

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Serine/threonine-protein kinase ATR (Homo sapiens (Human)) | BDBM50513177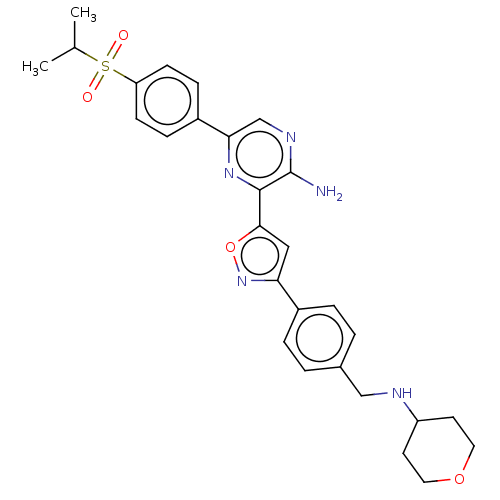 (CHEMBL4444602) | PDB Reactome pathway UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals (Europe) Ltd. Curated by ChEMBL | Assay Description Inhibition of recombinant human full-length N-terminal Flag epitope-tagged ATR expressed in HEK293T cells using ASELPASQPQPFSAKKK peptide as substrat... | J Med Chem 62: 5547-5561 (2019) Article DOI: 10.1021/acs.jmedchem.9b00426 BindingDB Entry DOI: 10.7270/Q2BZ69C9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A/Alpha-1B/Alpha-1D adrenergic receptor (Rattus norvegicus (rat)-Rattus norvegicus (Rat)) | BDBM50227376 (CHEMBL8689) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Binding affinity towards alpha-1 adrenergic receptor from rat brain homogenate preparation by displacement of [3H]prazosin | J Med Chem 31: 1031-5 (1988) BindingDB Entry DOI: 10.7270/Q29W0HPJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase ATR (Homo sapiens (Human)) | BDBM350085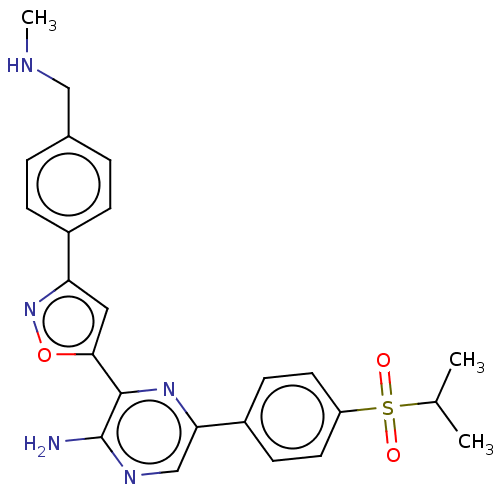 (3-[3-[4-[dideuterio(methylamino)methyl]phenyl]isox...) | PDB Reactome pathway UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 0.165 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals (Europe) Ltd. Curated by ChEMBL | Assay Description Inhibition of recombinant human full-length N-terminal Flag epitope-tagged ATR expressed in HEK293T cells using ASELPASQPQPFSAKKK peptide as substrat... | J Med Chem 62: 5547-5561 (2019) Article DOI: 10.1021/acs.jmedchem.9b00426 BindingDB Entry DOI: 10.7270/Q2BZ69C9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A/Alpha-1B/Alpha-1D adrenergic receptor (Rattus norvegicus (rat)-Rattus norvegicus (Rat)) | BDBM29568 (CHEMBL2 | PRAZOSIN | PRAZOSIN HYDROCHLORIDE | [3H]...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for its binding affinity for alpha-1 adrenergic receptor site by displacement of [3H]clonidine at 10e-6 M concentration | J Med Chem 30: 49-57 (1987) BindingDB Entry DOI: 10.7270/Q24J0HCH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A/Alpha-1B/Alpha-1D adrenergic receptor (Rattus norvegicus (rat)-Rattus norvegicus (Rat)) | BDBM29568 (CHEMBL2 | PRAZOSIN | PRAZOSIN HYDROCHLORIDE | [3H]...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Binding affinity towards alpha-1 adrenergic receptor from rat brain homogenate preparation by displacement of [3H]prazosin | J Med Chem 31: 1031-5 (1988) BindingDB Entry DOI: 10.7270/Q29W0HPJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nischarin (RAT) | BDBM50387827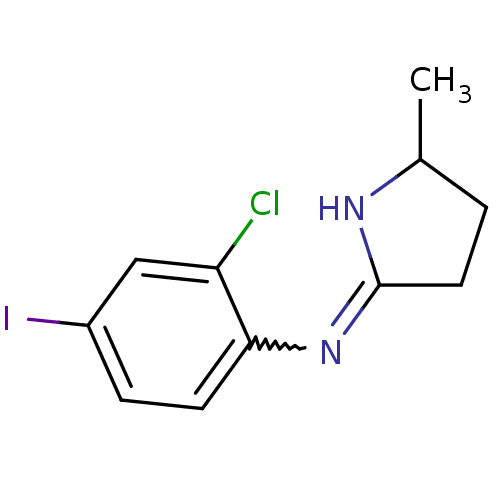 (CHEMBL2058635) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Strasbourg Curated by ChEMBL | Assay Description Displacement of [125I] PIC from I1 imidazoline receptor in rat PC12 cells after 45 mins by gamma counting | J Med Chem 58: 878-87 (2015) Article DOI: 10.1021/jm501456p BindingDB Entry DOI: 10.7270/Q2R21333 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50306153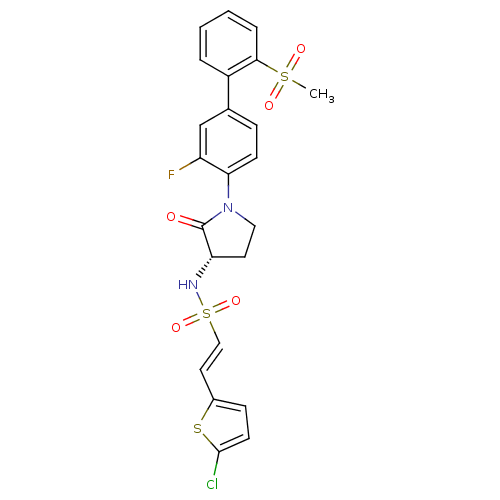 ((S)-2-(5-chlorothiophen-2-yl)-N-(1-(3-fluoro-2'-(m...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human factor 10a by fluorescence assay | Bioorg Med Chem Lett 20: 618-22 (2010) Article DOI: 10.1016/j.bmcl.2009.11.077 BindingDB Entry DOI: 10.7270/Q24M94NN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ITK/TSK (Homo sapiens (Human)) | BDBM50442142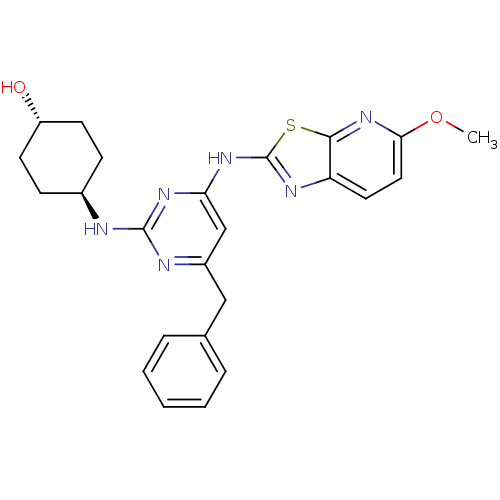 (CHEMBL2441275) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of recombinant human FLAG-tagged ITK using biotinylated GST-SAM68 as substrate after 30 mins | ACS Med Chem Lett 4: 948-52 (2013) Article DOI: 10.1021/ml400206q BindingDB Entry DOI: 10.7270/Q28P61Z7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50228676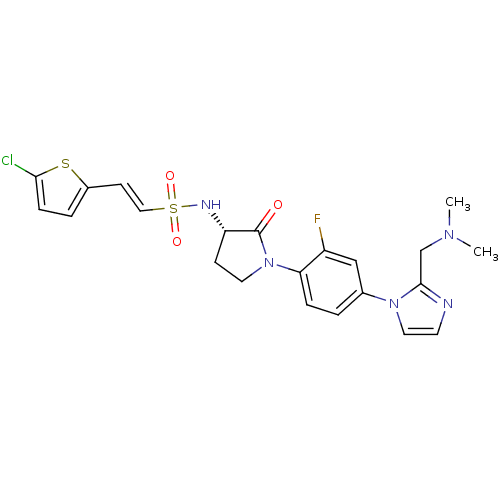 ((S)-2-(5-chlorothiophen-2-yl)-N-(1-(4-(2-((dimethy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human factor 10a by fluorescence assay | Bioorg Med Chem Lett 20: 618-22 (2010) Article DOI: 10.1016/j.bmcl.2009.11.077 BindingDB Entry DOI: 10.7270/Q24M94NN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase ATR (Homo sapiens (Human)) | BDBM420857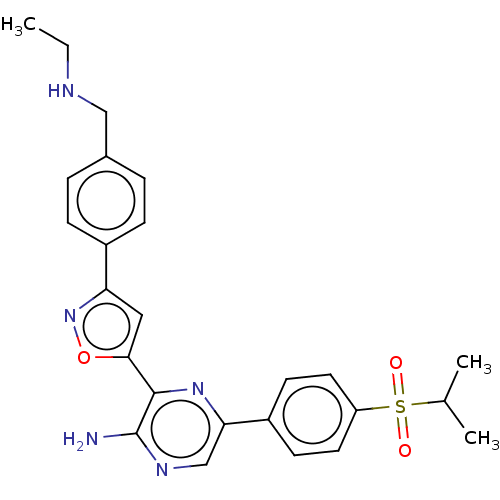 (US10479784, Compound IIA-10 | US10961232, Compound...) | PDB Reactome pathway UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | UniChem | Article PubMed | 0.210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals (Europe) Ltd. Curated by ChEMBL | Assay Description Inhibition of recombinant human full-length N-terminal Flag epitope-tagged ATR expressed in HEK293T cells using ASELPASQPQPFSAKKK peptide as substrat... | J Med Chem 62: 5547-5561 (2019) Article DOI: 10.1021/acs.jmedchem.9b00426 BindingDB Entry DOI: 10.7270/Q2BZ69C9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nischarin (RAT) | BDBM50387827 (CHEMBL2058635) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.219 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Strasbourg Curated by ChEMBL | Assay Description Displacement of [3H]paraiodoclonidine from imidazoline I1 receptor in rat PC12 cells after 30 mins by gamma counter | Bioorg Med Chem 20: 4710-5 (2012) Article DOI: 10.1016/j.bmc.2012.06.008 BindingDB Entry DOI: 10.7270/Q26Q1Z9Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nischarin (RAT) | BDBM50070374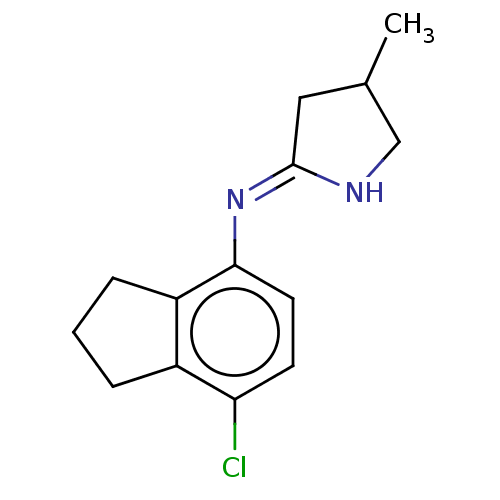 (CHEMBL3408293) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Strasbourg Curated by ChEMBL | Assay Description Displacement of [125I] PIC from I1 imidazoline receptor in rat PC12 cells after 45 mins by gamma counting | J Med Chem 58: 878-87 (2015) Article DOI: 10.1021/jm501456p BindingDB Entry DOI: 10.7270/Q2R21333 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nischarin (Homo sapiens (Human)) | BDBM215802 (US9303019, 8) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.220 | -57.3 | n/a | n/a | n/a | n/a | n/a | 7.35 | 37 |
UNIVERSITE DE STRASBOURG US Patent | Assay Description Binding assays were performed at 37° C. using [125I]LNP 911 as radioligand following the general procedure described but adapted to washed whole ... | US Patent US9303019 (2016) BindingDB Entry DOI: 10.7270/Q2N878NR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ITK/TSK (Homo sapiens (Human)) | BDBM50442143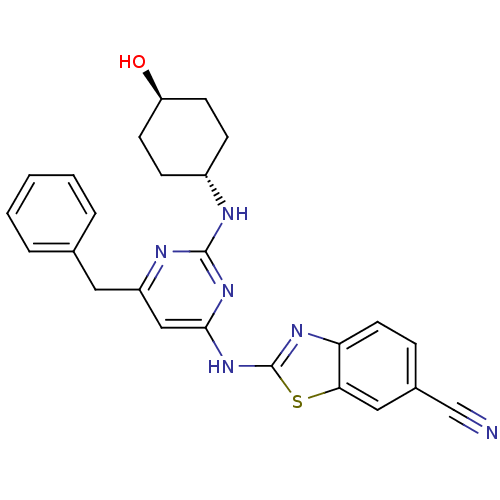 (CHEMBL2441274) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.251 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of recombinant human FLAG-tagged ITK using biotinylated GST-SAM68 as substrate after 30 mins | ACS Med Chem Lett 4: 948-52 (2013) Article DOI: 10.1021/ml400206q BindingDB Entry DOI: 10.7270/Q28P61Z7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ITK/TSK (Homo sapiens (Human)) | BDBM50442141 (CHEMBL2441276) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.251 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of recombinant human FLAG-tagged ITK using biotinylated GST-SAM68 as substrate after 30 mins | ACS Med Chem Lett 4: 948-52 (2013) Article DOI: 10.1021/ml400206q BindingDB Entry DOI: 10.7270/Q28P61Z7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase ATR (Homo sapiens (Human)) | BDBM420852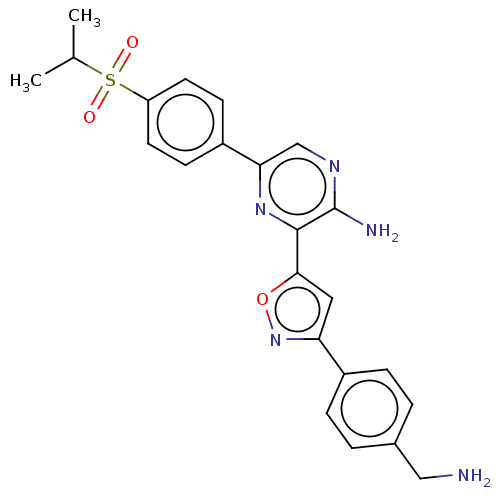 (US10479784, Compound IIA-5 | US10961232, Compound ...) | PDB Reactome pathway UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | UniChem | Article PubMed | 0.270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals (Europe) Ltd. Curated by ChEMBL | Assay Description Inhibition of recombinant human full-length N-terminal Flag epitope-tagged ATR expressed in HEK293T cells using ASELPASQPQPFSAKKK peptide as substrat... | J Med Chem 62: 5547-5561 (2019) Article DOI: 10.1021/acs.jmedchem.9b00426 BindingDB Entry DOI: 10.7270/Q2BZ69C9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A/Alpha-1B/Alpha-1D adrenergic receptor (Rattus norvegicus (rat)-Rattus norvegicus (Rat)) | BDBM50227379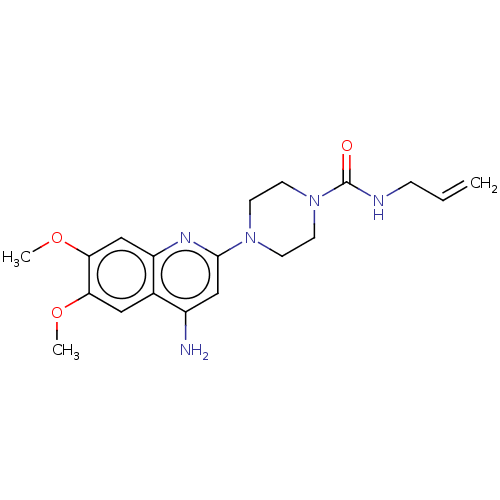 (CHEMBL267428) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Binding affinity towards alpha-1 adrenergic receptor from rat brain homogenate preparation by displacement of [3H]prazosin | J Med Chem 31: 1031-5 (1988) BindingDB Entry DOI: 10.7270/Q29W0HPJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50338686 ((R/S)-3-chloro-N-((3S)-1-(1-(methylamino)-2,3-dihy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of factor 10a activity measured using bis-(CBZ-glycylglycly)-L-arginine amide fluorogenic substrate | Bioorg Med Chem Lett 21: 1582-7 (2011) Article DOI: 10.1016/j.bmcl.2011.01.131 BindingDB Entry DOI: 10.7270/Q28052WG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ITK/TSK (Homo sapiens (Human)) | BDBM50442145 (CHEMBL2441271) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.316 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of recombinant human FLAG-tagged ITK using biotinylated GST-SAM68 as substrate after 30 mins | ACS Med Chem Lett 4: 948-52 (2013) Article DOI: 10.1021/ml400206q BindingDB Entry DOI: 10.7270/Q28P61Z7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A/Alpha-1B/Alpha-1D adrenergic receptor (Rattus norvegicus (rat)-Rattus norvegicus (Rat)) | BDBM50227369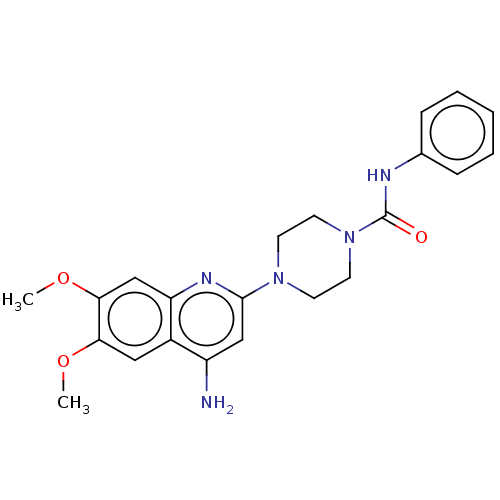 (CHEMBL268416) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Binding affinity towards alpha-1 adrenergic receptor from rat brain homogenate preparation by displacement of [3H]prazosin | J Med Chem 31: 1031-5 (1988) BindingDB Entry DOI: 10.7270/Q29W0HPJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ITK/TSK (Homo sapiens (Human)) | BDBM50442146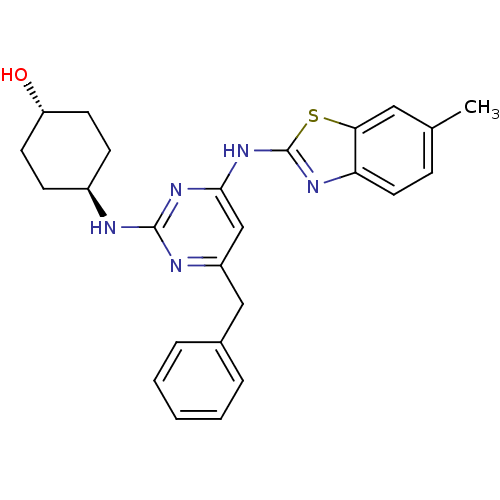 (CHEMBL2441270) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.398 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of recombinant human FLAG-tagged ITK using biotinylated GST-SAM68 as substrate after 30 mins | ACS Med Chem Lett 4: 948-52 (2013) Article DOI: 10.1021/ml400206q BindingDB Entry DOI: 10.7270/Q28P61Z7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A/Alpha-1B/Alpha-1D adrenergic receptor (Rattus norvegicus (rat)-Rattus norvegicus (Rat)) | BDBM50227368 (CHEMBL8603) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Binding affinity towards alpha-1 adrenergic receptor from rat brain homogenate preparation by displacement of [3H]prazosin | J Med Chem 31: 1031-5 (1988) BindingDB Entry DOI: 10.7270/Q29W0HPJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A/Alpha-1B/Alpha-1D adrenergic receptor (Rattus norvegicus (rat)-Rattus norvegicus (Rat)) | BDBM50227374 (CHEMBL8369) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.440 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Binding affinity towards alpha-1 adrenergic receptor from rat brain homogenate preparation by displacement of [3H]prazosin | J Med Chem 31: 1031-5 (1988) BindingDB Entry DOI: 10.7270/Q29W0HPJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A/Alpha-1B/Alpha-1D adrenergic receptor (Rattus norvegicus (rat)-Rattus norvegicus (Rat)) | BDBM50227372 (CHEMBL8585) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Binding affinity towards alpha-1 adrenergic receptor from rat brain homogenate preparation by displacement of [3H]prazosin | J Med Chem 31: 1031-5 (1988) BindingDB Entry DOI: 10.7270/Q29W0HPJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A/Alpha-1B/Alpha-1D adrenergic receptor (Rattus norvegicus (rat)-Rattus norvegicus (Rat)) | BDBM50227373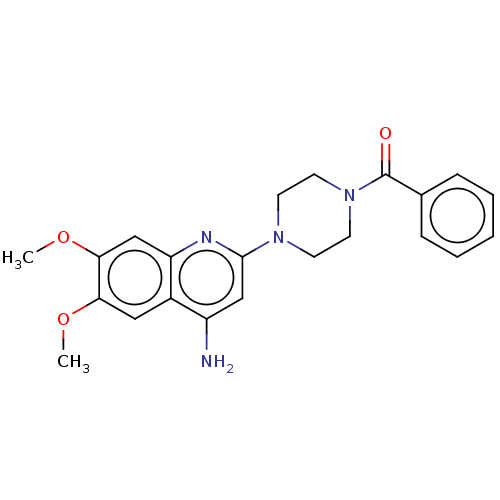 (CHEMBL266119) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.490 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Binding affinity towards alpha-1 adrenergic receptor from rat brain homogenate preparation by displacement of [3H]prazosin | J Med Chem 31: 1031-5 (1988) BindingDB Entry DOI: 10.7270/Q29W0HPJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase B (Homo sapiens (Human)) | BDBM50442149 (CHEMBL2441267) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of Aurora-B (unknown origin) using 5FAM-PKAtide as substrate after 120 mins | ACS Med Chem Lett 4: 948-52 (2013) Article DOI: 10.1021/ml400206q BindingDB Entry DOI: 10.7270/Q28P61Z7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase ATR (Homo sapiens (Human)) | BDBM97372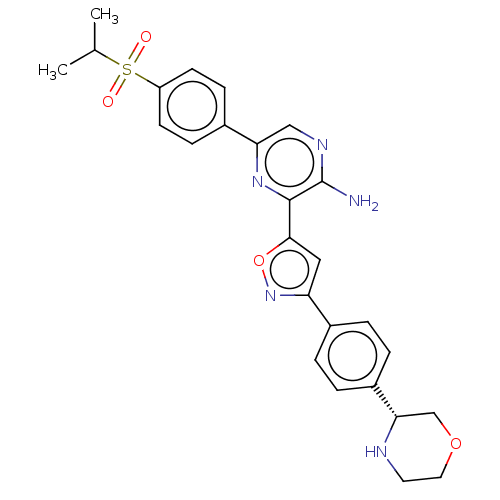 (US20130089624, 1) | PDB Reactome pathway UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.503 | -53.1 | 200 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Vertex Pharmaceuticals US Patent | Assay Description Compounds were screened for their ability to inhibit ATR kinase using a radioactive-phosphate incorporation assay. | US Patent US20130089624 (2013) BindingDB Entry DOI: 10.7270/Q2PZ57DN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase ATR (Homo sapiens (Human)) | BDBM50513173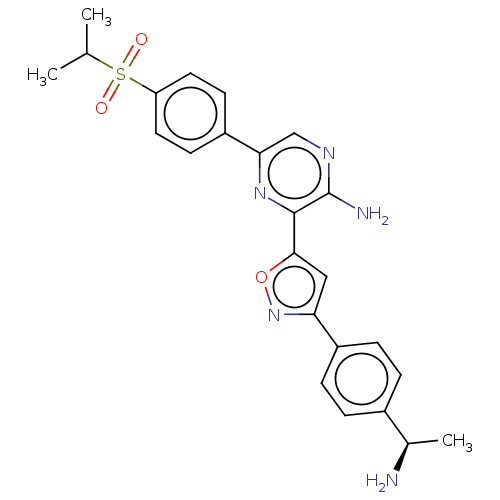 (CHEMBL4566104) | PDB Reactome pathway UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.530 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals (Europe) Ltd. Curated by ChEMBL | Assay Description Inhibition of recombinant human full-length N-terminal Flag epitope-tagged ATR expressed in HEK293T cells using ASELPASQPQPFSAKKK peptide as substrat... | J Med Chem 62: 5547-5561 (2019) Article DOI: 10.1021/acs.jmedchem.9b00426 BindingDB Entry DOI: 10.7270/Q2BZ69C9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase A (Homo sapiens (Human)) | BDBM13534 (CHEMBL572878 | N-[4-({4-[(3-methyl-1H-pyrazol-5-yl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals (Europe) Ltd Curated by ChEMBL | Assay Description Inhibition of Aurora-A by coupled assay | Bioorg Med Chem Lett 19: 3586-92 (2009) Article DOI: 10.1016/j.bmcl.2009.04.136 BindingDB Entry DOI: 10.7270/Q28K7944 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50339708 ((S)-2-(5-chlorothiophen-2-yl)-N-(1-(5-fluoro-1,2,3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of factor 10a using bis-(CBZ-glycylglycly)-L-arginine amide fluorogenic substrate | Bioorg Med Chem Lett 21: 1588-92 (2011) Article DOI: 10.1016/j.bmcl.2011.01.129 BindingDB Entry DOI: 10.7270/Q2RN385P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50339718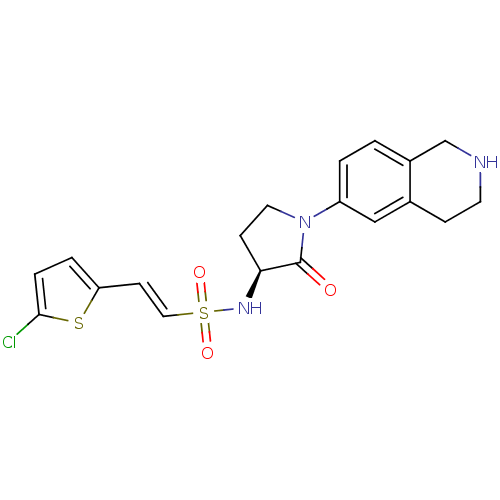 ((S)-2-(5-chlorothiophen-2-yl)-N-(2-oxo-1-(1,2,3,4-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of factor 10a using bis-(CBZ-glycylglycly)-L-arginine amide fluorogenic substrate | Bioorg Med Chem Lett 21: 1588-92 (2011) Article DOI: 10.1016/j.bmcl.2011.01.129 BindingDB Entry DOI: 10.7270/Q2RN385P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A/Alpha-1B/Alpha-1D adrenergic receptor (Rattus norvegicus (rat)-Rattus norvegicus (Rat)) | BDBM50227381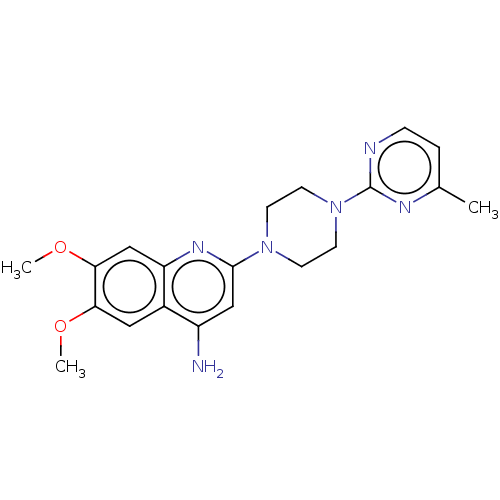 (CHEMBL267804) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.630 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Binding affinity towards alpha-1 adrenergic receptor from rat brain homogenate preparation by displacement of [3H]prazosin | J Med Chem 31: 1031-5 (1988) BindingDB Entry DOI: 10.7270/Q29W0HPJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ITK/TSK (Homo sapiens (Human)) | BDBM50442139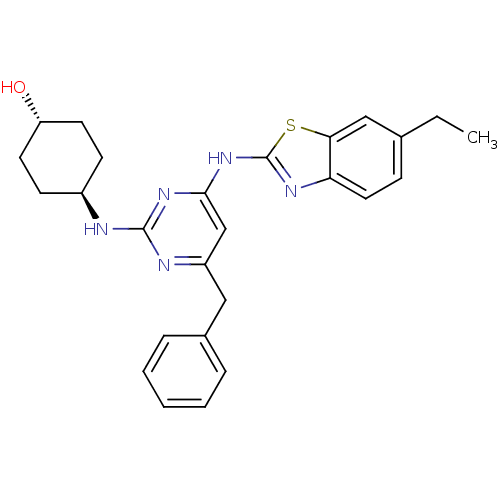 (CHEMBL2441273) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 0.631 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of recombinant human FLAG-tagged ITK using biotinylated GST-SAM68 as substrate after 30 mins | ACS Med Chem Lett 4: 948-52 (2013) Article DOI: 10.1021/ml400206q BindingDB Entry DOI: 10.7270/Q28P61Z7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase ATR (Homo sapiens (Human)) | BDBM50513176 (CHEMBL4448538) | PDB Reactome pathway UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.75 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals (Europe) Ltd. Curated by ChEMBL | Assay Description Inhibition of recombinant human full-length N-terminal Flag epitope-tagged ATR expressed in HEK293T cells using ASELPASQPQPFSAKKK peptide as substrat... | J Med Chem 62: 5547-5561 (2019) Article DOI: 10.1021/acs.jmedchem.9b00426 BindingDB Entry DOI: 10.7270/Q2BZ69C9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50306146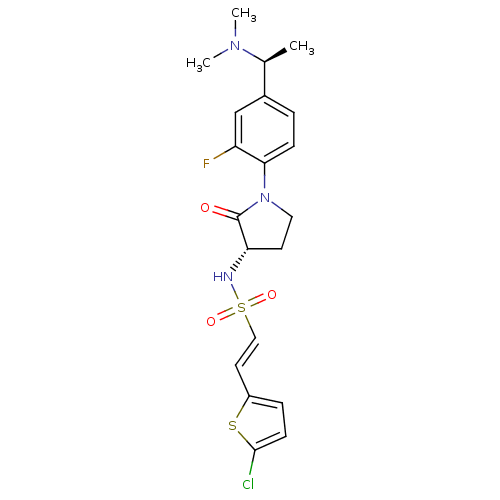 ((E)-2-(5-CHLOROTHIOPHEN-2-YL)-N-[(3S)-1-{4-[(1S)-1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human factor 10a by fluorescence assay | Bioorg Med Chem Lett 20: 618-22 (2010) Article DOI: 10.1016/j.bmcl.2009.11.077 BindingDB Entry DOI: 10.7270/Q24M94NN | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50306143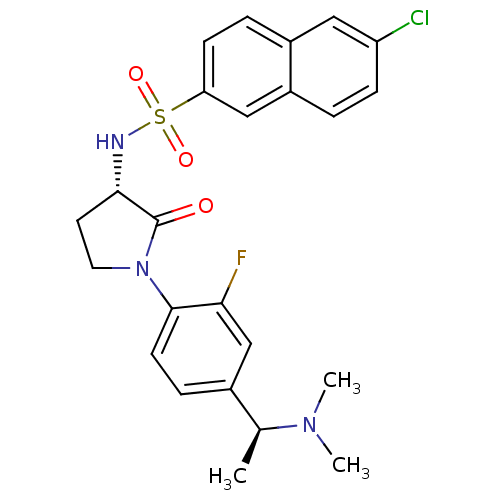 (6-chloro-N-((S)-1-(4-((S)-1-(dimethylamino)ethyl)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human factor 10a by fluorescence assay | Bioorg Med Chem Lett 20: 618-22 (2010) Article DOI: 10.1016/j.bmcl.2009.11.077 BindingDB Entry DOI: 10.7270/Q24M94NN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50306142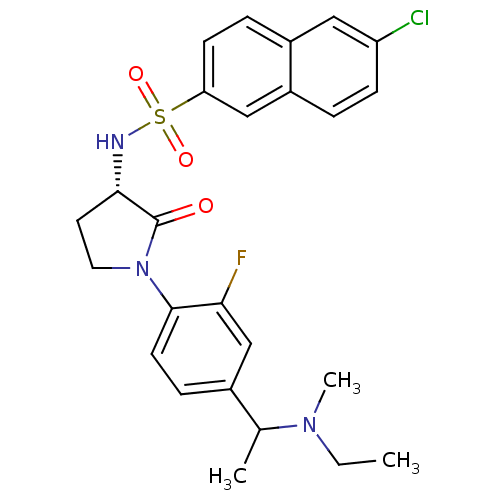 (6-chloro-N-((3S)-1-(4-(1-(ethyl(methyl)amino)ethyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human factor 10a by fluorescence assay | Bioorg Med Chem Lett 20: 618-22 (2010) Article DOI: 10.1016/j.bmcl.2009.11.077 BindingDB Entry DOI: 10.7270/Q24M94NN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50306138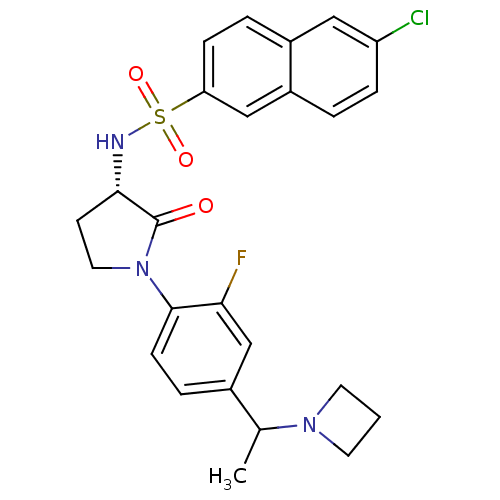 (CHEMBL604887 | N-((3S)-1-(4-(1-(azetidin-1-yl)ethy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human factor 10a by fluorescence assay | Bioorg Med Chem Lett 20: 618-22 (2010) Article DOI: 10.1016/j.bmcl.2009.11.077 BindingDB Entry DOI: 10.7270/Q24M94NN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50339716 ((S)-6-chloro-N-(1-(6-fluoro-2,3,4,5-tetrahydro-1H-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of factor 10a using bis-(CBZ-glycylglycly)-L-arginine amide fluorogenic substrate | Bioorg Med Chem Lett 21: 1588-92 (2011) Article DOI: 10.1016/j.bmcl.2011.01.129 BindingDB Entry DOI: 10.7270/Q2RN385P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50339714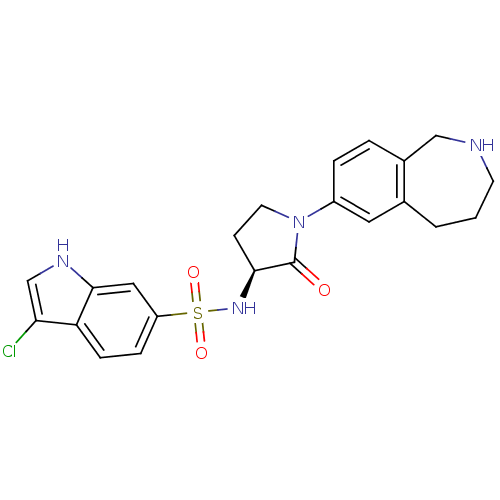 ((S)-3-chloro-N-(2-oxo-1-(2,3,4,5-tetrahydro-1H-ben...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of factor 10a using bis-(CBZ-glycylglycly)-L-arginine amide fluorogenic substrate | Bioorg Med Chem Lett 21: 1588-92 (2011) Article DOI: 10.1016/j.bmcl.2011.01.129 BindingDB Entry DOI: 10.7270/Q2RN385P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50339713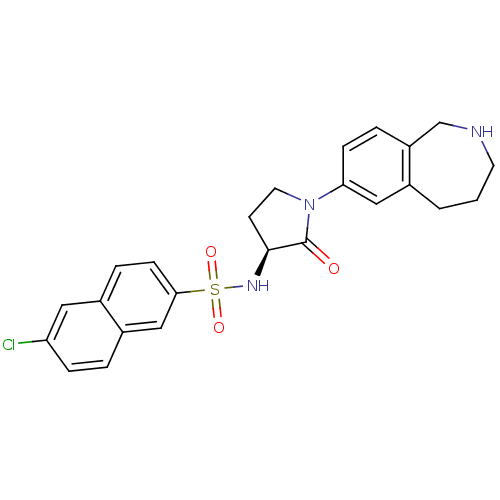 ((S)-6-chloro-N-(2-oxo-1-(2,3,4,5-tetrahydro-1H-ben...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of factor 10a using bis-(CBZ-glycylglycly)-L-arginine amide fluorogenic substrate | Bioorg Med Chem Lett 21: 1588-92 (2011) Article DOI: 10.1016/j.bmcl.2011.01.129 BindingDB Entry DOI: 10.7270/Q2RN385P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase B (Homo sapiens (Human)) | BDBM50277679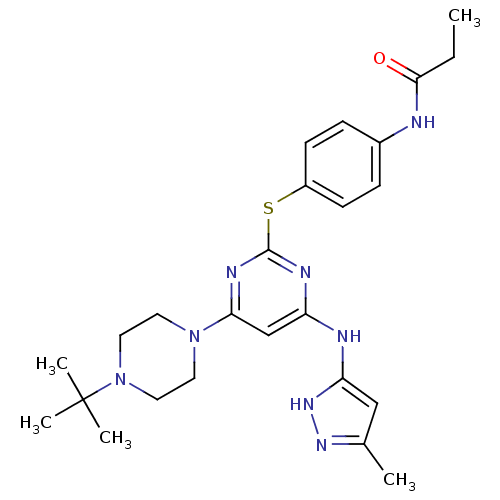 (CHEMBL484006 | N-(4-(4-(4-tert-butylpiperazin-1-yl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals (Europe) Ltd Curated by ChEMBL | Assay Description Inhibition of Aurora-B by time dependent kinetic study | Bioorg Med Chem Lett 19: 3586-92 (2009) Article DOI: 10.1016/j.bmcl.2009.04.136 BindingDB Entry DOI: 10.7270/Q28K7944 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A/Alpha-1B/Alpha-1D adrenergic receptor (Rattus norvegicus (rat)-Rattus norvegicus (Rat)) | BDBM50227380 (CHEMBL8632) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.810 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Binding affinity towards alpha-1 adrenergic receptor from rat brain homogenate preparation by displacement of [3H]prazosin | J Med Chem 31: 1031-5 (1988) BindingDB Entry DOI: 10.7270/Q29W0HPJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nischarin (RAT) | BDBM50387823 (CHEMBL2058631) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.930 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Strasbourg Curated by ChEMBL | Assay Description Displacement of [125I] PIC from I1 imidazoline receptor in rat PC12 cells after 45 mins by gamma counting | J Med Chem 58: 878-87 (2015) Article DOI: 10.1021/jm501456p BindingDB Entry DOI: 10.7270/Q2R21333 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nischarin (RAT) | BDBM50387823 (CHEMBL2058631) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.933 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Strasbourg Curated by ChEMBL | Assay Description Displacement of [3H]paraiodoclonidine from imidazoline I1 receptor in rat PC12 cells after 30 mins by gamma counter | Bioorg Med Chem 20: 4710-5 (2012) Article DOI: 10.1016/j.bmc.2012.06.008 BindingDB Entry DOI: 10.7270/Q26Q1Z9Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ITK/TSK (Homo sapiens (Human)) | BDBM50442147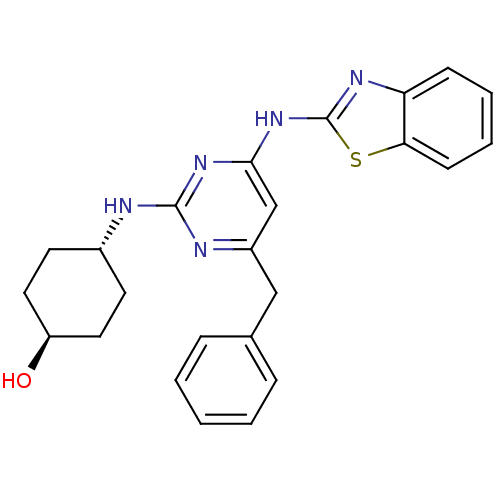 (CHEMBL2441269) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of recombinant human FLAG-tagged ITK using biotinylated GST-SAM68 as substrate after 30 mins | ACS Med Chem Lett 4: 948-52 (2013) Article DOI: 10.1021/ml400206q BindingDB Entry DOI: 10.7270/Q28P61Z7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50339720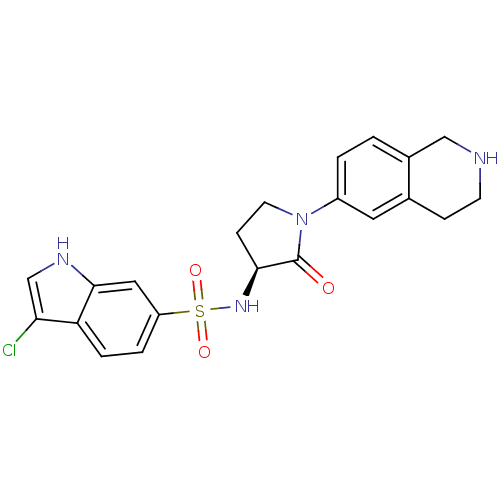 ((S)-3-chloro-N-(2-oxo-1-(1,2,3,4-tetrahydroisoquin...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of factor 10a using bis-(CBZ-glycylglycly)-L-arginine amide fluorogenic substrate | Bioorg Med Chem Lett 21: 1588-92 (2011) Article DOI: 10.1016/j.bmcl.2011.01.129 BindingDB Entry DOI: 10.7270/Q2RN385P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase ATR (Homo sapiens (Human)) | BDBM50513178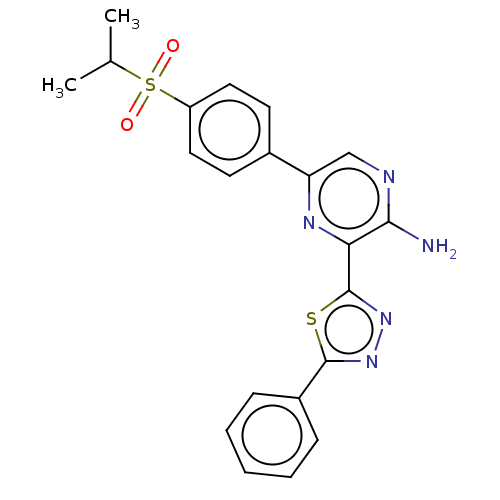 (CHEMBL4434665) | PDB Reactome pathway UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals (Europe) Ltd. Curated by ChEMBL | Assay Description Inhibition of recombinant human full-length N-terminal Flag epitope-tagged ATR expressed in HEK293T cells using ASELPASQPQPFSAKKK peptide as substrat... | J Med Chem 62: 5547-5561 (2019) Article DOI: 10.1021/acs.jmedchem.9b00426 BindingDB Entry DOI: 10.7270/Q2BZ69C9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase A (Homo sapiens (Human)) | BDBM50293671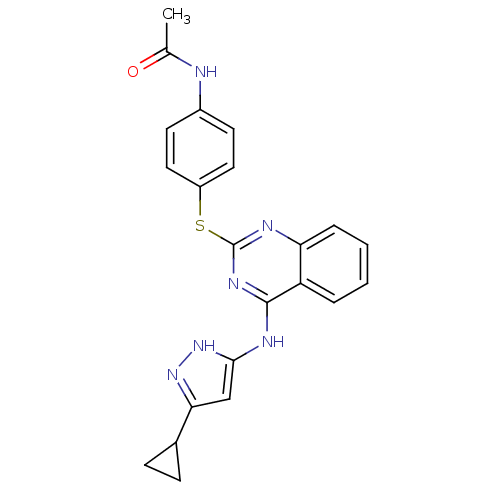 (CHEMBL561421 | N-(4-(4-(5-cyclopropyl-1H-pyrazol-3...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | <1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals (Europe) Ltd Curated by ChEMBL | Assay Description Inhibition of Aurora-A by coupled assay | Bioorg Med Chem Lett 19: 3586-92 (2009) Article DOI: 10.1016/j.bmcl.2009.04.136 BindingDB Entry DOI: 10.7270/Q28K7944 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase A (Homo sapiens (Human)) | BDBM50293699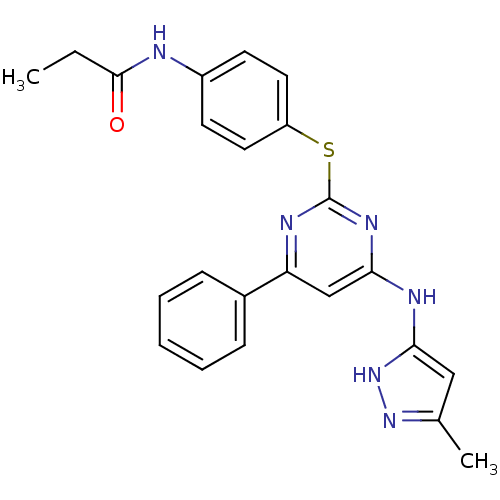 (CHEMBL554941 | N-(4-(4-(5-methyl-1H-pyrazol-3-ylam...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | <1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals (Europe) Ltd Curated by ChEMBL | Assay Description Inhibition of Aurora-A by coupled assay | Bioorg Med Chem Lett 19: 3586-92 (2009) Article DOI: 10.1016/j.bmcl.2009.04.136 BindingDB Entry DOI: 10.7270/Q28K7944 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 3440 total ) | Next | Last >> |