 Found 171 hits with Last Name = 'gruber' and Initial = 'j'
Found 171 hits with Last Name = 'gruber' and Initial = 'j' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Metabotropic glutamate receptor 1
(RAT) | BDBM50379862
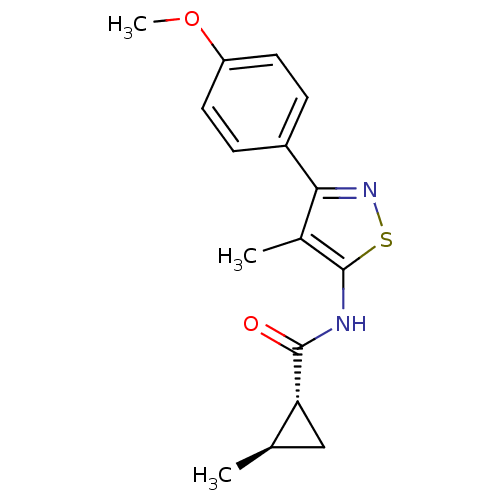
(CHEMBL2011870)Show SMILES COc1ccc(cc1)-c1nsc(NC(=O)[C@@H]2C[C@H]2C)c1C |r| Show InChI InChI=1S/C16H18N2O2S/c1-9-8-13(9)15(19)17-16-10(2)14(18-21-16)11-4-6-12(20-3)7-5-11/h4-7,9,13H,8H2,1-3H3,(H,17,19)/t9-,13-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Antagonist activity at rat mGluR1 |
Bioorg Med Chem Lett 22: 2514-7 (2012)
Article DOI: 10.1016/j.bmcl.2012.02.003
BindingDB Entry DOI: 10.7270/Q2RX9D3G |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Homo sapiens (Human)) | BDBM50535210
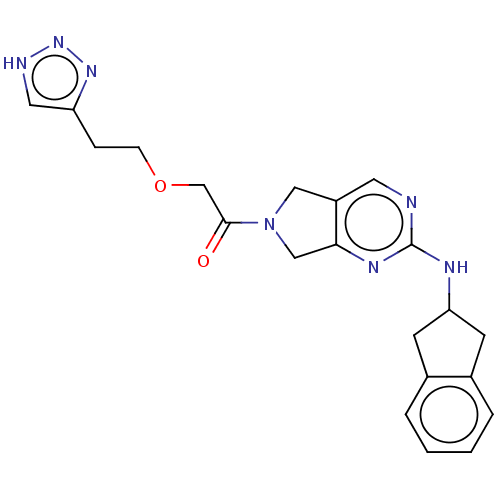
(CHEMBL4448598)Show SMILES O=C(COCCc1c[nH]nn1)N1Cc2cnc(NC3Cc4ccccc4C3)nc2C1 Show InChI InChI=1S/C21H23N7O2/c29-20(13-30-6-5-17-10-23-27-26-17)28-11-16-9-22-21(25-19(16)12-28)24-18-7-14-3-1-2-4-15(14)8-18/h1-4,9-10,18H,5-8,11-13H2,(H,22,24,25)(H,23,26,27) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of recombinant full length human C-terminal His-tagged autotaxin expressed in human 293E cells assessed as choline release using lysophosp... |
ACS Med Chem Lett 7: 857-61 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00207
BindingDB Entry DOI: 10.7270/Q2KD22D0 |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Homo sapiens (Human)) | BDBM50535210

(CHEMBL4448598)Show SMILES O=C(COCCc1c[nH]nn1)N1Cc2cnc(NC3Cc4ccccc4C3)nc2C1 Show InChI InChI=1S/C21H23N7O2/c29-20(13-30-6-5-17-10-23-27-26-17)28-11-16-9-22-21(25-19(16)12-28)24-18-7-14-3-1-2-4-15(14)8-18/h1-4,9-10,18H,5-8,11-13H2,(H,22,24,25)(H,23,26,27) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of autotaxin in healthy human plasma assessed as reduction in LPA level after 3 hrs by mass spectrometric analysis |
ACS Med Chem Lett 7: 857-61 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00207
BindingDB Entry DOI: 10.7270/Q2KD22D0 |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Homo sapiens (Human)) | BDBM50535213
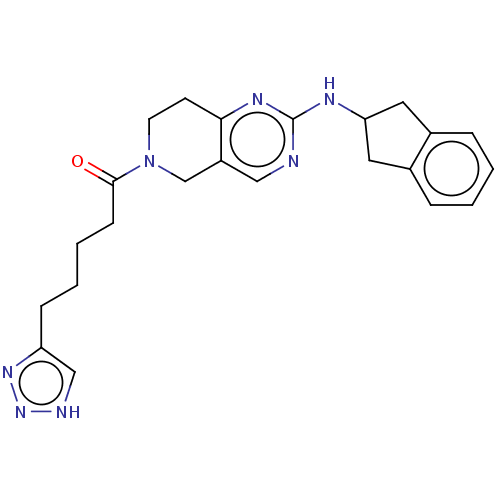
(CHEMBL4453084)Show SMILES O=C(CCCCc1c[nH]nn1)N1CCc2nc(NC3Cc4ccccc4C3)ncc2C1 Show InChI InChI=1S/C23H27N7O/c31-22(8-4-3-7-19-14-25-29-28-19)30-10-9-21-18(15-30)13-24-23(27-21)26-20-11-16-5-1-2-6-17(16)12-20/h1-2,5-6,13-14,20H,3-4,7-12,15H2,(H,24,26,27)(H,25,28,29) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of recombinant full length human C-terminal His-tagged autotaxin expressed in human 293E cells assessed as choline release using lysophosp... |
ACS Med Chem Lett 7: 857-61 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00207
BindingDB Entry DOI: 10.7270/Q2KD22D0 |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Homo sapiens (Human)) | BDBM50535214
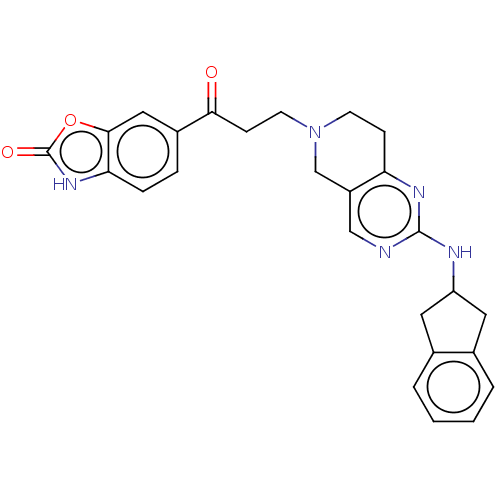
(CHEMBL4549771)Show SMILES O=C(CCN1CCc2nc(NC3Cc4ccccc4C3)ncc2C1)c1ccc2[nH]c(=O)oc2c1 Show InChI InChI=1S/C26H25N5O3/c32-23(18-5-6-22-24(13-18)34-26(33)30-22)8-10-31-9-7-21-19(15-31)14-27-25(29-21)28-20-11-16-3-1-2-4-17(16)12-20/h1-6,13-14,20H,7-12,15H2,(H,30,33)(H,27,28,29) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of recombinant full length human C-terminal His-tagged autotaxin expressed in human 293E cells assessed as choline release using lysophosp... |
ACS Med Chem Lett 7: 857-61 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00207
BindingDB Entry DOI: 10.7270/Q2KD22D0 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Homo sapiens (Human)) | BDBM50535215
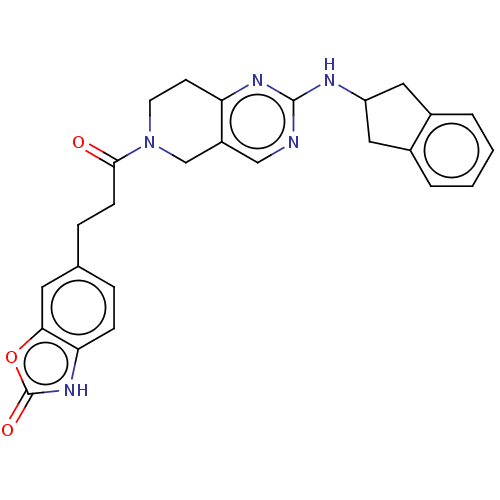
(CHEMBL4476558)Show SMILES O=C(CCc1ccc2[nH]c(=O)oc2c1)N1CCc2nc(NC3Cc4ccccc4C3)ncc2C1 Show InChI InChI=1S/C26H25N5O3/c32-24(8-6-16-5-7-22-23(11-16)34-26(33)30-22)31-10-9-21-19(15-31)14-27-25(29-21)28-20-12-17-3-1-2-4-18(17)13-20/h1-5,7,11,14,20H,6,8-10,12-13,15H2,(H,30,33)(H,27,28,29) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of recombinant full length human C-terminal His-tagged autotaxin expressed in human 293E cells assessed as choline release using lysophosp... |
ACS Med Chem Lett 7: 857-61 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00207
BindingDB Entry DOI: 10.7270/Q2KD22D0 |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Homo sapiens (Human)) | BDBM50535212

(CHEMBL4569141)Show SMILES Clc1ccc(CCNc2ncc3CN(CCc3n2)C(=O)CCc2ccc3[nH]c(=O)oc3c2)cc1 Show InChI InChI=1S/C25H24ClN5O3/c26-19-5-1-16(2-6-19)9-11-27-24-28-14-18-15-31(12-10-20(18)29-24)23(32)8-4-17-3-7-21-22(13-17)34-25(33)30-21/h1-3,5-7,13-14H,4,8-12,15H2,(H,30,33)(H,27,28,29) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of recombinant full length human C-terminal His-tagged autotaxin expressed in human 293E cells assessed as choline release using lysophosp... |
ACS Med Chem Lett 7: 857-61 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00207
BindingDB Entry DOI: 10.7270/Q2KD22D0 |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Homo sapiens (Human)) | BDBM50535220

(CHEMBL4454442)Show SMILES O=C(CCCCn1ccnc1)N1CCc2nc(NC3Cc4ccccc4C3)ncc2C1 Show InChI InChI=1S/C24H28N6O/c31-23(7-3-4-10-29-12-9-25-17-29)30-11-8-22-20(16-30)15-26-24(28-22)27-21-13-18-5-1-2-6-19(18)14-21/h1-2,5-6,9,12,15,17,21H,3-4,7-8,10-11,13-14,16H2,(H,26,27,28) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of recombinant full length human C-terminal His-tagged autotaxin expressed in human 293E cells assessed as choline release using lysophosp... |
ACS Med Chem Lett 7: 857-61 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00207
BindingDB Entry DOI: 10.7270/Q2KD22D0 |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Homo sapiens (Human)) | BDBM50535215

(CHEMBL4476558)Show SMILES O=C(CCc1ccc2[nH]c(=O)oc2c1)N1CCc2nc(NC3Cc4ccccc4C3)ncc2C1 Show InChI InChI=1S/C26H25N5O3/c32-24(8-6-16-5-7-22-23(11-16)34-26(33)30-22)31-10-9-21-19(15-31)14-27-25(29-21)28-20-12-17-3-1-2-4-18(17)13-20/h1-5,7,11,14,20H,6,8-10,12-13,15H2,(H,30,33)(H,27,28,29) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of autotaxin in healthy human plasma assessed as reduction in LPA level after 3 hrs by mass spectrometric analysis |
ACS Med Chem Lett 7: 857-61 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00207
BindingDB Entry DOI: 10.7270/Q2KD22D0 |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Homo sapiens (Human)) | BDBM50535213

(CHEMBL4453084)Show SMILES O=C(CCCCc1c[nH]nn1)N1CCc2nc(NC3Cc4ccccc4C3)ncc2C1 Show InChI InChI=1S/C23H27N7O/c31-22(8-4-3-7-19-14-25-29-28-19)30-10-9-21-18(15-30)13-24-23(27-21)26-20-11-16-5-1-2-6-17(16)12-20/h1-2,5-6,13-14,20H,3-4,7-12,15H2,(H,24,26,27)(H,25,28,29) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of autotaxin in healthy human plasma assessed as reduction in LPA level after 3 hrs by mass spectrometric analysis |
ACS Med Chem Lett 7: 857-61 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00207
BindingDB Entry DOI: 10.7270/Q2KD22D0 |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 1
(Homo sapiens (Human)) | BDBM50379866
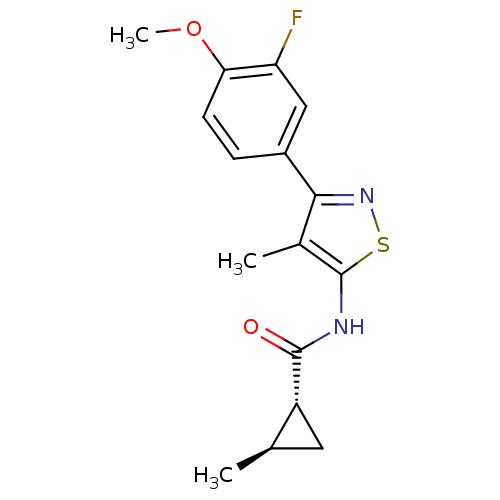
(CHEMBL2011874)Show SMILES COc1ccc(cc1F)-c1nsc(NC(=O)[C@@H]2C[C@H]2C)c1C |r| Show InChI InChI=1S/C16H17FN2O2S/c1-8-6-11(8)15(20)18-16-9(2)14(19-22-16)10-4-5-13(21-3)12(17)7-10/h4-5,7-8,11H,6H2,1-3H3,(H,18,20)/t8-,11-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Allosteric antagonist activity at human recombinant mGluR1 expressed in AV12 cells assessed as intracellular calcium concentration using Fluo-3 dye b... |
Bioorg Med Chem Lett 22: 2514-7 (2012)
Article DOI: 10.1016/j.bmcl.2012.02.003
BindingDB Entry DOI: 10.7270/Q2RX9D3G |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 1
(Homo sapiens (Human)) | BDBM50379867

(CHEMBL2011877)Show SMILES C[C@@H]1C[C@H]1C(=O)Nc1snc(c1C)-c1ccc(Br)cc1 |r| Show InChI InChI=1S/C15H15BrN2OS/c1-8-7-12(8)14(19)17-15-9(2)13(18-20-15)10-3-5-11(16)6-4-10/h3-6,8,12H,7H2,1-2H3,(H,17,19)/t8-,12-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Allosteric antagonist activity at human recombinant mGluR1 expressed in AV12 cells assessed as intracellular calcium concentration using Fluo-3 dye b... |
Bioorg Med Chem Lett 22: 2514-7 (2012)
Article DOI: 10.1016/j.bmcl.2012.02.003
BindingDB Entry DOI: 10.7270/Q2RX9D3G |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Homo sapiens (Human)) | BDBM50535220

(CHEMBL4454442)Show SMILES O=C(CCCCn1ccnc1)N1CCc2nc(NC3Cc4ccccc4C3)ncc2C1 Show InChI InChI=1S/C24H28N6O/c31-23(7-3-4-10-29-12-9-25-17-29)30-11-8-22-20(16-30)15-26-24(28-22)27-21-13-18-5-1-2-6-19(18)14-21/h1-2,5-6,9,12,15,17,21H,3-4,7-8,10-11,13-14,16H2,(H,26,27,28) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of autotaxin in healthy human plasma assessed as reduction in LPA level after 3 hrs by mass spectrometric analysis |
ACS Med Chem Lett 7: 857-61 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00207
BindingDB Entry DOI: 10.7270/Q2KD22D0 |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Homo sapiens (Human)) | BDBM50187693

(CHEMBL3186509)Show SMILES Clc1cc(Cl)cc(COC(=O)N2CCN(CCC(=O)c3ccc4[nH]c(=O)oc4c3)CC2)c1 Show InChI InChI=1S/C22H21Cl2N3O5/c23-16-9-14(10-17(24)12-16)13-31-22(30)27-7-5-26(6-8-27)4-3-19(28)15-1-2-18-20(11-15)32-21(29)25-18/h1-2,9-12H,3-8,13H2,(H,25,29) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 8.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of recombinant full length human C-terminal His-tagged autotaxin expressed in human 293E cells assessed as choline release using lysophosp... |
ACS Med Chem Lett 7: 857-61 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00207
BindingDB Entry DOI: 10.7270/Q2KD22D0 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Metabotropic glutamate receptor 1
(Homo sapiens (Human)) | BDBM50379861
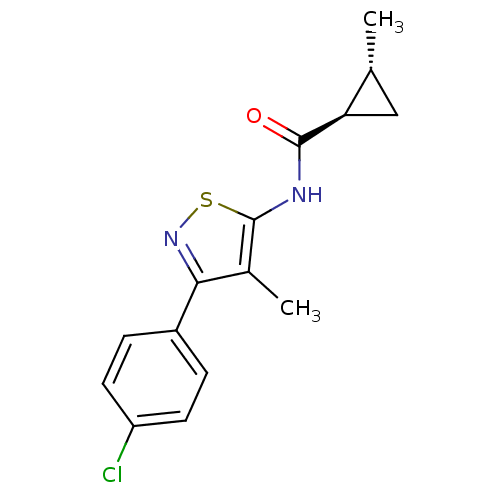
(CHEMBL2011876)Show SMILES C[C@@H]1C[C@H]1C(=O)Nc1snc(c1C)-c1ccc(Cl)cc1 |r| Show InChI InChI=1S/C15H15ClN2OS/c1-8-7-12(8)14(19)17-15-9(2)13(18-20-15)10-3-5-11(16)6-4-10/h3-6,8,12H,7H2,1-2H3,(H,17,19)/t8-,12-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Allosteric antagonist activity at human recombinant mGluR1 expressed in AV12 cells assessed as intracellular calcium concentration using Fluo-3 dye b... |
Bioorg Med Chem Lett 22: 2514-7 (2012)
Article DOI: 10.1016/j.bmcl.2012.02.003
BindingDB Entry DOI: 10.7270/Q2RX9D3G |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Homo sapiens (Human)) | BDBM50535221
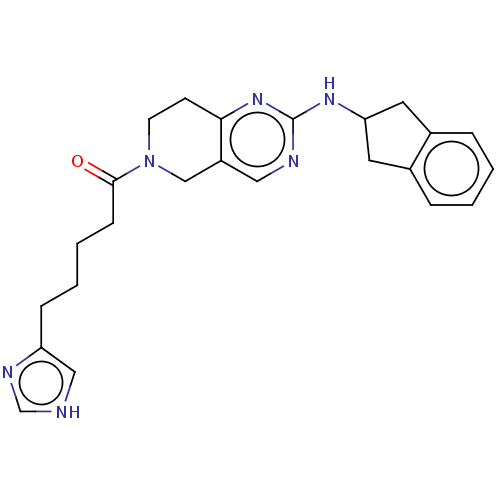
(CHEMBL4462537)Show SMILES O=C(CCCCc1c[nH]cn1)N1CCc2nc(NC3Cc4ccccc4C3)ncc2C1 Show InChI InChI=1S/C24H28N6O/c31-23(8-4-3-7-20-14-25-16-27-20)30-10-9-22-19(15-30)13-26-24(29-22)28-21-11-17-5-1-2-6-18(17)12-21/h1-2,5-6,13-14,16,21H,3-4,7-12,15H2,(H,25,27)(H,26,28,29) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of recombinant full length human C-terminal His-tagged autotaxin expressed in human 293E cells assessed as choline release using lysophosp... |
ACS Med Chem Lett 7: 857-61 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00207
BindingDB Entry DOI: 10.7270/Q2KD22D0 |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 5
(Homo sapiens (Human)) | BDBM50379865

(CHEMBL2011873)Show SMILES CCOc1ccc(cc1)-c1nsc(NC(=O)[C@@H]2C[C@H]2C)c1C |r| Show InChI InChI=1S/C17H20N2O2S/c1-4-21-13-7-5-12(6-8-13)15-11(3)17(22-19-15)18-16(20)14-9-10(14)2/h5-8,10,14H,4,9H2,1-3H3,(H,18,20)/t10-,14-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Antagonist activity at human mGluR5 |
Bioorg Med Chem Lett 22: 2514-7 (2012)
Article DOI: 10.1016/j.bmcl.2012.02.003
BindingDB Entry DOI: 10.7270/Q2RX9D3G |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Homo sapiens (Human)) | BDBM50535216
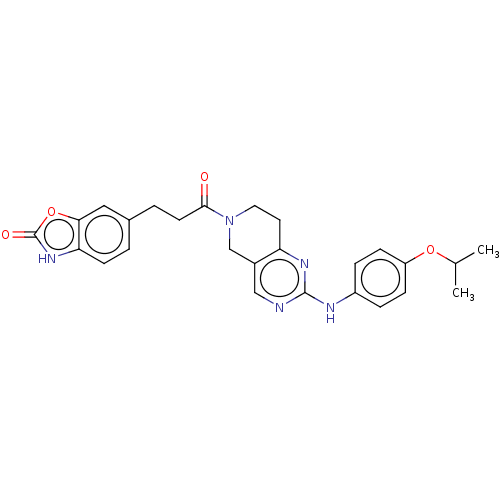
(CHEMBL4472840)Show SMILES CC(C)Oc1ccc(Nc2ncc3CN(CCc3n2)C(=O)CCc2ccc3[nH]c(=O)oc3c2)cc1 Show InChI InChI=1S/C26H27N5O4/c1-16(2)34-20-7-5-19(6-8-20)28-25-27-14-18-15-31(12-11-21(18)29-25)24(32)10-4-17-3-9-22-23(13-17)35-26(33)30-22/h3,5-9,13-14,16H,4,10-12,15H2,1-2H3,(H,30,33)(H,27,28,29) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of recombinant full length human C-terminal His-tagged autotaxin expressed in human 293E cells assessed as choline release using lysophosp... |
ACS Med Chem Lett 7: 857-61 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00207
BindingDB Entry DOI: 10.7270/Q2KD22D0 |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Homo sapiens (Human)) | BDBM50535219
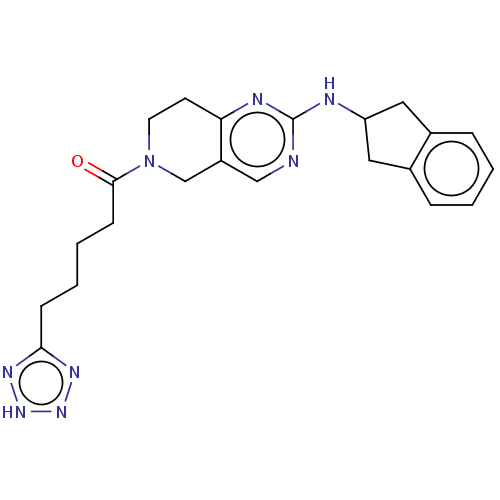
(CHEMBL4446322)Show SMILES O=C(CCCCc1nn[nH]n1)N1CCc2nc(NC3Cc4ccccc4C3)ncc2C1 Show InChI InChI=1S/C22H26N8O/c31-21(8-4-3-7-20-26-28-29-27-20)30-10-9-19-17(14-30)13-23-22(25-19)24-18-11-15-5-1-2-6-16(15)12-18/h1-2,5-6,13,18H,3-4,7-12,14H2,(H,23,24,25)(H,26,27,28,29) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of recombinant full length human C-terminal His-tagged autotaxin expressed in human 293E cells assessed as choline release using lysophosp... |
ACS Med Chem Lett 7: 857-61 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00207
BindingDB Entry DOI: 10.7270/Q2KD22D0 |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 1
(Homo sapiens (Human)) | BDBM50379862

(CHEMBL2011870)Show SMILES COc1ccc(cc1)-c1nsc(NC(=O)[C@@H]2C[C@H]2C)c1C |r| Show InChI InChI=1S/C16H18N2O2S/c1-9-8-13(9)15(19)17-16-10(2)14(18-21-16)11-4-6-12(20-3)7-5-11/h4-7,9,13H,8H2,1-3H3,(H,17,19)/t9-,13-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Allosteric antagonist activity at human recombinant mGluR1 expressed in AV12 cells assessed as intracellular calcium concentration using Fluo-3 dye b... |
Bioorg Med Chem Lett 22: 2514-7 (2012)
Article DOI: 10.1016/j.bmcl.2012.02.003
BindingDB Entry DOI: 10.7270/Q2RX9D3G |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 1
(Homo sapiens (Human)) | BDBM50379862

(CHEMBL2011870)Show SMILES COc1ccc(cc1)-c1nsc(NC(=O)[C@@H]2C[C@H]2C)c1C |r| Show InChI InChI=1S/C16H18N2O2S/c1-9-8-13(9)15(19)17-16-10(2)14(18-21-16)11-4-6-12(20-3)7-5-11/h4-7,9,13H,8H2,1-3H3,(H,17,19)/t9-,13-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Allosteric antagonist activity at human recombinant mGluR1 expressed in AV12 cells assessed as intracellular calcium concentration using Fluo-3 dye b... |
Bioorg Med Chem Lett 22: 2514-7 (2012)
Article DOI: 10.1016/j.bmcl.2012.02.003
BindingDB Entry DOI: 10.7270/Q2RX9D3G |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Homo sapiens (Human)) | BDBM50535221

(CHEMBL4462537)Show SMILES O=C(CCCCc1c[nH]cn1)N1CCc2nc(NC3Cc4ccccc4C3)ncc2C1 Show InChI InChI=1S/C24H28N6O/c31-23(8-4-3-7-20-14-25-16-27-20)30-10-9-22-19(15-30)13-26-24(29-22)28-21-11-17-5-1-2-6-18(17)12-21/h1-2,5-6,13-14,16,21H,3-4,7-12,15H2,(H,25,27)(H,26,28,29) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of autotaxin in healthy human plasma assessed as reduction in LPA level after 3 hrs by mass spectrometric analysis |
ACS Med Chem Lett 7: 857-61 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00207
BindingDB Entry DOI: 10.7270/Q2KD22D0 |
More data for this
Ligand-Target Pair | |
Multidrug resistance-associated protein 1
(Homo sapiens (Human)) | BDBM50174854
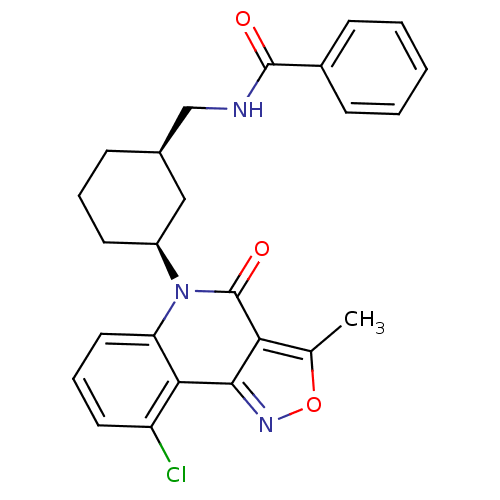
(CHEMBL200216 | N-(((1R,3S)-3-(9-chloro-3-methyl-4-...)Show SMILES Cc1onc2c1c(=O)n([C@H]1CCC[C@@H](CNC(=O)c3ccccc3)C1)c1cccc(Cl)c21 Show InChI InChI=1S/C25H24ClN3O3/c1-15-21-23(28-32-15)22-19(26)11-6-12-20(22)29(25(21)31)18-10-5-7-16(13-18)14-27-24(30)17-8-3-2-4-9-17/h2-4,6,8-9,11-12,16,18H,5,7,10,13-14H2,1H3,(H,27,30)/t16-,18+/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 38 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Inhibitory activity against MRP1-mediated LTC4 uptake into membrane vesicles from HeLa-T5 cells expressing MRP1 |
Bioorg Med Chem Lett 15: 5526-30 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.075
BindingDB Entry DOI: 10.7270/Q2VX0G1X |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 1
(Homo sapiens (Human)) | BDBM50379868
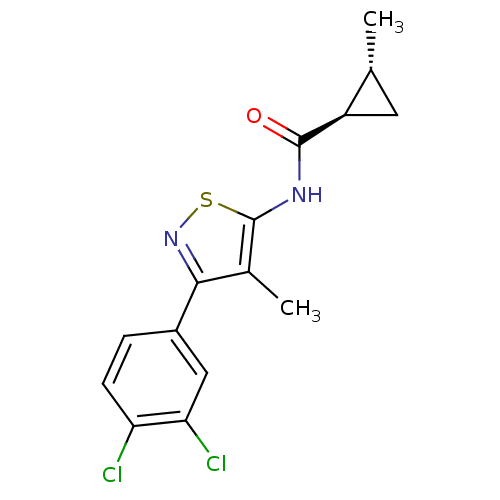
(CHEMBL2011878)Show SMILES C[C@@H]1C[C@H]1C(=O)Nc1snc(c1C)-c1ccc(Cl)c(Cl)c1 |r| Show InChI InChI=1S/C15H14Cl2N2OS/c1-7-5-10(7)14(20)18-15-8(2)13(19-21-15)9-3-4-11(16)12(17)6-9/h3-4,6-7,10H,5H2,1-2H3,(H,18,20)/t7-,10-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 39 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Allosteric antagonist activity at human recombinant mGluR1 expressed in AV12 cells assessed as intracellular calcium concentration using Fluo-3 dye b... |
Bioorg Med Chem Lett 22: 2514-7 (2012)
Article DOI: 10.1016/j.bmcl.2012.02.003
BindingDB Entry DOI: 10.7270/Q2RX9D3G |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Homo sapiens (Human)) | BDBM50535214

(CHEMBL4549771)Show SMILES O=C(CCN1CCc2nc(NC3Cc4ccccc4C3)ncc2C1)c1ccc2[nH]c(=O)oc2c1 Show InChI InChI=1S/C26H25N5O3/c32-23(18-5-6-22-24(13-18)34-26(33)30-22)8-10-31-9-7-21-19(15-31)14-27-25(29-21)28-20-11-16-3-1-2-4-17(16)12-20/h1-6,13-14,20H,7-12,15H2,(H,30,33)(H,27,28,29) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 42 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of autotaxin in healthy human plasma assessed as reduction in LPA level after 3 hrs by mass spectrometric analysis |
ACS Med Chem Lett 7: 857-61 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00207
BindingDB Entry DOI: 10.7270/Q2KD22D0 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Homo sapiens (Human)) | BDBM50187693

(CHEMBL3186509)Show SMILES Clc1cc(Cl)cc(COC(=O)N2CCN(CCC(=O)c3ccc4[nH]c(=O)oc4c3)CC2)c1 Show InChI InChI=1S/C22H21Cl2N3O5/c23-16-9-14(10-17(24)12-16)13-31-22(30)27-7-5-26(6-8-27)4-3-19(28)15-1-2-18-20(11-15)32-21(29)25-18/h1-2,9-12H,3-8,13H2,(H,25,29) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 44 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of autotaxin in healthy human plasma assessed as reduction in LPA level after 3 hrs by mass spectrometric analysis |
ACS Med Chem Lett 7: 857-61 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00207
BindingDB Entry DOI: 10.7270/Q2KD22D0 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Homo sapiens (Human)) | BDBM50535217

(CHEMBL4458386)Show SMILES Clc1cccc(CNc2ncc3CN(CCc3n2)C(=O)CCc2ccc3[nH]c(=O)oc3c2)c1 Show InChI InChI=1S/C24H22ClN5O3/c25-18-3-1-2-16(10-18)12-26-23-27-13-17-14-30(9-8-19(17)28-23)22(31)7-5-15-4-6-20-21(11-15)33-24(32)29-20/h1-4,6,10-11,13H,5,7-9,12,14H2,(H,29,32)(H,26,27,28) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 52 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of recombinant full length human C-terminal His-tagged autotaxin expressed in human 293E cells assessed as choline release using lysophosp... |
ACS Med Chem Lett 7: 857-61 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00207
BindingDB Entry DOI: 10.7270/Q2KD22D0 |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50229787

((4S,5R)-Nutlin-3 | (rac)-(4,5-bis(4-chlorophenyl)-...)Show SMILES COc1ccc(C2=N[C@H]([C@H](N2C(=O)N2CCNC(=O)C2)c2ccc(Cl)cc2)c2ccc(Cl)cc2)c(OC(C)C)c1 |t:6| Show InChI InChI=1S/C30H30Cl2N4O4/c1-18(2)40-25-16-23(39-3)12-13-24(25)29-34-27(19-4-8-21(31)9-5-19)28(20-6-10-22(32)11-7-20)36(29)30(38)35-15-14-33-26(37)17-35/h4-13,16,18,27-28H,14-15,17H2,1-3H3,(H,33,37)/t27-,28+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 61 | n/a | n/a | n/a | n/a | n/a | n/a |
Newcastle University
Curated by ChEMBL
| Assay Description
Inhibition of Mdm2 -p53 protein interaction by ELISA |
J Med Chem 54: 1233-43 (2011)
Article DOI: 10.1021/jm1011929
BindingDB Entry DOI: 10.7270/Q29K4BHN |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Metabotropic glutamate receptor 1
(Homo sapiens (Human)) | BDBM50379864
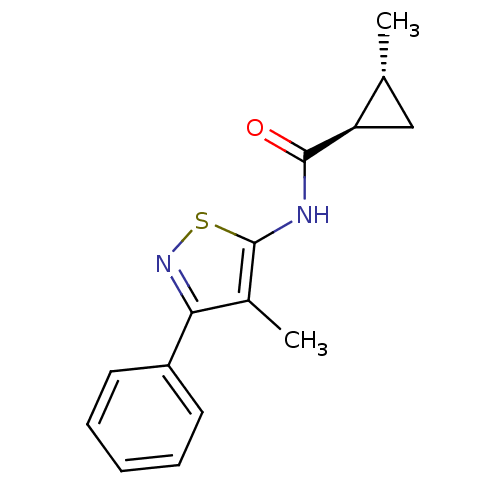
(CHEMBL2011872)Show SMILES C[C@@H]1C[C@H]1C(=O)Nc1snc(c1C)-c1ccccc1 |r| Show InChI InChI=1S/C15H16N2OS/c1-9-8-12(9)14(18)16-15-10(2)13(17-19-15)11-6-4-3-5-7-11/h3-7,9,12H,8H2,1-2H3,(H,16,18)/t9-,12-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 65 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Allosteric antagonist activity at human recombinant mGluR1 expressed in AV12 cells assessed as intracellular calcium concentration using Fluo-3 dye b... |
Bioorg Med Chem Lett 22: 2514-7 (2012)
Article DOI: 10.1016/j.bmcl.2012.02.003
BindingDB Entry DOI: 10.7270/Q2RX9D3G |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 1
(Homo sapiens (Human)) | BDBM50379869
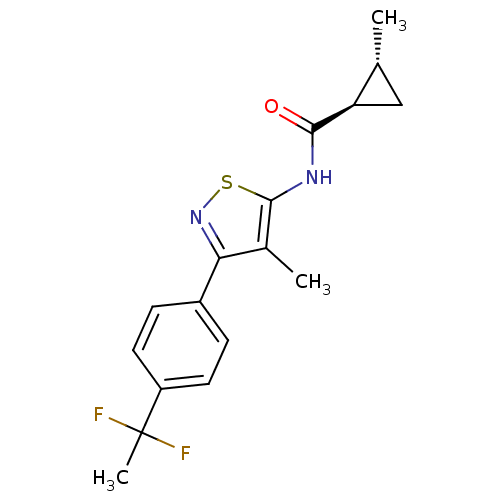
(CHEMBL2011879)Show SMILES C[C@@H]1C[C@H]1C(=O)Nc1snc(c1C)-c1ccc(cc1)C(C)(F)F |r| Show InChI InChI=1S/C17H18F2N2OS/c1-9-8-13(9)15(22)20-16-10(2)14(21-23-16)11-4-6-12(7-5-11)17(3,18)19/h4-7,9,13H,8H2,1-3H3,(H,20,22)/t9-,13-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 67 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Allosteric antagonist activity at human recombinant mGluR1 expressed in AV12 cells assessed as intracellular calcium concentration using Fluo-3 dye b... |
Bioorg Med Chem Lett 22: 2514-7 (2012)
Article DOI: 10.1016/j.bmcl.2012.02.003
BindingDB Entry DOI: 10.7270/Q2RX9D3G |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Homo sapiens (Human)) | BDBM50535211

(CHEMBL4561467)Show SMILES O=C(CCCCc1nnc[nH]1)N1CCc2nc(NC3Cc4ccccc4C3)ncc2C1 Show InChI InChI=1S/C23H27N7O/c31-22(8-4-3-7-21-25-15-26-29-21)30-10-9-20-18(14-30)13-24-23(28-20)27-19-11-16-5-1-2-6-17(16)12-19/h1-2,5-6,13,15,19H,3-4,7-12,14H2,(H,24,27,28)(H,25,26,29) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 79 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of recombinant full length human C-terminal His-tagged autotaxin expressed in human 293E cells assessed as choline release using lysophosp... |
ACS Med Chem Lett 7: 857-61 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00207
BindingDB Entry DOI: 10.7270/Q2KD22D0 |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Homo sapiens (Human)) | BDBM50535216

(CHEMBL4472840)Show SMILES CC(C)Oc1ccc(Nc2ncc3CN(CCc3n2)C(=O)CCc2ccc3[nH]c(=O)oc3c2)cc1 Show InChI InChI=1S/C26H27N5O4/c1-16(2)34-20-7-5-19(6-8-20)28-25-27-14-18-15-31(12-11-21(18)29-25)24(32)10-4-17-3-9-22-23(13-17)35-26(33)30-22/h3,5-9,13-14,16H,4,10-12,15H2,1-2H3,(H,30,33)(H,27,28,29) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 87 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of autotaxin in healthy human plasma assessed as reduction in LPA level after 3 hrs by mass spectrometric analysis |
ACS Med Chem Lett 7: 857-61 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00207
BindingDB Entry DOI: 10.7270/Q2KD22D0 |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Homo sapiens (Human)) | BDBM50535211

(CHEMBL4561467)Show SMILES O=C(CCCCc1nnc[nH]1)N1CCc2nc(NC3Cc4ccccc4C3)ncc2C1 Show InChI InChI=1S/C23H27N7O/c31-22(8-4-3-7-21-25-15-26-29-21)30-10-9-20-18(14-30)13-24-23(28-20)27-19-11-16-5-1-2-6-17(16)12-19/h1-2,5-6,13,15,19H,3-4,7-12,14H2,(H,24,27,28)(H,25,26,29) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 92 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of autotaxin in healthy human plasma assessed as reduction in LPA level after 3 hrs by mass spectrometric analysis |
ACS Med Chem Lett 7: 857-61 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00207
BindingDB Entry DOI: 10.7270/Q2KD22D0 |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 1
(Homo sapiens (Human)) | BDBM50379865

(CHEMBL2011873)Show SMILES CCOc1ccc(cc1)-c1nsc(NC(=O)[C@@H]2C[C@H]2C)c1C |r| Show InChI InChI=1S/C17H20N2O2S/c1-4-21-13-7-5-12(6-8-13)15-11(3)17(22-19-15)18-16(20)14-9-10(14)2/h5-8,10,14H,4,9H2,1-3H3,(H,18,20)/t10-,14-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 92 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Allosteric antagonist activity at human recombinant mGluR1 expressed in AV12 cells assessed as intracellular calcium concentration using Fluo-3 dye b... |
Bioorg Med Chem Lett 22: 2514-7 (2012)
Article DOI: 10.1016/j.bmcl.2012.02.003
BindingDB Entry DOI: 10.7270/Q2RX9D3G |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 1
(Homo sapiens (Human)) | BDBM50379860
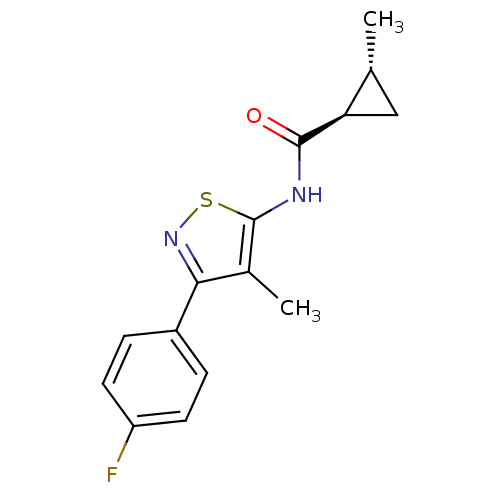
(CHEMBL2011875)Show SMILES C[C@@H]1C[C@H]1C(=O)Nc1snc(c1C)-c1ccc(F)cc1 |r| Show InChI InChI=1S/C15H15FN2OS/c1-8-7-12(8)14(19)17-15-9(2)13(18-20-15)10-3-5-11(16)6-4-10/h3-6,8,12H,7H2,1-2H3,(H,17,19)/t8-,12-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Allosteric antagonist activity at human recombinant mGluR1 expressed in AV12 cells assessed as intracellular calcium concentration using Fluo-3 dye b... |
Bioorg Med Chem Lett 22: 2514-7 (2012)
Article DOI: 10.1016/j.bmcl.2012.02.003
BindingDB Entry DOI: 10.7270/Q2RX9D3G |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Homo sapiens (Human)) | BDBM50187693

(CHEMBL3186509)Show SMILES Clc1cc(Cl)cc(COC(=O)N2CCN(CCC(=O)c3ccc4[nH]c(=O)oc4c3)CC2)c1 Show InChI InChI=1S/C22H21Cl2N3O5/c23-16-9-14(10-17(24)12-16)13-31-22(30)27-7-5-26(6-8-27)4-3-19(28)15-1-2-18-20(11-15)32-21(29)25-18/h1-2,9-12H,3-8,13H2,(H,25,29) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of autotaxin in healthy human whole blood assessed as reduction in LPA level after 2 hrs by LC-MS/MS analysis |
ACS Med Chem Lett 7: 857-61 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00207
BindingDB Entry DOI: 10.7270/Q2KD22D0 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Metabotropic glutamate receptor 1
(Homo sapiens (Human)) | BDBM50379863
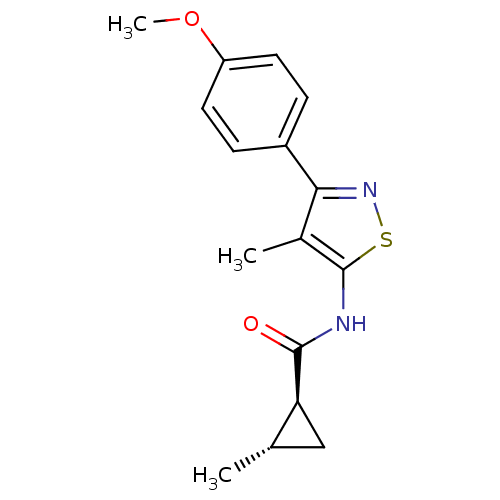
(CHEMBL2011871)Show SMILES COc1ccc(cc1)-c1nsc(NC(=O)[C@H]2C[C@@H]2C)c1C |r| Show InChI InChI=1S/C16H18N2O2S/c1-9-8-13(9)15(19)17-16-10(2)14(18-21-16)11-4-6-12(20-3)7-5-11/h4-7,9,13H,8H2,1-3H3,(H,17,19)/t9-,13-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Allosteric antagonist activity at human recombinant mGluR1 expressed in AV12 cells assessed as intracellular calcium concentration using Fluo-3 dye b... |
Bioorg Med Chem Lett 22: 2514-7 (2012)
Article DOI: 10.1016/j.bmcl.2012.02.003
BindingDB Entry DOI: 10.7270/Q2RX9D3G |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Homo sapiens (Human)) | BDBM50535219

(CHEMBL4446322)Show SMILES O=C(CCCCc1nn[nH]n1)N1CCc2nc(NC3Cc4ccccc4C3)ncc2C1 Show InChI InChI=1S/C22H26N8O/c31-21(8-4-3-7-20-26-28-29-27-20)30-10-9-19-17(14-30)13-23-22(25-19)24-18-11-15-5-1-2-6-16(15)12-18/h1-2,5-6,13,18H,3-4,7-12,14H2,(H,23,24,25)(H,26,27,28,29) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 118 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of autotaxin in healthy human plasma assessed as reduction in LPA level after 3 hrs by mass spectrometric analysis |
ACS Med Chem Lett 7: 857-61 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00207
BindingDB Entry DOI: 10.7270/Q2KD22D0 |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Homo sapiens (Human)) | BDBM50535209
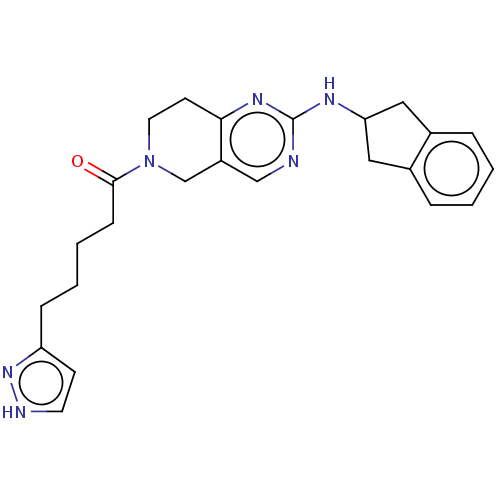
(CHEMBL4456849)Show SMILES O=C(CCCCc1cc[nH]n1)N1CCc2nc(NC3Cc4ccccc4C3)ncc2C1 Show InChI InChI=1S/C24H28N6O/c31-23(8-4-3-7-20-9-11-26-29-20)30-12-10-22-19(16-30)15-25-24(28-22)27-21-13-17-5-1-2-6-18(17)14-21/h1-2,5-6,9,11,15,21H,3-4,7-8,10,12-14,16H2,(H,26,29)(H,25,27,28) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 123 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of recombinant full length human C-terminal His-tagged autotaxin expressed in human 293E cells assessed as choline release using lysophosp... |
ACS Med Chem Lett 7: 857-61 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00207
BindingDB Entry DOI: 10.7270/Q2KD22D0 |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 1
(Homo sapiens (Human)) | BDBM50379870
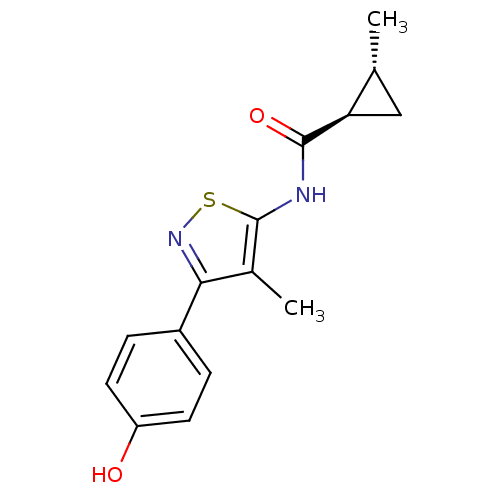
(CHEMBL2011880)Show SMILES C[C@@H]1C[C@H]1C(=O)Nc1snc(c1C)-c1ccc(O)cc1 |r| Show InChI InChI=1S/C15H16N2O2S/c1-8-7-12(8)14(19)16-15-9(2)13(17-20-15)10-3-5-11(18)6-4-10/h3-6,8,12,18H,7H2,1-2H3,(H,16,19)/t8-,12-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 138 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Allosteric antagonist activity at human recombinant mGluR1 expressed in AV12 cells assessed as intracellular calcium concentration using Fluo-3 dye b... |
Bioorg Med Chem Lett 22: 2514-7 (2012)
Article DOI: 10.1016/j.bmcl.2012.02.003
BindingDB Entry DOI: 10.7270/Q2RX9D3G |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 5
(Homo sapiens (Human)) | BDBM50379862

(CHEMBL2011870)Show SMILES COc1ccc(cc1)-c1nsc(NC(=O)[C@@H]2C[C@H]2C)c1C |r| Show InChI InChI=1S/C16H18N2O2S/c1-9-8-13(9)15(19)17-16-10(2)14(18-21-16)11-4-6-12(20-3)7-5-11/h4-7,9,13H,8H2,1-3H3,(H,17,19)/t9-,13-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 158 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Antagonist activity at human mGluR5 |
Bioorg Med Chem Lett 22: 2514-7 (2012)
Article DOI: 10.1016/j.bmcl.2012.02.003
BindingDB Entry DOI: 10.7270/Q2RX9D3G |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 5
(Homo sapiens (Human)) | BDBM50379866

(CHEMBL2011874)Show SMILES COc1ccc(cc1F)-c1nsc(NC(=O)[C@@H]2C[C@H]2C)c1C |r| Show InChI InChI=1S/C16H17FN2O2S/c1-8-6-11(8)15(20)18-16-9(2)14(19-22-16)10-4-5-13(21-3)12(17)7-10/h4-5,7-8,11H,6H2,1-3H3,(H,18,20)/t8-,11-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 159 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Antagonist activity at human mGluR5 |
Bioorg Med Chem Lett 22: 2514-7 (2012)
Article DOI: 10.1016/j.bmcl.2012.02.003
BindingDB Entry DOI: 10.7270/Q2RX9D3G |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50339398
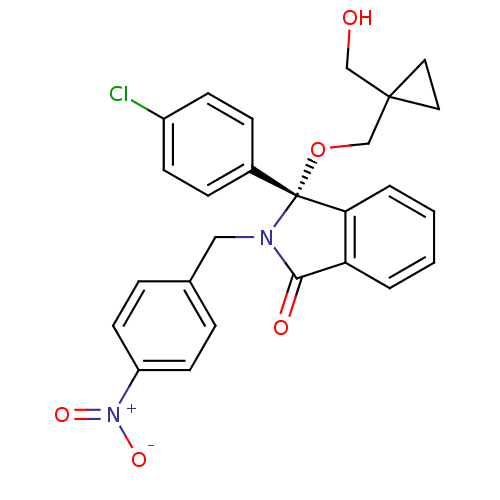
((+)-R-3-(4-Chlorophenyl)-3-(1-hydroxymethylcyclopr...)Show SMILES OCC1(CO[C@]2(N(Cc3ccc(cc3)[N+]([O-])=O)C(=O)c3ccccc23)c2ccc(Cl)cc2)CC1 |r| Show InChI InChI=1S/C26H23ClN2O5/c27-20-9-7-19(8-10-20)26(34-17-25(16-30)13-14-25)23-4-2-1-3-22(23)24(31)28(26)15-18-5-11-21(12-6-18)29(32)33/h1-12,30H,13-17H2/t26-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
Newcastle University
Curated by ChEMBL
| Assay Description
Inhibition of Mdm2 -p53 protein interaction by ELISA |
J Med Chem 54: 1233-43 (2011)
Article DOI: 10.1021/jm1011929
BindingDB Entry DOI: 10.7270/Q29K4BHN |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50339369
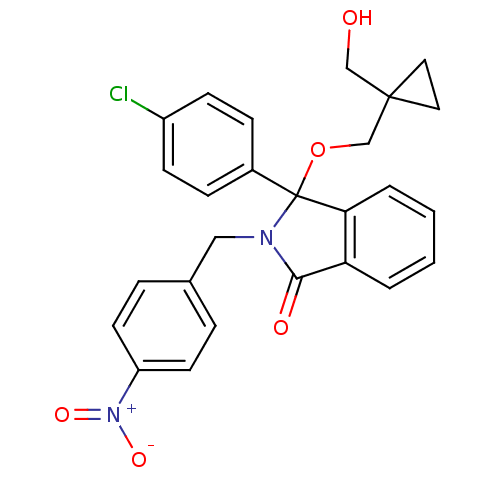
((+/-)-3-(4-chlorophenyl)-3-((1-(hydroxymethyl)cycl...)Show SMILES OCC1(COC2(N(Cc3ccc(cc3)[N+]([O-])=O)C(=O)c3ccccc23)c2ccc(Cl)cc2)CC1 Show InChI InChI=1S/C26H23ClN2O5/c27-20-9-7-19(8-10-20)26(34-17-25(16-30)13-14-25)23-4-2-1-3-22(23)24(31)28(26)15-18-5-11-21(12-6-18)29(32)33/h1-12,30H,13-17H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 230 | n/a | n/a | n/a | n/a | n/a | n/a |
Newcastle University
Curated by ChEMBL
| Assay Description
Inhibition of Mdm2 -p53 protein interaction by ELISA |
J Med Chem 54: 1233-43 (2011)
Article DOI: 10.1021/jm1011929
BindingDB Entry DOI: 10.7270/Q29K4BHN |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Homo sapiens (Human)) | BDBM50535212

(CHEMBL4569141)Show SMILES Clc1ccc(CCNc2ncc3CN(CCc3n2)C(=O)CCc2ccc3[nH]c(=O)oc3c2)cc1 Show InChI InChI=1S/C25H24ClN5O3/c26-19-5-1-16(2-6-19)9-11-27-24-28-14-18-15-31(12-10-20(18)29-24)23(32)8-4-17-3-7-21-22(13-17)34-25(33)30-21/h1-3,5-7,13-14H,4,8-12,15H2,(H,30,33)(H,27,28,29) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 262 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of autotaxin in healthy human plasma assessed as reduction in LPA level after 3 hrs by mass spectrometric analysis |
ACS Med Chem Lett 7: 857-61 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00207
BindingDB Entry DOI: 10.7270/Q2KD22D0 |
More data for this
Ligand-Target Pair | |
Multidrug resistance-associated protein 1
(Homo sapiens (Human)) | BDBM50174852
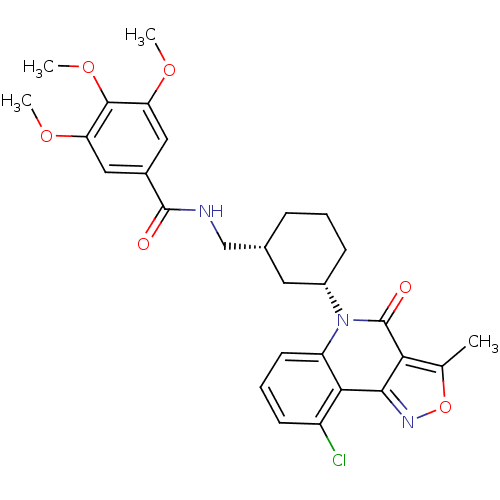
(CHEMBL198648 | rac-N-((3-(9-chloro-3-methyl-4-oxoi...)Show SMILES COc1cc(cc(OC)c1OC)C(=O)NC[C@@H]1CCC[C@@H](C1)n1c2cccc(Cl)c2c2noc(C)c2c1=O Show InChI InChI=1S/C28H30ClN3O6/c1-15-23-25(31-38-15)24-19(29)9-6-10-20(24)32(28(23)34)18-8-5-7-16(11-18)14-30-27(33)17-12-21(35-2)26(37-4)22(13-17)36-3/h6,9-10,12-13,16,18H,5,7-8,11,14H2,1-4H3,(H,30,33)/t16-,18+/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 270 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Inhibitory activity against MRP1-mediated LTC4 uptake into membrane vesicles from HeLa-T5 cells expressing MRP1 |
Bioorg Med Chem Lett 15: 5526-30 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.075
BindingDB Entry DOI: 10.7270/Q2VX0G1X |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50339371

((+/-)-trans-3-(4-Chlorophenyl)-3-(4-hydroxycyclohe...)Show SMILES O[C@H]1CC[C@H](OC2(N(Cc3ccc(cc3)[N+]([O-])=O)C(=O)c3ccccc23)c2ccc(Cl)cc2)C=C1 |r,c:37| Show InChI InChI=1S/C27H23ClN2O5/c28-20-9-7-19(8-10-20)27(35-23-15-13-22(31)14-16-23)25-4-2-1-3-24(25)26(32)29(27)17-18-5-11-21(12-6-18)30(33)34/h1-13,15,22-23,31H,14,16-17H2/t22-,23-,27?/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 310 | n/a | n/a | n/a | n/a | n/a | n/a |
Newcastle University
Curated by ChEMBL
| Assay Description
Inhibition of Mdm2 -p53 protein interaction by ELISA |
J Med Chem 54: 1233-43 (2011)
Article DOI: 10.1021/jm1011929
BindingDB Entry DOI: 10.7270/Q29K4BHN |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50339355

((+/-)-3-(4-Chlorophenyl)-3-(4-hydroxybutoxy)-2-(4-...)Show SMILES OCCCCOC1(N(Cc2ccc(cc2)[N+]([O-])=O)C(=O)c2ccccc12)c1ccc(Cl)cc1 Show InChI InChI=1S/C25H23ClN2O5/c26-20-11-9-19(10-12-20)25(33-16-4-3-15-29)23-6-2-1-5-22(23)24(30)27(25)17-18-7-13-21(14-8-18)28(31)32/h1-2,5-14,29H,3-4,15-17H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 360 | n/a | n/a | n/a | n/a | n/a | n/a |
Newcastle University
Curated by ChEMBL
| Assay Description
Inhibition of Mdm2 -p53 protein interaction by ELISA |
J Med Chem 54: 1233-43 (2011)
Article DOI: 10.1021/jm1011929
BindingDB Entry DOI: 10.7270/Q29K4BHN |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50339365

((+/-)-trans-3-(4-Chlorophenyl)-3-(5-hydroxycyclooc...)Show SMILES O[C@H]1CCC[C@@H](CCC1)OC1(N(Cc2ccc(cc2)[N+]([O-])=O)C(=O)c2ccccc12)c1ccc(Cl)cc1 |r| Show InChI InChI=1S/C29H29ClN2O5/c30-22-15-13-21(14-16-22)29(37-25-7-3-5-24(33)6-4-8-25)27-10-2-1-9-26(27)28(34)31(29)19-20-11-17-23(18-12-20)32(35)36/h1-2,9-18,24-25,33H,3-8,19H2/t24-,25-,29? | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 380 | n/a | n/a | n/a | n/a | n/a | n/a |
Newcastle University
Curated by ChEMBL
| Assay Description
Inhibition of Mdm2 -p53 protein interaction by ELISA |
J Med Chem 54: 1233-43 (2011)
Article DOI: 10.1021/jm1011929
BindingDB Entry DOI: 10.7270/Q29K4BHN |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50339370
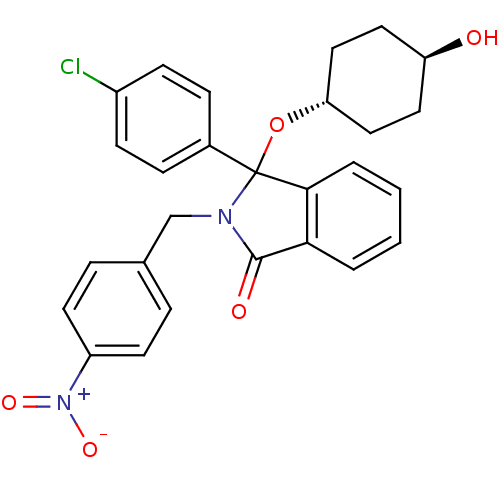
((+/-)-trans-3-(4-Chlorophenyl)-3-(4-hydroxycyclohe...)Show SMILES O[C@H]1CC[C@@H](CC1)OC1(N(Cc2ccc(cc2)[N+]([O-])=O)C(=O)c2ccccc12)c1ccc(Cl)cc1 |r,wU:4.7,wD:1.0,(-9.71,-36.66,;-8.4,-37.46,;-8.44,-39,;-7.12,-39.8,;-5.79,-39.06,;-5.74,-37.53,;-7.05,-36.73,;-4.48,-39.85,;-4.08,-41.35,;-3.16,-42.6,;-1.62,-42.6,;-.85,-43.93,;-1.62,-45.27,;-.86,-46.6,;.69,-46.6,;1.46,-45.26,;.68,-43.93,;1.47,-47.94,;.7,-49.28,;3.01,-47.94,;-4.08,-43.86,;-3.6,-45.32,;-5.55,-43.38,;-6.88,-44.15,;-8.22,-43.38,;-8.22,-41.83,;-6.89,-41.06,;-5.55,-41.82,;-2.76,-40.55,;-1.41,-41.31,;-.09,-40.52,;-.11,-38.97,;1.21,-38.18,;-1.47,-38.23,;-2.79,-39.02,)| Show InChI InChI=1S/C27H25ClN2O5/c28-20-9-7-19(8-10-20)27(35-23-15-13-22(31)14-16-23)25-4-2-1-3-24(25)26(32)29(27)17-18-5-11-21(12-6-18)30(33)34/h1-12,22-23,31H,13-17H2/t22-,23-,27? | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 390 | n/a | n/a | n/a | n/a | n/a | n/a |
Newcastle University
Curated by ChEMBL
| Assay Description
Inhibition of Mdm2 -p53 protein interaction by ELISA |
J Med Chem 54: 1233-43 (2011)
Article DOI: 10.1021/jm1011929
BindingDB Entry DOI: 10.7270/Q29K4BHN |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data