 Found 1073 hits with Last Name = 'gross' and Initial = 'j'
Found 1073 hits with Last Name = 'gross' and Initial = 'j' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50383163
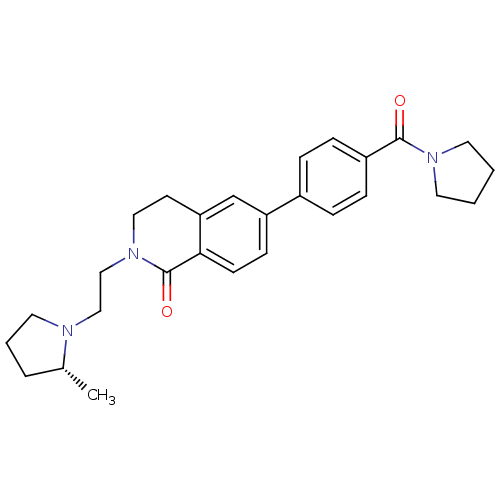
(CHEMBL2031885)Show SMILES C[C@@H]1CCCN1CCN1CCc2cc(ccc2C1=O)-c1ccc(cc1)C(=O)N1CCCC1 |r| Show InChI InChI=1S/C27H33N3O2/c1-20-5-4-15-28(20)17-18-30-16-12-24-19-23(10-11-25(24)27(30)32)21-6-8-22(9-7-21)26(31)29-13-2-3-14-29/h6-11,19-20H,2-5,12-18H2,1H3/t20-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-(R)-alpha-methylhistamine from human H3 receptor expressed in HEK293T cells after 120 mins by scintillation counting |
J Med Chem 55: 2452-68 (2012)
Article DOI: 10.1021/jm300011d
BindingDB Entry DOI: 10.7270/Q2736RZR |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 4
(GUINEA PIG) | BDBM29525

(3H-GR113808 | CHEMBL518682 | GR 113808 | [3H] GR 1...)Show SMILES Cn1cc(C(=O)OCC2CCN(CCNS(C)(=O)=O)CC2)c2ccccc12 Show InChI InChI=1S/C19H27N3O4S/c1-21-13-17(16-5-3-4-6-18(16)21)19(23)26-14-15-7-10-22(11-8-15)12-9-20-27(2,24)25/h3-6,13,15,20H,7-12,14H2,1-2H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Group Research Ltd.
Curated by PDSP Ki Database
| |
Br J Pharmacol 109: 618-24 (1993)
Article DOI: 10.1111/j.1476-5381.1993.tb13617.x
BindingDB Entry DOI: 10.7270/Q2CZ35PV |
More data for this
Ligand-Target Pair | |
Neprilysin
(Homo sapiens (Human)) | BDBM50411736
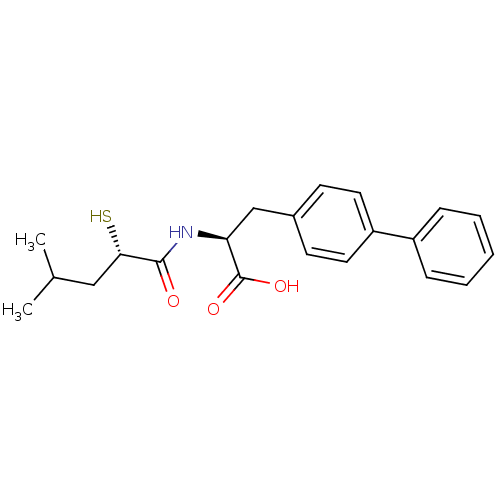
(CHEMBL271225)Show SMILES CC(C)C[C@H](S)C(=O)N[C@@H](Cc1ccc(cc1)-c1ccccc1)C(O)=O Show InChI InChI=1S/C21H25NO3S/c1-14(2)12-19(26)20(23)22-18(21(24)25)13-15-8-10-17(11-9-15)16-6-4-3-5-7-16/h3-11,14,18-19,26H,12-13H2,1-2H3,(H,22,23)(H,24,25)/t18-,19-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant NEP by fluorescence assay |
Bioorg Med Chem Lett 18: 732-7 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.048
BindingDB Entry DOI: 10.7270/Q2GT5PCB |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50383144
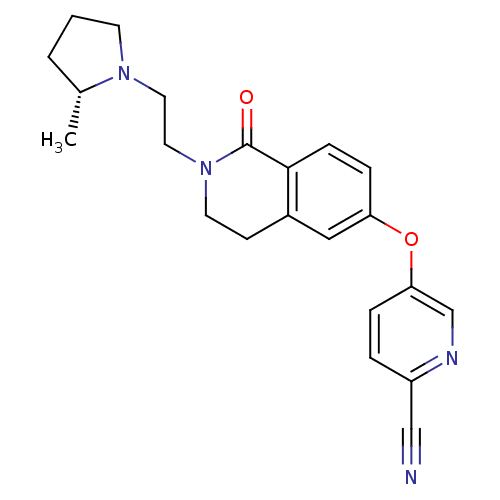
(CHEMBL2031864)Show SMILES C[C@@H]1CCCN1CCN1CCc2cc(Oc3ccc(nc3)C#N)ccc2C1=O |r| Show InChI InChI=1S/C22H24N4O2/c1-16-3-2-9-25(16)11-12-26-10-8-17-13-19(6-7-21(17)22(26)27)28-20-5-4-18(14-23)24-15-20/h4-7,13,15-16H,2-3,8-12H2,1H3/t16-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-(R)-alpha-methylhistamine from human H3 receptor expressed in HEK293T cells after 120 mins by scintillation counting |
J Med Chem 55: 2452-68 (2012)
Article DOI: 10.1021/jm300011d
BindingDB Entry DOI: 10.7270/Q2736RZR |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 4
(GUINEA PIG) | BDBM29525

(3H-GR113808 | CHEMBL518682 | GR 113808 | [3H] GR 1...)Show SMILES Cn1cc(C(=O)OCC2CCN(CCNS(C)(=O)=O)CC2)c2ccccc12 Show InChI InChI=1S/C19H27N3O4S/c1-21-13-17(16-5-3-4-6-18(16)21)19(23)26-14-15-7-10-22(11-8-15)12-9-20-27(2,24)25/h3-6,13,15,20H,7-12,14H2,1-2H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Group Research Ltd.
Curated by PDSP Ki Database
| |
Br J Pharmacol 109: 618-24 (1993)
Article DOI: 10.1111/j.1476-5381.1993.tb13617.x
BindingDB Entry DOI: 10.7270/Q2CZ35PV |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50354216
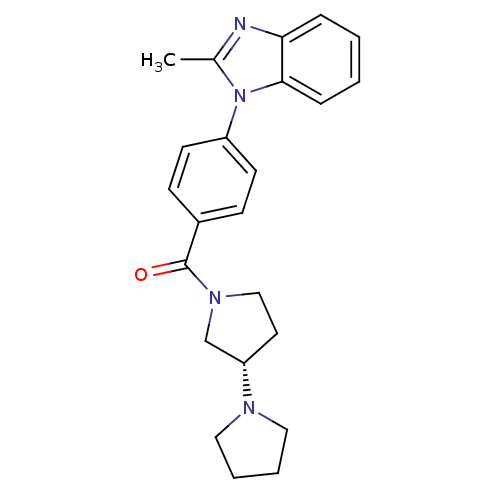
(CHEMBL1836004)Show SMILES Cc1nc2ccccc2n1-c1ccc(cc1)C(=O)N1CC[C@@H](C1)N1CCCC1 |r| Show InChI InChI=1S/C23H26N4O/c1-17-24-21-6-2-3-7-22(21)27(17)19-10-8-18(9-11-19)23(28)26-15-12-20(16-26)25-13-4-5-14-25/h2-3,6-11,20H,4-5,12-16H2,1H3/t20-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-(R)alpha-methylhistamine from human histamine H3 receptor expressed in human HEK293T cells |
Bioorg Med Chem Lett 21: 5957-60 (2011)
Article DOI: 10.1016/j.bmcl.2011.07.061
BindingDB Entry DOI: 10.7270/Q2QN6753 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50354216

(CHEMBL1836004)Show SMILES Cc1nc2ccccc2n1-c1ccc(cc1)C(=O)N1CC[C@@H](C1)N1CCCC1 |r| Show InChI InChI=1S/C23H26N4O/c1-17-24-21-6-2-3-7-22(21)27(17)19-10-8-18(9-11-19)23(28)26-15-12-20(16-26)25-13-4-5-14-25/h2-3,6-11,20H,4-5,12-16H2,1H3/t20-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-(R)-alpha-methylhistamine from human H3 receptor expressed in HEK293T cells after 120 mins by scintillation counting |
J Med Chem 55: 2452-68 (2012)
Article DOI: 10.1021/jm300011d
BindingDB Entry DOI: 10.7270/Q2736RZR |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50383142
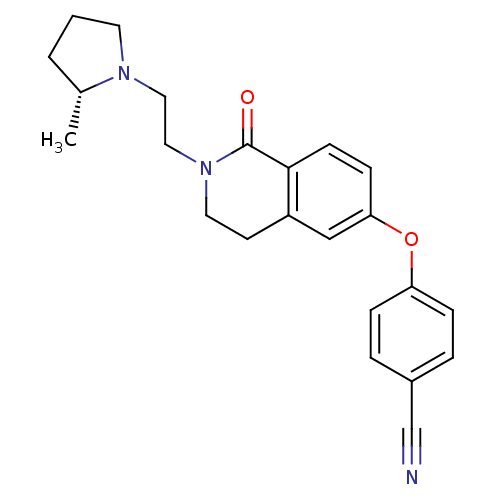
(CHEMBL2031862)Show SMILES C[C@@H]1CCCN1CCN1CCc2cc(Oc3ccc(cc3)C#N)ccc2C1=O |r| Show InChI InChI=1S/C23H25N3O2/c1-17-3-2-11-25(17)13-14-26-12-10-19-15-21(8-9-22(19)23(26)27)28-20-6-4-18(16-24)5-7-20/h4-9,15,17H,2-3,10-14H2,1H3/t17-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-(R)-alpha-methylhistamine from human H3 receptor expressed in HEK293T cells after 120 mins by scintillation counting |
J Med Chem 55: 2452-68 (2012)
Article DOI: 10.1021/jm300011d
BindingDB Entry DOI: 10.7270/Q2736RZR |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50383167
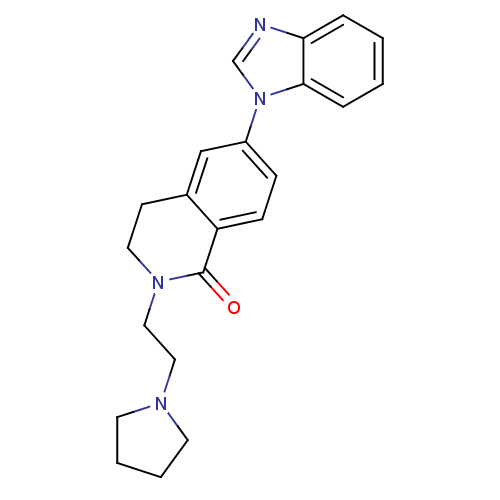
(CHEMBL2031741)Show SMILES O=C1N(CCN2CCCC2)CCc2cc(ccc12)-n1cnc2ccccc12 Show InChI InChI=1S/C22H24N4O/c27-22-19-8-7-18(26-16-23-20-5-1-2-6-21(20)26)15-17(19)9-12-25(22)14-13-24-10-3-4-11-24/h1-2,5-8,15-16H,3-4,9-14H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-(R)-alpha-methylhistamine from human H3 receptor expressed in HEK293T cells after 120 mins by scintillation counting |
J Med Chem 55: 2452-68 (2012)
Article DOI: 10.1021/jm300011d
BindingDB Entry DOI: 10.7270/Q2736RZR |
More data for this
Ligand-Target Pair | |
Neprilysin
(Homo sapiens (Human)) | BDBM50411731
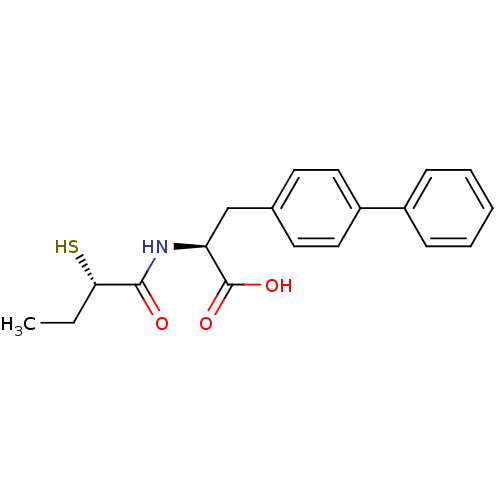
(CHEMBL257726)Show SMILES CC[C@H](S)C(=O)N[C@@H](Cc1ccc(cc1)-c1ccccc1)C(O)=O Show InChI InChI=1S/C19H21NO3S/c1-2-17(24)18(21)20-16(19(22)23)12-13-8-10-15(11-9-13)14-6-4-3-5-7-14/h3-11,16-17,24H,2,12H2,1H3,(H,20,21)(H,22,23)/t16-,17-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant NEP by fluorescence assay |
Bioorg Med Chem Lett 18: 732-7 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.048
BindingDB Entry DOI: 10.7270/Q2GT5PCB |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50383138
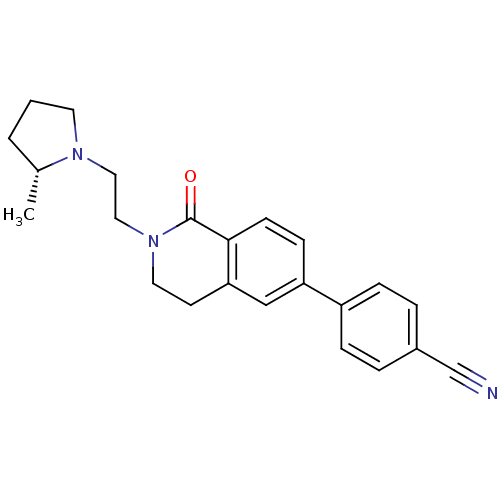
(CHEMBL2031762)Show SMILES C[C@@H]1CCCN1CCN1CCc2cc(ccc2C1=O)-c1ccc(cc1)C#N |r| Show InChI InChI=1S/C23H25N3O/c1-17-3-2-11-25(17)13-14-26-12-10-21-15-20(8-9-22(21)23(26)27)19-6-4-18(16-24)5-7-19/h4-9,15,17H,2-3,10-14H2,1H3/t17-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-(R)-alpha-methylhistamine from human H3 receptor expressed in HEK293T cells after 120 mins by scintillation counting |
J Med Chem 55: 2452-68 (2012)
Article DOI: 10.1021/jm300011d
BindingDB Entry DOI: 10.7270/Q2736RZR |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50383153
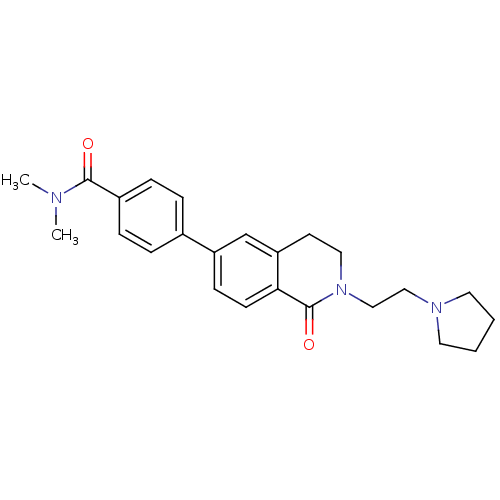
(CHEMBL2031874)Show SMILES CN(C)C(=O)c1ccc(cc1)-c1ccc2C(=O)N(CCN3CCCC3)CCc2c1 Show InChI InChI=1S/C24H29N3O2/c1-25(2)23(28)19-7-5-18(6-8-19)20-9-10-22-21(17-20)11-14-27(24(22)29)16-15-26-12-3-4-13-26/h5-10,17H,3-4,11-16H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-(R)-alpha-methylhistamine from human H3 receptor expressed in HEK293T cells after 120 mins by scintillation counting |
J Med Chem 55: 2452-68 (2012)
Article DOI: 10.1021/jm300011d
BindingDB Entry DOI: 10.7270/Q2736RZR |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50383162
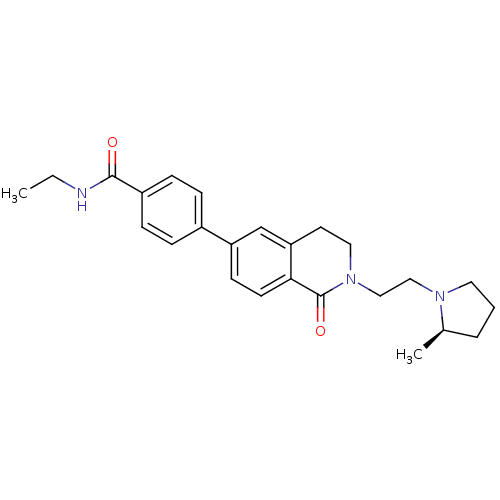
(CHEMBL2031884)Show SMILES CCNC(=O)c1ccc(cc1)-c1ccc2C(=O)N(CCN3CCC[C@H]3C)CCc2c1 |r| Show InChI InChI=1S/C25H31N3O2/c1-3-26-24(29)20-8-6-19(7-9-20)21-10-11-23-22(17-21)12-14-28(25(23)30)16-15-27-13-4-5-18(27)2/h6-11,17-18H,3-5,12-16H2,1-2H3,(H,26,29)/t18-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-(R)-alpha-methylhistamine from human H3 receptor expressed in HEK293T cells after 120 mins by scintillation counting |
J Med Chem 55: 2452-68 (2012)
Article DOI: 10.1021/jm300011d
BindingDB Entry DOI: 10.7270/Q2736RZR |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50383161
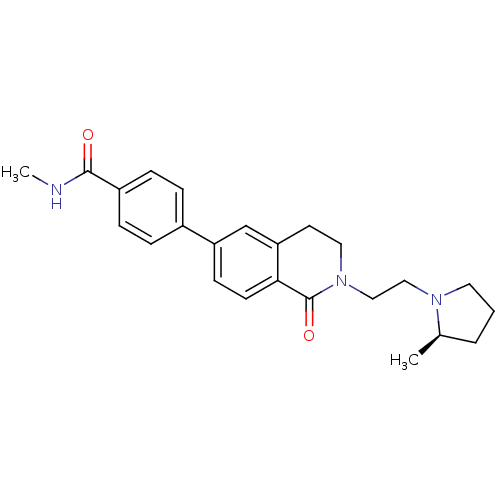
(CHEMBL2031883)Show SMILES CNC(=O)c1ccc(cc1)-c1ccc2C(=O)N(CCN3CCC[C@H]3C)CCc2c1 |r| Show InChI InChI=1S/C24H29N3O2/c1-17-4-3-12-26(17)14-15-27-13-11-21-16-20(9-10-22(21)24(27)29)18-5-7-19(8-6-18)23(28)25-2/h5-10,16-17H,3-4,11-15H2,1-2H3,(H,25,28)/t17-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-(R)-alpha-methylhistamine from human H3 receptor expressed in HEK293T cells after 120 mins by scintillation counting |
J Med Chem 55: 2452-68 (2012)
Article DOI: 10.1021/jm300011d
BindingDB Entry DOI: 10.7270/Q2736RZR |
More data for this
Ligand-Target Pair | |
Neprilysin
(Homo sapiens (Human)) | BDBM50286715
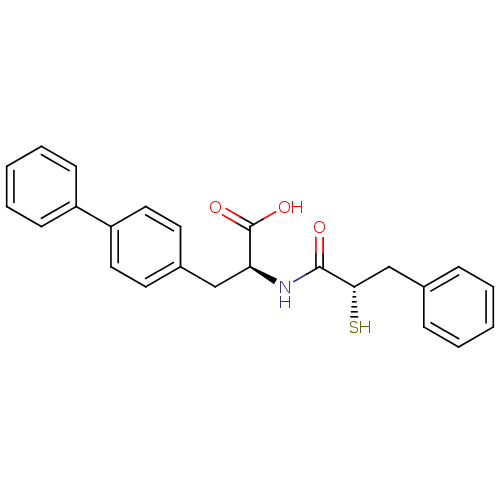
((S)-3-biphenyl-4-yl-2-((S)-2-mercapto-3-phenyl-pro...)Show SMILES OC(=O)[C@H](Cc1ccc(cc1)-c1ccccc1)NC(=O)[C@@H](S)Cc1ccccc1 Show InChI InChI=1S/C24H23NO3S/c26-23(22(29)16-17-7-3-1-4-8-17)25-21(24(27)28)15-18-11-13-20(14-12-18)19-9-5-2-6-10-19/h1-14,21-22,29H,15-16H2,(H,25,26)(H,27,28)/t21-,22-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant NEP by fluorescence assay |
Bioorg Med Chem Lett 18: 732-7 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.048
BindingDB Entry DOI: 10.7270/Q2GT5PCB |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Rattus norvegicus (rat)) | BDBM50209674
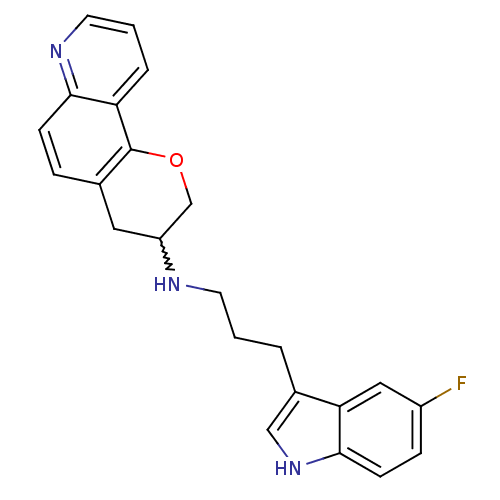
(CHEMBL391531 | N-(3-(5-fluoro-1H-indol-3-yl)propyl...)Show SMILES Fc1ccc2[nH]cc(CCCNC3COc4c(C3)ccc3ncccc43)c2c1 |w:12.11| Show InChI InChI=1S/C23H22FN3O/c24-17-6-8-22-20(12-17)16(13-27-22)3-1-9-25-18-11-15-5-7-21-19(4-2-10-26-21)23(15)28-14-18/h2,4-8,10,12-13,18,25,27H,1,3,9,11,14H2 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]paroxetine from 5HT reuptake site of rat cortical 5HTT |
Bioorg Med Chem Lett 17: 3117-21 (2007)
Article DOI: 10.1016/j.bmcl.2007.03.049
BindingDB Entry DOI: 10.7270/Q2125SBH |
More data for this
Ligand-Target Pair | |
Neprilysin
(Homo sapiens (Human)) | BDBM50411730
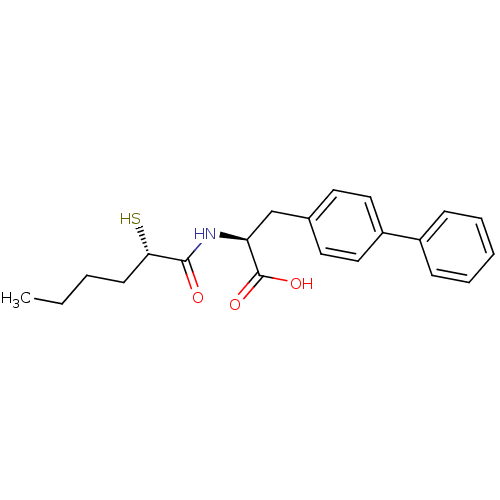
(CHEMBL257270)Show SMILES CCCC[C@H](S)C(=O)N[C@@H](Cc1ccc(cc1)-c1ccccc1)C(O)=O Show InChI InChI=1S/C21H25NO3S/c1-2-3-9-19(26)20(23)22-18(21(24)25)14-15-10-12-17(13-11-15)16-7-5-4-6-8-16/h4-8,10-13,18-19,26H,2-3,9,14H2,1H3,(H,22,23)(H,24,25)/t18-,19-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant NEP by fluorescence assay |
Bioorg Med Chem Lett 18: 732-7 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.048
BindingDB Entry DOI: 10.7270/Q2GT5PCB |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Rattus norvegicus (rat)) | BDBM50209674

(CHEMBL391531 | N-(3-(5-fluoro-1H-indol-3-yl)propyl...)Show SMILES Fc1ccc2[nH]cc(CCCNC3COc4c(C3)ccc3ncccc43)c2c1 |w:12.11| Show InChI InChI=1S/C23H22FN3O/c24-17-6-8-22-20(12-17)16(13-27-22)3-1-9-25-18-11-15-5-7-21-19(4-2-10-26-21)23(15)28-14-18/h2,4-8,10,12-13,18,25,27H,1,3,9,11,14H2 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]paroxetine from 5HT reuptake site of rat cortical 5HTT |
Bioorg Med Chem Lett 17: 3117-21 (2007)
Article DOI: 10.1016/j.bmcl.2007.03.049
BindingDB Entry DOI: 10.7270/Q2125SBH |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50383149
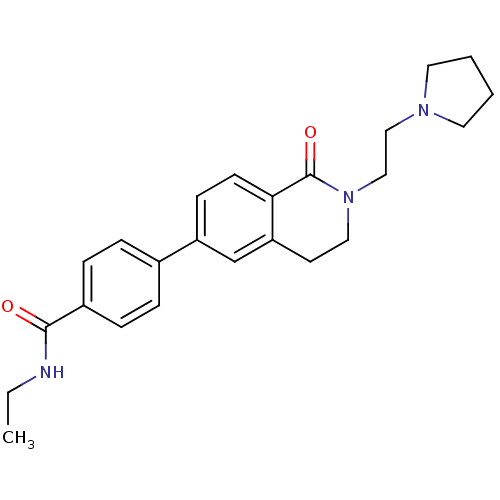
(CHEMBL2031869)Show SMILES CCNC(=O)c1ccc(cc1)-c1ccc2C(=O)N(CCN3CCCC3)CCc2c1 Show InChI InChI=1S/C24H29N3O2/c1-2-25-23(28)19-7-5-18(6-8-19)20-9-10-22-21(17-20)11-14-27(24(22)29)16-15-26-12-3-4-13-26/h5-10,17H,2-4,11-16H2,1H3,(H,25,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-(R)-alpha-methylhistamine from human H3 receptor expressed in HEK293T cells after 120 mins by scintillation counting |
J Med Chem 55: 2452-68 (2012)
Article DOI: 10.1021/jm300011d
BindingDB Entry DOI: 10.7270/Q2736RZR |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50383143
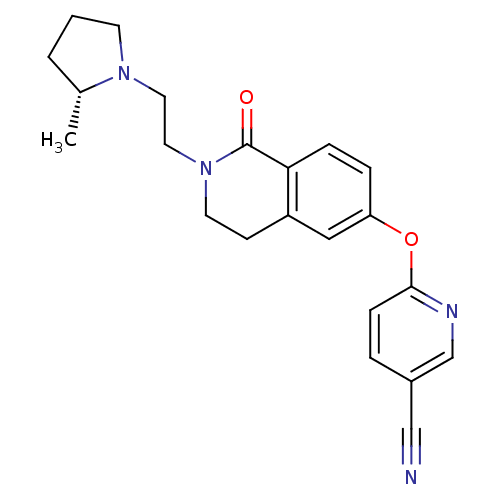
(CHEMBL2031863)Show SMILES C[C@@H]1CCCN1CCN1CCc2cc(Oc3ccc(cn3)C#N)ccc2C1=O |r| Show InChI InChI=1S/C22H24N4O2/c1-16-3-2-9-25(16)11-12-26-10-8-18-13-19(5-6-20(18)22(26)27)28-21-7-4-17(14-23)15-24-21/h4-7,13,15-16H,2-3,8-12H2,1H3/t16-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-(R)-alpha-methylhistamine from human H3 receptor expressed in HEK293T cells after 120 mins by scintillation counting |
J Med Chem 55: 2452-68 (2012)
Article DOI: 10.1021/jm300011d
BindingDB Entry DOI: 10.7270/Q2736RZR |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50383136

(CHEMBL2031760)Show SMILES C[C@@H]1CCCN1CCN1CCc2cc(ccc2C1=O)-c1ccc(F)cc1 |r| Show InChI InChI=1S/C22H25FN2O/c1-16-3-2-11-24(16)13-14-25-12-10-19-15-18(6-9-21(19)22(25)26)17-4-7-20(23)8-5-17/h4-9,15-16H,2-3,10-14H2,1H3/t16-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-(R)-alpha-methylhistamine from human H3 receptor expressed in HEK293T cells after 120 mins by scintillation counting |
J Med Chem 55: 2452-68 (2012)
Article DOI: 10.1021/jm300011d
BindingDB Entry DOI: 10.7270/Q2736RZR |
More data for this
Ligand-Target Pair | |
Neprilysin
(Homo sapiens (Human)) | BDBM50286724
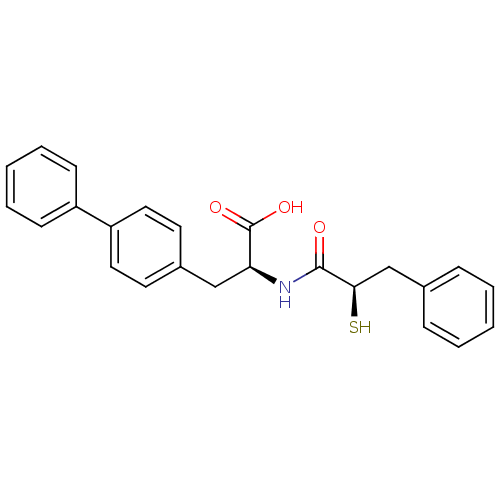
((S)-3-Biphenyl-4-yl-2-((R)-2-mercapto-3-phenyl-pro...)Show SMILES OC(=O)[C@H](Cc1ccc(cc1)-c1ccccc1)NC(=O)[C@H](S)Cc1ccccc1 Show InChI InChI=1S/C24H23NO3S/c26-23(22(29)16-17-7-3-1-4-8-17)25-21(24(27)28)15-18-11-13-20(14-12-18)19-9-5-2-6-10-19/h1-14,21-22,29H,15-16H2,(H,25,26)(H,27,28)/t21-,22+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant NEP by fluorescence assay |
Bioorg Med Chem Lett 18: 732-7 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.048
BindingDB Entry DOI: 10.7270/Q2GT5PCB |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Rattus norvegicus (rat)) | BDBM50209679
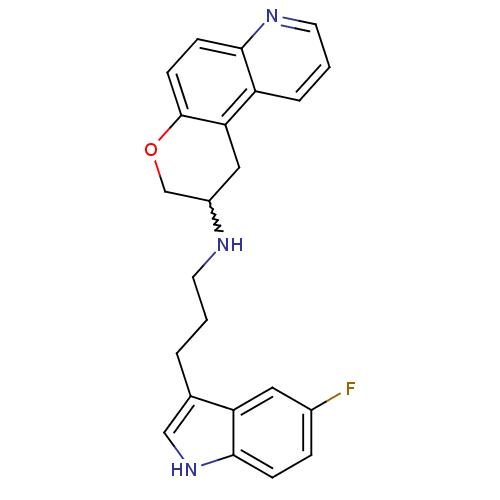
(CHEMBL234853 | N-(3-(5-fluoro-1H-indol-3-yl)propyl...)Show SMILES Fc1ccc2[nH]cc(CCCNC3COc4ccc5ncccc5c4C3)c2c1 |w:12.11| Show InChI InChI=1S/C23H22FN3O/c24-16-5-6-22-19(11-16)15(13-27-22)3-1-9-25-17-12-20-18-4-2-10-26-21(18)7-8-23(20)28-14-17/h2,4-8,10-11,13,17,25,27H,1,3,9,12,14H2 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]paroxetine from 5HT reuptake site of rat cortical 5HTT |
Bioorg Med Chem Lett 17: 3117-21 (2007)
Article DOI: 10.1016/j.bmcl.2007.03.049
BindingDB Entry DOI: 10.7270/Q2125SBH |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50383158
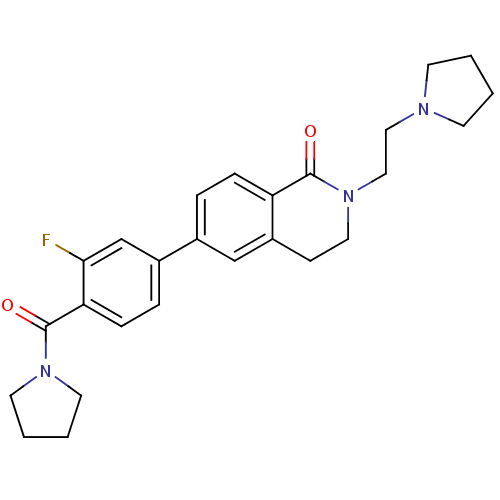
(CHEMBL2031879)Show SMILES Fc1cc(ccc1C(=O)N1CCCC1)-c1ccc2C(=O)N(CCN3CCCC3)CCc2c1 Show InChI InChI=1S/C26H30FN3O2/c27-24-18-20(6-8-23(24)26(32)29-12-3-4-13-29)19-5-7-22-21(17-19)9-14-30(25(22)31)16-15-28-10-1-2-11-28/h5-8,17-18H,1-4,9-16H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-(R)-alpha-methylhistamine from human H3 receptor expressed in HEK293T cells after 120 mins by scintillation counting |
J Med Chem 55: 2452-68 (2012)
Article DOI: 10.1021/jm300011d
BindingDB Entry DOI: 10.7270/Q2736RZR |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50383148
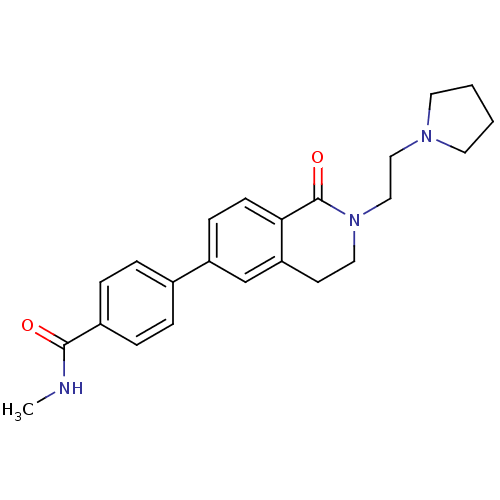
(CHEMBL2031868)Show SMILES CNC(=O)c1ccc(cc1)-c1ccc2C(=O)N(CCN3CCCC3)CCc2c1 Show InChI InChI=1S/C23H27N3O2/c1-24-22(27)18-6-4-17(5-7-18)19-8-9-21-20(16-19)10-13-26(23(21)28)15-14-25-11-2-3-12-25/h4-9,16H,2-3,10-15H2,1H3,(H,24,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-(R)-alpha-methylhistamine from human H3 receptor expressed in HEK293T cells after 120 mins by scintillation counting |
J Med Chem 55: 2452-68 (2012)
Article DOI: 10.1021/jm300011d
BindingDB Entry DOI: 10.7270/Q2736RZR |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme 2
(Homo sapiens (Human)) | BDBM50411731

(CHEMBL257726)Show SMILES CC[C@H](S)C(=O)N[C@@H](Cc1ccc(cc1)-c1ccccc1)C(O)=O Show InChI InChI=1S/C19H21NO3S/c1-2-17(24)18(21)20-16(19(22)23)12-13-8-10-15(11-9-13)14-6-4-3-5-7-14/h3-11,16-17,24H,2,12H2,1H3,(H,20,21)(H,22,23)/t16-,17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant ACE2 by fluorescence assay |
Bioorg Med Chem Lett 18: 732-7 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.048
BindingDB Entry DOI: 10.7270/Q2GT5PCB |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme 2
(Homo sapiens (Human)) | BDBM50411736

(CHEMBL271225)Show SMILES CC(C)C[C@H](S)C(=O)N[C@@H](Cc1ccc(cc1)-c1ccccc1)C(O)=O Show InChI InChI=1S/C21H25NO3S/c1-14(2)12-19(26)20(23)22-18(21(24)25)13-15-8-10-17(11-9-15)16-6-4-3-5-7-16/h3-11,14,18-19,26H,12-13H2,1-2H3,(H,22,23)(H,24,25)/t18-,19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant ACE2 by fluorescence assay |
Bioorg Med Chem Lett 18: 732-7 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.048
BindingDB Entry DOI: 10.7270/Q2GT5PCB |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Rattus norvegicus (rat)) | BDBM50209682
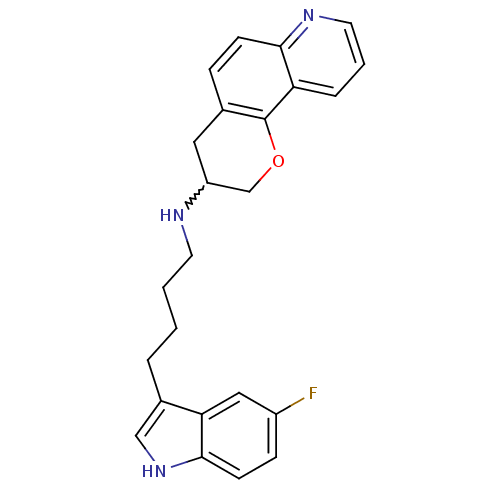
(CHEMBL233624 | N-(4-(5-fluoro-1H-indol-3-yl)butyl)...)Show SMILES Fc1ccc2[nH]cc(CCCCNC3COc4c(C3)ccc3ncccc43)c2c1 |w:13.12| Show InChI InChI=1S/C24H24FN3O/c25-18-7-9-23-21(13-18)17(14-28-23)4-1-2-10-26-19-12-16-6-8-22-20(5-3-11-27-22)24(16)29-15-19/h3,5-9,11,13-14,19,26,28H,1-2,4,10,12,15H2 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]paroxetine from 5HT reuptake site of rat cortical 5HTT |
Bioorg Med Chem Lett 17: 3117-21 (2007)
Article DOI: 10.1016/j.bmcl.2007.03.049
BindingDB Entry DOI: 10.7270/Q2125SBH |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme 2
(Homo sapiens (Human)) | BDBM50411605
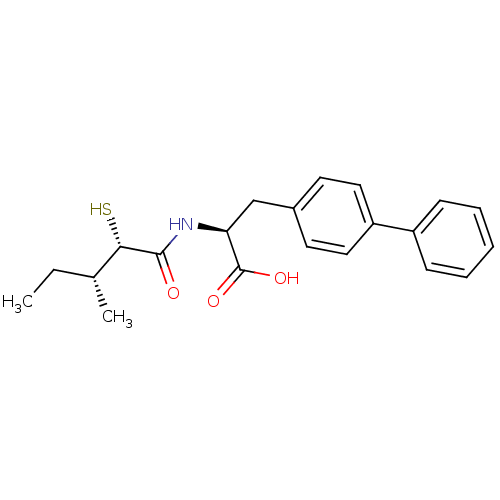
(CHEMBL252391)Show SMILES CC[C@@H](C)[C@H](S)C(=O)N[C@@H](Cc1ccc(cc1)-c1ccccc1)C(O)=O Show InChI InChI=1S/C21H25NO3S/c1-3-14(2)19(26)20(23)22-18(21(24)25)13-15-9-11-17(12-10-15)16-7-5-4-6-8-16/h4-12,14,18-19,26H,3,13H2,1-2H3,(H,22,23)(H,24,25)/t14-,18+,19+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant ACE2 by fluorescence assay |
Bioorg Med Chem Lett 18: 732-7 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.048
BindingDB Entry DOI: 10.7270/Q2GT5PCB |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme 2
(Homo sapiens (Human)) | BDBM50411733
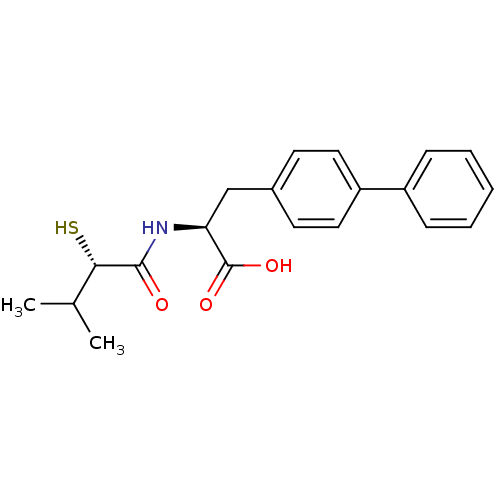
(CHEMBL269997)Show SMILES CC(C)[C@H](S)C(=O)N[C@@H](Cc1ccc(cc1)-c1ccccc1)C(O)=O Show InChI InChI=1S/C20H23NO3S/c1-13(2)18(25)19(22)21-17(20(23)24)12-14-8-10-16(11-9-14)15-6-4-3-5-7-15/h3-11,13,17-18,25H,12H2,1-2H3,(H,21,22)(H,23,24)/t17-,18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant ACE2 by fluorescence assay |
Bioorg Med Chem Lett 18: 732-7 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.048
BindingDB Entry DOI: 10.7270/Q2GT5PCB |
More data for this
Ligand-Target Pair | |
Relaxin-3 receptor 2
(Homo sapiens (Human)) | BDBM50558038
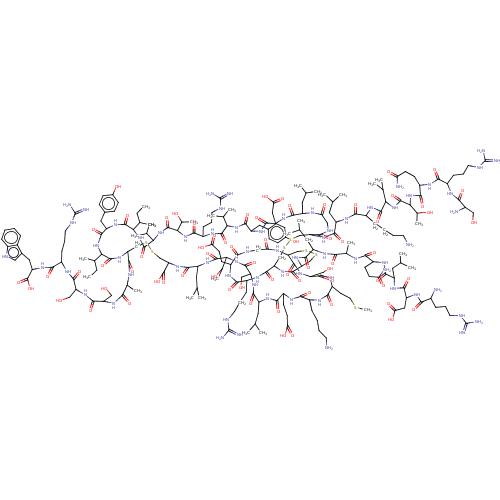
(CHEMBL4798311)Show SMILES CC[C@H](C)[C@@H]1NC(=O)[C@@H](Cc2ccc(O)cc2)NC(=O)[C@@H](NC(=O)[C@@H](NC(=O)[C@@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H](NC(=O)[C@H](Cc2ccc(O)cc2)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC(C)C)NC(=O)CNC(=O)[C@H](CSSC[C@@H]2NC(=O)[C@H](CSSC[C@H](NC(=O)CNC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CCCNC(N)=N)NC2=O)C(=O)N[C@@H](CO)C(=O)N[C@@H](CCSC)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CO)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CSSC[C@H](NC1=O)C(=O)N[C@@H](C)C(=O)N[C@@H](CO)C(=O)N[C@@H](CO)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(O)=O)C(O)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](C)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC(O)=O)NC(=O)[C@@H](N)CCCNC(N)=N)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CCCCN)NC(=O)[C@@H](NC(=O)[C@@H](NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H](N)CO)[C@@H](C)O)C(C)C)C(C)C)[C@@H](C)O)C(C)C)[C@@H](C)CC |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of europium-labeled Eu(A)-mINSL5 from human RXFP4 expressed in CHO-K1 cells |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.5b01786
BindingDB Entry DOI: 10.7270/Q2PC3624 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50354214
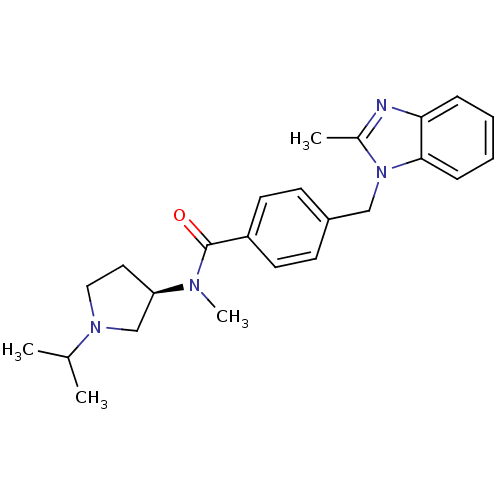
(CHEMBL1835992)Show SMILES CC(C)N1CC[C@H](C1)N(C)C(=O)c1ccc(Cn2c(C)nc3ccccc23)cc1 |r| Show InChI InChI=1S/C24H30N4O/c1-17(2)27-14-13-21(16-27)26(4)24(29)20-11-9-19(10-12-20)15-28-18(3)25-22-7-5-6-8-23(22)28/h5-12,17,21H,13-16H2,1-4H3/t21-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-(R)alpha-methylhistamine from human histamine H3 receptor expressed in human HEK293T cells |
Bioorg Med Chem Lett 21: 5957-60 (2011)
Article DOI: 10.1016/j.bmcl.2011.07.061
BindingDB Entry DOI: 10.7270/Q2QN6753 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50383159
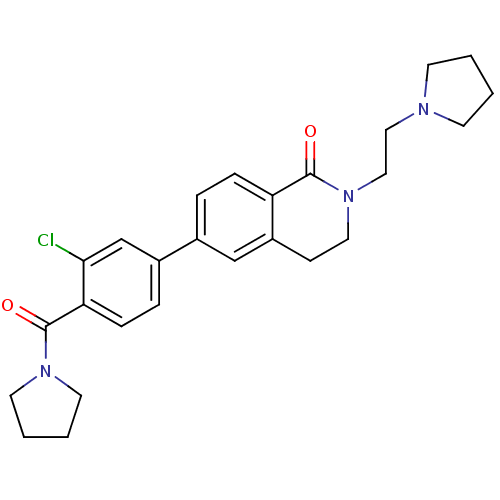
(CHEMBL2031880)Show SMILES Clc1cc(ccc1C(=O)N1CCCC1)-c1ccc2C(=O)N(CCN3CCCC3)CCc2c1 Show InChI InChI=1S/C26H30ClN3O2/c27-24-18-20(6-8-23(24)26(32)29-12-3-4-13-29)19-5-7-22-21(17-19)9-14-30(25(22)31)16-15-28-10-1-2-11-28/h5-8,17-18H,1-4,9-16H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-(R)-alpha-methylhistamine from human H3 receptor expressed in HEK293T cells after 120 mins by scintillation counting |
J Med Chem 55: 2452-68 (2012)
Article DOI: 10.1021/jm300011d
BindingDB Entry DOI: 10.7270/Q2736RZR |
More data for this
Ligand-Target Pair | |
Relaxin-3 receptor 2
(Homo sapiens (Human)) | BDBM50558038

(CHEMBL4798311)Show SMILES CC[C@H](C)[C@@H]1NC(=O)[C@@H](Cc2ccc(O)cc2)NC(=O)[C@@H](NC(=O)[C@@H](NC(=O)[C@@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H](NC(=O)[C@H](Cc2ccc(O)cc2)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC(C)C)NC(=O)CNC(=O)[C@H](CSSC[C@@H]2NC(=O)[C@H](CSSC[C@H](NC(=O)CNC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CCCNC(N)=N)NC2=O)C(=O)N[C@@H](CO)C(=O)N[C@@H](CCSC)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CO)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CSSC[C@H](NC1=O)C(=O)N[C@@H](C)C(=O)N[C@@H](CO)C(=O)N[C@@H](CO)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(O)=O)C(O)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](C)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC(O)=O)NC(=O)[C@@H](N)CCCNC(N)=N)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CCCCN)NC(=O)[C@@H](NC(=O)[C@@H](NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H](N)CO)[C@@H](C)O)C(C)C)C(C)C)[C@@H](C)O)C(C)C)[C@@H](C)CC |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of europium-labeled Eu(A)-mINSL5 from human RXFP4 expressed in CHO-K1 cells |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.5b01786
BindingDB Entry DOI: 10.7270/Q2PC3624 |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme 2
(Homo sapiens (Human)) | BDBM50411729
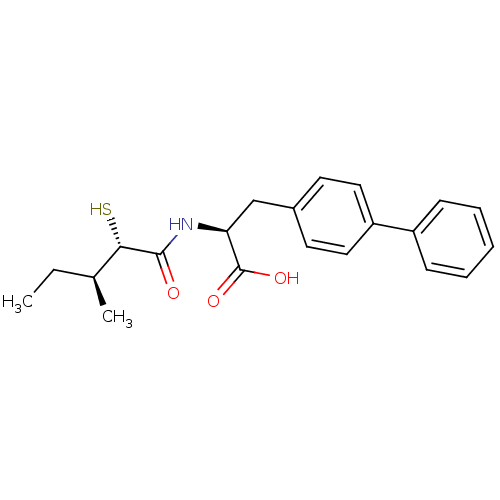
(CHEMBL269996)Show SMILES CC[C@H](C)[C@H](S)C(=O)N[C@@H](Cc1ccc(cc1)-c1ccccc1)C(O)=O Show InChI InChI=1S/C21H25NO3S/c1-3-14(2)19(26)20(23)22-18(21(24)25)13-15-9-11-17(12-10-15)16-7-5-4-6-8-16/h4-12,14,18-19,26H,3,13H2,1-2H3,(H,22,23)(H,24,25)/t14-,18-,19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant ACE2 by fluorescence assay |
Bioorg Med Chem Lett 18: 732-7 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.048
BindingDB Entry DOI: 10.7270/Q2GT5PCB |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50383160

(CHEMBL2031881)Show SMILES CNC(=O)c1ccc(c(F)c1)-c1ccc2C(=O)N(CCN3CCCC3)CCc2c1 Show InChI InChI=1S/C23H26FN3O2/c1-25-22(28)18-5-6-19(21(24)15-18)16-4-7-20-17(14-16)8-11-27(23(20)29)13-12-26-9-2-3-10-26/h4-7,14-15H,2-3,8-13H2,1H3,(H,25,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-(R)-alpha-methylhistamine from human H3 receptor expressed in HEK293T cells after 120 mins by scintillation counting |
J Med Chem 55: 2452-68 (2012)
Article DOI: 10.1021/jm300011d
BindingDB Entry DOI: 10.7270/Q2736RZR |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50383156

(CHEMBL2031877)Show SMILES C[C@H]1CCCN1C(=O)c1ccc(cc1)-c1ccc2C(=O)N(CCN3CCCC3)CCc2c1 |r| Show InChI InChI=1S/C27H33N3O2/c1-20-5-4-15-30(20)26(31)22-8-6-21(7-9-22)23-10-11-25-24(19-23)12-16-29(27(25)32)18-17-28-13-2-3-14-28/h6-11,19-20H,2-5,12-18H2,1H3/t20-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-(R)-alpha-methylhistamine from human H3 receptor expressed in HEK293T cells after 120 mins by scintillation counting |
J Med Chem 55: 2452-68 (2012)
Article DOI: 10.1021/jm300011d
BindingDB Entry DOI: 10.7270/Q2736RZR |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50383155
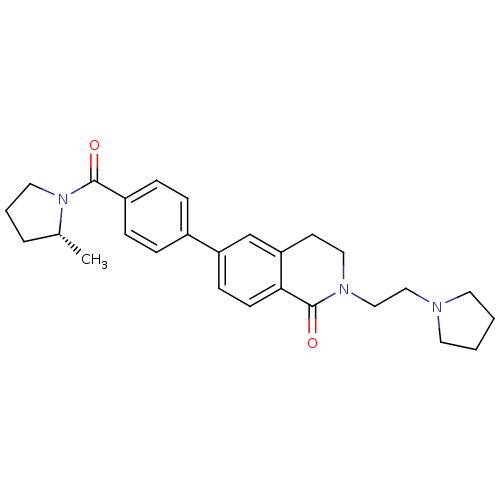
(CHEMBL2031876)Show SMILES C[C@@H]1CCCN1C(=O)c1ccc(cc1)-c1ccc2C(=O)N(CCN3CCCC3)CCc2c1 |r| Show InChI InChI=1S/C27H33N3O2/c1-20-5-4-15-30(20)26(31)22-8-6-21(7-9-22)23-10-11-25-24(19-23)12-16-29(27(25)32)18-17-28-13-2-3-14-28/h6-11,19-20H,2-5,12-18H2,1H3/t20-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-(R)-alpha-methylhistamine from human H3 receptor expressed in HEK293T cells after 120 mins by scintillation counting |
J Med Chem 55: 2452-68 (2012)
Article DOI: 10.1021/jm300011d
BindingDB Entry DOI: 10.7270/Q2736RZR |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme 2
(Homo sapiens (Human)) | BDBM50411737
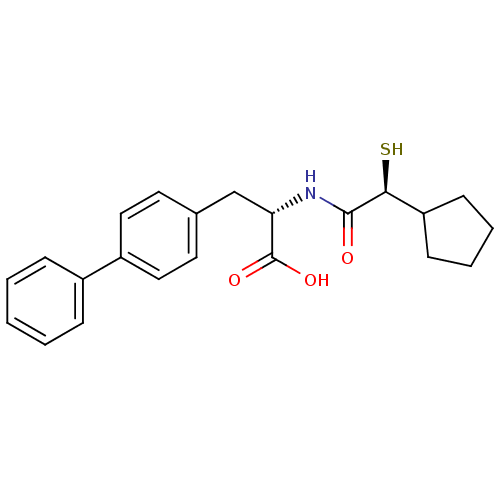
(CHEMBL404117)Show SMILES OC(=O)[C@H](Cc1ccc(cc1)-c1ccccc1)NC(=O)[C@@H](S)C1CCCC1 Show InChI InChI=1S/C22H25NO3S/c24-21(20(27)18-8-4-5-9-18)23-19(22(25)26)14-15-10-12-17(13-11-15)16-6-2-1-3-7-16/h1-3,6-7,10-13,18-20,27H,4-5,8-9,14H2,(H,23,24)(H,25,26)/t19-,20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant ACE2 by fluorescence assay |
Bioorg Med Chem Lett 18: 732-7 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.048
BindingDB Entry DOI: 10.7270/Q2GT5PCB |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50354205

(CHEMBL1835999)Show SMILES CN([C@@H]1CCN(C1)C1CCC1)C(=O)c1ccc(cc1)-n1cccn1 |r| Show InChI InChI=1S/C19H24N4O/c1-21(18-10-13-22(14-18)16-4-2-5-16)19(24)15-6-8-17(9-7-15)23-12-3-11-20-23/h3,6-9,11-12,16,18H,2,4-5,10,13-14H2,1H3/t18-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-(R)alpha-methylhistamine from human histamine H3 receptor expressed in human HEK293T cells |
Bioorg Med Chem Lett 21: 5957-60 (2011)
Article DOI: 10.1016/j.bmcl.2011.07.061
BindingDB Entry DOI: 10.7270/Q2QN6753 |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme 2
(Homo sapiens (Human)) | BDBM50411730

(CHEMBL257270)Show SMILES CCCC[C@H](S)C(=O)N[C@@H](Cc1ccc(cc1)-c1ccccc1)C(O)=O Show InChI InChI=1S/C21H25NO3S/c1-2-3-9-19(26)20(23)22-18(21(24)25)14-15-10-12-17(13-11-15)16-7-5-4-6-8-16/h4-8,10-13,18-19,26H,2-3,9,14H2,1H3,(H,22,23)(H,24,25)/t18-,19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant ACE2 by fluorescence assay |
Bioorg Med Chem Lett 18: 732-7 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.048
BindingDB Entry DOI: 10.7270/Q2GT5PCB |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50383165
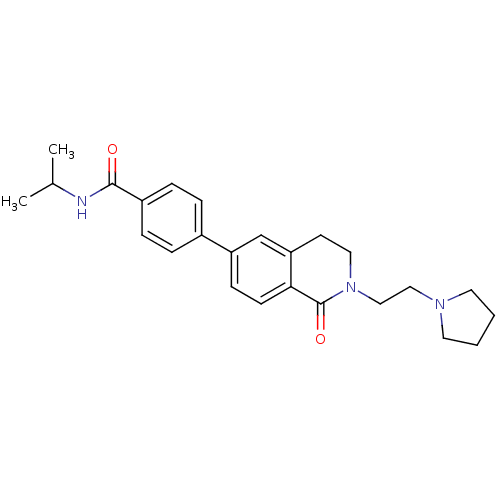
(CHEMBL2031870)Show SMILES CC(C)NC(=O)c1ccc(cc1)-c1ccc2C(=O)N(CCN3CCCC3)CCc2c1 Show InChI InChI=1S/C25H31N3O2/c1-18(2)26-24(29)20-7-5-19(6-8-20)21-9-10-23-22(17-21)11-14-28(25(23)30)16-15-27-12-3-4-13-27/h5-10,17-18H,3-4,11-16H2,1-2H3,(H,26,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-(R)-alpha-methylhistamine from human H3 receptor expressed in HEK293T cells after 120 mins by scintillation counting |
J Med Chem 55: 2452-68 (2012)
Article DOI: 10.1021/jm300011d
BindingDB Entry DOI: 10.7270/Q2736RZR |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50383152
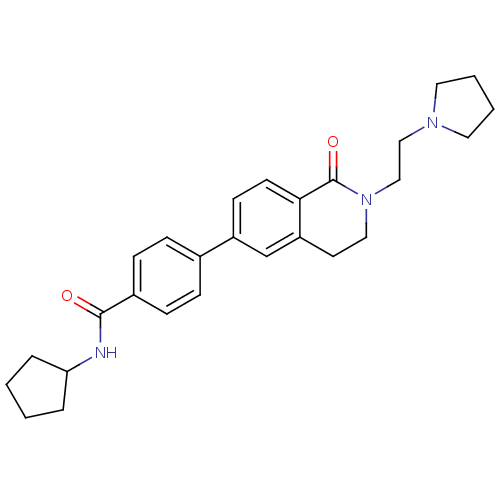
(CHEMBL2031873)Show SMILES O=C(NC1CCCC1)c1ccc(cc1)-c1ccc2C(=O)N(CCN3CCCC3)CCc2c1 Show InChI InChI=1S/C27H33N3O2/c31-26(28-24-5-1-2-6-24)21-9-7-20(8-10-21)22-11-12-25-23(19-22)13-16-30(27(25)32)18-17-29-14-3-4-15-29/h7-12,19,24H,1-6,13-18H2,(H,28,31) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-(R)-alpha-methylhistamine from human H3 receptor expressed in HEK293T cells after 120 mins by scintillation counting |
J Med Chem 55: 2452-68 (2012)
Article DOI: 10.1021/jm300011d
BindingDB Entry DOI: 10.7270/Q2736RZR |
More data for this
Ligand-Target Pair | |
Sigma non-opioid intracellular receptor 1
(Homo sapiens (Human)) | BDBM50251208
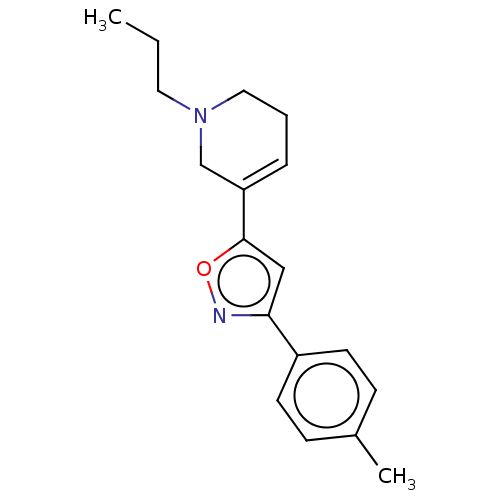
(CHEMBL4088272)Show InChI InChI=1S/C18H22N2O/c1-3-10-20-11-4-5-16(13-20)18-12-17(19-21-18)15-8-6-14(2)7-9-15/h5-9,12H,3-4,10-11,13H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
QBI COVID-19 Research Group (QCRG)
Curated by ChEMBL
| Assay Description
Displacement of [3H]-pentazocin from the Sigma1 receptor |
Nature 583: 459-468 (2020)
Article DOI: 10.1038/s41586-020-2286-9
BindingDB Entry DOI: 10.7270/Q29Z984K |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Sigma non-opioid intracellular receptor 1
(Homo sapiens (Human)) | BDBM50048866

(1-Cyclohexyl-4-[3-(5-methoxy-1,2,3,4-tetrahydro-na...)Show InChI InChI=1S/C24H38N2O/c1-27-24-14-6-12-22-20(8-5-13-23(22)24)9-7-15-25-16-18-26(19-17-25)21-10-3-2-4-11-21/h6,12,14,20-21H,2-5,7-11,13,15-19H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
QBI COVID-19 Research Group (QCRG)
Curated by ChEMBL
| Assay Description
Displacement of [3H]-pentazocin from the Sigma1 receptor |
Nature 583: 459-468 (2020)
Article DOI: 10.1038/s41586-020-2286-9
BindingDB Entry DOI: 10.7270/Q29Z984K |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50383151
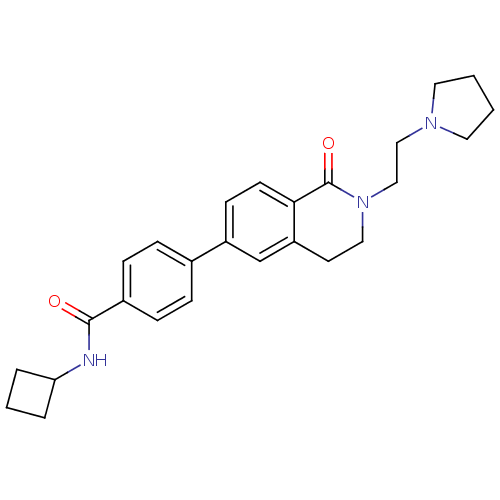
(CHEMBL2031872)Show SMILES O=C(NC1CCC1)c1ccc(cc1)-c1ccc2C(=O)N(CCN3CCCC3)CCc2c1 Show InChI InChI=1S/C26H31N3O2/c30-25(27-23-4-3-5-23)20-8-6-19(7-9-20)21-10-11-24-22(18-21)12-15-29(26(24)31)17-16-28-13-1-2-14-28/h6-11,18,23H,1-5,12-17H2,(H,27,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-(R)-alpha-methylhistamine from human H3 receptor expressed in HEK293T cells after 120 mins by scintillation counting |
J Med Chem 55: 2452-68 (2012)
Article DOI: 10.1021/jm300011d
BindingDB Entry DOI: 10.7270/Q2736RZR |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50383137
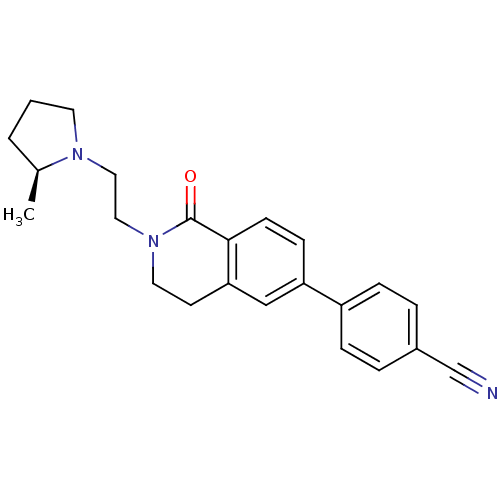
(CHEMBL2031761)Show SMILES C[C@H]1CCCN1CCN1CCc2cc(ccc2C1=O)-c1ccc(cc1)C#N |r| Show InChI InChI=1S/C23H25N3O/c1-17-3-2-11-25(17)13-14-26-12-10-21-15-20(8-9-22(21)23(26)27)19-6-4-18(16-24)5-7-19/h4-9,15,17H,2-3,10-14H2,1H3/t17-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-(R)-alpha-methylhistamine from human H3 receptor expressed in HEK293T cells after 120 mins by scintillation counting |
J Med Chem 55: 2452-68 (2012)
Article DOI: 10.1021/jm300011d
BindingDB Entry DOI: 10.7270/Q2736RZR |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50383140
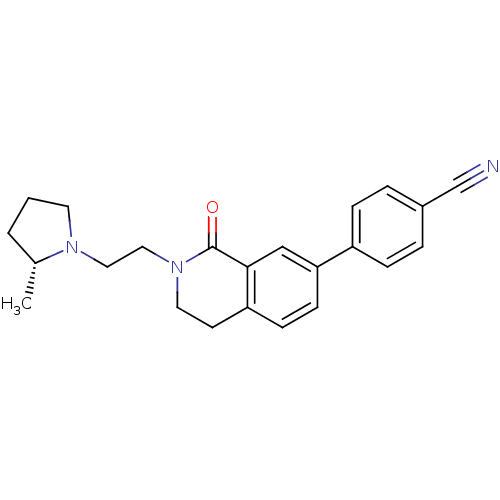
(CHEMBL2031764)Show SMILES C[C@@H]1CCCN1CCN1CCc2ccc(cc2C1=O)-c1ccc(cc1)C#N |r| Show InChI InChI=1S/C23H25N3O/c1-17-3-2-11-25(17)13-14-26-12-10-20-8-9-21(15-22(20)23(26)27)19-6-4-18(16-24)5-7-19/h4-9,15,17H,2-3,10-14H2,1H3/t17-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-(R)-alpha-methylhistamine from human H3 receptor expressed in HEK293T cells after 120 mins by scintillation counting |
J Med Chem 55: 2452-68 (2012)
Article DOI: 10.1021/jm300011d
BindingDB Entry DOI: 10.7270/Q2736RZR |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50383154
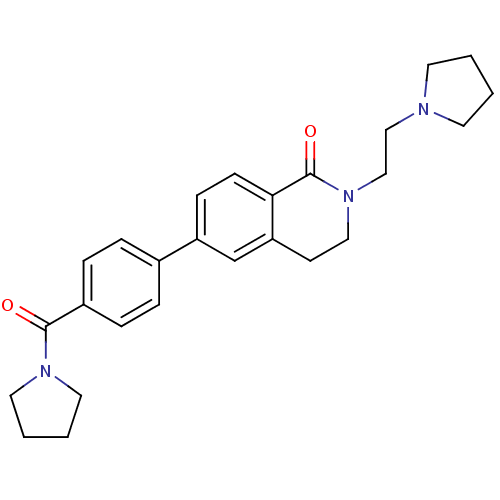
(CHEMBL2031875)Show SMILES O=C(N1CCCC1)c1ccc(cc1)-c1ccc2C(=O)N(CCN3CCCC3)CCc2c1 Show InChI InChI=1S/C26H31N3O2/c30-25(28-14-3-4-15-28)21-7-5-20(6-8-21)22-9-10-24-23(19-22)11-16-29(26(24)31)18-17-27-12-1-2-13-27/h5-10,19H,1-4,11-18H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-(R)-alpha-methylhistamine from human H3 receptor expressed in HEK293T cells after 120 mins by scintillation counting |
J Med Chem 55: 2452-68 (2012)
Article DOI: 10.1021/jm300011d
BindingDB Entry DOI: 10.7270/Q2736RZR |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme 2
(Homo sapiens (Human)) | BDBM50411728
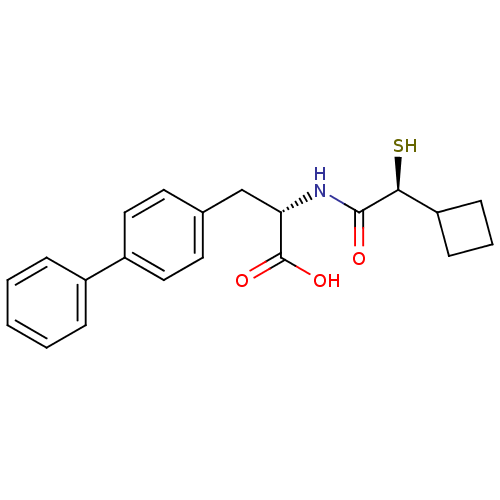
(CHEMBL257229)Show SMILES OC(=O)[C@H](Cc1ccc(cc1)-c1ccccc1)NC(=O)[C@@H](S)C1CCC1 Show InChI InChI=1S/C21H23NO3S/c23-20(19(26)17-7-4-8-17)22-18(21(24)25)13-14-9-11-16(12-10-14)15-5-2-1-3-6-15/h1-3,5-6,9-12,17-19,26H,4,7-8,13H2,(H,22,23)(H,24,25)/t18-,19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant ACE2 by fluorescence assay |
Bioorg Med Chem Lett 18: 732-7 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.048
BindingDB Entry DOI: 10.7270/Q2GT5PCB |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data