

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM472 (5-tert-butyl-4-{[(6S)-4-hydroxy-6-[2-(4-hydroxyphe...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research | Assay Description Sensitivity of HIV-1 protease activity to protease inhibitors was determined by a peptide substrate cleavage assay. Protease products were analyzed o... | J Med Chem 43: 843-58 (2000) Article DOI: 10.1021/jm990281p BindingDB Entry DOI: 10.7270/Q21N7Z9R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM430 (3-[(2-tert-butyl-4-hydroxy-5-methylphenyl)sulfanyl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research | Assay Description Sensitivity of HIV-1 protease activity to protease inhibitors was determined by a peptide substrate cleavage assay. Protease products were analyzed o... | J Med Chem 43: 843-58 (2000) Article DOI: 10.1021/jm990281p BindingDB Entry DOI: 10.7270/Q21N7Z9R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM430 (3-[(2-tert-butyl-4-hydroxy-5-methylphenyl)sulfanyl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.0300 | -62.5 | n/a | n/a | n/a | n/a | n/a | 6.2 | 37 |
Parke-Davis Pharmaceutical Research | Assay Description For determination of IC50 values, HIV-1 protease was added to assay buffer containing inhibitor and the substrate (H-His-Lys-Ala-Arg-Val-Leu- (p-NO2)... | Bioorg Med Chem 7: 2775-800 (1999) Article DOI: 10.1016/s0968-0896(99)00215-1 BindingDB Entry DOI: 10.7270/Q21C1V2B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50409174 (CHEMBL169119) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research | Assay Description Tested for binding affinity against HIV protease | J Med Chem 43: 843-58 (2000) Article DOI: 10.1021/jm990281p BindingDB Entry DOI: 10.7270/Q21N7Z9R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM472 (5-tert-butyl-4-{[(6S)-4-hydroxy-6-[2-(4-hydroxyphe...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research | Assay Description Tested for binding affinity against HIV protease | J Med Chem 43: 843-58 (2000) Article DOI: 10.1021/jm990281p BindingDB Entry DOI: 10.7270/Q21N7Z9R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM465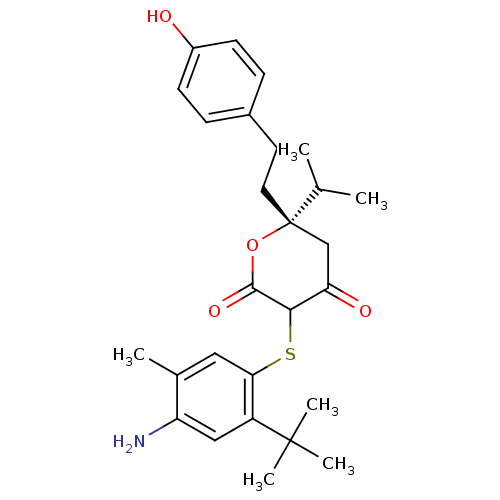 ((6S)-3-[(4-amino-2-tert-butyl-5-methylphenyl)sulfa...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Binding affinity of the compound towards HIV protease was determined | Bioorg Med Chem Lett 9: 1481-6 (1999) BindingDB Entry DOI: 10.7270/Q2668CC2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM465 ((6S)-3-[(4-amino-2-tert-butyl-5-methylphenyl)sulfa...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | 6.2 | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Binding affinity of the compound towards HIV protease was determined | Bioorg Med Chem Lett 9: 1481-6 (1999) BindingDB Entry DOI: 10.7270/Q2668CC2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM465 ((6S)-3-[(4-amino-2-tert-butyl-5-methylphenyl)sulfa...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research | Assay Description Sensitivity of HIV-1 protease activity to protease inhibitors was determined by a peptide substrate cleavage assay. Protease products were analyzed o... | J Med Chem 43: 843-58 (2000) Article DOI: 10.1021/jm990281p BindingDB Entry DOI: 10.7270/Q21N7Z9R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM2208 ((6S)-3-{[2-tert-butyl-4-(hydroxymethyl)-5-methylph...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research | Assay Description For determination of IC50 values, HIV-1 protease was added to assay buffer containing inhibitor and the substrate (H-His-Lys-Ala-Arg-Val-Leu- (p-NO2)... | Bioorg Med Chem 7: 2775-800 (1999) Article DOI: 10.1016/s0968-0896(99)00215-1 BindingDB Entry DOI: 10.7270/Q21C1V2B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50078088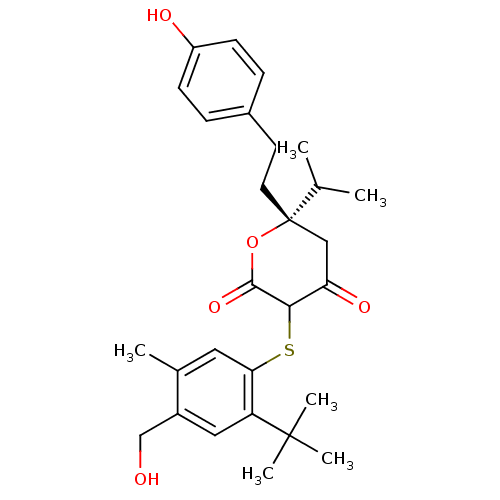 ((S)-3-(2-tert-Butyl-4-hydroxymethyl-5-methyl-pheny...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | 4.7 | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibitory activity of the compound was evaluated against human immunodeficiency virus type 1(HIV-1) protease at pH 4.7 | Bioorg Med Chem Lett 9: 2019-24 (1999) BindingDB Entry DOI: 10.7270/Q2PZ5814 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50078088 ((S)-3-(2-tert-Butyl-4-hydroxymethyl-5-methyl-pheny...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | 6.2 | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description In vitro binding affinity of the compound against HIV protease at pH 6.2 was determined | Bioorg Med Chem Lett 9: 1481-6 (1999) BindingDB Entry DOI: 10.7270/Q2668CC2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM2204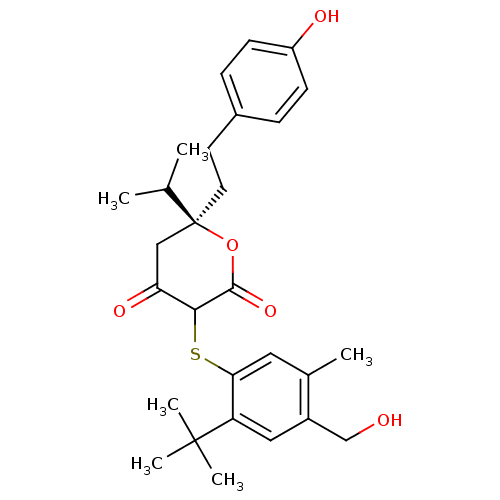 ((3-(2-tert-Butyl-4-hydroxymethyl-5-methyl-phenylsu...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research | Assay Description For determination of IC50 values, HIV-1 protease was added to assay buffer containing inhibitor and the substrate (H-His-Lys-Ala-Arg-Val-Leu- (p-NO2)... | Bioorg Med Chem 7: 2775-800 (1999) Article DOI: 10.1016/s0968-0896(99)00215-1 BindingDB Entry DOI: 10.7270/Q21C1V2B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50216785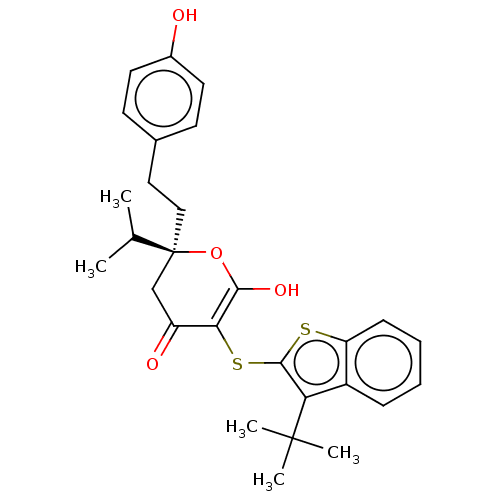 (CHEMBL61756) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | <0.100 | n/a | n/a | n/a | n/a | n/a | n/a | 4.7 | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibitory activity of the compound was evaluated against human immunodeficiency virus type 1(HIV-1) protease at pH 6.2 | Bioorg Med Chem Lett 9: 2019-24 (1999) BindingDB Entry DOI: 10.7270/Q2PZ5814 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM2206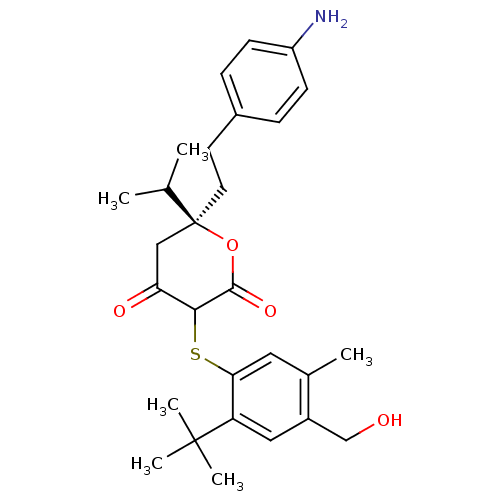 ((6S)-6-[2-(4-aminophenyl)ethyl]-3-{[2-tert-butyl-4...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research | Assay Description For determination of IC50 values, HIV-1 protease was added to assay buffer containing inhibitor and the substrate (H-His-Lys-Ala-Arg-Val-Leu- (p-NO2)... | Bioorg Med Chem 7: 2775-800 (1999) Article DOI: 10.1016/s0968-0896(99)00215-1 BindingDB Entry DOI: 10.7270/Q21C1V2B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50078087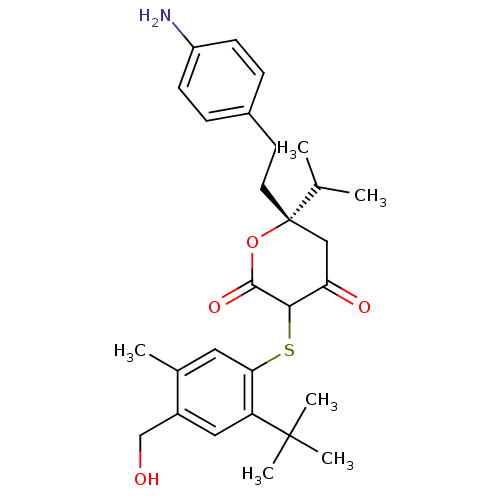 ((S)-6-[2-(4-Amino-phenyl)-ethyl]-3-(2-tert-butyl-4...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | 6.2 | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description In vitro binding affinity of the compound against HIV protease at pH 6.2 was determined | Bioorg Med Chem Lett 9: 1481-6 (1999) BindingDB Entry DOI: 10.7270/Q2668CC2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM2533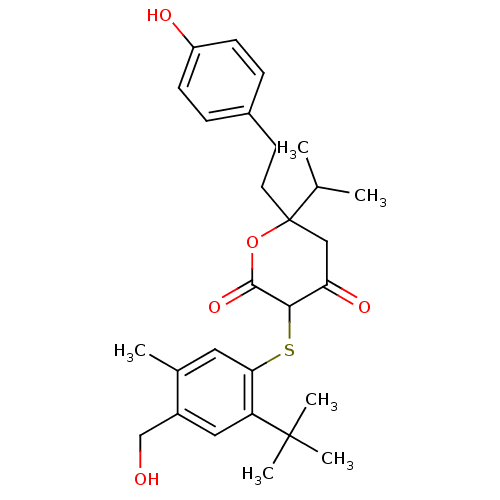 (3-{[2-tert-butyl-4-(hydroxymethyl)-5-methylphenyl]...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.170 | -58.0 | n/a | n/a | n/a | n/a | n/a | 6.2 | 37 |
Parke-Davis Pharmaceutical Research | Assay Description For determination of IC50 values, HIV-1 protease was added to assay buffer containing inhibitor and the substrate (H-His-Lys-Ala-Arg-Val-Leu- (p-NO2)... | Bioorg Med Chem 7: 2775-800 (1999) Article DOI: 10.1016/s0968-0896(99)00215-1 BindingDB Entry DOI: 10.7270/Q21C1V2B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM469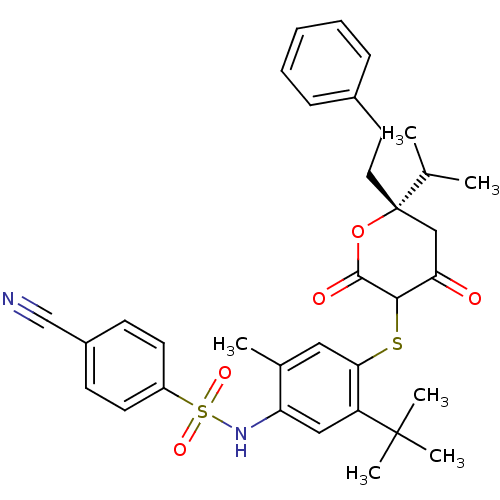 (CHEMBL2110206 | Dihydropyran-2-one deriv. 74 | N-(...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research | Assay Description Tested for binding affinity against HIV protease | J Med Chem 43: 843-58 (2000) Article DOI: 10.1021/jm990281p BindingDB Entry DOI: 10.7270/Q21N7Z9R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM469 (CHEMBL2110206 | Dihydropyran-2-one deriv. 74 | N-(...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research | Assay Description Sensitivity of HIV-1 protease activity to protease inhibitors was determined by a peptide substrate cleavage assay. Protease products were analyzed o... | J Med Chem 43: 843-58 (2000) Article DOI: 10.1021/jm990281p BindingDB Entry DOI: 10.7270/Q21N7Z9R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM402 (CHEMBL354027 | Dihydropyran-2-one deriv. 7 | N-[5-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research | Assay Description Tested for binding affinity against HIV protease | J Med Chem 43: 843-58 (2000) Article DOI: 10.1021/jm990281p BindingDB Entry DOI: 10.7270/Q21N7Z9R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM402 (CHEMBL354027 | Dihydropyran-2-one deriv. 7 | N-[5-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.210 | -57.5 | n/a | n/a | n/a | n/a | n/a | 6.2 | 37 |
Parke-Davis Pharmaceutical Research | Assay Description Sensitivity of HIV-1 protease activity to protease inhibitors was determined by a peptide substrate cleavage assay. Protease products were analyzed o... | J Med Chem 43: 843-58 (2000) Article DOI: 10.1021/jm990281p BindingDB Entry DOI: 10.7270/Q21N7Z9R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50580518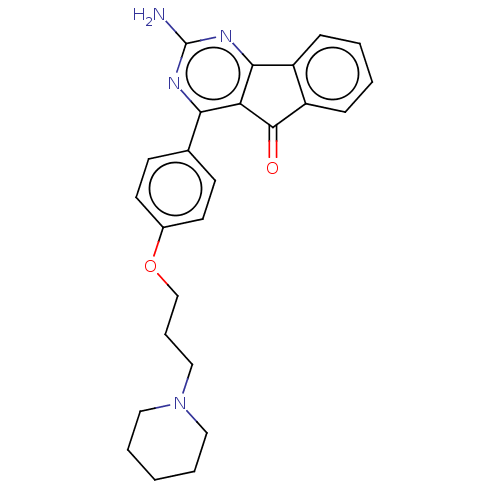 (CHEMBL5089580) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H] N-alpha methylhistamine from human recombinant histamine H3 receptor expressed in HEK293 cells measured after 90 mins | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00914 BindingDB Entry DOI: 10.7270/Q22F7S8G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50247053 (1-(3-(3-(4-chlorophenyl)propoxy)propyl)piperidine ...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College Curated by ChEMBL | Assay Description Inhibition of human H3 receptor | Eur J Med Chem 152: 223-234 (2018) Article DOI: 10.1016/j.ejmech.2018.04.043 BindingDB Entry DOI: 10.7270/Q2697632 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM470 (CHEMBL2110205 | Dihydropyran-2-one deriv. 75 | N-(...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research | Assay Description Sensitivity of HIV-1 protease activity to protease inhibitors was determined by a peptide substrate cleavage assay. Protease products were analyzed o... | J Med Chem 43: 843-58 (2000) Article DOI: 10.1021/jm990281p BindingDB Entry DOI: 10.7270/Q21N7Z9R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50580523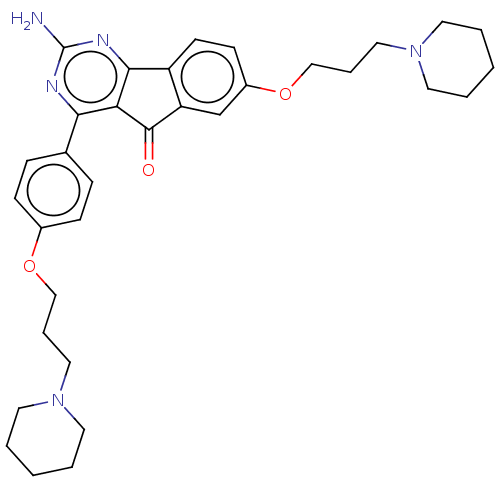 (CHEMBL5093850) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H] N-alpha methylhistamine from human recombinant histamine H3 receptor expressed in HEK293 cells measured after 90 mins | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00914 BindingDB Entry DOI: 10.7270/Q22F7S8G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM467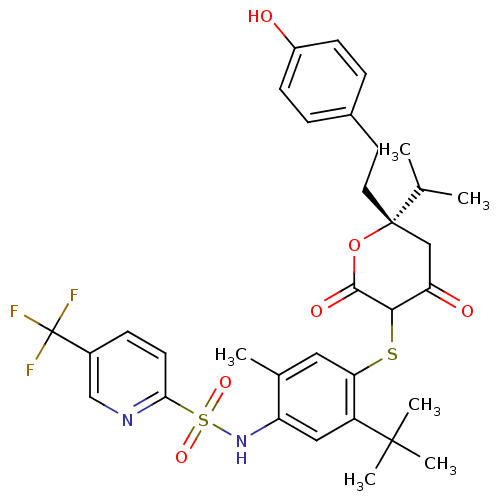 ((S)-N-(5-tert-Butyl-4-{4-hydroxy-6-[2-(4-hydroxyph...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research | Assay Description Tested for binding affinity against HIV protease | J Med Chem 43: 843-58 (2000) Article DOI: 10.1021/jm990281p BindingDB Entry DOI: 10.7270/Q21N7Z9R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM467 ((S)-N-(5-tert-Butyl-4-{4-hydroxy-6-[2-(4-hydroxyph...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research | Assay Description Sensitivity of HIV-1 protease activity to protease inhibitors was determined by a peptide substrate cleavage assay. Protease products were analyzed o... | J Med Chem 43: 843-58 (2000) Article DOI: 10.1021/jm990281p BindingDB Entry DOI: 10.7270/Q21N7Z9R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM403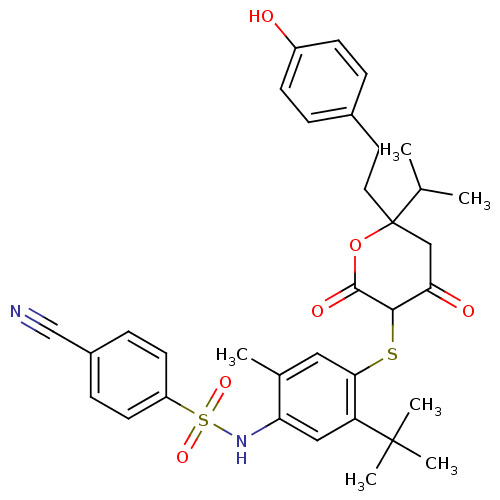 (CHEMBL169391 | Dihydropyran-2-one deriv. 8 | N-[5-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.410 | -55.7 | 2.20 | n/a | n/a | n/a | n/a | 6.2 | 37 |
Parke-Davis Pharmaceutical Research | Assay Description Sensitivity of HIV-1 protease activity to protease inhibitors was determined by a peptide substrate cleavage assay. Protease products were analyzed o... | J Med Chem 43: 843-58 (2000) Article DOI: 10.1021/jm990281p BindingDB Entry DOI: 10.7270/Q21N7Z9R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM403 (CHEMBL169391 | Dihydropyran-2-one deriv. 8 | N-[5-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.410 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research | Assay Description Tested for binding affinity against HIV protease | J Med Chem 43: 843-58 (2000) Article DOI: 10.1021/jm990281p BindingDB Entry DOI: 10.7270/Q21N7Z9R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM2536 (6-Alkyl-6-phenethyldihydropyrone 13y | 6-[2-(4-ami...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research | Assay Description For determination of IC50 values, HIV-1 protease was added to assay buffer containing inhibitor and the substrate (H-His-Lys-Ala-Arg-Val-Leu- (p-NO2)... | Bioorg Med Chem 7: 2775-800 (1999) Article DOI: 10.1016/s0968-0896(99)00215-1 BindingDB Entry DOI: 10.7270/Q21C1V2B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50580512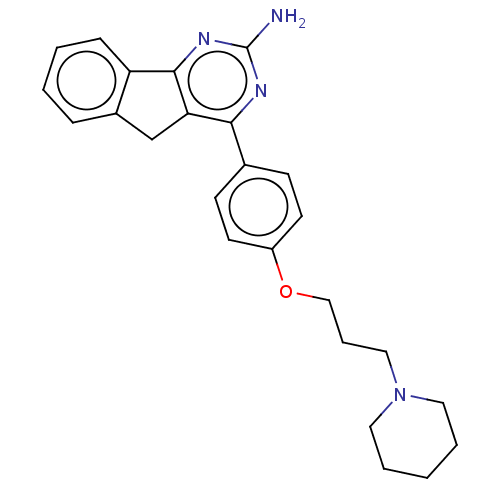 (CHEMBL5072724) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.490 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H] N-alpha methylhistamine from human recombinant histamine H3 receptor expressed in HEK293 cells measured after 90 mins | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00914 BindingDB Entry DOI: 10.7270/Q22F7S8G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM398 (CHEMBL287361 | Dihydropyran-2-one deriv. 3 | N-[5-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.530 | -55.1 | 1.80 | n/a | n/a | n/a | n/a | 6.2 | 37 |
Parke-Davis Pharmaceutical Research | Assay Description Sensitivity of HIV-1 protease activity to protease inhibitors was determined by a peptide substrate cleavage assay. Protease products were analyzed o... | J Med Chem 43: 843-58 (2000) Article DOI: 10.1021/jm990281p BindingDB Entry DOI: 10.7270/Q21N7Z9R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM398 (CHEMBL287361 | Dihydropyran-2-one deriv. 3 | N-[5-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.530 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research | Assay Description Tested for binding affinity against HIV protease | J Med Chem 43: 843-58 (2000) Article DOI: 10.1021/jm990281p BindingDB Entry DOI: 10.7270/Q21N7Z9R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50580514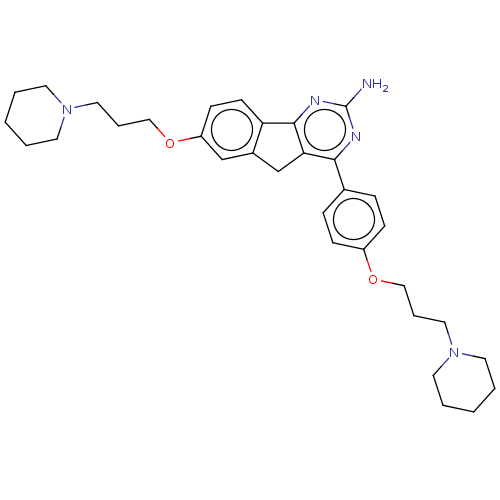 (CHEMBL5093935) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H] N-alpha methylhistamine from human recombinant histamine H3 receptor expressed in HEK293 cells measured after 90 mins | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00914 BindingDB Entry DOI: 10.7270/Q22F7S8G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM396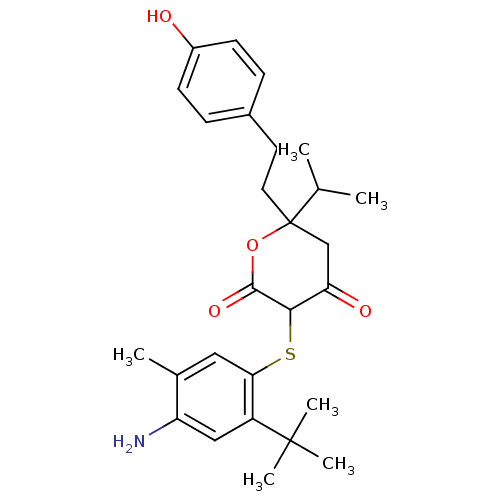 (3-[(4-amino-2-tert-butyl-5-methylphenyl)sulfanyl]-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.670 | -54.5 | 2.70 | n/a | n/a | n/a | n/a | 6.2 | 37 |
Parke-Davis Pharmaceutical Research | Assay Description Sensitivity of HIV-1 protease activity to protease inhibitors was determined by a peptide substrate cleavage assay. Protease products were analyzed o... | J Med Chem 43: 843-58 (2000) Article DOI: 10.1021/jm990281p BindingDB Entry DOI: 10.7270/Q21N7Z9R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM396 (3-[(4-amino-2-tert-butyl-5-methylphenyl)sulfanyl]-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.670 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research | Assay Description Tested for binding affinity against HIV protease | J Med Chem 43: 843-58 (2000) Article DOI: 10.1021/jm990281p BindingDB Entry DOI: 10.7270/Q21N7Z9R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM396 (3-[(4-amino-2-tert-butyl-5-methylphenyl)sulfanyl]-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.670 | n/a | n/a | n/a | n/a | n/a | n/a | 6.2 | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Binding affinity of the compound towards HIV protease was determined | Bioorg Med Chem Lett 9: 1481-6 (1999) BindingDB Entry DOI: 10.7270/Q2668CC2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM464 (5-tert-butyl-4-{[(6R)-4-hydroxy-2-oxo-6-(2-phenyle...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research | Assay Description Sensitivity of HIV-1 protease activity to protease inhibitors was determined by a peptide substrate cleavage assay. Protease products were analyzed o... | J Med Chem 43: 843-58 (2000) Article DOI: 10.1021/jm990281p BindingDB Entry DOI: 10.7270/Q21N7Z9R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM464 (5-tert-butyl-4-{[(6R)-4-hydroxy-2-oxo-6-(2-phenyle...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research | Assay Description Tested for binding affinity against HIV protease | J Med Chem 43: 843-58 (2000) Article DOI: 10.1021/jm990281p BindingDB Entry DOI: 10.7270/Q21N7Z9R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM421 (CHEMBL169849 | Dihydropyran-2-one deriv. 26 | N-(5...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.830 | -53.9 | n/a | n/a | n/a | n/a | n/a | 6.2 | 37 |
Parke-Davis Pharmaceutical Research | Assay Description Sensitivity of HIV-1 protease activity to protease inhibitors was determined by a peptide substrate cleavage assay. Protease products were analyzed o... | J Med Chem 43: 843-58 (2000) Article DOI: 10.1021/jm990281p BindingDB Entry DOI: 10.7270/Q21N7Z9R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM421 (CHEMBL169849 | Dihydropyran-2-one deriv. 26 | N-(5...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.830 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research | Assay Description Tested for binding affinity against HIV protease | J Med Chem 43: 843-58 (2000) Article DOI: 10.1021/jm990281p BindingDB Entry DOI: 10.7270/Q21N7Z9R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50202986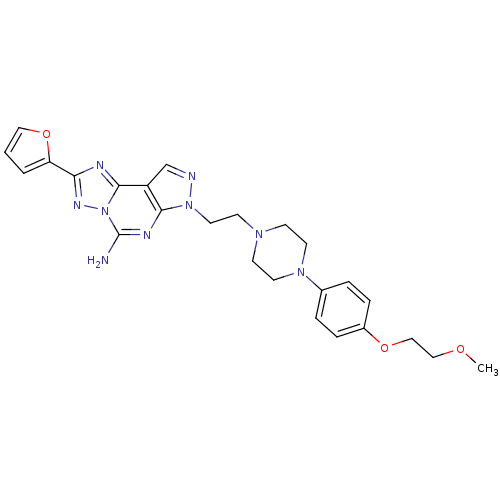 (2-furan-2-yl-7-(2-{4-[4-(2-methoxy-ethoxy)-phenyl]...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.880 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]MSX-2 from human recombinant adenosine A2A receptor expressed in CHO cells by liquid scintillation counting analysis | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00914 BindingDB Entry DOI: 10.7270/Q22F7S8G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM429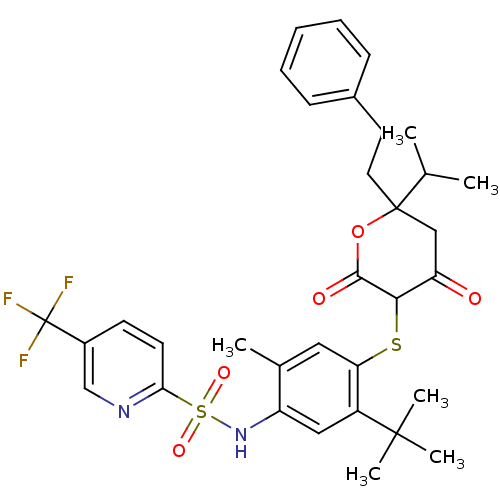 (Dihydropyran-2-one deriv. 34 | N-(5-tert-butyl-4-{...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.04 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research | Assay Description Sensitivity of HIV-1 protease activity to protease inhibitors was determined by a peptide substrate cleavage assay. Protease products were analyzed o... | J Med Chem 43: 843-58 (2000) Article DOI: 10.1021/jm990281p BindingDB Entry DOI: 10.7270/Q21N7Z9R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM448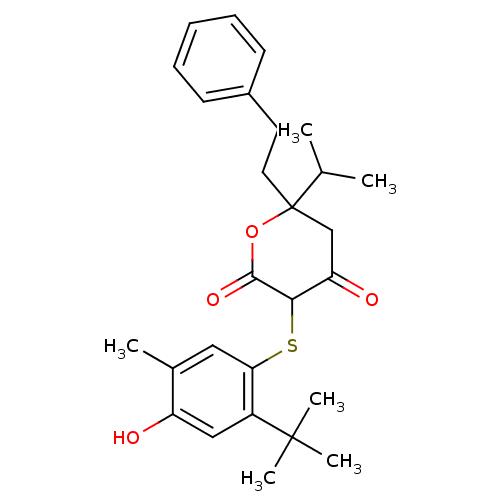 (3-[(2-tert-butyl-4-hydroxy-5-methylphenyl)sulfanyl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research | Assay Description For determination of IC50 values, HIV-1 protease was added to assay buffer containing inhibitor and the substrate (H-His-Lys-Ala-Arg-Val-Leu- (p-NO2)... | Bioorg Med Chem 7: 2775-800 (1999) Article DOI: 10.1016/s0968-0896(99)00215-1 BindingDB Entry DOI: 10.7270/Q21C1V2B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM448 (3-[(2-tert-butyl-4-hydroxy-5-methylphenyl)sulfanyl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research | Assay Description Sensitivity of HIV-1 protease activity to protease inhibitors was determined by a peptide substrate cleavage assay. Protease products were analyzed o... | J Med Chem 43: 843-58 (2000) Article DOI: 10.1021/jm990281p BindingDB Entry DOI: 10.7270/Q21N7Z9R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM448 (3-[(2-tert-butyl-4-hydroxy-5-methylphenyl)sulfanyl...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research | Assay Description Tested for binding affinity against HIV protease | J Med Chem 43: 843-58 (2000) Article DOI: 10.1021/jm990281p BindingDB Entry DOI: 10.7270/Q21N7Z9R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM423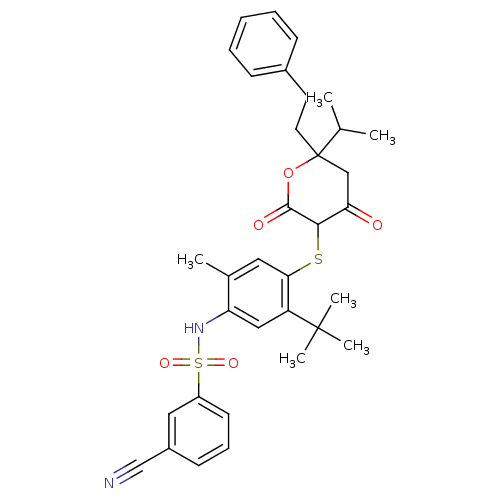 (CHEMBL263028 | Dihydropyran-2-one deriv. 28 | N-(5...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research | Assay Description Tested for binding affinity against HIV protease | J Med Chem 43: 843-58 (2000) Article DOI: 10.1021/jm990281p BindingDB Entry DOI: 10.7270/Q21N7Z9R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM423 (CHEMBL263028 | Dihydropyran-2-one deriv. 28 | N-(5...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.40 | -52.6 | n/a | n/a | n/a | n/a | n/a | 6.2 | 37 |
Parke-Davis Pharmaceutical Research | Assay Description Sensitivity of HIV-1 protease activity to protease inhibitors was determined by a peptide substrate cleavage assay. Protease products were analyzed o... | J Med Chem 43: 843-58 (2000) Article DOI: 10.1021/jm990281p BindingDB Entry DOI: 10.7270/Q21N7Z9R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50458224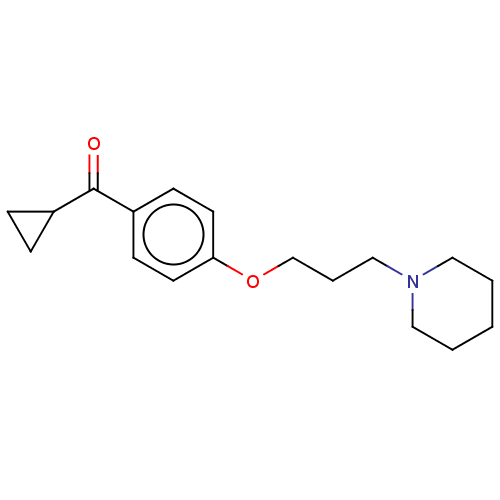 (CHEMBL2297871) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Heinrich Heine University Duesseldorf Curated by ChEMBL | Assay Description Displacement of [125I]iodoproxyfan from human H3R expressed in CHOK1 cell membranes after 60 mins by gamma counting method | Eur J Med Chem 148: 487-497 (2018) Article DOI: 10.1016/j.ejmech.2018.02.015 BindingDB Entry DOI: 10.7270/Q2Z89G1Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM22914 (CHEMBL260374 | N-cyclohexyl-4-(1H-imidazol-5-yl)pi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College Curated by ChEMBL | Assay Description Displacement of [3H]-(R)alpha-MeHA from rat brain H3 receptor | Bioorg Med Chem 26: 2573-2585 (2018) Article DOI: 10.1016/j.bmc.2018.04.023 BindingDB Entry DOI: 10.7270/Q2TQ641P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50458219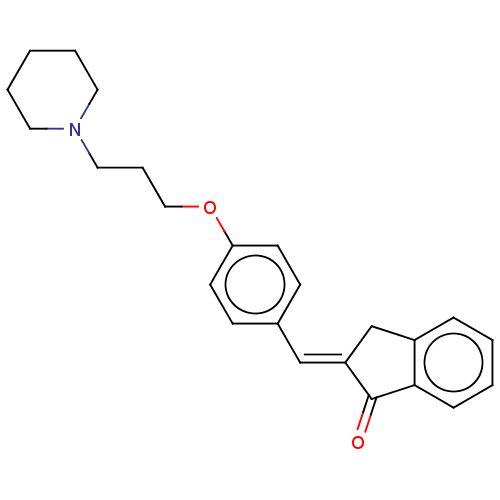 (CHEMBL4209497) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Heinrich Heine University Duesseldorf Curated by ChEMBL | Assay Description Displacement of [3H]-N-alpha-methylhistamine from human H3R expressed in HEK293 cell membranes after 90 mins by liquid scintillation counting method | Eur J Med Chem 148: 487-497 (2018) Article DOI: 10.1016/j.ejmech.2018.02.015 BindingDB Entry DOI: 10.7270/Q2Z89G1Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1013 total ) | Next | Last >> |