 Found 526 hits with Last Name = 'gauuan' and Initial = 'j'
Found 526 hits with Last Name = 'gauuan' and Initial = 'j' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Coagulation factor X
(Homo sapiens (Human)) | BDBM50377655
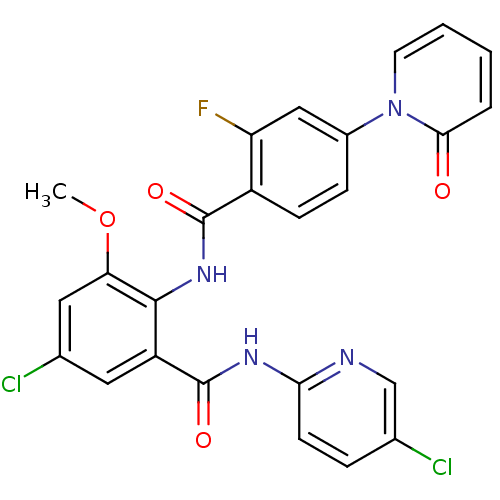
(CHEMBL260160)Show SMILES COc1cc(Cl)cc(C(=O)Nc2ccc(Cl)cn2)c1NC(=O)c1ccc(cc1F)-n1ccccc1=O Show InChI InChI=1S/C25H17Cl2FN4O4/c1-36-20-11-15(27)10-18(25(35)30-21-8-5-14(26)13-29-21)23(20)31-24(34)17-7-6-16(12-19(17)28)32-9-3-2-4-22(32)33/h2-13H,1H3,(H,31,34)(H,29,30,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.00500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 18: 2845-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.03.092
BindingDB Entry DOI: 10.7270/Q2611169 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50377635
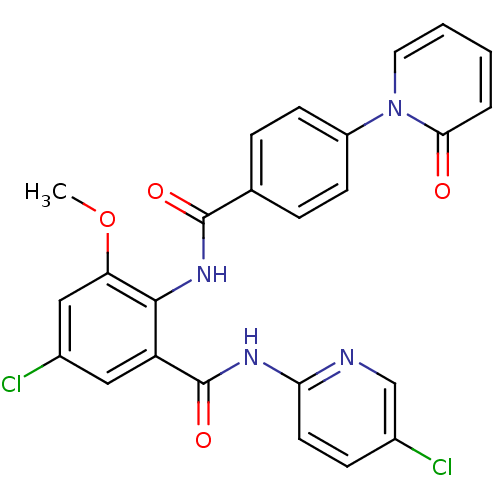
(CHEMBL402980)Show SMILES COc1cc(Cl)cc(C(=O)Nc2ccc(Cl)cn2)c1NC(=O)c1ccc(cc1)-n1ccccc1=O Show InChI InChI=1S/C25H18Cl2N4O4/c1-35-20-13-17(27)12-19(25(34)29-21-10-7-16(26)14-28-21)23(20)30-24(33)15-5-8-18(9-6-15)31-11-3-2-4-22(31)32/h2-14H,1H3,(H,30,33)(H,28,29,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 18: 2845-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.03.092
BindingDB Entry DOI: 10.7270/Q2611169 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50377637
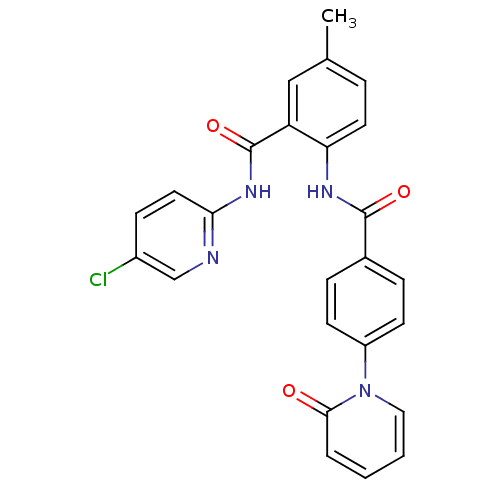
(CHEMBL257398)Show SMILES Cc1ccc(NC(=O)c2ccc(cc2)-n2ccccc2=O)c(c1)C(=O)Nc1ccc(Cl)cn1 Show InChI InChI=1S/C25H19ClN4O3/c1-16-5-11-21(20(14-16)25(33)29-22-12-8-18(26)15-27-22)28-24(32)17-6-9-19(10-7-17)30-13-3-2-4-23(30)31/h2-15H,1H3,(H,28,32)(H,27,29,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 18: 2845-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.03.092
BindingDB Entry DOI: 10.7270/Q2611169 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50377629
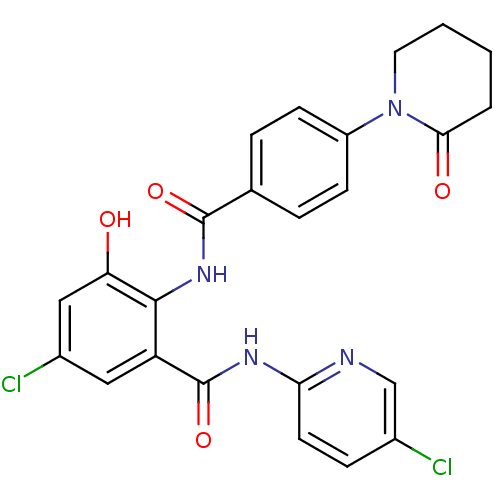
(CHEMBL260086)Show SMILES Oc1cc(Cl)cc(C(=O)Nc2ccc(Cl)cn2)c1NC(=O)c1ccc(cc1)N1CCCCC1=O Show InChI InChI=1S/C24H20Cl2N4O4/c25-15-6-9-20(27-13-15)28-24(34)18-11-16(26)12-19(31)22(18)29-23(33)14-4-7-17(8-5-14)30-10-2-1-3-21(30)32/h4-9,11-13,31H,1-3,10H2,(H,29,33)(H,27,28,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 18: 2845-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.03.092
BindingDB Entry DOI: 10.7270/Q2611169 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50328717

(5-Chloro-N-(5-chloro-pyridin-2-yl)-3-methoxy-2-[4-...)Show SMILES COc1cc(Cl)cc(C(=O)Nc2ccc(Cl)cn2)c1NC(=O)c1ccc(cc1)N1CCCCC1=O Show InChI InChI=1S/C25H22Cl2N4O4/c1-35-20-13-17(27)12-19(25(34)29-21-10-7-16(26)14-28-21)23(20)30-24(33)15-5-8-18(9-6-15)31-11-3-2-4-22(31)32/h5-10,12-14H,2-4,11H2,1H3,(H,30,33)(H,28,29,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0570 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 18: 2845-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.03.092
BindingDB Entry DOI: 10.7270/Q2611169 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50377638

(CHEMBL257400)Show SMILES Clc1ccc(NC(=O)c2cc(Cl)ccc2NC(=O)c2ccc(cc2)-n2ccccc2=O)nc1 Show InChI InChI=1S/C24H16Cl2N4O3/c25-16-6-10-20(19(13-16)24(33)29-21-11-7-17(26)14-27-21)28-23(32)15-4-8-18(9-5-15)30-12-2-1-3-22(30)31/h1-14H,(H,28,32)(H,27,29,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 18: 2845-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.03.092
BindingDB Entry DOI: 10.7270/Q2611169 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50377628
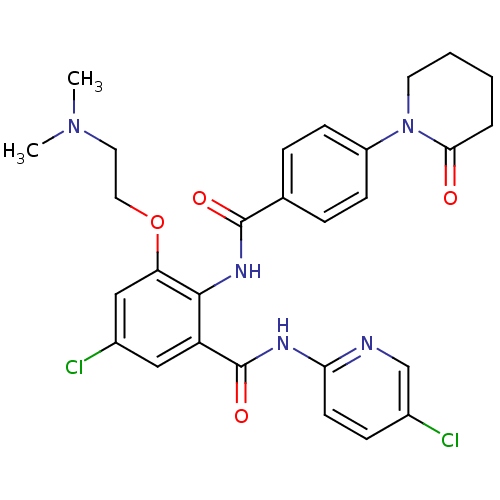
(CHEMBL261536)Show SMILES CN(C)CCOc1cc(Cl)cc(C(=O)Nc2ccc(Cl)cn2)c1NC(=O)c1ccc(cc1)N1CCCCC1=O Show InChI InChI=1S/C28H29Cl2N5O4/c1-34(2)13-14-39-23-16-20(30)15-22(28(38)32-24-11-8-19(29)17-31-24)26(23)33-27(37)18-6-9-21(10-7-18)35-12-4-3-5-25(35)36/h6-11,15-17H,3-5,12-14H2,1-2H3,(H,33,37)(H,31,32,38) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0650 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 18: 2845-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.03.092
BindingDB Entry DOI: 10.7270/Q2611169 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM19023

(1-(4-methoxyphenyl)-7-oxo-6-[4-(2-oxopiperidin-1-y...)Show SMILES COc1ccc(cc1)-n1nc(C(N)=O)c2CCN(C(=O)c12)c1ccc(cc1)N1CCCCC1=O Show InChI InChI=1S/C25H25N5O4/c1-34-19-11-9-18(10-12-19)30-23-20(22(27-30)24(26)32)13-15-29(25(23)33)17-7-5-16(6-8-17)28-14-3-2-4-21(28)31/h5-12H,2-4,13-15H2,1H3,(H2,26,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 18: 2845-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.03.092
BindingDB Entry DOI: 10.7270/Q2611169 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50377640
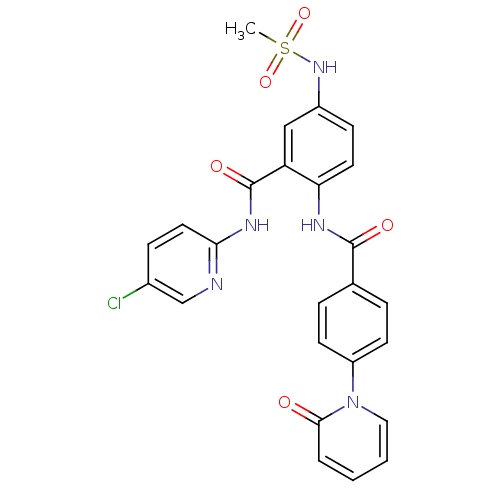
(CHEMBL258196)Show SMILES CS(=O)(=O)Nc1ccc(NC(=O)c2ccc(cc2)-n2ccccc2=O)c(c1)C(=O)Nc1ccc(Cl)cn1 Show InChI InChI=1S/C25H20ClN5O5S/c1-37(35,36)30-18-8-11-21(20(14-18)25(34)29-22-12-7-17(26)15-27-22)28-24(33)16-5-9-19(10-6-16)31-13-3-2-4-23(31)32/h2-15,30H,1H3,(H,28,33)(H,27,29,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 18: 2845-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.03.092
BindingDB Entry DOI: 10.7270/Q2611169 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50377656

(CHEMBL259534)Show SMILES Clc1ccc(NC(=O)c2ccccc2NC(=O)c2ccc(cc2)-n2ccccc2=O)nc1 Show InChI InChI=1S/C24H17ClN4O3/c25-17-10-13-21(26-15-17)28-24(32)19-5-1-2-6-20(19)27-23(31)16-8-11-18(12-9-16)29-14-4-3-7-22(29)30/h1-15H,(H,27,31)(H,26,28,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 18: 2845-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.03.092
BindingDB Entry DOI: 10.7270/Q2611169 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50377631

(CHEMBL256152)Show SMILES Clc1ccc(NC(=O)c2cc(Br)ccc2NC(=O)c2ccc(cc2)N2CCCCC2=O)nc1 Show InChI InChI=1S/C24H20BrClN4O3/c25-16-6-10-20(19(13-16)24(33)29-21-11-7-17(26)14-27-21)28-23(32)15-4-8-18(9-5-15)30-12-2-1-3-22(30)31/h4-11,13-14H,1-3,12H2,(H,28,32)(H,27,29,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 18: 2845-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.03.092
BindingDB Entry DOI: 10.7270/Q2611169 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50377636
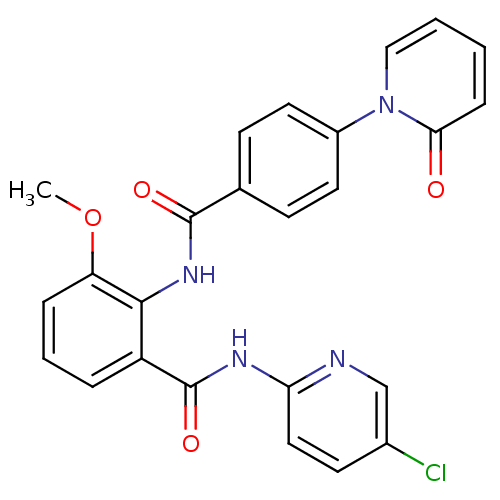
(CHEMBL257399)Show SMILES COc1cccc(C(=O)Nc2ccc(Cl)cn2)c1NC(=O)c1ccc(cc1)-n1ccccc1=O Show InChI InChI=1S/C25H19ClN4O4/c1-34-20-6-4-5-19(25(33)28-21-13-10-17(26)15-27-21)23(20)29-24(32)16-8-11-18(12-9-16)30-14-3-2-7-22(30)31/h2-15H,1H3,(H,29,32)(H,27,28,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 18: 2845-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.03.092
BindingDB Entry DOI: 10.7270/Q2611169 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50377630
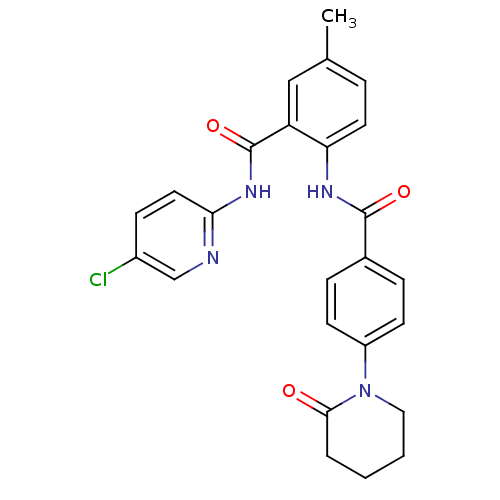
(CHEMBL404448)Show SMILES Cc1ccc(NC(=O)c2ccc(cc2)N2CCCCC2=O)c(c1)C(=O)Nc1ccc(Cl)cn1 Show InChI InChI=1S/C25H23ClN4O3/c1-16-5-11-21(20(14-16)25(33)29-22-12-8-18(26)15-27-22)28-24(32)17-6-9-19(10-7-17)30-13-3-2-4-23(30)31/h5-12,14-15H,2-4,13H2,1H3,(H,28,32)(H,27,29,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 18: 2845-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.03.092
BindingDB Entry DOI: 10.7270/Q2611169 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50377643
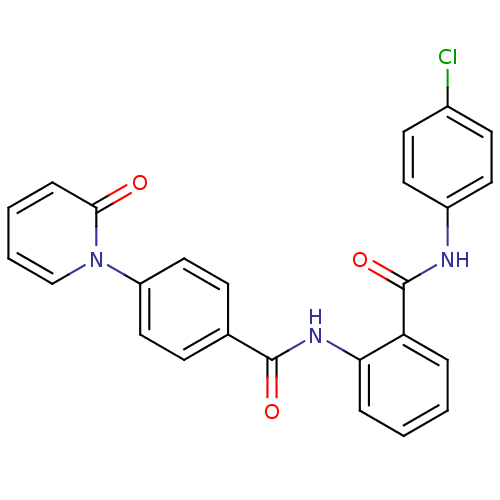
(CHEMBL257966)Show SMILES Clc1ccc(NC(=O)c2ccccc2NC(=O)c2ccc(cc2)-n2ccccc2=O)cc1 Show InChI InChI=1S/C25H18ClN3O3/c26-18-10-12-19(13-11-18)27-25(32)21-5-1-2-6-22(21)28-24(31)17-8-14-20(15-9-17)29-16-4-3-7-23(29)30/h1-16H,(H,27,32)(H,28,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 18: 2845-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.03.092
BindingDB Entry DOI: 10.7270/Q2611169 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50377627

(CHEMBL260369)Show SMILES COc1cc(Cl)cc(C(=O)Nc2ccc(Cl)cn2)c1NC(=O)c1ccc(nc1)-n1ccccc1=O Show InChI InChI=1S/C24H17Cl2N5O4/c1-35-18-11-16(26)10-17(24(34)29-19-7-6-15(25)13-27-19)22(18)30-23(33)14-5-8-20(28-12-14)31-9-3-2-4-21(31)32/h2-13H,1H3,(H,30,33)(H,27,29,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 18: 2845-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.03.092
BindingDB Entry DOI: 10.7270/Q2611169 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50377632
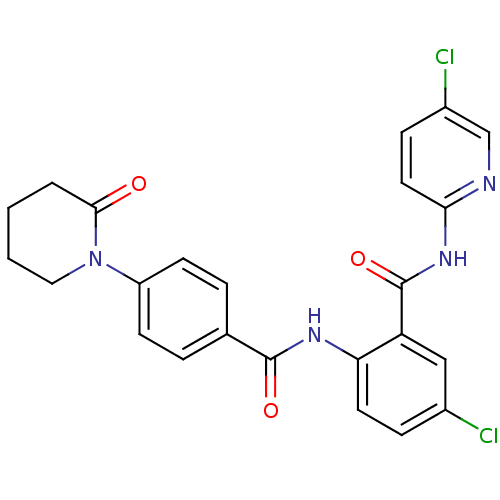
(CHEMBL404449)Show SMILES Clc1ccc(NC(=O)c2cc(Cl)ccc2NC(=O)c2ccc(cc2)N2CCCCC2=O)nc1 Show InChI InChI=1S/C24H20Cl2N4O3/c25-16-6-10-20(19(13-16)24(33)29-21-11-7-17(26)14-27-21)28-23(32)15-4-8-18(9-5-15)30-12-2-1-3-22(30)31/h4-11,13-14H,1-3,12H2,(H,28,32)(H,27,29,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 18: 2845-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.03.092
BindingDB Entry DOI: 10.7270/Q2611169 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50377633

(CHEMBL403075)Show SMILES Clc1ccc(NC(=O)c2cc(ccc2NC(=O)c2ccc(cc2)N2CCCCC2=O)C#N)nc1 Show InChI InChI=1S/C25H20ClN5O3/c26-18-7-11-22(28-15-18)30-25(34)20-13-16(14-27)4-10-21(20)29-24(33)17-5-8-19(9-6-17)31-12-2-1-3-23(31)32/h4-11,13,15H,1-3,12H2,(H,29,33)(H,28,30,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 18: 2845-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.03.092
BindingDB Entry DOI: 10.7270/Q2611169 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50377646
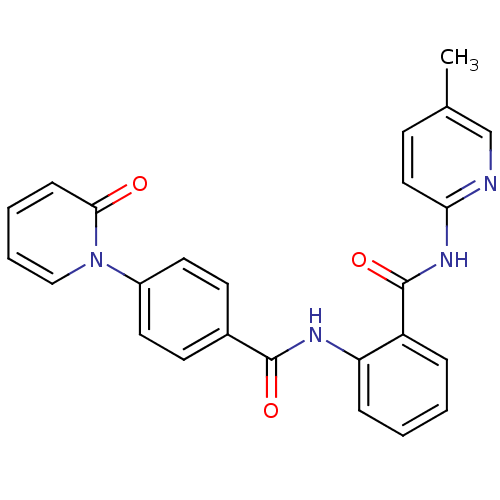
(CHEMBL259535)Show SMILES Cc1ccc(NC(=O)c2ccccc2NC(=O)c2ccc(cc2)-n2ccccc2=O)nc1 Show InChI InChI=1S/C25H20N4O3/c1-17-9-14-22(26-16-17)28-25(32)20-6-2-3-7-21(20)27-24(31)18-10-12-19(13-11-18)29-15-5-4-8-23(29)30/h2-16H,1H3,(H,27,31)(H,26,28,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.580 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 18: 2845-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.03.092
BindingDB Entry DOI: 10.7270/Q2611169 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50377641

(CHEMBL402836)Show SMILES Clc1ccc(NCc2ccccc2NC(=O)c2ccc(cc2)-n2ccccc2=O)nc1 Show InChI InChI=1S/C24H19ClN4O2/c25-19-10-13-22(27-16-19)26-15-18-5-1-2-6-21(18)28-24(31)17-8-11-20(12-9-17)29-14-4-3-7-23(29)30/h1-14,16H,15H2,(H,26,27)(H,28,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.620 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 18: 2845-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.03.092
BindingDB Entry DOI: 10.7270/Q2611169 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50377647

(CHEMBL411044)Show SMILES Fc1ccc(NC(=O)c2ccccc2NC(=O)c2ccc(cc2)-n2ccccc2=O)nc1 Show InChI InChI=1S/C24H17FN4O3/c25-17-10-13-21(26-15-17)28-24(32)19-5-1-2-6-20(19)27-23(31)16-8-11-18(12-9-16)29-14-4-3-7-22(29)30/h1-15H,(H,27,31)(H,26,28,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 18: 2845-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.03.092
BindingDB Entry DOI: 10.7270/Q2611169 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50377652
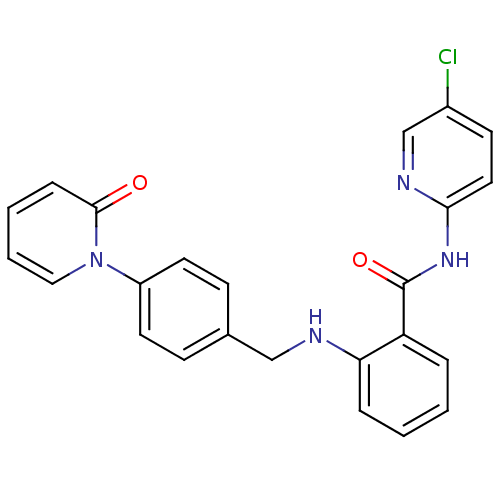
(CHEMBL256573)Show SMILES Clc1ccc(NC(=O)c2ccccc2NCc2ccc(cc2)-n2ccccc2=O)nc1 Show InChI InChI=1S/C24H19ClN4O2/c25-18-10-13-22(27-16-18)28-24(31)20-5-1-2-6-21(20)26-15-17-8-11-19(12-9-17)29-14-4-3-7-23(29)30/h1-14,16,26H,15H2,(H,27,28,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 18: 2845-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.03.092
BindingDB Entry DOI: 10.7270/Q2611169 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50377650

(CHEMBL260354)Show SMILES Clc1ccc(NC(=O)c2ccccc2NC(=O)c2ccc(cc2)N2CCCCC2=O)nc1 Show InChI InChI=1S/C24H21ClN4O3/c25-17-10-13-21(26-15-17)28-24(32)19-5-1-2-6-20(19)27-23(31)16-8-11-18(12-9-16)29-14-4-3-7-22(29)30/h1-2,5-6,8-13,15H,3-4,7,14H2,(H,27,31)(H,26,28,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 18: 2845-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.03.092
BindingDB Entry DOI: 10.7270/Q2611169 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50377644

(CHEMBL1162978)Show SMILES Clc1cnc(NC(=O)c2ccccc2NC(=O)c2ccc(cc2)-n2ccccc2=O)nc1 Show InChI InChI=1S/C23H16ClN5O3/c24-16-13-25-23(26-14-16)28-22(32)18-5-1-2-6-19(18)27-21(31)15-8-10-17(11-9-15)29-12-4-3-7-20(29)30/h1-14H,(H,27,31)(H,25,26,28,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 18: 2845-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.03.092
BindingDB Entry DOI: 10.7270/Q2611169 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50377634

(CHEMBL428432)Show SMILES CS(=O)(=O)Nc1ccc(NC(=O)c2ccc(cc2)N2CCCCC2=O)c(c1)C(=O)Nc1ccc(Cl)cn1 Show InChI InChI=1S/C25H24ClN5O5S/c1-37(35,36)30-18-8-11-21(20(14-18)25(34)29-22-12-7-17(26)15-27-22)28-24(33)16-5-9-19(10-6-16)31-13-3-2-4-23(31)32/h5-12,14-15,30H,2-4,13H2,1H3,(H,28,33)(H,27,29,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 18: 2845-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.03.092
BindingDB Entry DOI: 10.7270/Q2611169 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50377639
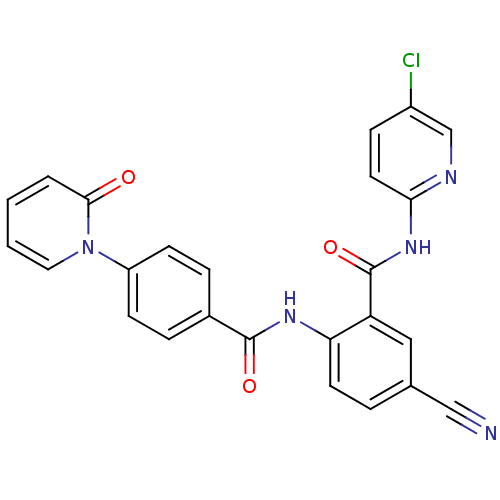
(CHEMBL258188)Show SMILES Clc1ccc(NC(=O)c2cc(ccc2NC(=O)c2ccc(cc2)-n2ccccc2=O)C#N)nc1 Show InChI InChI=1S/C25H16ClN5O3/c26-18-7-11-22(28-15-18)30-25(34)20-13-16(14-27)4-10-21(20)29-24(33)17-5-8-19(9-6-17)31-12-2-1-3-23(31)32/h1-13,15H,(H,29,33)(H,28,30,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 18: 2845-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.03.092
BindingDB Entry DOI: 10.7270/Q2611169 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50377649
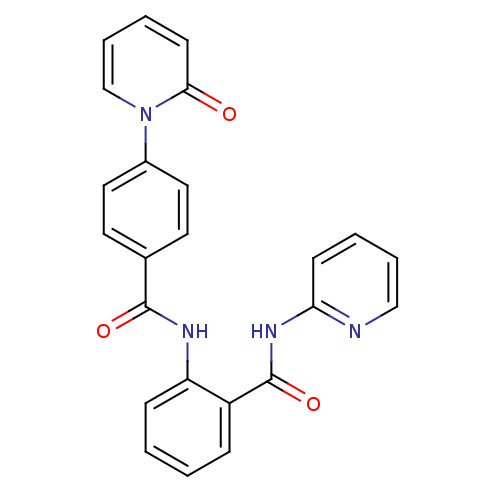
(CHEMBL260912)Show SMILES O=C(Nc1ccccc1C(=O)Nc1ccccn1)c1ccc(cc1)-n1ccccc1=O Show InChI InChI=1S/C24H18N4O3/c29-22-10-4-6-16-28(22)18-13-11-17(12-14-18)23(30)26-20-8-2-1-7-19(20)24(31)27-21-9-3-5-15-25-21/h1-16H,(H,26,30)(H,25,27,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 18: 2845-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.03.092
BindingDB Entry DOI: 10.7270/Q2611169 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50377642
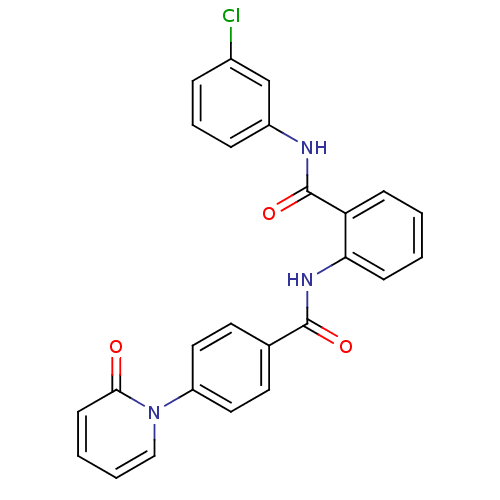
(CHEMBL257967)Show SMILES Clc1cccc(NC(=O)c2ccccc2NC(=O)c2ccc(cc2)-n2ccccc2=O)c1 Show InChI InChI=1S/C25H18ClN3O3/c26-18-6-5-7-19(16-18)27-25(32)21-8-1-2-9-22(21)28-24(31)17-11-13-20(14-12-17)29-15-4-3-10-23(29)30/h1-16H,(H,27,32)(H,28,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 18: 2845-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.03.092
BindingDB Entry DOI: 10.7270/Q2611169 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50377653
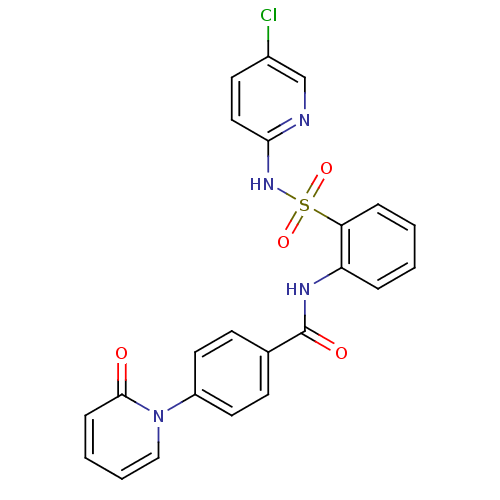
(CHEMBL402761)Show SMILES Clc1ccc(NS(=O)(=O)c2ccccc2NC(=O)c2ccc(cc2)-n2ccccc2=O)nc1 Show InChI InChI=1S/C23H17ClN4O4S/c24-17-10-13-21(25-15-17)27-33(31,32)20-6-2-1-5-19(20)26-23(30)16-8-11-18(12-9-16)28-14-4-3-7-22(28)29/h1-15H,(H,25,27)(H,26,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
| MMDB
PDB
Article
PubMed
| 5.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 18: 2845-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.03.092
BindingDB Entry DOI: 10.7270/Q2611169 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50377648
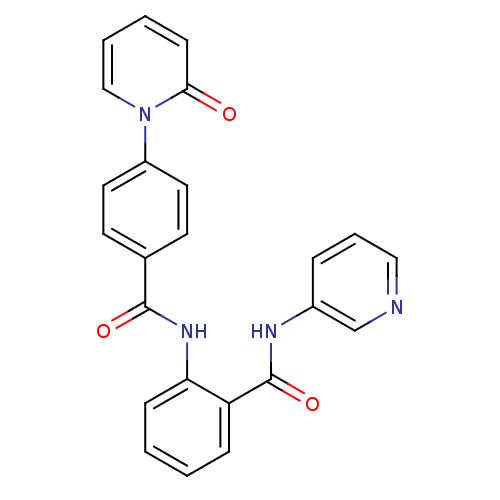
(CHEMBL260911)Show SMILES O=C(Nc1ccccc1C(=O)Nc1cccnc1)c1ccc(cc1)-n1ccccc1=O Show InChI InChI=1S/C24H18N4O3/c29-22-9-3-4-15-28(22)19-12-10-17(11-13-19)23(30)27-21-8-2-1-7-20(21)24(31)26-18-6-5-14-25-16-18/h1-16H,(H,26,31)(H,27,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 43 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 18: 2845-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.03.092
BindingDB Entry DOI: 10.7270/Q2611169 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50377654

(CHEMBL256574)Show SMILES CC(Nc1ccc(Cl)cn1)c1ccccc1NC(=O)c1ccc(cc1)-n1ccccc1=O |w:1.0| Show InChI InChI=1S/C25H21ClN4O2/c1-17(28-23-14-11-19(26)16-27-23)21-6-2-3-7-22(21)29-25(32)18-9-12-20(13-10-18)30-15-5-4-8-24(30)31/h2-17H,1H3,(H,27,28)(H,29,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 59 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 18: 2845-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.03.092
BindingDB Entry DOI: 10.7270/Q2611169 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50377645

(CHEMBL259533)Show SMILES FC(F)(F)c1ccc(NC(=O)c2ccccc2NC(=O)c2ccc(cc2)-n2ccccc2=O)nc1 Show InChI InChI=1S/C25H17F3N4O3/c26-25(27,28)17-10-13-21(29-15-17)31-24(35)19-5-1-2-6-20(19)30-23(34)16-8-11-18(12-9-16)32-14-4-3-7-22(32)33/h1-15H,(H,30,34)(H,29,31,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.56E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 18: 2845-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.03.092
BindingDB Entry DOI: 10.7270/Q2611169 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM50007296

(CHEMBL3238246)Show SMILES CC(C)(C)CC[C@@H](N1C(=O)C(=N[C@@]11CC[C@@H](CC1)C(C)(C)C)c1cc(Cl)cc(Cl)c1)c1ccc(cc1)C(=O)NCc1nn[nH]n1 |r,wU:6.5,15.19,wD:12.13,c:10,(14.39,-29.64,;15.92,-29.51,;16.8,-30.77,;15.33,-30.92,;16.51,-28.08,;15.58,-26.85,;16.18,-25.43,;15.24,-24.21,;15.69,-22.73,;17.14,-22.22,;14.42,-21.86,;13.2,-22.79,;13.71,-24.25,;12.17,-24.12,;11.29,-25.39,;11.95,-26.78,;13.48,-26.9,;14.36,-25.64,;11.08,-28.04,;9.54,-28.18,;11.96,-29.31,;10.19,-29.31,;14.39,-20.32,;15.7,-19.52,;15.67,-17.98,;16.98,-17.18,;14.32,-17.24,;13,-18.03,;11.65,-17.3,;13.04,-19.57,;17.71,-25.32,;18.39,-23.94,;19.92,-23.82,;20.79,-25.1,;20.12,-26.48,;18.58,-26.59,;22.32,-24.98,;23.19,-26.25,;22.99,-23.59,;24.53,-23.47,;25.2,-22.08,;26.72,-21.81,;26.92,-20.29,;25.53,-19.61,;24.47,-20.73,)| Show InChI InChI=1S/C34H43Cl2N7O2/c1-32(2,3)14-13-27(21-7-9-22(10-8-21)30(44)37-20-28-39-41-42-40-28)43-31(45)29(23-17-25(35)19-26(36)18-23)38-34(43)15-11-24(12-16-34)33(4,5)6/h7-10,17-19,24,27H,11-16,20H2,1-6H3,(H,37,44)(H,39,40,41,42)/t24-,27-,34-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.43E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to adenosine A3 receptor (unknown origin) by agonist displacement assay |
J Med Chem 57: 2601-10 (2014)
Article DOI: 10.1021/jm401858f
BindingDB Entry DOI: 10.7270/Q20V8F9P |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50377651

(CHEMBL404745)Show SMILES Clc1ccc(NC(=O)c2ccccc2NS(=O)(=O)c2ccc(cc2)-n2ccccc2=O)nc1 Show InChI InChI=1S/C23H17ClN4O4S/c24-16-8-13-21(25-15-16)26-23(30)19-5-1-2-6-20(19)27-33(31,32)18-11-9-17(10-12-18)28-14-4-3-7-22(28)29/h1-15,27H,(H,25,26,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 7.67E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 18: 2845-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.03.092
BindingDB Entry DOI: 10.7270/Q2611169 |
More data for this
Ligand-Target Pair | |
Lymphokine-activated killer T-cell-originated protein kinase
(Homo sapiens (Human)) | BDBM148993

(US8962648, 72 | US8962648, 73)Show InChI InChI=1S/C18H14N2O2S/c19-9-10-1-3-11(4-2-10)15-14(21)6-5-13-16(15)12-7-8-23-17(12)18(22)20-13/h1-8,21H,9,19H2,(H,20,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.380 | n/a | n/a | n/a | n/a | 7.4 | 25 |
OncoTherapy Science, Inc.
US Patent
| Assay Description
PBK activity was determined in the presence or absence of compounds using fluorescein isothiocyanate-labeled (FITC-labeled) histone H3 peptide as a s... |
US Patent US8962648 (2015)
BindingDB Entry DOI: 10.7270/Q27H1H9K |
More data for this
Ligand-Target Pair | |
Lymphokine-activated killer T-cell-originated protein kinase
(Homo sapiens (Human)) | BDBM149054

(US8962648, 319)Show SMILES CC(C)(N)c1ccc(cc1)-c1c(O)ccc2[nH]c(=O)c3sccc3c12 Show InChI InChI=1S/C20H18N2O2S/c1-20(2,21)12-5-3-11(4-6-12)16-15(23)8-7-14-17(16)13-9-10-25-18(13)19(24)22-14/h3-10,23H,21H2,1-2H3,(H,22,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.430 | n/a | n/a | n/a | n/a | 7.4 | 25 |
OncoTherapy Science, Inc.
US Patent
| Assay Description
PBK activity was determined in the presence or absence of compounds using fluorescein isothiocyanate-labeled (FITC-labeled) histone H3 peptide as a s... |
US Patent US8962648 (2015)
BindingDB Entry DOI: 10.7270/Q27H1H9K |
More data for this
Ligand-Target Pair | |
Lymphokine-activated killer T-cell-originated protein kinase
(Homo sapiens (Human)) | BDBM149042
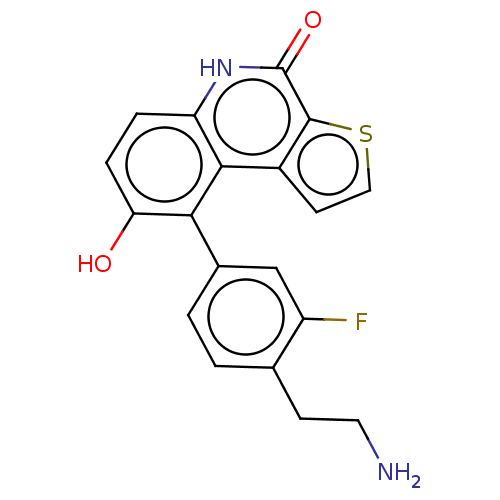
(US8962648, 276)Show SMILES NCCc1ccc(cc1F)-c1c(O)ccc2[nH]c(=O)c3sccc3c12 Show InChI InChI=1S/C19H15FN2O2S/c20-13-9-11(2-1-10(13)5-7-21)16-15(23)4-3-14-17(16)12-6-8-25-18(12)19(24)22-14/h1-4,6,8-9,23H,5,7,21H2,(H,22,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | 7.4 | 25 |
OncoTherapy Science, Inc.
US Patent
| Assay Description
PBK activity was determined in the presence or absence of compounds using fluorescein isothiocyanate-labeled (FITC-labeled) histone H3 peptide as a s... |
US Patent US8962648 (2015)
BindingDB Entry DOI: 10.7270/Q27H1H9K |
More data for this
Ligand-Target Pair | |
Lymphokine-activated killer T-cell-originated protein kinase
(Homo sapiens (Human)) | BDBM149041
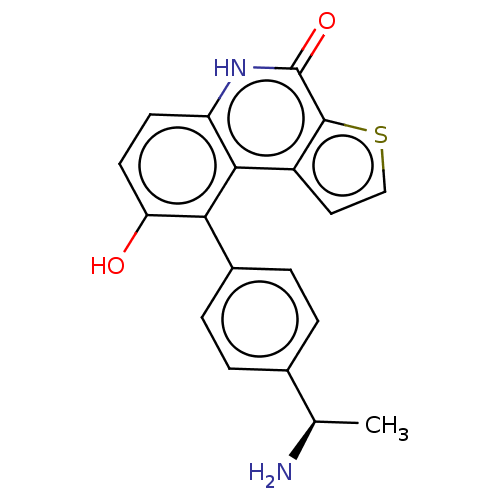
(US8962648, 275)Show SMILES C[C@@H](N)c1ccc(cc1)-c1c(O)ccc2[nH]c(=O)c3sccc3c12 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.540 | n/a | n/a | n/a | n/a | 7.4 | 25 |
OncoTherapy Science, Inc.
US Patent
| Assay Description
PBK activity was determined in the presence or absence of compounds using fluorescein isothiocyanate-labeled (FITC-labeled) histone H3 peptide as a s... |
US Patent US8962648 (2015)
BindingDB Entry DOI: 10.7270/Q27H1H9K |
More data for this
Ligand-Target Pair | |
Lymphokine-activated killer T-cell-originated protein kinase
(Homo sapiens (Human)) | BDBM149027

(US8962648, 235)Show SMILES NCC1CCN(Cc2ccc(cc2F)-c2c(O)ccc3[nH]c(=O)c4sccc4c23)CC1 Show InChI InChI=1S/C24H24FN3O2S/c25-18-11-15(1-2-16(18)13-28-8-5-14(12-26)6-9-28)21-20(29)4-3-19-22(21)17-7-10-31-23(17)24(30)27-19/h1-4,7,10-11,14,29H,5-6,8-9,12-13,26H2,(H,27,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.570 | n/a | n/a | n/a | n/a | 7.4 | 25 |
OncoTherapy Science, Inc.
US Patent
| Assay Description
PBK activity was determined in the presence or absence of compounds using fluorescein isothiocyanate-labeled (FITC-labeled) histone H3 peptide as a s... |
US Patent US8962648 (2015)
BindingDB Entry DOI: 10.7270/Q27H1H9K |
More data for this
Ligand-Target Pair | |
Lymphokine-activated killer T-cell-originated protein kinase
(Homo sapiens (Human)) | BDBM148993

(US8962648, 72 | US8962648, 73)Show InChI InChI=1S/C18H14N2O2S/c19-9-10-1-3-11(4-2-10)15-14(21)6-5-13-16(15)12-7-8-23-17(12)18(22)20-13/h1-8,21H,9,19H2,(H,20,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.630 | n/a | n/a | n/a | n/a | 7.4 | 25 |
OncoTherapy Science, Inc.
US Patent
| Assay Description
PBK activity was determined in the presence or absence of compounds using fluorescein isothiocyanate-labeled (FITC-labeled) histone H3 peptide as a s... |
US Patent US8962648 (2015)
BindingDB Entry DOI: 10.7270/Q27H1H9K |
More data for this
Ligand-Target Pair | |
Lymphokine-activated killer T-cell-originated protein kinase
(Homo sapiens (Human)) | BDBM149118

(US8962648, 1160)Show SMILES C[C@@H](CN)c1ccc(cc1)-c1c(O)ccc2[nH]c(=O)c3sccc3c12 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.640 | n/a | n/a | n/a | n/a | 7.4 | 25 |
OncoTherapy Science, Inc.
US Patent
| Assay Description
PBK activity was determined in the presence or absence of compounds using fluorescein isothiocyanate-labeled (FITC-labeled) histone H3 peptide as a s... |
US Patent US8962648 (2015)
BindingDB Entry DOI: 10.7270/Q27H1H9K |
More data for this
Ligand-Target Pair | |
Lymphokine-activated killer T-cell-originated protein kinase
(Homo sapiens (Human)) | BDBM149043
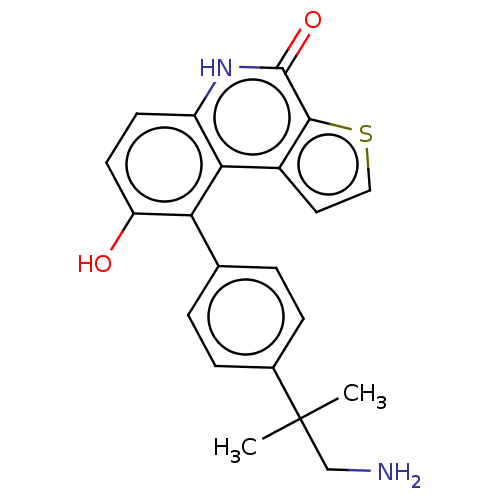
(US8962648, 277)Show SMILES CC(C)(CN)c1ccc(cc1)-c1c(O)ccc2[nH]c(=O)c3sccc3c12 Show InChI InChI=1S/C21H20N2O2S/c1-21(2,11-22)13-5-3-12(4-6-13)17-16(24)8-7-15-18(17)14-9-10-26-19(14)20(25)23-15/h3-10,24H,11,22H2,1-2H3,(H,23,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.670 | n/a | n/a | n/a | n/a | 7.4 | 25 |
OncoTherapy Science, Inc.
US Patent
| Assay Description
PBK activity was determined in the presence or absence of compounds using fluorescein isothiocyanate-labeled (FITC-labeled) histone H3 peptide as a s... |
US Patent US8962648 (2015)
BindingDB Entry DOI: 10.7270/Q27H1H9K |
More data for this
Ligand-Target Pair | |
Lymphokine-activated killer T-cell-originated protein kinase
(Homo sapiens (Human)) | BDBM149016
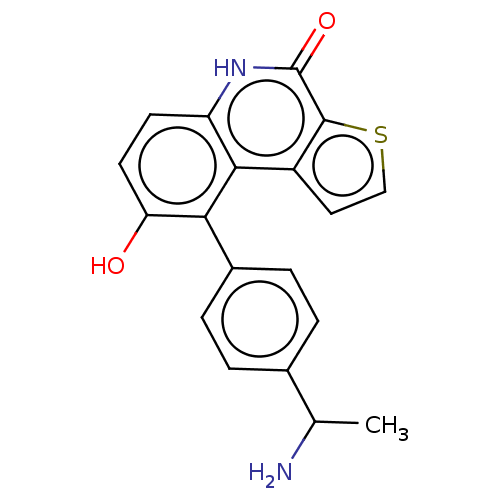
(US8962648, 195)Show SMILES CC(N)c1ccc(cc1)-c1c(O)ccc2[nH]c(=O)c3sccc3c12 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.730 | n/a | n/a | n/a | n/a | 7.4 | 25 |
OncoTherapy Science, Inc.
US Patent
| Assay Description
PBK activity was determined in the presence or absence of compounds using fluorescein isothiocyanate-labeled (FITC-labeled) histone H3 peptide as a s... |
US Patent US8962648 (2015)
BindingDB Entry DOI: 10.7270/Q27H1H9K |
More data for this
Ligand-Target Pair | |
Lymphokine-activated killer T-cell-originated protein kinase
(Homo sapiens (Human)) | BDBM149192
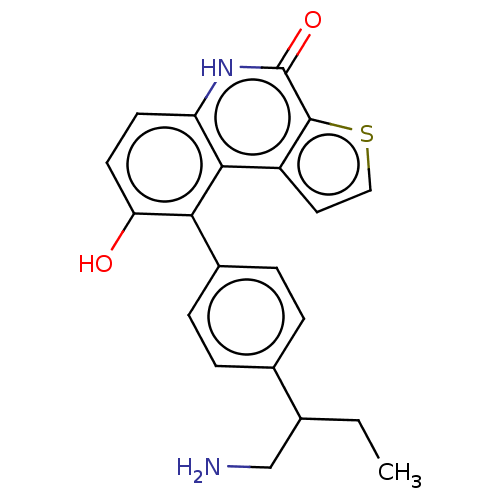
(US8962648, 1309)Show SMILES CCC(CN)c1ccc(cc1)-c1c(O)ccc2[nH]c(=O)c3sccc3c12 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.740 | n/a | n/a | n/a | n/a | 7.4 | 25 |
OncoTherapy Science, Inc.
US Patent
| Assay Description
PBK activity was determined in the presence or absence of compounds using fluorescein isothiocyanate-labeled (FITC-labeled) histone H3 peptide as a s... |
US Patent US8962648 (2015)
BindingDB Entry DOI: 10.7270/Q27H1H9K |
More data for this
Ligand-Target Pair | |
Lymphokine-activated killer T-cell-originated protein kinase
(Homo sapiens (Human)) | BDBM149039
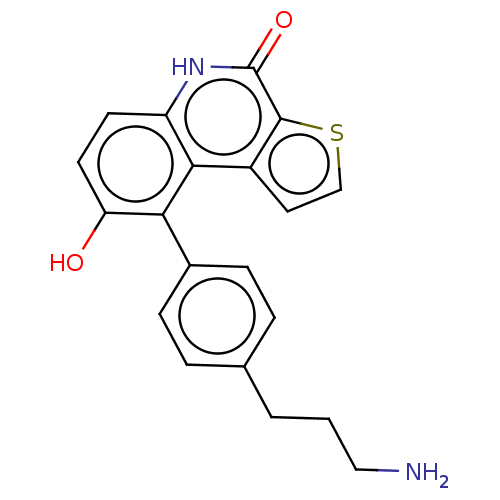
(US8962648, 273)Show SMILES NCCCc1ccc(cc1)-c1c(O)ccc2[nH]c(=O)c3sccc3c12 Show InChI InChI=1S/C20H18N2O2S/c21-10-1-2-12-3-5-13(6-4-12)17-16(23)8-7-15-18(17)14-9-11-25-19(14)20(24)22-15/h3-9,11,23H,1-2,10,21H2,(H,22,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.740 | n/a | n/a | n/a | n/a | 7.4 | 25 |
OncoTherapy Science, Inc.
US Patent
| Assay Description
PBK activity was determined in the presence or absence of compounds using fluorescein isothiocyanate-labeled (FITC-labeled) histone H3 peptide as a s... |
US Patent US8962648 (2015)
BindingDB Entry DOI: 10.7270/Q27H1H9K |
More data for this
Ligand-Target Pair | |
Lymphokine-activated killer T-cell-originated protein kinase
(Homo sapiens (Human)) | BDBM148997
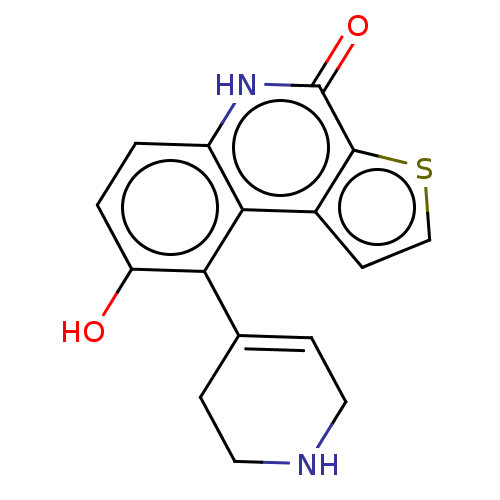
(US8962648, 84)Show SMILES Oc1ccc2[nH]c(=O)c3sccc3c2c1C1=CCNCC1 |t:18| Show InChI InChI=1S/C16H14N2O2S/c19-12-2-1-11-14(13(12)9-3-6-17-7-4-9)10-5-8-21-15(10)16(20)18-11/h1-3,5,8,17,19H,4,6-7H2,(H,18,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.780 | n/a | n/a | n/a | n/a | 7.4 | 25 |
OncoTherapy Science, Inc.
US Patent
| Assay Description
PBK activity was determined in the presence or absence of compounds using fluorescein isothiocyanate-labeled (FITC-labeled) histone H3 peptide as a s... |
US Patent US8962648 (2015)
BindingDB Entry DOI: 10.7270/Q27H1H9K |
More data for this
Ligand-Target Pair | |
Lymphokine-activated killer T-cell-originated protein kinase
(Homo sapiens (Human)) | BDBM149034
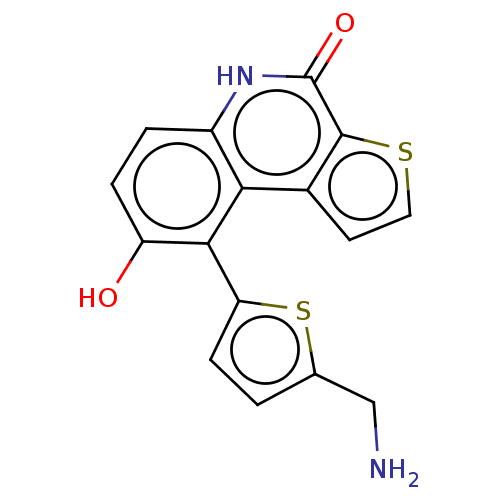
(US8962648, 266)Show InChI InChI=1S/C16H12N2O2S2/c17-7-8-1-4-12(22-8)14-11(19)3-2-10-13(14)9-5-6-21-15(9)16(20)18-10/h1-6,19H,7,17H2,(H,18,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.780 | n/a | n/a | n/a | n/a | 7.4 | 25 |
OncoTherapy Science, Inc.
US Patent
| Assay Description
PBK activity was determined in the presence or absence of compounds using fluorescein isothiocyanate-labeled (FITC-labeled) histone H3 peptide as a s... |
US Patent US8962648 (2015)
BindingDB Entry DOI: 10.7270/Q27H1H9K |
More data for this
Ligand-Target Pair | |
Lymphokine-activated killer T-cell-originated protein kinase
(Homo sapiens (Human)) | BDBM149026
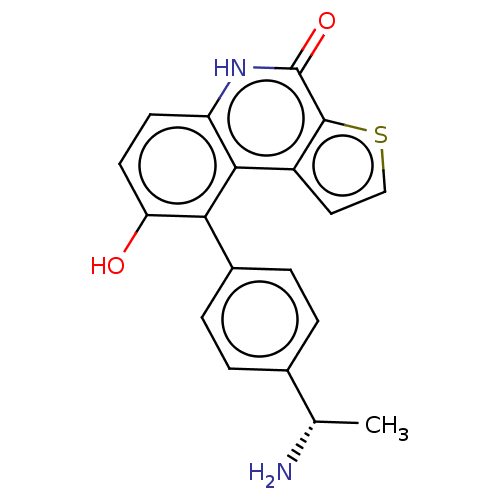
(US8962648, 233)Show SMILES C[C@H](N)c1ccc(cc1)-c1c(O)ccc2[nH]c(=O)c3sccc3c12 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.810 | n/a | n/a | n/a | n/a | 7.4 | 25 |
OncoTherapy Science, Inc.
US Patent
| Assay Description
PBK activity was determined in the presence or absence of compounds using fluorescein isothiocyanate-labeled (FITC-labeled) histone H3 peptide as a s... |
US Patent US8962648 (2015)
BindingDB Entry DOI: 10.7270/Q27H1H9K |
More data for this
Ligand-Target Pair | |
Lymphokine-activated killer T-cell-originated protein kinase
(Homo sapiens (Human)) | BDBM149062
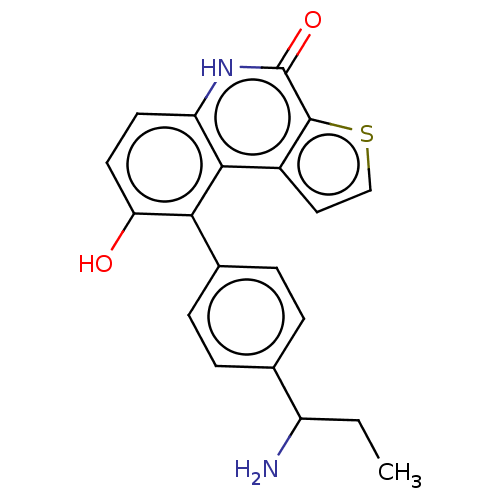
(US8962648, 336)Show SMILES CCC(N)c1ccc(cc1)-c1c(O)ccc2[nH]c(=O)c3sccc3c12 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.820 | n/a | n/a | n/a | n/a | 7.4 | 25 |
OncoTherapy Science, Inc.
US Patent
| Assay Description
PBK activity was determined in the presence or absence of compounds using fluorescein isothiocyanate-labeled (FITC-labeled) histone H3 peptide as a s... |
US Patent US8962648 (2015)
BindingDB Entry DOI: 10.7270/Q27H1H9K |
More data for this
Ligand-Target Pair | |
Lymphokine-activated killer T-cell-originated protein kinase
(Homo sapiens (Human)) | BDBM149097
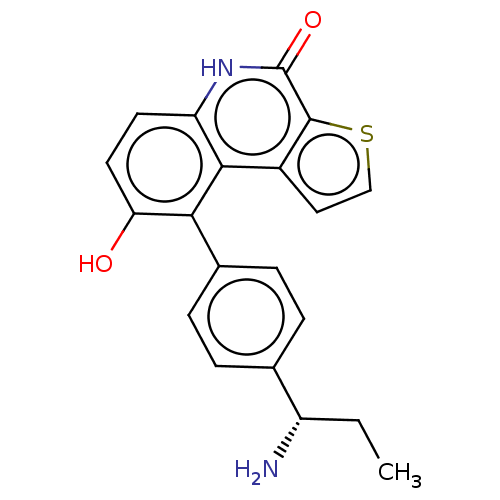
(US8962648, 1120)Show SMILES CC[C@H](N)c1ccc(cc1)-c1c(O)ccc2[nH]c(=O)c3sccc3c12 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.880 | n/a | n/a | n/a | n/a | 7.4 | 25 |
OncoTherapy Science, Inc.
US Patent
| Assay Description
PBK activity was determined in the presence or absence of compounds using fluorescein isothiocyanate-labeled (FITC-labeled) histone H3 peptide as a s... |
US Patent US8962648 (2015)
BindingDB Entry DOI: 10.7270/Q27H1H9K |
More data for this
Ligand-Target Pair | |
Lymphokine-activated killer T-cell-originated protein kinase
(Homo sapiens (Human)) | BDBM149038
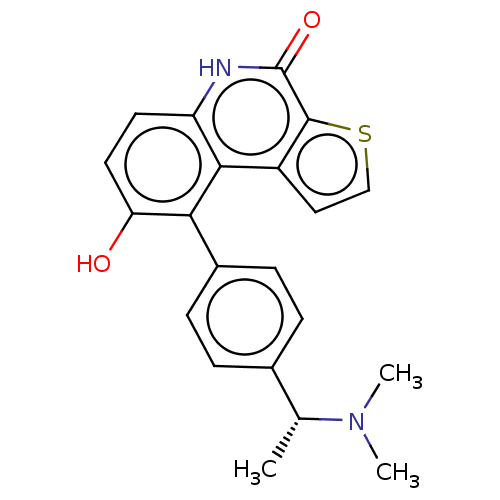
(US8962648, 272)Show SMILES C[C@@H](N(C)C)c1ccc(cc1)-c1c(O)ccc2[nH]c(=O)c3sccc3c12 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.880 | n/a | n/a | n/a | n/a | 7.4 | 25 |
OncoTherapy Science, Inc.
US Patent
| Assay Description
PBK activity was determined in the presence or absence of compounds using fluorescein isothiocyanate-labeled (FITC-labeled) histone H3 peptide as a s... |
US Patent US8962648 (2015)
BindingDB Entry DOI: 10.7270/Q27H1H9K |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data