens (Human)) | BDBM24910
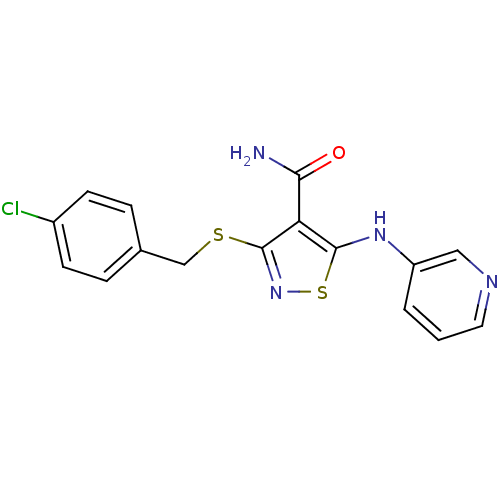
(3-{[(4-chlorophenyl)methyl]sulfanyl}-5-(pyridin-3-...)Show InChI InChI=1S/C16H13ClN4OS2/c17-11-5-3-10(4-6-11)9-23-16-13(14(18)22)15(24-21-16)20-12-2-1-7-19-8-12/h1-8,20H,9H2,(H2,18,22) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Pfizer
| Assay Description
The kinase domain of the human TrkA receptor in phosphorylation buffer with 0.5 uM ATP is incubated in plates coated with PGT substrate. Compounds ar... |
Bioorg Med Chem Lett 16: 3444-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.003
BindingDB Entry DOI: 10.7270/Q26Q1VH5 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 5
(Homo sapiens (Human)) | BDBM50155225
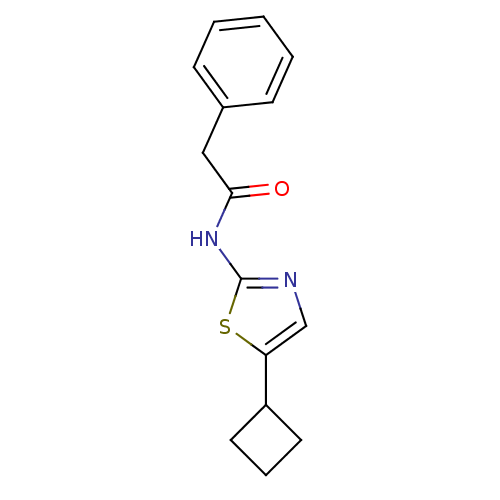
(CHEMBL365855 | N-(5-Cyclobutyl-thiazol-2-yl)-2-phe...)Show InChI InChI=1S/C15H16N2OS/c18-14(9-11-5-2-1-3-6-11)17-15-16-10-13(19-15)12-7-4-8-12/h1-3,5-6,10,12H,4,7-9H2,(H,16,17,18) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Concentration required for inhibition of cyclin-dependent kinase 5 was measured by scintillation proximity assay |
Bioorg Med Chem Lett 14: 5521-5 (2004)
Article DOI: 10.1016/j.bmcl.2004.09.006
BindingDB Entry DOI: 10.7270/Q2D50MFB |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 2/G1/S-specific cyclin-E1
(Homo sapiens (Human)) | BDBM50155230

(CHEMBL187903 | N-(5-Cyclobutyl-thiazol-2-yl)-2-(5,...)Show InChI InChI=1S/C18H20N4OS/c1-11-6-14-15(7-12(11)2)22(10-20-14)9-17(23)21-18-19-8-16(24-18)13-4-3-5-13/h6-8,10,13H,3-5,9H2,1-2H3,(H,19,21,23) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Concentration required for inhibition of cyclin dependant kinase 2-cyclin E was measured by scintillation proximity assay |
Bioorg Med Chem Lett 14: 5521-5 (2004)
Article DOI: 10.1016/j.bmcl.2004.09.006
BindingDB Entry DOI: 10.7270/Q2D50MFB |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 2/G1/S-specific cyclin-E1
(Homo sapiens (Human)) | BDBM50155236

(CHEMBL363954 | N-(5-Cyclobutyl-thiazol-2-yl)-2-qui...)Show InChI InChI=1S/C18H17N3OS/c22-17(21-18-20-11-16(23-18)13-3-1-4-13)10-12-6-7-15-14(9-12)5-2-8-19-15/h2,5-9,11,13H,1,3-4,10H2,(H,20,21,22) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Concentration required for inhibition of cyclin dependant kinase 2-cyclin E was measured by scintillation proximity assay |
Bioorg Med Chem Lett 14: 5521-5 (2004)
Article DOI: 10.1016/j.bmcl.2004.09.006
BindingDB Entry DOI: 10.7270/Q2D50MFB |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 5 activator 1
(Homo sapiens (Human)) | BDBM50415042

(CHEMBL582813)Show SMILES O=C(Cc1cccc2ccccc12)Nc1cn(cn1)[C@@H]1C[C@@H](C1)NC(=O)c1ccncc1 |r,wU:21.26,19.21,(31.19,-43.99,;32.52,-43.23,;33.86,-44,;33.85,-45.54,;35.18,-46.31,;35.18,-47.84,;33.84,-48.62,;32.5,-47.84,;31.18,-48.6,;29.86,-47.84,;29.86,-46.3,;31.19,-45.54,;32.51,-46.31,;32.53,-41.68,;31.2,-40.91,;29.96,-41.83,;28.71,-40.93,;29.17,-39.47,;30.71,-39.45,;27.25,-41.42,;25.86,-40.74,;25.17,-42.12,;26.55,-42.81,;23.85,-42.9,;22.51,-42.15,;22.49,-40.61,;21.18,-42.93,;19.85,-42.17,;18.52,-42.95,;18.54,-44.49,;19.89,-45.25,;21.21,-44.46,)| Show InChI InChI=1S/C25H23N5O2/c31-24(12-19-6-3-5-17-4-1-2-7-22(17)19)29-23-15-30(16-27-23)21-13-20(14-21)28-25(32)18-8-10-26-11-9-18/h1-11,15-16,20-21H,12-14H2,(H,28,32)(H,29,31)/t20-,21+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of CDK5/p25 by scintillation proximity assay |
Bioorg Med Chem Lett 19: 5703-7 (2009)
Article DOI: 10.1016/j.bmcl.2009.08.019
BindingDB Entry DOI: 10.7270/Q29G5P29 |
More data for this
Ligand-Target Pair | |
High affinity nerve growth factor receptor
(Homo sapiens (Human)) | BDBM24914
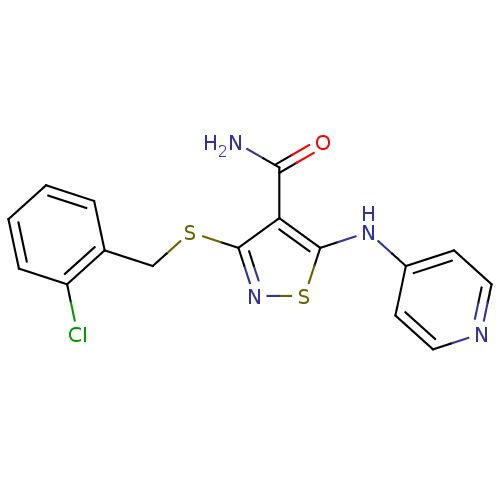
(3-{[(2-chlorophenyl)methyl]sulfanyl}-5-(pyridin-4-...)Show InChI InChI=1S/C16H13ClN4OS2/c17-12-4-2-1-3-10(12)9-23-16-13(14(18)22)15(24-21-16)20-11-5-7-19-8-6-11/h1-8H,9H2,(H2,18,22)(H,19,20) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 28 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Pfizer
| Assay Description
The kinase domain of the human TrkA receptor in phosphorylation buffer with 0.5 uM ATP is incubated in plates coated with PGT substrate. Compounds ar... |
Bioorg Med Chem Lett 16: 3444-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.003
BindingDB Entry DOI: 10.7270/Q26Q1VH5 |
More data for this
Ligand-Target Pair | |
High affinity nerve growth factor receptor
(Homo sapiens (Human)) | BDBM24911
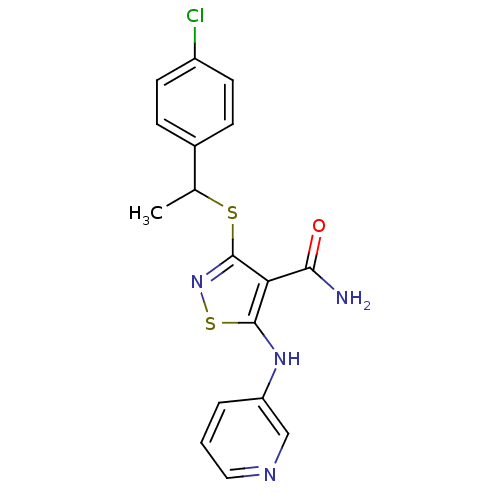
(3-{[1-(4-chlorophenyl)ethyl]sulfanyl}-5-(pyridin-3...)Show InChI InChI=1S/C17H15ClN4OS2/c1-10(11-4-6-12(18)7-5-11)24-17-14(15(19)23)16(25-22-17)21-13-3-2-8-20-9-13/h2-10,21H,1H3,(H2,19,23) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 29 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Pfizer
| Assay Description
The kinase domain of the human TrkA receptor in phosphorylation buffer with 0.5 uM ATP is incubated in plates coated with PGT substrate. Compounds ar... |
Bioorg Med Chem Lett 16: 3444-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.003
BindingDB Entry DOI: 10.7270/Q26Q1VH5 |
More data for this
Ligand-Target Pair | |
High affinity nerve growth factor receptor
(Homo sapiens (Human)) | BDBM24909

(3-{[(4-chlorophenyl)methyl]sulfanyl}-5-(pyridin-2-...)Show InChI InChI=1S/C16H13ClN4OS2/c17-11-6-4-10(5-7-11)9-23-16-13(14(18)22)15(24-21-16)20-12-3-1-2-8-19-12/h1-8H,9H2,(H2,18,22)(H,19,20) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 33 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Pfizer
| Assay Description
The kinase domain of the human TrkA receptor in phosphorylation buffer with 0.5 uM ATP is incubated in plates coated with PGT substrate. Compounds ar... |
Bioorg Med Chem Lett 16: 3444-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.003
BindingDB Entry DOI: 10.7270/Q26Q1VH5 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 2/G1/S-specific cyclin-E1
(Homo sapiens (Human)) | BDBM50155231

(2-(1H-Benzoimidazol-5-yl)-N-(5-cyclobutyl-thiazol-...)Show InChI InChI=1S/C16H16N4OS/c21-15(7-10-4-5-12-13(6-10)19-9-18-12)20-16-17-8-14(22-16)11-2-1-3-11/h4-6,8-9,11H,1-3,7H2,(H,18,19)(H,17,20,21) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Concentration required for inhibition of cyclin dependant kinase 2-cyclin E was measured by scintillation proximity assay |
Bioorg Med Chem Lett 14: 5521-5 (2004)
Article DOI: 10.1016/j.bmcl.2004.09.006
BindingDB Entry DOI: 10.7270/Q2D50MFB |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data