

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50206229 (3-(9-(3-(piperidin-1-yl)propoxy)-1,2,3,5,6,10b-hex...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development L.L.C. Curated by ChEMBL | Assay Description Antagonist activity at human histamine H3 receptor expressed in SK-N-MC cells assessed as effect on cAMP accumulation | Bioorg Med Chem Lett 17: 2603-7 (2007) Article DOI: 10.1016/j.bmcl.2007.01.106 BindingDB Entry DOI: 10.7270/Q2HH6JR6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50216250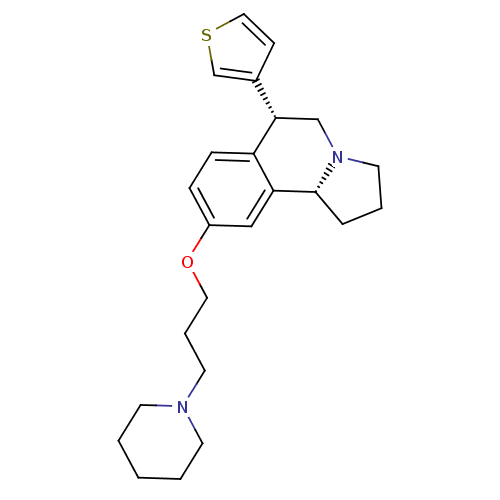 ((6R,10bR)-9-(3-(piperidin-1-yl)propoxy)-6-(thiophe...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development L.L.C. Curated by ChEMBL | Assay Description Binding affinity to human histamine H3 receptor | Bioorg Med Chem Lett 17: 4374-7 (2007) Article DOI: 10.1016/j.bmcl.2007.03.043 BindingDB Entry DOI: 10.7270/Q22Z157G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50216250 ((6R,10bR)-9-(3-(piperidin-1-yl)propoxy)-6-(thiophe...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development L.L.C. Curated by ChEMBL | Assay Description Binding affinity to rat SERT | Bioorg Med Chem Lett 17: 4374-7 (2007) Article DOI: 10.1016/j.bmcl.2007.03.043 BindingDB Entry DOI: 10.7270/Q22Z157G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50198598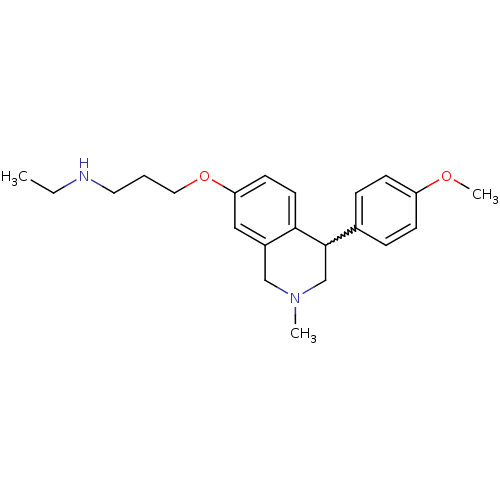 (CHEMBL396945 | N-ethyl-3-(4-(4-methoxyphenyl)-2-me...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.730 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development LLC Curated by ChEMBL | Assay Description Binding affinity to rat SERT | Bioorg Med Chem Lett 17: 702-6 (2007) Article DOI: 10.1016/j.bmcl.2006.10.089 BindingDB Entry DOI: 10.7270/Q23B5ZSS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50216245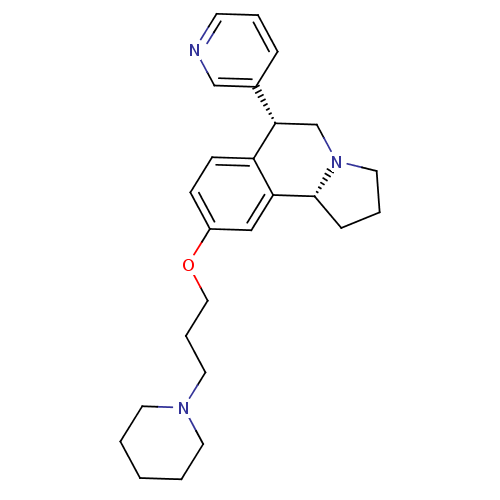 ((6R,10bR)-9-(3-(piperidin-1-yl)propoxy)-6-(pyridin...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development L.L.C. Curated by ChEMBL | Assay Description Binding affinity to human histamine H3 receptor | Bioorg Med Chem Lett 17: 4374-7 (2007) Article DOI: 10.1016/j.bmcl.2007.03.043 BindingDB Entry DOI: 10.7270/Q22Z157G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50216249 ((6S,10bR)-9-(3-(piperidin-1-yl)propoxy)-6-(pyridin...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development L.L.C. Curated by ChEMBL | Assay Description Binding affinity to human histamine H3 receptor | Bioorg Med Chem Lett 17: 4374-7 (2007) Article DOI: 10.1016/j.bmcl.2007.03.043 BindingDB Entry DOI: 10.7270/Q22Z157G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50206218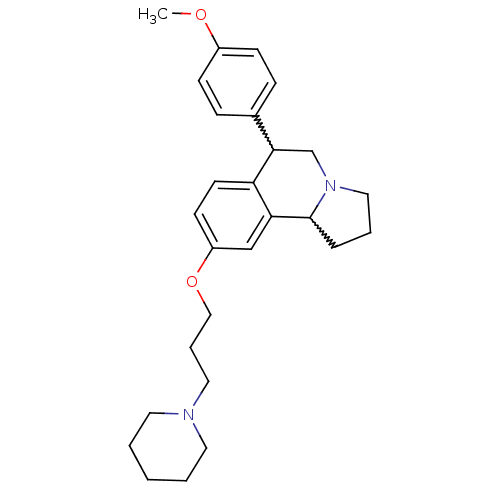 (6-(4-methoxyphenyl)-9-(3-(piperidin-1-yl)propoxy)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development L.L.C. Curated by ChEMBL | Assay Description Antagonist activity at human histamine H3 receptor expressed in SK-N-MC cells assessed as effect on cAMP accumulation | Bioorg Med Chem Lett 17: 2603-7 (2007) Article DOI: 10.1016/j.bmcl.2007.01.106 BindingDB Entry DOI: 10.7270/Q2HH6JR6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50206222 (6-phenyl-9-(3-(piperidin-1-yl)propoxy)-1,2,3,5,6,1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development L.L.C. Curated by ChEMBL | Assay Description Antagonist activity at human histamine H3 receptor expressed in SK-N-MC cells assessed as effect on cAMP accumulation | Bioorg Med Chem Lett 17: 2603-7 (2007) Article DOI: 10.1016/j.bmcl.2007.01.106 BindingDB Entry DOI: 10.7270/Q2HH6JR6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50198609 (7-(3-(azetidin-1-yl)propoxy)-4-(4-methoxyphenyl)-2...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development LLC Curated by ChEMBL | Assay Description Binding affinity to rat SERT | Bioorg Med Chem Lett 17: 702-6 (2007) Article DOI: 10.1016/j.bmcl.2006.10.089 BindingDB Entry DOI: 10.7270/Q23B5ZSS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50216252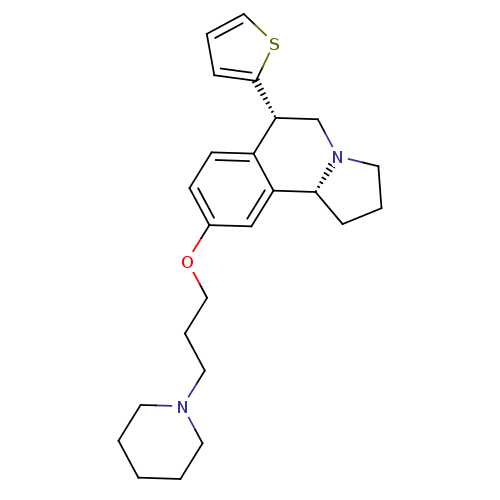 ((6S,10bR)-9-(3-(piperidin-1-yl)propoxy)-6-(thiophe...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development L.L.C. Curated by ChEMBL | Assay Description Binding affinity to human histamine H3 receptor | Bioorg Med Chem Lett 17: 4374-7 (2007) Article DOI: 10.1016/j.bmcl.2007.03.043 BindingDB Entry DOI: 10.7270/Q22Z157G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50216251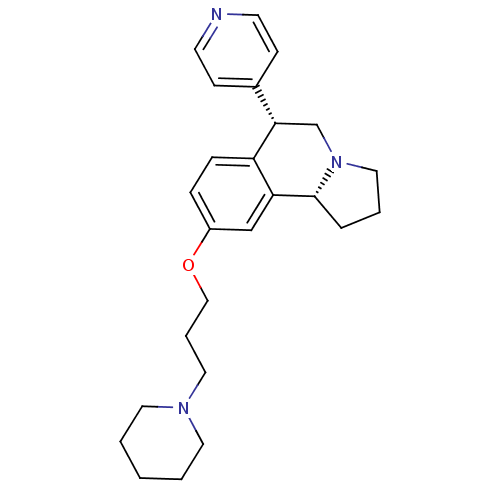 ((6S,10bR)-9-(3-(piperidin-1-yl)propoxy)-6-(pyridin...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development L.L.C. Curated by ChEMBL | Assay Description Binding affinity to human histamine H3 receptor | Bioorg Med Chem Lett 17: 4374-7 (2007) Article DOI: 10.1016/j.bmcl.2007.03.043 BindingDB Entry DOI: 10.7270/Q22Z157G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50206228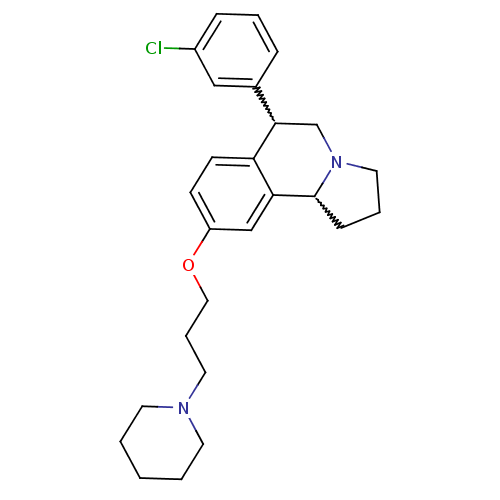 (6-(3-chlorophenyl)-9-(3-(piperidin-1-yl)propoxy)-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development L.L.C. Curated by ChEMBL | Assay Description Antagonist activity at human histamine H3 receptor expressed in SK-N-MC cells assessed as effect on cAMP accumulation | Bioorg Med Chem Lett 17: 2603-7 (2007) Article DOI: 10.1016/j.bmcl.2007.01.106 BindingDB Entry DOI: 10.7270/Q2HH6JR6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50206235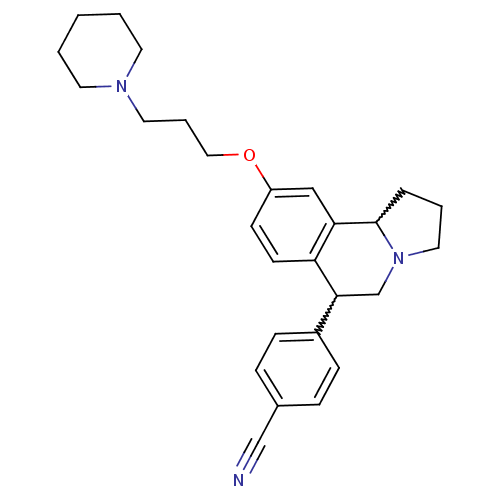 (4-(9-(3-(piperidin-1-yl)propoxy)-1,2,3,5,6,10b-hex...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development L.L.C. Curated by ChEMBL | Assay Description Antagonist activity at human histamine H3 receptor expressed in SK-N-MC cells assessed as effect on cAMP accumulation | Bioorg Med Chem Lett 17: 2603-7 (2007) Article DOI: 10.1016/j.bmcl.2007.01.106 BindingDB Entry DOI: 10.7270/Q2HH6JR6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50216252 ((6S,10bR)-9-(3-(piperidin-1-yl)propoxy)-6-(thiophe...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development L.L.C. Curated by ChEMBL | Assay Description Binding affinity to rat SERT | Bioorg Med Chem Lett 17: 4374-7 (2007) Article DOI: 10.1016/j.bmcl.2007.03.043 BindingDB Entry DOI: 10.7270/Q22Z157G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Homo sapiens (Human)) | BDBM50216250 ((6R,10bR)-9-(3-(piperidin-1-yl)propoxy)-6-(thiophe...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development L.L.C. Curated by ChEMBL | Assay Description Binding affinity to human SERT | Bioorg Med Chem Lett 17: 4374-7 (2007) Article DOI: 10.1016/j.bmcl.2007.03.043 BindingDB Entry DOI: 10.7270/Q22Z157G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50198585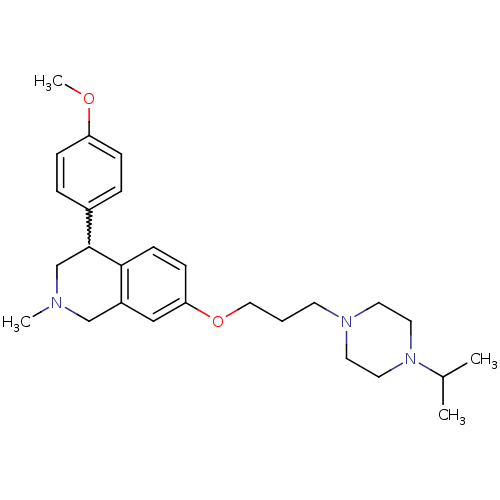 (7-(3-(4-isopropylpiperazin-1-yl)propoxy)-4-(4-meth...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development LLC Curated by ChEMBL | Assay Description Binding affinity to rat SERT | Bioorg Med Chem Lett 17: 702-6 (2007) Article DOI: 10.1016/j.bmcl.2006.10.089 BindingDB Entry DOI: 10.7270/Q23B5ZSS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50206227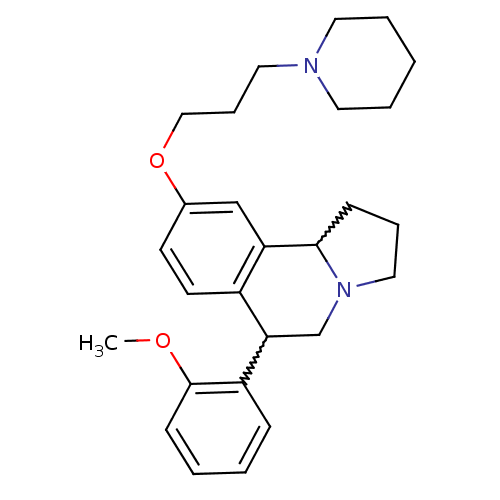 (6-(2-methoxyphenyl)-9-(3-(piperidin-1-yl)propoxy)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development L.L.C. Curated by ChEMBL | Assay Description Antagonist activity at human histamine H3 receptor expressed in SK-N-MC cells assessed as effect on cAMP accumulation | Bioorg Med Chem Lett 17: 2603-7 (2007) Article DOI: 10.1016/j.bmcl.2007.01.106 BindingDB Entry DOI: 10.7270/Q2HH6JR6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50206225 (6-(4-nitrophenyl)-9-(3-(piperidin-1-yl)propoxy)-1,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development L.L.C. Curated by ChEMBL | Assay Description Antagonist activity at human histamine H3 receptor expressed in SK-N-MC cells assessed as effect on cAMP accumulation | Bioorg Med Chem Lett 17: 2603-7 (2007) Article DOI: 10.1016/j.bmcl.2007.01.106 BindingDB Entry DOI: 10.7270/Q2HH6JR6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50206229 (3-(9-(3-(piperidin-1-yl)propoxy)-1,2,3,5,6,10b-hex...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development L.L.C. Curated by ChEMBL | Assay Description Displacement of [3H]citalopram from rat brain SERT | Bioorg Med Chem Lett 17: 2603-7 (2007) Article DOI: 10.1016/j.bmcl.2007.01.106 BindingDB Entry DOI: 10.7270/Q2HH6JR6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50198599 (1-(3-(4-(4-methoxyphenyl)-2-methyl-1,2,3,4-tetrahy...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development LLC Curated by ChEMBL | Assay Description Binding affinity to rat SERT | Bioorg Med Chem Lett 17: 702-6 (2007) Article DOI: 10.1016/j.bmcl.2006.10.089 BindingDB Entry DOI: 10.7270/Q23B5ZSS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50198607 (7-(3-(4-ethylpiperazin-1-yl)propoxy)-4-(4-methoxyp...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development LLC Curated by ChEMBL | Assay Description Binding affinity to rat SERT | Bioorg Med Chem Lett 17: 702-6 (2007) Article DOI: 10.1016/j.bmcl.2006.10.089 BindingDB Entry DOI: 10.7270/Q23B5ZSS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50206233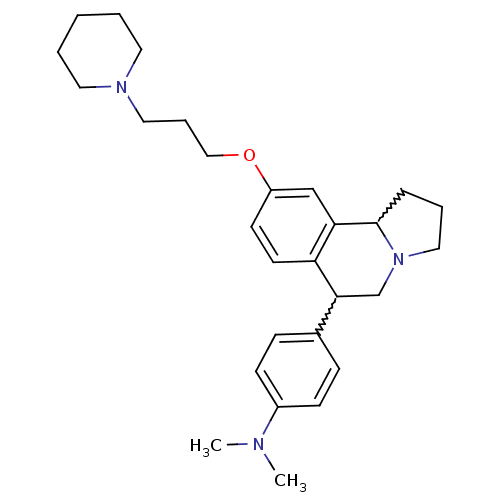 (CHEMBL238951 | N,N-dimethyl-4-(9-(3-(piperidin-1-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development L.L.C. Curated by ChEMBL | Assay Description Antagonist activity at human histamine H3 receptor expressed in SK-N-MC cells assessed as effect on cAMP accumulation | Bioorg Med Chem Lett 17: 2603-7 (2007) Article DOI: 10.1016/j.bmcl.2007.01.106 BindingDB Entry DOI: 10.7270/Q2HH6JR6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50198593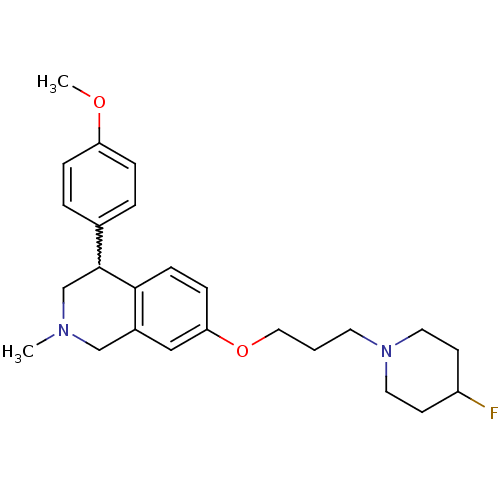 (7-(3-(4-fluoropiperidin-1-yl)propoxy)-4-(4-methoxy...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development L.L.C. Curated by ChEMBL | Assay Description Binding affinity at rat SERT | Bioorg Med Chem Lett 17: 1047-51 (2007) Article DOI: 10.1016/j.bmcl.2006.11.036 BindingDB Entry DOI: 10.7270/Q2VM4BXB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50198593 (7-(3-(4-fluoropiperidin-1-yl)propoxy)-4-(4-methoxy...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development LLC Curated by ChEMBL | Assay Description Binding affinity to rat SERT | Bioorg Med Chem Lett 17: 702-6 (2007) Article DOI: 10.1016/j.bmcl.2006.10.089 BindingDB Entry DOI: 10.7270/Q23B5ZSS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50216248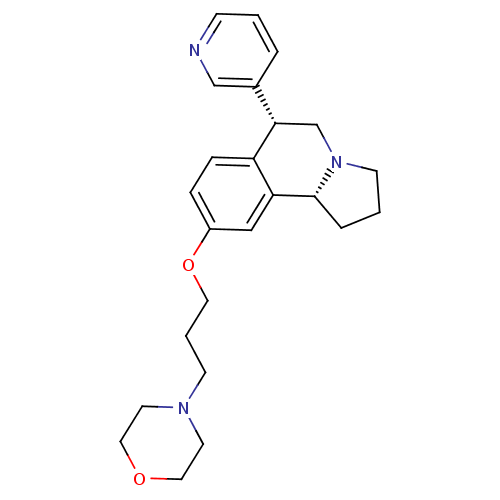 ((6R,10bR)-9-(3-morpholinopropoxy)-6-(pyridin-3-yl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development L.L.C. Curated by ChEMBL | Assay Description Binding affinity to human histamine H3 receptor | Bioorg Med Chem Lett 17: 4374-7 (2007) Article DOI: 10.1016/j.bmcl.2007.03.043 BindingDB Entry DOI: 10.7270/Q22Z157G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50198593 (7-(3-(4-fluoropiperidin-1-yl)propoxy)-4-(4-methoxy...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development L.L.C. Curated by ChEMBL | Assay Description Binding affinity at rat SERT | Bioorg Med Chem Lett 17: 1047-51 (2007) Article DOI: 10.1016/j.bmcl.2006.11.036 BindingDB Entry DOI: 10.7270/Q2VM4BXB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50206236 (6-(4-fluorophenyl)-9-(3-(piperidin-1-yl)propoxy)-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development L.L.C. Curated by ChEMBL | Assay Description Antagonist activity at human histamine H3 receptor expressed in SK-N-MC cells assessed as effect on cAMP accumulation | Bioorg Med Chem Lett 17: 2603-7 (2007) Article DOI: 10.1016/j.bmcl.2007.01.106 BindingDB Entry DOI: 10.7270/Q2HH6JR6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50198596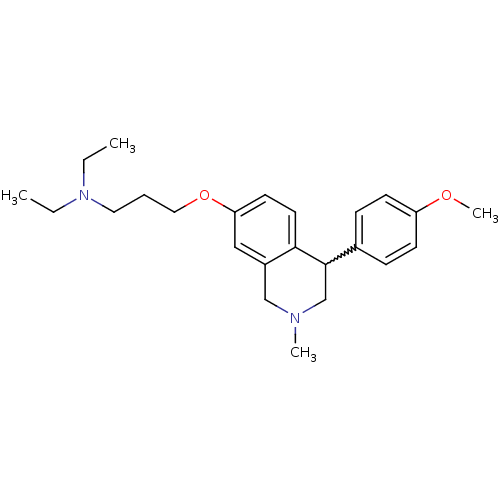 (CHEMBL427845 | N,N-diethyl-3-(4-(4-methoxyphenyl)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development LLC Curated by ChEMBL | Assay Description Binding affinity to human histamine H3 receptor | Bioorg Med Chem Lett 17: 702-6 (2007) Article DOI: 10.1016/j.bmcl.2006.10.089 BindingDB Entry DOI: 10.7270/Q23B5ZSS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50206221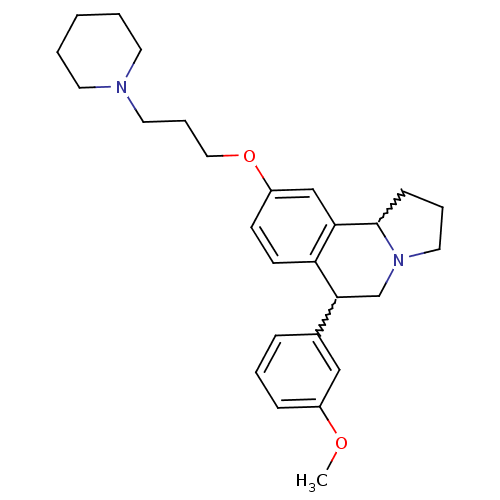 (6-(3-methoxyphenyl)-9-(3-(piperidin-1-yl)propoxy)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development L.L.C. Curated by ChEMBL | Assay Description Antagonist activity at human histamine H3 receptor expressed in SK-N-MC cells assessed as effect on cAMP accumulation | Bioorg Med Chem Lett 17: 2603-7 (2007) Article DOI: 10.1016/j.bmcl.2007.01.106 BindingDB Entry DOI: 10.7270/Q2HH6JR6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50206217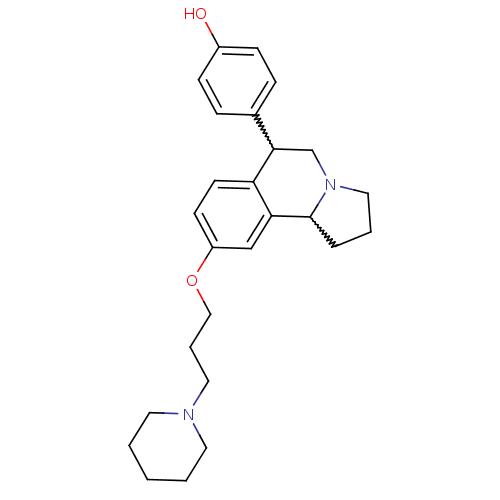 (4-(9-(3-(piperidin-1-yl)propoxy)-1,2,3,5,6,10b-hex...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development L.L.C. Curated by ChEMBL | Assay Description Antagonist activity at human histamine H3 receptor expressed in SK-N-MC cells assessed as effect on cAMP accumulation | Bioorg Med Chem Lett 17: 2603-7 (2007) Article DOI: 10.1016/j.bmcl.2007.01.106 BindingDB Entry DOI: 10.7270/Q2HH6JR6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50198593 (7-(3-(4-fluoropiperidin-1-yl)propoxy)-4-(4-methoxy...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development L.L.C. Curated by ChEMBL | Assay Description Binding affinity at rat SERT | Bioorg Med Chem Lett 17: 1047-51 (2007) Article DOI: 10.1016/j.bmcl.2006.11.036 BindingDB Entry DOI: 10.7270/Q2VM4BXB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Homo sapiens (Human)) | BDBM50198585 (7-(3-(4-isopropylpiperazin-1-yl)propoxy)-4-(4-meth...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development LLC Curated by ChEMBL | Assay Description Binding affinity to human SERT | Bioorg Med Chem Lett 17: 702-6 (2007) Article DOI: 10.1016/j.bmcl.2006.10.089 BindingDB Entry DOI: 10.7270/Q23B5ZSS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50199593 (7-(3-(4-fluoropiperidin-1-yl)propoxy)-2-methyl-4-(...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development L.L.C. Curated by ChEMBL | Assay Description Binding affinity at rat SERT | Bioorg Med Chem Lett 17: 1047-51 (2007) Article DOI: 10.1016/j.bmcl.2006.11.036 BindingDB Entry DOI: 10.7270/Q2VM4BXB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50216242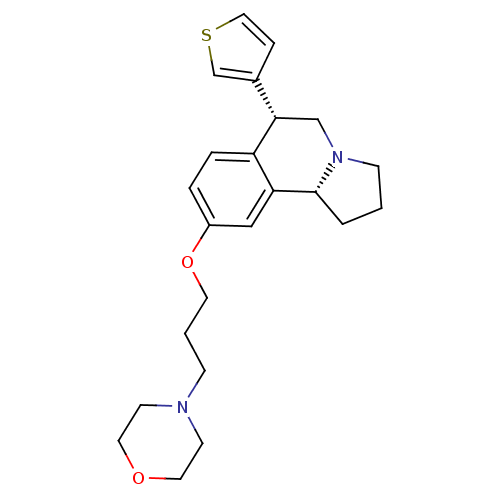 ((6R,10bR)-9-(3-morpholinopropoxy)-6-(thiophen-3-yl...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development L.L.C. Curated by ChEMBL | Assay Description Binding affinity to rat SERT | Bioorg Med Chem Lett 17: 4374-7 (2007) Article DOI: 10.1016/j.bmcl.2007.03.043 BindingDB Entry DOI: 10.7270/Q22Z157G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50216245 ((6R,10bR)-9-(3-(piperidin-1-yl)propoxy)-6-(pyridin...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development L.L.C. Curated by ChEMBL | Assay Description Binding affinity to rat SERT | Bioorg Med Chem Lett 17: 4374-7 (2007) Article DOI: 10.1016/j.bmcl.2007.03.043 BindingDB Entry DOI: 10.7270/Q22Z157G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50216256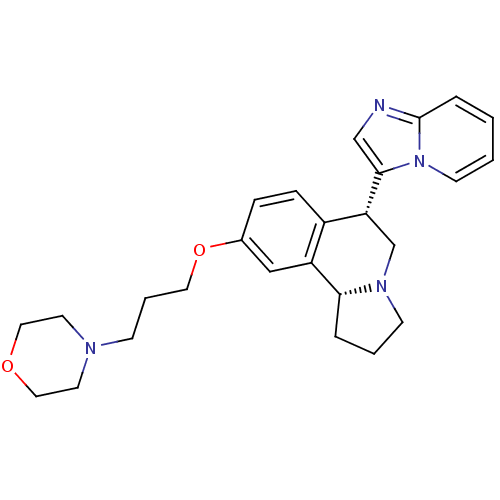 ((6S,10bR)-6-(H-imidazo[1,2-a]pyridin-3-yl)-9-(3-mo...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development L.L.C. Curated by ChEMBL | Assay Description Binding affinity to human histamine H3 receptor | Bioorg Med Chem Lett 17: 4374-7 (2007) Article DOI: 10.1016/j.bmcl.2007.03.043 BindingDB Entry DOI: 10.7270/Q22Z157G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50198590 (4-(4-methoxyphenyl)-2-methyl-7-(3-(piperidin-1-yl)...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development LLC Curated by ChEMBL | Assay Description Binding affinity to rat SERT | Bioorg Med Chem Lett 17: 702-6 (2007) Article DOI: 10.1016/j.bmcl.2006.10.089 BindingDB Entry DOI: 10.7270/Q23B5ZSS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Homo sapiens (Human)) | BDBM30130 (CHEMBL1201082 | CHEMBL41 | Fluoxetin | Fluoxetine ...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development LLC Curated by ChEMBL | Assay Description Binding affinity to human SERT | Bioorg Med Chem Lett 17: 702-6 (2007) Article DOI: 10.1016/j.bmcl.2006.10.089 BindingDB Entry DOI: 10.7270/Q23B5ZSS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50198604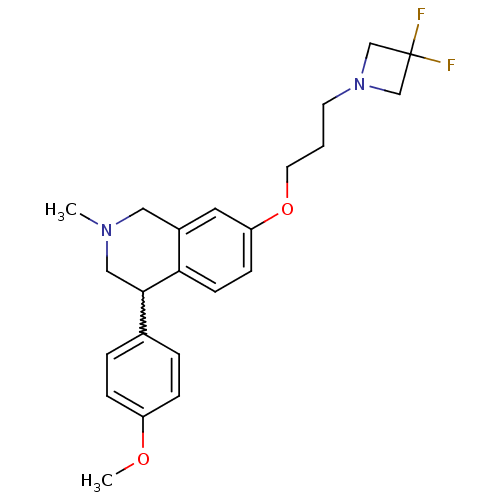 (7-(3-(3,3-difluoroazetidin-1-yl)propoxy)-4-(4-meth...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development LLC Curated by ChEMBL | Assay Description Binding affinity to rat SERT | Bioorg Med Chem Lett 17: 702-6 (2007) Article DOI: 10.1016/j.bmcl.2006.10.089 BindingDB Entry DOI: 10.7270/Q23B5ZSS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50198602 ((1-(3-(4-(4-methoxyphenyl)-2-methyl-1,2,3,4-tetrah...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development LLC Curated by ChEMBL | Assay Description Binding affinity to rat SERT | Bioorg Med Chem Lett 17: 702-6 (2007) Article DOI: 10.1016/j.bmcl.2006.10.089 BindingDB Entry DOI: 10.7270/Q23B5ZSS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Homo sapiens (Human)) | BDBM50198607 (7-(3-(4-ethylpiperazin-1-yl)propoxy)-4-(4-methoxyp...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development LLC Curated by ChEMBL | Assay Description Binding affinity to human SERT | Bioorg Med Chem Lett 17: 702-6 (2007) Article DOI: 10.1016/j.bmcl.2006.10.089 BindingDB Entry DOI: 10.7270/Q23B5ZSS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50198593 (7-(3-(4-fluoropiperidin-1-yl)propoxy)-4-(4-methoxy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development L.L.C. Curated by ChEMBL | Assay Description Binding affinity at human histamine H3 receptor | Bioorg Med Chem Lett 17: 1047-51 (2007) Article DOI: 10.1016/j.bmcl.2006.11.036 BindingDB Entry DOI: 10.7270/Q2VM4BXB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50199593 (7-(3-(4-fluoropiperidin-1-yl)propoxy)-2-methyl-4-(...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development L.L.C. Curated by ChEMBL | Assay Description Binding affinity at rat SERT | Bioorg Med Chem Lett 17: 1047-51 (2007) Article DOI: 10.1016/j.bmcl.2006.11.036 BindingDB Entry DOI: 10.7270/Q2VM4BXB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50199600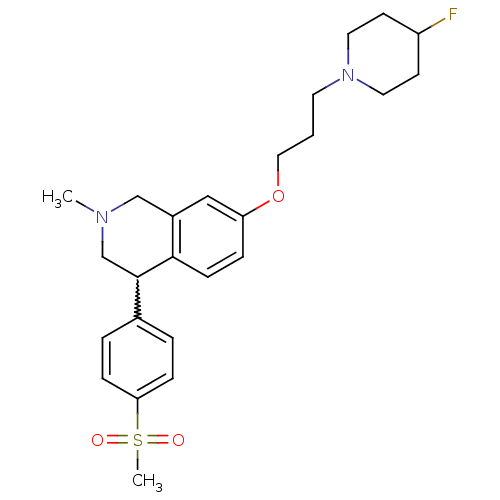 (7-(3-(4-fluoropiperidin-1-yl)propoxy)-2-methyl-4-(...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development L.L.C. Curated by ChEMBL | Assay Description Binding affinity at human histamine H3 receptor | Bioorg Med Chem Lett 17: 1047-51 (2007) Article DOI: 10.1016/j.bmcl.2006.11.036 BindingDB Entry DOI: 10.7270/Q2VM4BXB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50199601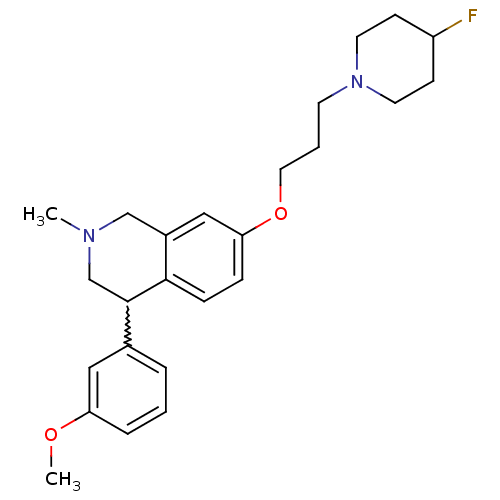 (7-(3-(4-fluoropiperidin-1-yl)propoxy)-4-(3-methoxy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development L.L.C. Curated by ChEMBL | Assay Description Binding affinity at human histamine H3 receptor | Bioorg Med Chem Lett 17: 1047-51 (2007) Article DOI: 10.1016/j.bmcl.2006.11.036 BindingDB Entry DOI: 10.7270/Q2VM4BXB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Homo sapiens (Human)) | BDBM50199593 (7-(3-(4-fluoropiperidin-1-yl)propoxy)-2-methyl-4-(...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development L.L.C. Curated by ChEMBL | Assay Description Binding affinity at human SERT | Bioorg Med Chem Lett 17: 1047-51 (2007) Article DOI: 10.1016/j.bmcl.2006.11.036 BindingDB Entry DOI: 10.7270/Q2VM4BXB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Homo sapiens (Human)) | BDBM50199593 (7-(3-(4-fluoropiperidin-1-yl)propoxy)-2-methyl-4-(...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development L.L.C. Curated by ChEMBL | Assay Description Binding affinity at human SERT | Bioorg Med Chem Lett 17: 1047-51 (2007) Article DOI: 10.1016/j.bmcl.2006.11.036 BindingDB Entry DOI: 10.7270/Q2VM4BXB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50198593 (7-(3-(4-fluoropiperidin-1-yl)propoxy)-4-(4-methoxy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development L.L.C. Curated by ChEMBL | Assay Description Binding affinity at human histamine H3 receptor | Bioorg Med Chem Lett 17: 1047-51 (2007) Article DOI: 10.1016/j.bmcl.2006.11.036 BindingDB Entry DOI: 10.7270/Q2VM4BXB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50198590 (4-(4-methoxyphenyl)-2-methyl-7-(3-(piperidin-1-yl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development LLC Curated by ChEMBL | Assay Description Binding affinity to human histamine H3 receptor | Bioorg Med Chem Lett 17: 702-6 (2007) Article DOI: 10.1016/j.bmcl.2006.10.089 BindingDB Entry DOI: 10.7270/Q23B5ZSS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50198590 (4-(4-methoxyphenyl)-2-methyl-7-(3-(piperidin-1-yl)...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development L.L.C. Curated by ChEMBL | Assay Description Binding affinity at rat SERT | Bioorg Med Chem Lett 17: 1047-51 (2007) Article DOI: 10.1016/j.bmcl.2006.11.036 BindingDB Entry DOI: 10.7270/Q2VM4BXB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 306 total ) | Next | Last >> |