

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Serine/threonine-protein kinase Chk1 (Homo sapiens (Human)) | BDBM50222915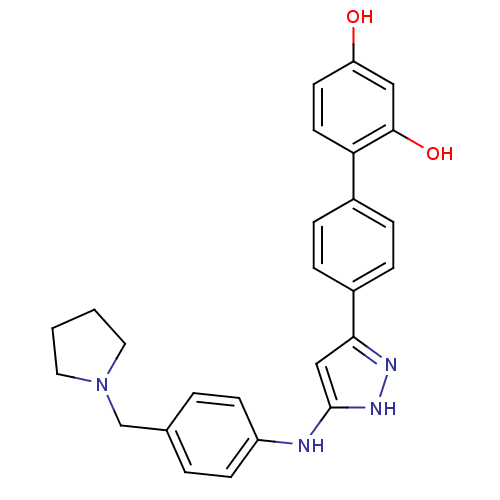 (4'-[5-(4-pyrrolidin-1-ylmethyl-phenylamino)-1H-pyr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of human CHK1 expressed in baculovirus/insect cell system | J Med Chem 50: 5253-6 (2007) Article DOI: 10.1021/jm0704604 BindingDB Entry DOI: 10.7270/Q2K0754D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase Chk1 (Homo sapiens (Human)) | BDBM50222916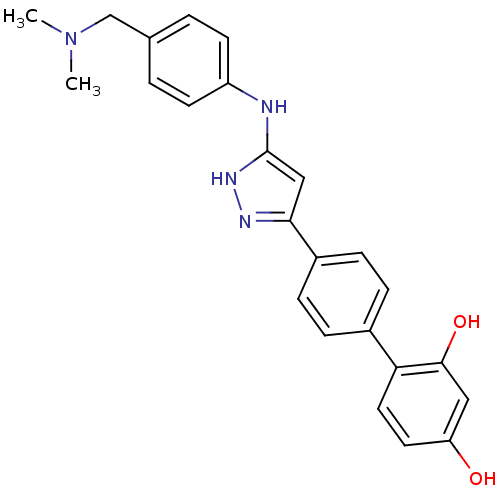 (4'-{5-[4-(dimethylamino-methyl)-phenylamino]-2H-py...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of human CHK1 expressed in baculovirus/insect cell system | J Med Chem 50: 5253-6 (2007) Article DOI: 10.1021/jm0704604 BindingDB Entry DOI: 10.7270/Q2K0754D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase Chk1 (Homo sapiens (Human)) | BDBM50222920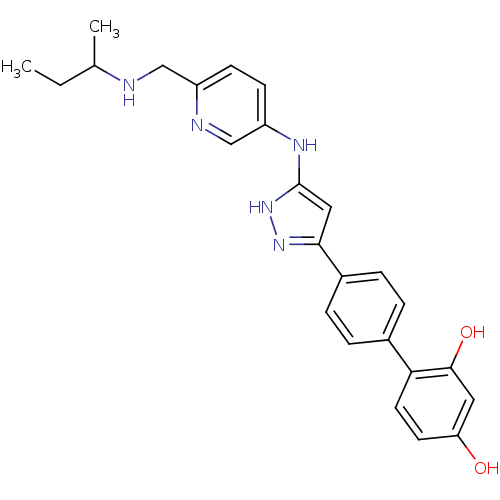 (4'-{5-[6-(sec-butylamino-methyl)-pyridin-3-ylamino...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of human CHK1 expressed in baculovirus/insect cell system | J Med Chem 50: 5253-6 (2007) Article DOI: 10.1021/jm0704604 BindingDB Entry DOI: 10.7270/Q2K0754D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase Chk1 (Homo sapiens (Human)) | BDBM50222917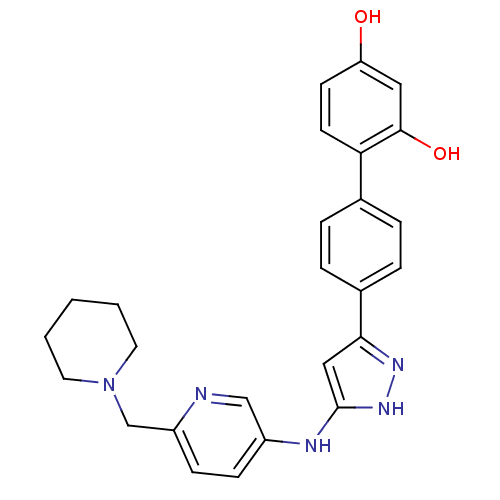 (4'-[5-(6-piperidin-1-ylmethyl-pyridin-3-ylamino)-1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of human CHK1 expressed in baculovirus/insect cell system | J Med Chem 50: 5253-6 (2007) Article DOI: 10.1021/jm0704604 BindingDB Entry DOI: 10.7270/Q2K0754D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase Chk1 (Homo sapiens (Human)) | BDBM50222913 (4'-{5-[4-(isopropylamino-methyl)-phenylamino]-2H-p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of human CHK1 expressed in baculovirus/insect cell system | J Med Chem 50: 5253-6 (2007) Article DOI: 10.1021/jm0704604 BindingDB Entry DOI: 10.7270/Q2K0754D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase Chk1 (Homo sapiens (Human)) | BDBM50222914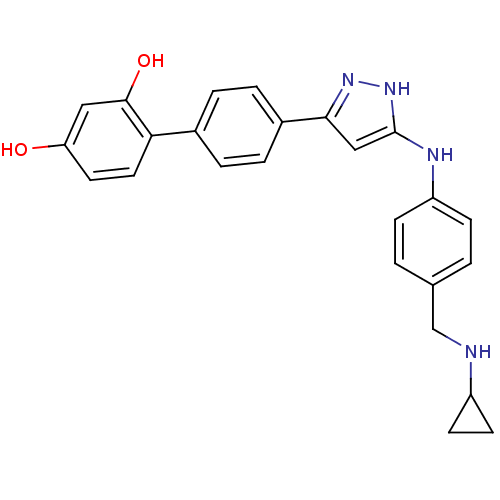 (4'-[5-(4-cyclopropylaminomethyl-phenylamino)-2H-py...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid UniChem Patents Similars | Article PubMed | 0.380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of human CHK1 expressed in baculovirus/insect cell system | J Med Chem 50: 5253-6 (2007) Article DOI: 10.1021/jm0704604 BindingDB Entry DOI: 10.7270/Q2K0754D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase Chk1 (Homo sapiens (Human)) | BDBM50222921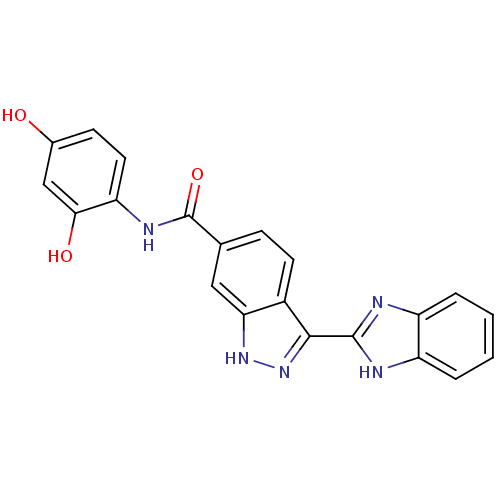 (3-(1H-benzo[d]imidazol-2-yl)-N-(2,4-dihydroxypheny...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of human CHK1 expressed in baculovirus/insect cell system | J Med Chem 50: 5253-6 (2007) Article DOI: 10.1021/jm0704604 BindingDB Entry DOI: 10.7270/Q2K0754D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase Chk1 (Homo sapiens (Human)) | BDBM50222919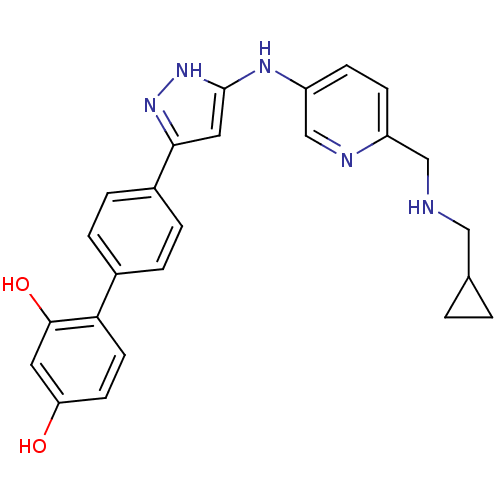 (4'-(5-{6-[(cyclopropylmethyl-amino)-methyl]-pyridi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of human CHK1 expressed in baculovirus/insect cell system | J Med Chem 50: 5253-6 (2007) Article DOI: 10.1021/jm0704604 BindingDB Entry DOI: 10.7270/Q2K0754D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50517230 (CHEMBL4467984) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.840 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland Curated by ChEMBL | Assay Description Binding affinity to human D2R | Bioorg Med Chem Lett 29: (2019) Article DOI: 10.1016/j.bmcl.2019.126644 BindingDB Entry DOI: 10.7270/Q2QV3QW7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50517232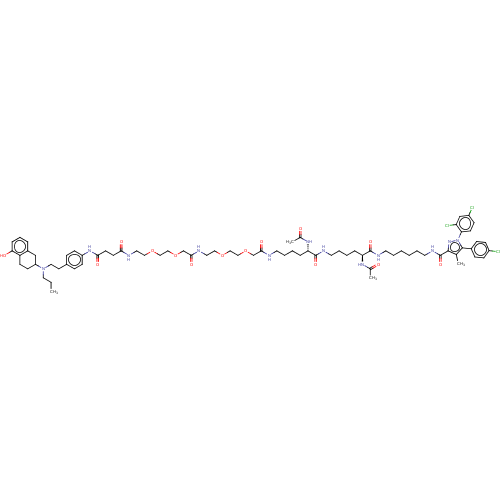 (CHEMBL4546839) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland Curated by ChEMBL | Assay Description Binding affinity to human D2R | Bioorg Med Chem Lett 29: (2019) Article DOI: 10.1016/j.bmcl.2019.126644 BindingDB Entry DOI: 10.7270/Q2QV3QW7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase Chk1 (Homo sapiens (Human)) | BDBM50222918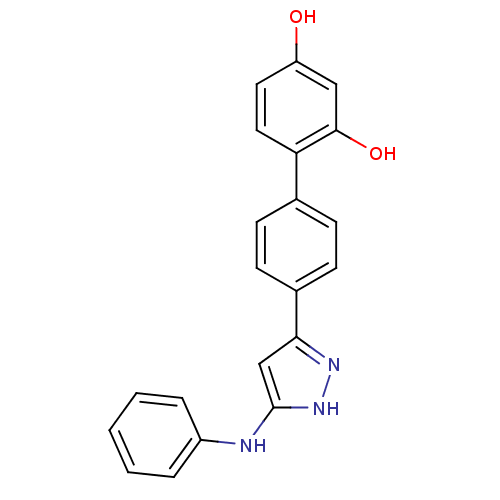 (4'-(5-phenylamino-2H-pyrazol-3-yl)-biphenyl-2,4-di...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of human CHK1 expressed in baculovirus/insect cell system | J Med Chem 50: 5253-6 (2007) Article DOI: 10.1021/jm0704604 BindingDB Entry DOI: 10.7270/Q2K0754D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase Chk1 (Homo sapiens (Human)) | BDBM92906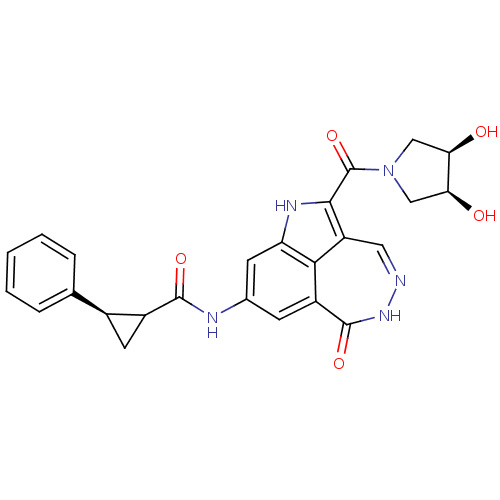 (CHK1 compound 1) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.75 | -49.5 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Pfizer | Assay Description The inhibitors reported in this study bind to CHK1 according to a general mechanism illustrated in Scheme 1 where E, S, and I stand for enzyme, subst... | Biochemistry 48: 9823-30 (2009) Article DOI: 10.1021/bi900258v BindingDB Entry DOI: 10.7270/Q25M649B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50517229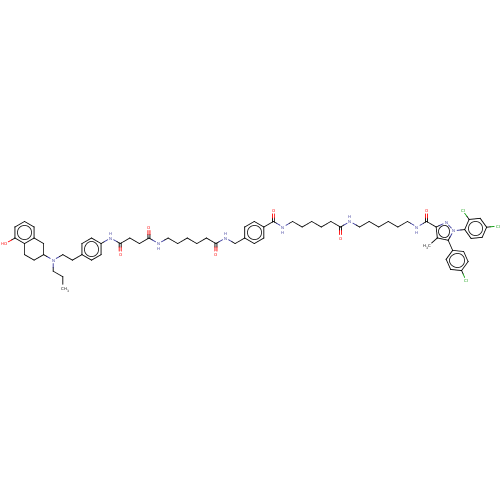 (CHEMBL4541515) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland Curated by ChEMBL | Assay Description Binding affinity to human D2R | Bioorg Med Chem Lett 29: (2019) Article DOI: 10.1016/j.bmcl.2019.126644 BindingDB Entry DOI: 10.7270/Q2QV3QW7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50517236 (CHEMBL4465127) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland Curated by ChEMBL | Assay Description Binding affinity to human D2R | Bioorg Med Chem Lett 29: (2019) Article DOI: 10.1016/j.bmcl.2019.126644 BindingDB Entry DOI: 10.7270/Q2QV3QW7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50517222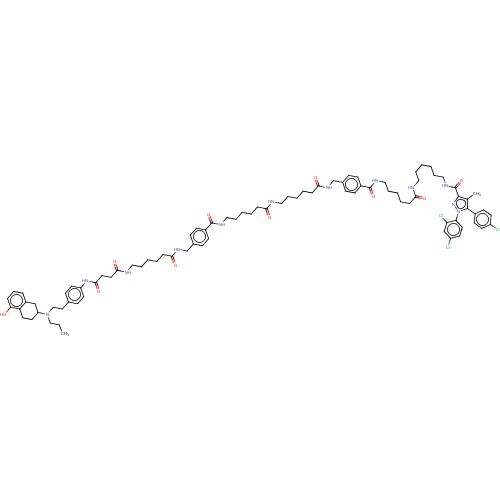 (CHEMBL4579585) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland Curated by ChEMBL | Assay Description Binding affinity to human D2R | Bioorg Med Chem Lett 29: (2019) Article DOI: 10.1016/j.bmcl.2019.126644 BindingDB Entry DOI: 10.7270/Q2QV3QW7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase Chk1 (Homo sapiens (Human)) | BDBM92906 (CHK1 compound 1) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 5.14 | -46.5 | n/a | n/a | n/a | n/a | n/a | 8.0 | 20 |
Pfizer | Assay Description Surface plasmon resonance (SPR) biosensor binding studies were conducted using a Biacore 3000 instrument (GE Healtchare). | Biochemistry 48: 9823-30 (2009) Article DOI: 10.1021/bi900258v BindingDB Entry DOI: 10.7270/Q25M649B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50517233 (CHEMBL4568756) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 8.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from human CB1R in HEK293 cell membranes after 60 mins liquid scintillation analysis | Bioorg Med Chem Lett 29: (2019) Article DOI: 10.1016/j.bmcl.2019.126644 BindingDB Entry DOI: 10.7270/Q2QV3QW7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50517221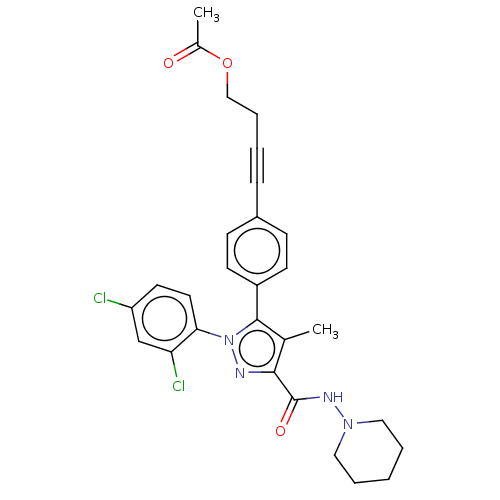 (CHEMBL4554135) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from human CB1R in HEK293 cell membranes after 60 mins liquid scintillation analysis | Bioorg Med Chem Lett 29: (2019) Article DOI: 10.1016/j.bmcl.2019.126644 BindingDB Entry DOI: 10.7270/Q2QV3QW7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50517223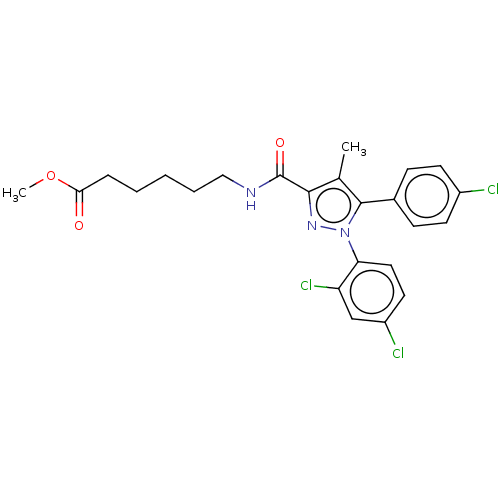 (CHEMBL4471116) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from human CB1R in HEK293 cell membranes after 60 mins liquid scintillation analysis | Bioorg Med Chem Lett 29: (2019) Article DOI: 10.1016/j.bmcl.2019.126644 BindingDB Entry DOI: 10.7270/Q2QV3QW7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50010289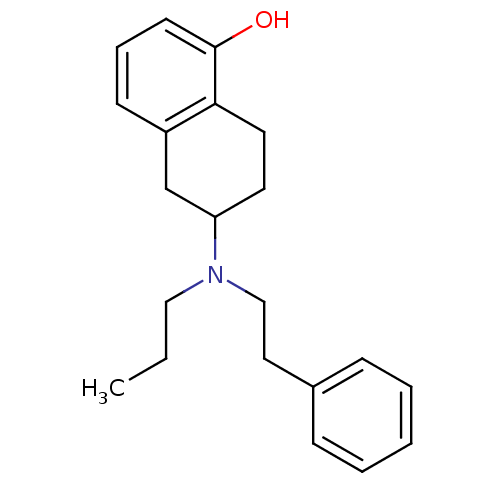 ((R)6-(Phenethyl-propyl-amino)-5,6,7,8-tetrahydro-n...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland Curated by ChEMBL | Assay Description Displacement of [3H] spiperone from human D2 dopamine receptor expressed in monkey caudate-putamen membranes | Bioorg Med Chem Lett 29: (2019) Article DOI: 10.1016/j.bmcl.2019.126644 BindingDB Entry DOI: 10.7270/Q2QV3QW7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50517226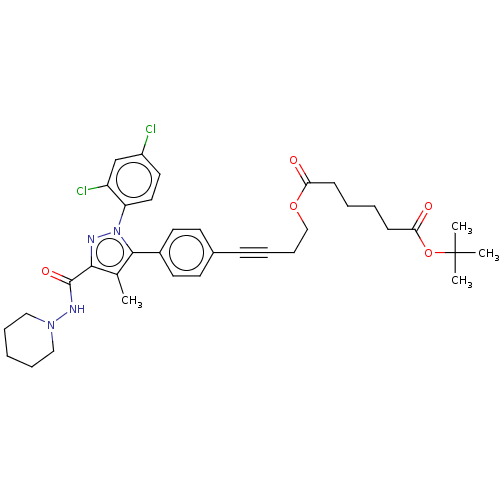 (CHEMBL4449666) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 45 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from human CB1R in HEK293 cell membranes after 60 mins liquid scintillation analysis | Bioorg Med Chem Lett 29: (2019) Article DOI: 10.1016/j.bmcl.2019.126644 BindingDB Entry DOI: 10.7270/Q2QV3QW7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50517227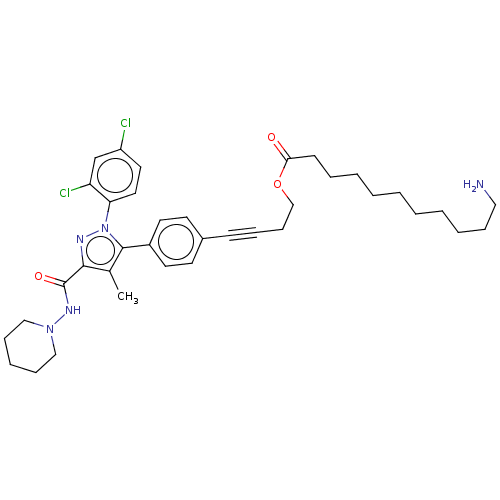 (CHEMBL4562705) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 97 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from human CB1R in HEK293 cell membranes after 60 mins liquid scintillation analysis | Bioorg Med Chem Lett 29: (2019) Article DOI: 10.1016/j.bmcl.2019.126644 BindingDB Entry DOI: 10.7270/Q2QV3QW7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50517225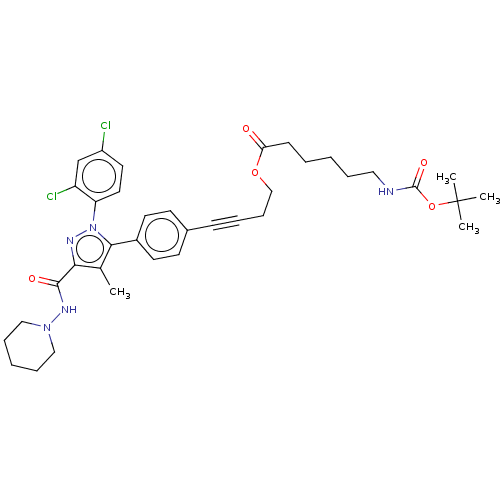 (CHEMBL4453578) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 106 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from human CB1R in HEK293 cell membranes after 60 mins liquid scintillation analysis | Bioorg Med Chem Lett 29: (2019) Article DOI: 10.1016/j.bmcl.2019.126644 BindingDB Entry DOI: 10.7270/Q2QV3QW7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50517224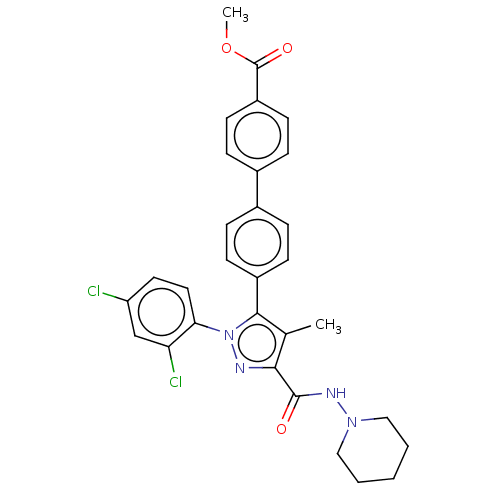 (CHEMBL4443273) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 131 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from human CB1R in HEK293 cell membranes after 60 mins liquid scintillation analysis | Bioorg Med Chem Lett 29: (2019) Article DOI: 10.1016/j.bmcl.2019.126644 BindingDB Entry DOI: 10.7270/Q2QV3QW7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase Chk1 (Homo sapiens (Human)) | BDBM92908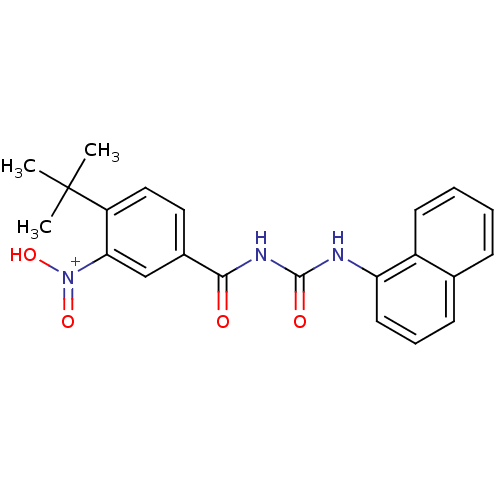 (CHK1 compound 3) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 146 | -38.6 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Pfizer | Assay Description The inhibitors reported in this study bind to CHK1 according to a general mechanism illustrated in Scheme 1 where E, S, and I stand for enzyme, subst... | Biochemistry 48: 9823-30 (2009) Article DOI: 10.1021/bi900258v BindingDB Entry DOI: 10.7270/Q25M649B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50517234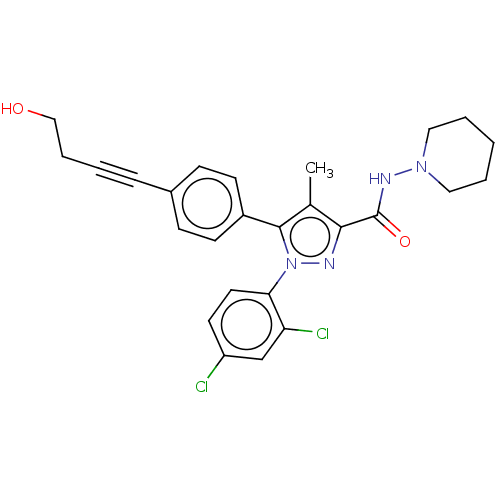 (CHEMBL4446228) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from human CB1R in HEK293 cell membranes after 60 mins liquid scintillation analysis | Bioorg Med Chem Lett 29: (2019) Article DOI: 10.1016/j.bmcl.2019.126644 BindingDB Entry DOI: 10.7270/Q2QV3QW7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50517235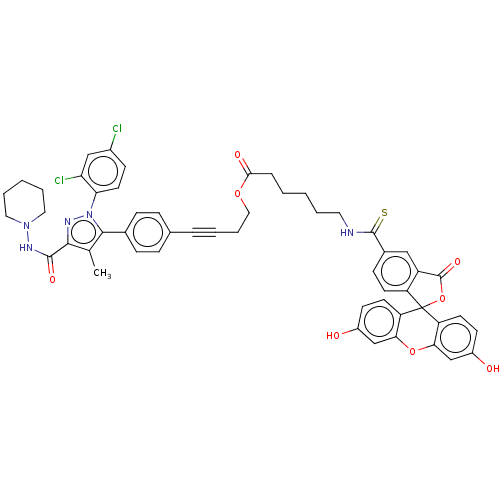 (CHEMBL4444520) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from human CB1R in HEK293 cell membranes after 60 mins liquid scintillation analysis | Bioorg Med Chem Lett 29: (2019) Article DOI: 10.1016/j.bmcl.2019.126644 BindingDB Entry DOI: 10.7270/Q2QV3QW7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase Chk1 (Homo sapiens (Human)) | BDBM92908 (CHK1 compound 3) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 290 | -36.7 | n/a | n/a | n/a | n/a | n/a | 8.0 | 20 |
Pfizer | Assay Description Surface plasmon resonance (SPR) biosensor binding studies were conducted using a Biacore 3000 instrument (GE Healtchare). | Biochemistry 48: 9823-30 (2009) Article DOI: 10.1021/bi900258v BindingDB Entry DOI: 10.7270/Q25M649B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase Chk1 (Homo sapiens (Human)) | BDBM50222922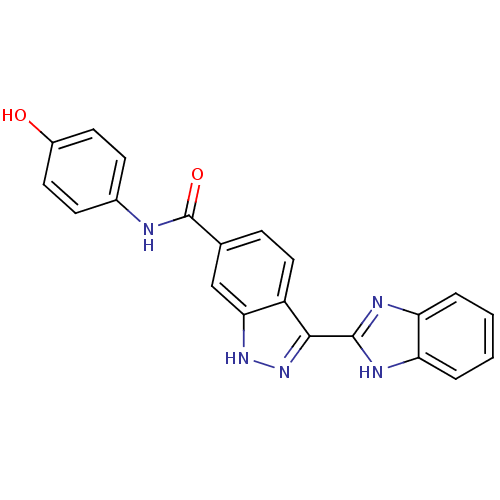 (3-(1H-benzo[d]imidazol-2-yl)-N-(4-hydroxyphenyl)-1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 301 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of human CHK1 expressed in baculovirus/insect cell system | J Med Chem 50: 5253-6 (2007) Article DOI: 10.1021/jm0704604 BindingDB Entry DOI: 10.7270/Q2K0754D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-A2 [171-432]/Cyclin-dependent kinase 2 (Homo sapiens (Human)) | BDBM5566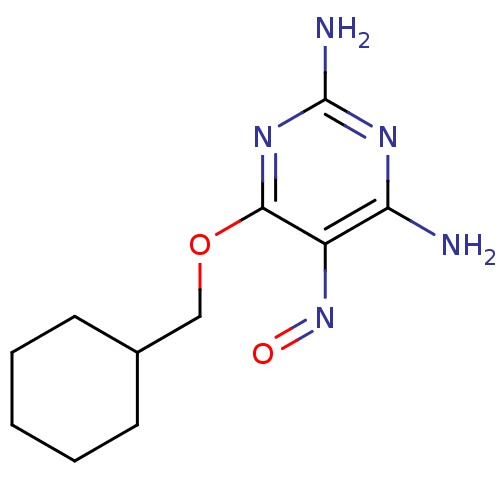 (2,6-Diamino-4-cyclohexylmethoxy-5-nitrosopyrimidin...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 1.30E+3 | -34.2 | n/a | n/a | n/a | n/a | n/a | 7.5 | 30 |
University of Newcastle | Assay Description The enzyme was assayed with substrate histone H1 in the presence of 12.5 uM ATP/[gamma-32P] ATP. IC50 is the inhibitor concentration, which inhibits ... | J Med Chem 43: 2797-804 (2000) Article DOI: 10.1021/jm990628o BindingDB Entry DOI: 10.7270/Q20R9MKP | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50517231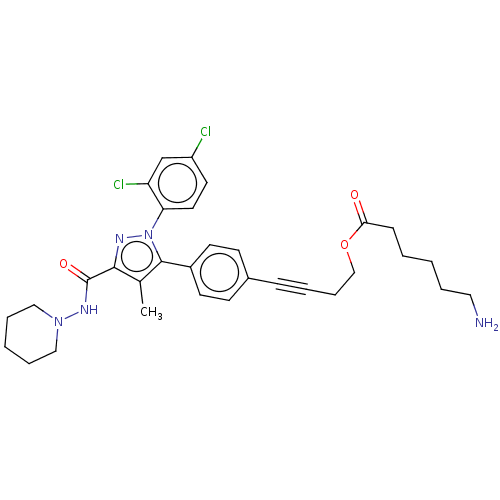 (CHEMBL4517197) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.81E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from human CB1R in HEK293 cell membranes after 60 mins liquid scintillation analysis | Bioorg Med Chem Lett 29: (2019) Article DOI: 10.1016/j.bmcl.2019.126644 BindingDB Entry DOI: 10.7270/Q2QV3QW7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase Chk1 (Homo sapiens (Human)) | BDBM92907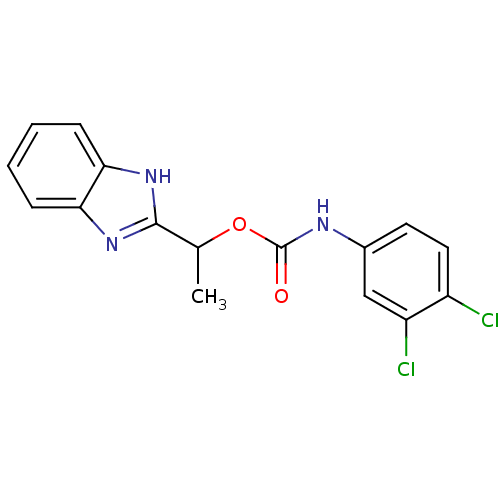 (CHK1 compound 2) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | 1.89E+3 | -32.3 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Pfizer | Assay Description The inhibitors reported in this study bind to CHK1 according to a general mechanism illustrated in Scheme 1 where E, S, and I stand for enzyme, subst... | Biochemistry 48: 9823-30 (2009) Article DOI: 10.1021/bi900258v BindingDB Entry DOI: 10.7270/Q25M649B | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50517228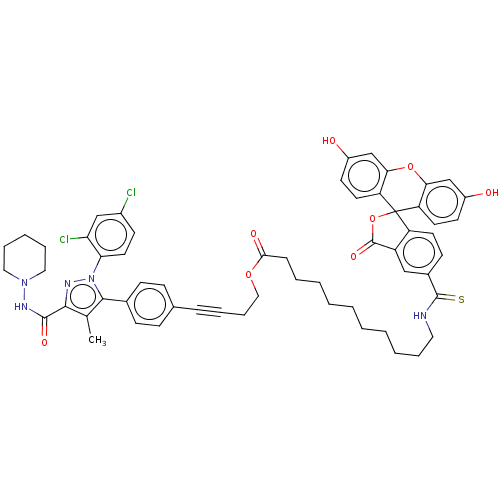 (CHEMBL4461338) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.11E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from human CB1R in HEK293 cell membranes after 60 mins liquid scintillation analysis | Bioorg Med Chem Lett 29: (2019) Article DOI: 10.1016/j.bmcl.2019.126644 BindingDB Entry DOI: 10.7270/Q2QV3QW7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 1/G2/mitotic-specific cyclin-B (Marthasterias glacialis (starfish)) | BDBM5566 (2,6-Diamino-4-cyclohexylmethoxy-5-nitrosopyrimidin...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 2.50E+3 | -32.5 | n/a | n/a | n/a | n/a | n/a | 7.5 | 30 |
University of Newcastle | Assay Description The enzyme was assayed with substrate histone H1 in the presence of 12.5 uM ATP/[gamma-32P] ATP. IC50 is the inhibitor concentration, which inhibits ... | J Med Chem 43: 2797-804 (2000) Article DOI: 10.1021/jm990628o BindingDB Entry DOI: 10.7270/Q20R9MKP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 1/G2/mitotic-specific cyclin-B (Marthasterias glacialis (starfish)) | BDBM5485 (6-(cyclohexylmethoxy)-9H-purin-2-amine | CHEMBL269...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 5.00E+3 | -30.8 | n/a | n/a | n/a | n/a | n/a | 7.5 | 30 |
University of Newcastle | Assay Description The enzyme was assayed with substrate histone H1 in the presence of 12.5 uM ATP/[gamma-32P] ATP. IC50 is the inhibitor concentration, which inhibits ... | J Med Chem 43: 2797-804 (2000) Article DOI: 10.1021/jm990628o BindingDB Entry DOI: 10.7270/Q20R9MKP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-A2 [171-432]/Cyclin-dependent kinase 2 (Homo sapiens (Human)) | BDBM5485 (6-(cyclohexylmethoxy)-9H-purin-2-amine | CHEMBL269...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 1.20E+4 | -28.6 | n/a | n/a | n/a | n/a | n/a | 7.5 | 30 |
University of Newcastle | Assay Description The enzyme was assayed with substrate histone H1 in the presence of 12.5 uM ATP/[gamma-32P] ATP. IC50 is the inhibitor concentration, which inhibits ... | J Med Chem 43: 2797-804 (2000) Article DOI: 10.1021/jm990628o BindingDB Entry DOI: 10.7270/Q20R9MKP | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Peptide deformylase (Streptococcus pneumoniae serotype 4 (strain ATCC B...) | BDBM50383996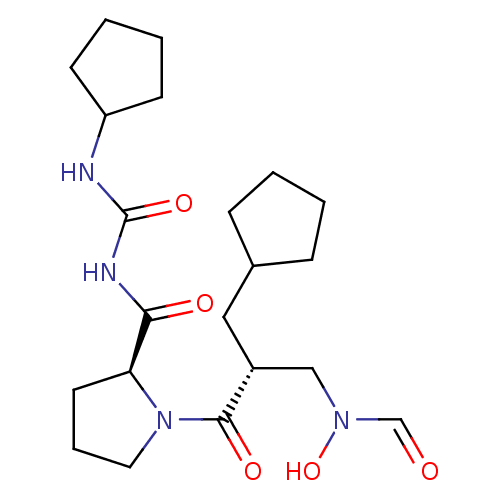 (CHEMBL2032134) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of Streptococcus pneumoniae PDF assessed as formate release from fMAS peptide substrate after 20 mins by formate dehydrogenase coupled ass... | Bioorg Med Chem Lett 22: 4028-32 (2012) Article DOI: 10.1016/j.bmcl.2012.04.086 BindingDB Entry DOI: 10.7270/Q2K64K3V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptide deformylase (Streptococcus pneumoniae serotype 4 (strain ATCC B...) | BDBM50383999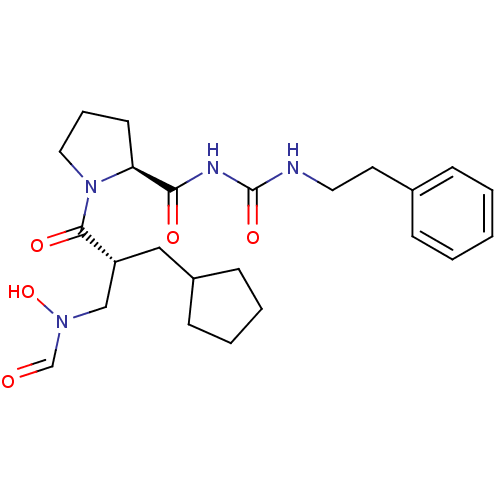 (CHEMBL2032137) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of Streptococcus pneumoniae PDF assessed as formate release from fMAS peptide substrate after 20 mins by formate dehydrogenase coupled ass... | Bioorg Med Chem Lett 22: 4028-32 (2012) Article DOI: 10.1016/j.bmcl.2012.04.086 BindingDB Entry DOI: 10.7270/Q2K64K3V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptide deformylase (Streptococcus pneumoniae serotype 4 (strain ATCC B...) | BDBM50383966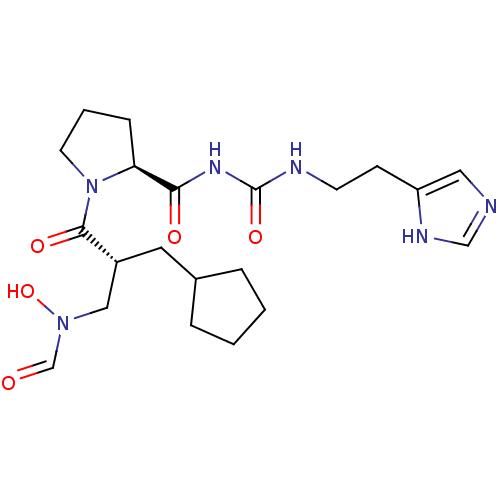 (CHEMBL2032148) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of Streptococcus pneumoniae PDF assessed as formate release from fMAS peptide substrate after 20 mins by formate dehydrogenase coupled ass... | Bioorg Med Chem Lett 22: 4028-32 (2012) Article DOI: 10.1016/j.bmcl.2012.04.086 BindingDB Entry DOI: 10.7270/Q2K64K3V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptide deformylase (Streptococcus pneumoniae serotype 4 (strain ATCC B...) | BDBM50384000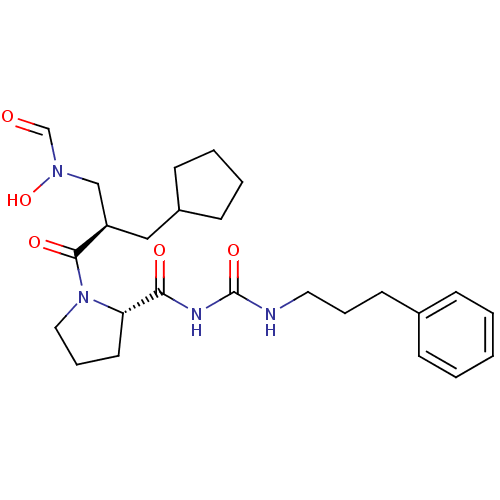 (CHEMBL2032138) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of Streptococcus pneumoniae PDF assessed as formate release from fMAS peptide substrate after 20 mins by formate dehydrogenase coupled ass... | Bioorg Med Chem Lett 22: 4028-32 (2012) Article DOI: 10.1016/j.bmcl.2012.04.086 BindingDB Entry DOI: 10.7270/Q2K64K3V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptide deformylase (Streptococcus pneumoniae serotype 4 (strain ATCC B...) | BDBM50383998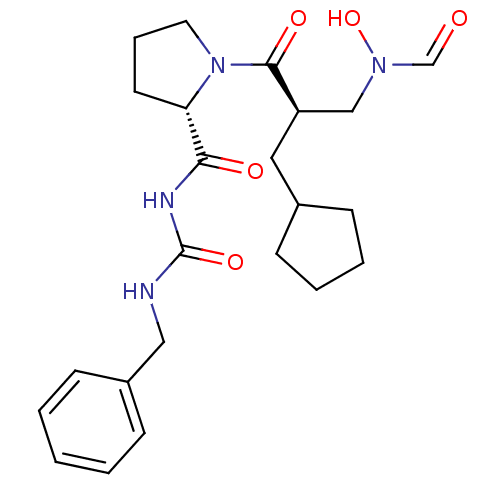 (CHEMBL2032136) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of Streptococcus pneumoniae PDF assessed as formate release from fMAS peptide substrate after 20 mins by formate dehydrogenase coupled ass... | Bioorg Med Chem Lett 22: 4028-32 (2012) Article DOI: 10.1016/j.bmcl.2012.04.086 BindingDB Entry DOI: 10.7270/Q2K64K3V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Eukaryotic translation initiation factor 2-alpha kinase 3 (Homo sapiens (Human)) | BDBM50396542 (CHEMBL2171141) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Inhibition of GST tagged PERK cytoplasmic domain mediated EIF2alpha phosphorylation | J Med Chem 55: 7193-207 (2012) Article DOI: 10.1021/jm300713s BindingDB Entry DOI: 10.7270/Q2222VWP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Eukaryotic translation initiation factor 2-alpha kinase 3 (Homo sapiens (Human)) | BDBM50396536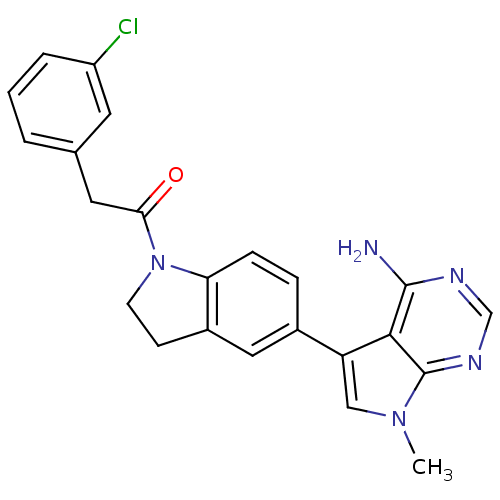 (CHEMBL2171122) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Inhibition of GST tagged PERK cytoplasmic domain mediated EIF2alpha phosphorylation | J Med Chem 55: 7193-207 (2012) Article DOI: 10.1021/jm300713s BindingDB Entry DOI: 10.7270/Q2222VWP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Eukaryotic translation initiation factor 2-alpha kinase 3 (Homo sapiens (Human)) | BDBM50396532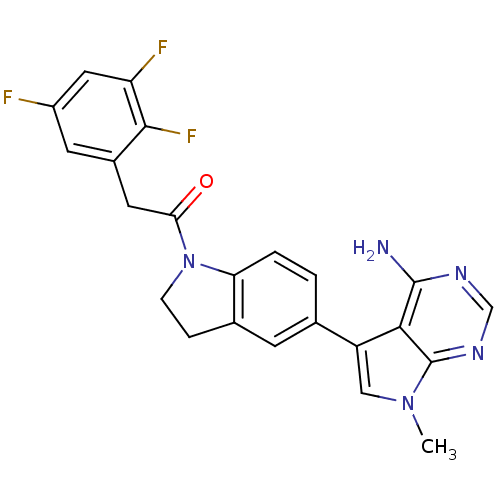 (CHEMBL2171126) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Inhibition of GST tagged PERK cytoplasmic domain mediated EIF2alpha phosphorylation | J Med Chem 55: 7193-207 (2012) Article DOI: 10.1021/jm300713s BindingDB Entry DOI: 10.7270/Q2222VWP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Eukaryotic translation initiation factor 2-alpha kinase 3 (Homo sapiens (Human)) | BDBM50396539 (CHEMBL2171144) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Inhibition of GST tagged PERK cytoplasmic domain mediated EIF2alpha phosphorylation | J Med Chem 55: 7193-207 (2012) Article DOI: 10.1021/jm300713s BindingDB Entry DOI: 10.7270/Q2222VWP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Eukaryotic translation initiation factor 2-alpha kinase 3 (Homo sapiens (Human)) | BDBM50396540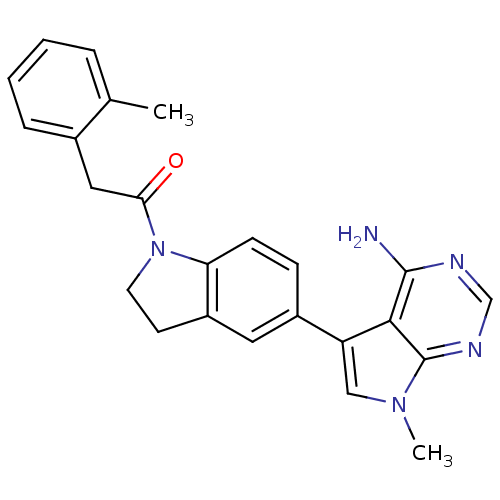 (CHEMBL2171143) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Inhibition of GST tagged PERK cytoplasmic domain mediated EIF2alpha phosphorylation | J Med Chem 55: 7193-207 (2012) Article DOI: 10.1021/jm300713s BindingDB Entry DOI: 10.7270/Q2222VWP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptide deformylase (Streptococcus pneumoniae serotype 4 (strain ATCC B...) | BDBM50383997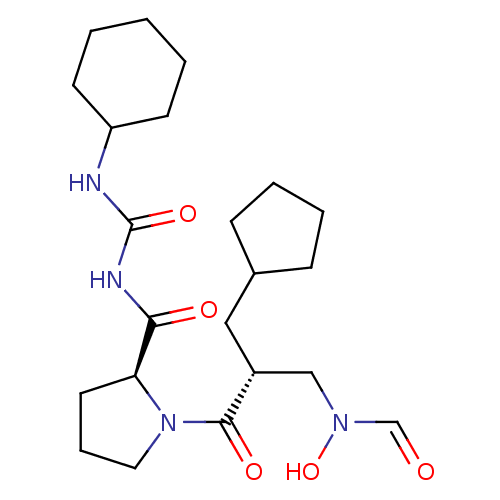 (CHEMBL2032135) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of Streptococcus pneumoniae PDF assessed as formate release from fMAS peptide substrate after 20 mins by formate dehydrogenase coupled ass... | Bioorg Med Chem Lett 22: 4028-32 (2012) Article DOI: 10.1016/j.bmcl.2012.04.086 BindingDB Entry DOI: 10.7270/Q2K64K3V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptide deformylase (Streptococcus pneumoniae serotype 4 (strain ATCC B...) | BDBM50383994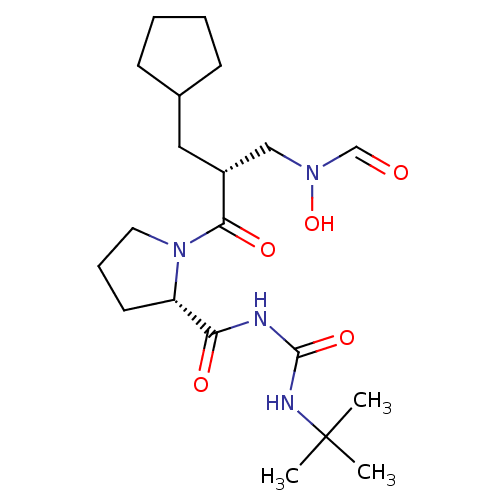 (CHEMBL2032132) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.220 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of Streptococcus pneumoniae PDF assessed as formate release from fMAS peptide substrate after 20 mins by formate dehydrogenase coupled ass... | Bioorg Med Chem Lett 22: 4028-32 (2012) Article DOI: 10.1016/j.bmcl.2012.04.086 BindingDB Entry DOI: 10.7270/Q2K64K3V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptide deformylase (Streptococcus pneumoniae serotype 4 (strain ATCC B...) | BDBM50383987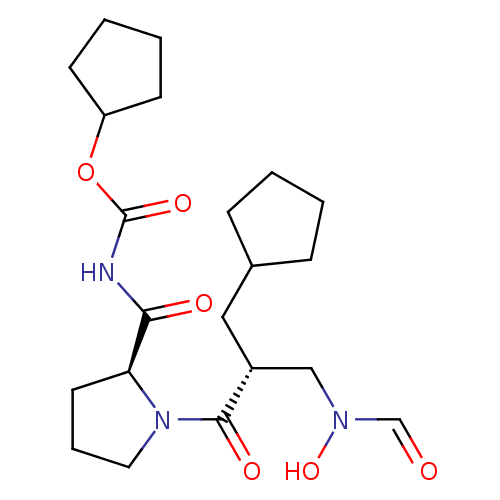 (CHEMBL2032125) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of Streptococcus pneumoniae PDF assessed as formate release from fMAS peptide substrate after 20 mins by formate dehydrogenase coupled ass... | Bioorg Med Chem Lett 22: 4028-32 (2012) Article DOI: 10.1016/j.bmcl.2012.04.086 BindingDB Entry DOI: 10.7270/Q2K64K3V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptide deformylase (Streptococcus pneumoniae serotype 4 (strain ATCC B...) | BDBM50383982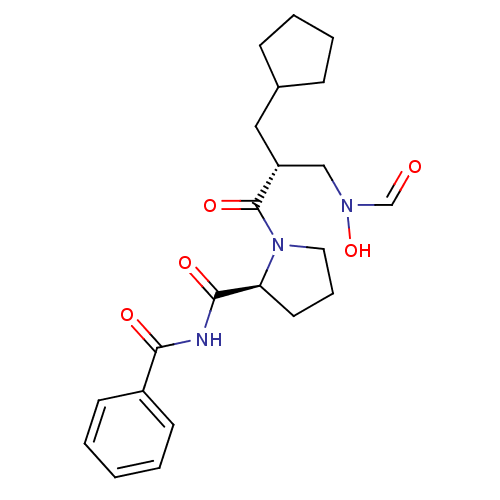 (CHEMBL2032119) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 0.280 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of Streptococcus pneumoniae PDF assessed as formate release from fMAS peptide substrate after 20 mins by formate dehydrogenase coupled ass... | Bioorg Med Chem Lett 22: 4028-32 (2012) Article DOI: 10.1016/j.bmcl.2012.04.086 BindingDB Entry DOI: 10.7270/Q2K64K3V | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Displayed 1 to 50 (of 1079 total ) | Next | Last >> |