

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| E3 ubiquitin-protein ligase Mdm2 (Homo sapiens (Human)) | BDBM31225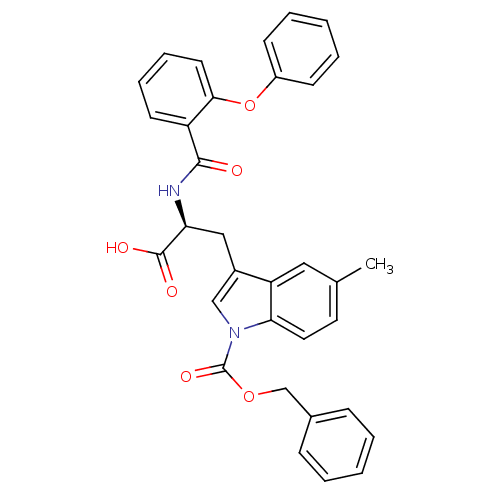 (2-phenoxybenzoyltryptophan derivative, R5C3) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 100 | -39.7 | n/a | n/a | n/a | n/a | n/a | 7.4 | 23 |
Schering-Plough Research Institute | Assay Description Ki values were determined from a competition experiment in which serial dilutions of inhibitor were added to compete against a fixed concentration (1... | Anal Biochem 331: 138-46 (2004) Article DOI: 10.1016/j.ab.2004.03.009 BindingDB Entry DOI: 10.7270/Q2VX0DVV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 (Homo sapiens (Human)) | BDBM31224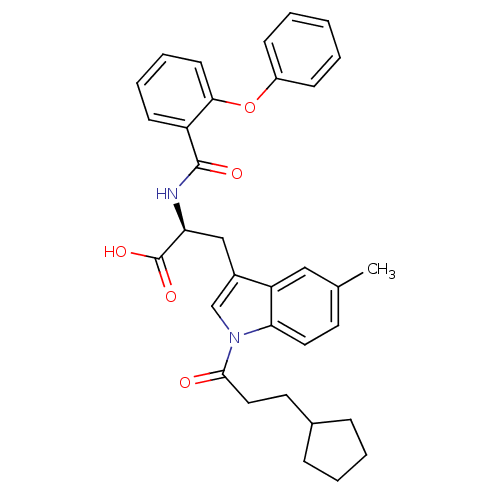 (2-phenoxybenzoyltryptophan derivative, R5C2) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 200 | -38.0 | n/a | n/a | n/a | n/a | n/a | 7.4 | 23 |
Schering-Plough Research Institute | Assay Description Ki values were determined from a competition experiment in which serial dilutions of inhibitor were added to compete against a fixed concentration (1... | Anal Biochem 331: 138-46 (2004) Article DOI: 10.1016/j.ab.2004.03.009 BindingDB Entry DOI: 10.7270/Q2VX0DVV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 (Homo sapiens (Human)) | BDBM31216 (2-phenoxybenzoyltryptophan derivative, R3C2) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 400 | -36.3 | n/a | n/a | n/a | n/a | n/a | 7.4 | 23 |
Schering-Plough Research Institute | Assay Description Ki values were determined from a competition experiment in which serial dilutions of inhibitor were added to compete against a fixed concentration (1... | Anal Biochem 331: 138-46 (2004) Article DOI: 10.1016/j.ab.2004.03.009 BindingDB Entry DOI: 10.7270/Q2VX0DVV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 (Homo sapiens (Human)) | BDBM31209 (2-phenoxybenzoyltryptophan derivative, R1C3) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 500 | -35.7 | n/a | n/a | n/a | n/a | n/a | 7.4 | 23 |
Schering-Plough Research Institute | Assay Description Ki values were determined from a competition experiment in which serial dilutions of inhibitor were added to compete against a fixed concentration (1... | Anal Biochem 331: 138-46 (2004) Article DOI: 10.1016/j.ab.2004.03.009 BindingDB Entry DOI: 10.7270/Q2VX0DVV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 (Homo sapiens (Human)) | BDBM31210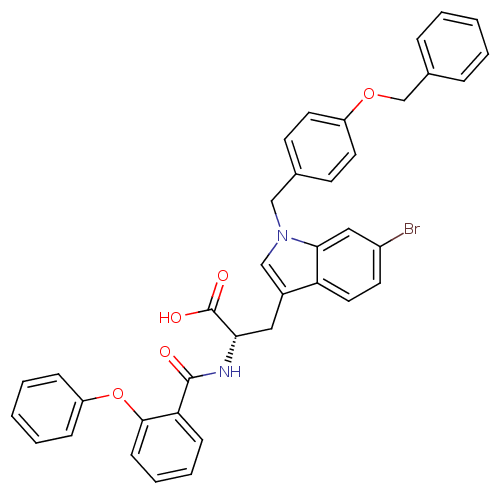 (2-phenoxybenzoyltryptophan derivative, R1C4) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 600 | -35.3 | n/a | n/a | n/a | n/a | n/a | 7.4 | 23 |
Schering-Plough Research Institute | Assay Description Ki values were determined from a competition experiment in which serial dilutions of inhibitor were added to compete against a fixed concentration (1... | Anal Biochem 331: 138-46 (2004) Article DOI: 10.1016/j.ab.2004.03.009 BindingDB Entry DOI: 10.7270/Q2VX0DVV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 (Homo sapiens (Human)) | BDBM31220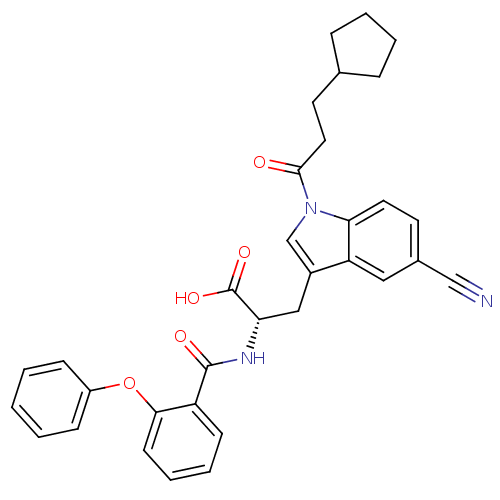 (2-phenoxybenzoyltryptophan derivative, R4C2) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 600 | -35.3 | n/a | n/a | n/a | n/a | n/a | 7.4 | 23 |
Schering-Plough Research Institute | Assay Description Ki values were determined from a competition experiment in which serial dilutions of inhibitor were added to compete against a fixed concentration (1... | Anal Biochem 331: 138-46 (2004) Article DOI: 10.1016/j.ab.2004.03.009 BindingDB Entry DOI: 10.7270/Q2VX0DVV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 (Homo sapiens (Human)) | BDBM31226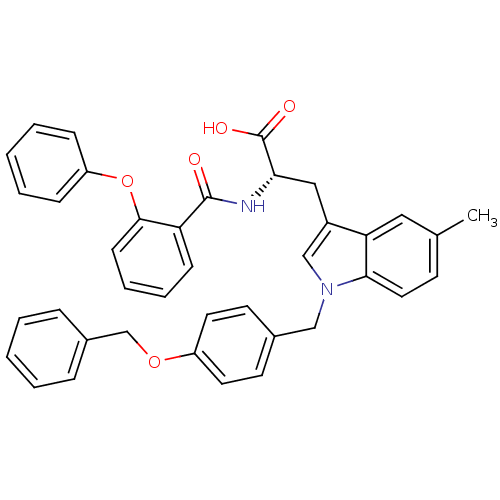 (2-phenoxybenzoyltryptophan derivative, R5C4) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 600 | -35.3 | n/a | n/a | n/a | n/a | n/a | 7.4 | 23 |
Schering-Plough Research Institute | Assay Description Ki values were determined from a competition experiment in which serial dilutions of inhibitor were added to compete against a fixed concentration (1... | Anal Biochem 331: 138-46 (2004) Article DOI: 10.1016/j.ab.2004.03.009 BindingDB Entry DOI: 10.7270/Q2VX0DVV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 (Homo sapiens (Human)) | BDBM31213 (2-phenoxybenzoyltryptophan derivative, R2C3) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 600 | -35.3 | n/a | n/a | n/a | n/a | n/a | 7.4 | 23 |
Schering-Plough Research Institute | Assay Description Ki values were determined from a competition experiment in which serial dilutions of inhibitor were added to compete against a fixed concentration (1... | Anal Biochem 331: 138-46 (2004) Article DOI: 10.1016/j.ab.2004.03.009 BindingDB Entry DOI: 10.7270/Q2VX0DVV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 (Homo sapiens (Human)) | BDBM31221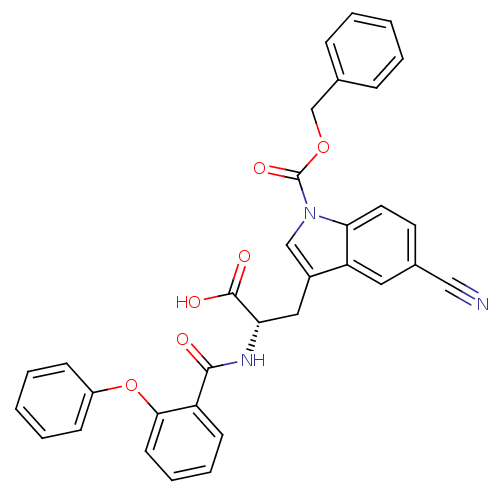 (2-phenoxybenzoyltryptophan derivative, R4C3) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 600 | -35.3 | n/a | n/a | n/a | n/a | n/a | 7.4 | 23 |
Schering-Plough Research Institute | Assay Description Ki values were determined from a competition experiment in which serial dilutions of inhibitor were added to compete against a fixed concentration (1... | Anal Biochem 331: 138-46 (2004) Article DOI: 10.1016/j.ab.2004.03.009 BindingDB Entry DOI: 10.7270/Q2VX0DVV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 (Homo sapiens (Human)) | BDBM31217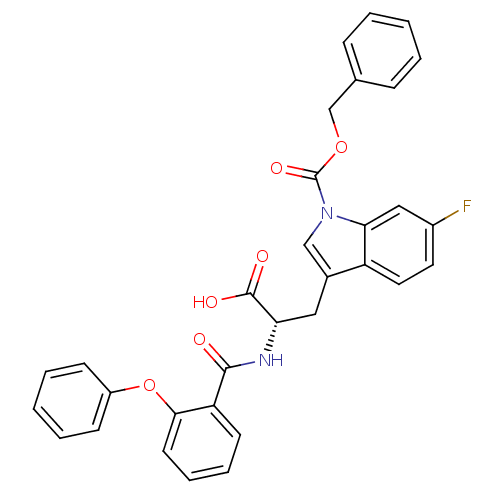 (2-phenoxybenzoyltryptophan derivative, R3C3) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 800 | -34.6 | n/a | n/a | n/a | n/a | n/a | 7.4 | 23 |
Schering-Plough Research Institute | Assay Description Ki values were determined from a competition experiment in which serial dilutions of inhibitor were added to compete against a fixed concentration (1... | Anal Biochem 331: 138-46 (2004) Article DOI: 10.1016/j.ab.2004.03.009 BindingDB Entry DOI: 10.7270/Q2VX0DVV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 (Homo sapiens (Human)) | BDBM31212 (2-phenoxybenzoyltryptophan derivative, R2C2) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 800 | -34.6 | n/a | n/a | n/a | n/a | n/a | 7.4 | 23 |
Schering-Plough Research Institute | Assay Description Ki values were determined from a competition experiment in which serial dilutions of inhibitor were added to compete against a fixed concentration (1... | Anal Biochem 331: 138-46 (2004) Article DOI: 10.1016/j.ab.2004.03.009 BindingDB Entry DOI: 10.7270/Q2VX0DVV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 (Homo sapiens (Human)) | BDBM31208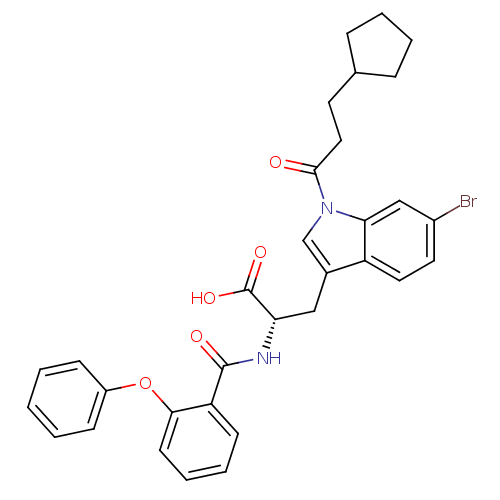 (2-phenoxybenzoyltryptophan derivative, R1C2) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 800 | -34.6 | n/a | n/a | n/a | n/a | n/a | 7.4 | 23 |
Schering-Plough Research Institute | Assay Description Ki values were determined from a competition experiment in which serial dilutions of inhibitor were added to compete against a fixed concentration (1... | Anal Biochem 331: 138-46 (2004) Article DOI: 10.1016/j.ab.2004.03.009 BindingDB Entry DOI: 10.7270/Q2VX0DVV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 (Homo sapiens (Human)) | BDBM31214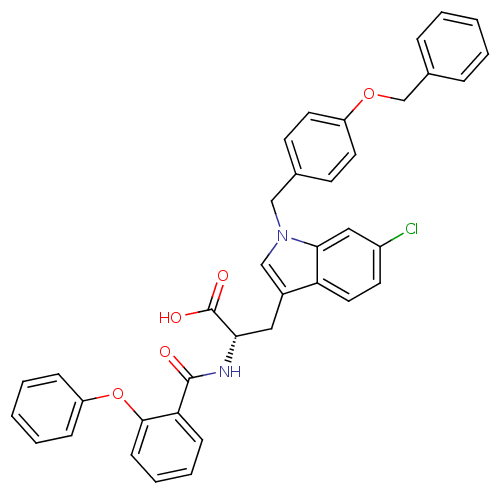 (2-phenoxybenzoyltryptophan derivative, R2C4) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.00E+3 | -34.0 | n/a | n/a | n/a | n/a | n/a | 7.4 | 23 |
Schering-Plough Research Institute | Assay Description Ki values were determined from a competition experiment in which serial dilutions of inhibitor were added to compete against a fixed concentration (1... | Anal Biochem 331: 138-46 (2004) Article DOI: 10.1016/j.ab.2004.03.009 BindingDB Entry DOI: 10.7270/Q2VX0DVV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 (Homo sapiens (Human)) | BDBM31222 (2-phenoxybenzoyltryptophan derivative, R4C4) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.00E+3 | -34.0 | n/a | n/a | n/a | n/a | n/a | 7.4 | 23 |
Schering-Plough Research Institute | Assay Description Ki values were determined from a competition experiment in which serial dilutions of inhibitor were added to compete against a fixed concentration (1... | Anal Biochem 331: 138-46 (2004) Article DOI: 10.1016/j.ab.2004.03.009 BindingDB Entry DOI: 10.7270/Q2VX0DVV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 (Homo sapiens (Human)) | BDBM31218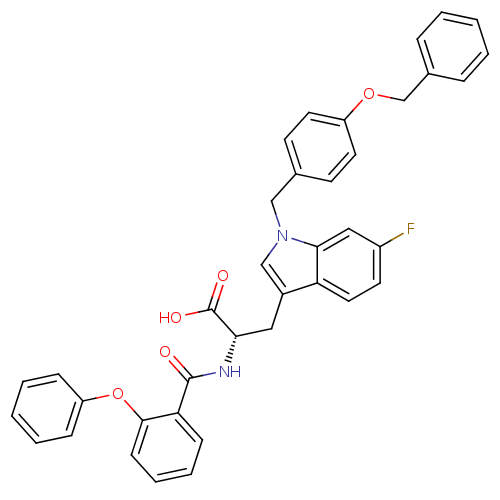 (2-phenoxybenzoyltryptophan derivative, R3C4) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 2.00E+3 | -32.3 | n/a | n/a | n/a | n/a | n/a | 7.4 | 23 |
Schering-Plough Research Institute | Assay Description Ki values were determined from a competition experiment in which serial dilutions of inhibitor were added to compete against a fixed concentration (1... | Anal Biochem 331: 138-46 (2004) Article DOI: 10.1016/j.ab.2004.03.009 BindingDB Entry DOI: 10.7270/Q2VX0DVV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 (Homo sapiens (Human)) | BDBM31207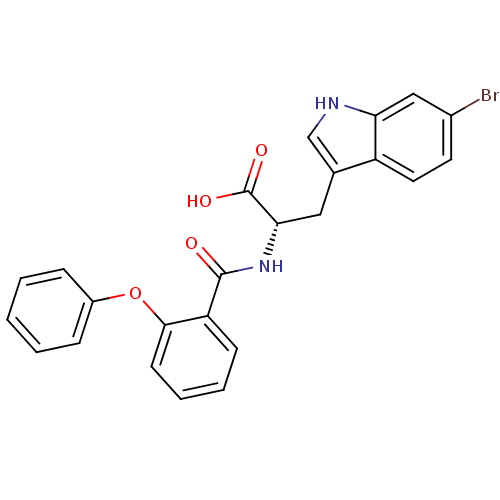 (2-phenoxybenzoyltryptophan derivative, R1C1) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 3.00E+3 | -31.3 | n/a | n/a | n/a | n/a | n/a | 7.4 | 23 |
Schering-Plough Research Institute | Assay Description Ki values were determined from a competition experiment in which serial dilutions of inhibitor were added to compete against a fixed concentration (1... | Anal Biochem 331: 138-46 (2004) Article DOI: 10.1016/j.ab.2004.03.009 BindingDB Entry DOI: 10.7270/Q2VX0DVV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 (Homo sapiens (Human)) | BDBM31211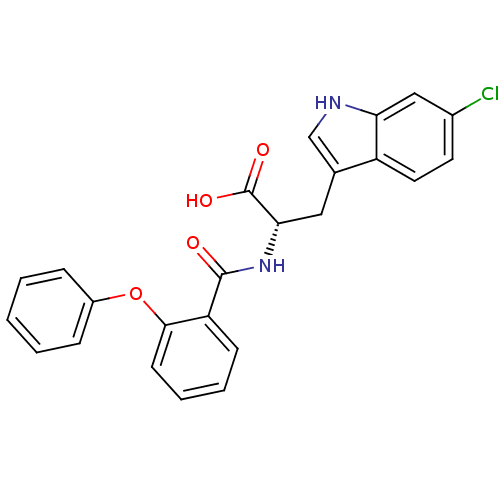 (2-phenoxybenzoyltryptophan derivative, R2C1) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 6.00E+3 | -29.6 | n/a | n/a | n/a | n/a | n/a | 7.4 | 23 |
Schering-Plough Research Institute | Assay Description Ki values were determined from a competition experiment in which serial dilutions of inhibitor were added to compete against a fixed concentration (1... | Anal Biochem 331: 138-46 (2004) Article DOI: 10.1016/j.ab.2004.03.009 BindingDB Entry DOI: 10.7270/Q2VX0DVV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 (Homo sapiens (Human)) | BDBM31219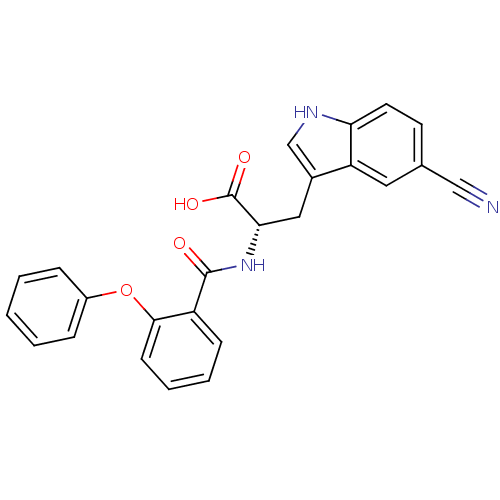 (2-phenoxybenzoyltryptophan derivative, R4C1) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.00E+4 | -28.3 | n/a | n/a | n/a | n/a | n/a | 7.4 | 23 |
Schering-Plough Research Institute | Assay Description Ki values were determined from a competition experiment in which serial dilutions of inhibitor were added to compete against a fixed concentration (1... | Anal Biochem 331: 138-46 (2004) Article DOI: 10.1016/j.ab.2004.03.009 BindingDB Entry DOI: 10.7270/Q2VX0DVV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 (Homo sapiens (Human)) | BDBM31223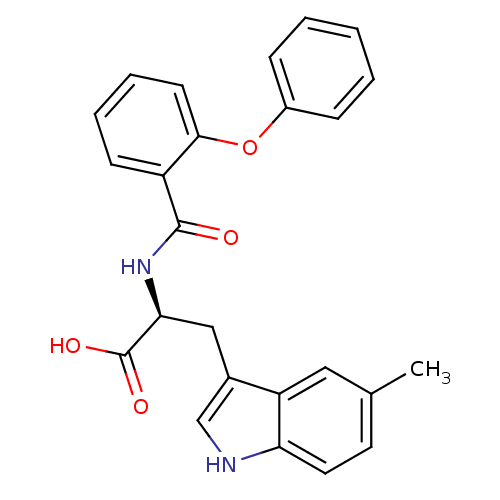 (2-phenoxybenzoyltryptophan derivative, R5C1) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.00E+4 | -28.3 | n/a | n/a | n/a | n/a | n/a | 7.4 | 23 |
Schering-Plough Research Institute | Assay Description Ki values were determined from a competition experiment in which serial dilutions of inhibitor were added to compete against a fixed concentration (1... | Anal Biochem 331: 138-46 (2004) Article DOI: 10.1016/j.ab.2004.03.009 BindingDB Entry DOI: 10.7270/Q2VX0DVV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 (Homo sapiens (Human)) | BDBM31215 (2-phenoxybenzoyltryptophan derivative, R3C1) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 2.00E+4 | -26.6 | n/a | n/a | n/a | n/a | n/a | 7.4 | 23 |
Schering-Plough Research Institute | Assay Description Ki values were determined from a competition experiment in which serial dilutions of inhibitor were added to compete against a fixed concentration (1... | Anal Biochem 331: 138-46 (2004) Article DOI: 10.1016/j.ab.2004.03.009 BindingDB Entry DOI: 10.7270/Q2VX0DVV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM13934 (Atazanavir | BMS 232632 | CGP 73547 | CHEMBL1163 |...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of wild-type HIV1 protease expressed in Escherichia coli assessed as reduction in product formation preincubated for 30 mins followed by a... | ACS Med Chem Lett 7: 702-7 (2016) Article DOI: 10.1021/acsmedchemlett.6b00135 BindingDB Entry DOI: 10.7270/Q28W3G74 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50190623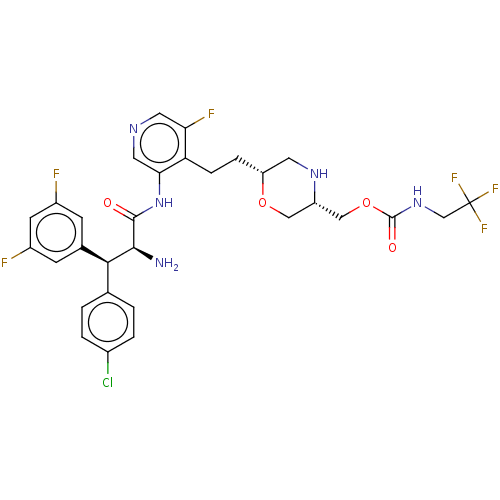 (CHEMBL3828743) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of wild-type HIV1 protease expressed in Escherichia coli assessed as reduction in product formation preincubated for 30 mins followed by a... | ACS Med Chem Lett 7: 702-7 (2016) Article DOI: 10.1021/acsmedchemlett.6b00135 BindingDB Entry DOI: 10.7270/Q28W3G74 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50190624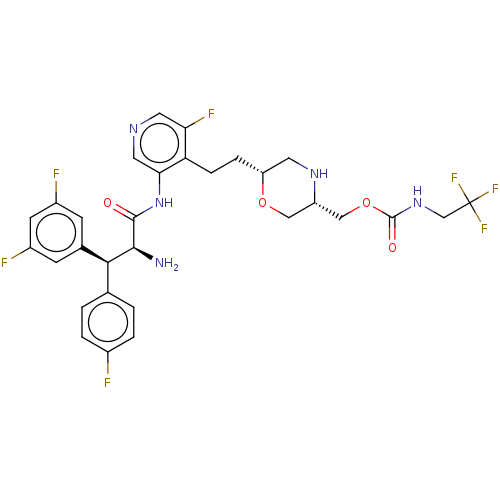 (CHEMBL3828417) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of wild-type HIV1 protease expressed in Escherichia coli assessed as reduction in product formation preincubated for 30 mins followed by a... | ACS Med Chem Lett 7: 702-7 (2016) Article DOI: 10.1021/acsmedchemlett.6b00135 BindingDB Entry DOI: 10.7270/Q28W3G74 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50190625 (CHEMBL3828552) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of wild-type HIV1 protease expressed in Escherichia coli assessed as reduction in product formation preincubated for 30 mins followed by a... | ACS Med Chem Lett 7: 702-7 (2016) Article DOI: 10.1021/acsmedchemlett.6b00135 BindingDB Entry DOI: 10.7270/Q28W3G74 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50190638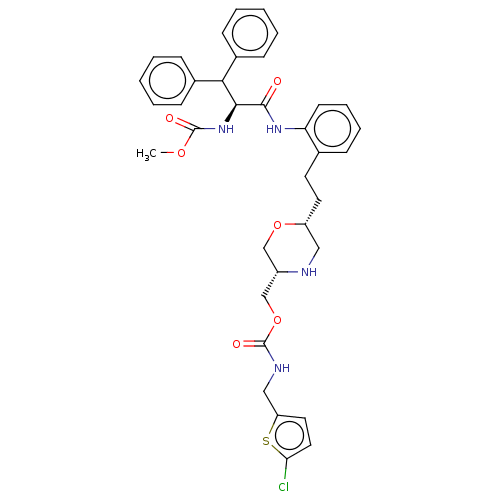 (CHEMBL3828119) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of wild-type HIV1 protease expressed in Escherichia coli assessed as reduction in product formation preincubated for 30 mins followed by a... | ACS Med Chem Lett 7: 702-7 (2016) Article DOI: 10.1021/acsmedchemlett.6b00135 BindingDB Entry DOI: 10.7270/Q28W3G74 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50190631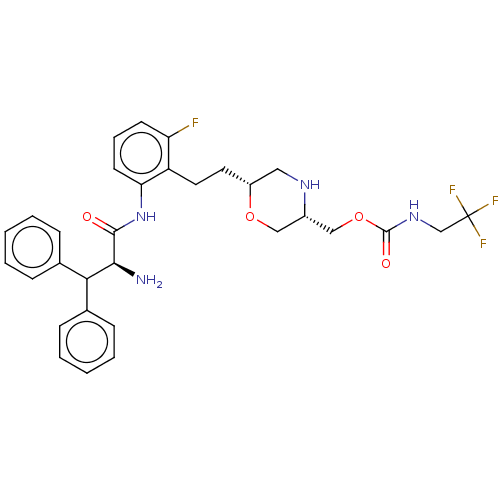 (CHEMBL3828166) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of wild-type HIV1 protease expressed in Escherichia coli assessed as reduction in product formation preincubated for 30 mins followed by a... | ACS Med Chem Lett 7: 702-7 (2016) Article DOI: 10.1021/acsmedchemlett.6b00135 BindingDB Entry DOI: 10.7270/Q28W3G74 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50190639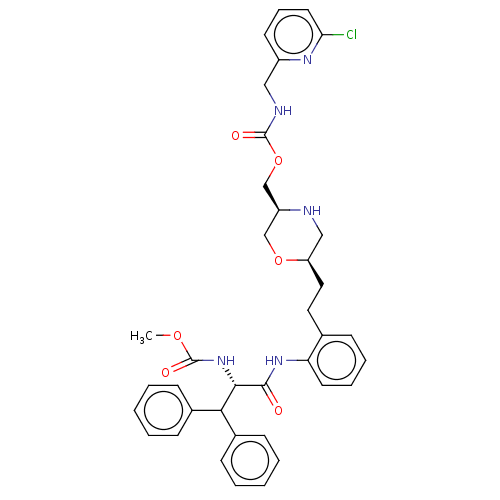 (CHEMBL3827524) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of wild-type HIV1 protease expressed in Escherichia coli assessed as reduction in product formation preincubated for 30 mins followed by a... | ACS Med Chem Lett 7: 702-7 (2016) Article DOI: 10.1021/acsmedchemlett.6b00135 BindingDB Entry DOI: 10.7270/Q28W3G74 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50190629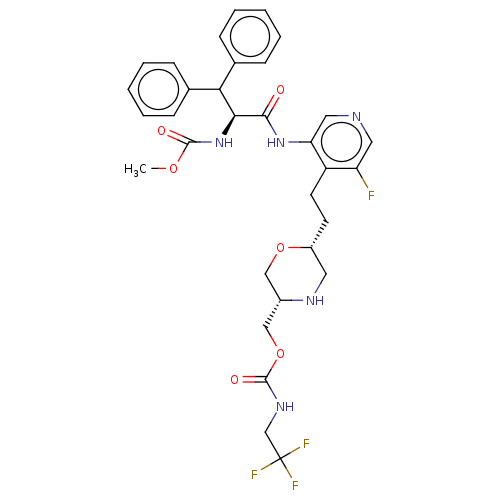 (CHEMBL3828678) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of wild-type HIV1 protease expressed in Escherichia coli assessed as reduction in product formation preincubated for 30 mins followed by a... | ACS Med Chem Lett 7: 702-7 (2016) Article DOI: 10.1021/acsmedchemlett.6b00135 BindingDB Entry DOI: 10.7270/Q28W3G74 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50190636 (CHEMBL3828275) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of wild-type HIV1 protease expressed in Escherichia coli assessed as reduction in product formation preincubated for 30 mins followed by a... | ACS Med Chem Lett 7: 702-7 (2016) Article DOI: 10.1021/acsmedchemlett.6b00135 BindingDB Entry DOI: 10.7270/Q28W3G74 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50190641 (CHEMBL3827205) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of wild-type HIV1 protease expressed in Escherichia coli assessed as reduction in product formation preincubated for 30 mins followed by a... | ACS Med Chem Lett 7: 702-7 (2016) Article DOI: 10.1021/acsmedchemlett.6b00135 BindingDB Entry DOI: 10.7270/Q28W3G74 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50190640 (CHEMBL3828355) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of wild-type HIV1 protease expressed in Escherichia coli assessed as reduction in product formation preincubated for 30 mins followed by a... | ACS Med Chem Lett 7: 702-7 (2016) Article DOI: 10.1021/acsmedchemlett.6b00135 BindingDB Entry DOI: 10.7270/Q28W3G74 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50190642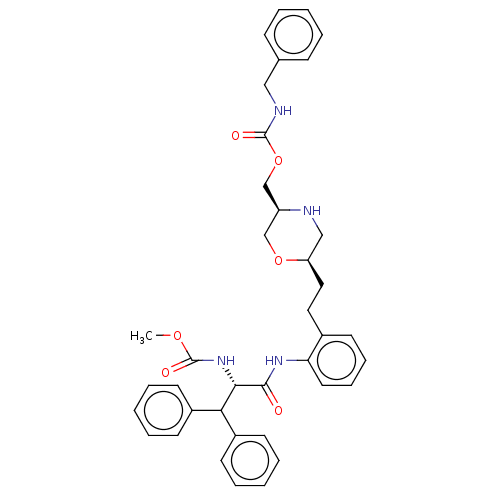 (CHEMBL3827353) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 6.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of wild-type HIV1 protease expressed in Escherichia coli assessed as reduction in product formation preincubated for 30 mins followed by a... | ACS Med Chem Lett 7: 702-7 (2016) Article DOI: 10.1021/acsmedchemlett.6b00135 BindingDB Entry DOI: 10.7270/Q28W3G74 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50190627 (CHEMBL3827958) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of wild-type HIV1 protease expressed in Escherichia coli assessed as reduction in product formation preincubated for 30 mins followed by a... | ACS Med Chem Lett 7: 702-7 (2016) Article DOI: 10.1021/acsmedchemlett.6b00135 BindingDB Entry DOI: 10.7270/Q28W3G74 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50190626 (CHEMBL3827319) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of wild-type HIV1 protease expressed in Escherichia coli assessed as reduction in product formation preincubated for 30 mins followed by a... | ACS Med Chem Lett 7: 702-7 (2016) Article DOI: 10.1021/acsmedchemlett.6b00135 BindingDB Entry DOI: 10.7270/Q28W3G74 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50190637 (CHEMBL3827348) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of wild-type HIV1 protease expressed in Escherichia coli assessed as reduction in product formation preincubated for 30 mins followed by a... | ACS Med Chem Lett 7: 702-7 (2016) Article DOI: 10.1021/acsmedchemlett.6b00135 BindingDB Entry DOI: 10.7270/Q28W3G74 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50190628 (CHEMBL3827975) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of wild-type HIV1 protease expressed in Escherichia coli assessed as reduction in product formation preincubated for 30 mins followed by a... | ACS Med Chem Lett 7: 702-7 (2016) Article DOI: 10.1021/acsmedchemlett.6b00135 BindingDB Entry DOI: 10.7270/Q28W3G74 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50190632 (CHEMBL3827450) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of wild-type HIV1 protease expressed in Escherichia coli assessed as reduction in product formation preincubated for 30 mins followed by a... | ACS Med Chem Lett 7: 702-7 (2016) Article DOI: 10.1021/acsmedchemlett.6b00135 BindingDB Entry DOI: 10.7270/Q28W3G74 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50190630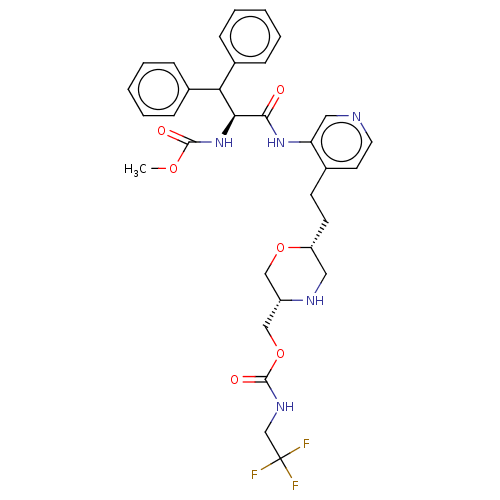 (CHEMBL3827960) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of wild-type HIV1 protease expressed in Escherichia coli assessed as reduction in product formation preincubated for 30 mins followed by a... | ACS Med Chem Lett 7: 702-7 (2016) Article DOI: 10.1021/acsmedchemlett.6b00135 BindingDB Entry DOI: 10.7270/Q28W3G74 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50190633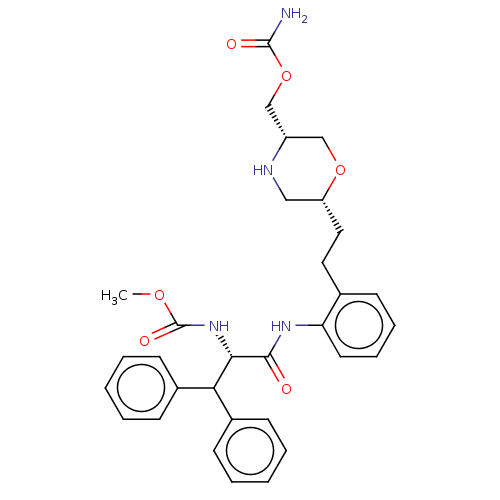 (CHEMBL3827407) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of wild-type HIV1 protease expressed in Escherichia coli assessed as reduction in product formation preincubated for 30 mins followed by a... | ACS Med Chem Lett 7: 702-7 (2016) Article DOI: 10.1021/acsmedchemlett.6b00135 BindingDB Entry DOI: 10.7270/Q28W3G74 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50190634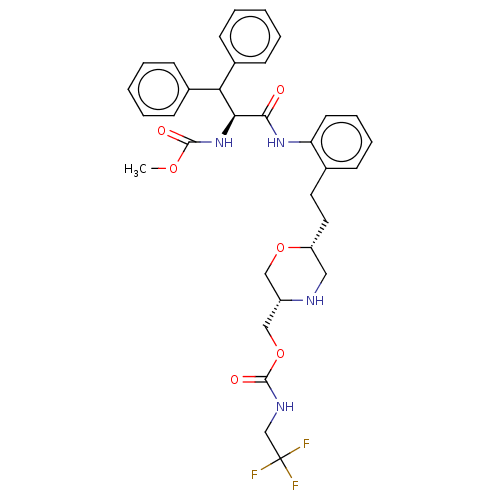 (CHEMBL3828494) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of wild-type HIV1 protease expressed in Escherichia coli assessed as reduction in product formation preincubated for 30 mins followed by a... | ACS Med Chem Lett 7: 702-7 (2016) Article DOI: 10.1021/acsmedchemlett.6b00135 BindingDB Entry DOI: 10.7270/Q28W3G74 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50190635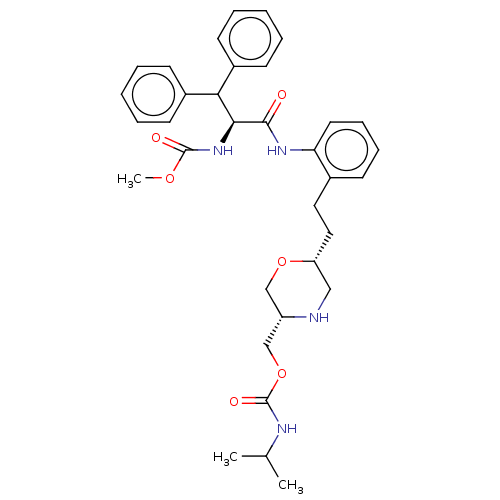 (CHEMBL3827668) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of wild-type HIV1 protease expressed in Escherichia coli assessed as reduction in product formation preincubated for 30 mins followed by a... | ACS Med Chem Lett 7: 702-7 (2016) Article DOI: 10.1021/acsmedchemlett.6b00135 BindingDB Entry DOI: 10.7270/Q28W3G74 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C8 (Homo sapiens (Human)) | BDBM50444436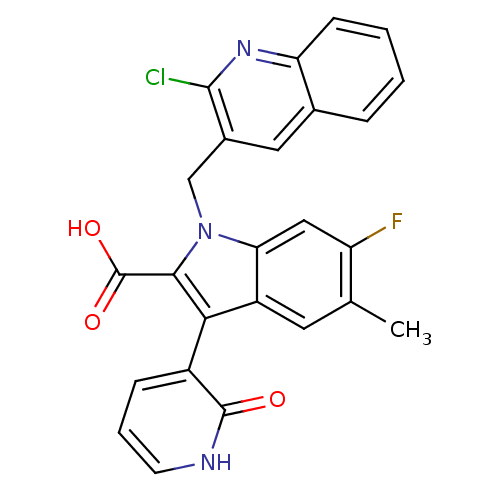 (CHEMBL3092124) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 2.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of CYP2C8 in human liver microsomes measured after concurrent incubation | Bioorg Med Chem Lett 23: 6585-7 (2013) Article DOI: 10.1016/j.bmcl.2013.10.060 BindingDB Entry DOI: 10.7270/Q2GF0W06 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C8 (Homo sapiens (Human)) | BDBM50444436 (CHEMBL3092124) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of CYP2C8 in human liver microsomes measured after compound pre-incubation | Bioorg Med Chem Lett 23: 6585-7 (2013) Article DOI: 10.1016/j.bmcl.2013.10.060 BindingDB Entry DOI: 10.7270/Q2GF0W06 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C19 (Homo sapiens (Human)) | BDBM50444436 (CHEMBL3092124) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of CYP2CJ in human liver microsomes measured after compound pre-incubation | Bioorg Med Chem Lett 23: 6585-7 (2013) Article DOI: 10.1016/j.bmcl.2013.10.060 BindingDB Entry DOI: 10.7270/Q2GF0W06 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM50444436 (CHEMBL3092124) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of CYP2C9 in human liver microsomes measured after compound pre-incubation | Bioorg Med Chem Lett 23: 6585-7 (2013) Article DOI: 10.1016/j.bmcl.2013.10.060 BindingDB Entry DOI: 10.7270/Q2GF0W06 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A2 (Homo sapiens (Human)) | BDBM50444436 (CHEMBL3092124) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of CYP1A2 in human liver microsomes measured after compound pre-incubation | Bioorg Med Chem Lett 23: 6585-7 (2013) Article DOI: 10.1016/j.bmcl.2013.10.060 BindingDB Entry DOI: 10.7270/Q2GF0W06 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50444436 (CHEMBL3092124) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of CYP2D6 in human liver microsomes measured after concurrent incubation | Bioorg Med Chem Lett 23: 6585-7 (2013) Article DOI: 10.1016/j.bmcl.2013.10.060 BindingDB Entry DOI: 10.7270/Q2GF0W06 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50444436 (CHEMBL3092124) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of CYP2D6 in human liver microsomes measured after compound pre-incubation | Bioorg Med Chem Lett 23: 6585-7 (2013) Article DOI: 10.1016/j.bmcl.2013.10.060 BindingDB Entry DOI: 10.7270/Q2GF0W06 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C19 (Homo sapiens (Human)) | BDBM50444436 (CHEMBL3092124) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of CYP2CJ in human liver microsomes measured after concurrent incubation | Bioorg Med Chem Lett 23: 6585-7 (2013) Article DOI: 10.1016/j.bmcl.2013.10.060 BindingDB Entry DOI: 10.7270/Q2GF0W06 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM50444436 (CHEMBL3092124) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of CYP2C9 in human liver microsomes measured after concurrent incubation | Bioorg Med Chem Lett 23: 6585-7 (2013) Article DOI: 10.1016/j.bmcl.2013.10.060 BindingDB Entry DOI: 10.7270/Q2GF0W06 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 53 total ) | Next | Last >> |