 Found 2310 hits with Last Name = 'gordon' and Initial = 'j'
Found 2310 hits with Last Name = 'gordon' and Initial = 'j' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50036480

(2,6-Dichloro-3-(2-morpholin-4-yl-ethoxy)-benzoic a...)Show SMILES CC(C)c1cccc2c1C(=O)N(COC(=O)c1c(Cl)ccc(OCCN3CCOCC3)c1Cl)S2(=O)=O Show InChI InChI=1S/C24H26Cl2N2O7S/c1-15(2)16-4-3-5-19-20(16)23(29)28(36(19,31)32)14-35-24(30)21-17(25)6-7-18(22(21)26)34-13-10-27-8-11-33-12-9-27/h3-7,15H,8-14H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.00800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling Winthrop Inc.
Curated by ChEMBL
| Assay Description
Potency of inhibition against human leukocyte elastase (HLE) expressed as an apparent binding constant |
J Med Chem 38: 739-44 (1995)
BindingDB Entry DOI: 10.7270/Q2W66JTD |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50036476

(2,6-Dichloro-3-(4-methyl-piperazine-1-sulfonyl)-be...)Show SMILES COc1cc2c(C(=O)N(COC(=O)c3c(Cl)ccc(c3Cl)S(=O)(=O)N3CCN(C)CC3)S2(=O)=O)c(c1)C(C)C Show InChI InChI=1S/C24H27Cl2N3O8S2/c1-14(2)16-11-15(36-4)12-19-20(16)23(30)29(39(19,34)35)13-37-24(31)21-17(25)5-6-18(22(21)26)38(32,33)28-9-7-27(3)8-10-28/h5-6,11-12,14H,7-10,13H2,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling Winthrop Inc.
Curated by ChEMBL
| Assay Description
Potency of inhibition against human leukocyte elastase (HLE) expressed as an apparent binding constant |
J Med Chem 38: 739-44 (1995)
BindingDB Entry DOI: 10.7270/Q2W66JTD |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50036478

(3-Carboxymethoxy-2,6-dichloro-benzoic acid 4-isopr...)Show SMILES COc1cc2c(C(=O)N(COC(=O)c3c(Cl)ccc(OCC(O)=O)c3Cl)S2(=O)=O)c(c1)C(C)C Show InChI InChI=1S/C21H19Cl2NO9S/c1-10(2)12-6-11(31-3)7-15-17(12)20(27)24(34(15,29)30)9-33-21(28)18-13(22)4-5-14(19(18)23)32-8-16(25)26/h4-7,10H,8-9H2,1-3H3,(H,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling Winthrop Inc.
Curated by ChEMBL
| Assay Description
Potency of inhibition against human leukocyte elastase (HLE) expressed as an apparent binding constant |
J Med Chem 38: 739-44 (1995)
BindingDB Entry DOI: 10.7270/Q2W66JTD |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50036481

(2,6-Dichloro-3-[(2-dimethylamino-ethyl)-methyl-sul...)Show SMILES COc1cc2c(C(=O)N(COC(=O)c3c(Cl)ccc(c3Cl)S(=O)(=O)N(C)CCN(C)C)S2(=O)=O)c(c1)C(C)C Show InChI InChI=1S/C24H29Cl2N3O8S2/c1-14(2)16-11-15(36-6)12-19-20(16)23(30)29(39(19,34)35)13-37-24(31)21-17(25)7-8-18(22(21)26)38(32,33)28(5)10-9-27(3)4/h7-8,11-12,14H,9-10,13H2,1-6H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling Winthrop Inc.
Curated by ChEMBL
| Assay Description
Potency of inhibition against human leukocyte elastase (HLE) expressed as an apparent binding constant |
J Med Chem 38: 739-44 (1995)
BindingDB Entry DOI: 10.7270/Q2W66JTD |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50036477

(2,6-Dichloro-3-(2-morpholin-4-yl-ethoxy)-benzoic a...)Show SMILES COc1cc2c(C(=O)N(COC(=O)c3c(Cl)ccc(OCCN4CCOCC4)c3Cl)S2(=O)=O)c(c1)C(C)C Show InChI InChI=1S/C25H28Cl2N2O8S/c1-15(2)17-12-16(34-3)13-20-21(17)24(30)29(38(20,32)33)14-37-25(31)22-18(26)4-5-19(23(22)27)36-11-8-28-6-9-35-10-7-28/h4-5,12-13,15H,6-11,14H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling Winthrop Inc.
Curated by ChEMBL
| Assay Description
Potency of inhibition against human leukocyte elastase (HLE) expressed as an apparent binding constant |
J Med Chem 38: 739-44 (1995)
BindingDB Entry DOI: 10.7270/Q2W66JTD |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50036475

(2,6-Dichloro-3-(2-pyrrolidin-1-yl-ethoxy)-benzoic ...)Show SMILES COc1cc2c(C(=O)N(COC(=O)c3c(Cl)ccc(OCCN4CCCC4)c3Cl)S2(=O)=O)c(c1)C(C)C Show InChI InChI=1S/C25H28Cl2N2O7S/c1-15(2)17-12-16(34-3)13-20-21(17)24(30)29(37(20,32)33)14-36-25(31)22-18(26)6-7-19(23(22)27)35-11-10-28-8-4-5-9-28/h6-7,12-13,15H,4-5,8-11,14H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling Winthrop Inc.
Curated by ChEMBL
| Assay Description
Potency of inhibition against human leukocyte elastase (HLE) expressed as an apparent binding constant |
J Med Chem 38: 739-44 (1995)
BindingDB Entry DOI: 10.7270/Q2W66JTD |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50029699
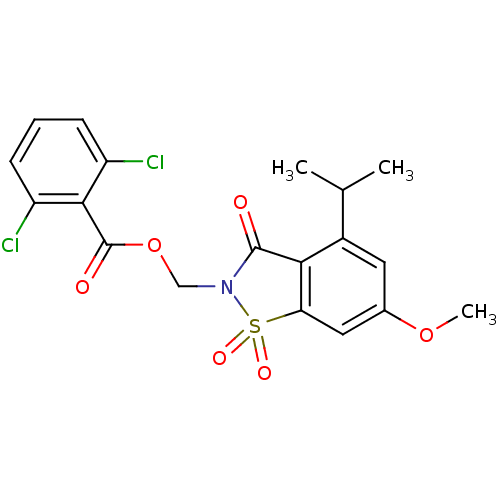
(2,6-Dichloro-benzoic acid 4-isopropyl-6-methoxy-1,...)Show SMILES COc1cc2c(C(=O)N(COC(=O)c3c(Cl)cccc3Cl)S2(=O)=O)c(c1)C(C)C Show InChI InChI=1S/C19H17Cl2NO6S/c1-10(2)12-7-11(27-3)8-15-16(12)18(23)22(29(15,25)26)9-28-19(24)17-13(20)5-4-6-14(17)21/h4-8,10H,9H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 0.0230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Evaluated for inhibitory activity against Human leukocyte elastase (HLE) |
Bioorg Med Chem Lett 5: 331-336 (1995)
Article DOI: 10.1016/0960-894X(95)00030-W
BindingDB Entry DOI: 10.7270/Q2SX6D60 |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50286326
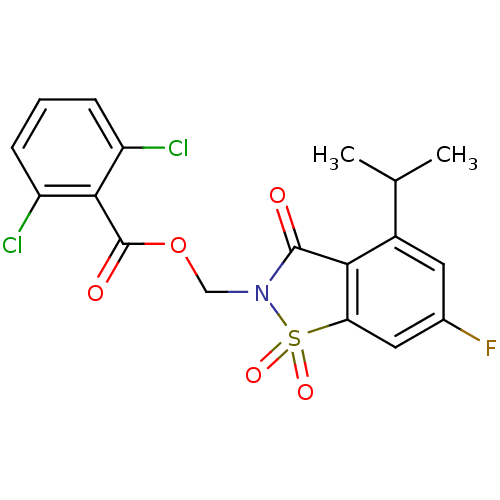
(2,6-Dichloro-benzoic acid 6-fluoro-4-isopropyl-1,1...)Show SMILES CC(C)c1cc(F)cc2c1C(=O)N(COC(=O)c1c(Cl)cccc1Cl)S2(=O)=O Show InChI InChI=1S/C18H14Cl2FNO5S/c1-9(2)11-6-10(21)7-14-15(11)17(23)22(28(14,25)26)8-27-18(24)16-12(19)4-3-5-13(16)20/h3-7,9H,8H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Evaluated for inhibitory activity against Human leukocyte elastase (HLE) |
Bioorg Med Chem Lett 5: 331-336 (1995)
Article DOI: 10.1016/0960-894X(95)00030-W
BindingDB Entry DOI: 10.7270/Q2SX6D60 |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50039631
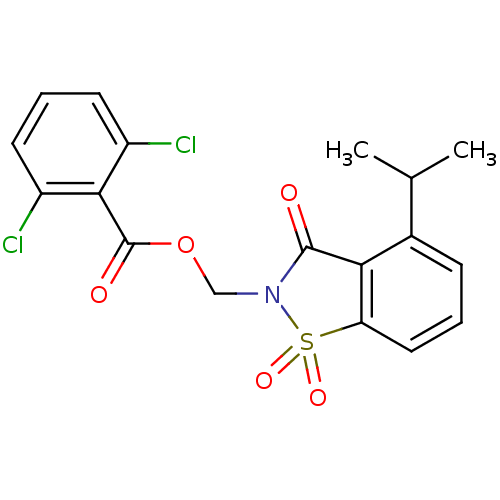
(2,6-Dichloro-benzoic acid 4-isopropyl-1,1,3-trioxo...)Show SMILES CC(C)c1cccc2c1C(=O)N(COC(=O)c1c(Cl)cccc1Cl)S2(=O)=O Show InChI InChI=1S/C18H15Cl2NO5S/c1-10(2)11-5-3-8-14-15(11)17(22)21(27(14,24)25)9-26-18(23)16-12(19)6-4-7-13(16)20/h3-8,10H,9H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Evaluated for inhibitory activity against Human leukocyte elastase (HLE) |
Bioorg Med Chem Lett 5: 331-336 (1995)
Article DOI: 10.1016/0960-894X(95)00030-W
BindingDB Entry DOI: 10.7270/Q2SX6D60 |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50034671
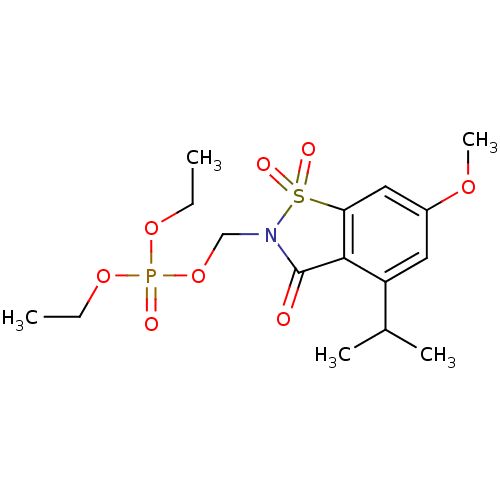
(CHEMBL41327 | Phosphoric acid diethyl ester 4-isop...)Show SMILES CCOP(=O)(OCC)OCN1C(=O)c2c(cc(OC)cc2C(C)C)S1(=O)=O Show InChI InChI=1S/C16H24NO8PS/c1-6-23-26(19,24-7-2)25-10-17-16(18)15-13(11(3)4)8-12(22-5)9-14(15)27(17,20)21/h8-9,11H,6-7,10H2,1-5H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling Winthrop Pharmaceuticals Research Division
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Human leukocyte elastase |
J Med Chem 38: 1571-4 (1995)
BindingDB Entry DOI: 10.7270/Q2M32TS7 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1D
(Homo sapiens (Human)) | BDBM50413549
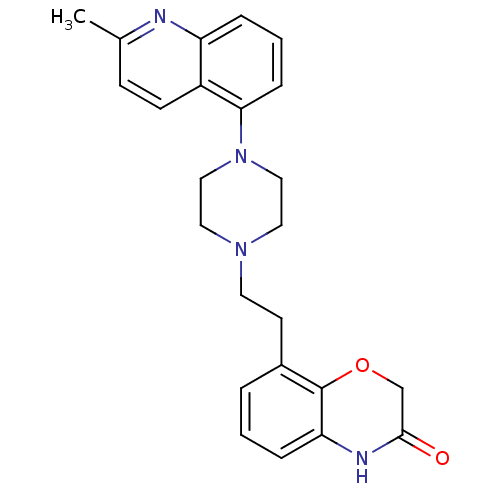
(CHEMBL513715)Show SMILES Cc1ccc2c(cccc2n1)N1CCN(CCc2cccc3NC(=O)COc23)CC1 Show InChI InChI=1S/C24H26N4O2/c1-17-8-9-19-20(25-17)5-3-7-22(19)28-14-12-27(13-15-28)11-10-18-4-2-6-21-24(18)30-16-23(29)26-21/h2-9H,10-16H2,1H3,(H,26,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Antagonist activity at human 5-HT1D receptor expressed in CHO cells assessed as inhibition of GTPgammaS binding by scintillation proximity assay |
Bioorg Med Chem Lett 20: 7092-6 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.085
BindingDB Entry DOI: 10.7270/Q2MC919V |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1D
(Homo sapiens (Human)) | BDBM50413549

(CHEMBL513715)Show SMILES Cc1ccc2c(cccc2n1)N1CCN(CCc2cccc3NC(=O)COc23)CC1 Show InChI InChI=1S/C24H26N4O2/c1-17-8-9-19-20(25-17)5-3-7-22(19)28-14-12-27(13-15-28)11-10-18-4-2-6-21-24(18)30-16-23(29)26-21/h2-9H,10-16H2,1H3,(H,26,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Antagonist activity at human 5HT1D assessed as GTPgammaS binding by scintillation proximity assay in presence of 5-HT |
Bioorg Med Chem Lett 19: 2338-42 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.056
BindingDB Entry DOI: 10.7270/Q2J967MX |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50412441
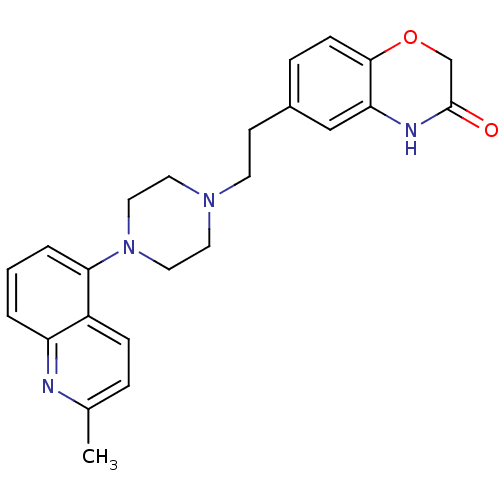
(CHEMBL490417 | SB-744185)Show SMILES Cc1ccc2c(cccc2n1)N1CCN(CCc2ccc3OCC(=O)Nc3c2)CC1 Show InChI InChI=1S/C24H26N4O2/c1-17-5-7-19-20(25-17)3-2-4-22(19)28-13-11-27(12-14-28)10-9-18-6-8-23-21(15-18)26-24(29)16-30-23/h2-8,15H,9-14,16H2,1H3,(H,26,29) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0631 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Antagonist activity at human 5HT1A assessed as GTPgammaS binding by scintillation proximity assay in presence of 5-HT |
Bioorg Med Chem Lett 19: 2338-42 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.056
BindingDB Entry DOI: 10.7270/Q2J967MX |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50034676
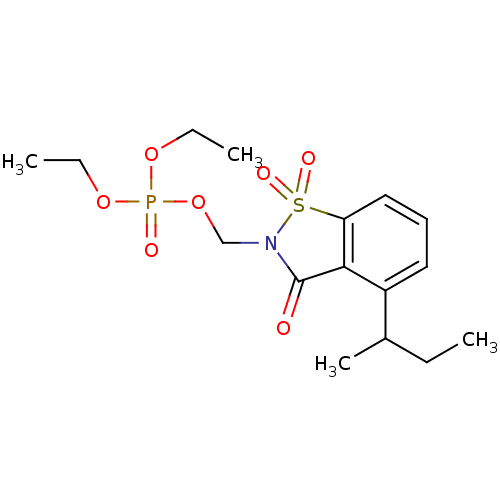
(CHEMBL41881 | Phosphoric acid 4-sec-butyl-1,1,3-tr...)Show SMILES CCOP(=O)(OCC)OCN1C(=O)c2c(cccc2C(C)CC)S1(=O)=O Show InChI InChI=1S/C16H24NO7PS/c1-5-12(4)13-9-8-10-14-15(13)16(18)17(26(14,20)21)11-24-25(19,22-6-2)23-7-3/h8-10,12H,5-7,11H2,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling Winthrop Pharmaceuticals Research Division
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Human leukocyte elastase |
J Med Chem 38: 1571-4 (1995)
BindingDB Entry DOI: 10.7270/Q2M32TS7 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50413077
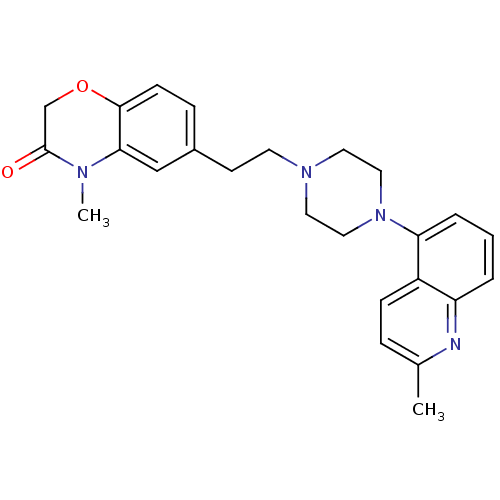
(CHEMBL522257)Show SMILES CN1C(=O)COc2ccc(CCN3CCN(CC3)c3cccc4nc(C)ccc34)cc12 Show InChI InChI=1S/C25H28N4O2/c1-18-6-8-20-21(26-18)4-3-5-22(20)29-14-12-28(13-15-29)11-10-19-7-9-24-23(16-19)27(2)25(30)17-31-24/h3-9,16H,10-15,17H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Antagonist activity at human 5HT1A assessed as GTPgammaS binding by scintillation proximity assay in presence of 5-HT |
Bioorg Med Chem Lett 19: 2338-42 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.056
BindingDB Entry DOI: 10.7270/Q2J967MX |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1D
(Homo sapiens (Human)) | BDBM50417409

(CHEMBL1290487)Show SMILES CN1C(=O)CCc2c(CCN3CCN(CC3)c3cccc4nc(C)ccc34)cccc12 Show InChI InChI=1S/C26H30N4O/c1-19-9-10-22-23(27-19)6-4-8-25(22)30-17-15-29(16-18-30)14-13-20-5-3-7-24-21(20)11-12-26(31)28(24)2/h3-10H,11-18H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Antagonist activity at human 5-HT1D receptor expressed in CHO cells assessed as inhibition of GTPgammaS binding by scintillation proximity assay |
Bioorg Med Chem Lett 20: 7092-6 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.085
BindingDB Entry DOI: 10.7270/Q2MC919V |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1D
(Homo sapiens (Human)) | BDBM50413560
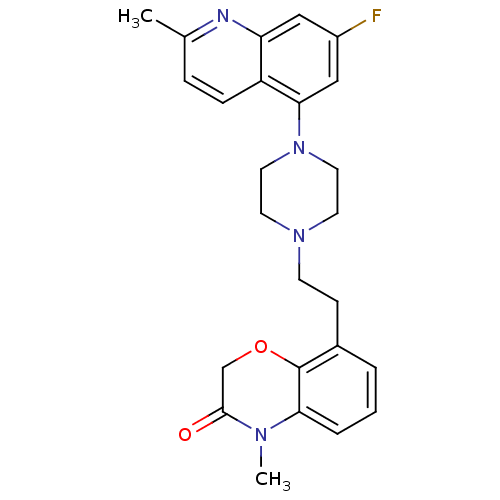
(CHEMBL469374)Show SMILES CN1C(=O)COc2c(CCN3CCN(CC3)c3cc(F)cc4nc(C)ccc34)cccc12 Show InChI InChI=1S/C25H27FN4O2/c1-17-6-7-20-21(27-17)14-19(26)15-23(20)30-12-10-29(11-13-30)9-8-18-4-3-5-22-25(18)32-16-24(31)28(22)2/h3-7,14-15H,8-13,16H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Antagonist activity at human 5HT1D assessed as GTPgammaS binding by scintillation proximity assay in presence of 5-HT |
Bioorg Med Chem Lett 19: 2338-42 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.056
BindingDB Entry DOI: 10.7270/Q2J967MX |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50413555
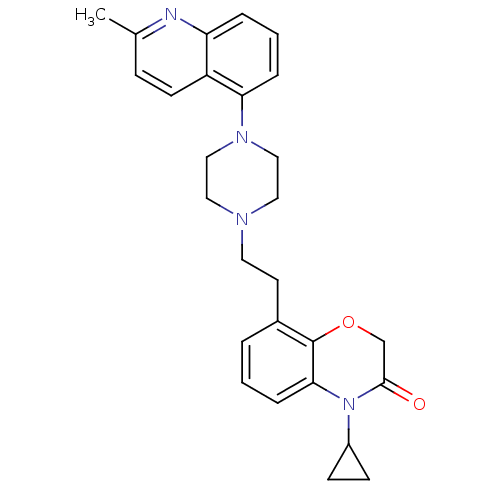
(CHEMBL469568)Show SMILES Cc1ccc2c(cccc2n1)N1CCN(CCc2cccc3N(C4CC4)C(=O)COc23)CC1 Show InChI InChI=1S/C27H30N4O2/c1-19-8-11-22-23(28-19)5-3-6-24(22)30-16-14-29(15-17-30)13-12-20-4-2-7-25-27(20)33-18-26(32)31(25)21-9-10-21/h2-8,11,21H,9-10,12-18H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Antagonist activity at human 5HT1A assessed as GTPgammaS binding by scintillation proximity assay in presence of 5-HT |
Bioorg Med Chem Lett 19: 2338-42 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.056
BindingDB Entry DOI: 10.7270/Q2J967MX |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1D
(Homo sapiens (Human)) | BDBM50413077

(CHEMBL522257)Show SMILES CN1C(=O)COc2ccc(CCN3CCN(CC3)c3cccc4nc(C)ccc34)cc12 Show InChI InChI=1S/C25H28N4O2/c1-18-6-8-20-21(26-18)4-3-5-22(20)29-14-12-28(13-15-29)11-10-19-7-9-24-23(16-19)27(2)25(30)17-31-24/h3-9,16H,10-15,17H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Antagonist activity at human 5HT1D assessed as GTPgammaS binding by scintillation proximity assay in presence of 5-HT |
Bioorg Med Chem Lett 19: 2338-42 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.056
BindingDB Entry DOI: 10.7270/Q2J967MX |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1D
(Homo sapiens (Human)) | BDBM50417420
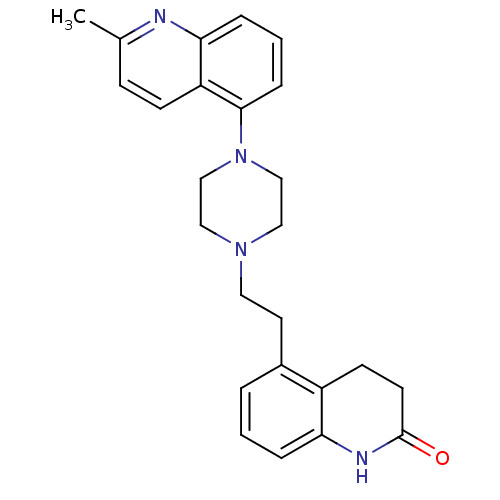
(CHEMBL1290486)Show SMILES Cc1ccc2c(cccc2n1)N1CCN(CCc2cccc3NC(=O)CCc23)CC1 Show InChI InChI=1S/C25H28N4O/c1-18-8-9-21-23(26-18)6-3-7-24(21)29-16-14-28(15-17-29)13-12-19-4-2-5-22-20(19)10-11-25(30)27-22/h2-9H,10-17H2,1H3,(H,27,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Antagonist activity at human 5-HT1D receptor expressed in CHO cells assessed as inhibition of GTPgammaS binding by scintillation proximity assay |
Bioorg Med Chem Lett 20: 7092-6 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.085
BindingDB Entry DOI: 10.7270/Q2MC919V |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1D
(Homo sapiens (Human)) | BDBM50413550
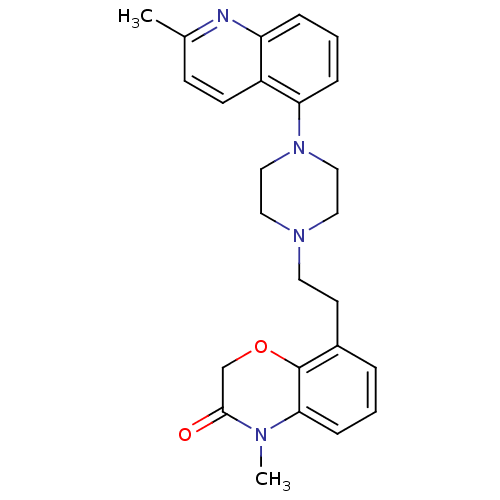
(CHEMBL469345)Show SMILES CN1C(=O)COc2c(CCN3CCN(CC3)c3cccc4nc(C)ccc34)cccc12 Show InChI InChI=1S/C25H28N4O2/c1-18-9-10-20-21(26-18)6-4-7-22(20)29-15-13-28(14-16-29)12-11-19-5-3-8-23-25(19)31-17-24(30)27(23)2/h3-10H,11-17H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Antagonist activity at human 5-HT1D receptor expressed in CHO cells assessed as inhibition of GTPgammaS binding by scintillation proximity assay |
Bioorg Med Chem Lett 20: 7092-6 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.085
BindingDB Entry DOI: 10.7270/Q2MC919V |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50034677
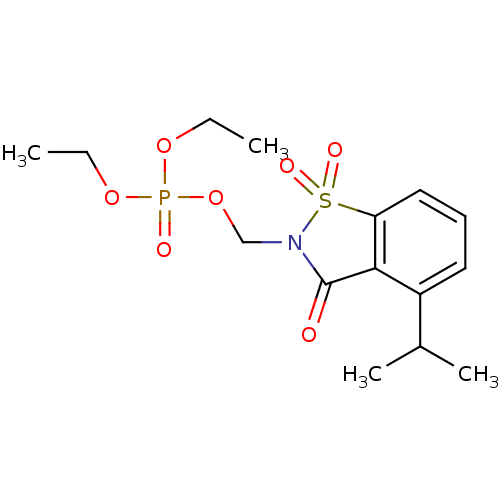
(CHEMBL288810 | Phosphoric acid diethyl ester 4-iso...)Show SMILES CCOP(=O)(OCC)OCN1C(=O)c2c(cccc2C(C)C)S1(=O)=O Show InChI InChI=1S/C15H22NO7PS/c1-5-21-24(18,22-6-2)23-10-16-15(17)14-12(11(3)4)8-7-9-13(14)25(16,19)20/h7-9,11H,5-6,10H2,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling Winthrop Pharmaceuticals Research Division
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Human leukocyte elastase |
J Med Chem 38: 1571-4 (1995)
BindingDB Entry DOI: 10.7270/Q2M32TS7 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50413086
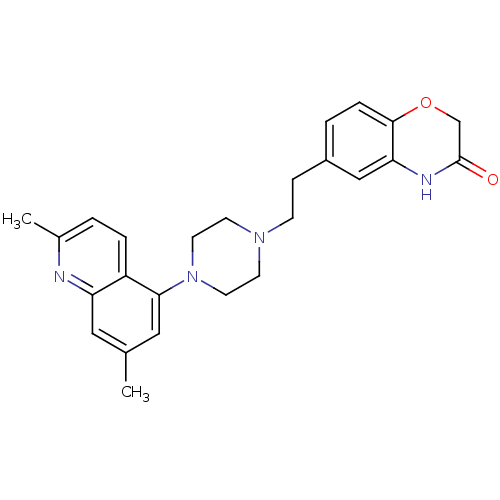
(CHEMBL484260)Show SMILES Cc1cc(N2CCN(CCc3ccc4OCC(=O)Nc4c3)CC2)c2ccc(C)nc2c1 Show InChI InChI=1S/C25H28N4O2/c1-17-13-21-20(5-3-18(2)26-21)23(14-17)29-11-9-28(10-12-29)8-7-19-4-6-24-22(15-19)27-25(30)16-31-24/h3-6,13-15H,7-12,16H2,1-2H3,(H,27,30) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.126 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]WAY-100635 from human 5HT1A receptor expressed in CHO cells |
Bioorg Med Chem Lett 18: 5653-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.08.084
BindingDB Entry DOI: 10.7270/Q2BR8TC6 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1D
(Homo sapiens (Human)) | BDBM50417424
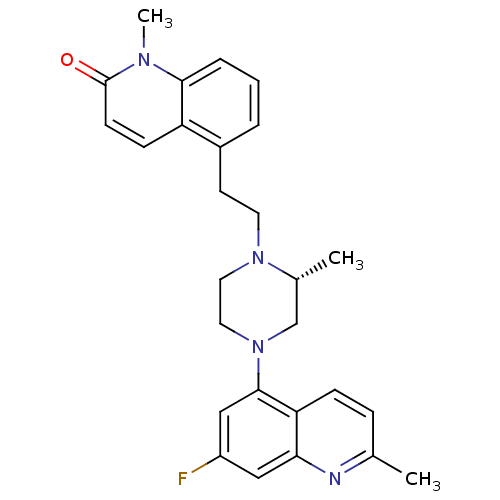
(CHEMBL1289394)Show SMILES C[C@@H]1CN(CCN1CCc1cccc2n(C)c(=O)ccc12)c1cc(F)cc2nc(C)ccc12 |r| Show InChI InChI=1S/C27H29FN4O/c1-18-7-8-23-24(29-18)15-21(28)16-26(23)32-14-13-31(19(2)17-32)12-11-20-5-4-6-25-22(20)9-10-27(33)30(25)3/h4-10,15-16,19H,11-14,17H2,1-3H3/t19-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.126 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Antagonist activity at human 5-HT1D receptor expressed in CHO cells assessed as inhibition of GTPgammaS binding by scintillation proximity assay |
Bioorg Med Chem Lett 20: 7092-6 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.085
BindingDB Entry DOI: 10.7270/Q2MC919V |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1D
(Homo sapiens (Human)) | BDBM50417411
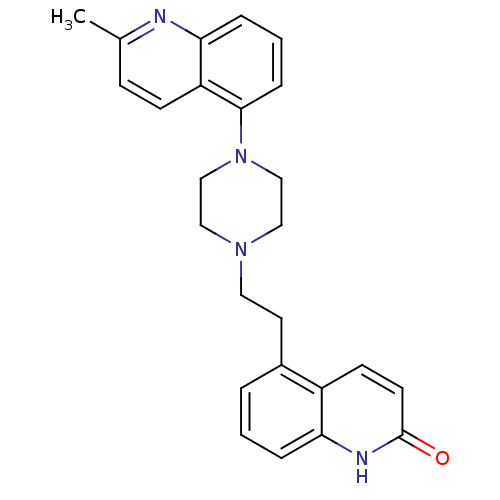
(CHEMBL1290715)Show SMILES Cc1ccc2c(cccc2n1)N1CCN(CCc2cccc3[nH]c(=O)ccc23)CC1 Show InChI InChI=1S/C25H26N4O/c1-18-8-9-21-23(26-18)6-3-7-24(21)29-16-14-28(15-17-29)13-12-19-4-2-5-22-20(19)10-11-25(30)27-22/h2-11H,12-17H2,1H3,(H,27,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.126 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Antagonist activity at human 5-HT1D receptor expressed in CHO cells assessed as inhibition of GTPgammaS binding by scintillation proximity assay |
Bioorg Med Chem Lett 20: 7092-6 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.085
BindingDB Entry DOI: 10.7270/Q2MC919V |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50413553
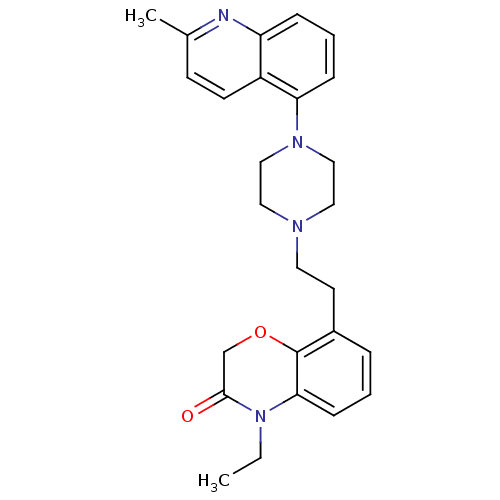
(CHEMBL472290)Show SMILES CCN1C(=O)COc2c(CCN3CCN(CC3)c3cccc4nc(C)ccc34)cccc12 Show InChI InChI=1S/C26H30N4O2/c1-3-30-24-9-4-6-20(26(24)32-18-25(30)31)12-13-28-14-16-29(17-15-28)23-8-5-7-22-21(23)11-10-19(2)27-22/h4-11H,3,12-18H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.126 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Antagonist activity at human 5HT1A assessed as GTPgammaS binding by scintillation proximity assay in presence of 5-HT |
Bioorg Med Chem Lett 19: 2338-42 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.056
BindingDB Entry DOI: 10.7270/Q2J967MX |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1D
(Homo sapiens (Human)) | BDBM50413555

(CHEMBL469568)Show SMILES Cc1ccc2c(cccc2n1)N1CCN(CCc2cccc3N(C4CC4)C(=O)COc23)CC1 Show InChI InChI=1S/C27H30N4O2/c1-19-8-11-22-23(28-19)5-3-6-24(22)30-16-14-29(15-17-30)13-12-20-4-2-7-25-27(20)33-18-26(32)31(25)21-9-10-21/h2-8,11,21H,9-10,12-18H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.158 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Antagonist activity at human 5HT1D assessed as GTPgammaS binding by scintillation proximity assay in presence of 5-HT |
Bioorg Med Chem Lett 19: 2338-42 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.056
BindingDB Entry DOI: 10.7270/Q2J967MX |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1D
(Homo sapiens (Human)) | BDBM50412441

(CHEMBL490417 | SB-744185)Show SMILES Cc1ccc2c(cccc2n1)N1CCN(CCc2ccc3OCC(=O)Nc3c2)CC1 Show InChI InChI=1S/C24H26N4O2/c1-17-5-7-19-20(25-17)3-2-4-22(19)28-13-11-27(12-14-28)10-9-18-6-8-23-21(15-18)26-24(29)16-30-23/h2-8,15H,9-14,16H2,1H3,(H,26,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.158 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]5HT from human 5HT1D receptor expressed in CHO cells |
Bioorg Med Chem Lett 18: 5653-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.08.084
BindingDB Entry DOI: 10.7270/Q2BR8TC6 |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50413083
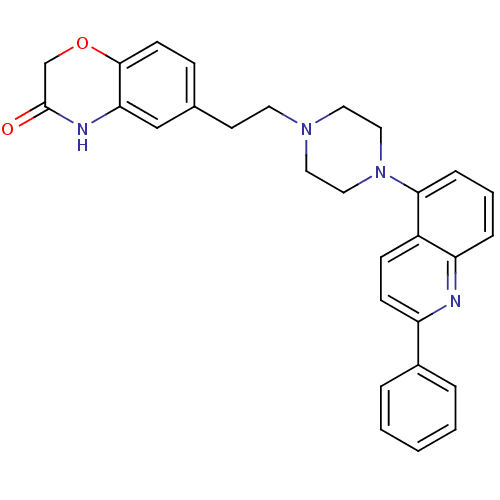
(CHEMBL484059)Show SMILES O=C1COc2ccc(CCN3CCN(CC3)c3cccc4nc(ccc34)-c3ccccc3)cc2N1 Show InChI InChI=1S/C29H28N4O2/c34-29-20-35-28-12-9-21(19-26(28)31-29)13-14-32-15-17-33(18-16-32)27-8-4-7-25-23(27)10-11-24(30-25)22-5-2-1-3-6-22/h1-12,19H,13-18,20H2,(H,31,34) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.158 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]citalopram from human SerT expressed pig LLCPK cells |
Bioorg Med Chem Lett 18: 5653-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.08.084
BindingDB Entry DOI: 10.7270/Q2BR8TC6 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1D
(Homo sapiens (Human)) | BDBM50413084
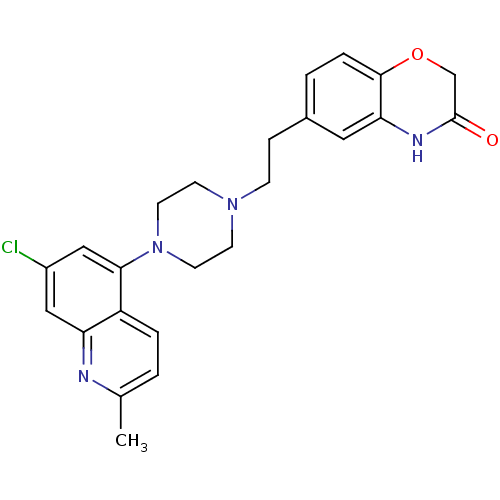
(CHEMBL521506)Show SMILES Cc1ccc2c(cc(Cl)cc2n1)N1CCN(CCc2ccc3OCC(=O)Nc3c2)CC1 Show InChI InChI=1S/C24H25ClN4O2/c1-16-2-4-19-20(26-16)13-18(25)14-22(19)29-10-8-28(9-11-29)7-6-17-3-5-23-21(12-17)27-24(30)15-31-23/h2-5,12-14H,6-11,15H2,1H3,(H,27,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.158 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]5HT from human 5HT1D receptor expressed in CHO cells |
Bioorg Med Chem Lett 18: 5653-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.08.084
BindingDB Entry DOI: 10.7270/Q2BR8TC6 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50413550

(CHEMBL469345)Show SMILES CN1C(=O)COc2c(CCN3CCN(CC3)c3cccc4nc(C)ccc34)cccc12 Show InChI InChI=1S/C25H28N4O2/c1-18-9-10-20-21(26-18)6-4-7-22(20)29-15-13-28(14-16-29)12-11-19-5-3-8-23-25(19)31-17-24(30)27(23)2/h3-10H,11-17H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.158 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Antagonist activity at human 5HT1A assessed as GTPgammaS binding by scintillation proximity assay in presence of 5-HT |
Bioorg Med Chem Lett 19: 2338-42 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.056
BindingDB Entry DOI: 10.7270/Q2J967MX |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50413550

(CHEMBL469345)Show SMILES CN1C(=O)COc2c(CCN3CCN(CC3)c3cccc4nc(C)ccc34)cccc12 Show InChI InChI=1S/C25H28N4O2/c1-18-9-10-20-21(26-18)6-4-7-22(20)29-15-13-28(14-16-29)12-11-19-5-3-8-23-25(19)31-17-24(30)27(23)2/h3-10H,11-17H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.158 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Antagonist activity at human 5-HT1A receptor expressed in HEK293 cells assessed as inhibition of GTPgammaS binding by scintillation proximity assay |
Bioorg Med Chem Lett 20: 7092-6 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.085
BindingDB Entry DOI: 10.7270/Q2MC919V |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1D
(Homo sapiens (Human)) | BDBM50413078
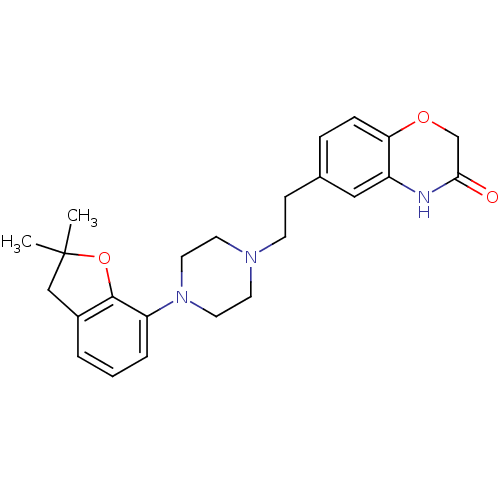
(CHEMBL491839)Show SMILES CC1(C)Cc2cccc(N3CCN(CCc4ccc5OCC(=O)Nc5c4)CC3)c2O1 Show InChI InChI=1S/C24H29N3O3/c1-24(2)15-18-4-3-5-20(23(18)30-24)27-12-10-26(11-13-27)9-8-17-6-7-21-19(14-17)25-22(28)16-29-21/h3-7,14H,8-13,15-16H2,1-2H3,(H,25,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.158 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]5HT from human 5HT1D receptor expressed in CHO cells |
Bioorg Med Chem Lett 18: 5653-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.08.084
BindingDB Entry DOI: 10.7270/Q2BR8TC6 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM86708

(CAS_146714-97-8 | CHEMBL31354 | CHEMBL514874 | CHE...)Show SMILES COc1ccccc1N1CCN(CCN(C(=O)C2CCCCC2)c2ccccn2)CC1 Show InChI InChI=1S/C25H34N4O2/c1-31-23-12-6-5-11-22(23)28-18-15-27(16-19-28)17-20-29(24-13-7-8-14-26-24)25(30)21-9-3-2-4-10-21/h5-8,11-14,21H,2-4,9-10,15-20H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.158 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]8-OH-DPAT from human recombinant 5HT1A receptor |
J Med Chem 51: 2887-90 (2008)
Article DOI: 10.1021/jm8001444
BindingDB Entry DOI: 10.7270/Q2DV1M4K |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50339685
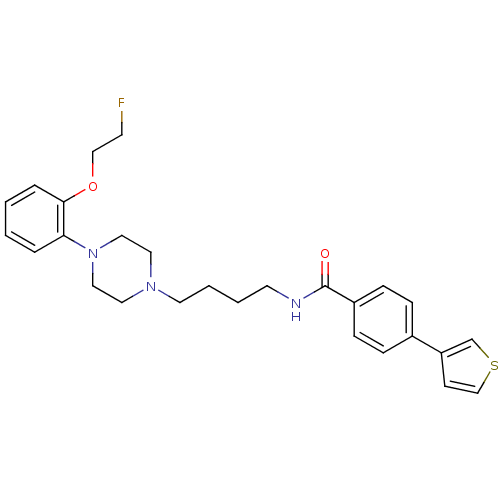
(4-(Thiophen-3-yl)-N-(4-(4-(2-(2-fluoroethoxy)pheny...)Show SMILES FCCOc1ccccc1N1CCN(CCCCNC(=O)c2ccc(cc2)-c2ccsc2)CC1 Show InChI InChI=1S/C27H32FN3O2S/c28-12-19-33-26-6-2-1-5-25(26)31-17-15-30(16-18-31)14-4-3-13-29-27(32)23-9-7-22(8-10-23)24-11-20-34-21-24/h1-2,5-11,20-21H,3-4,12-19H2,(H,29,32) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Temple University School of Pharmacy
Curated by ChEMBL
| Assay Description
Displacement of 2,3-dimethoxy-5-[125I]-iodo-N-[9-benzyl-9-azabicyclo[3.3.1]nonan-3beta-yl]benzamide from human dopamine D2L receptor expressed in HEK... |
Bioorg Med Chem Lett 29: 2690-2694 (2019)
Article DOI: 10.1016/j.bmcl.2019.07.020
BindingDB Entry DOI: 10.7270/Q2JQ14CS |
More data for this
Ligand-Target Pair | |
Transcription initiation factor TFIID subunit 1
(Homo sapiens (Human)) | BDBM50572134
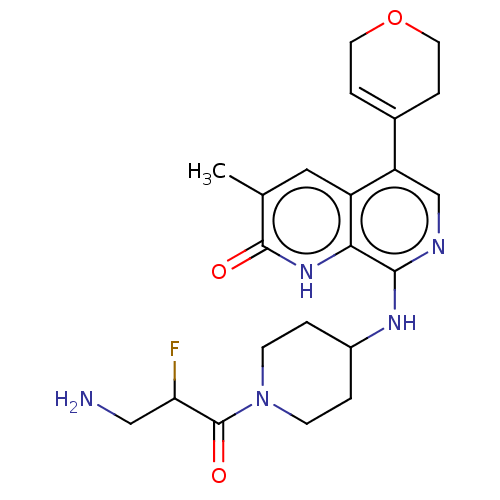
(CHEMBL4868363)Show SMILES Cc1cc2c(cnc(NC3CCN(CC3)C(=O)C(F)CN)c2[nH]c1=O)C1=CCOCC1 |t:28| | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human partial length TAF1 bromodomain 2 (D1521 to D1656 residues) expressed in bacterial expression system by BROMOscan assay |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00294
BindingDB Entry DOI: 10.7270/Q21V5JRX |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50413560

(CHEMBL469374)Show SMILES CN1C(=O)COc2c(CCN3CCN(CC3)c3cc(F)cc4nc(C)ccc34)cccc12 Show InChI InChI=1S/C25H27FN4O2/c1-17-6-7-20-21(27-17)14-19(26)15-23(20)30-12-10-29(11-13-30)9-8-18-4-3-5-22-25(18)32-16-24(31)28(22)2/h3-7,14-15H,8-13,16H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Antagonist activity at human 5HT1A assessed as GTPgammaS binding by scintillation proximity assay in presence of 5-HT |
Bioorg Med Chem Lett 19: 2338-42 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.056
BindingDB Entry DOI: 10.7270/Q2J967MX |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50413561
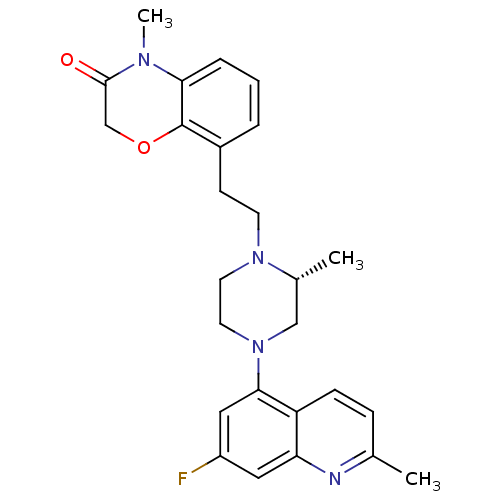
(CHEMBL513875)Show SMILES C[C@@H]1CN(CCN1CCc1cccc2N(C)C(=O)COc12)c1cc(F)cc2nc(C)ccc12 |r| Show InChI InChI=1S/C26H29FN4O2/c1-17-7-8-21-22(28-17)13-20(27)14-24(21)31-12-11-30(18(2)15-31)10-9-19-5-4-6-23-26(19)33-16-25(32)29(23)3/h4-8,13-14,18H,9-12,15-16H2,1-3H3/t18-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Antagonist activity at human 5HT1A assessed as GTPgammaS binding by scintillation proximity assay in presence of 5-HT |
Bioorg Med Chem Lett 19: 2338-42 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.056
BindingDB Entry DOI: 10.7270/Q2J967MX |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1D
(Homo sapiens (Human)) | BDBM50413553

(CHEMBL472290)Show SMILES CCN1C(=O)COc2c(CCN3CCN(CC3)c3cccc4nc(C)ccc34)cccc12 Show InChI InChI=1S/C26H30N4O2/c1-3-30-24-9-4-6-20(26(24)32-18-25(30)31)12-13-28-14-16-29(17-15-28)23-8-5-7-22-21(23)11-10-19(2)27-22/h4-11H,3,12-18H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Antagonist activity at human 5HT1D assessed as GTPgammaS binding by scintillation proximity assay in presence of 5-HT |
Bioorg Med Chem Lett 19: 2338-42 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.056
BindingDB Entry DOI: 10.7270/Q2J967MX |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50286333
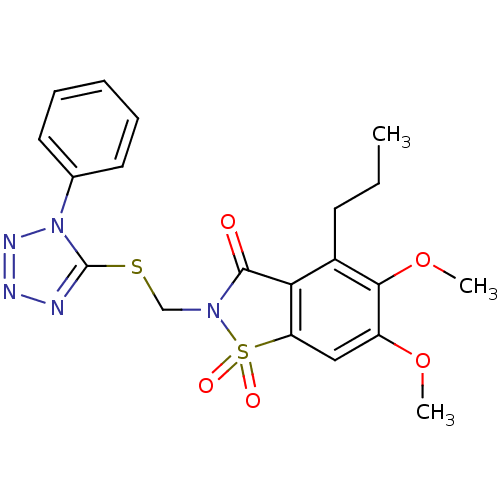
(5,6-Dimethoxy-1,1-dioxo-2-(1-phenyl-1H-tetrazol-5-...)Show SMILES CCCc1c2C(=O)N(CSc3nnnn3-c3ccccc3)S(=O)(=O)c2cc(OC)c1OC Show InChI InChI=1S/C20H21N5O5S2/c1-4-8-14-17-16(11-15(29-2)18(14)30-3)32(27,28)24(19(17)26)12-31-20-21-22-23-25(20)13-9-6-5-7-10-13/h5-7,9-11H,4,8,12H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Evaluated for inhibitory activity against Human leukocyte elastase (HLE) |
Bioorg Med Chem Lett 5: 331-336 (1995)
Article DOI: 10.1016/0960-894X(95)00030-W
BindingDB Entry DOI: 10.7270/Q2SX6D60 |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50036482
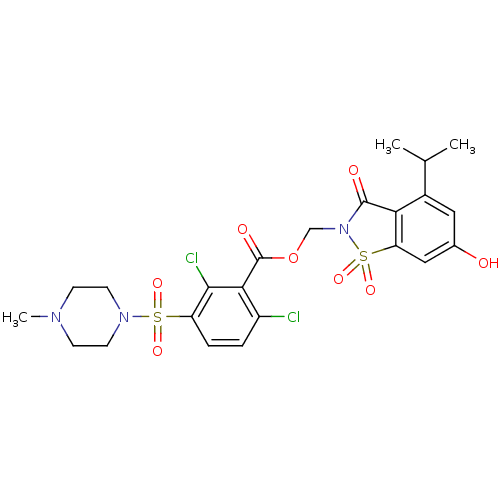
(2,6-Dichloro-3-(4-methyl-piperazine-1-sulfonyl)-be...)Show SMILES CC(C)c1cc(O)cc2c1C(=O)N(COC(=O)c1c(Cl)ccc(c1Cl)S(=O)(=O)N1CCN(C)CC1)S2(=O)=O Show InChI InChI=1S/C23H25Cl2N3O8S2/c1-13(2)15-10-14(29)11-18-19(15)22(30)28(38(18,34)35)12-36-23(31)20-16(24)4-5-17(21(20)25)37(32,33)27-8-6-26(3)7-9-27/h4-5,10-11,13,29H,6-9,12H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling Winthrop Inc.
Curated by ChEMBL
| Assay Description
Potency of inhibition against human leukocyte elastase (HLE) expressed as an apparent binding constant |
J Med Chem 38: 739-44 (1995)
BindingDB Entry DOI: 10.7270/Q2W66JTD |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1D
(Homo sapiens (Human)) | BDBM50413085
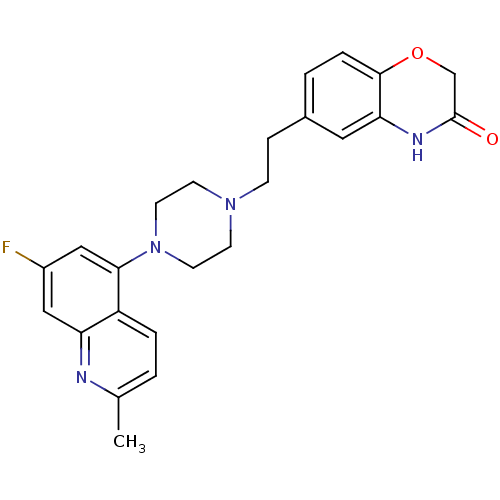
(CHEMBL485491)Show SMILES Cc1ccc2c(cc(F)cc2n1)N1CCN(CCc2ccc3OCC(=O)Nc3c2)CC1 Show InChI InChI=1S/C24H25FN4O2/c1-16-2-4-19-20(26-16)13-18(25)14-22(19)29-10-8-28(9-11-29)7-6-17-3-5-23-21(12-17)27-24(30)15-31-23/h2-5,12-14H,6-11,15H2,1H3,(H,27,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]5HT from human 5HT1D receptor expressed in CHO cells |
Bioorg Med Chem Lett 18: 5653-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.08.084
BindingDB Entry DOI: 10.7270/Q2BR8TC6 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1B
(Homo sapiens (Human)) | BDBM50412441

(CHEMBL490417 | SB-744185)Show SMILES Cc1ccc2c(cccc2n1)N1CCN(CCc2ccc3OCC(=O)Nc3c2)CC1 Show InChI InChI=1S/C24H26N4O2/c1-17-5-7-19-20(25-17)3-2-4-22(19)28-13-11-27(12-14-28)10-9-18-6-8-23-21(15-18)26-24(29)16-30-23/h2-8,15H,9-14,16H2,1H3,(H,26,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]5HT from human 5HT1B receptor expressed in CHO cells |
Bioorg Med Chem Lett 18: 5653-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.08.084
BindingDB Entry DOI: 10.7270/Q2BR8TC6 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1D
(Homo sapiens (Human)) | BDBM50413075
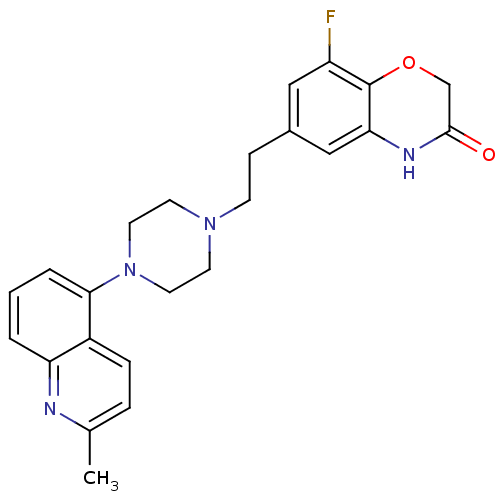
(CHEMBL489394)Show SMILES Cc1ccc2c(cccc2n1)N1CCN(CCc2cc(F)c3OCC(=O)Nc3c2)CC1 Show InChI InChI=1S/C24H25FN4O2/c1-16-5-6-18-20(26-16)3-2-4-22(18)29-11-9-28(10-12-29)8-7-17-13-19(25)24-21(14-17)27-23(30)15-31-24/h2-6,13-14H,7-12,15H2,1H3,(H,27,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]5HT from human 5HT1D receptor expressed in CHO cells |
Bioorg Med Chem Lett 18: 5653-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.08.084
BindingDB Entry DOI: 10.7270/Q2BR8TC6 |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM50512803

(CHEMBL4567895)Show SMILES COc1ccccc1N1CCN(CCCCNC(=O)c2ccc(cc2)-c2ccsc2)CC1 Show InChI InChI=1S/C26H31N3O2S/c1-31-25-7-3-2-6-24(25)29-17-15-28(16-18-29)14-5-4-13-27-26(30)22-10-8-21(9-11-22)23-12-19-32-20-23/h2-3,6-12,19-20H,4-5,13-18H2,1H3,(H,27,30) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Temple University School of Pharmacy
Curated by ChEMBL
| Assay Description
Displacement of 2,3-dimethoxy-5-[125I]-iodo-N-[9-benzyl-9-azabicyclo[3.3.1]nonan-3beta-yl]benzamide from human dopamine D3 receptor expressed in HEK2... |
Bioorg Med Chem Lett 29: 2690-2694 (2019)
Article DOI: 10.1016/j.bmcl.2019.07.020
BindingDB Entry DOI: 10.7270/Q2JQ14CS |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1D
(Homo sapiens (Human)) | BDBM50413079
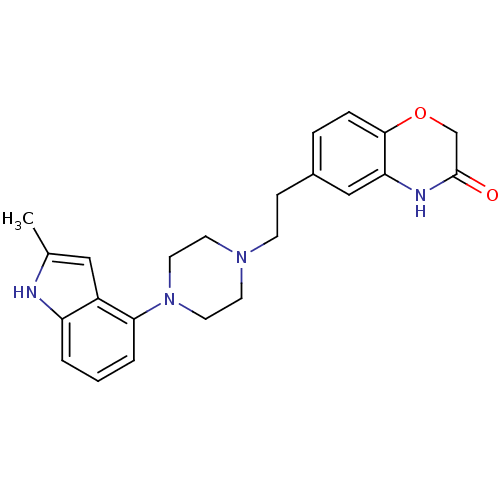
(CHEMBL444398)Show SMILES Cc1cc2c(cccc2[nH]1)N1CCN(CCc2ccc3OCC(=O)Nc3c2)CC1 Show InChI InChI=1S/C23H26N4O2/c1-16-13-18-19(24-16)3-2-4-21(18)27-11-9-26(10-12-27)8-7-17-5-6-22-20(14-17)25-23(28)15-29-22/h2-6,13-14,24H,7-12,15H2,1H3,(H,25,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]5HT from human 5HT1D receptor expressed in CHO cells |
Bioorg Med Chem Lett 18: 5653-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.08.084
BindingDB Entry DOI: 10.7270/Q2BR8TC6 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1D
(Homo sapiens (Human)) | BDBM50413087
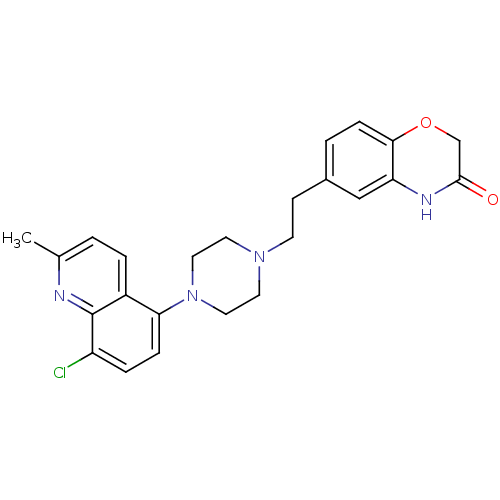
(CHEMBL497980)Show SMILES Cc1ccc2c(ccc(Cl)c2n1)N1CCN(CCc2ccc3OCC(=O)Nc3c2)CC1 Show InChI InChI=1S/C24H25ClN4O2/c1-16-2-4-18-21(6-5-19(25)24(18)26-16)29-12-10-28(11-13-29)9-8-17-3-7-22-20(14-17)27-23(30)15-31-22/h2-7,14H,8-13,15H2,1H3,(H,27,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]5HT from human 5HT1D receptor expressed in CHO cells |
Bioorg Med Chem Lett 18: 5653-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.08.084
BindingDB Entry DOI: 10.7270/Q2BR8TC6 |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM50512799
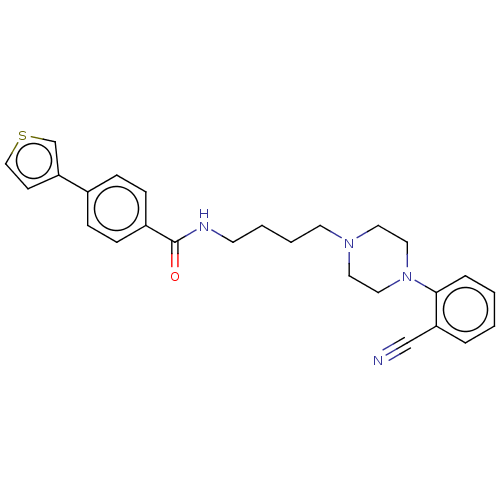
(CHEMBL4571568)Show SMILES O=C(NCCCCN1CCN(CC1)c1ccccc1C#N)c1ccc(cc1)-c1ccsc1 Show InChI InChI=1S/C26H28N4OS/c27-19-23-5-1-2-6-25(23)30-16-14-29(15-17-30)13-4-3-12-28-26(31)22-9-7-21(8-10-22)24-11-18-32-20-24/h1-2,5-11,18,20H,3-4,12-17H2,(H,28,31) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Temple University School of Pharmacy
Curated by ChEMBL
| Assay Description
Displacement of 2,3-dimethoxy-5-[125I]-iodo-N-[9-benzyl-9-azabicyclo[3.3.1]nonan-3beta-yl]benzamide from human dopamine D3 receptor expressed in HEK2... |
Bioorg Med Chem Lett 29: 2690-2694 (2019)
Article DOI: 10.1016/j.bmcl.2019.07.020
BindingDB Entry DOI: 10.7270/Q2JQ14CS |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50413068
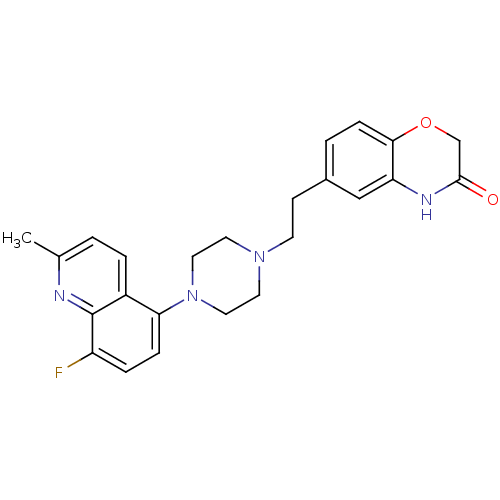
(CHEMBL497979)Show SMILES Cc1ccc2c(ccc(F)c2n1)N1CCN(CCc2ccc3OCC(=O)Nc3c2)CC1 Show InChI InChI=1S/C24H25FN4O2/c1-16-2-4-18-21(6-5-19(25)24(18)26-16)29-12-10-28(11-13-29)9-8-17-3-7-22-20(14-17)27-23(30)15-31-22/h2-7,14H,8-13,15H2,1H3,(H,27,30) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.251 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]WAY-100635 from human 5HT1A receptor expressed in CHO cells |
Bioorg Med Chem Lett 18: 5653-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.08.084
BindingDB Entry DOI: 10.7270/Q2BR8TC6 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50413073
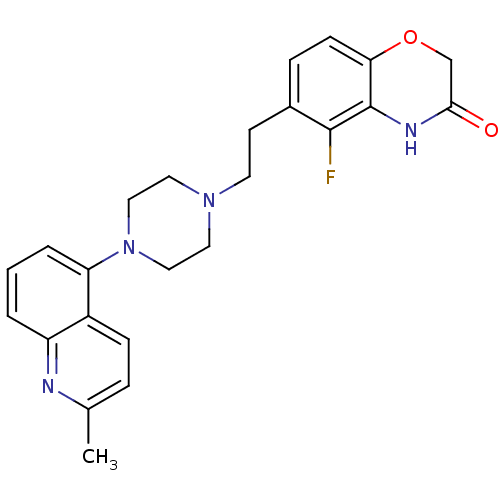
(CHEMBL496520)Show SMILES Cc1ccc2c(cccc2n1)N1CCN(CCc2ccc3OCC(=O)Nc3c2F)CC1 Show InChI InChI=1S/C24H25FN4O2/c1-16-5-7-18-19(26-16)3-2-4-20(18)29-13-11-28(12-14-29)10-9-17-6-8-21-24(23(17)25)27-22(30)15-31-21/h2-8H,9-15H2,1H3,(H,27,30) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.251 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]WAY-100635 from human 5HT1A receptor expressed in CHO cells |
Bioorg Med Chem Lett 18: 5653-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.08.084
BindingDB Entry DOI: 10.7270/Q2BR8TC6 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data