 Found 62 hits with Last Name = 'gomha' and Initial = 'sm'
Found 62 hits with Last Name = 'gomha' and Initial = 'sm' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Serine/threonine-protein kinase Chk1
(Homo sapiens (Human)) | BDBM50072313

(CHEMBL3408393)Show SMILES O=C1N(\C(S\C1=C1/SC(=NN1c1ccccc1)c1ccccc1)=N\c1nc(cc(-c2ccccc2)c1C#N)-c1ccccc1)c1ccccc1 |c:9| Show InChI InChI=1S/C41H26N6OS2/c42-27-34-33(28-16-6-1-7-17-28)26-35(29-18-8-2-9-19-29)43-37(34)44-41-46(31-22-12-4-13-23-31)39(48)36(49-41)40-47(32-24-14-5-15-25-32)45-38(50-40)30-20-10-3-11-21-30/h1-26H/b40-36-,44-41- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Cairo University
Curated by ChEMBL
| Assay Description
Inhibition of CHK1 (unknown origin) using FAM-KKKVSRSGLYRSPSMPENLNRPR-COOH peptide substrate incubated for 1 hr by caliper microfluidic assay |
Eur J Med Chem 92: 459-70 (2015)
Article DOI: 10.1016/j.ejmech.2015.01.019
BindingDB Entry DOI: 10.7270/Q2QV3P5D |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase Chk1
(Homo sapiens (Human)) | BDBM50072316
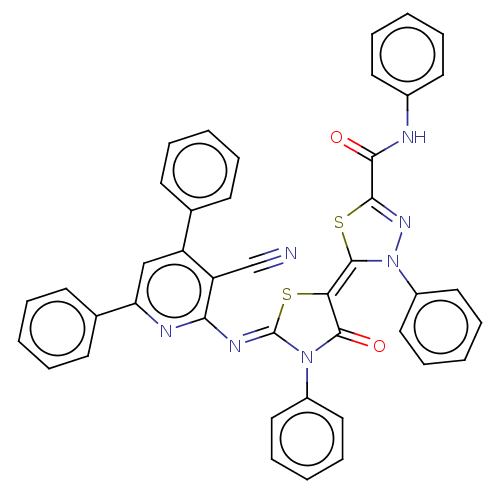
(CHEMBL3408396)Show SMILES O=C(Nc1ccccc1)C1=NN(\C(S1)=C1\S\C(=N/c2nc(cc(-c3ccccc3)c2C#N)-c2ccccc2)N(C1=O)c1ccccc1)c1ccccc1 |t:10| Show InChI InChI=1S/C42H27N7O2S2/c43-27-34-33(28-16-6-1-7-17-28)26-35(29-18-8-2-9-19-29)45-37(34)46-42-48(31-22-12-4-13-23-31)40(51)36(52-42)41-49(32-24-14-5-15-25-32)47-39(53-41)38(50)44-30-20-10-3-11-21-30/h1-26H,(H,44,50)/b41-36-,46-42- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Cairo University
Curated by ChEMBL
| Assay Description
Inhibition of CHK1 (unknown origin) using FAM-KKKVSRSGLYRSPSMPENLNRPR-COOH peptide substrate incubated for 1 hr by caliper microfluidic assay |
Eur J Med Chem 92: 459-70 (2015)
Article DOI: 10.1016/j.ejmech.2015.01.019
BindingDB Entry DOI: 10.7270/Q2QV3P5D |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase Chk1
(Homo sapiens (Human)) | BDBM50072315
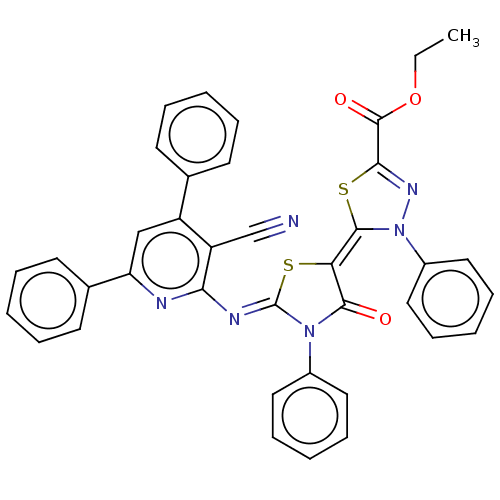
(CHEMBL3408395)Show SMILES CCOC(=O)C1=NN(\C(S1)=C1\S\C(=N/c2nc(cc(-c3ccccc3)c2C#N)-c2ccccc2)N(C1=O)c1ccccc1)c1ccccc1 |t:5| Show InChI InChI=1S/C38H26N6O3S2/c1-2-47-37(46)34-42-44(28-21-13-6-14-22-28)36(49-34)32-35(45)43(27-19-11-5-12-20-27)38(48-32)41-33-30(24-39)29(25-15-7-3-8-16-25)23-31(40-33)26-17-9-4-10-18-26/h3-23H,2H2,1H3/b36-32-,41-38- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Cairo University
Curated by ChEMBL
| Assay Description
Inhibition of CHK1 (unknown origin) using FAM-KKKVSRSGLYRSPSMPENLNRPR-COOH peptide substrate incubated for 1 hr by caliper microfluidic assay |
Eur J Med Chem 92: 459-70 (2015)
Article DOI: 10.1016/j.ejmech.2015.01.019
BindingDB Entry DOI: 10.7270/Q2QV3P5D |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase Chk1
(Homo sapiens (Human)) | BDBM50072314
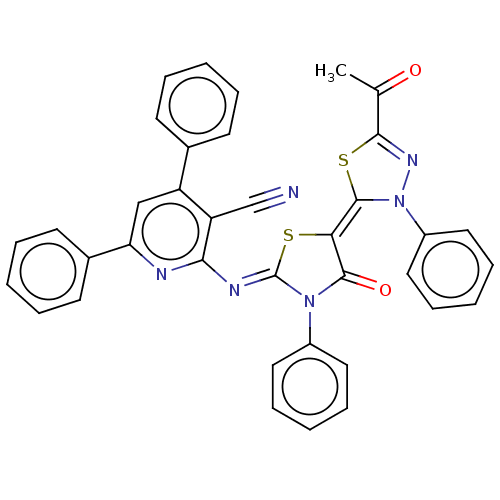
(CHEMBL3408394)Show SMILES CC(=O)C1=NN(\C(S1)=C1\S\C(=N/c2nc(cc(-c3ccccc3)c2C#N)-c2ccccc2)N(C1=O)c1ccccc1)c1ccccc1 |t:3| Show InChI InChI=1S/C37H24N6O2S2/c1-24(44)34-41-43(28-20-12-5-13-21-28)36(47-34)32-35(45)42(27-18-10-4-11-19-27)37(46-32)40-33-30(23-38)29(25-14-6-2-7-15-25)22-31(39-33)26-16-8-3-9-17-26/h2-22H,1H3/b36-32-,40-37- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Cairo University
Curated by ChEMBL
| Assay Description
Inhibition of CHK1 (unknown origin) using FAM-KKKVSRSGLYRSPSMPENLNRPR-COOH peptide substrate incubated for 1 hr by caliper microfluidic assay |
Eur J Med Chem 92: 459-70 (2015)
Article DOI: 10.1016/j.ejmech.2015.01.019
BindingDB Entry DOI: 10.7270/Q2QV3P5D |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase Chk1
(Homo sapiens (Human)) | BDBM50072312

(CHEMBL3408392)Show SMILES N#Cc1c(\N=c2/scc(-c3ccccc3)n2-c2ccccc2)nc(cc1-c1ccccc1)-c1ccccc1 Show InChI InChI=1S/C33H22N4S/c34-22-29-28(24-13-5-1-6-14-24)21-30(25-15-7-2-8-16-25)35-32(29)36-33-37(27-19-11-4-12-20-27)31(23-38-33)26-17-9-3-10-18-26/h1-21,23H/b36-33- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Cairo University
Curated by ChEMBL
| Assay Description
Inhibition of CHK1 (unknown origin) using FAM-KKKVSRSGLYRSPSMPENLNRPR-COOH peptide substrate incubated for 1 hr by caliper microfluidic assay |
Eur J Med Chem 92: 459-70 (2015)
Article DOI: 10.1016/j.ejmech.2015.01.019
BindingDB Entry DOI: 10.7270/Q2QV3P5D |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase Chk1
(Homo sapiens (Human)) | BDBM17054
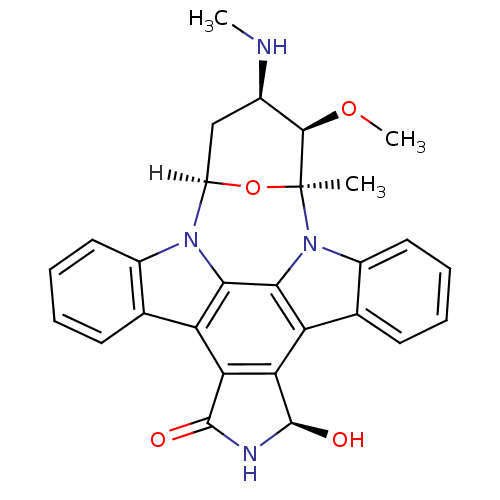
((2S,3R,4R,6R,18R)-18-hydroxy-3-methoxy-2-methyl-4-...)Show SMILES [H][C@]12C[C@@H](NC)[C@@H](OC)[C@](C)(O1)n1c3ccccc3c3c4[C@@H](O)NC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O4/c1-28-25(35-3)15(29-2)12-18(36-28)31-16-10-6-4-8-13(16)19-21-22(27(34)30-26(21)33)20-14-9-5-7-11-17(14)32(28)24(20)23(19)31/h4-11,15,18,25,27,29,34H,12H2,1-3H3,(H,30,33)/t15-,18-,25-,27-,28+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 9.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Cairo University
Curated by ChEMBL
| Assay Description
Inhibition of CHK1 (unknown origin) using FAM-KKKVSRSGLYRSPSMPENLNRPR-COOH peptide substrate incubated for 1 hr by caliper microfluidic assay |
Eur J Med Chem 92: 459-70 (2015)
Article DOI: 10.1016/j.ejmech.2015.01.019
BindingDB Entry DOI: 10.7270/Q2QV3P5D |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Serine/threonine-protein kinase Chk1
(Homo sapiens (Human)) | BDBM50072281
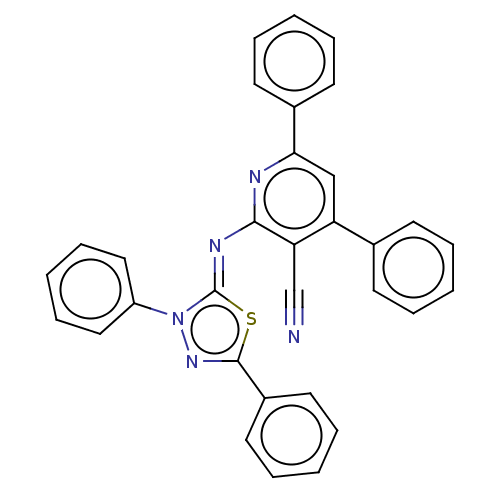
(CHEMBL3408382)Show SMILES N#Cc1c(\N=c2/sc(nn2-c2ccccc2)-c2ccccc2)nc(cc1-c1ccccc1)-c1ccccc1 Show InChI InChI=1S/C32H21N5S/c33-22-28-27(23-13-5-1-6-14-23)21-29(24-15-7-2-8-16-24)34-30(28)35-32-37(26-19-11-4-12-20-26)36-31(38-32)25-17-9-3-10-18-25/h1-21H/b35-32- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Cairo University
Curated by ChEMBL
| Assay Description
Inhibition of CHK1 (unknown origin) using FAM-KKKVSRSGLYRSPSMPENLNRPR-COOH peptide substrate incubated for 1 hr by caliper microfluidic assay |
Eur J Med Chem 92: 459-70 (2015)
Article DOI: 10.1016/j.ejmech.2015.01.019
BindingDB Entry DOI: 10.7270/Q2QV3P5D |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4 [K6R,T557I]
(Homo sapiens (Human)) | BDBM11162

((1R)-3-oxo-3-[3-(trifluoroethyl)-5,6-dihydro[1,2,4...)Show SMILES N[C@@H](CC(=O)N1CCn2c(C1)nnc2C(F)(F)F)Cc1cc(F)c(F)cc1F |r| Show InChI InChI=1S/C16H15F6N5O/c17-10-6-12(19)11(18)4-8(10)3-9(23)5-14(28)26-1-2-27-13(7-26)24-25-15(27)16(20,21)22/h4,6,9H,1-3,5,7,23H2/t9-/m1/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | 25 |
Cairo University
| Assay Description
A 200-無 reaction system containing DPP-IV (Sigma), a test compound, and 25 mmol/L HEPES buffer (containing 140 mmol/L NaCl, 1% BSA, and 80 mmol/L Mg... |
Chem Biol Drug Des 86: 1292-303 (2015)
Article DOI: 10.1111/cbdd.12593
BindingDB Entry DOI: 10.7270/Q2M32THN |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Serine/threonine-protein kinase Chk1
(Homo sapiens (Human)) | BDBM50072310
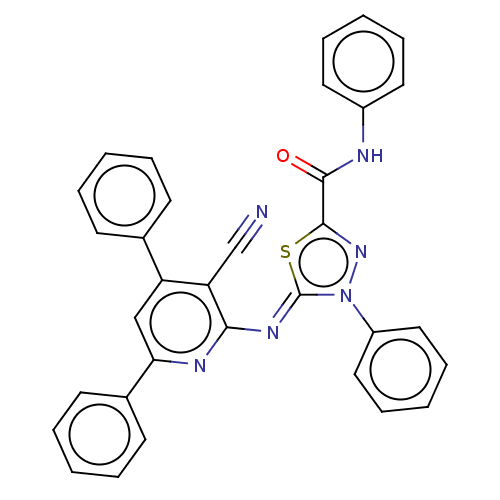
(CHEMBL3408390)Show SMILES O=C(Nc1ccccc1)c1nn(-c2ccccc2)\c(=N\c2nc(cc(-c3ccccc3)c2C#N)-c2ccccc2)s1 Show InChI InChI=1S/C33H22N6OS/c34-22-28-27(23-13-5-1-6-14-23)21-29(24-15-7-2-8-16-24)36-30(28)37-33-39(26-19-11-4-12-20-26)38-32(41-33)31(40)35-25-17-9-3-10-18-25/h1-21H,(H,35,40)/b37-33- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Cairo University
Curated by ChEMBL
| Assay Description
Inhibition of CHK1 (unknown origin) using FAM-KKKVSRSGLYRSPSMPENLNRPR-COOH peptide substrate incubated for 1 hr by caliper microfluidic assay |
Eur J Med Chem 92: 459-70 (2015)
Article DOI: 10.1016/j.ejmech.2015.01.019
BindingDB Entry DOI: 10.7270/Q2QV3P5D |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase Chk1
(Homo sapiens (Human)) | BDBM50072309

(CHEMBL3408389)Show SMILES CCOC(=O)c1nn(-c2ccc(Cl)cc2)\c(=N\c2nc(cc(-c3ccccc3)c2C#N)-c2ccccc2)s1 Show InChI InChI=1S/C29H20ClN5O2S/c1-2-37-28(36)27-34-35(22-15-13-21(30)14-16-22)29(38-27)33-26-24(18-31)23(19-9-5-3-6-10-19)17-25(32-26)20-11-7-4-8-12-20/h3-17H,2H2,1H3/b33-29- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Cairo University
Curated by ChEMBL
| Assay Description
Inhibition of CHK1 (unknown origin) using FAM-KKKVSRSGLYRSPSMPENLNRPR-COOH peptide substrate incubated for 1 hr by caliper microfluidic assay |
Eur J Med Chem 92: 459-70 (2015)
Article DOI: 10.1016/j.ejmech.2015.01.019
BindingDB Entry DOI: 10.7270/Q2QV3P5D |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase Chk1
(Homo sapiens (Human)) | BDBM50072308

(CHEMBL3408388)Show SMILES CCOC(=O)c1nn(-c2ccc(C)cc2)\c(=N\c2nc(cc(-c3ccccc3)c2C#N)-c2ccccc2)s1 Show InChI InChI=1S/C30H23N5O2S/c1-3-37-29(36)28-34-35(23-16-14-20(2)15-17-23)30(38-28)33-27-25(19-31)24(21-10-6-4-7-11-21)18-26(32-27)22-12-8-5-9-13-22/h4-18H,3H2,1-2H3/b33-30- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Cairo University
Curated by ChEMBL
| Assay Description
Inhibition of CHK1 (unknown origin) using FAM-KKKVSRSGLYRSPSMPENLNRPR-COOH peptide substrate incubated for 1 hr by caliper microfluidic assay |
Eur J Med Chem 92: 459-70 (2015)
Article DOI: 10.1016/j.ejmech.2015.01.019
BindingDB Entry DOI: 10.7270/Q2QV3P5D |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4 [K6R,T557I]
(Homo sapiens (Human)) | BDBM167935
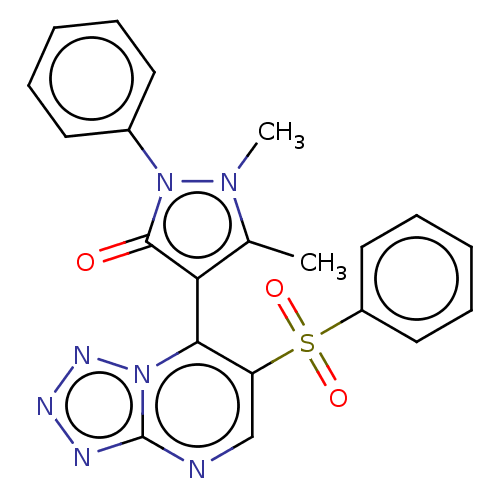
(1,5-Dimethyl-2-phenyl-4-(6-(phenylsulfonyl)tetrazo...)Show SMILES Cc1c(-c2c(cnc3nnnn23)S(=O)(=O)c2ccccc2)c(=O)n(-c2ccccc2)n1C |(2.21,6.35,;3.68,6.83,;4.92,5.92,;4.92,4.38,;6.26,3.61,;6.26,2.07,;4.92,1.3,;3.59,2.07,;2.13,1.6,;1.22,2.84,;2.13,4.09,;3.59,3.61,;7.59,4.38,;6.82,5.72,;8.36,3.05,;8.92,5.15,;8.92,6.69,;10.26,7.46,;11.59,6.69,;11.59,5.15,;10.26,4.38,;6.17,6.83,;7.63,6.35,;5.69,8.29,;6.6,9.54,;5.97,10.95,;6.88,12.19,;8.41,12.03,;9.04,10.62,;8.13,9.38,;4.15,8.29,;3.25,9.54,)| Show InChI InChI=1S/C21H17N7O3S/c1-14-18(20(29)28(26(14)2)15-9-5-3-6-10-15)19-17(13-22-21-23-24-25-27(19)21)32(30,31)16-11-7-4-8-12-16/h3-13H,1-2H3 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | 25 |
Cairo University
| Assay Description
A 200-無 reaction system containing DPP-IV (Sigma), a test compound, and 25 mmol/L HEPES buffer (containing 140 mmol/L NaCl, 1% BSA, and 80 mmol/L Mg... |
Chem Biol Drug Des 86: 1292-303 (2015)
Article DOI: 10.1111/cbdd.12593
BindingDB Entry DOI: 10.7270/Q2M32THN |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4 [K6R,T557I]
(Homo sapiens (Human)) | BDBM167938

(1',5'-Dimethyl-2'-phenyl-5-(phenylsulf...)Show SMILES Cc1c(-c2cc(n[nH]2)S(=O)(=O)c2ccccc2)c(=O)n(-c2ccccc2)n1C Show InChI InChI=1S/C20H18N4O3S/c1-14-19(20(25)24(23(14)2)15-9-5-3-6-10-15)17-13-18(22-21-17)28(26,27)16-11-7-4-8-12-16/h3-13H,1-2H3,(H,21,22) | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | 25 |
Cairo University
| Assay Description
A 200-無 reaction system containing DPP-IV (Sigma), a test compound, and 25 mmol/L HEPES buffer (containing 140 mmol/L NaCl, 1% BSA, and 80 mmol/L Mg... |
Chem Biol Drug Des 86: 1292-303 (2015)
Article DOI: 10.1111/cbdd.12593
BindingDB Entry DOI: 10.7270/Q2M32THN |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase Chk1
(Homo sapiens (Human)) | BDBM50072307
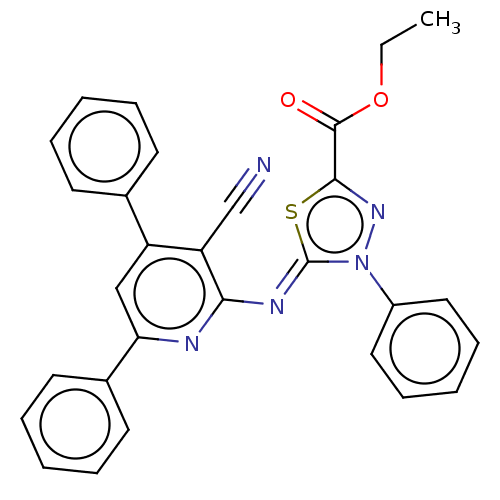
(CHEMBL3408387)Show SMILES CCOC(=O)c1nn(-c2ccccc2)\c(=N\c2nc(cc(-c3ccccc3)c2C#N)-c2ccccc2)s1 Show InChI InChI=1S/C29H21N5O2S/c1-2-36-28(35)27-33-34(22-16-10-5-11-17-22)29(37-27)32-26-24(19-30)23(20-12-6-3-7-13-20)18-25(31-26)21-14-8-4-9-15-21/h3-18H,2H2,1H3/b32-29- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Cairo University
Curated by ChEMBL
| Assay Description
Inhibition of CHK1 (unknown origin) using FAM-KKKVSRSGLYRSPSMPENLNRPR-COOH peptide substrate incubated for 1 hr by caliper microfluidic assay |
Eur J Med Chem 92: 459-70 (2015)
Article DOI: 10.1016/j.ejmech.2015.01.019
BindingDB Entry DOI: 10.7270/Q2QV3P5D |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase Chk1
(Homo sapiens (Human)) | BDBM50072306

(CHEMBL3408386)Show SMILES CC(=O)c1nn(-c2ccc(Cl)cc2)\c(=N\c2nc(cc(-c3ccccc3)c2C#N)-c2ccccc2)s1 Show InChI InChI=1S/C28H18ClN5OS/c1-18(35)27-33-34(22-14-12-21(29)13-15-22)28(36-27)32-26-24(17-30)23(19-8-4-2-5-9-19)16-25(31-26)20-10-6-3-7-11-20/h2-16H,1H3/b32-28- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Cairo University
Curated by ChEMBL
| Assay Description
Inhibition of CHK1 (unknown origin) using FAM-KKKVSRSGLYRSPSMPENLNRPR-COOH peptide substrate incubated for 1 hr by caliper microfluidic assay |
Eur J Med Chem 92: 459-70 (2015)
Article DOI: 10.1016/j.ejmech.2015.01.019
BindingDB Entry DOI: 10.7270/Q2QV3P5D |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase Chk1
(Homo sapiens (Human)) | BDBM50072305

(CHEMBL3408385)Show SMILES COc1ccc(cc1)-n1nc(s\c1=N/c1nc(cc(-c2ccccc2)c1C#N)-c1ccccc1)C(C)=O Show InChI InChI=1S/C29H21N5O2S/c1-19(35)28-33-34(22-13-15-23(36-2)16-14-22)29(37-28)32-27-25(18-30)24(20-9-5-3-6-10-20)17-26(31-27)21-11-7-4-8-12-21/h3-17H,1-2H3/b32-29- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Cairo University
Curated by ChEMBL
| Assay Description
Inhibition of CHK1 (unknown origin) using FAM-KKKVSRSGLYRSPSMPENLNRPR-COOH peptide substrate incubated for 1 hr by caliper microfluidic assay |
Eur J Med Chem 92: 459-70 (2015)
Article DOI: 10.1016/j.ejmech.2015.01.019
BindingDB Entry DOI: 10.7270/Q2QV3P5D |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase Chk1
(Homo sapiens (Human)) | BDBM50072304
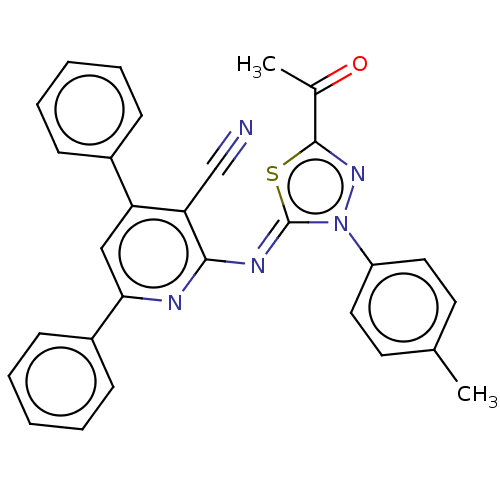
(CHEMBL3408384)Show SMILES CC(=O)c1nn(-c2ccc(C)cc2)\c(=N\c2nc(cc(-c3ccccc3)c2C#N)-c2ccccc2)s1 Show InChI InChI=1S/C29H21N5OS/c1-19-13-15-23(16-14-19)34-29(36-28(33-34)20(2)35)32-27-25(18-30)24(21-9-5-3-6-10-21)17-26(31-27)22-11-7-4-8-12-22/h3-17H,1-2H3/b32-29- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Cairo University
Curated by ChEMBL
| Assay Description
Inhibition of CHK1 (unknown origin) using FAM-KKKVSRSGLYRSPSMPENLNRPR-COOH peptide substrate incubated for 1 hr by caliper microfluidic assay |
Eur J Med Chem 92: 459-70 (2015)
Article DOI: 10.1016/j.ejmech.2015.01.019
BindingDB Entry DOI: 10.7270/Q2QV3P5D |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase Chk1
(Homo sapiens (Human)) | BDBM50072303
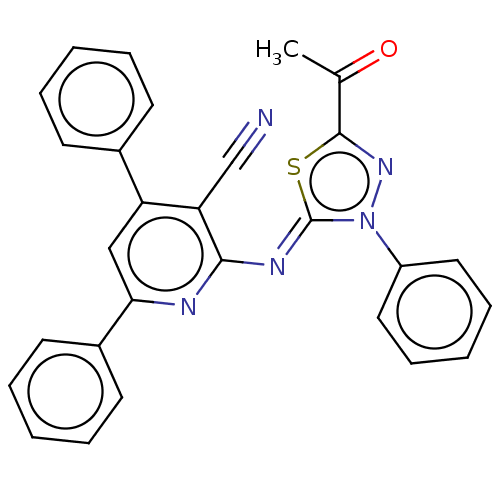
(CHEMBL3408383)Show SMILES CC(=O)c1nn(-c2ccccc2)\c(=N\c2nc(cc(-c3ccccc3)c2C#N)-c2ccccc2)s1 Show InChI InChI=1S/C28H19N5OS/c1-19(34)27-32-33(22-15-9-4-10-16-22)28(35-27)31-26-24(18-29)23(20-11-5-2-6-12-20)17-25(30-26)21-13-7-3-8-14-21/h2-17H,1H3/b31-28- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Cairo University
Curated by ChEMBL
| Assay Description
Inhibition of CHK1 (unknown origin) using FAM-KKKVSRSGLYRSPSMPENLNRPR-COOH peptide substrate incubated for 1 hr by caliper microfluidic assay |
Eur J Med Chem 92: 459-70 (2015)
Article DOI: 10.1016/j.ejmech.2015.01.019
BindingDB Entry DOI: 10.7270/Q2QV3P5D |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase Chk1
(Homo sapiens (Human)) | BDBM50072311
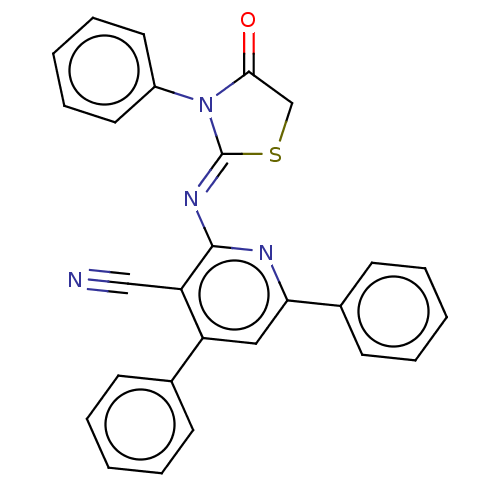
(CHEMBL3408391)Show SMILES O=C1CS\C(=N/c2nc(cc(-c3ccccc3)c2C#N)-c2ccccc2)N1c1ccccc1 Show InChI InChI=1S/C27H18N4OS/c28-17-23-22(19-10-4-1-5-11-19)16-24(20-12-6-2-7-13-20)29-26(23)30-27-31(25(32)18-33-27)21-14-8-3-9-15-21/h1-16H,18H2/b30-27- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Cairo University
Curated by ChEMBL
| Assay Description
Inhibition of CHK1 (unknown origin) using FAM-KKKVSRSGLYRSPSMPENLNRPR-COOH peptide substrate incubated for 1 hr by caliper microfluidic assay |
Eur J Med Chem 92: 459-70 (2015)
Article DOI: 10.1016/j.ejmech.2015.01.019
BindingDB Entry DOI: 10.7270/Q2QV3P5D |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase Chk1
(Homo sapiens (Human)) | BDBM50072280
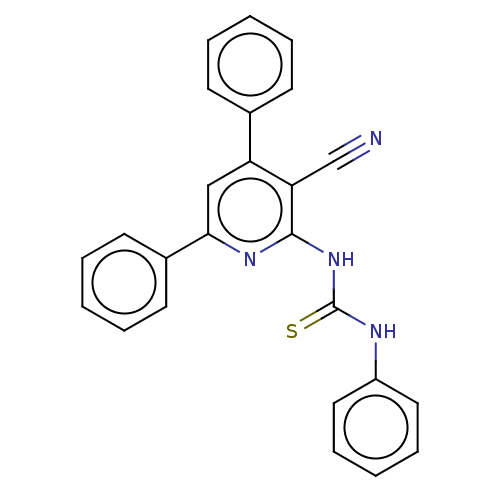
(CHEMBL3408381)Show SMILES S=C(Nc1ccccc1)Nc1nc(cc(-c2ccccc2)c1C#N)-c1ccccc1 Show InChI InChI=1S/C25H18N4S/c26-17-22-21(18-10-4-1-5-11-18)16-23(19-12-6-2-7-13-19)28-24(22)29-25(30)27-20-14-8-3-9-15-20/h1-16H,(H2,27,28,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Cairo University
Curated by ChEMBL
| Assay Description
Inhibition of CHK1 (unknown origin) using FAM-KKKVSRSGLYRSPSMPENLNRPR-COOH peptide substrate incubated for 1 hr by caliper microfluidic assay |
Eur J Med Chem 92: 459-70 (2015)
Article DOI: 10.1016/j.ejmech.2015.01.019
BindingDB Entry DOI: 10.7270/Q2QV3P5D |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4 [K6R,T557I]
(Homo sapiens (Human)) | BDBM167943
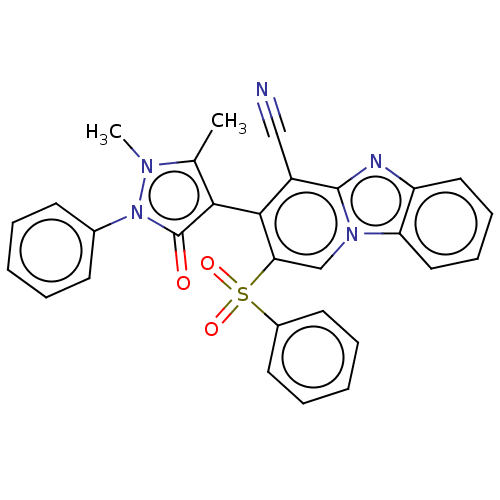
(3-(1,5-Dimethyl-3-oxo-2-phenyl-2,3-dihydro-1Hpyraz...)Show SMILES Cc1c(-c2c(C#N)c3nc4ccccc4n3cc2S(=O)(=O)c2ccccc2)c(=O)n(-c2ccccc2)n1C |(19.46,-10.69,;19.94,-9.22,;19.03,-7.97,;17.49,-7.97,;16.72,-9.31,;17.49,-10.64,;18.26,-11.98,;15.18,-9.31,;14.15,-10.45,;12.74,-9.83,;11.34,-10.45,;10.09,-9.55,;10.25,-8.02,;11.66,-7.39,;12.9,-8.3,;14.41,-7.97,;15.18,-6.64,;16.72,-6.64,;17.49,-5.31,;18.82,-6.08,;16.16,-4.54,;18.26,-3.97,;19.8,-3.97,;20.57,-2.64,;19.8,-1.31,;18.26,-1.31,;17.49,-2.64,;19.94,-6.73,;19.46,-5.26,;21.4,-7.2,;22.65,-6.3,;24.05,-6.93,;25.3,-6.02,;25.14,-4.49,;23.73,-3.86,;22.48,-4.77,;21.4,-8.74,;22.65,-9.65,)| Show InChI InChI=1S/C29H21N5O3S/c1-19-26(29(35)34(32(19)2)20-11-5-3-6-12-20)27-22(17-30)28-31-23-15-9-10-16-24(23)33(28)18-25(27)38(36,37)21-13-7-4-8-14-21/h3-16,18H,1-2H3 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >50 | n/a | n/a | n/a | n/a | n/a | 25 |
Cairo University
| Assay Description
A 200-無 reaction system containing DPP-IV (Sigma), a test compound, and 25 mmol/L HEPES buffer (containing 140 mmol/L NaCl, 1% BSA, and 80 mmol/L Mg... |
Chem Biol Drug Des 86: 1292-303 (2015)
Article DOI: 10.1111/cbdd.12593
BindingDB Entry DOI: 10.7270/Q2M32THN |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4 [K6R,T557I]
(Homo sapiens (Human)) | BDBM167944
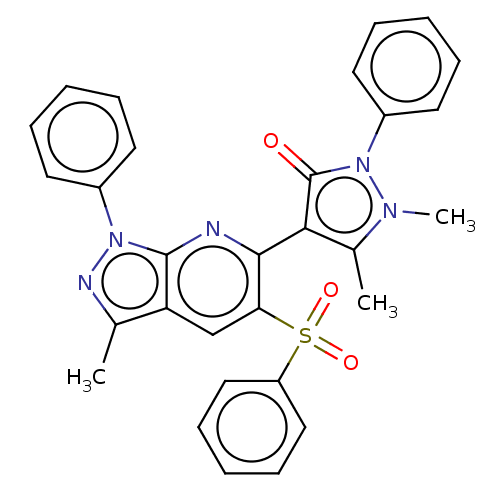
(1,5-Dimethyl-4-(3-methyl-1-phenyl-5-(phenylsulfony...)Show SMILES Cc1nn(-c2ccccc2)c2nc(-c3c(C)n(C)n(-c4ccccc4)c3=O)c(cc12)S(=O)(=O)c1ccccc1 |(-1.94,3.48,;-1.46,2.02,;-2.37,.77,;-1.46,-.48,;-1.94,-1.94,;-.91,-3.09,;-1.39,-4.55,;-2.89,-4.87,;-3.92,-3.73,;-3.45,-2.26,;;1.33,-.77,;2.67,,;4,-.77,;5.41,-.14,;5.73,1.36,;6.44,-1.29,;7.97,-1.13,;5.67,-2.62,;6.29,-4.03,;7.83,-4.19,;8.45,-5.6,;7.55,-6.84,;6.02,-6.68,;5.39,-5.27,;4.16,-2.3,;3.02,-3.33,;2.67,1.54,;1.33,2.31,;,1.54,;4,2.31,;4.77,.98,;5.33,3.08,;3.23,3.64,;1.69,3.64,;.92,4.98,;1.69,6.31,;3.23,6.31,;4,4.98,)| Show InChI InChI=1S/C30H25N5O3S/c1-20-25-19-26(39(37,38)24-17-11-6-12-18-24)28(31-29(25)34(32-20)22-13-7-4-8-14-22)27-21(2)33(3)35(30(27)36)23-15-9-5-10-16-23/h4-19H,1-3H3 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >50 | n/a | n/a | n/a | n/a | n/a | 25 |
Cairo University
| Assay Description
A 200-無 reaction system containing DPP-IV (Sigma), a test compound, and 25 mmol/L HEPES buffer (containing 140 mmol/L NaCl, 1% BSA, and 80 mmol/L Mg... |
Chem Biol Drug Des 86: 1292-303 (2015)
Article DOI: 10.1111/cbdd.12593
BindingDB Entry DOI: 10.7270/Q2M32THN |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4 [K6R,T557I]
(Homo sapiens (Human)) | BDBM167936

(1,5-Dimethyl-2-phenyl-4-(3-(phenylsulfonyl)benzo[4...)Show SMILES Cc1c(-c2c(cnc3nc4ccccc4n23)S(=O)(=O)c2ccccc2)c(=O)n(-c2ccccc2)n1C |(9.96,8.33,;8.42,8.41,;7.45,7.21,;7.85,5.72,;9.34,5.32,;9.74,3.84,;8.65,2.75,;7.16,3.15,;5.87,2.31,;4.67,3.28,;3.15,3.04,;2.18,4.23,;2.73,5.67,;4.26,5.91,;5.22,4.71,;6.76,4.63,;10.43,6.41,;9.34,7.5,;11.52,5.32,;11.52,7.5,;13,7.1,;14.09,8.19,;13.69,9.68,;12.21,10.08,;11.12,8.99,;6.02,7.76,;4.72,6.92,;6.1,9.3,;4.9,10.27,;5.14,11.79,;3.94,12.76,;2.51,12.21,;2.26,10.69,;3.46,9.72,;7.58,9.7,;8.14,11.14,)| Show InChI InChI=1S/C27H21N5O3S/c1-18-24(26(33)32(30(18)2)19-11-5-3-6-12-19)25-23(36(34,35)20-13-7-4-8-14-20)17-28-27-29-21-15-9-10-16-22(21)31(25)27/h3-17H,1-2H3 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 73 | n/a | n/a | n/a | n/a | n/a | 25 |
Cairo University
| Assay Description
A 200-無 reaction system containing DPP-IV (Sigma), a test compound, and 25 mmol/L HEPES buffer (containing 140 mmol/L NaCl, 1% BSA, and 80 mmol/L Mg... |
Chem Biol Drug Des 86: 1292-303 (2015)
Article DOI: 10.1111/cbdd.12593
BindingDB Entry DOI: 10.7270/Q2M32THN |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4 [K6R,T557I]
(Homo sapiens (Human)) | BDBM167932

(1,5-Dimethyl-2-phenyl-4-(6-(phenylsulfonyl)-[1,2,4...)Show SMILES Cc1c(-c2c(cnc3ncnn23)S(=O)(=O)c2ccccc2)c(=O)n(-c2ccccc2)n1C |(2.21,6.35,;3.68,6.83,;4.92,5.92,;4.92,4.38,;6.26,3.61,;6.26,2.07,;4.92,1.3,;3.59,2.07,;2.13,1.6,;1.22,2.84,;2.13,4.09,;3.59,3.61,;7.59,4.38,;6.82,5.72,;8.36,3.05,;8.92,5.15,;8.92,6.69,;10.26,7.46,;11.59,6.69,;11.59,5.15,;10.26,4.38,;6.17,6.83,;7.63,6.35,;5.69,8.29,;6.6,9.54,;5.97,10.95,;6.88,12.19,;8.41,12.03,;9.04,10.62,;8.13,9.38,;4.15,8.29,;3.25,9.54,)| Show InChI InChI=1S/C22H18N6O3S/c1-15-19(21(29)28(26(15)2)16-9-5-3-6-10-16)20-18(13-23-22-24-14-25-27(20)22)32(30,31)17-11-7-4-8-12-17/h3-14H,1-2H3 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 107 | n/a | n/a | n/a | n/a | n/a | 25 |
Cairo University
| Assay Description
A 200-無 reaction system containing DPP-IV (Sigma), a test compound, and 25 mmol/L HEPES buffer (containing 140 mmol/L NaCl, 1% BSA, and 80 mmol/L Mg... |
Chem Biol Drug Des 86: 1292-303 (2015)
Article DOI: 10.1111/cbdd.12593
BindingDB Entry DOI: 10.7270/Q2M32THN |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4 [K6R,T557I]
(Homo sapiens (Human)) | BDBM167933
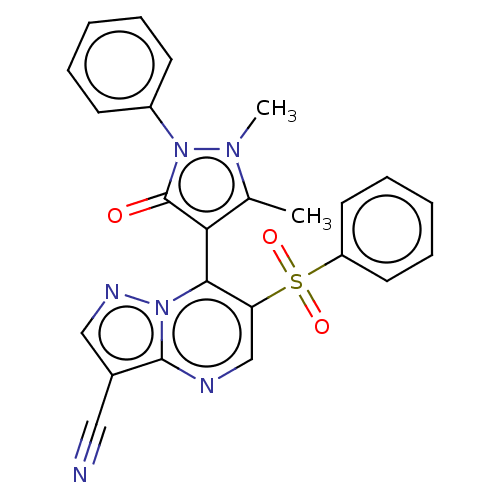
(7-(1,5-Dimethyl-3-oxo-2-phenyl-2,3-dihydro-1H-pyra...)Show SMILES Cc1c(-c2c(cnc3c(cnn23)C#N)S(=O)(=O)c2ccccc2)c(=O)n(-c2ccccc2)n1C |(2.21,6.35,;3.68,6.83,;4.92,5.92,;4.92,4.38,;6.26,3.61,;6.26,2.07,;4.92,1.3,;3.59,2.07,;2.13,1.6,;1.22,2.84,;2.13,4.09,;3.59,3.61,;1.65,.13,;1.17,-1.33,;7.59,4.38,;6.82,5.72,;8.36,3.05,;8.92,5.15,;8.92,6.69,;10.26,7.46,;11.59,6.69,;11.59,5.15,;10.26,4.38,;6.17,6.83,;7.63,6.35,;5.69,8.29,;6.6,9.54,;5.97,10.95,;6.88,12.19,;8.41,12.03,;9.04,10.62,;8.13,9.38,;4.15,8.29,;3.25,9.54,)| Show InChI InChI=1S/C24H18N6O3S/c1-16-21(24(31)30(28(16)2)18-9-5-3-6-10-18)22-20(34(32,33)19-11-7-4-8-12-19)15-26-23-17(13-25)14-27-29(22)23/h3-12,14-15H,1-2H3 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 112 | n/a | n/a | n/a | n/a | n/a | 25 |
Cairo University
| Assay Description
A 200-無 reaction system containing DPP-IV (Sigma), a test compound, and 25 mmol/L HEPES buffer (containing 140 mmol/L NaCl, 1% BSA, and 80 mmol/L Mg... |
Chem Biol Drug Des 86: 1292-303 (2015)
Article DOI: 10.1111/cbdd.12593
BindingDB Entry DOI: 10.7270/Q2M32THN |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50020725
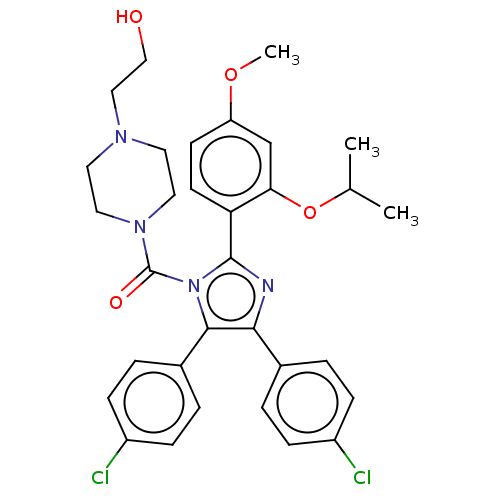
(CHEMBL3291439)Show SMILES COc1ccc(-c2nc(c(-c3ccc(Cl)cc3)n2C(=O)N2CCN(CCO)CC2)-c2ccc(Cl)cc2)c(OC(C)C)c1 Show InChI InChI=1S/C32H34Cl2N4O4/c1-21(2)42-28-20-26(41-3)12-13-27(28)31-35-29(22-4-8-24(33)9-5-22)30(23-6-10-25(34)11-7-23)38(31)32(40)37-16-14-36(15-17-37)18-19-39/h4-13,20-21,39H,14-19H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 260 | n/a | n/a | n/a | n/a | n/a | n/a |
Cairo University
Curated by ChEMBL
| Assay Description
Inhibition of GST-tagged HDM2 (unknown origin) expressed in Escherichia coli BL21 cells assessed as p53 activation by measuring inhibition of His-p53... |
Eur J Med Chem 82: 472-9 (2014)
Article DOI: 10.1016/j.ejmech.2014.05.082
BindingDB Entry DOI: 10.7270/Q2GT5PR8 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4 [K6R,T557I]
(Homo sapiens (Human)) | BDBM167934
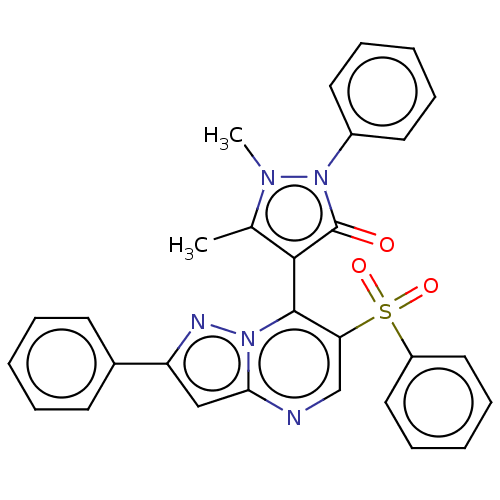
(1,5-Dimethyl-2-phenyl-4-(2-phenyl-6-(phenylsulfony...)Show SMILES Cc1c(-c2c(cnc3cc(nn23)-c2ccccc2)S(=O)(=O)c2ccccc2)c(=O)n(-c2ccccc2)n1C |(2.21,6.35,;3.68,6.83,;4.92,5.92,;4.92,4.38,;6.26,3.61,;6.26,2.07,;4.92,1.3,;3.59,2.07,;2.13,1.6,;1.22,2.84,;2.13,4.09,;3.59,3.61,;-.32,2.84,;-1.09,1.51,;-2.63,1.51,;-3.4,2.84,;-2.63,4.18,;-1.09,4.18,;7.59,4.38,;6.82,5.72,;8.36,3.05,;8.92,5.15,;8.92,6.69,;10.26,7.46,;11.59,6.69,;11.59,5.15,;10.26,4.38,;6.17,6.83,;7.63,6.35,;5.69,8.29,;6.6,9.54,;5.97,10.95,;6.88,12.19,;8.41,12.03,;9.04,10.62,;8.13,9.38,;4.15,8.29,;3.25,9.54,)| Show InChI InChI=1S/C29H23N5O3S/c1-20-27(29(35)34(32(20)2)22-14-8-4-9-15-22)28-25(38(36,37)23-16-10-5-11-17-23)19-30-26-18-24(31-33(26)28)21-12-6-3-7-13-21/h3-19H,1-2H3 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 313 | n/a | n/a | n/a | n/a | n/a | 25 |
Cairo University
| Assay Description
A 200-無 reaction system containing DPP-IV (Sigma), a test compound, and 25 mmol/L HEPES buffer (containing 140 mmol/L NaCl, 1% BSA, and 80 mmol/L Mg... |
Chem Biol Drug Des 86: 1292-303 (2015)
Article DOI: 10.1111/cbdd.12593
BindingDB Entry DOI: 10.7270/Q2M32THN |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50020716
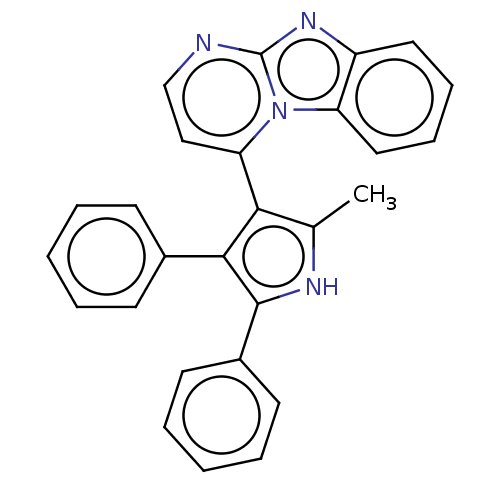
(CHEMBL3286447)Show SMILES Cc1[nH]c(c(c1-c1ccnc2nc3ccccc3n12)-c1ccccc1)-c1ccccc1 |(23.91,-24.16,;22.42,-23.76,;21.24,-24.74,;19.94,-23.91,;20.33,-22.42,;21.86,-22.33,;22.7,-21.04,;21.99,-19.67,;22.82,-18.37,;24.36,-18.44,;25.07,-19.81,;26.56,-20.2,;26.64,-21.74,;27.85,-22.7,;27.62,-24.22,;26.18,-24.78,;24.98,-23.82,;25.21,-22.3,;24.24,-21.11,;19.35,-21.23,;19.89,-19.78,;18.92,-18.6,;17.4,-18.85,;16.85,-20.28,;17.83,-21.47,;18.5,-24.47,;17.3,-23.51,;15.86,-24.07,;15.63,-25.59,;16.84,-26.55,;18.27,-25.99,)| Show InChI InChI=1S/C27H20N4/c1-18-24(23-16-17-28-27-30-21-14-8-9-15-22(21)31(23)27)25(19-10-4-2-5-11-19)26(29-18)20-12-6-3-7-13-20/h2-17,29H,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 360 | n/a | n/a | n/a | n/a | n/a | n/a |
Cairo University
Curated by ChEMBL
| Assay Description
Inhibition of GST-tagged HDM2 (unknown origin) expressed in Escherichia coli BL21 cells assessed as p53 activation by measuring inhibition of His-p53... |
Eur J Med Chem 82: 472-9 (2014)
Article DOI: 10.1016/j.ejmech.2014.05.082
BindingDB Entry DOI: 10.7270/Q2GT5PR8 |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50020717

(CHEMBL3291433)Show SMILES Cc1[nH]c(c(c1-c1ccnc2[nH]c(=S)[nH]c(=O)c12)-c1ccccc1)-c1ccccc1 |(21.9,-22.75,;20.41,-22.34,;19.21,-23.29,;17.93,-22.44,;18.34,-20.96,;19.87,-20.89,;20.72,-19.61,;19.79,-18.37,;20.44,-16.96,;21.96,-16.79,;22.88,-18.03,;24.4,-17.85,;25.32,-19.09,;26.84,-18.92,;24.69,-20.5,;23.17,-20.67,;22.8,-22.17,;22.24,-19.43,;17.38,-19.76,;17.95,-18.32,;16.99,-17.11,;15.46,-17.35,;14.9,-18.78,;15.85,-19.98,;16.48,-22.98,;15.29,-22,;13.85,-22.54,;13.6,-24.06,;14.78,-25.04,;16.22,-24.5,)| Show InChI InChI=1S/C24H18N4OS/c1-14-18(17-12-13-25-22-20(17)23(29)28-24(30)27-22)19(15-8-4-2-5-9-15)21(26-14)16-10-6-3-7-11-16/h2-13,26H,1H3,(H2,25,27,28,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 440 | n/a | n/a | n/a | n/a | n/a | n/a |
Cairo University
Curated by ChEMBL
| Assay Description
Inhibition of GST-tagged HDM2 (unknown origin) expressed in Escherichia coli BL21 cells assessed as p53 activation by measuring inhibition of His-p53... |
Eur J Med Chem 82: 472-9 (2014)
Article DOI: 10.1016/j.ejmech.2014.05.082
BindingDB Entry DOI: 10.7270/Q2GT5PR8 |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50020724
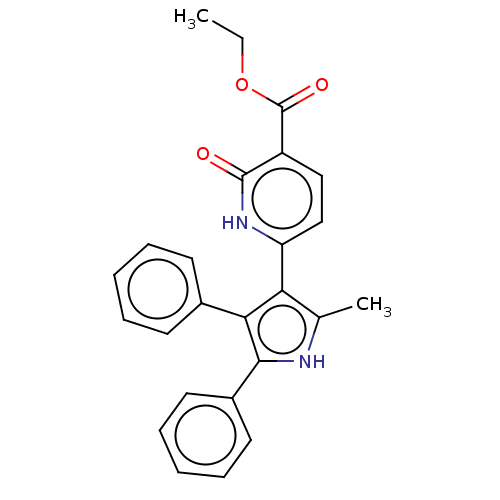
(CHEMBL3291438)Show SMILES CCOC(=O)c1ccc([nH]c1=O)-c1c(C)[nH]c(c1-c1ccccc1)-c1ccccc1 Show InChI InChI=1S/C25H22N2O3/c1-3-30-25(29)19-14-15-20(27-24(19)28)21-16(2)26-23(18-12-8-5-9-13-18)22(21)17-10-6-4-7-11-17/h4-15,26H,3H2,1-2H3,(H,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 550 | n/a | n/a | n/a | n/a | n/a | n/a |
Cairo University
Curated by ChEMBL
| Assay Description
Inhibition of GST-tagged HDM2 (unknown origin) expressed in Escherichia coli BL21 cells assessed as p53 activation by measuring inhibition of His-p53... |
Eur J Med Chem 82: 472-9 (2014)
Article DOI: 10.1016/j.ejmech.2014.05.082
BindingDB Entry DOI: 10.7270/Q2GT5PR8 |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50020723

(CHEMBL3291437)Show SMILES Cc1[nH]c(c(c1-c1ccc(C#N)c(=O)[nH]1)-c1ccccc1)-c1ccccc1 Show InChI InChI=1S/C23H17N3O/c1-15-20(19-13-12-18(14-24)23(27)26-19)21(16-8-4-2-5-9-16)22(25-15)17-10-6-3-7-11-17/h2-13,25H,1H3,(H,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 570 | n/a | n/a | n/a | n/a | n/a | n/a |
Cairo University
Curated by ChEMBL
| Assay Description
Inhibition of GST-tagged HDM2 (unknown origin) expressed in Escherichia coli BL21 cells assessed as p53 activation by measuring inhibition of His-p53... |
Eur J Med Chem 82: 472-9 (2014)
Article DOI: 10.1016/j.ejmech.2014.05.082
BindingDB Entry DOI: 10.7270/Q2GT5PR8 |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50020722
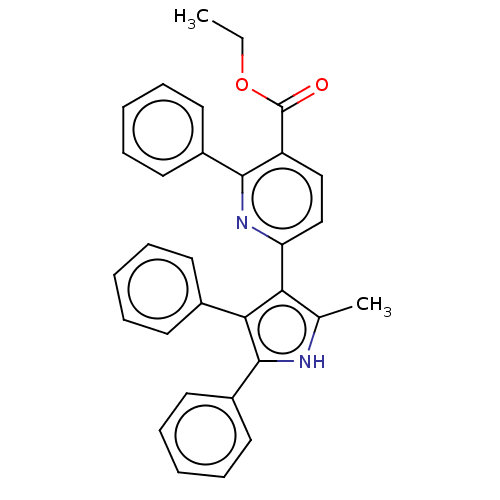
(CHEMBL3291436)Show SMILES CCOC(=O)c1ccc(nc1-c1ccccc1)-c1c(C)[nH]c(c1-c1ccccc1)-c1ccccc1 Show InChI InChI=1S/C31H26N2O2/c1-3-35-31(34)25-19-20-26(33-29(25)23-15-9-5-10-16-23)27-21(2)32-30(24-17-11-6-12-18-24)28(27)22-13-7-4-8-14-22/h4-20,32H,3H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 610 | n/a | n/a | n/a | n/a | n/a | n/a |
Cairo University
Curated by ChEMBL
| Assay Description
Inhibition of GST-tagged HDM2 (unknown origin) expressed in Escherichia coli BL21 cells assessed as p53 activation by measuring inhibition of His-p53... |
Eur J Med Chem 82: 472-9 (2014)
Article DOI: 10.1016/j.ejmech.2014.05.082
BindingDB Entry DOI: 10.7270/Q2GT5PR8 |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50020721
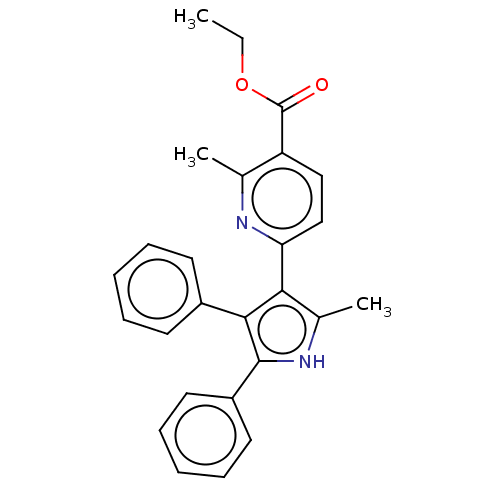
(CHEMBL3291435)Show SMILES CCOC(=O)c1ccc(nc1C)-c1c(C)[nH]c(c1-c1ccccc1)-c1ccccc1 Show InChI InChI=1S/C26H24N2O2/c1-4-30-26(29)21-15-16-22(27-17(21)2)23-18(3)28-25(20-13-9-6-10-14-20)24(23)19-11-7-5-8-12-19/h5-16,28H,4H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 780 | n/a | n/a | n/a | n/a | n/a | n/a |
Cairo University
Curated by ChEMBL
| Assay Description
Inhibition of GST-tagged HDM2 (unknown origin) expressed in Escherichia coli BL21 cells assessed as p53 activation by measuring inhibition of His-p53... |
Eur J Med Chem 82: 472-9 (2014)
Article DOI: 10.1016/j.ejmech.2014.05.082
BindingDB Entry DOI: 10.7270/Q2GT5PR8 |
More data for this
Ligand-Target Pair | |
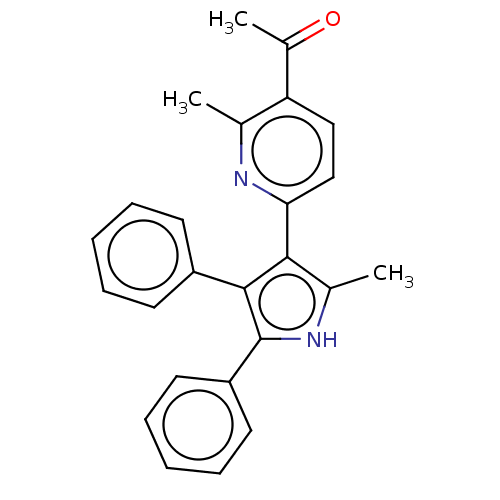
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50020720
(CHEMBL3291434)Show SMILES CC(=O)c1ccc(nc1C)-c1c(C)[nH]c(c1-c1ccccc1)-c1ccccc1 Show InChI InChI=1S/C25H22N2O/c1-16-21(18(3)28)14-15-22(26-16)23-17(2)27-25(20-12-8-5-9-13-20)24(23)19-10-6-4-7-11-19/h4-15,27H,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 810 | n/a | n/a | n/a | n/a | n/a | n/a |
Cairo University
Curated by ChEMBL
| Assay Description
Inhibition of GST-tagged HDM2 (unknown origin) expressed in Escherichia coli BL21 cells assessed as p53 activation by measuring inhibition of His-p53... |
Eur J Med Chem 82: 472-9 (2014)
Article DOI: 10.1016/j.ejmech.2014.05.082
BindingDB Entry DOI: 10.7270/Q2GT5PR8 |
More data for this
Ligand-Target Pair | |
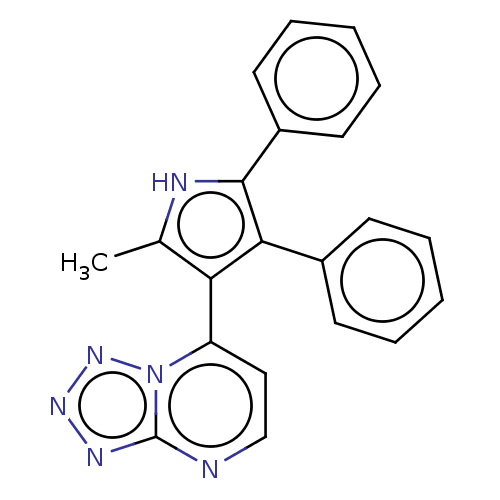
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50020715
(CHEMBL3291222)Show SMILES Cc1[nH]c(c(c1-c1ccnc2nnnn12)-c1ccccc1)-c1ccccc1 |(19.28,-24.27,;17.81,-23.8,;16.55,-24.7,;15.32,-23.8,;15.79,-22.34,;17.33,-22.34,;18.29,-21.14,;17.72,-19.71,;18.69,-18.5,;20.21,-18.74,;20.77,-20.18,;22.21,-20.72,;22.14,-22.25,;20.66,-22.65,;19.81,-21.37,;14.83,-21.14,;15.4,-19.71,;14.43,-18.5,;12.91,-18.74,;12.35,-20.17,;13.31,-21.37,;13.83,-24.21,;12.73,-23.12,;11.26,-23.53,;10.87,-25.02,;11.95,-26.11,;13.44,-25.7,)| Show InChI InChI=1S/C21H16N6/c1-14-18(17-12-13-22-21-24-25-26-27(17)21)19(15-8-4-2-5-9-15)20(23-14)16-10-6-3-7-11-16/h2-13,23H,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 840 | n/a | n/a | n/a | n/a | n/a | n/a |
Cairo University
Curated by ChEMBL
| Assay Description
Inhibition of GST-tagged HDM2 (unknown origin) expressed in Escherichia coli BL21 cells assessed as p53 activation by measuring inhibition of His-p53... |
Eur J Med Chem 82: 472-9 (2014)
Article DOI: 10.1016/j.ejmech.2014.05.082
BindingDB Entry DOI: 10.7270/Q2GT5PR8 |
More data for this
Ligand-Target Pair | |
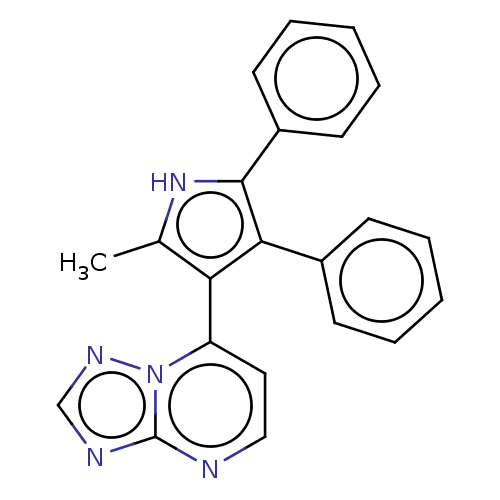
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50020726
(CHEMBL3291221)Show SMILES Cc1[nH]c(c(c1-c1ccnc2ncnn12)-c1ccccc1)-c1ccccc1 |(17.31,-23.85,;15.91,-23.21,;14.57,-23.98,;13.43,-22.95,;14.06,-21.54,;15.59,-21.7,;16.62,-20.57,;16.15,-19.1,;17.19,-17.96,;18.7,-18.28,;19.17,-19.75,;20.57,-20.37,;20.41,-21.91,;18.9,-22.23,;18.13,-20.89,;13.29,-20.2,;14.07,-18.87,;13.3,-17.54,;11.76,-17.54,;10.99,-18.86,;11.75,-20.2,;11.92,-23.26,;10.89,-22.12,;9.39,-22.43,;8.91,-23.9,;9.94,-25.04,;11.44,-24.72,)| Show InChI InChI=1S/C22H17N5/c1-15-19(18-12-13-23-22-24-14-25-27(18)22)20(16-8-4-2-5-9-16)21(26-15)17-10-6-3-7-11-17/h2-14,26H,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 890 | n/a | n/a | n/a | n/a | n/a | n/a |
Cairo University
Curated by ChEMBL
| Assay Description
Inhibition of GST-tagged HDM2 (unknown origin) expressed in Escherichia coli BL21 cells assessed as p53 activation by measuring inhibition of His-p53... |
Eur J Med Chem 82: 472-9 (2014)
Article DOI: 10.1016/j.ejmech.2014.05.082
BindingDB Entry DOI: 10.7270/Q2GT5PR8 |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50020727
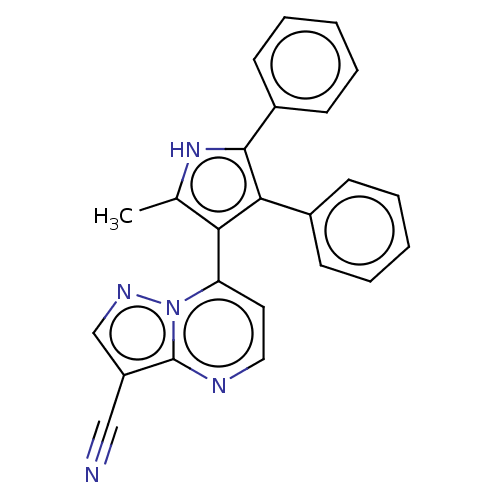
(CHEMBL3291220)Show SMILES Cc1[nH]c(c(c1-c1ccnc2c(cnn12)C#N)-c1ccccc1)-c1ccccc1 |(17.11,-21.51,;15.65,-21.02,;14.4,-21.91,;13.16,-20.99,;13.65,-19.53,;15.19,-19.55,;16.1,-18.31,;15.49,-16.9,;16.41,-15.66,;17.94,-15.83,;18.55,-17.25,;20.01,-17.73,;20,-19.27,;18.53,-19.74,;17.63,-18.49,;21.27,-16.84,;22.52,-15.94,;12.75,-18.28,;13.4,-16.88,;12.5,-15.63,;10.97,-15.78,;10.33,-17.17,;11.22,-18.43,;11.69,-21.46,;10.56,-20.42,;9.09,-20.88,;8.75,-22.39,;9.89,-23.42,;11.36,-22.96,)| Show InChI InChI=1S/C24H17N5/c1-16-21(20-12-13-26-24-19(14-25)15-27-29(20)24)22(17-8-4-2-5-9-17)23(28-16)18-10-6-3-7-11-18/h2-13,15,28H,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 910 | n/a | n/a | n/a | n/a | n/a | n/a |
Cairo University
Curated by ChEMBL
| Assay Description
Inhibition of GST-tagged HDM2 (unknown origin) expressed in Escherichia coli BL21 cells assessed as p53 activation by measuring inhibition of His-p53... |
Eur J Med Chem 82: 472-9 (2014)
Article DOI: 10.1016/j.ejmech.2014.05.082
BindingDB Entry DOI: 10.7270/Q2GT5PR8 |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50020728
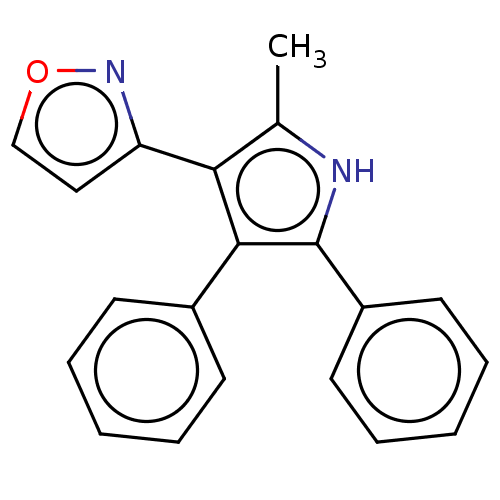
(CHEMBL3291219)Show InChI InChI=1S/C20H16N2O/c1-14-18(17-12-13-23-22-17)19(15-8-4-2-5-9-15)20(21-14)16-10-6-3-7-11-16/h2-13,21H,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 940 | n/a | n/a | n/a | n/a | n/a | n/a |
Cairo University
Curated by ChEMBL
| Assay Description
Inhibition of GST-tagged HDM2 (unknown origin) expressed in Escherichia coli BL21 cells assessed as p53 activation by measuring inhibition of His-p53... |
Eur J Med Chem 82: 472-9 (2014)
Article DOI: 10.1016/j.ejmech.2014.05.082
BindingDB Entry DOI: 10.7270/Q2GT5PR8 |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50020729
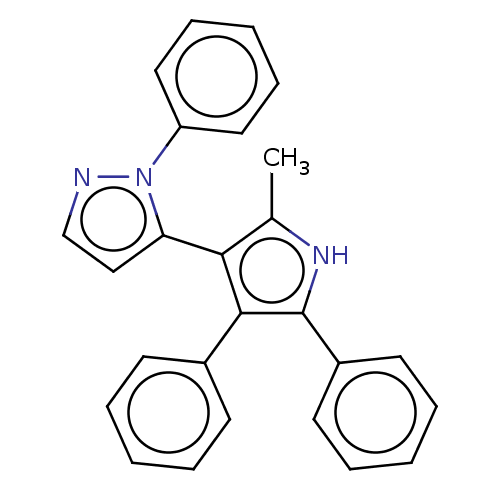
(CHEMBL3291218)Show SMILES Cc1[nH]c(c(c1-c1ccnn1-c1ccccc1)-c1ccccc1)-c1ccccc1 |(19.77,-21.87,;18.32,-21.38,;17.07,-22.28,;15.82,-21.37,;16.3,-19.91,;17.84,-19.91,;18.75,-18.68,;18.29,-17.21,;19.54,-16.3,;20.77,-17.22,;20.29,-18.68,;21.2,-19.92,;20.56,-21.33,;21.47,-22.58,;23,-22.43,;23.63,-21.02,;22.73,-19.77,;15.4,-18.66,;16.03,-17.25,;15.14,-16.01,;13.61,-16.16,;12.97,-17.56,;13.87,-18.81,;14.35,-21.84,;13.21,-20.81,;11.75,-21.28,;11.42,-22.78,;12.56,-23.81,;14.03,-23.34,)| Show InChI InChI=1S/C26H21N3/c1-19-24(23-17-18-27-29(23)22-15-9-4-10-16-22)25(20-11-5-2-6-12-20)26(28-19)21-13-7-3-8-14-21/h2-18,28H,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 970 | n/a | n/a | n/a | n/a | n/a | n/a |
Cairo University
Curated by ChEMBL
| Assay Description
Inhibition of GST-tagged HDM2 (unknown origin) expressed in Escherichia coli BL21 cells assessed as p53 activation by measuring inhibition of His-p53... |
Eur J Med Chem 82: 472-9 (2014)
Article DOI: 10.1016/j.ejmech.2014.05.082
BindingDB Entry DOI: 10.7270/Q2GT5PR8 |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50020714
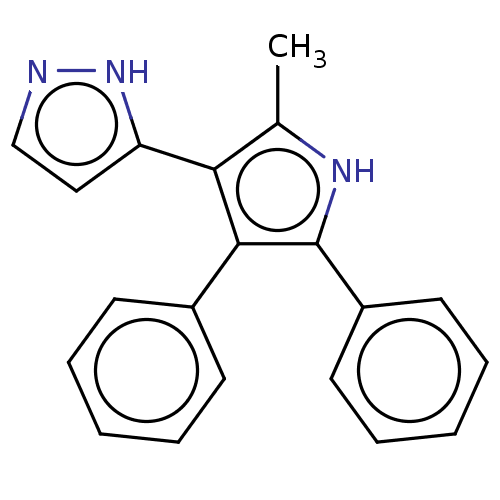
(CHEMBL3291217)Show SMILES Cc1[nH]c(c(c1-c1ccn[nH]1)-c1ccccc1)-c1ccccc1 Show InChI InChI=1S/C20H15N3/c1-14-18(17-12-13-21-23-17)19(15-8-4-2-5-9-15)20(22-14)16-10-6-3-7-11-16/h2-13H,1H3/b18-17+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 990 | n/a | n/a | n/a | n/a | n/a | n/a |
Cairo University
Curated by ChEMBL
| Assay Description
Inhibition of GST-tagged HDM2 (unknown origin) expressed in Escherichia coli BL21 cells assessed as p53 activation by measuring inhibition of His-p53... |
Eur J Med Chem 82: 472-9 (2014)
Article DOI: 10.1016/j.ejmech.2014.05.082
BindingDB Entry DOI: 10.7270/Q2GT5PR8 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4 [K6R,T557I]
(Homo sapiens (Human)) | BDBM167939

(1',5'-Dimethyl-2,2'-diphenyl-5-(phenyl...)Show SMILES Cc1c(-c2cc(nn2-c2ccccc2)S(=O)(=O)c2ccccc2)c(=O)n(-c2ccccc2)n1C |(4.04,-11.23,;2.58,-10.76,;2.1,-9.29,;3.01,-8.05,;2.53,-6.58,;3.78,-5.68,;5.02,-6.58,;4.55,-8.05,;5.45,-9.29,;6.99,-9.13,;7.89,-10.38,;7.26,-11.78,;5.73,-11.94,;4.83,-10.7,;3.78,-4.14,;2.24,-4.14,;5.32,-4.14,;3.78,-2.6,;2.45,-1.83,;2.45,-.29,;3.78,.48,;5.11,-.29,;5.11,-1.83,;.56,-9.29,;-.34,-8.05,;.09,-10.76,;-1.38,-11.23,;-1.7,-12.74,;-3.16,-13.21,;-4.31,-12.18,;-3.99,-10.68,;-2.52,-10.2,;1.33,-11.66,;1.33,-13.2,)| Show InChI InChI=1S/C26H22N4O3S/c1-19-25(26(31)30(28(19)2)21-14-8-4-9-15-21)23-18-24(27-29(23)20-12-6-3-7-13-20)34(32,33)22-16-10-5-11-17-22/h3-18H,1-2H3 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.64E+3 | n/a | n/a | n/a | n/a | n/a | 25 |
Cairo University
| Assay Description
A 200-無 reaction system containing DPP-IV (Sigma), a test compound, and 25 mmol/L HEPES buffer (containing 140 mmol/L NaCl, 1% BSA, and 80 mmol/L Mg... |
Chem Biol Drug Des 86: 1292-303 (2015)
Article DOI: 10.1111/cbdd.12593
BindingDB Entry DOI: 10.7270/Q2M32THN |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50072313

(CHEMBL3408393)Show SMILES O=C1N(\C(S\C1=C1/SC(=NN1c1ccccc1)c1ccccc1)=N\c1nc(cc(-c2ccccc2)c1C#N)-c1ccccc1)c1ccccc1 |c:9| Show InChI InChI=1S/C41H26N6OS2/c42-27-34-33(28-16-6-1-7-17-28)26-35(29-18-8-2-9-19-29)43-37(34)44-41-46(31-22-12-4-13-23-31)39(48)36(49-41)40-47(32-24-14-5-15-25-32)45-38(50-40)30-20-10-3-11-21-30/h1-26H/b40-36-,44-41- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Cairo University
Curated by ChEMBL
| Assay Description
Inhibition of human ERG assessed as reduction in channel current expressed in HEK cells by patch clamp assay |
Eur J Med Chem 92: 459-70 (2015)
Article DOI: 10.1016/j.ejmech.2015.01.019
BindingDB Entry DOI: 10.7270/Q2QV3P5D |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50072316

(CHEMBL3408396)Show SMILES O=C(Nc1ccccc1)C1=NN(\C(S1)=C1\S\C(=N/c2nc(cc(-c3ccccc3)c2C#N)-c2ccccc2)N(C1=O)c1ccccc1)c1ccccc1 |t:10| Show InChI InChI=1S/C42H27N7O2S2/c43-27-34-33(28-16-6-1-7-17-28)26-35(29-18-8-2-9-19-29)45-37(34)46-42-48(31-22-12-4-13-23-31)40(51)36(52-42)41-49(32-24-14-5-15-25-32)47-39(53-41)38(50)44-30-20-10-3-11-21-30/h1-26H,(H,44,50)/b41-36-,46-42- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Cairo University
Curated by ChEMBL
| Assay Description
Inhibition of human ERG assessed as reduction in channel current expressed in HEK cells by patch clamp assay |
Eur J Med Chem 92: 459-70 (2015)
Article DOI: 10.1016/j.ejmech.2015.01.019
BindingDB Entry DOI: 10.7270/Q2QV3P5D |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50072315

(CHEMBL3408395)Show SMILES CCOC(=O)C1=NN(\C(S1)=C1\S\C(=N/c2nc(cc(-c3ccccc3)c2C#N)-c2ccccc2)N(C1=O)c1ccccc1)c1ccccc1 |t:5| Show InChI InChI=1S/C38H26N6O3S2/c1-2-47-37(46)34-42-44(28-21-13-6-14-22-28)36(49-34)32-35(45)43(27-19-11-5-12-20-27)38(48-32)41-33-30(24-39)29(25-15-7-3-8-16-25)23-31(40-33)26-17-9-4-10-18-26/h3-23H,2H2,1H3/b36-32-,41-38- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Cairo University
Curated by ChEMBL
| Assay Description
Inhibition of human ERG assessed as reduction in channel current expressed in HEK cells by patch clamp assay |
Eur J Med Chem 92: 459-70 (2015)
Article DOI: 10.1016/j.ejmech.2015.01.019
BindingDB Entry DOI: 10.7270/Q2QV3P5D |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50072314

(CHEMBL3408394)Show SMILES CC(=O)C1=NN(\C(S1)=C1\S\C(=N/c2nc(cc(-c3ccccc3)c2C#N)-c2ccccc2)N(C1=O)c1ccccc1)c1ccccc1 |t:3| Show InChI InChI=1S/C37H24N6O2S2/c1-24(44)34-41-43(28-20-12-5-13-21-28)36(47-34)32-35(45)42(27-18-10-4-11-19-27)37(46-32)40-33-30(23-38)29(25-14-6-2-7-15-25)22-31(39-33)26-16-8-3-9-17-26/h2-22H,1H3/b36-32-,40-37- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Cairo University
Curated by ChEMBL
| Assay Description
Inhibition of human ERG assessed as reduction in channel current expressed in HEK cells by patch clamp assay |
Eur J Med Chem 92: 459-70 (2015)
Article DOI: 10.1016/j.ejmech.2015.01.019
BindingDB Entry DOI: 10.7270/Q2QV3P5D |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50072312

(CHEMBL3408392)Show SMILES N#Cc1c(\N=c2/scc(-c3ccccc3)n2-c2ccccc2)nc(cc1-c1ccccc1)-c1ccccc1 Show InChI InChI=1S/C33H22N4S/c34-22-29-28(24-13-5-1-6-14-24)21-30(25-15-7-2-8-16-25)35-32(29)36-33-37(27-19-11-4-12-20-27)31(23-38-33)26-17-9-3-10-18-26/h1-21,23H/b36-33- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Cairo University
Curated by ChEMBL
| Assay Description
Inhibition of human ERG assessed as reduction in channel current expressed in HEK cells by patch clamp assay |
Eur J Med Chem 92: 459-70 (2015)
Article DOI: 10.1016/j.ejmech.2015.01.019
BindingDB Entry DOI: 10.7270/Q2QV3P5D |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50072281

(CHEMBL3408382)Show SMILES N#Cc1c(\N=c2/sc(nn2-c2ccccc2)-c2ccccc2)nc(cc1-c1ccccc1)-c1ccccc1 Show InChI InChI=1S/C32H21N5S/c33-22-28-27(23-13-5-1-6-14-23)21-29(24-15-7-2-8-16-24)34-30(28)35-32-37(26-19-11-4-12-20-26)36-31(38-32)25-17-9-3-10-18-25/h1-21H/b35-32- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Cairo University
Curated by ChEMBL
| Assay Description
Inhibition of human ERG assessed as reduction in channel current expressed in HEK cells by patch clamp assay |
Eur J Med Chem 92: 459-70 (2015)
Article DOI: 10.1016/j.ejmech.2015.01.019
BindingDB Entry DOI: 10.7270/Q2QV3P5D |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50072310

(CHEMBL3408390)Show SMILES O=C(Nc1ccccc1)c1nn(-c2ccccc2)\c(=N\c2nc(cc(-c3ccccc3)c2C#N)-c2ccccc2)s1 Show InChI InChI=1S/C33H22N6OS/c34-22-28-27(23-13-5-1-6-14-23)21-29(24-15-7-2-8-16-24)36-30(28)37-33-39(26-19-11-4-12-20-26)38-32(41-33)31(40)35-25-17-9-3-10-18-25/h1-21H,(H,35,40)/b37-33- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Cairo University
Curated by ChEMBL
| Assay Description
Inhibition of human ERG assessed as reduction in channel current expressed in HEK cells by patch clamp assay |
Eur J Med Chem 92: 459-70 (2015)
Article DOI: 10.1016/j.ejmech.2015.01.019
BindingDB Entry DOI: 10.7270/Q2QV3P5D |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50072309

(CHEMBL3408389)Show SMILES CCOC(=O)c1nn(-c2ccc(Cl)cc2)\c(=N\c2nc(cc(-c3ccccc3)c2C#N)-c2ccccc2)s1 Show InChI InChI=1S/C29H20ClN5O2S/c1-2-37-28(36)27-34-35(22-15-13-21(30)14-16-22)29(38-27)33-26-24(18-31)23(19-9-5-3-6-10-19)17-25(32-26)20-11-7-4-8-12-20/h3-17H,2H2,1H3/b33-29- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Cairo University
Curated by ChEMBL
| Assay Description
Inhibition of human ERG assessed as reduction in channel current expressed in HEK cells by patch clamp assay |
Eur J Med Chem 92: 459-70 (2015)
Article DOI: 10.1016/j.ejmech.2015.01.019
BindingDB Entry DOI: 10.7270/Q2QV3P5D |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50072308

(CHEMBL3408388)Show SMILES CCOC(=O)c1nn(-c2ccc(C)cc2)\c(=N\c2nc(cc(-c3ccccc3)c2C#N)-c2ccccc2)s1 Show InChI InChI=1S/C30H23N5O2S/c1-3-37-29(36)28-34-35(23-16-14-20(2)15-17-23)30(38-28)33-27-25(19-31)24(21-10-6-4-7-11-21)18-26(32-27)22-12-8-5-9-13-22/h4-18H,3H2,1-2H3/b33-30- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Cairo University
Curated by ChEMBL
| Assay Description
Inhibition of human ERG assessed as reduction in channel current expressed in HEK cells by patch clamp assay |
Eur J Med Chem 92: 459-70 (2015)
Article DOI: 10.1016/j.ejmech.2015.01.019
BindingDB Entry DOI: 10.7270/Q2QV3P5D |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data